Abstract
Chondroitin sulfate (CS) has shown either ameliorating or aggravating effects on osteoarthritis (OA) in separately conducted clinical trials. Because CS is usually administered orally, it should be affected by or would impact on the individual gut microbiota. Evidence is accumulating that CS can nourish sulfatase-secreting bacteria (SSB) and sulfate-reducing bacteria (SRB). To decipher how can an individual gut microbiota determine the clinical values of CS for treatment on OA, we suggest here that CS would give distinct outcomes for OA treatment depending on Akkermansia muciniphila, a gut commensal probiotic bacterial species as optimal presence albeit also behaving as mucus-eroding bacteria (MEB) when abundant presence. Briefly, CS would ameliorate OA if A. muciniphila is present due to without overgrowth of SSB and SRB, whereas CS would aggravate OA if A. muciniphila is absent because of failure in or lack of competition with abundant SSB and SRB. By noting such a frequently ignored phenomenon, we urge the development of non-orally administering CS to minimize its side-effects and extend it to other medicinal applications.
Keywords: chondroitin sulfate, osteoarthritis, gut microbiota, probiotic, antibiotic, opportunistic infection, pro-inflammation, anti-inflammation
Chondroitin sulfate (CS), also known as polysaccharide constituted of alternated residues of N-acetylgalactosamine and glucuronic acid, is a naturally O-sulfated glycan in cartilage and bones. CS extracted from cow or pig cartilage is prescribed as a symptomatic slow-acting drug for osteoarthritis (OA) in 22 countries, including the European Union, but it is still regulated in the USA as a dietary supplement for improving OA (Jordan, 2003). The United States Food and Drug Administration (FDA) denied a request that CS be labeled as reducing the risk of OA and OA-related joint pain, tenderness, and swelling (FDA, 2004). Despite compelling evidence that CS interferes with OA progression (Vergés and Castañeda-Hernández, 2004; Clegg et al., 2006; Reginster et al., 2007), a systematic review of 20 clinical trials concluded that CS had not demonstrated any symptomatic benefit and discouraged its use in routine clinical practice (Reichenbach et al., 2007). A recent Cochrane clinical trial review concluded that CS appeared to alleviate pain in the short term, but did not improve or maintain the health of joints affected by OA (Singh et al., 2015).
Because of this shortcoming in pain relief, CS was not recommended for the symptomatic treatment of knee OA (Jevseva et al., 2013). Combined treatment with CS and glucosamine sulfate or glucosamine hydrochloride did not reduce joint damage in a rabbit model of knee OA (Roman-Blas et al., 2017b). CS plus glucosamine sulfate was not superior to placebo in alleviating articular lesions in a randomized, double-blind, placebo-controlled clinical trial in patients with knee OA (Roman-Blas et al., 2017a).
The effectiveness, benefits, and harms of CS for treating OA are not fully understood, which is possibly attributed to a “good” or “bad” study design. Contamination of heparin with CS has been associated with adverse clinical events, such as allergy and hypertension (Kishimoto et al., 2008), and the potential effect of CS-mediated gut dysbiosis on the differential pharmaceutical outcomes of CS must be considered. Here we aim to give a perspective on the clinical assessment of the impact of CS on OA and other inflammatory disorders.
Explaining inconsistent outcomes of CS on OA by the individual-specificity of gut microbiota symbionts
Considering the oral administration, gastrointestinal uptake, and structural similarity of CS with mucin-type O-glycans of the colonic epithelium, we propose a hypothesis of an Akkermansia muciniphila-dependent CS effect on OA, in which CS improves OA if A. muciniphila is present, and CS aggravates OA if A. muciniphila is absent. A. muciniphila is a species of mucin-eroding bacteria (MEB) that competes with sulfatase-secreting bacteria (SSB) and the population should increase when SSB are rare and decrease when SSB are abundant. MEB depend on mucin-type O-glycans as a sole carbon source; SSB are mucin generalists that can utilize other polysaccharides including those in dietary fiber (Desai et al., 2016).
Colonization of A. muciniphila in the gastrointestinal tract varies among individuals (Bressa et al., 2017), and affected by many variables including consumption of fruits and vegetables, drugs, obesity, and surgical history. A. muciniphila has been shown to increase following restriction in dietary fiber and a shift from fiber to mucin consumption (Desai et al., 2016) and increase of Akkermansia spp. has been associated with the antiobesity activity of a polyphenol-rich cranberry extract (Anhê et al., 2015). The antiobesity activity of capsaicin observed in mice fed a high-fat diet was also associated with increase of A. muciniphila (Shen et al., 2017). Enrichment of gut A. muciniphila populations has been observed in diabetes patients treated with metformin (de la Cuesta-Zuluaga et al., 2017), and following weight loss in obese and/or type 2 diabetes patients (Remely et al., 2016). Roux-en-Y gastric bypass surgery has also been found to increase A. muciniphila populations (Yan et al., 2016).
How can A. muciniphila residing in the gut confer a beneficial effect of CS on OA? Why is CS ineffective or even harmful when SSB preferentially occupy the gut? A. muciniphila may act as a gatekeeper of the mucosa (de Vos, 2017) or stimulate the mucosal immunity to prevent pathogenic invasion, and prompt thickening of the colonic mucosa to reduce the susceptibility to infection. Overgrowth of A. muciniphila is thought to cause mucin degradation, severe damage of the colonic mucosa, extensive endotoxin leakage, and unrecoverable tissue damage. A. muciniphila and B. caccae have been shown to stimulate epithelial access and initiate lethal colitis by the mucosal pathogen Citrobacter rodentium in gnotobiotic mice with a synthetic gut microbiota community (Desai et al., 2016).
Although a small A. muciniphila population may induce mucin turnover and a large population may induce colonic mucosal injury, a threshold level has not been identified. If bacterial consumption of mucin-derived nutrients exceeds production, then the integrity of the colonic barrier might be compromised (Rey et al., 2013). The overgrowth of A. muciniphila might derive from genetic immune deficiency. That is supported by the observation that A. muciniphila promoted intestinal inflammation in genetically susceptible germ-free Il10−/− mice (Seregin et al., 2017).
Evidence for the probiotic value of A. muciniphila
Evidence of a dose-dependent benefit of A. muciniphila on human health is lacking, but antibiotics capable of eliminating Akkermansia reinforce the colonic mucus barrier and prevent heme-dependent cytotoxic micelles from reaching the epithelium (Ijssennager et al., 2015). A. muciniphila may also be a novel functional microbe with probiotic properties (Dao et al., 2016). Evidence for the therapeutic values of A. muciniphila in metabolic diseases includes an inverse correlation with metabolic changes occurring with obesity and type 1 diabetes in mice and humans (Shin et al., 2014; Gomes et al., 2017) and prevention of obesity induced by changes in adipose tissue metabolism and gut barrier function caused by a high-fat diet in mice (Everard et al., 2013).
Favorable effects of A. muciniphila on improved glucose tolerance and maintained high interferon (IFN) γ levels for controlling various pathogen infections, primarily those caused by intracellular pathogens, have been described by Greer et al. (2016) and Ottman et al. (2017) attributed a positive correlation of A. muciniphila and gut health to immune modulatory effects and immune tolerance to this commensal bacterium. A. muciniphila has been reported to promote intestinal barrier integrity and ameliorate experimental alcoholic liver disease by enhancing colonic mucus thickness and tight-junction expression (Grander et al., 2017). Bifidobacterium that resemble A. muciniphila and degrade mucins by secreting endo-α-N-acetylgalactosaminidase and 1,2-α-L-fucosidase (Ruas-Madiedo et al., 2008) have probiotic behavior that might stimulate mucin overproduction and accelerate replenishment of lost mucins.
The anti-inflammatory activity of CS has been demonstrated in vitro. When CS was immobilized on a scaffold and exposed to proinflammatory cytokines or cytokine-stimulated peripheral blood mononuclear cells, significant increases in the production of molecules with immunosuppressive activity and the expression of their inducible enzymes were observed (Corradetti et al., 2016). Subcutaneous implantation of CS-immobilized scaffold in rats reduced leukocyte infiltration and upregulated genes encoding proteins associated with apoptosis of inflammatory cells (Corradetti et al., 2016). CS was found to suppress nuclear NF-κB translocation in vitro (Tan and Tabata, 2014), and to inhibit NF-κB activation by pathogen- and damage-associated molecules in cultured THP-1 macrophages (Stabler et al., 2017).
Proinflammatory activity of CS-promoted SSB and anti- inflammatory activity of antibiotic-resistant A. muciniphila
CS supports the overgrowth of Bacteroides sp. strain 3452A (an unnamed DNA homology group), B. ovatus, and B. thetaiotaomicron (Lipeski et al., 1986) in the colon and can promote the growth of B. thetaiotaomicron, which is an SSB, and Desulfovibrio piger, a species of sulfate-reducing bacteria (SRB) (Rey et al., 2013). SRB may thus thrive if supported by a food chain-like lifestyle, i.e., SRB growth depends on sulfate-containing CS. A number of SSB species, including B. thetaiotaomicron J1 and 82, B. ovitus E3, and Clostridium hathewayi R4, have been isolated from healthy subjects (Shang et al., 2016). Theoretically, oral CS administration should support the proportional expansion of SSB and SRB in the distal gut.
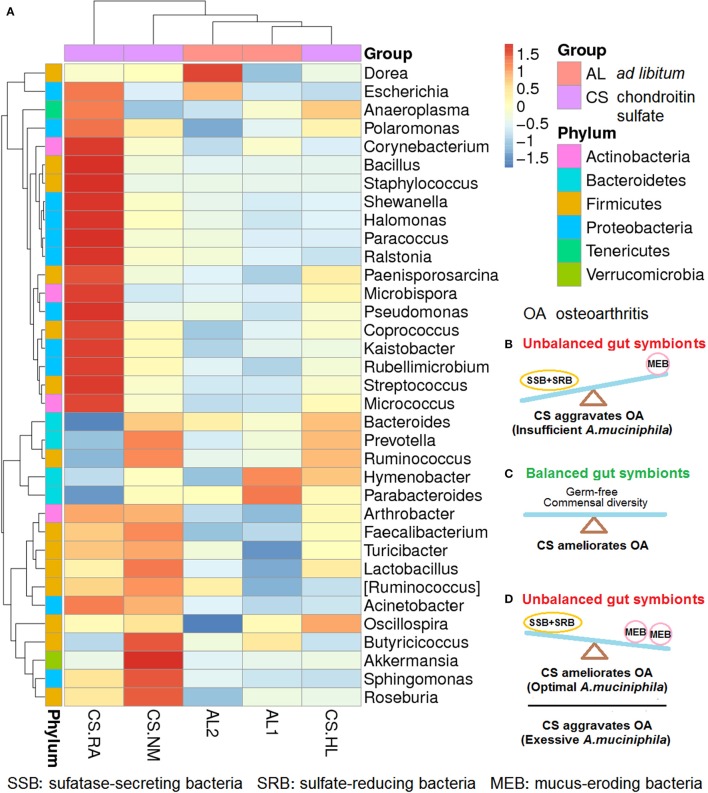
We previously reported that daily CS feeding alters the gut microbiota spectrum of mice, but the response varied with individual differences (Figure 1A). In mice with symptoms of early-phase rheumatoid arthritis (RA), Bacillus cereus, an SSB was abundant and A. muciniphila was rare compared with normal mice. CS.NM with a normal phenotype has more A. muciniphila, but less B. cereus. CS.HL suffering from slight hairless shows no overgrowth of B. cereus and A. muciniphila (Liao et al., 2017b). Distinct gut microflora communities might be rapidly established in mice fed CS at identical doses and schedules.
Figure 1.
Changes in the gut microbiota profiles of mice fed ad libitum (AL) and chondroitin sulfate (CS) (n = 3 for mice fed CS; n = 2 for mice fed AL; CS.RA, CS.NM, and CS.HL represent three mice fed CS; AL1 and AL2 represent two mice fed AL). (A) CS-induced gut microbiota shifts that varied in individual mice. (B) Unbalanced gut symbionts with more SSB+SRB than MEB species that aggravate OA due to insufficient A. muciniphila; (C) balanced gut symbionts in germ-free conditions or with commensal diversity that ameliorate OA because of no gut microbiota interference. (D) Unbalanced gut symbionts with more MEB than SSB+SRB that ameliorate OA when the abundance of A. muciniphila is optimal or aggravate OA if the colonization of A. muciniphila is excessive. The relative abundance of bacteria is represented by red (increased) to blue (decreased) colors accounting for Z values from −1.5 to 1.5.
As shown in Figure 1B, when the proliferation of SSB plus SRB exceeds that of MEB because of unbalanced gut symbionts, CS would aggravate OA because of an A. muciniphila population insufficient to stimulate mucin induction and mucus repair. If the gut is kept permanently germ-free, or a commensal diversity of SSB, SRB, MEB, and other symbions is maintained, CS would ameliorate OA (Figure 1C). CS might also ameliorate OA in the presence of normal mucosal immunity and an optimum gut milieu including A. muciniphila. However, CS would aggravate OA given an excess of A. muciniphila in a gut with decreased mucosal immunity such as in the immunocompromised, elderly and ill, or patients who are immunosuppressed following organ transplantation (Figure 1D).
We previously reported that CS increased the abundance of Rikenella, a genus of SSB, and Desulfovibrio, a genus of SRB, but did not significantly change the abundance of A. muciniphila. Surprisingly, Rikenella and Desulfovibrio were both eradicated by cephalosporin, but A. muciniphila increased. Conversely, Rikenella and Desulfovibrio thrived on exposure to berberine, but A. muciniphila was eliminated by this antibiotic analog. Consequently, cephalosporin significantly mitigated colonic mucus lesions and delayed the early pathogenesis of dementia, steatohepatitis, and atherosclerosis. Berberine significantly aggravated colonic mucus lesions and enhanced multi-systemic pathogenesis (Liao et al., 2017a). It has also been observed that germ-free rats had thinner colonic mucus layers, suggesting that microbial colonization is required for mucin production (Desai et al., 2016).
CS has found to upregulate expression of secretory leukocyte protease inhibitor (SLPI), a marker of tissue damage, and mucin 1 (MUC1), a marker of mucus repair; cephalosporin decreased SLPI and MUC1 expression to normal levels (Liao et al., 2017a). The SRB that flourish in association with CS administration could cause a related increase in mucin degradation compared with mucin biosynthesis. In line with the study results, ablation of MUC2 secretion brings bacteria into close contact with the gut epithelium, leading to inflammation and colon cancer (Van der Sluis et al., 2006). The results support the assumption that SSB compete with MEB, and that CS significantly increases the abundance of SSB and restores the A. muciniphila population after SSB are killed by antibiotics.
A pro- and anti-inflammatory activities of CS might reflect the temporal and spatial changes of lipopolysaccharide (LPS) content. We previously reported that peripheral (serum and muscle) LPSs declined to normal levels, and that visceral (hepatic and cardiac tissues) LPSs were elevated to maintain a pro-inflammatory state, or adipose and cerebral LPSs declined to maintain an anti-inflammatory state (Liao et al., 2017a,b). Administration of of a relative high 1.2 mg/kg LPS dose led to anti-inflammatory peritoneal changes, and a relative low 0.25 mg/kg LPS dose caused pro-inflammatory visceral changes, suggesting that anti-LPS treatment reduced LPS in peritoneal but not visceral tissues (Gao et al., 2015). A trend of decrease of anti-inflammatory indicators in the serum following oral CS administration might thus lead to erroneous conclusions of the anti-inflammatory effects of CS.
Perspectives
The composition of the gut microbiome influences the pro- and anti-inflammatory activity of CS on aggravation or amelioration of OA via compromise or reinforcement of the colonic mucus barrier. Individual gut microbiota spectra should be carefully considered when evaluating the in vivo effects of CS on OA. The contribution of A.muciniphila in relation to dietary and other etiological variables must be considered to when evaluating the therapeutic values of CS in OA.
Caution should be exercised when administering CS orally to elderly OA patients, who may have decreased mucosal immunity. Similarly, OA patients with a history of organ transplantation and using immunosuppressive drugs might not maintain an A. muciniphila population optimal for avoiding persistent colonic mucus erosion and prolonged OA deterioration.
Development of next-generation CS formulations such as injectable lyophilized CS preparations not only minimizes CS-related side-effects, but also expands the indication of CS to a broad range of inflammatory disorders including inflammatory bowel disease. Alternatively, it should be an option that CS is used in combination with probiotics, prebiotics, or emerging dietary intervention (Segarra et al., 2016; Holleran et al., 2017).
Author contributions
QZ and QW wrote the manuscript. QX, CL, and SH critically reviewed the manuscript. All authors have read and approved the final version of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jiang He, Tao Liao, and Yan-Ping Chen, and Li-Li Tan for their assistance in manuscript preparation. This work was supported by the National Natural Science Foundation of China (NSFC, No. 81273817 and No. 81473740 to QW; No. 81673861 to CL) and Guangdong Science and Technology Plan Project (No. 20150404042 to QX).
References
- Anhê F. F., Roy D., Pilon G., Dudonné S., Matamoros S., Varin T. V., et al. (2015). A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64, 872–883. 10.1136/gutjnl-2014-307142 [DOI] [PubMed] [Google Scholar]
- Bressa C., Bailén-Andrino M., Pérez-Santiago J., González-Soltero R., Pérez M., Montalvo-Lominchar M. G., et al. (2017). Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 12:e0171352. 10.1371/journal.pone.0171352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg D. O., Reda D. J., Harris C. L., Klein M. A., O'Dell J. R., Hooper M. M., et al. (2006). Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. New Eng. J. Med. 354, 795–808. 10.1056/NEJMoa052771 [DOI] [PubMed] [Google Scholar]
- Corradetti B., Taraballi F., Minardi S., Van Eps J., Cabrera F., Francis L. W., et al. (2016). Chondroitin sulfate immobilized on a biomimetic scaffold modulates inflammation while driving chondrogenesis. Stem Cells Transl. Med. 5, 670–682. 10.5966/sctm.2015-0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao M. C., Everard A., Aron-Wisnewsky J., Sokolovska N., Prifti E., Verger E. O., et al. (2016). Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436. 10.1136/gutjnl-2014-308778 [DOI] [PubMed] [Google Scholar]
- de la Cuesta-Zuluaga J., Mueller N. T., Corrales-Agudelo V., Velásquez-Mejía E. P., Carmona J. A., Abad J. M., et al. (2017). Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 40, 54–62. 10.2337/dc16-1324 [DOI] [PubMed] [Google Scholar]
- de Vos W. M. (2017). Akkermansia muciniphila: a conserved intestinal symbiont that acts as the gatekeeper of our mucosa. Microbiology 163, 646–648. 10.1099/mic.0.000444 [DOI] [PubMed] [Google Scholar]
- Desai M. S., Seekatz A. M., Koropatkin N. M., Kamada N., Hickey C. A., Wolter M., et al. (2016). A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339, e21–1353. e21. 10.1016/j.cell.2016.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A., Belzer C., Geurts L., Ouwerkerk J. P., Druart C., Bindels L. B., et al. (2013). Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U.S.A. 110, 9066–9071. 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2004). Letter Regarding the Relationship Between the Consumption of Glucosamine and/or Chondroitin Sulfate and a Reduced Risk of: Osteoarthritis; Osteoarthritis-Related Joint Pain, Joint Tenderness, and Joint Swelling; Joint Degeneration; and Cartilage Deterioration (Docket No. 2004P-0059). Addressed to John W. Emford, Esq. William K. Hubbard. Available online at: http://www.fda.gov/food/ingredientspackaginglabeling/labelingnutrition/ucm073400.htm
- Gao Q., He J., Liao T., Zeng Q. P. (2015). 2,4-dinitrophenol downregulates genes for diabetes and fatty liver in obese mice. J. Biosci. Med. 3, 44–51. 10.4236/jbm.2015.39007 [DOI] [Google Scholar]
- Gomes J. M., Costa J. A., Alfenas R. C. (2017). Metabolic endotoxemia and diabetes mellitus: a systematic review. Metab. Clin. Exp. 68, 133–144. 10.1016/j.metabol.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Grander C., Adolph T. E., Wieser V., Lowe P., Wrzosek L., Gyongyosi B., et al. (2017). Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. [Epub ahead of print]. 10.1136/gutjnl-2016-313432 [DOI] [PubMed] [Google Scholar]
- Greer R. L., Dong X., Moraes A. C. F., Ryszard A., Zielke R. A., Fernandes G. R., et al. (2016). Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat. Commun. 7:13329. 10.1038/ncomms13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran G., Lopetuso L. R., Ianiro G., Pecere S., Pizzoferrato M., Petito V., et al. (2017). Gut microbiota and inflammatory bowel disease: an update. Minerva. Gastroenterol. Dietol. 63, 373–384. 10.23736/S1121-421X.17.02386-8 [DOI] [PubMed] [Google Scholar]
- Ijssennager N., Belzer C., Hooiveld G. J., Dekker J., van Mil S. W., Müller M., et al. (2015). Gut microbiota facilitates dietary heme-induced epithelial hyper-proliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. U.S.A. 112, 10038–10043. 10.1073/pnas.1507645112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevseva D. S., Brown G. A., Jones D. L., Matzkin E. G., Manner P. A., Mooar P., et al. (2013). The American Academy of orthopaediac surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J. Bone Joint Surg. Am. 95, 1885–1886. 10.5435/JAAOS-21-09-571 [DOI] [PubMed] [Google Scholar]
- Jordan K. M. (2003). An evidence based approach to the management of knee osteoarthritis: report of a task force of the standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 62, 1145–1155. 10.1136/ard.2003.011742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K., Viswanathan K., Ganguly T., Elankumaran S., Smith S., Pelzer K., et al. (2008). Contaminated heparin associated with adverse clinical events and activation of the contact system. New Engl. J. Med. 358, 2457–2467. 10.1056/NEJMoa0803200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T., Chen Y. P., Huang S. Q., Tan L. L., Li C. Q., Huang X. A., et al. (2017a). Chondroitin sulfate elicits systemic pathogenesis in mice by interfering with gut microbiota homeostasis. BioRxiv 10.1101/142588 [DOI] [Google Scholar]
- Liao T., Chen Y. P., Tan L. L., Li C. Q., Wang Q., Huang S. Q., et al. (2017b). Chondroitin sulfate flourishes gut sulfatase-secreting bacteria to damage mucus layers, leak bacterial debris, and trigger inflammatory lesions in mice. BioRxiv 10.1101/145714 [DOI] [Google Scholar]
- Lipeski L., Guthrie E. P., O'Brien M., Kotarski S. F., Salyers A. A. (1986). Comparison of proteins involved in chondroitin sulfate utilization by three colonic Bacteroides species. Appl. Environ. Microbiol. 51, 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman N., Reunanen J., Meijerink M., Pietilä T. E., Kainulainen V., Klievink J., et al. (2017). Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 12:e0173004 10.1371/journal.pone.0173004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster J. Y., Heraud F., Zegels B., Bruyere O. (2007). Symptom and structure modifying properties of chondroitin sulfate in osteoarthritis. Mini Rev. Med. Chem. 7, 1051–1061. 10.2174/138955707782110088 [DOI] [PubMed] [Google Scholar]
- Reichenbach S., Sterchi R., Scherer M., Trelle S., Bürgi E., Bürgi U., et al. (2007). Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann. Intern. Med. 146, 580–590. 10.7326/0003-4819-146-8-200704170-00009 [DOI] [PubMed] [Google Scholar]
- Remely M., Hippe B., Zanner J., Aumueller E., Brath H., Haslberger A. G. (2016). Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr. Metab. Immune Disord. Drug. Targets 17, 99–106. 10.2174/1871530316666160831093813 [DOI] [PubMed] [Google Scholar]
- Rey F. E., Gonzalez M. D., Cheng J., Ahern P. P., Gordon J. I. (2013). Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc. Natl. Acad. Sci. U.S.A. 110, 13582–13587. 10.1073/pnas.1312524110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Blas J. A., Castaneda S., Sanchez-Pernaute O., Largo R., Herrero-Beaumont G., et al. (2017a). Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-bland, placebo-controlled clinical trial. Arthritis Rheumatol. 69, 77–85. 10.1002/art.39819 [DOI] [PubMed] [Google Scholar]
- Roman-Blas J. A., Mediero A., Tardio L., Portal-Nunez S., Gratal P., Herrero-Beaumont G., et al. (2017b). The combined therapy with chondroitin sulfate plus glucosamine sulfate or chondroitin sulfate plus glucosamine hydrochloride does not improve joint damage in an experimental model of knee osteoarthritis in rabbits. Eur. J. Pharmacol. 794, 8–14. 10.1016/j.ejphar.2016.11.015 [DOI] [PubMed] [Google Scholar]
- Ruas-Madiedo P., Gueimonde M., Fernández-Garcia M., de los Reyes-Gavilán C. G., Margolles A. (2008). Mucin degradation by Bifidobacterium strains isolated from the human intestinal microbiota. App. Environ. Microbiol. 74, 1936–1940. 10.1128/AEM.02509-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra S., Martinez-Subiela S., Cerda-Cuellar M., Martinez-Puig D., Munoz-Prieto A., Rodriguez-Franco F., et al. (2016). Oral chondroitin sulfate and probiotics for the treatment of cannie inflammatory bowel disease: a randomized, controlled clinical trial. BMC Vet. Res. 12:49 10.1186/s12917-016-0676-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregin S. S., Golovchenko N., Schaf B., Chen J., Pudlo N. A., Mitchell J., et al. (2017). NLRP6 protects Il10-/- mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Rep. 19:2174 10.1016/j.celrep.2017.05.074 [DOI] [PubMed] [Google Scholar]
- Shang Q., Yin Y., Zhu L., Li G., Yu G., Wang X. (2016). Degradation of chondroitin sulfate by the gut microbiota of Chinese individuals. Int. J. Biol. Micromol. 86, 112–118. 10.1016/j.ijbiomac.2016.01.055 [DOI] [PubMed] [Google Scholar]
- Shen W., Shen M., Zhao X., Zhu H., Yang Y., Lu S., et al. (2017). Anti-obesity effect of capsaicin in mice fed with high-fat diet is associated with an increase in population of the gut bacterium Akkermansia muciniphila. Front. Microbiol. 8:272. 10.3389/fmicb.2017.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N. R., Lee J. C., Lee H. Y., Kim M. S., Whon T. W., Lee M. S., et al. (2014). An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63, 727–735. 10.1136/gutjnl-2012-303839 [DOI] [PubMed] [Google Scholar]
- Singh J. A., Noorbaloochi S., MacDonald R., Maxwell L. J. (2015). Chondroitin for osteoarthritis. Cochrane Data. Sys. Rev. 1:CD005614. 10.1002/14651858.CD005614.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler T. V., Huang Z., Montell E., Vergés J., Kraus V. B. (2017). Chondroitin sulphate inhibits NF-κB activity induced by interaction of pathogenic and damage associated molecules. Osteoarthr. Cartil. 25, 166–174. 10.1016/j.joca.2016.08.012 [DOI] [PubMed] [Google Scholar]
- Tan G. K., Tabata Y. (2014). Chondroitin-6-sulfate attenuates inflammatory responses in murine macrophages via suppression of NF-κB nuclear translocation. Acta Biomater. 10, 2684–2692. 10.1016/j.actbio.2014.02.025 [DOI] [PubMed] [Google Scholar]
- Van der Sluis M., De Koning B. A., De Bruijn A. C., Velcich A., Meijerink J. P., Van Goudoever J. B., et al. (2006). Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129. 10.1053/j.gastro.2006.04.020 [DOI] [PubMed] [Google Scholar]
- Vergés J., Castañeda-Hernández G. (2004). On the bioavailability of oral chondroitin sulfate formulations: proposed criteria for bioequivalence studies. Proc. West. Pharmacol. Soc. 47, 50–53. [PubMed] [Google Scholar]
- Yan M., Song M. M., Bai R. X., Cheng S., Yan W. M. (2016). Effect of Roux-en-Y gastric bypass surgery on intestinal Akkermansia muciniphila. World J. Gastrointest. Surg. 8, 301–307. 10.4240/wjgs.v8.i4.301 [DOI] [PMC free article] [PubMed] [Google Scholar]