Abstract
Objective
Angiopoietin-like protein-4 (ANGPTL4) is a circulating protein that is highly expressed in liver and implicated in regulation of plasma triglyceride levels. Systemic ANGPTL4 increases during prolonged fasting and is suggested to be secreted from skeletal muscle following exercise.
Methods
We investigated the origin of exercise-induced ANGPTL4 in humans by measuring the arterial-to-venous difference over the leg and the hepato-splanchnic bed during an acute bout of exercise. Furthermore, the impact of the glucagon-to-insulin ratio on plasma ANGPTL4 was studied in healthy individuals. The regulation of ANGPTL4 was investigated in both hepatic and muscle cells.
Results
The hepato-splanchnic bed, but not the leg, contributed to exercise-induced plasma ANGPTL4. Further studies using hormone infusions revealed that the glucagon-to-insulin ratio is an important regulator of plasma ANGPTL4 as elevated glucagon in the absence of elevated insulin increased plasma ANGPTL4 in resting subjects, whereas infusion of somatostatin during exercise blunted the increase of both glucagon and ANGPTL4. Moreover, activation of the cAMP/PKA signaling cascade let to an increase in ANGPTL4 mRNA levels in hepatic cells, which was prevented by inhibition of PKA. In humans, muscle ANGPTL4 mRNA increased during fasting, with only a marginal further induction by exercise. In human muscle cells, no inhibitory effect of AMPK activation could be demonstrated on ANGPTL4 expression.
Conclusions
The data suggest that exercise-induced ANGPTL4 is secreted from the liver and driven by a glucagon-cAMP-PKA pathway in humans. These findings link the liver, insulin/glucagon, and lipid metabolism together, which could implicate a role of ANGPTL4 in metabolic diseases.
Keywords: Diabetes, Insulin, Liver, Muscle, Myokine
Highlights
-
•
Release of Angiopoietin-like Protein 4 from the hepato-splanchnic bed is induced by exercise.
-
•
It is regulated by the glucagon-to-insulin ratio in vivo in humans.
-
•
In vitro in hepatocytes Angiopoietin-like Protein 4 is stimulated by cAMP.
-
•
Angiopoietin-like Protein 4 is not released from the exercising nor resting leg.
1. Introduction
Angiopoietin-like protein-4 (ANGPTL4) was identified in 2000 as a secreted plasma protein expressed in adipose tissue [1] and liver [2]. ANGPTL4 is induced by fasting in both white adipose tissue and liver under the regulation of peroxisome proliferator-activated receptors (PPAR) [1]. ANGPTL4 regulates plasma triglyceride levels by decreasing lipoprotein lipase (LPL) activity [3], [4], [5], [6], promotes adipose tissue lipolysis [7], and is suggested to improve glucose tolerance [8]. Population studies identify gene variants leading to inactivation of ANGPTL4 associated with low plasma triglycerides [9], [10], which is substantiated by the finding that mice lacking ANGPTL4 have low concentrations of triglycerides in plasma and increased LPL activity [11].
In humans, ANGPTL4 is expressed in adipose tissue [12], intestine [13], skeletal muscle [14], and, most abundantly, in liver [15]. Little is known, however, about the origin of circulating ANGPTL4 in humans. The liver may be the main contributor because of the high hepatic ANGPTL4 mRNA level and a pronounced hepatic response to fasting and exercise [14], [15], but adipose tissue and skeletal muscle have also been suggested [16], [17]. Increased plasma ANGPTL4 in response to exercise is considered to be muscle-derived, leading to the designation “myokine” [17], but clear evidence for the release of ANGPTL4 from muscles during or following exercise in humans is lacking.
Several regulatory mechanisms are suggested to increase the expression of ANGPTL4, including free fatty acids (FFA) via PPAR activation [18], glucocorticoid receptor signaling [19], and hypoxia [20]. Fasting and exercise are important inducers of plasma ANGPTL4 attributed to the increase in circulating FFA [15]. Interestingly, insulin has an inhibitory effect on both ANGPTL4 gene transcription in rodent liver [21], [22] and plasma levels of ANGPTL4 in humans [23]. However, it is not known whether the inhibitory effect of insulin is direct or secondary to its anti-lipolytic effect. A negative regulation of ANGPTL4 expression upon activation of AMPK is suggested in skeletal muscle [17].
By assessing arterial-to-venous differences over the leg and the hepato-splanchnic bed, we demonstrated that the increase in plasma follistatin and FGF21 in exercising humans is liver-derived with no contribution of the exercising leg [24], [25], [26]. Inspired by these observations, we investigated the origin of circulating ANGPTL4 in response to acute exercise in healthy humans. We evaluated the regulation of ANGPTL4 mRNA content in resting and exercising muscle and the contribution of fasting. Finally, the glucagon-to-insulin ratio and cAMP-dependent signaling as a potential driver of the exercise-induced increase in circulating ANGPTL4 was examined.
2. Material and methods
2.1. One-legged exercise
As described [26], nine healthy young males participated in this study. A femoral artery catheter and bilateral femoral vein catheters were inserted, and 2 h of knee-extensor exercise at 50% of maximum power was followed by 3 h of rest. Blood samples were obtained regularly and vastus lateralis needle biopsies obtained at time points 0, 2, 5 h, and on the following morning. Femoral arterial blood flow was measured by Doppler ultrasound. The subjects fasted from 10 pm the day before and remained fasted throughout the 5 h experimental period. The next morning, subjects returned after an overnight fast for a muscle biopsy and blood sample (24 h).
2.2. Exercise vs. fasting
Eighteen healthy male subjects were recruited and underwent a medical examination. The subjects performed an incremental exercise test on a cycle ergometer (Monark Ergomedic 839 E, Monark Ltd., Varberg, Sweden), and maximum oxygen uptake (VO2 max) was determined by indirect calorimetry (Quark b2, CosMed, Rome). The subjects were divided into an exercise group (n = 10) and a rest group (n = 8). Characteristics of the exercise group were: age 23.3 ± 0.5 yrs., BMI 23.3 ± 0.4 kg/m2, VO2 max 53.1 ± 2.2 ml/kg/min, and for the resting group: age 22.8 ± 0.4 yrs., BMI 23.9 ± 0.6 kg/m2, VO2 max 53.8 ± 2.3 ml/kg/min.
The participants were asked to refrain from strenuous exercise 24 h prior to the experimental day. On the experimental day, after an overnight fast, a catheter was placed in an antecubital vein and baseline blood samples drawn. The exercise trial (exercise group) included 3 h of ergometer cycling exercise at an intensity corresponding to 50% of VO2 max. Heart rate and VO2 were measured every hour during exercise to control intensity. After the exercise, the subjects rested supine for 6 h. Blood samples were drawn regularly and muscle biopsies were obtained from the vastus lateralis muscle at time 0, 3, 6, 9 h, and the following morning (24 h). During the experimental day, subjects remained fasted until the last blood sample was obtained, but they had free access to water. In the control trial, the subjects rested in the supine position throughout the 9 h of the trial with blood samples and biopsies obtained at time points as described for the exercise trial.
2.3. Hepatic venous catheter
This study is described in Ref. [24]. In brief, ten healthy males performed 2 h of ergometer cycling at 60% of VO2 max in a semi-supine position followed by 4 h of rest with catheters placed in an antecubital vein, a hepatic vein, and a brachial artery, which allowed for arterial-venous measurements over the liver. Hepatic plasma flow was measured as indocyanine green clearance before, during, and in recovery from exercise, and the production/clearance of ANGPTL4 was calculated as described for FGF21 [24]. The subjects arrived after an overnight fast and remained fasted throughout the trial, but they had free access to water.
2.4. Hormone infusion
This study is also described in Ref. [24]. Ten healthy males went through four experimental protocols after an overnight fast (test days 1–4). Trial 1: 1 h glucagon infusion (GlucaGen, Novo Nordisk) at 6 ng/kg/min. Trial 2: 2 h somatostatin infusion (Octreotide, Hospira Nordic) at 100 ng/kg/min (started 10 min prior to the glucagon infusion, in total 130 min) and 1 h glucagon infusion at 6 ng/kg/min. Trial 3: 2 h (130 min) somatostatin infusion at 100 ng/kg/min (same as test day 2). Trial 4: saline infusion at the same rate as the glucagon infusion rate. In all four trials, the subjects remained fasted but had free access to water.
2.5. Exercise with pancreatic clamp
As described in Ref. [27], six healthy male subjects underwent two experimental protocols in randomized order: 1) 2 h of cycling at 60% of VO2 max; 2) 2 h of cycling at 60% of VO2 max with a “pancreatic clamp” (2 h of somatostatin infusion at 100 ng/kg/min with replacement of glucagon 0.60 ng/kg/min and insulin 0.05 mU/kg/min). A variable glucose infusion was applied to maintain a stable glucose level. Data from one participant were removed from the analysis due to unmeasurable high concentration of ANGPTL4 despite attempts with multiple dilutions. A possible explanation is the presence of unspecific autoantibodies in the samples.
2.6. Ethical committee approval
The studies were approved by the Scientific Ethics Committee of the capital region of Denmark (one-legged exercise study [26]; acute exercise vs. fasting: H-4-2011-055; hormone infusion trial, and exercise trial with liver vein catheterization [24] and exercise with the pancreatic clamp [27]) in accordance with the Helsinki Declaration. All subjects provided oral and written informed consent to participate.
2.7. Laboratory analysis
Blood samples were collected in tubes containing aprotinin for analysis of glucagon and in tubes containing EDTA for other analyses. All blood samples were spun immediately at 4 °C at 3000 g for 15 min and the plasma fraction was stored at −80 °C until analyses. Plasma ANGPTL4 was measured by commercially available Enzyme-Linked Immunosorbant Assay (ELISA)-kit (BioVendor RD191073200R, Biovendor, Brno, Czech Republic) with an intra-assay coefficient of variation (CV) of 3.2% and inter-assay CV of 7.0%. All samples were run in duplicate in accordance with the protocol from the manufacturer. Free fatty acids were measured by a commercially available enzymatic NEFA kit (Wako Diagnostics, Richmond, USA) with intra-assay CV of 4.2% and inter-assay CV of 8.5%. RNA was extracted from muscle biopsies as described in Ref. [26]. Muscle ANGPTL4 mRNA content in the biopsies was quantified by real time PCR using primers from Applied Biosystem: cat# Hs01101127_m1 and was related to 18S RNA.
2.8. Cell cultures
Primary human skeletal muscle cells were obtained from the needle biopsies of the vastus lateralis muscle and cultivated and fused to myotubes [28]. The Ethical Committee of Tübingen University Medical Department approved the protocol. For siRNA-mediated knock-down, cells were transfected with siRNA oligonucleotides using Viromer Blue (Lipocalyx, Halle, Germany) on day 5 of differentiation. The ON-TARGET plus SMART-pool for PRKAA1, PRKAA2 (Dharmacon, Thermo-Fisher Scientific, Waltham, MS, USA), and non-targeting oligonucleotides for bacterial luciferase (sense: 5′-cguacgcggaauacuucga-3′; antisense: 5′-ucgaaguauuccgcguacg-3′; eurofins MWG/Operon; Ebersberg, Germany) were used at 20 nM. Medium was changed after 24 h and cells were stimulated as indicated. HepG2 cells (DMSZ, Braunschweig, Germany) were cultivated in RPMI 1640 containing 11 mM glucose and 10% FBS, 1% glutamine, and 1% penicillin/streptomycin. Cells were starved 3 h in RPMI 1640 before substances were added. RNA isolation is described in reference [29]. Quantitative real-time PCR (qRT-PCR) was performed on a Roche LightCycler 480 using QuantiTect Primer Assays Hs_ANGPTL4_1_SG, Hs_PRKAA1_1_SG, Hs_PRKAA2_1_SG, Hs_PCK1_1_SG, HsG6PC_1_SG, Hs_ACTB_2_SG, and Hs_TBP_1_SG (Qiagen, Hilden, Germany). Proteins were separated by SDS polyacrylamide (7.5–15%) gradient gel electrophoresis. Immunodetection on nitrocellulose membranes was performed with antibodies against phospho-Thr-172 of AMPKα and phospho-Ser-79 of ACC (Cell Signaling Technology, Frankfurt, Germany), AMPKα1 and 2 (Upstate Biotechnology, Lake Placid, NY), ACC (Merck Millipore, Billerica, MA).
2.9. Statistical analysis
Data are presented as mean ± SEM. Differences between groups were evaluated by a two- or three-way ANOVA. The group effect was evaluated using the slice function by time in the mixed model. Significant effect of time was evaluated by a one-way ANOVA followed by a Dunnett's post hoc test. Release or uptake was calculated as arterial-to-venous difference and production/clearance was calculated as the arterial-to-venous difference multiplied with plasma flow. These curves were further evaluated according to the area under the curve by t-test as were the in vitro data. The statistical analyses were performed by SAS 9.4 (Institute Inc., Cary, NC, USA) and a P < 0.05 was considered statistically significant.
3. Results
3.1. One-legged exercise increases muscle ANGPTL4 transcripts but not protein release from the leg
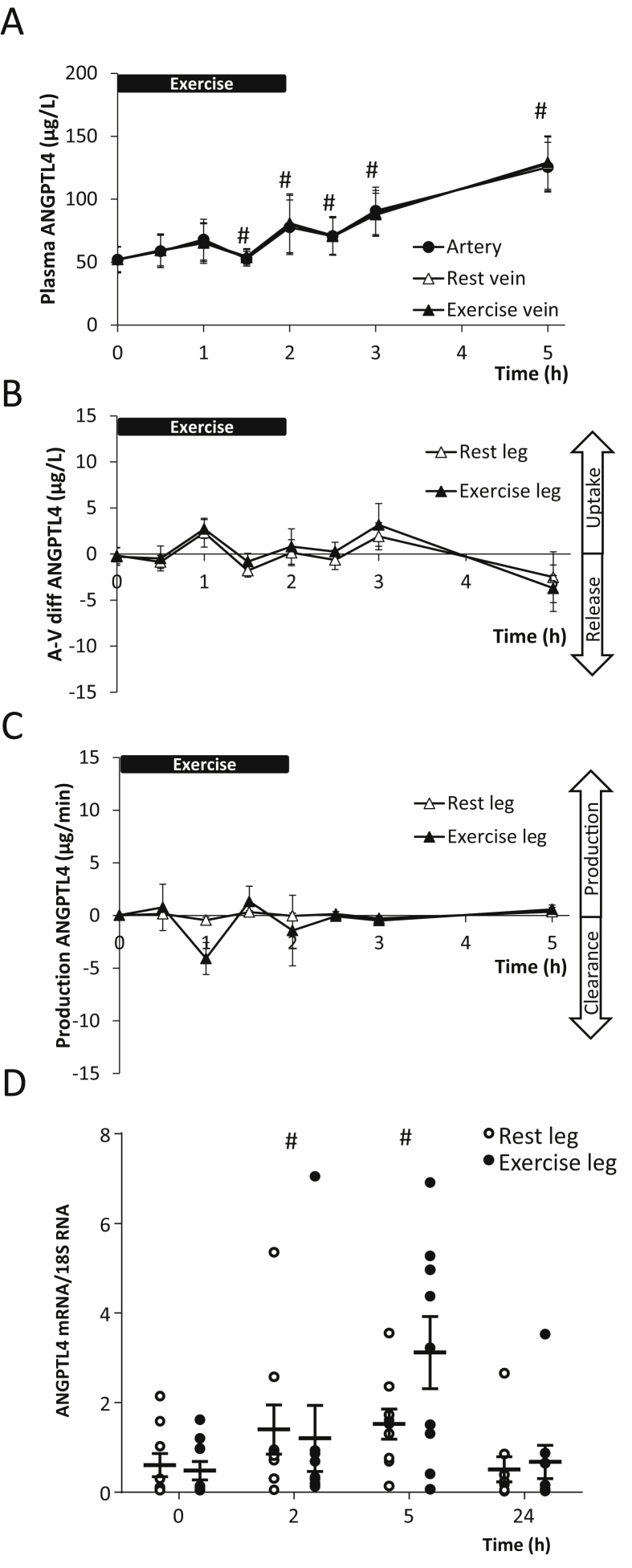
Two hours of one-legged exercise induced a 1.5-fold (P < 0.05) increase in plasma ANGPTL4 followed by a further increase into the recovery reaching 2.5-fold (P < 0.05) with no difference between arterial and femoral venous blood (Figure 1A). The arterial-to-venous difference showed no release or uptake of ANGPTL4 in the resting or the exercising leg at any time (Figure 1B) and thus, no production or clearance could be demonstrated (Figure 1C). ANGPTL4 mRNA content increased in skeletal muscle; however, no difference could be detected between the ANGPTL4 mRNA content in the exercising and resting leg (Figure 1D). Yet during recovery (time point 5 h), ANGPTL4 mRNA in the exercised leg tended to increase further (7.4-fold versus 3.1-fold) compared to the resting leg. The mRNA level was back to baseline in both legs on the following morning (time point 24 h).
Figure 1.
Nine healthy male subjects performed 2 h (0–2 h) of one-legged knee extensor exercise. A: Arterial (●) and femoral venous concentrations of angiopoietin-like protein 4 (ANGPTL4) from both the resting (Δ) and the exercising (▲) leg (the curves are superimposed) (MANOVA, Time: P < 0.0001, Group: P = 0.9929, Time × Group: P = 1.000). B: Arterial to venous differences over the resting (Δ) and the exercising (▲) leg of ANGPTL4 (Two-way ANOVA, Time: P = 0.0073, Group: P = 0.6064, Time × Group: P = 0.9954). C: Production (μg/min) or clearance of ANGPTL4 from the resting (Δ) and the exercising (▲) leg (Two-way ANOVA, Time: P = 0.1734, Group: P = 0.4460, Time × Group: P = 0.5284). D: ANGPTL4 mRNA content in the skeletal muscle biopsies from both the resting (○) and the exercising (●) leg (Two-way ANOVA, Time: P < 0.0001, Group: P = 0.9761, Time × Group: P = 0.7075). #: Significant changes from time point 0 h by a 2-way ANOVA followed by a Dunnett's post hoc test. P < 0.05 was considered significant.
To evaluate the separate effect of exercise and fasting on muscle ANGPTL4 mRNA content, an overnight fast followed by 3 h of exercise was compared with an overnight fast followed by rest. Irrespective of preceding exercise or rest, muscle ANGPTL4 mRNA content increased at 6 and 9 h of the trial (Supplemental Figure 1A) with no difference detected between groups. The mRNA level returned to baseline in both groups the following morning (24 h). Plasma FFA peaked with a 4.1-fold (P < 0.05) increase at the end of exercise (3 h). The exercise group had markedly higher plasma FFA during exercise and in the early recovery, whereas during the resting trial a gradual increase was observed reaching 1.7-fold (P < 0.05) after 9 h (Supplemental Figure 1B). Plasma glucagon peaked at 2.3-fold at end of the exercise (P < 0.05), whereas no change in glucagon could be detected during the rest trial (Supplemental Figure 1C). In contrast, plasma insulin decreased ∼40% (P < 0.05) during exercise, whereas in the rest trial insulin gradually decreased with a nadir at 9 h (Supplemental Figure 1D).
3.2. The hepato-splanchnic bed contributes to circulating ANGPTL4 during exercise in humans
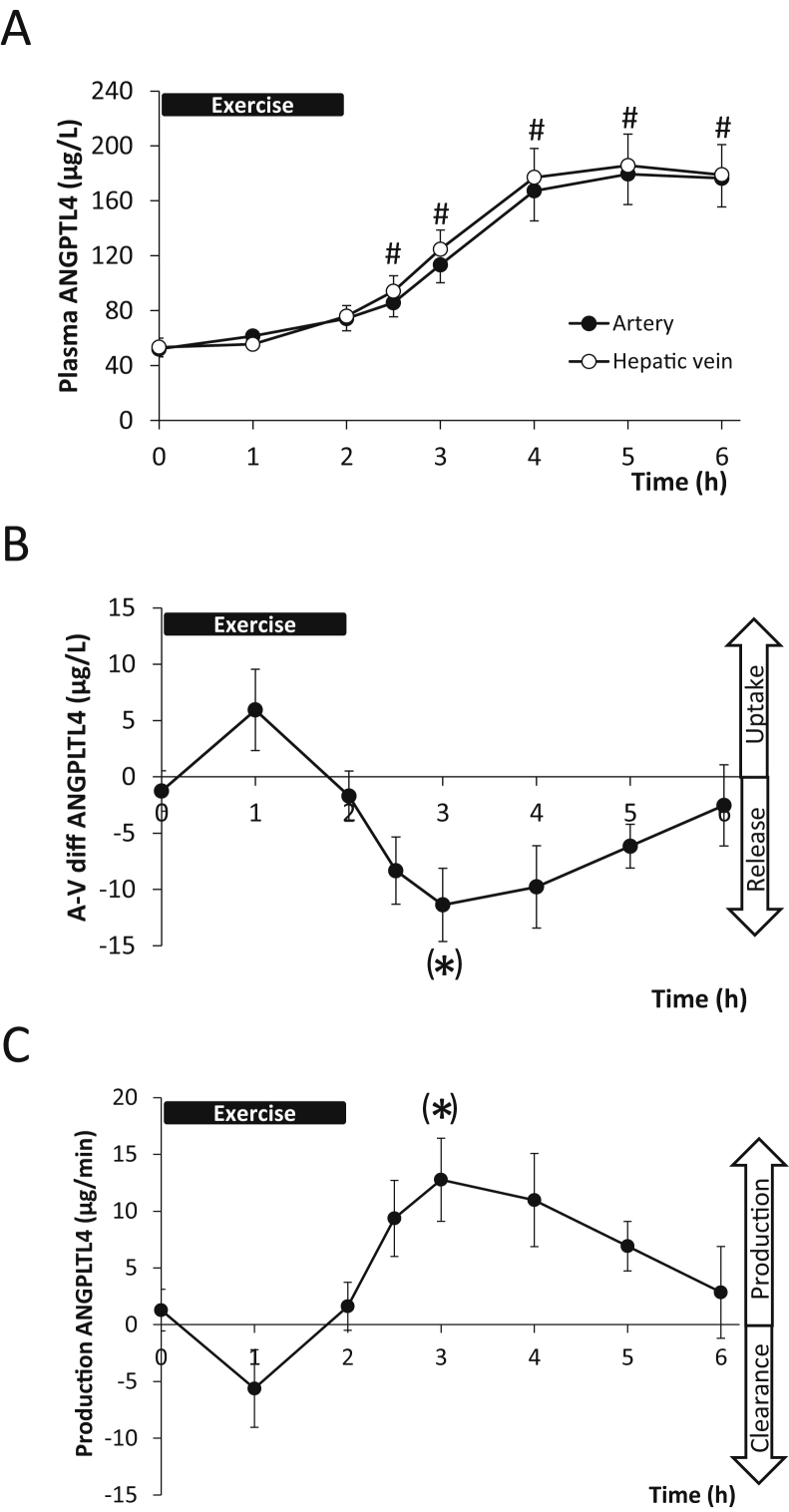
Since no release of ANGPTL4 from the legs could be demonstrated, we investigated the contribution from the hepato-splanchnic bed to exercise-induced plasma ANGPTL4 levels. ANGPTL4 increased 3.5-fold (P < 0.05) during recovery from 2 h semi-supine cycling (Figure 2A). Calculation of the arterial-to-venous difference (Figure 2B) and the production (Figure 2C) over the hepato-splanchnic bed revealed a release during recovery after exercise (one-way ANOVA (P < 0.01)). The post hoc Dunnett's test did reach borderline significance (P = 0.08 and P = 0.06, respectively, at time point 3 h). In addition, the area under the curve (AUC) revealed a significant exercise-induced release 1820 ± 426 μg × min/L (P < 0.05) and production 2038 ± 474 μg (P < 0.05).
Figure 2.
Hepato-splanchnic release of angiopoietin-like protein 4 (ANGPTL4) in healthy human subjects during exercise. A: ANGPTL4 concentration in the artery (●) and the hepatic vein (○) before, during (0–2 h) and into recovery after exercise (2–6 h) (Two-way ANOVA, Time: P < 0.0001, Group: P = 0.3727, Time × Group: P = 0.9883). B: Arterial-to-venous (μg/l) difference over the hepato-splanchnic bed. A negative value indicates a release whereas a positive value indicates an uptake (One-way ANOVA, Time: P = 0.0017). C: Hepato-splanchnic production of ANGPTL4 is calculated as arterial-to-venous difference multiplied by hepatic plasma flow (μg/min) (One-way ANOVA, Time: P = 0.0014). A positive value indicates a release into the circulation and a negative an uptake. #: Significant changes from time point 0 h after an 2-way ANOVA followed by a Dunnett's post hoc test. (*) designates a borderline significance of the post hoc Dunnett's test at P = 0.08 and P = 0.06 respectively at time point 3 h. The area under the curve for both arterial-to-venous difference (B) and production (C) were significantly different from zero (P < 0.05) by a t-test.
3.3. Inhibition of glucagon-to-insulin ratio and FFA blunts exercise-induced increase in plasma ANGPTL4
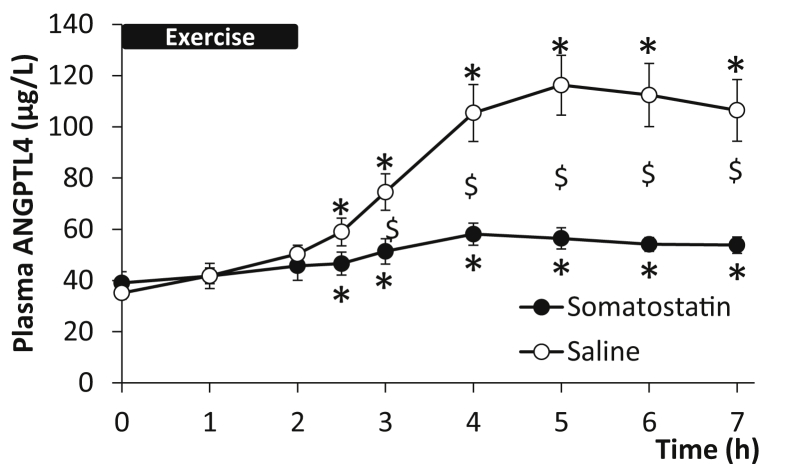
During exercise, both the increase in glucagon-to-insulin ratio and FFA are blunted by a pancreatic clamp [27]. Young healthy male volunteers performed a 2 h cycling exercise with a pancreatic clamp and – on a different day – the same exercise with saline infused as control. During the saline infusion, plasma ANGPTL4 increased 3.2-fold (P < 0.05) in the recovery from exercise while the increase in plasma ANGPTL4 was markedly reduced (a 1.4-fold; P < 0.05) with the pancreatic clamp (Figure 3).
Figure 3.
Inhibition of the increase in glucagon-to-insulin ratio by somatostatin infusion in healthy male subjects (n = 5) blunts the exercise-induced increase in plasma angiopoietin-like protein 4 (ANGPLT4) (Two-way ANOVA, Time: P < 0.0001, Group: P = 0.0002, Time × Group: P < 0.0001). The subjects performed 2 h of bicycling exercise (0–2 h) on two separate days. On the control day saline was infused (○) and on the pancreatic clamp day somatostatin (●) was infused, see Ref. [27] for details. $: Significant difference between groups by 2-way ANOVA; *: Significant change from the 0 h time point by one-way ANOVA. P < 0.05 was considered significant.
3.4. Regulation of plasma ANGPTL4 by the glucagon-to-insulin ratio at rest
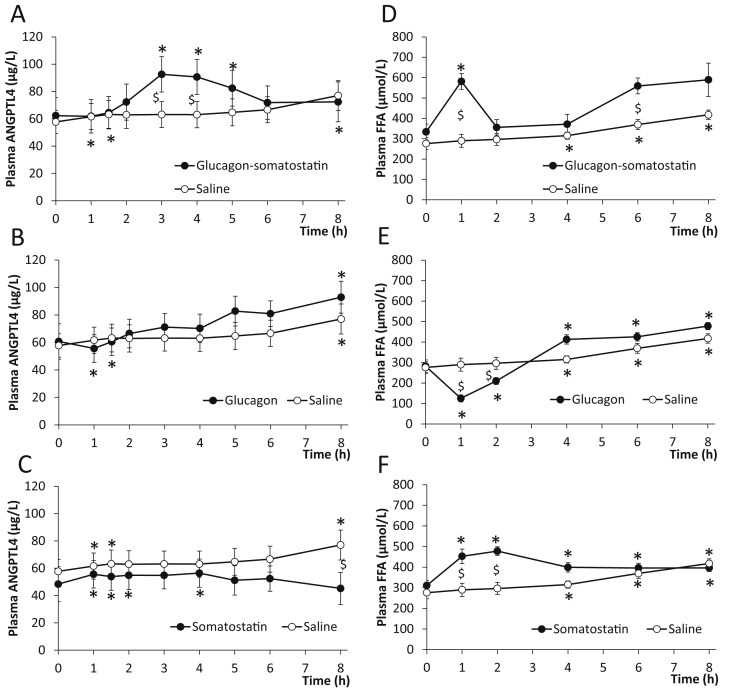
To evaluate whether an elevated glucagon-to-insulin ratio stimulates exercise-induced increase in plasma ANGPTL4 levels, ANGPTL4 was measured in samples from a hormone infusion study where the exercise-induced increase in the glucagon-to-insulin ratio was mimicked in resting healthy subjects [24]. When increasing the glucagon-to-insulin ratio for 1 h by infusion of glucagon to a plasma concentration of ∼100 pmol/L and reducing the insulin level by infusion of somatostatin, an increase in plasma ANGPTL4 manifested with similar kinetics to that developed in the recovery from exercise (Figure 4A). 1 h of saline infusion resulted in a small gradual increase in circulating ANGPTL4 (Figure 4A). When glucagon was administered without somatostatin co-infusion, insulin increased following blood glucose peak at ∼120 pmol/L [24] and no difference in plasma ANGPTL4 between the glucagon and saline infusion was observed (Figure 4B). Finally, infusion of only somatostatin that decreases circulating insulin [24] resulted in a similar minor change in plasma ANGPTL4 as the saline infusion (Figure 4C). The plasma FFA response was assessed as a further stimulus for ANGPTL4. Increasing glucagon for 1 h together with lowering of insulin by somatostatin resulted in a FFA peak at 1 h. During the saline infusion, FFA increased gradually (Figure 4D). When glucagon was administered without insulin inhibition, FFA was reduced due to the anti-lipolytic effect of insulin (Figure 4E). Finally, with infusing somatostatin alone plasma FFA increased (Figure 4F).
Figure 4.
Effect of increasing the glucagon-to-insulin ratio on plasma angiopoietin-like protein 4 (ANGPTL4) and free fatty acids (FFA) in resting healthy subjects (n = 10). For details, including the glucagon-to-insulin ratio of the study, see Ref. [24]. A–C: Changes in plasma ANGPTL4 concentration by infusion of glucagon and somatostatin (●) (Two-way ANOVA, Time: P < 0.0001, Group: P = 0.5002, Time × Group: P < 0.0001) (A), by infusion glucagon (●) (Two-way ANOVA, Time: P < 0.0001, Group: P = 0.6127, Time × Group: P < 0.0001) (B), by infusion of somatostatin (●) (Two-way ANOVA, Time: P = 0.0004, Group: P = 0.4160, Time × Group: P < 0.0001) (C) and by saline infusion as control (○) (A–C). Plasma concentrations of glucagon and insulin are presented in Ref. [24]. D–F: Changes in FFA concentration by infusion of glucagon and somatostatin (●) (Two-way ANOVA, Time: P < 0.0001, Group: P = 0.0152, Time × Group: P = 0.0515) (D), by infusion of glucagon (●) (Two-way ANOVA, Time: P < 0.0001, Group: P = 0.2870, Time × Group: P < 0.0001) (E), by somatostatin (●) (Two-way ANOVA, Time: P < 0.0001, Group: P = 0.0179, Time × Group: P < 0.0001) (F), and by saline (○) (D–F). $: Significant difference between groups by 2-way ANOVA. *: Significant change from the 0 h time point by one-way ANOVA. P < 0.05 was considered significant.
3.5. cAMP-protein kinase A signaling in HepG2 cells induces ANGPTL4 mRNA expression
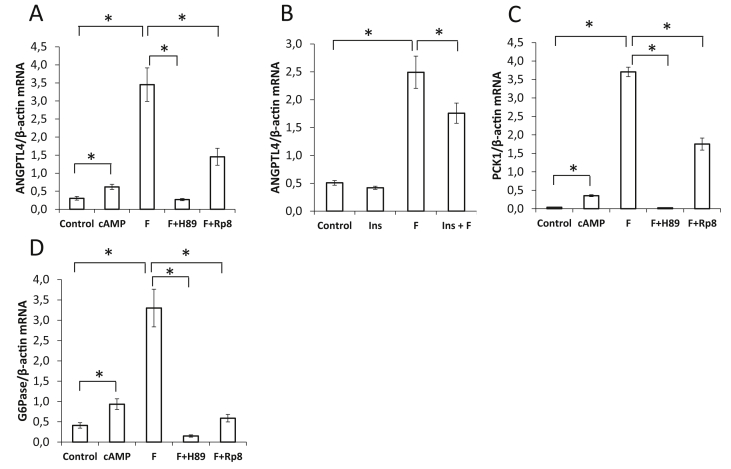
As glucagon increases cAMP and activates protein kinase A (PKA) in hepatocytes, the response of ANGPTL4 mRNA to stimulation of this pathway was evaluated using HepG2 cells. A cell-permeable cAMP analogue and the adenylate cyclase activator forskolin increased ANGPTL4 mRNA, which was reduced by two competitive inhibitors of PKA (Figure 5A). Insulin also inhibited the forskolin-induced ANGPTL4 mRNA induction (Figure 5B). Regulation pattern of ANGPTL4 mRNA by activators and inhibitors of the cAMP-PKA pathway is similar to the regulation of the known cAMP target genes phosphoenolpyruvate carboxykinase (Figure 5C) and glucose-6-phosphatase (Figure 5D). Thus the hepatic ANGPTL4 production can be stimulated via the cAMP-PKA pathway.
Figure 5.
Regulation of angiopoietin-like protein 4 (ANGPTL4), phosphoenolpyruvate carboxykinase (PCK1), and glucose-6-phosphatase (G6PC) mRNA content in HepG2 cells by the cAMP-protein kinase A (PKA) pathway. A–D: Cells were treated with 100 μM 8-(4-chlorophenylthio) (CPT)-cAMP (cAMP), 20 μM forskolin (F) 30 μM H89, 100 μM Rp8-8-bromoadenosine-3′,5′-cyclic monophosporothioate (Rp-8), or 100 nM insulin (Ins) as indicated for 2 h. mRNA abundance related to β-actin is shown as mean ± SEM. *: Significant difference evaluated by t-test. P < 0.05 was considered significant.
3.6. Influence of AMPK on ANGPTL4 mRNA regulation in human muscle cells
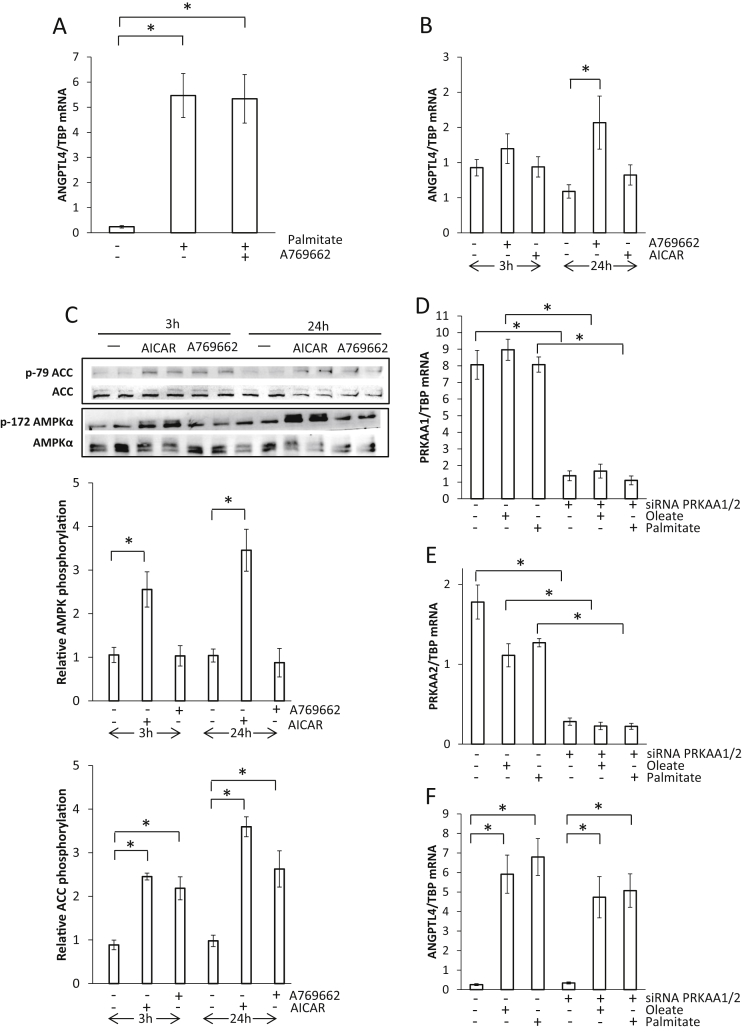
It has been suggested that activation of AMPK suppresses the fatty acid-dependent up-regulation of ANGPTL4 mRNA in skeletal muscle following exercise resulting in lower ANGPTL4 mRNA content in the exercising compared to the resting leg [17]. Since the present data do not indicate such difference, the effect of AMPK activation on ANGPTL4 abundance was studied in human primary muscle cells. Cells were stimulated with palmitate for 24 h leading to induction of ANGPTL4 mRNA (Figure 6A); however, this induction was not suppressed by co-stimulating with the AMPK activator (A769662) (Figure 6A). Treating muscle cells with only the AMPK activators A769662 and AICAR for 3 or 24 h did not decrease but rather induced a small increase in ANGPTL4 mRNA (Figure 6B). Activation of AMPK was visualized as phosphorylation of AMPK and the AMPK substrate ACC (Figure 6C). As reported previously, both AMPK activators increase phosphorylation of ACC to a similar extend, while AICAR is more effective than 100 μM A769662 to stimulate phosphorylation of AMPK [30]. To further investigate the role of AMPK, the catalytic subunits α1 and α2 were knocked-down by siRNA, and muscle cells were stimulated with either palmitate or oleate. Figure 6D and E display the efficiency of siRNA knock-down of AMPK catalytic subunit α1 and α2, respectively. The reduction of both AMPK catalytic subunits α1 and α2, did not affect the palmitate- or oleate-mediated induction of ANGPTL4 mRNA (Figure 6F). Taken together, the data do not indicate that activation of AMPK influences ANGPTL4 mRNA abundance in skeletal muscle.
Figure 6.
The effect of AMPK activation on angiopoietin-like protein 4 (ANGPTL4) mRNA abundance in differentiated human skeletal muscle cells. A: Cells were treated with 250 μM palmitate and 100 μM A769662 for 24 h. B: Cells were treated with 100 μM A769662 or 1 mM 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) for 3 or 24 h. C: Representative immunoblots of protein lysates (in duplicate) using the indicated antibodies. Relative band intensities normalized to total protein are shown as fold change compared with vehicle-treated cells (mean ± SEM). D–F: Cells were transfected with siRNA oligonucleotides against AMPK catalytic subunit α1 (PRKAA1) and α2 (PRKAA2) and treated with 250 μM palmitate and oleate for 24 h. ANGPTL4 mRNA abundance related to TATA box binding protein (TBP) measured by qPCR is shown as mean ± SEM. *: Significant t-test. P < 0.05 was considered significant.
4. Discussion
This study demonstrates that – despite the fact that ANGPTL4 mRNA levels increase in skeletal muscle after exercise – the protein is not released to the circulation from either an exercising or a resting leg. In contrast, the hepato-splanchnic bed contributed to the increase in plasma ANGPTL4 after exercise. The hormone infusion and “pancreatic clamp” studies suggest that increased glucagon-to-insulin ratio is a potent stimulus for production of ANGPTL4 in the hepato-splanchnic bed, and the in vitro HepG2 studies support that hepato-splanchnic ANGPTL4 release is mediated by a cAMP-PKA dependent up-regulation of ANGPTL4 mRNA abundance in hepatocytes.
During exercise pronounced up-regulation of ANGPTL4 mRNA takes place in both liver [14], [31] and skeletal muscle [14], [17] of mice. Here we provide in vivo evidence in humans for a release of ANGPTL4 from the liver during exercise, whereas the leg (e.g., skeletal muscle) does not contribute. We did not evaluate the participation of other tissues, e.g. the intestine, the spleen, or adipose tissue to the exercise-induced increase in plasma ANGPTL4. The liver as source for exercise-induced ANGPLT4 is in line with the finding of glucagon as the stimulus, particularly because skeletal muscle does not exhibit relevant glucagon receptor expression [32]. The finding adds ANGPTL4 to the list of exercise-regulated human “hepatokines”, including FGF21 [24] and follistatin [25], and mRNA data in mice suggest that exercise is a profound stimulus for the liver to secrete metabolically active factors – factors which may act on other tissues in an endocrine fashion [33]. Based on hepatic mRNA abundance in mice, the liver is also likely to be an important source of plasma ANGPTL4 at rest and during fasting [15]. However, prior to exercise no detectable release of ANGPTL4 was demonstrated in this study, suggesting that the hepato-splanchnic bed is not the only tissue releasing ANGPTL4 to the circulation. Due to the high mRNA content of ANGPTL4 in subcutaneous adipose tissue [1], [12], it could be that fat tissue is a source of circulating ANGPTL4 during fasting.
We provide evidence for activation of the cAMP/PKA pathway regulating ANGPTL4 expression. In experimental models, PPARα, PPARδ [1], and glucocorticoid receptor signaling [19] up-regulate ANGPTL4 mRNA. The data obtained both in vivo and in vitro provide evidence for a contribution of glucagon-dependent activation of the cAMP/PKA pathway to exercise-induced increase in ANGPTL4. Since an elevated glucagon-to-insulin ratio in vivo is accompanied by increased circulating FFA we could not differentiate between the two stimuli. However, regulation and kinetics of plasma FFA, were not paralleled by changes in plasma ANGPTL4. Of note, increasing FFA in 6 h in resting subjects has been demonstrated to increase plasma ANGPTL4 [15]; however, elevation of FFA for 6 h also induces insulin resistance.
Infusion of somatostatin alone resulted in increased FFA (Figure 4F), but had no effect on plasma ANGPTL4 (Figure 4C). The up-regulation of plasma ANGPTL4 occurs 1–2 h after the peak in the glucagon-to-insulin ratio (Figure 4A), pointing to transcriptional regulation. The cell culture experiments support that cAMP-mediated activation of PKA increases ANGPTL4 mRNA. The results are in accordance with data obtained in white and brown adipocytes, showing that β-adrenergic stimulation increases cAMP and ANGPTL4 mRNA and protein abundance [34]. There is little information about how the cAMP/PKA-dependent pathway activates ANGPTL4 expression. Activation of PKA increases the transcriptional activity of PPARs by mechanisms involving stabilization of the PPAR/RXR/DNA binding, but also by potential interaction with other cofactors [35]. During exercise, epinephrine activates the adenylyl cyclase and also leads to increased cAMP in hepatocytes, which may add to ANGPTL4 stimulation. The inhibitory effect of insulin, which is suppressed during exercise, seems to play an additional role in up-regulation of ANGPTL4, as glucagon infused without somatostatin does not induce an increase in ANGPTL4. Also, cortisol increases during exercise, but in humans prednisolone seems to reduce circulating ANGPTL4 [23], making cortisol an unlikely mediator of exercise-induced ANGPTL4.
The increase in ANGPTL4 mRNA in skeletal muscle without a release to the circulation suggests a para/autocrine function. ANGPTL4 protein is detected in muscle tissue by ELISA and with immunofluorescence staining [17] and is released to the media of primary human myotubes upon electric pulse stimulation [29]. ANGPTL4 may interact locally with LPL, which is also increased in skeletal muscle biopsies after a short-term fast together with PDK4, UCP3, and CPT1 [36]. ANGPTL4 inhibits LPL activity on the surface of capillary endothelial cells within muscle tissue [37], and interactions between ANGPTL4 and LPL were also observed intracellularly [6], [12]. Another possible function of intramuscular ANGPTL4 production is regulation of angiogenesis as suggested in relation to neovascularization and tendon healing [38]. Of note, increased muscle mRNA or enhanced intramuscular protein release has been shown for other secreted factors such as IL-8 or VEGF which do not necessarily translate into higher systemic levels [39], [40]. On the other hand, we cannot exclude a contribution of muscle ANGPTL4 to plasma concentrations under other circumstances.
In vitro, we did not observe the previously reported inhibitory effect of AMPK activation on ANGPTL4 mRNA in skeletal muscle cells [17]. With a similar approach, using the same concentration of AICAR (1 mM) and short-term (3 h) as well as long-term (24 h) treatment, no reduction of ANGPTL4 mRNA content was observed. A comparable conclusion was reached using the AMPK activator A769662 and knocking-down the catalytic subunits of AMPK. In contrast to cell culture experiments in murine C2C12 myotubes, we studied human myotubes. Down-regulation of ANGPTL4 by AMPK activation is tissue-specific, as demonstrated in brown but not white adipose tissue [34]. Thus, different results obtained in human and murine myotubes could be expected. We conclude that AMPK activation in exercising human muscles does not interfere with ANGPTL4 mRNA expression.
Data on regulation of ANGPTL4 mRNA in human skeletal muscle during exercise are inconsistent [14], [17]. Norheim et al. found increased ANGPTL4 mRNA in response to exercise before and after 12 weeks of training [14]. Catoire et al. also applying one-legged exercise, but without assessment of the arterial-to-venous difference – found no increase in ANGPTL4 mRNA in muscle in the exercising leg but only in the resting leg [17]. In both studies, no control for the effect of the short-term fasting was included. The present data underline the importance of the control setting because an overnight fast followed by exercise led to an almost identical increase in skeletal muscle ANGPTL4 mRNA as that of an overnight fast followed by rest. ANGPTL4 is increased by short-term fasting in skeletal muscle and when combined with acute exercise no major additive or inhibitory effects are observed.
ANGPTL4 is a mediator of hyperplasia of pancreatic α-cells [41], suggesting a feedback loop to the endocrine pancreas. Hence, conditions affecting the sensitivity to insulin and glucagon such as the metabolic syndrome and type 2 diabetes could be expected to dysregulate ANGPTL4. The metabolic syndrome and type 2 diabetes are characterized by insulin resistance that could increase ANGPTL4. Notably, ANGPTL4 mRNA abundance in adipose tissue is reduced in response to a hyperinsulinemic-euglycemic clamp in healthy subjects but not in patients with type 2 diabetes [42]. Circulating levels of ANGPTL4 are also related to markers of the metabolic syndrome [43] and are elevated in type 2 diabetes [44]. Thus, impaired insulin sensitivity is associated with elevated circulating ANGPTL4.
5. Conclusion
In conclusion, ANGPTL4 is released from the hepato-splanchnic bed but not the leg during exercise. The glucagon-to-insulin ratio is identified as an important regulator of ANGPTL4 plasma in humans, probably involving cAMP-PKA-driven hepatic ANGPTL4 expression. Further insight into the effects of hepatic release of ANGPTL4 could be of great value to understand exercise-induced inter-organ crosstalk.
Author contributions
BI, JSH, and PP designed and executed the human studies, analyzed data, and wrote and revised manuscript. JOC and NHS executed the human studies and wrote and revised manuscript. CH, MS, and CW executed the HEPG2 and primary muscle cell studies, analyzed data and wrote and revised manuscript. HUH, MHdA, and BKP designed the study and wrote and revised manuscript. All authors made substantial contributions to conception and design and revised the manuscript critically for important intellectual content. All authors have given their final approval of the manuscript to be published.
Acknowledgments
The authors are grateful for the excellent technical support provided by Heike Runge from the University Hospital Tübingen, Tübingen, Germany. The Centre for Physical Activity Research (CFAS) is supported by a grant from TrygFonden (BKP). During the study period, the Centre of Inflammation and Metabolism (CIM) was supported by a grant from the Danish National Research Foundation (DNRF55) (BKP). CIM/CFAS is a member of DD2 – the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant no. 09-067009 and 09-075724) (BKP). This study was further supported by a grant from the Danish Diabetes Academy supported by the Novo Nordisk Foundation to CW, AP Møller Fonden til Lægevidenskabens Fremme, Rigshospitalet Forskningsfond (BI), and by the Augustinus Foundation (PP), and in part by grants from the German Federal Ministry of Education and Research (BMBF) to the German Centre for Diabetes Research (DZD e.V.; No. 01GI0925).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.06.018.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Kersten S., Mandard S., Tan N.S., Escher P., Metzger D., Chambon P. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. Journal of Biological Chemistry. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 2.Kim I., Kim H.G., Kim H., Kim H.H., Park S.K., Uhm C.S. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochemical Journal. 2000;346(Pt 3):603–610. [PMC free article] [PubMed] [Google Scholar]
- 3.Koster A., Chao Y.B., Mosior M., Ford A., Gonzalez-DeWhitt P.A., Hale J.E. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 4.Yau M.H., Wang Y., Lam K.S., Zhang J., Wu D., Xu A. A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. Journal of Biological Chemistry. 2009;284:11942–11952. doi: 10.1074/jbc.M809802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukonina V., Lookene A., Olivecrona T., Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dijk W., Beigneux A.P., Larsson M., Bensadoun A., Young S.G., Kersten S. Angiopoietin-like 4 promotes intracellular degradation of lipoprotein lipase in adipocytes. The Journal of Lipid Research. 2016;57:1670–1683. doi: 10.1194/jlr.M067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray N.E., Lam L.N., Yang K., Zhou A.Y., Koliwad S., Wang J.C. Angiopoietin-like 4 (Angptl4) protein is a physiological mediator of intracellular lipolysis in murine adipocytes. Journal of Biological Chemistry. 2012;287:8444–8456. doi: 10.1074/jbc.M111.294124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Liu L.M., Wei L., Ye W.W., Meng X.Y., Chen F. Angiopoietin-like protein 4 improves glucose tolerance and insulin resistance but induces liver steatosis in high-fat-diet mice. Molecular Medicine Reports. 2016;14:3293–3300. doi: 10.3892/mmr.2016.5637. [DOI] [PubMed] [Google Scholar]
- 9.Romeo S., Pennacchio L.A., Fu Y., Boerwinkle E., Tybjaerg-Hansen A., Hobbs H.H. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nature Genetics. 2007;39:513–516. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo S., Yin W., Kozlitina J., Pennacchio L.A., Boerwinkle E., Hobbs H.H. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. Journal of Clinical Investigation. 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H.K., Youn B.S., Shin M.S., Namkoong C., Park K.H., Baik J.H. Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes. 2010;59:2772–2780. doi: 10.2337/db10-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robciuc M.R., Skrobuk P., Anisimov A., Olkkonen V.M., Alitalo K., Eckel R.H. Angiopoietin-like 4 mediates PPAR delta effect on lipoprotein lipase-dependent fatty acid uptake but not on beta-oxidation in myotubes. PLoS One. 2012;7:e46212. doi: 10.1371/journal.pone.0046212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alex S., Lichtenstein L., Dijk W., Mensink R.P., Tan N.S., Kersten S. ANGPTL4 is produced by entero-endocrine cells in the human intestinal tract. Histochemistry and Cell Biology. 2014;141:383–391. doi: 10.1007/s00418-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 14.Norheim F., Hjorth M., Langleite T.M., Lee S., Holen T., Bindesboll C. Regulation of angiopoietin-like protein 4 production during and after exercise. Physiological Reports. 2014;2:e12109. doi: 10.14814/phy2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kersten S., Lichtenstein L., Steenbergen E., Mudde K., Hendriks H.F., Hesselink M.K. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arteriosclerosis Thrombosis and Vascular Biology. 2009;29:969–974. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 16.van der Kolk B.W., Goossens G.H., Jocken J.W., Kersten S., Blaak E.E. Angiopoietin-like protein 4 and postprandial skeletal muscle lipid metabolism in overweight and obese prediabetics. Journal of Clinical Endocrinology and Metabolism. 2016;101:2332–2339. doi: 10.1210/jc.2015-4285. [DOI] [PubMed] [Google Scholar]
- 17.Catoire M., Alex S., Paraskevopulos N., Mattijssen F., Evers-van Gogh I., Schaart G. Fatty acid-inducible ANGPTL4 governs lipid metabolic response to exercise. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1043–E1052. doi: 10.1073/pnas.1400889111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staiger H., Haas C., Machann J., Werner R., Weisser M., Schick F. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 2009;58:579–589. doi: 10.2337/db07-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koliwad S.K., Kuo T., Shipp L.E., Gray N.E., Backhed F., So A.Y. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. Journal of Biological Chemistry. 2009;284:25593–25601. doi: 10.1074/jbc.M109.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin X., Rodrigues M., Umapathi M., Kashiwabuchi F., Ma T., Babapoor-Farrokhran S. Hypoxic retinal Muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3425–E3434. doi: 10.1073/pnas.1217091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang C.C., Yang M.W., Lin S.M., Kuo T.B., Chan K.H., Lin C.Y. Power spectral analysis of systemic arterial pressure signals during open heart surgery. Zhonghua Yi Xue Za Zhi (Taipei) 1995;55:421–426. [PubMed] [Google Scholar]
- 22.Mizutani N., Ozaki N., Seino Y., Fukami A., Sakamoto E., Fukuyama T. Reduction of insulin signaling upregulates angiopoietin-like protein 4 through elevated free fatty acids in diabetic mice. Experimental and Clinical Endocrinology and Diabetes. 2012;120:139–144. doi: 10.1055/s-0031-1291258. [DOI] [PubMed] [Google Scholar]
- 23.van Raalte D.H., Brands M., Serlie M.J., Mudde K., Stienstra R., Sauerwein H.P. Angiopoietin-like protein 4 is differentially regulated by glucocorticoids and insulin in vitro and in vivo in healthy humans. Experimental and Clinical Endocrinology and Diabetes. 2012;120:598–603. doi: 10.1055/s-0032-1321864. [DOI] [PubMed] [Google Scholar]
- 24.Hansen J.S., Clemmesen J.O., Secher N.H., Hoene M., Drescher A., Weigert C. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Molecular Metabolism. 2015;4:551–560. doi: 10.1016/j.molmet.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen J.S., Rutti S., Arous C., Clemmesen J.O., Secher N.H., Drescher A. Circulating follistatin is liver-derived and regulated by the glucagon-to-insulin ratio. Journal of Clinical Endocrinology and Metabolism. 2015;101:550–560. doi: 10.1210/jc.2015-3668. [DOI] [PubMed] [Google Scholar]
- 26.Hansen J., Brandt C., Nielsen A.R., Hojman P., Whitham M., Febbraio M.A. Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology. 2010;152:164–171. doi: 10.1210/en.2010-0868. [DOI] [PubMed] [Google Scholar]
- 27.Hansen J.S., Pedersen B.K., Xu G., Lehmann R., Weigert C., Plomgaard P. Exercise-induced secretion of FGF21 and follistatin are blocked by pancreatic clamp and impaired in type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2016;101:2816–2825. doi: 10.1210/jc.2016-1681. [DOI] [PubMed] [Google Scholar]
- 28.Wolf M., Chen S., Zhao X., Scheler M., Irmler M., Staiger H. Production and release of acylcarnitines by primary myotubes reflect the differences in fasting fat oxidation of the donors. Journal of Clinical Endocrinology and Metabolism. 2013;98:E1137–E1142. doi: 10.1210/jc.2012-3976. [DOI] [PubMed] [Google Scholar]
- 29.Scheler M., Irmler M., Lehr S., Hartwig S., Staiger H., Al-Hasani H. Cytokine response of primary human myotubes in an in vitro exercise model. American Journal of Physiology. Cell Physiology. 2013;305:C877–C886. doi: 10.1152/ajpcell.00043.2013. [DOI] [PubMed] [Google Scholar]
- 30.Goransson O., McBride A., Hawley S.A., Ross F.A., Shpiro N., Foretz M. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. Journal of Biological Chemistry. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoene M., Lehmann R., Hennige A.M., Pohl A.K., Haring H.U., Schleicher E.D. Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. The Journal of Physiology. 2009;587:241–252. doi: 10.1113/jphysiol.2008.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charron M.J., Vuguin P.M. Lack of glucagon receptor signaling and its implications beyond glucose homeostasis. Journal of Endocrinology. 2015;224:R123–R130. doi: 10.1530/JOE-14-0614. [DOI] [PubMed] [Google Scholar]
- 33.Hoene M., Weigert C. The stress response of the liver to physical exercise. Exercise Immunology Review. 2010;16:163–183. [PubMed] [Google Scholar]
- 34.Dijk W., Heine M., Vergnes L., Boon M.R., Schaart G., Hesselink M.K. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. Elife. 2015;4 doi: 10.7554/eLife.08428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazennec G., Canaple L., Saugy D., Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Molecular Endocrinology. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilegaard H., Saltin B., Neufer P.D. Effect of short-term fasting and refeeding on transcriptional regulation of metabolic genes in human skeletal muscle. Diabetes. 2003;52:657–662. doi: 10.2337/diabetes.52.3.657. [DOI] [PubMed] [Google Scholar]
- 37.Chi X., Shetty S.K., Shows H.W., Hjelmaas A.J., Malcolm E.K., Davies B.S. Angiopoietin-like 4 modifies the interactions between lipoprotein lipase and its endothelial cell transporter GPIHBP1. Journal of Biological Chemistry. 2015;290:11865–11877. doi: 10.1074/jbc.M114.623769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mousavizadeh R., Scott A., Lu A., Ardekani G.S., Behzad H., Lundgreen K. Angiopoietin-like 4 promotes angiogenesis in the tendon and is increased in cyclically loaded tendon fibroblasts. The Journal of Physiology. 2016;594:2971–2983. doi: 10.1113/JP271752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoier B., Prats C., Qvortrup K., Pilegaard H., Bangsbo J., Hellsten Y. Subcellular localization and mechanism of secretion of vascular endothelial growth factor in human skeletal muscle. FASEB Journal. 2013;27:3496–3504. doi: 10.1096/fj.12-224618. [DOI] [PubMed] [Google Scholar]
- 40.Della Gatta P.A., Cameron-Smith D., Peake J.M. Acute resistance exercise increases the expression of chemotactic factors within skeletal muscle. European Journal of Applied Physiology. 2014;114:2157–2167. doi: 10.1007/s00421-014-2936-4. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Zvi D., Barrandon O., Hadley S., Blum B., Peterson Q.P., Melton D.A. Angptl4 links alpha-cell proliferation following glucagon receptor inhibition with adipose tissue triglyceride metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:15498–15503. doi: 10.1073/pnas.1513872112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruge T., Sukonina V., Kroupa O., Makoveichuk E., Lundgren M., Svensson M.K. Effects of hyperinsulinemia on lipoprotein lipase, angiopoietin-like protein 4, and glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 in subjects with and without type 2 diabetes mellitus. Metabolism. 2012;61:652–660. doi: 10.1016/j.metabol.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Mehta N., Qamar A., Qu L., Qasim A.N., Mehta N.N., Reilly M.P. Differential association of plasma angiopoietin-like proteins 3 and 4 with lipid and metabolic traits. Arteriosclerosis Thrombosis and Vascular Biology. 2014;34:1057–1063. doi: 10.1161/ATVBAHA.113.302802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abu-Farha M., Al-Khairi I., Cherian P., Chandy B., Sriraman D., Alhubail A. Increased ANGPTL3, 4 and ANGPTL8/betatrophin expression levels in obesity and T2D. Lipids in Health and Disease. 2016;15:181. doi: 10.1186/s12944-016-0337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.