Abstract
Objective
The Japan Society of Gynecologic Oncology (JSGO) published the first practice guideline for endometrial cancer in 2006. The JSGO guideline evaluation committee assessed the effect of this guideline introduction on clinical practice and patient outcome using data provided by the Japan Society of Obstetrics and Gynecology (JSOG) cancer registration system.
Methods
Data of patients with endometrial cancer registered between 2000 and 2012 were analyzed, and epidemiological and clinical trends were assessed. The influence of guideline introduction on survival was determined by analyzing data of patients registered between 2004 and 2009 using competing risk model.
Results
In total, 65,241 cases of endometrial cancer were registered. Total number of patients registered each year increased about 3 times in the analyzed period, and the proportion of older patients with type II endometrial cancer rapidly increased. The frequency of lymphadenectomy had decreased not only among the low-recurrence risk group but also among the intermediate- or high-recurrence risk group. Adjuvant therapy was integrated into chemotherapy (p<0.001). Overall survival did not significantly differ before and after the guideline introduction (hazard ratio [HR]=0.891; p=0.160). Additional analyses revealed patients receiving adjuvant chemotherapy showed better prognosis than those receiving adjuvant radiation therapy when limited to stage I or II (HR= 0.598; p=0.003).
Conclusion
It was suggested that guideline introduction influenced the management of endometrial cancer at several aspects. Better organized information and continuous evaluation are necessary to understand the causal relationship between the guideline and patient outcome.
Keywords: Practice Guideline; Endometrial Neoplasms; Chemotherapy, Adjuvant; Lymph Node Excision; Survival
INTRODUCTION
The number of patients with endometrial cancer, which is becoming the most frequent disease among gynecologic malignancies in Japan, has been increasing [1]. It is estimated that about 13,600 women were newly diagnosed with endometrial cancer in Japan in 2012 [2]. Although the management of endometrial cancer was based on surgery and adjuvant therapies, the types of hysterectomy, the selection criteria for lymphadenectomy or adjuvant therapies highly varied among facilities and clinicians. To standardize and integrate the management of endometrial cancer, the Japan Society of Gynecologic Oncology (JSGO) evaluated clinical evidences, discussed in consensus meetings, and published the first guideline for the treatment of endometrial cancer in 2006 [3]. This first edition guideline consisted of 7 chapters with 41 clinical questions (CQs) and answers accompanied by detailed comments and recommendation grade. The guideline has been revised periodically in 2009 and 2013 with minor changes and additional categories, but major indications are the same as the first edition (refer to the previous articles for minor information [3,4]). The JSGO also published the first guideline for ovarian cancer in 2004 and for uterine cervical cancer in 2007. Although these guidelines are now commonly referred to by gynecologic oncologists in diverse institutes all over Japan, there is no nationwide evaluation as yet about their influence on the management or outcome of patients with gynecologic cancer. To assess the effect of guideline introduction in our nation, the JSGO established a guideline evaluation committee comprising the JSGO members.
With collaboration between the JSGO and the Japan Society of Obstetrics and Gynecology (JSOG), the JSGO guideline evaluation committee has analyzed the data of the JSOG cancer registration system to evaluate the change of clinical practice or patient outcome brought about by the guideline introduction in endometrial, ovarian, and uterine cervical cancers, respectively. In this article, we report the results of endometrial cancer focusing on the publication of the first edition guideline in 2006 as a part of the JSGO guideline evaluation committee project. Epidemiological inspection was also performed to comprehend the trend of patient characteristics in Japan.
MATERIALS AND METHODS
The JSOG cancer registration system is the most detailed and one of the largest registration project about gynecologic malignant neoplasm in our country with well-organized inquiry system to minimize any errors or missing data. Data of all patients with endometrial cancer registered in the JSOG cancer registration system from 2000 to 2012 were provided and analyzed based on the approval by the ethics board of the Tokai University Hospital (13R-199), where the chief director of this project Mikio Mikami belongs.
The data consist of only epithelial malignancies including carcinosarcoma, which has been basically considered and treated as an aggressive subtype of endometrial carcinoma in Japan in concordance with accumulated evidence and the International Federation of Gynecology and Obstetrics (FIGO) statement [5,6]. Any of mesenchymal tumors or other nonepithelial neoplasms were excluded from the registration criteria. The data include age; histological type; the differentiation grade for endometrioid adenocarcinoma; both FIGO stage and tumor, node, and metastasis (TNM) stage; treatment methods listed in time order; information about lymphadenectomy; status of lymph node metastasis; and outcome with overall survival (OS). If the first treatment method was surgery, the treatment listed right after the surgery was considered as adjuvant therapy. Radiation therapy was registered as either extra-beam radiation therapy (EBRT) or intracavitary brachytherapy (ICBT). No information about chemotherapy regimen was available. Outcome was surveyed 3 years and 5 years after initial registration. Only 3-year outcome was reported among cases registered from 2008 to 2010, and no outcome information was available for patients registered after 2011.
All the data were adopted for epidemiological survey and clinical trend analysis. The influence of guideline introduction on patients' OS was evaluated using the data reported between 2004 and 2009.
Cochran-Armitage trend test was adopted for all the trend tests. Competing risk model described by Fine and Gray [7] was employed for comparison of patients' OS, which was performed at a statistics center established in Clinical Research, Innovation and Education Center, Tohoku University Hospital. Outcome was analyzed using SAS® version 9.4 (SAS Institute, Cary, NC, USA) and other statistical analyses were performed using SAS® version 9.4 or JMP® Pro 12.2.0 (SAS Institute). p-values less than 0.05 were considered to be significant in this study.
RESULTS
1. Patient characteristics
A total of 65,241 cases of endometrial cancer were registered in the JSOG cancer registration system between 2000 and 2012. Patient characteristics are shown in Table 1. Either FIGO clinical staging criteria published in 1982 or FIGO postoperative staging criteria published in 1988 were adopted according to each case except for 2012 [8]. Postoperative staging criteria were employed for a majority of the patients whose primary treatment was surgery. The novel FIGO staging criteria published in 2009 were adopted for all the cases in 2012 [9].
Table 1. Patient characteristics.
| Parameters | No. of patients (%) | ||
|---|---|---|---|
| Age (yr) | |||
| ≤29 | 514 (0.8) | ||
| 30–39 | 3,474 (5.3) | ||
| 40–49 | 9,043 (13.9) | ||
| 50–59 | 22,791 (34.9) | ||
| 60–69 | 17,371 (26.6) | ||
| 70–79 | 9,451 (14.5) | ||
| ≥80 | 2,597 (4.0) | ||
| FIGO stage* | |||
| I | 42,562 (65.2) | ||
| II | 5,458 (8.4) | ||
| III | 12,700 (19.5) | ||
| IV | 4,517 (6.9) | ||
| Unknown | 4 (0.0) | ||
| Histology | |||
| Endometrioid | |||
| Grade 1 | 32,245 (49.4) | ||
| Grade 2 | 14,497 (22.2) | ||
| Grade 3 | 6,980 (10.7) | ||
| Unknown | 1,418 (2.2) | ||
| Serous | 2,582 (4.0) | ||
| Clear cell | 1,493 (2.3) | ||
| Mucinous | 367 (0.6) | ||
| Others/unknown | 5,659 (8.7) | ||
| Primary treatment | |||
| Surgery | |||
| With LA | 44,525 (68.2) | ||
| Without LA | 16,740 (25.7) | ||
| Chemotherapy | 2,237 (3.4) | ||
| EBRT | 684 (1.0) | ||
| ICBT | 101 (0.2) | ||
| Hormonal therapy | 742 (1.1) | ||
| Others/unknown | 212 (0.3) | ||
EBRT, extra-beam radiation therapy; FIGO, International Federation of Gynecology and Obstetrics; ICBT, intracavitary brachytherapy; LA, lymphadenectomy.
*For postoperative staging, 1988 FIGO staging criteria were adopted from 2000 to 2011. FIGO clinical staging criteria were also employed from 2000 to 2011. 2009 FIGO staging criteria were adopted for the all cases registered in 2012.
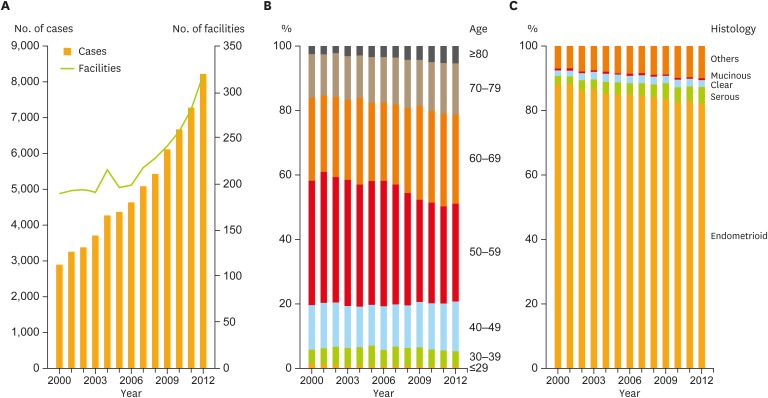
The number of registered cases increased over the years, resulting in approximately 3-fold increase in 2012 compared to that in 2000 (Fig. 1A). This trend was similar to the estimated incidence of endometrial cancer in our country reported by the Monitoring of Cancer Incidence in Japan (MCIJ) project (Supplementary Fig. 1A) [2]. Comparing these, it was found that about half of patients with endometrial cancer were registered to the JSOG cancer registration system each year. The number of facilities participating in the JSOG registration system also increased, especially after the first guideline publication in 2006 (Fig. 1A).
Fig. 1.
Trends of patient characteristics. (A) Number of patients and facilities per year. (B) Age distribution per year. Patients aged >60 years significantly increased (p<0.001). (C) Histological subtype distribution per year. Trend test revealed significant increases in the frequency of serous adenocarcinoma, and in the population of both serous and clear cell carcinoma (p<0.001).
When stratified by age, it was obvious that the proportion of older patients showed an increasing trend (Fig. 1B). The trend test revealed that the number of patients aged >60 years had significantly increased during the analyzed period (p<0.001). In parallel, it is also worth mentioning that the proportion of cases with serous adenocarcinoma showed a statistically significant increase (p<0.001), as shown in Fig. 1C, even though endometrioid adenocarcinoma still occupied >80% of the cases in 2012. As serous adenocarcinoma and clear cell carcinoma are the representative subtypes of the so-called “type II” endometrial cancer [10,11,12], the trend of the total proportion of both subtypes was also assessed and a significant difference was observed (p<0.001). This result indicated that type II endometrial cancer has been rapidly increasing in Japan.
Because type II endometrial cancer is more common among older patients [12], an additional trend test was performed to validate the relationship between age distribution and the total proportion of serous/clear cell carcinoma. A significant difference was observed in this trend test, suggesting that age and frequency of serous/clear cell carcinoma positively correlated with each other (p<0.001, Supplementary Fig. 1B). Taken together, it is plausible to consider that the incidence of type II endometrial cancer in older patients is drastically increasing in the Japanese population.
There were no obvious proportional changes in FIGO stages except for 2012, where the different staging criteria were adopted (Supplementary Fig. 1C).
2. Clinical trends in treatment
Treatment strategies presented in the guideline are well documented in the articles published previously [3,4]. Simplified summary is also available in Supplementary Fig. 2.
1) Primary treatment and lymphadenectomy
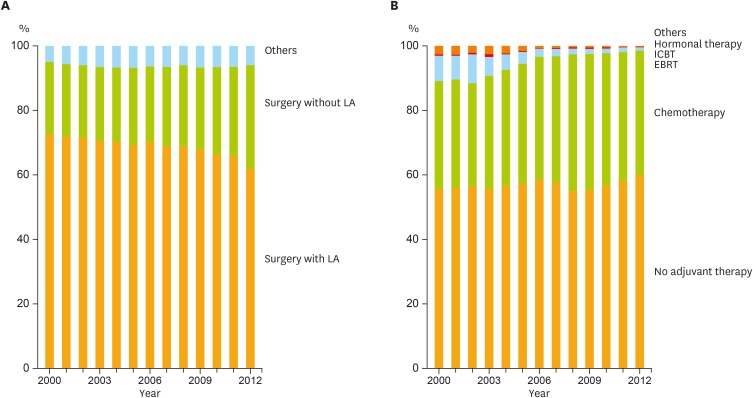
Fig. 2A demonstrates the distribution of primary treatment by year. Although surgery was applied for >90% of patients as their primary treatment through the period, the proportion of lymphadenectomy procedures had significantly decreased among patients who received surgery (Cochran-Armitage trend test, p<0.001).
Fig. 2.
Trends of clinical practice in endometrial cancer management. (A) Distribution of initial treatment by year. Among surgeries, the proportion of LA procedures had significantly decreased (Cochran-Armitage trend test, p<0.001). (B) Trend of adjuvant therapy in each year. The proportion of adjuvant chemotherapy had significantly increased among the adjuvant therapies (Cochran-Armitage trend test, p<0.001).
EBRT, extra-beam radiation therapy; ICBT, intracavitary brachytherapy; LA, lymphadenectomy.
CQs and answers in the guideline recommend hysterectomy with salpingo-oophorectomy as an essential surgical procedure. In terms of lymphadenectomy, the guideline mentions that lymphadenectomy is informative and meaningful to determine accurate cancer stage by histological inspection, but does not guarantee the advantage of lymphadenectomy on patient survival with reference to some conflicting clinical research results [13,14,15,16,17]. On the other hand, the guideline also indicates that lymphadenectomy can be omitted for patients with only low risk of lymph node metastasis or recurrence in the context.
To assess the influence of guideline introduction on the frequency of lymphadenectomy, patients who initially received surgery were divided into pre- (2000–2006) and post- (2007–2012) guideline publication periods. The χ2 test revealed a significant difference in the frequency of lymphadenectomy between these 2 groups (Table 2). To further assess the relationship between the risks of recurrence/lymph node metastasis and the frequency of lymphadenectomy, these patients were distributed according to their clinical stages as shown in Table 2. Patients registered in 2012 were excluded from this assay because staging criteria were different from others. Cases without detailed stage information were also excluded. The proportion of lymphadenectomy procedures tended to decline in all stages after the guideline publication. Significant differences were observed in stages Ia, Ib, IIa, and importantly in IIIc by the χ2 test (Table 2).
Table 2. Frequency of LA between pre- and post-guideline publication.
| Stages | No. of patients (%) | |||
|---|---|---|---|---|
| 2000–2006 | 2007–2012 (All) | |||
| With LA | Without LA | With LA | Without LA | |
| All* | 18,777 (75.5) | 6,101 (24.5) | 25,747 (70.8) | 10,636 (29.2) |
| Ia† | 2,801 (61.1) | 1,788 (38.9) | 3,049 (55.9) | 2,406 (44.1) |
| Ib† | 6,731 (78.1) | 1,886 (21.9) | 7,167 (75.1) | 2,373 (24.9) |
| Ic | 2,609 (78.8) | 701 (21.2) | 2,952 (77.0) | 883 (23.0) |
| IIa† | 663 (79.4) | 172 (20.6) | 760 (73.1) | 279 (26.9) |
| IIb | 1,032 (81.9) | 228 (18.1) | 1,193 (79.6) | 305 (20.4) |
| IIIa | 2,180 (79.4) | 564 (20.6) | 2,494 (78.1) | 698 (21.9) |
| IIIb | 73 (66.4) | 37 (33.6) | 67 (60.9) | 43 (39.1) |
| IIIc† | 2,132 (93.8) | 142 (6.2) | 2,280 (92.0) | 197 (8.0) |
| IVa | 45 (48.9) | 47 (51.1) | 41 (45.1) | 50 (54.9) |
| IVb | 460 (50.4) | 453 (49.6) | 584 (46.3) | 676 (53.7) |
LA, lymphadenectomy.
*It shows significant difference between 2000–2006 and 2007–2012 using χ2 test (p<0.001); †It shows significant difference between 2000–2006 and 2007–2011 using χ2 test (p<0.050).
2) Adjuvant therapy
In contrast to the United States (US) and other countries, adjuvant chemotherapy was traditionally more common than adjuvant radiation therapy in Japan after 1990s [1]. Based on the result of a randomized controlled trial (RCT) targeting stage III or IV endometrial cancer patients [18], the guideline recommends adjuvant chemotherapy with higher level of evidence than adjuvant radiation therapy for the high-recurrence risk cases defined as the Supplementary Table 1. The guideline also mentions that adjuvant therapy should be considered for intermediate-recurrence risk group, showing a more favorable attitude toward adjuvant chemotherapy than adjuvant radiation therapy through its comment. In addition, the guideline does not recommend adjuvant hormonal therapy. The proportion of adjuvant therapies from 2000 to 2012 is shown in Fig. 2B. Chemotherapy significantly increased among adjuvant therapies (p<0.001). It was observed that adjuvant therapy was integrated into chemotherapy in our country, which is concordant with the guideline statements.
3) Treatment for advanced stage patients
The guideline suggests multiple treatment options for advanced stage endometrial cancer patients including hysterectomy, cytoreductive or palliative surgery, chemotherapy, and radiation therapy. However, the indication is not as consistent as the one for stage I or II patients because cancer progression sites vary among cases and less evidence is available. When looking at the distribution of primary treatment by clinical stage, it is understood that surgery was selected in more than 90% of stage III cases, and even in around 60% of stage IVb patients (Supplementary Fig. 3A). Significant proportion of stage IV patients also received lymphadenectomy, which suggests surgical tumor reduction has been valued in the treatment of endometrial cancer in our nation. Chemotherapy was mostly selected as a primary treatment in stage III or IV patients who did not receive surgical treatment initially (Supplementary Fig. 3B). Further evaluation about multidisciplinary cancer management for advanced stage endometrial cancer patients was difficult with the current database.
3. Influence of the guideline on patient outcome
Patient outcome was analyzed to explore the contribution of guideline publication. Competing risk model was employed, and death by other diseases was considered to be the competing risk. Survival curve of each stage based on cumulative incidence rate is shown in Supplementary Fig. 4. The OS rates between 2004–2006 and 2007–2009 were compared by multivariate analysis. A total of 13,258 and 16,606 cases were registered in each period. Information regarding OS was missing in 2,835 (21.4%) and 3,425 (20.6%) cases, respectively. Age, year of registration, FIGO stage, histological type, and types of primary treatment were employed as independent variables. As ICBT is available only in a limited number of institutes in Japan, both EBRT and ICBT are integrated into radiation therapy to minimize possible institutional bias. The results are summarized in Table 3. No significant difference was observed in terms of guideline introduction (hazard ratio [HR]=0.891; p=0.160). Univariate analyses for subgroups (FIGO stage, histological subtypes, or absence/presence of lymphadenectomy) were additionally performed, but no significant difference was found in any of the subgroups (data not shown).
Table 3. Multivariate analysis of the influence of the guideline publication.
| Parameters | HR | 95% CI | p-value | |
|---|---|---|---|---|
| 2004–2006 | 1.000 (reference) | - | - | |
| 2007–2009 | 0.891 | 0.759–1.046 | 0.160 | |
| Year | 1.020 | 0.972–1.069 | 0.423 | |
| Age | 1.002 | 1.001–1.002 | <0.001 | |
| Stage | ||||
| I | 1.000 (reference) | - | - | |
| II | 2.281 | 1.913–2.718 | <0.001 | |
| III | 5.695 | 5.079–6.387 | <0.001 | |
| IV | 14.017 | 12.287–15.990 | <0.001 | |
| Histology | ||||
| Endometrioid grade 1 | 1.000 (reference) | - | - | |
| Endometrioid grade 2 | 2.481 | 2.166–2.842 | <0.001 | |
| Endometrioid grade 3 | 4.149 | 3.594–4.790 | <0.001 | |
| Endometrioid grade unknown | 3.372 | 2.595–4.381 | <0.001 | |
| Serous/clear-cell | 5.424 | 4.690–6.272 | <0.001 | |
| Others | 6.712 | 5.833–7.723 | <0.001 | |
| Primary treatment | ||||
| Surgery with LA | 1.000 (reference) | - | - | |
| Surgery without LA | 1.992 | 1.810–2.192 | <0.001 | |
| Radiation therapy* | 4.969 | 3.842–6.427 | <0.001 | |
| Chemotherapy | 2.508 | 2.198–2.862 | <0.001 | |
| Others | 4.828 | 3.228–7.221 | <0.001 | |
CI, confidence interval; EBRT, extra-beam radiation therapy; HR, hazard ratio; ICBT, intracavitary brachytherapy; LA, lymphadenectomy.
*Including both EBRT and ICBT.
Focusing on the trend of adjuvant therapy demonstrated in the section “Adjuvant therapy” of Result, we further compared OS among patients who received adjuvant chemotherapy or adjuvant radiation therapy by multivariate analysis. Only FIGO stage I or II cases were determined to be eligible for this analysis to minimize potential confounding factors, as cancerous lesions were considered to be completely removed by surgery in most of these cases. Independent variables were age, year of registration, FIGO stage, histological subtype, and the influence of guideline publication. The results are shown in Table 4. HR (0.598) of adjuvant chemotherapy against adjuvant radiation therapy was significantly low (p=0.003).
Table 4. Multivariate analysis of adjuvant therapy.
| Parameters | HR | 95% CI | p-value |
|---|---|---|---|
| Surgery | 1.000 (reference) | - | - |
| Surgery+adjuvant chemotherapy | 1.102 | 0.916–1.325 | 0.302 |
| Surgery+radiation therapy* | 1.841 | 1.316–2.577 | <0.001 |
| Surgery+others | 1.340 | 0.591–3.037 | 0.483 |
CI, confidence interval; EBRT, extra-beam radiation therapy; HR, hazard ratio; ICBT, intracavitary brachytherapy.
*Including both EBRT and ICBT.
DISCUSSION
Together with the JSGO guideline evaluation projects for uterine cervical cancer and ovarian cancer (unpublished), this is one of the first reports to assess the influence of guideline introduction on gynecologic cancer in Japan. The JSOG cancer registration database was found to be composed of approximately half of all endometrial cancer cases in Japan. The database also contained few missing information regarding patient characteristics owing to the inquiry system. These aspects make this nationwide evaluation meaningful and reliable.
It is estimated that westernization of lifestyle has affected the increase in the incidences of endometrial cancer, especially estrogen-related type I cancer in Japan and other countries [19,20,21]. Here we also indicated that the number of patients with type II endometrial cancer aged >60 years is rapidly increasing, which may be related to the growing aging society in Japan. The same observation has been reported in the US population [22]. Needless to say, type II endometrial cancer is more aggressive than type I cancer, but older patients are sometimes obliged to choose less invasive treatments according to their performance status or complications. Even though the number is still small compared with the endometrioid adenocarcinoma, it must be alerted that the future guideline should respond appropriately to this drastic increase in type II cancer as well as the increase in the total number of endometrial cancer cases.
Our investigation demonstrated that the frequency of lymphadenectomy had decreased during the analyzed period regardless of the risk of lymph node metastasis or recurrence, which makes an interesting contrast with the trend in the US. Melamed et al. [23] reported that the frequency of lymphadenectomy in the US declined after 2007 primarily among patients with low risk of lymph node metastasis, but no significant change was observed in the patients who were found to have lymph node metastasis. We could not conclude to what extent the guideline had influenced the selection of lymphadenectomy in our country because the guideline has neutral attitudes toward lymphadenectomy for patient outcome. Some important clinical studies on lymphadenectomy have been reported which can affect the trend of lymphadenectomy after the first edition guideline publication [24,25,26], but it is still controversial, even in the guideline of the latest version, as to who will truly obtain benefits by lymphadenectomy and who will not. At least, the results in this study suggest that the indication for lymphadenectomy was judged based not on the risk evaluation but on other factors in a significant number of cases. The increase in the number of older patients with poor performance status or severe complications can be one possible factor. The growing diversity of participating facilities may also affect the total proportion of lymphadenectomy procedures. Unfortunately, our data do not harbor enough information to examine these possible confounding factors. Overall, we estimate the influence of guideline publication is limited in terms of frequency of lymphadenectomy.
The trend of adjuvant therapy is another interesting issue. In contrast to the guidelines in other countries, which are based more on adjuvant radiation therapy [27,28,29,30], our guideline emphasizes on the benefits of adjuvant chemotherapy. The results of this study revealed that adjuvant therapies have been integrated into chemotherapy as recommended in the guideline. It should be highlighted that adjuvant chemotherapy was common in Japan even before the guideline publication [1]. Several studies pointed out the shortage of radiation oncologists or facilities in Japan in comparison with the US [31,32,33]. Besides evidences from clinical trials mentioned in the results section, the limited accessibility to radiation therapy might also be in the background.
We showed the possible advantage of adjuvant chemotherapy against adjuvant radiation therapy, implying indirect influence of the guideline on patient survival. After the publication of the guideline in 2006, several RCTs comparing adjuvant radiation therapy and chemotherapy for intermediate- or high-recurrence risk groups have been reported, in which no significant difference was observed [34,35]. However, a meta-analysis reported in 2011 supports our result by indicating a small benefit of adjuvant chemotherapy over radiation therapy [36]. At the same time, it should be emphasized again that the direct influence of the guideline publication on patient outcome was not verified in this study, and our retrospective examination missed several information indispensable to minimize confounding factors such as performance status, types of hysterectomy, cytoreductive status in primary surgery, area of lymphadenectomy (except for 2012), status of venous or lymphatic invasions, regimens of chemotherapy or radiation therapy, and types of facilities. The result of this study should be interpreted very carefully.
Guidelines should reflect the global trend brought about by the progression of novel treatment strategies and updated evidences. Thus, guidelines are required to be revised periodically. Simultaneously, we should evaluate the guideline at a certain interval to confirm its influence on the clinical practice. Furthermore, these retrospective inspections will uncover the conflicting issue that should be discussed in future prospective studies. The long-term purpose of this guideline evaluation is to establish novel evidence-based guideline through proposing clinical trials based on highly qualified retrospective analyses.
The JSOG cancer registration project has established unique, one and only database in our nation. Here, we would like to propose some modifications as there were several limitations in the data available. The current database does not contain aforementioned information which can reduce unfavorable biases and lead to more reliable results for future evaluation. In addition, the proportion of patients without outcome information should be smaller for more trustworthy assessment. It would be ideal if all this information is involved in the future registration. However, it can be easily understood that if the registration system gets more complicated, then more effort will be required for all participants. It is desirable to modify the registration system and obtain a more sophisticated database with detailed information in collaboration with JSOG, JSGO, and every participant for a more reliable guideline evaluation and better cancer management.
In conclusion, although this is a retrospective study with limited information, we demonstrated the possible influence of guideline introduction on clinical trends and patient survival through nationwide assessment. Continuous evaluation with improved database is desirable to comprehend the effect of guidelines more precisely, and to contribute to better patient outcome.
ACKNOWLEDGMENTS
We thank the committee and all the participants of the Japan Society of Obstetrics and Gynecology (JSOG) cancer registration project.
Footnotes
Funding: This project was financially supported by Health and Labour Sciences Research Grants by Ministry of Health, Labour and Welfare in Japan.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: S.S., N.S., M.M., I.M., S.M., S.I., U.N., T.F., Y.W., Y.N., U.Y., K.H.
- Data curation: T.F.
- Formal analysis: S.S., T.F.
- Funding acquisition: M.M.
- Investigation: S.S., N.S., M.M., T.F.
- Methodology: S.S., N.S., M.M., I.M., S.M., S.I., U.N., T.F., Y.W., Y.N., U.Y., K.H.
- Project administration: M.M.
- Resources: S.S., T.F.
- Software: S.S., T.F., Y.N., U.Y., K.H.
- Supervision: N.S., M.M.
- Validation: S.S., T.F.
- Visualization: S.S., T.F.
- Writing - original draft: S.S., N.S., M.M., T.F.
- Writing - review & editing: S.S., N.S., M.M., I.M., S.M., S.I., U.N., T.F., Y.W., Y.N., U.Y., K.H.
Presentation: This article has been presented as 68th Annual Congress of the Japan Society of Obstetrics and Gynecology, April 21–24, 2016, Tokyo, Japan and 58th Annual Congress of the Japan Society of Gynecologic Oncology, July 8–10, 2016, Yonago, Japan.
Supplementary Materials
Classification of postoperative recurrence risk of endometrial cancer in the 2006 and 2009 editions
Patient characteristics. (A) Estimated number of endometrial cancer cases per year in Japan. Data quoted from the MCIJ project [2]. (B) Trend test between age and histological subtypes. Serous/clear represents the total proportion of serous and clear cell carcinomas, showing a significantly increasing trend with increase in age (p<0.001). (C) Trend of stage distribution per year.
A summary of treatment strategies presented in the guideline published in 2006. To be advised that the revised editions were published in 2009 and 2013, which have some minor changes from this flowchart. Several differences are summarized in the annotation. More information for treatment options or detailed strategies in each edition is available in the references published elsewhere [3,4].
Trends of clinical practice in advanced stage patients. (A) Distribution of initial treatment in advanced stage patients by stage and by year. (B) Distribution of non-surgical primary treatment among stage III or IV patients by year. Radiation therapy includes both EBRT and ICBT.
Survival curves of each stage. Survival curves are drawn based on cumulative incidence rate. Data comprises cases registered from 2004 to 2009.
References
- 1.Ushijima K. Current status of gynecologic cancer in Japan. J Gynecol Oncol. 2009;20:67–71. doi: 10.3802/jgo.2009.20.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 3.Nagase S, Katabuchi H, Hiura M, Sakuragi N, Aoki Y, Kigawa J, et al. Evidence-based guidelines for treatment of uterine body neoplasm in Japan: Japan Society of Gynecologic Oncology (JSGO) 2009 edition. Int J Clin Oncol. 2010;15:531–542. doi: 10.1007/s10147-010-0138-6. [DOI] [PubMed] [Google Scholar]
- 4.Ebina Y, Katabuchi H, Mikami M, Nagase S, Yaegashi N, Udagawa Y, et al. Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. Int J Clin Oncol. 2016;21:419–434. doi: 10.1007/s10147-016-0981-1. [DOI] [PubMed] [Google Scholar]
- 5.McCluggage WG. Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomas? J Clin Pathol. 2002;55:321–325. doi: 10.1136/jcp.55.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104:177–178. doi: 10.1016/j.ijgo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 8.Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989;96:889–892. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 9.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 11.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 12.Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129:277–284. doi: 10.1016/j.ygyno.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cragun JM, Havrilesky LJ, Calingaert B, Synan I, Secord AA, Soper JT, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–3675. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]
- 14.Candiani GB, Belloni C, Maggi R, Colombo G, Frigoli A, Carinelli SG. Evaluation of different surgical approaches in the treatment of endometrial cancer at FIGO stage I. Gynecol Oncol. 1990;37:6–8. doi: 10.1016/0090-8258(90)90297-x. [DOI] [PubMed] [Google Scholar]
- 15.Trimble EL, Kosary C, Park RC. Lymph node sampling and survival in endometrial cancer. Gynecol Oncol. 1998;71:340–343. doi: 10.1006/gyno.1998.5254. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Am A, Ron IG, Kuperminc M, Gal I, Jaffa A, Kovner F, et al. The role of routine pelvic lymph node sampling in patients with stage I endometrial carcinoma: second thoughts. Acta Obstet Gynecol Scand. 1998;77:347–350. [PubMed] [Google Scholar]
- 17.Sartori E, Gadducci A, Landoni F, Lissoni A, Maggino T, Zola P, et al. Clinical behavior of 203 stage II endometrial cancer cases: the impact of primary surgical approach and of adjuvant radiation therapy. Int J Gynecol Cancer. 2001;11:430–437. doi: 10.1046/j.1525-1438.2001.01061.x. [DOI] [PubMed] [Google Scholar]
- 18.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 19.Takayama S, Monma Y, Tsubota-Utsugi M, Nagase S, Tsubono Y, Numata T, et al. Food intake and the risk of endometrial endometrioid adenocarcinoma in Japanese women. Nutr Cancer. 2013;65:954–960. doi: 10.1080/01635581.2013.818158. [DOI] [PubMed] [Google Scholar]
- 20.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 21.Dunn JE. Cancer epidemiology in populations of the United States--with emphasis on Hawaii and California--and Japan. Cancer Res. 1975;35:3240–3245. [PubMed] [Google Scholar]
- 22.Ueda SM, Kapp DS, Cheung MK, Shin JY, Osann K, Husain A, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198:218.e1–218.e6. doi: 10.1016/j.ajog.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 23.Melamed A, Rauh-Hain JA, Clemmer JT, Diver EJ, Hall TR, Clark RM, et al. Changing trends in lymphadenectomy for endometrioid adenocarcinoma of the endometrium. Obstet Gynecol. 2015;126:815–822. doi: 10.1097/AOG.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 24.ASTEC study group Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 26.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–1172. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]
- 27.National Comprehensive Cancer Network (US) NCCN Clinical Practice Guidelines in Oncology. Uterine neoplasms, version 2. 2016 [Internet] Fort Washington, PA: National Comprehensive Cancer Network; 2016. [cited 2016 Oct 28]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. [Google Scholar]
- 28.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi33–vi38. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 30.Lee SW, Lee TS, Hong DG, No JH, Park DC, Bae JM, et al. Practice guidelines for management of uterine corpus cancer in Korea: a Korean Society of Gynecologic Oncology Consensus Statement. J Gynecol Oncol. 2017;28:e12. doi: 10.3802/jgo.2017.28.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano T. Status of Japanese radiation oncology. Radiat Med. 2004;22:17–19. [PubMed] [Google Scholar]
- 32.Teshima T, Owen JB, Hanks GE, Sato S, Tsunemoto H, Inoue T. A comparison of the structure of radiation oncology in the United States and Japan. Int J Radiat Oncol Biol Phys. 1996;34:235–242. doi: 10.1016/0360-3016(95)02046-2. [DOI] [PubMed] [Google Scholar]
- 33.Tanikawa T, Ohba H, Ogasawara K, Okuda Y, Ando Y. Geographical distribution of radiotherapy resources in Japan: investigating the inequitable distribution of human resources by using the Gini coefficient. J Radiat Res (Tokyo) 2012;53:489–491. doi: 10.1269/jrr.11103. [DOI] [PubMed] [Google Scholar]
- 34.Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226–233. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson N, Bryant A, Miles T, Hogberg T, Cornes P. Adjuvant chemotherapy for endometrial cancer after hysterectomy. Cochrane Database Syst Rev. 2011:CD003175. doi: 10.1002/14651858.CD003175.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Classification of postoperative recurrence risk of endometrial cancer in the 2006 and 2009 editions
Patient characteristics. (A) Estimated number of endometrial cancer cases per year in Japan. Data quoted from the MCIJ project [2]. (B) Trend test between age and histological subtypes. Serous/clear represents the total proportion of serous and clear cell carcinomas, showing a significantly increasing trend with increase in age (p<0.001). (C) Trend of stage distribution per year.
A summary of treatment strategies presented in the guideline published in 2006. To be advised that the revised editions were published in 2009 and 2013, which have some minor changes from this flowchart. Several differences are summarized in the annotation. More information for treatment options or detailed strategies in each edition is available in the references published elsewhere [3,4].
Trends of clinical practice in advanced stage patients. (A) Distribution of initial treatment in advanced stage patients by stage and by year. (B) Distribution of non-surgical primary treatment among stage III or IV patients by year. Radiation therapy includes both EBRT and ICBT.
Survival curves of each stage. Survival curves are drawn based on cumulative incidence rate. Data comprises cases registered from 2004 to 2009.