ABSTRACT
Environmental conditions play crucial roles in modulating immunity and disease in plants. For instance, many bacterial disease outbreaks occur after periods of high humidity and rain. A critical step in bacterial infection is entry into the plant interior through wounds or natural openings, such as stomata. Bacterium-triggered stomatal closure is an integral part of the plant immune response to reduce pathogen invasion. Recently, we found that high humidity compromises stomatal defense, which is accompanied by regulation of the salicylic acid and jasmonic acid pathways in guard cells. Periods of darkness, when most stomata are closed, are effective in decreasing pathogen penetration into leaves. However, coronatine produced by Pseudomonas syringae pv. tomato (Pst) DC3000 cells can open dark-closed stomata facilitating infection. Thus, a well-known disease-promoting environmental condition (high humidity) acts in part by suppressing stomatal defense, whereas an anti-stomatal defense factor such as coronatine, may provide epidemiological advantages to ensure bacterial infection when environmental conditions (darkness and insufficient humidity) favor stomatal defense.
KEYWORDS: Abiotic stress, abscisic acid, air relative humidity, Arabidopsis, bacterial diseases, biotic stress, darkness, hormone balance, jasmonate, salicylic acid
Plant disease is a successful culmination of three important factors viz. high pathogen virulence, ineffective plant immunity, and favorable environmental conditions. This central dogma of plant pathology is a 50-year-old concept of the disease triangle1 and is relevant in all aspects of plant-pathogen interactions2. Environmental abiotic factors such as relative humidity (RH) and light conditions have a drastic effect on prevalence of disease in different geographical regions. Plants need to adapt to simultaneous exposure to variable biotic and abiotic stresses, sometimes with opposing effects, for maintenance of healthy whole plant physiology. For instance, high disease incidence can be explained by the occurrence of climatic conditions that favor pathogen growth and weaken the plant immune system3. It is well known that the outbreak of late blight of potato caused by Phytophthora infestans that lead to the unfortunate Irish potato famine of 1845 was initiated and spread rapidly mainly because of the unusually wet and cool climatic conditions chronicled for that year2. Still, current knowledge on the molecular basis of environment-mediated regulation of plant responses to pathogens is still in its infancy. Moreover, we have gathered evidence that different cell types (e.g., guard cell and mesophyll cell) may have variable molecular responses to the same environmental condition3 adding additional levels of complexity in plant immune responses.
Plant immune system consists of a complex network of signals tuned to respond to specific types of biotic stresses. One of the first outputs of pattern-triggered immunity (PTI) consists of stomatal defense4. The microscopic stomatal pores in the leaves are important not only for transpiration and exchange of gases, but also as entry points for some pathogenic microbes, which otherwise could not transit from the phylloplane to the leaf apoplast. However, recognition of microbe-associated molecular patterns (MAMPs) by plant pattern-recognition receptors (PRRs) is a signal to close stomata that serve as guarding gates against microbe invasion5. A rapid (< 2h) bacterium-triggered stomatal closure is also observed when the plant perceives non-pathogens such as Escherichia coli, Salmonella enterica, and Bacillus subtilis4,6-8.
Molecular mechanisms underlying stomatal defense have been studied mostly in the Arabidopsis-Pst pathosystem. This well-studied system has been very useful to decipher both stomatal defense and counter-defense mainly due to the initial PTI response and subsequent induction of coronatine production in the bacterium that overrides PTI9,10. This temporal response in the Arabidopsis guard cell is mediated by phytohormones5. For instance, abscisic acid (ABA), salicylic acid (SA), and jasmonic acid (JA) play important roles in guard cell signaling during Arabidopsis/P. syringae interaction.
Endogenous ABA and SA are important for stomatal closure in response to bacteria or purified MAMPs4,11-17. By contrast, strong evidence suggests that, similar to its structural and functional mimic coronatine, jasmonoyl-L-isoleucine (JA-Ile) mediates stomatal opening3,18. Intriguingly, control of stomatal movement by air RH also seems to operate through hormone signaling. As an example, low RH induced-stomatal closure is associated with ABA biosynthesis19, whereas activation of stomatal opening by high RH is associated with ABA catabolism20. However, we have found that exogenous treatment of ABA does not close stomata to the full extent under high RH as compared with plants at moderate RH3. This finding indicates that while ABA has a prominent role in RH-mediated stomatal movement, it does not seem to be the only target of high RH in guard cells.
Previously, SA-dependent phenotypes have also been shown to be suppressed under high RH21, including the suppression of SA-dependent activation of PR genes in Arabidopsis leaves at 24 h after shifting plants to high RH22. As SA signaling is required for stomatal closure4,13, we performed guard cell-specific analysis and determined that high RH also repressed the expression of PR1 gene in this cell type3 (Fig. 1). On the other hand, JA-responsive genes are upregulated in guard cells within 1h of plant exposure to high RH3. However, this regulation is independent of the JA-Ile receptor, COI1. COI1-independent and JA-dependent signaling pathway has been previously proposed and induction of some JAZ genes in coi1 plants has been reported when Arabidopsis leaves are infected with Sclerotinia sclerotiorum23. In addition, P. syringae pv. maculicola ES4326 infection in coi1–1 plants also leads to induction of JA-regulated genes, indicating that JA response can be activated downstream or independent of COI124. Moreover, an effector from Pst DC3000, HopX1 triggers degradation of JAZ proteins in a COI1-independent manner and promotes stomatal opening25. Consistent with this, we observed that the JA biosynthesis genes, LOX3 and OPR3 are repressed within 1 h of exposure to high RH3. This finding suggests that JA-Ile replenishment may not be required as the signaling occurs independent of COI1 in guard cells. Specific branches of the SA and JA signaling pathways regulated by RH are yet to be determined.
Figure 1.
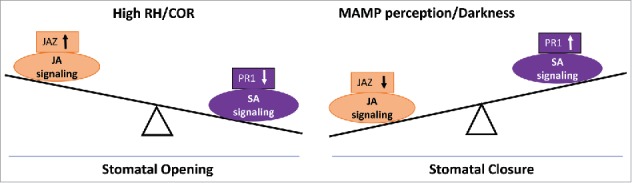
A simplified model depicting distinct modulation of JA and SA signaling pathways by different external factors in guard cells. Left: stomatal defense is reduced under high air relative humidity (RH) or in the presence of the phytotoxin coronatine by repressing SA signaling and activation of JA signaling. Right: stomatal defense is enhanced in natural conditions (darkness) or induced by MAMPs, which is accompanied by upregulation of SA signaling and downregulation of JA signaling.
In several circumstances, JA and SA act antagonistically and some key regulators in this crosstalk have been identified. SA inhibits JA signaling through the regulatory protein, NONEXPRESSOR OF PR GENES 1 (NPR1)26. By contrast, JA and coronatine inhibit SA biosynthesis genes (isochorismate synthase, ICS1) and activate SA degradation genes (benzoic acid/SA carboxyl methyltransferase 1, BSMT1) through 3 NAC transcription factors, ANAC019, ANAC055, and ANAC07227. However, we observed that both activation of JA and suppression of SA occur simultaneously in guard cells of plants exposed to high RH3 and hence these pathways are likely to be regulated independently by RH. Guard cell response to RH is much quicker (< 1h) than that of whole leaves (> 8h) suggesting the existence of an independent regulation of guard cell signaling by RH. However, it is possible that JA/SA antagonism exist in guard cell under high RH at a step downstream of the signaling components tested so far, which still needs further investigation. Based on current evidence, we propose that the shift of balance between SA and JA signaling leads to repression of bacterium-triggered stomatal closure and consequently bacteria that are otherwise unable to overcome PTI can still penetrate leaf tissue under high RH (Fig. 1).
High humidity also promotes rapid proliferation of bacteria in the epiphytic phase28. However, in general, phyllosphere is a water-limiting environment29 that imposes a challenge for epiphytic survival of pathogens in this niche. To counter this challenge, bacteria produce extracellular polymeric substances (EPS) to maintain hydration and form aggregates on the leaf surface30,31. High humidity positively affects such aggregate formation of P. syringae pv syringae B728a on bean leaf surface and aids in rapid proliferation of the bacteria and subsequent entry into the endophytic phase30. To maintain epiphytic fitness, virulent bacteria can physically alter the wettability of the leaf surface by producing biosurfactants32,33. Furthermore, bacterial-dependency on high RH to establish apoplastic infection while suppressing host immunity has also been demonstrated recently34. These observations emphasize that RH participates in multiple steps of molecular plant-pathogen interaction and influences its outcome.
In contrast to high RH that aids plant susceptibility and counteracts stomatal defense, several other abiotic factors may favor a robust stomatal defense. In particular, absence of light may lead to stomatal closure; indeed, most stomata of C3 and C4 plants are closed at night. This suggests that bacterial penetration of leaves through stomata would be minimal at night. Interestingly, the clock proteins CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) not only control the circadian stomatal movement, but they are also required for flagellin-mediated immune response35. Disruption of the clock activity through CCA1 and LHY resulted in stomata that are less responsive to dark and P. syringae pv. maculicola, thus rendering Arabidopsis plants more susceptible to infection at night. Furthermore, surface-inoculated plants, but not syringe-infiltrated plants, are more resistant to bacterium infection at dusk than at dawn35. These findings mechanistically link stomatal defense and the circadian clock.
Interestingly, the levels of the two most well-known hormones associated with biotic stress, JA and SA, naturally oscillate throughout a 24 h cycle. While the JA level peaks in the daytime, the SA level is highest during the night in whole leaves36,37. These oscillations are under the control of the clock and several clock-associated proteins37. If the JA/SA hormone balance determines the opening and closing of stomata (Fig. 1), then one would assume that inducing JA signaling at night could promote stomatal opening. Previously, others and we have determined that coronatine, a molecular mimic of JA-Ile, overcomes bacterium-triggered stomatal closure by upregulating JA signaling and repressing SA signaling4,38. Consistently, Pst DC3000 senses the leaf surface, produces coronatine, and opens dark-closed stomata39. It remains to be determined whether coronatine disrupts the natural guard cell circadian movement by actively suppressing CCA and LHY1 mediated signaling. Nonetheless, it is evident that a stomatal defense-favoring environmental condition such as darkness can be overcome by a virulent pathogen that shifts the hormone balance in guard cell toward JA3,39 (Fig. 2).
Figure 2.
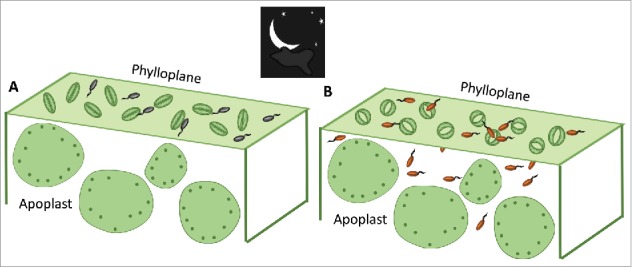
Stomata can be closed in the dark, a condition that may prevent bacterial internalization of leaves (A). However, virulent pathogens, such as the COR-producing bacterium Pst DC3000, can open dark-closed stomata and gain entry into the leaf apoplast (B). Colored bacterial cells in (B) indicate induction of coronatine biosynthesis.
Acknowledgments
This research topic in the M. Melotto Lab is supported by grants from the U.S. National Institute of Allergy and Infectious Disease (5R01AI068718), the U.S. Department of Agriculture – National Institute of Food and Agriculture (2015–67017–23360 and 2017–67017–26180), Center for Produce Safety (CPF43206), and UC Davis-FAPESP SPRINT (Award 40747474).
References
- 1.Stevens RB. Plant Pathology, an Advanced Treatise. Vol. 3, 357–429 (Academic, 1960). [Google Scholar]
- 2.Scholthof KB. The disease triangle: Pathogens, the environment and society. Nat Rev Microbiol. 2007;5(2):152–6. doi: 10.1038/nrmicro1596. [DOI] [PubMed] [Google Scholar]
- 3.Panchal S, Chitrakar R, Thompson B, Obulareddy N, Roy D, Hambright WS, Melotto M. Regulation of stomatal defense by air relative humidity. Plant Physiol. 2016;172:2021–32. doi: 10.1104/pp.16.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–80. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Arnaud D, Hwang I. A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol Plant. 2015;8(4):566–81. doi: 10.1016/j.molp.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Kroupitski Y, Golberg D, Belausov E, Pinto R, Swartzberg D, Granot D, Sela S. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microbiol. 2009;75:6076–86. doi: 10.1128/AEM.01084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy D, Panchal S, Rosa BA, Melotto M. Escherichia coli O157:H7 induces stronger plant immunity than Salmonella enterica Typhimurium SL1344. Phytopathology. 2013;103:326–32. doi: 10.1094/PHYTO-09-12-0230-FI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar AS, Lakshmanan V, Caplan JL, Powell D, Czymmek KJ, Levia DF, Bais HP. Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata. Plant J. 2012;72:694–706. doi: 10.1111/j.1365-313X.2012.05116.x. [DOI] [PubMed] [Google Scholar]
- 9.Melotto M, Zhang L, Oblessuc PR, He SY. Stomatal defense a decade later. Plant Physiol. 2017;174:561–71. doi: 10.1104/pp.16.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin XF, He SY. Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu Rev Phytopathol. 2013;51:473–98. doi: 10.1146/annurev-phyto-082712-102321. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, He SY, Assmann SM. The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J. 2008;56: 984–96. doi: 10.1111/j.1365-313X.2008.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng W, He SY. A Prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. Plant Physiol. 2010;153:1188–98. doi: 10.1104/pp.110.157016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng W, Brutus A, Kremer JM, Withers JC, Gao X, Jones AD, He SY. A genetic screen reveals Arabidopsis stomatal and/or apoplastic defenses against Pseudomonas syringae pv. tomato DC3000. PLoS Pathog. 2011;7:e1002291. doi: 10.1371/journal.ppat.1002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montillet JL, Hirt H. New checkpoints in stomatal defense. Trends Plant Sci. 2013;18:295–7. doi: 10.1016/j.tplants.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Du M, Zhai Q, Deng L, Li S, Li H, Yan L, Huang Z, Wang B, Jiang H, Huang T, Li CB. Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. Plant Cell. 2014;26:3167–84. doi: 10.1105/tpc.114.128272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim CW, Luan S, Lee SC. A prominent role for RCAR3-mediated ABA signaling in response to Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis. Plant Cell Physiol. 2014;55:1691–1703. doi: 10.1093/pcp/pcu100. [DOI] [PubMed] [Google Scholar]
- 17.Deger AG, Scherzer S, Nuhkat M, Kedzierska J, Kollist H, Brosché M, Unyayar S, Boudsocq M, Hedrich R, Roelfsema MR. Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytologist. 2015;208:162–73. doi: 10.1111/nph.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada M, Ito S, Marsubara A, Iwakura I, Egoshi S, Ueda M. Total syntheses of coronatine by exo-selective Diels-Alder reaction and their biological activities on stomatal opening. Org Biolomol Chem. 2009;7:3065–73. doi: 10.1039/b905159g. [DOI] [Google Scholar]
- 19.Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N. The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol. 2013;23:53–57. doi: 10.1016/j.cub.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto M, Tanaka Y, Abrams SR, Kamiya Y, Seki M, Nambara E. High humidity induces abscisic acid 8'-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiol. 2009;149:825–34. doi: 10.1104/pp.108.130823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshioka K, Kachroo P, Tsiu F, Sharma SB, Shah J, Klessig DF. Environmentally sensitive, SA-dependent defense responses in the cpr22 mutant of Arabidopsis. Plant J. 2001;26:227–59. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F, Menke FL, Yoshioka K, Moder W, Shirano Y, Klessig DF. High humidity suppresses ssi4-mediated cell death and disease resistance upstream of MAP kinase activation, H2O2 production and defense gene expression. Plant J. 2004;39:920–32. doi: 10.1111/j.1365-313X.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 23.Stotz HU, Jikumaru Y, Shimada Y, Sasaki E, Stingl N, Mueller MJ, Kamiya Y. Jasmonate-dependent and COI1-independent defense responses against Sclerotinia sclerotiorum in Arabidopsis thaliana: auxin is part of COI1-independent defense signaling. Plant Cell Physiol. 2011;52(11):1941–56. doi: 10.1093/pcp/pcr127. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell. 2002;14(3):559–74. doi: 10.1105/tpc.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimenez-Ibanez S, Boter M, Fernández-Barbero G, Chini A, Rathjen JP, Solano R. The bacterial effector Hopx1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol. 2014;12(2):e1001792. doi: 10.1371/journal.pbio.1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. The Plant Cell. 2003;15:760–70. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng X-Y, Spivey NW, Zeng W, Liu P-P, Fu ZQ, Klessig DF, He SY, Dong X. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe. 2012;11:587–96. doi: 10.1016/j.chom.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirano SS, Upper CD. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae – a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev. 2000;64:624–53. doi: 10.1128/MMBR.64.3.624-653.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beattie GA. Water relations in the interaction of foliar bacterial pathogens with plants. Annu Rev Phytopathol. 2011;49:533–55. doi: 10.1146/annurev-phyto-073009-114436. [DOI] [PubMed] [Google Scholar]
- 30.Monier JM, Lindow SE. Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc Natl Acad Sci USA. 2003;100:15977–82. doi: 10.1073/pnas.2436560100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Penaloza-Vazquez A, Chakrabarty AM, Bender CL. Involvement of the exopolysaccharide alginate in the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Mol Microbiol. 1999;33:712–720. doi: 10.1046/j.1365-2958.1999.01516.x. [DOI] [PubMed] [Google Scholar]
- 32.Bunster L, Fokkema NJ, Schippers B. Effect of surface-active Pseudomonas spp. on leaf wettability. Appl Environ Microbiol. 1989;55:1340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiber L, Krimm U, Knoll D, Sayed M, Auling G, Kroppenstedt RM. Plant–microbe interactions: identification of epiphytic bacteria and their ability to alter leaf surface permeability. New Phytol. 2005;166:589–94. doi: 10.1111/j.1469-8137.2005.01343.x. [DOI] [PubMed] [Google Scholar]
- 34.Xin XF, Nomura K, Aung K, Velásquez AC, Yao J, Boutrot F, JH4 Chang, C3 Zipfel, He SY. Bacteria establish an aqueous living space in plants crucial for virulence. Nature. 2016;539(7630):524–9. doi: 10.1038/nature20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, Xie Q, Anderson RG, Ng G, Seitz NC, Peterson T, McClung CR, McDowell JM, Kong D, Kwak JM, et al.. Crosstalk between the circadian clock and innate immunity in Arabidopsis. PLoS Pathogens. 2013;9(6):e1003370. doi: 10.1371/journal.ppat.1003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodspeed D, Chehab EW, Min-Venditti A, Braam J, Covington MF. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc Natl Acad Sci USA. 2012;109:4674–7. doi: 10.1073/pnas.1116368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grundy J, Stoker C, Carré IA. Circadian regulation of abiotic stress tolerance in plants. Front Plant Sci. 2015;6:648. doi: 10.3389/fpls.2015.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Yao J, Withers J, Xin XF, Banerjee R, Fariduddin Q, Nakamura Y, Nomura K, Howe GA, Boland W, et al.. Host target modification as a strategy to counter pathogen hijacking of the jasmonate hormone receptor. Proc Natl Acad Sci USA. 2015;112:14354–9. doi: 10.1073/pnas.1510745112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panchal S, Roy D, Chitrakar R, Price L, Breitbach ZS, Armstrong DW, Melotto M. Coronatine facilitates Pseudomonas syringae infection of Arabidopsis leaves at night. Front. Plant Sci. 2016;7:880. doi: 10.3389/fpls.2016.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]


