Abstract
The complex pathology of spinal cord injury (SCI), involving a cascade of secondary events and the formation of inhibitory barriers, hampers regeneration across the lesion site and often results in irreversible loss of motor function. The limited regenerative capacity of endogenous cells after SCI has led to a focus on the development of cell therapies that can confer both neuroprotective and neuroregenerative benefits. Stem cells have emerged as a candidate cell source because of their ability to self-renew and differentiate into a multitude of specialized cell types. While ethical and safety concerns impeded the use of stem cells in the past, advances in isolation and differentiation methods have largely mitigated these issues. A confluence of work in stem cell biology, genetics, and developmental neurobiology has informed the directed differentiation of specific spinal cell types. After transplantation, these stem cell-derived populations can replace lost cells, provide trophic support, remyelinate surviving axons, and form relay circuits that contribute to functional recovery. Further refining stem cell differentiation and transplantation methods, including combinatorial strategies that involve biomaterial scaffolds and drug delivery, is critical as stem cell-based treatments enter clinical trials.
Keywords: Induced pluripotent stem cells, Embryonic stem cells, Neuronal differentiation, Biomaterial scaffolds, Transcriptional reprogramming, Combination therapy
1. Introduction
1.1 Epidemiology of Spinal Cord Injury
The consequences of spinal cord injury (SCI) extend beyond the initial trauma, disrupting a variety of normal sensorimotor behaviors and having far reaching psychological and economic impacts to patients and healthcare systems. SCI disables more than 270,000 people in the United States with 12,500 new cases annually and estimated lifetime medical costs exceeding several million dollars per individual depending on the severity of functional loss (DeVivo et al. 2011, Singh et al. 2014, National Spinal Cord Injury Statistical Center 2014). SCI patients have lower life expectancies, lower employment rates, and lower chances for successful marriage compared to uninjured peers, trends which have not changed significantly in the last 40 years and which transcend international borders (Singh et al. 2014, National Spinal Cord Injury Statistical Center 2014).
1.2 Pathophysiology of Spinal Cord Injury
Each traumatic SCI is unique and often occurs alongside multiple systemic injuries. This complicates both immediate and long-term management, thus making a one-treatment-fits-all strategy difficult. The primary mechanical trauma results in the immediate compression and disruption of axons and vasculature, triggering a secondary cascade of events including ischemia and excitotoxic chemical release, which exacerbate local cell death and significantly expand the injury site (Tator and Fehlings 1991). The rapid necrosis results in a cystic cavity that is infiltrated by inflammatory cells, microglia, fibroblasts and reactive astrocytes. These form a dense, fibrous glial scar that expresses a multitude of inhibitory chemical cues and serves as a physical and chemical barrier that prevents regeneration across the lesion (Schwab and Bartholdi 1996, Fawcett and Asher 1999, Silver and Miller 2004, Donnelly and Popovich 2008). Wallerian degeneration, chronic demyelination, and muscle atrophy persist months or even years after the injury, limiting the potential for functional recovery over time (Totoiu and Keirstead 2005).
1.3 Remodeling after Spinal Cord Injury
While spinal circuits were previously thought to be rigid with limited regenerative capacity after injury, research in the past few decades has uncovered remodeling in spared tissue that can result in spontaneous functional recovery. Regeneration of descending tracts from the brain is not necessarily or solely responsible for regained function; plasticity has been observed at various hierarchal levels in the central nervous system (CNS), including the cerebral cortex, limbic structures, corticospinal tracts (CST), propriospinal neurons (PNs), motor neurons (MNs) and segmental interneurons (INs) (Courtine et al. 2009, Isa and Nishimura 2014). Detour circuits formed by CST fibers, PNs, and local IN networks have been attributed to renewed innervation of intact central pattern generators (CPGs) (Flynn et al. 2011, Filli and Schwab 2015). Indeed, cell populations around the lesion are well placed to receive and respond to the host of pro-regenerative molecular cues that are upregulated after injury. These include molecules that are also expressed during development and signal intrinsic axon regeneration pathways as well as direct connectivity and synaptogenesis (Hollis 2015). Perhaps as a result of these cues, endogenous neurogenesis in ependymal cells, resulting in the proliferation of oligodendrocytes and astrocytes, has been observed in both rodents and primates after spinal cord injury (Johansson et al. 1999, McTigue et al. 2001, Yang et al. 2006, Barnabe-Heider et al. 2010).
1.4 Stem Cell Therapy
Although early surgical interventions focus on reducing the amount of damage done by secondary processes and stabilizing the spinal cord, most SCI treatments emphasize neuroprotection, neuroregeneration, and rehabilitation. Cell-based therapies have gained popularity as a research focus because they can provide multiple benefits, including replenishment of lost cell types, scaffolding for axon regeneration, and the delivery of immuno-modulatory, neurotrophic and anti-inhibitory factors. Neural progenitor cells, mesenchymal stromal cells (MSCs), olfactory ensheathing glia, Schwann cells, and various pluripotent stem cells are among those currently investigated for their utility after SCI. In this review, we focus specifically on stem cells and strategies that have been used to differentiate and deliver them after SCI.
2. Stem Cell Sources
2.1 Multipotent Stem Cells
Two types of multipotent stem cells, unspecialized cells capable of differentiating into a discrete population of specific cell types of the same germ layer, are primarily investigated for treatment after SCI: MSCs and neural stem cells (NSCs) (Figure 1). MSCs are appealing clinically because they can differentiate into many non- hematopoietic lineages, but are also easily isolated from patient bone marrow, thus allowing for autologous transplantation. However, several studies have demonstrated that their utility is confined to trophic and immunomodulatory effects after SCI in both animals and patients (Hofstetter et al. 2002, Parr et al. 2007, Tetzlaff et al. 2011, Urdzikova et al. 2014). High variability between studies, limited functional recovery, poor engraftment, and questionable neuronal differentiation in vivo limit the use of MSCs for cell replacement (Tetzlaff et al. 2011).
Figure 1.
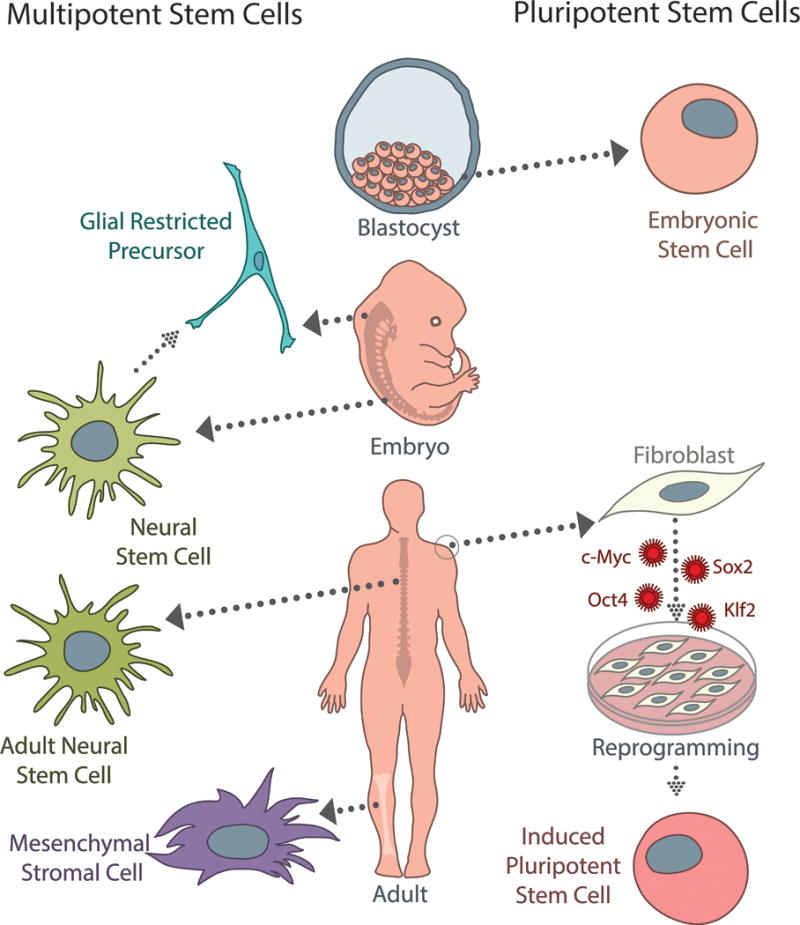
There are several sources of multipotent (left) and pluripotent (right) stem cells currently used for spinal cord injury. Neural stem cells (NSCs) can be derived from fetal or adult tissue, and are capable of differentiating into neurons, oligodendrocytes, and astrocytes. While not typically considered stem cells, glial-restricted precursors (GRPs) are a commonly studied, tri-potent population that can be isolated from neural stem cells or fetal tissue directly. GRPs differentiate into oligodendrocyte progenitor cells and two types of astrocytes. Mesenchymal stromal cells (MSCs) are an appealing population clinically because they can be isolated from adult bone marrow or peripheral blood; however, while they are capable of differentiating into a wide variety of cells types, the efficacy of neuronal differentiation is a specific concern for SCI treatment. Embryonic stem cells (ESCs) are a pluripotent population, which can give rise to cell types from all three germ layers; however, because they are derived from the inner cell mass of early blastocysts, ethical considerations limit their clinical potential. Induced pluripotent stem cells (iPSCs) can be generated from adult somatic cells (fibroblasts, melanocytes, cord or peripheral blood cells, adipose stem cells, etc.) by several different reprogramming methods using the Yamanaka factors (c-Myc, Sox2, Oct4, Klf2). While induction and reprogramming efficiencies remain a concern, iPSCs represent an autologous, patient-specific population that has significant clinical potential as the field progresses.
NSCs have been widely studied for transplantation after SCI because their maturation is restricted to glial and neuronal subtypes, thus reducing tumorgenicity while replenishing lost cells, aiding in remyelination and trophic factor secretion, and promoting axon regeneration. NSCs can be harvested from either adult or fetal spinal cord tissue and expanded as neurospheres in the presence of growth factors, including epidermal growth factor (EGF) and/or basic fibroblast growth factor (FGF2), prior to transplantation (Weiss et al. 1996, Shihabuddin et al. 1997, Uchida et al. 2000, Brewer and Torricelli 2007) (Figure 1). Fetal NSCs are generally heterogeneous, containing a mixture of neuronal and glial restricted progenitor cells, as well as self-renewing stem cells (Tetzlaff et al. 2011); in adults, ependymal cells along the central canal are NSCs that respond dramatically after SCI and constitute an endogenous source of stem cells to target (Weiss et al. 1996, Johansson et al. 1999, McTigue et al. 2001, Yang et al. 2006, Barnabe-Heider et al. 2010). Because NSCs can retain their positional identity through in vitro expansion, anatomical origin is an important consideration for cell replacement therapy and can be exploited to maximize integration into host spinal circuits (Hitoshi et al. 2002, Philippidou and Dasen 2013).
Functional recovery after NSC transplantation has been observed in a variety of animal models and can be enhanced by co-treatments with trophic factors (Tetzlaff et al. 2011). Though NSCs are capable of differentiating into all CNS types, both endogenous and transplanted NSCs in the spinal cord overwhelmingly become astrocytes and oligodendrocytes, with variable neuronal differentiation (Cao et al. 2001, Karimi-Abdolrezaee et al. 2006, Parr et al. 2008, Kriegstein and Alvarez-Buylla 2009, Barnabe-Heider et al. 2010). Furthermore, despite their many positive attributes, NSCs cannot be used for autologous transplantation and may be excluded from clinical use by contentions deriving them from fetal or post-mortem patient tissue. To circumvent this issue, many labs generate NSCs from pluripotent stem cells or directly reprogram them from somatic cells, such as fibroblasts.
2.2 Pluripotent Stem Cells
Pluripotent stem cells (PSCs) are characterized by their ability to replicate indefinitely while maintaining the ability to differentiate into specialized cell lineages from all three embryonic germ layers. Embryonic stem cells (ESCs) derived from the inner cell mass of pre-implantation blastocysts were the first isolated, differentiated, and proposed for use in cell therapy after SCI (Evans and Kaufman 1981, Thomson et al. 1998, McDonald et al. 1999)(Figure 1). In the absence of factors that maintain pluripotency, such as leukemia inhibitory factor (LIF), FGFs, or rho-associated protein kinase (ROCK) inhibitors (Smith et al. 1988, Williams et al. 1988, Watanabe et al. 2007), ESCs spontaneously differentiate; many strategies have been developed to preferentially induce differentiation along spinal neural lineages and even specify a positional identity along the neuraxis, discussed below. While several studies have shown benefits associated with ESC-derived neuronal transplants (Hatami et al. 2009, Johnson et al. 2010b), ethical concerns and potential side effects including teratoma formation caused by the presence of undifferentiated ESCs prevent clinical application.
Induced pluripotent stem cells (iPSCs) have become an appealing alternative to ESCs and allow for autologous transplantation of patient-derived cells. By reprogramming somatic cells with a defined set of transcription factors, the Yamanaka factors, adult mouse and human cells revert to a pluripotent state, thus alleviating ethical impediments caused by the destruction of embryos (Takahashi and Yamanaka 2006, Takahashi et al. 2007) (Figure 1). Differentiation strategies initially developed for ESCs have been translated to iPSCs. However, similar to ESCs, transplantation of iPSCs retains a high risk of immune rejection and tumorgenesis, the latter of which can be compounded by virally overexpressing oncogenic transcription factors for iPSC generation. Safer, non-viral methods have been developed, but spontaneous reversion to a pluripotent state is a unique concern for iPSC-derived cells and contributes to the tumorgenic potential (Saric et al. 2008, Chen et al. 2013). Considerations including the mechanism of reprogramming, the cell source, and the differentiation process are important to weigh in designing therapeutic strategies involving pluripotent stem cells (Khazaei et al. 2014).
3. Directing Neural Cell Fate
3.1 Cell-Cell Interactions
Neural induction is a default pathway for PSCs, but differentiation efficiency varies depending on both intrinsic cell line qualities, as well as the extracellular environment (Bain et al. 1995, Munoz-Sanjuan and Brivanlou 2002, Hu et al. 2010). Spatial cues play an important role in cell fate determination and can dramatically alter the efficiency of neural induction. Two dimensional (2D) adherent monolayer cultures or co-culture with feeder cells have been used with varying success, though the use of micropatterned surfaces or manual selection of spontaneous rosette structures can help direct neuronal morphogenesis (Zhang et al. 2001, Knight et al. 2015). Allowing cultures to grow in three dimensions (3D), instead of in 2D, generates environmental conditions that more closely mimic those found in vivo, and can consequently enhance neuronal differentiation. Non-adherent cultures that allow PSCs to aggregate into embryoid bodies (EBs) have traditionally been used, since EBs develop similarly to the pre-gastrulation embryo and are primed for application of patterning factors that can direct more specific cell types (Weitzer 2006). A problem with EBs is that they are heterogeneous and disorganized, which frequently results in variable neural induction. To improve differentiation consistency, several methods have been developed to control EB dimensions beyond static suspension culture, including the hanging drop method, cell encapsulation, microwell or microfabrication methods, and the use of bioreactors (Rungarunlert et al. 2009).
New 3D culture systems are now gaining traction. PSC-derived organoid cultures, evolved from EBs, have become appealing for in vitro disease modeling and as a source for complex cell populations (Lancaster and Knoblich 2014, Huch and Koo 2015). Several protocols exist to generate organoids for individual brain regions or heterogeneous cerebral organoids containing multiple regions (Lancaster and Knoblich 2014). The reliance on random structure formation can be avoided by using defined 3D scaffolds. Using Matrigel or a synthetic matrix separately, the Tanaka group was able to generate neuroepithelial cysts from single mouse ESCs that undergo classic dorsoventral patterning when in the presence of retinoic acid (RA)(Meinhardt et al. 2014). These studies demonstrate not only the feasibility of creating complex, organized spinal tissue, but also how bioengineered 3D scaffolds can be used to finely control the stem cell microenvironment. Such scaffolds can also be designed as transplantation vehicles for SCI treatment (Willerth and Sakiyama-Elbert 2008, Murphy and Atala 2014). We have done significant work optimizing neural differentiation within fibrin gels that have been modified for controlled release of growth factors or co-delivery of anti-inhibitory molecules. After transplantation, these combinatorial treatments are able to improve cell survival, differentiation, and neurite growth at the host-graft interface (Willerth et al. 2006, Willerth et al. 2007, Willerth et al. 2008, Johnson et al. 2010b, McCreedy et al. 2014b, Wilems et al. 2015).
3.2 Mimicking Development to Drive Differentiation
The traditional method of converting PSCs to CNS cells involves recapitulating the developmental environment of the neural tube via exposure to growth factors and morphogens (Figure 2). Induction efficiency and lineage specification can be adjusted by modulating the concentration and exposure duration of these factors. Early induction protocols called for the use of harvested or recombinant protein, but a more sophisticated understanding of the signaling, transcriptional, and epigenetic cues responsible for cell fate specification in recent years has enabled the use of small molecules to direct differentiation in lieu of proteins, which can be expensive and labile.
Figure 2.
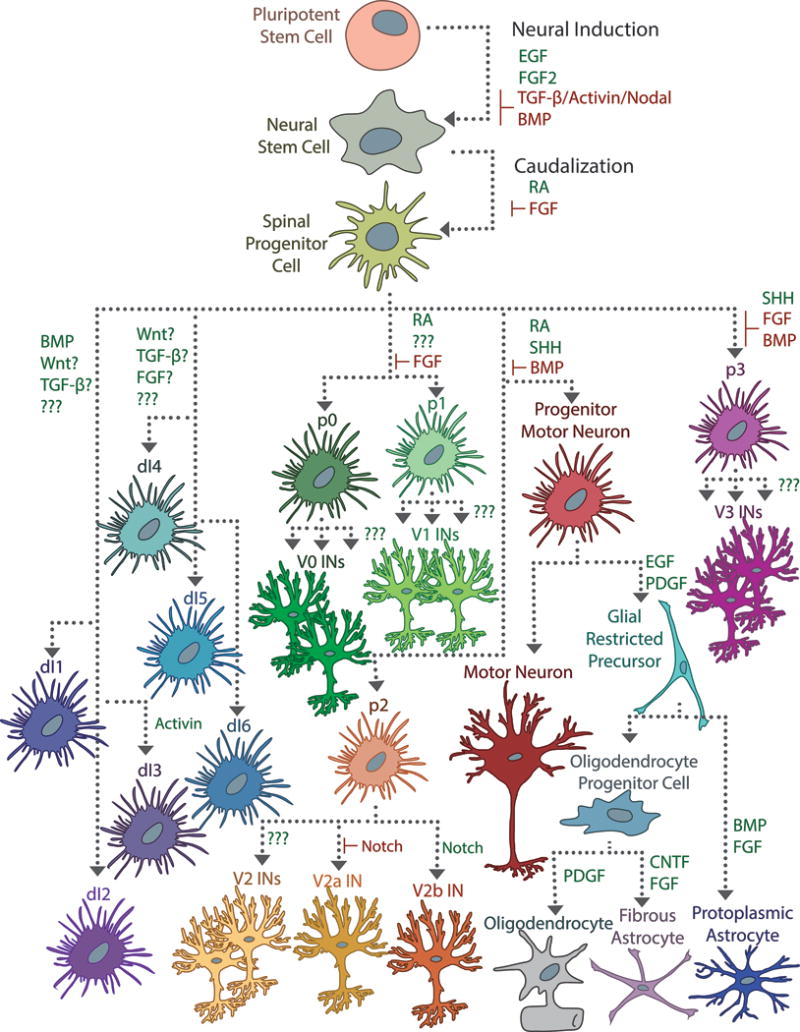
Directing differentiation of pluripotent stem cells is traditionally achieved by triggering the signaling cascades responsible for cell fate determination. Neural induction from pluripotent cells typically begins with conversion into a neural stem cell (NSC), which may involve stimulating EGF and FGF2 signaling and inhibiting BMP and TGF-β/Activin/Nodal signaling. Subsequent exposure with RA caudalizes the NSCs and ensures a spinal identity. A complex interplay of signaling events is responsible for the dorsoventral patterning of the spinal cord, made more opaque by the importance of exposure duration and cell-cell signaling events. In the developing spinal cord, Shh from the notochord and floor plate interact with RA to induce differentiation of ventral progenitor cells (p0–p3, pMN), which mature into motor neurons, oligodendrocytes, astrocytes, and a variety of ventral interneuron populations. A combination of BMP, Wnt, FGF, and TGF-β signaling induces differentiation of the dorsal progenitor populations (dI1–dI6), but the precise mechanisms are poorly understood. Some specific factors that influence progenitor subspecialization have been determined experimentally in vitro, especially with regards to glial-restricted progenitor cells (GRPs), but for most interneuron populations, these are unknown.
3.2.1 Neural Induction
Neural induction and the generation of nestin+ NSCs is the first step towards differentiating specific spinal cell types from PSCs. While the spinal cord is a caudal CNS structure, the neural cells from which it is formed initially acquire a rostral character through a combination of transforming growth factor (TGF)-β, bone morphogenetic protein (BMP), FGF, and Wnt signaling (Wilson and Edlund 2001, Munoz-Sanjuan and Brivanlou 2002). Forming EBs results in spontaneous NSC production, but as previously discussed, the process can give variable neuronal yields (Bain et al. 1995). Taking cues from development, several groups have efficiently induced a neural fate by inhibiting TGF-β/Activin/Nodal and BMP signaling pathways in vitro using recombinant inhibitors or small molecule antagonists (Smith et al. 2008, Chambers et al. 2009, Patani et al. 2009, Chambers et al. 2012). EGF and FGF2 have been used to isolate NSCs from primary tissue, but can also promote survival and induction of NSCs from PSCs (Okabe et al. 1996, Shihabuddin et al. 1997, Reubinoff et al. 2001, Joannides et al. 2007, Chambers et al. 2012). The timing of growth factor exposure is important; after NSCs have developed, inhibition of FGF2 in vitro aids in the differentiation of NSCs into neurons and thus improves differentiation efficiency (Joannides et al. 2007, Chambers et al. 2012).
3.2.2 Caudalization with Retinoic Acid
Endogenous neural progenitors acquire a spinal identity in response to caudalizing signals, predominantly RA, which is involved in both anteroposterior and dorsoventral patterning of the neural tube (Durston et al. 1998, Muhr et al. 1999). RA inhibits FGF signaling and triggers differentiation in the neuroepithelium (Diez del Corral et al. 2003, Novitch et al. 2003). Treatment of ESCs with RA was among the first efforts to obtain spinal neurons; however, when used for prolonged durations, RA functions as a differentiation agent that significantly impacts neuronal subtype specification in the ventral spinal cord (Bain et al. 1995, Jessell 2000, Wilson and Maden 2005, Maden 2007). It is of note that RA serves many neuroprotective and neuroregenerative roles in the adult CNS and could have value for SCI therapy beyond its use in neural induction (Maden 2007).
3.2.3 Growth Factor-Mediated Patterning
A combination of factors released from mesodermal structures around the neural tube is responsible for neuronal differentiation: RA from the somites, sonic hedgehog (Shh) from the notochord, BMPs, EGFs, and Wnts from the ectoderm and roof plate, and FGFs from the posterior of the neural tube (Lee and Jessell 1999, Jessell 2000). While research parsing endogenous differentiation pathways has been ongoing for more than two decades, new genes and transcription factors are continually added to the current models as spinal neuron subtypes are being defined. The signaling involved in cell fate specification is synergistic, time-dependent, and concentration-dependent. The sensitivity of differentiation means slight changes to PSC induction protocols can significantly alter the PSC-derived neuronal population distribution, but also allows for optimization of specific subpopulations.
In the ventral spinal cord, RA and Shh work together to induce four classes of ventral interneuron progenitors (p0–p3), which give rise to various ventral interneurons, as well as a progenitor motor neuron (pMN) domain, which gives rise to motor neurons, astrocytes, and oligodendrocytes (Briscoe and Ericson 2001, Wilson and Maden 2005). These neuronal populations contribute to central pattern generator networks in the spinal cord that are critical for normal motor function and coordination (Arber 2012). A significant body of work by the Jessell lab has demonstrated the importance of Shh-dependent, differential homeobox transcription factor expression in generating the discrete ventral progenitor domains, as well as specification of neuronal subtypes (Briscoe and Ericson 2001). RA and Shh are necessary and sufficient to induce ventral spinal differentiation from PSCs; by modulating the relative concentrations and durations of signaling factors, they and others have been able to optimize induction protocols to enrich for specific ventral populations (Wichterle et al. 2002, Okada et al. 2004, Li et al. 2005, Kim et al. 2009, Dessaud et al. 2010, McCreedy et al. 2012, Brown et al. 2014, Xu and Sakiyama-Elbert 2015).
Little work has been done to develop PSC-derived populations from the dorsal spinal cord, in part because the molecular mechanisms involved in diversification are not as well established as those in the ventral cord (Lee and Jessell 1999, Helms and Johnson 2003). Dorsal interneurons are primarily associated with sensory circuits, though dI3 and dI6 interneurons have been implicated in motor coordination (Dyck et al. 2012, Bui et al. 2013). There are six classes of early-born dorsal interneurons (dI1–dI6) as well as two late-born ones (dILA, dILB). These domains can be further subdivided by their dependence on BMP signaling from the roof-plate for development: dependent Class A (dI1–dI3) and independent Class B (dI4–dI6, dILA/B). Similar to Shh signaling in the ventral spinal cord, gradients of BMP activity contribute to the formation of discrete homeobox transcription factor expression domains that give rise to Class A progenitor cells (Timmer et al. 2002). Treatment of EBs with RA alone induces differentiation of dorsal Class B interneurons and V0 and V1 interneurons, which are less dependent on roof plate and floor plate signaling for development (Kim et al. 2009). Wnt, FGF, and EGF signaling are important for initial neural crest development, but their roles in dorsal interneuron diversification are less well understood (Lee and Jessell 1999, Lee et al. 2000, Muller et al. 2002, Helms and Johnson 2003). At least one group has shown that a combination of these developmental factors directs dorsal interneuron differentiation in mouse embryonic stem cells (Murashov et al. 2005). Others have bypassed neural induction entirely and used transcriptional programming to obtain sensory neurons from fibroblasts (Blanchard et al. 2015).
3.2.4 Refining Inductions for Subtype Specification
There are many subpopulations of neurons and glia beyond the cardinal classes defined during spinal cord development. While spinal oligodendrocytes and astrocytes arise from the pMN domain, which requires RA/Shh signaling, they also occur elsewhere in Shh independent ways and can require the addition of FGFs, BMPs, tumor necrosis factors (TNF), interleukins (ILs), platelet-derived growth factor (PDGF) and/or ciliary neurotrophic factor (CNTF) signaling to direct their differentiation in vitro and in vivo (Benveniste et al. 2005, Nistor et al. 2005, Krencik and Zhang 2011, Roybon et al. 2013, Douvaras and Fossati 2015, Goldman and Kuypers 2015). Many interneuron populations have not yet been classified by unique transcription factor profiles, and the populations that have been characterized are certain to be even more subdivided. As additional spinal subtypes and their differentiation mechanisms are identified, it will be important to translate those to optimize PSC induction protocols. For example, Notch signaling acts as a transcriptional switch between inhibitory and excitatory interneuron subpopulations in both dIL and V2 interneurons, and possibly contributes to diversification elsewhere in the spinal cord (Mizuguchi et al. 2006, Del Barrio et al. 2007). By adding a Notch inhibitor to an RA/Shh induction, V2a interneurons can be selectively enriched in PSC-derived cultures (Brown et al. 2014).
There has recently been interest in developing PSC-derived populations that not only comprise of defined neuronal subtypes, but also have a defined positional identity, as these are important for migration and integration into host motor circuits (Philippidou and Dasen 2013). Much of this work has been done in motor neurons, which have genetically defined subtypes that are anatomically and functionally distinct. Subtle changes to the induction can alter Hox signaling and thus positional identity, including the type of signaling agent used (inhibitor versus recombinant protein), the exposure time and sequence of induction factors, and the addition factors that stimulate FGF and Wnt/β-catenin signaling (Maden 2007, Patani et al. 2009, Peljto et al. 2010, Patani et al. 2011, Davis-Dusenbery et al. 2014, Lippmann et al. 2015). Stem cell therapies that match host cell populations as closely as possible will provide the best opportunity for success.
3.3 Transcriptional Reprogramming
Driving differentiation of PSCs in a chemically defined environment has limitations, especially in human cells where induction and maturation can take months. Despite efforts to generate specific spinal cell types, the co-dependence in signaling involved in neuronal diversification necessarily leads to heterogeneity in cultures that use common induction factors. Overexpressing transcription factors directly responsible for neuronal subtype specification is a way to obtain more homogeneous cultures while minimizing induction time. Overexpression of just Ngn2 or NeuroD1 in PSCs results in efficient conversion into functionally active neurons (Zhang et al. 2013). Precise manipulation of transcription factor activation is important for cell type specificity; spinal motor neurons can be generated using a combination of Ngn2, Isl1, and Lhx3 (LIN factors), but replacement of Lhx3 with Phox2a yields cranial motor neurons (Hester et al. 2011, Mazzoni et al. 2013).
Direct reprogramming from one somatic cell type to another has become possible, bypassing an intermediate pluripotent state. Avoiding iPSC generation can potentially reduce the time to generate a specific cell type and minimizes the risk of teratoma formation upon transplantation. Several combinations of transcription factors convert fibroblasts to directly reprogrammed NSCs (iNSCs), though it is possible to generate tripotent iNSCs that can become neurons, astrocytes and oligodendrocytes by expressing just Sox2 or Oct4 (Kim et al. 2011, Han et al. 2012, Lujan et al. 2012, Ring et al. 2012, Mitchell et al. 2014). Vierbuchen et al. demonstrated that expression of Brn2 (Pou3f2), Ascl1, and Myt1l (BAM factors) were sufficient to convert mouse fibroblasts to functionally mature, excitatory neurons (iNs) (Vierbuchen et al. 2010). A variety of distinct neuronal and glial populations have since been generated from both mouse and human somatic cells, including dopaminergic neurons, sensory neurons, motor neurons, oligodendrocyte precursor cells (OPCs), and astrocytes. (Caiazzo et al. 2011, Son et al. 2011, Najm et al. 2013, Yang et al. 2013, Marro and Yang 2014, Blanchard et al. 2015, Caiazzo et al. 2015). Of interest for SCI, astrocytes have been directly converted to iNSCs and functional neurons both in vitro and in vivo (Corti et al. 2012, Guo et al. 2014).
Very low neuronal conversion efficiencies—hovering near 1% using certain protocols— remain a hurdle facing direct reprogramming as a clinically relevant method for cell replacement. Depending on the protocol efficiency, mitotic cells, such as fibroblasts, must be significantly expanded in order to obtain a sufficient quantity of desirable cells, and then purified prior to transplantation, which can be expensive and time consuming. The sub-optimal conversion rates may not induce therapeutic outcomes that overcome the risks associated with reprogramming. Several ways to enhance the reprogramming process are in development. For example, microRNAs can behave synergistically with the BAM factors to more efficiently convert iNs (Ambasudhan et al. 2011, Yoo et al. 2011). iPSC reprogramming can be optimized by blocking DNA and histone methylation or modulating chromatin structure to open transcriptional binding sites (Huangfu et al. 2008, Lin et al. 2009, Luna-Zurita and Bruneau 2013, Tso and McKinnon 2015). Direct reprogramming has recently been achieved by delivering small molecules (Hu et al. 2015, Li et al. 2015); this is clinically relevant, as it avoids potentially hazardous viral transductions and presents the opportunity to bioengineer delivery systems to convert endogenous cells into cell types of interest. This approach may be more viable to replenish neurons by transcriptional reprogramming, since transplanted iNSCs suffer from poor engraftment (Hong et al. 2014).
4. Transplantation Outcomes of Stem Cell-Derived Spinal Types
4.1 Considerations for Transplantation
The optimal window for cell therapy is generally thought to be within two to four weeks of the initial trauma, when the acute phase of the secondary injury has abated and while severed axons and surviving interneuron populations are still capable of responding to the host of axon guidance molecules and neurotrophins upregulated after SCI (Schwab and Bartholdi 1996, Hayashi et al. 2000, Coumans et al. 2001, Widenfalk et al. 2001, Bareyre et al. 2004, Conta and Stelzner 2004, Courtine et al. 2008, Lang et al. 2012, Hollis 2015). Multiple groups have observed that delayed transplantation into a more chronic injury is beneficial compared to acute treatment (Coumans et al. 2001, Karimi-Abdolrezaee et al. 2006), possibly because of alterations in the inflammatory response that allow for better cell survival in both endogenous and transplanted populations (Donnelly and Popovich 2008, Moreno-Manzano et al. 2009, Rolls et al. 2009, David et al. 2012). Beyond that timeframe, the glial scar stabilizes and the upregulation of pro-regenerative factors attenuates, thus reducing the potential for regeneration (Satake et al. 2000, Widenfalk et al. 2001, Silver and Miller 2004, Cregg et al. 2014). The intended mechanism by which the transplant stimulates recovery is an important consideration when choosing both the time of intervention and the specific cell population for transplantation (Figure 3) (Bradbury and McMahon 2006). For example, the replenishment of neuronal populations is critical to take advantage of functional relay mechanisms (Bareyre et al. 2004, Courtine et al. 2008), but post-mitotic neurons suffer upon transplantation, so using plastic progenitor populations may be a more reasonable approach. At the same time, inhibitory proteoglycans and trophic factors secreted by reactive astrocytes have differential effects on stem cell differentiation and survival, which may significantly alter the composition of the transplant. (Silver and Miller 2004, Rolls et al. 2009). Transplanting glia, which have roles in trophic support or immunomodulation, may mitigate some inhibition from the lesion (Zeis et al. 2015, Liddelow and Hoyer 2016). Anecdotal evidence from Asterias’ clinical trial suggests that, even after cessation of immunosuppression, human ESC-derived OPCs that successfully integrated into host networks can survive years after transplantation (Asterias 2016).
Figure 3.
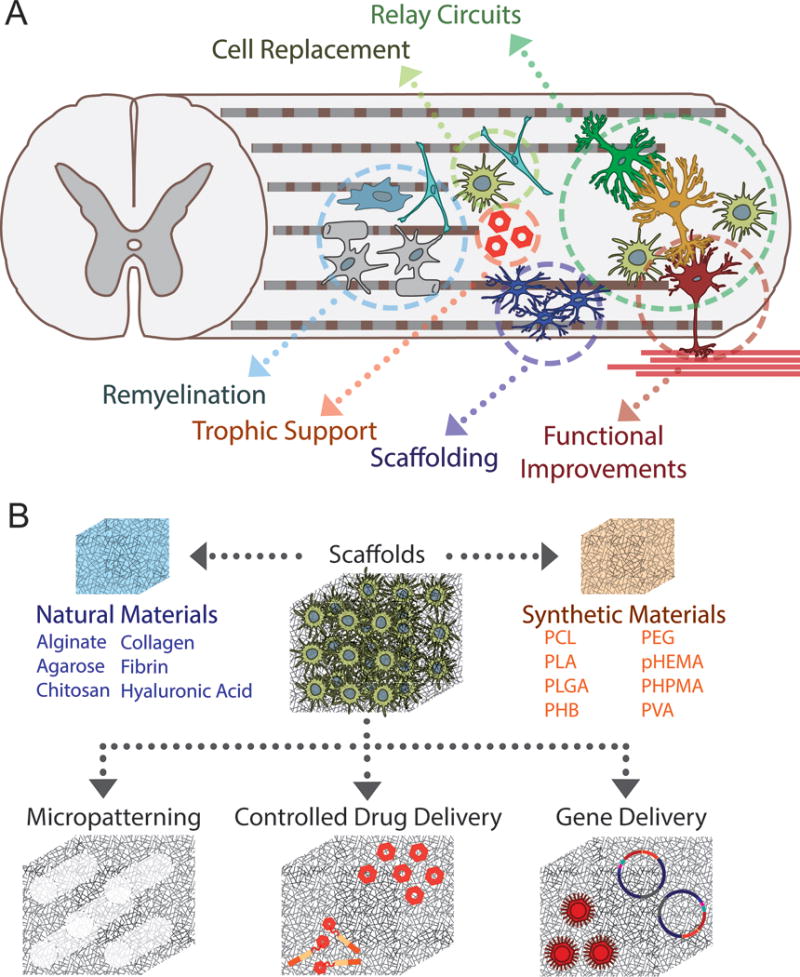
(A) Transplantation of stem cell-derived spinal populations into the SCI lesion has multiple benefits. NSCs can differentiate into mature neuronal and glial populations to replace lost or damaged cells. These populations can serve as relay circuits between intact tissue across the lesion, and thus promote functional improvements as determined by electrophysiological and behavioral means. Stem cell-derived oligodendrocytes and astrocytes can remyelinate damaged axons and provide scaffolding for regenerating axons, as well provide trophic support through the secretion of growth factors. (B) Compared to direct injection of cells into the lesion or the intrathecal space or the delivery of micro-encapsulated cells, biomaterial scaffolds represent a better opportunity for a combinatorial therapy. Modifications including micropatterning, drug delivery, and gene delivery can be achieved to support and guide regeneration at the site of injury.
4.2 PSC-derived NSCs
PSC-derived NSC transplants have numerous neuroprotective and regenerative benefits beyond cell replacement including immunomodulation, the secretion of neurotrophic factors to enhance endogenous cell survival, axon regeneration, and remyelination initiated by NSC-derived oligodendrocytes. Most studies demonstrate that transplants are capable of differentiating into neurons, astrocytes, and oligodendrocytes in vivo, though the ratios may differ depending on the method of NSC differentiation, the SCI model, and time point of investigation (Nori et al. 2011, Kobayashi et al. 2012). The extent of functional recovery also varies between studies. Significant recovery has been observed after human iPSC-derived NSC transplantation with no tumorgenicity and integration into host neural circuits, prompting consideration for clinical trials (Nori et al. 2011, Kobayashi et al. 2012). However, others have shown only marginal behavioral improvements despite good cell survival, differentiation, migration, and integration (Nutt et al. 2013, Khazaei et al. 2014, Lu et al. 2014, Pomeshchik et al. 2015, Romanyuk et al. 2015).
4.3 PSC-Derived Oligodendrocyte Precursor Cells (OPCs)
Oligodendrocyte death and chronic demyelination are features of the secondary injury cascade that follows the primary trauma after SCI (Kakulas 1999, Totoiu and Keirstead 2005). Adult OPCs have a limited capacity to regenerate or migrate, but a variety of stem cells, including endogenous adult NSCs, preferentially differentiate into oligodendrocytes that are capable of providing trophic support, remyelination and functional repair (Gensert and Goldman 1997, Faulkner and Keirstead 2005, Meletis et al. 2008). Functional recovery after NSC transplantation has been attributed in large part to OPC differentiation. ESC-derived OPCs have been transplanted after SCI with success, resulting in remyelination of host axons, improved electrophysiological and motor functions, and greater white and gray matter sparing (Brustle et al. 1999, Keirstead et al. 2005, Nistor et al. 2005, Sharp et al. 2010). Phase I/2 clinical trials for the transplantation of human ESC-derived OPCs into SCI patients with thoracic and high cervical injuries are currently underway, with demonstrable neurological improvements observed in patients from the initial safety cohort (Khazaei et al. 2014).
4.4 PSC-Derived Astrocytes
Astrocyte diversity and the role of astrocyte phenotype on neural circuit formation and regeneration remains poorly understood (Zhang and Barres 2010, Clarke and Barres 2013). While reactive astrocytes actively inhibit regeneration through the glial scar, other endogenous astrocytes have pro-regenerative roles, including the formation of astrocyte bridges that precede axons extending through the inhibitory environment (Kawaja and Gage 1991, Taylor et al. 2006, Nicaise et al. 2015). It is thus important to consider how the preparation of stem cell-derived astrocytes affects phenotype prior to transplantation; glial-restricted precursors that are pre-differentiated into astrocytes using different growth factor cocktails have opposite effects on recovery after transplantation (Davies et al. 2008). Astrocytes that differentiate from PSC-derived NSCs in vivo may contribute to or deter from the regenerative effects seen after transplantation depending on the method of NSC derivation, but the outcome is rarely linked to a specific astrocyte subtype. Protocols to obtain pro-regenerative astrocyte subtypes are still being developed, so studies that investigate the specific effect of PSC-derived astrocytes on spinal cord regeneration have not yet been published (Benveniste et al. 2005, Emdad et al. 2012, Roybon et al. 2013, Falnikar et al. 2015).
4.5 PSC-Derived Motor Neurons
Motor neurons have some potential for cell replacement because their loss significantly impairs behavioral recovery in several traumatic and neurodegenerative conditions. Many protocols have been developed to obtain enriched, subtype-specific motor neuron pools that closely resemble endogenous neurons and retain the ability to engraft in the developing spinal cord (Davis-Dusenbery et al. 2014). Efforts to transplant enriched motor neurons after SCI have been confined to embryonic or PSC-derived pMNs, since maturation comes with an intrinsic loss in plasticity needed to survive the hostile SCI environment. PSC-derived pMNs are able to survive, migrate, and differentiate after SCI transplantation in vivo, but the gliogenic nature of the injury site limits neuronal differentiation despite the ability of these pMNs to robustly differentiate into motor neurons in vitro (Erceg et al. 2010, Rossi et al. 2010). PSC-derived pMN transplantation also confers neuroprotective and neuroregenerative benefits akin to OPC transplantation; pMNs secrete neurotrophic factors that enhance endogenous axon sprouting and can result in functional improvements (Erceg et al. 2010, Rossi et al. 2010). Methods to direct maturation of progenitor populations or augment survival of mature neuronal transplants in vivo need improvement to take advantage of new PSC derivation methods.
4.6 PSC-Derived Interneurons
Local interneurons have been implicated in the formation of detour circuits that significantly contribute to functional recovery after partial SCI (Courtine et al. 2008, Flynn et al. 2011). Transplanted PSC-derived NSCs differentiate into a variety of interneurons that are capable of reconstructing functional neural relays, but, like astrocytes, the specific identity of those interneurons is rarely investigated beyond neurotransmitter expression profiles. Identifying the contributions of unique subtypes towards regeneration is important, since they respond differentially after SCI (Flynn et al. 2011, Husch et al. 2012). The recent availability of protocols and transgenic ESC lines that enrich for specific interneuron populations should improve access to these subtypes for modeling and transplantation (Murashov et al. 2005, Kim et al. 2009, Brown et al. 2014, Xu et al. 2015, Xu and Sakiyama-Elbert 2015, Iyer et al. 2016).
5. Transplantation Strategies
5.1 Biomaterials Scaffolds
Despite the many benefits of cell transplantation, the hostile, toxic environment of the injury site is a significant detriment to cell survival (Figure 3). A wide variety of biomaterial scaffolds have been investigated as transplantation vehicles and are typically chosen to accomplish one or more goals such as protecting transplanted populations, providing a structural bridge between injured areas, promoting axonal extension and cell migration, and mediating sustained delivery of pharmacological therapies (Raspa et al. 2016).
Protein-based biomaterials tend to have superior biocompatibility and an abundance of cell adhesion sites, which are critically important because they assist in cell survival, migration, proliferation, and differentiation. Collagen not only displays a number of cell adhesion sites, but also has mechanical properties similar to that of most soft tissues (Yoshii et al. 2004). When combined with growth factors, ESC-derived NSCs, or Schwann cells, collagen scaffolds promote CST and axon regeneration, remyelination and functional recovery (Hatami et al. 2009, Han et al. 2010, Patel et al. 2010). Fibrin based scaffolds have a particularly high therapeutic potential; they are biodegradable, can be injectable or formed in situ, have a multitude of cell adhesion sites, and can easily be modified for growth factor delivery (Itosaka et al. 2009, Kim et al. 2014). Our lab and others have done significant work demonstrating the effectiveness of using fibrin scaffolds to deliver growth factors, anti-inhibitory molecules, and ESC-derived NSCs or pMNs (Johnson et al. 2009, Johnson et al. 2010a, Johnson et al. 2010b, Johnson et al. 2010c, McCreedy et al. 2014b, Wilems et al. 2015, Wilems and Sakiyama-Elbert 2015).
Other natural materials that have been used for SCI can be heavily glycosylated, and are comparatively less adhesive than collagen or fibrin. Agarose has been shown to guide neurite outgrowth in vitro (Luo and Shoichet 2004) and to increase axonal regeneration following SCI (Stokols and Tuszynski 2006). Templated agarose scaffolds containing autologous MSCs that ubiquitously express brain-derived neurotrophic factor (BDNF) significantly increase long-range axonal growth after complete transection of the thoracic column (Gao et al. 2013). Alginate is a highly porous material widely used for both cell transplantation and drug delivery, but while alginate scaffolds have shown promise after SCI when seeded with BDNF-expressing MSCs, they may have cytocompatibilty issues and a high degradation rate (Zimmermann et al. 2001, Tobias et al. 2005, Ashton et al. 2007, Gunther et al. 2015). Hyaluronic acid (HA) is a major component of the extracellular matrix within the spinal cord and is commonly used as a foundation for hybrid biomaterial blends (Gupta et al. 2006, Horn et al. 2007, Park et al. 2010, Khaing et al. 2011), HA is often modified to incorporate additional cell adhesion sites or to alter its mechanical properties. For example, chemical modification of HA to allow for covalent crosslinking reduces the degradation rate compared to unmodified HA gels, thus providing utility over a longer period (Fuhrmann et al. 2015). OPCs transplanted in HA scaffolds crosslinked with thiol-functionalized gelatin and poly(ethylene glycol) diacrylate remyelinate endogenous axons in an ethidium bromide-induced demyelination lesion model (Li et al. 2013b).
Compared to natural materials, synthetic scaffolds offer more customizable attributes and greater control over mechanical properties, but their use can be limited by enhanced inflammatory and immune responses, few cell adhesion sites, and poor engraftment into the host tissue (Straley et al. 2010, Shrestha et al. 2014). For instance, poly(lactic-co-glycolic acid) (PLGA) scaffolds embedded with NSCs and Schwann cells demonstrate robust axonal extension following a complete thoracic transection one month post-transplantation (Olson et al. 2009). However, acidic PLGA degradation byproducts reduce the pH of the local environment, which can lead to reactive gliosis and/or a heightened inflammatory response (Oh et al. 1995, Sung et al. 2004, Kim et al. 2007). Poly(hydroxyethyl methacrylate) (pHEMA) scaffolds can mimic the mechanical properties of the spinal cord and improve axon regeneration, but syringomyelia development and limited integration at the host-transplant interface have been observed (Bakshi et al. 2004, Nomura et al. 2006, Li et al. 2013a).
Balancing the positive and negative properties of a biomaterial is important when making scaffold design decisions. Scaffolds that combine several materials may emphasize positive attributes while masking negative ones, and therefore optimize the efficacy of a treatment. For example, a study using pHEMA-co-methyl methacrylate channels filled with different materials and growth factors showed that fibrin filled channels led to the greatest regeneration of reticular neuron axons and improved functional recovery compared to unfilled pHEMA controls (Tsai et al. 2006). The incorporation of bioactive peptide sequences into hydrogels is a promising approach that promotes specific cell-material or material-growth factor interactions to prompt regeneration (Woerly et al. 2001, Taylor et al. 2006, Taylor and Sakiyama-Elbert 2006, Park et al. 2010, Kubinova et al. 2015). In one combinatorial study, NSCs were seeded into conduits containing growth factors that were immobilized onto polycaprolactone (PCL) scaffolds using silk fibroin coatings; improved functional outcomes and axonal regeneration were observed 8 weeks after transplantation into a rat thoracic SCI model (Tang et al. 2014). These findings and others indicate that hybrid matrices combined with controlled drug delivery vehicles and cell transplantation can be manipulated to enhance regeneration following injury.
5.2 Controlled Drug Delivery
Systemic delivery, scaffold-based delivery, and micro- and nano-particle based delivery systems are the three main strategies to deliver pharmacological factors after SCI (Willerth and Sakiyama-Elbert 2007, Lee et al. 2010, Wang et al. 2011, Tyler et al. 2013, Zhao et al. 2013)(Figure 3). Neurotrophins and growth factors are often investigated, as well as anti-inhibitory molecules, anti-convulsants, immunotherapeutic molecules, hormones, and antibody treatments, many of which are in current use or in clinical trials (Mohtaram et al. 2013, Silva et al. 2014, Kabu et al. 2015). Commonly used methods such as direct injection, intrathecal infusion, or simple diffusion-based scaffold systems can be invasive, costly, and incapable of delivering bioactive molecules over the time-scale necessary for effectiveness (Jones and Tuszynski 2001). However, because more controllable drug delivery systems are not necessarily compatible with cells—harsh synthesis methods can result cell toxicity or a heightened immune response— combinatorial systems that are able to co-deliver drugs and stem cell-derived populations are still in the early stages of development. We and others have demonstrated that simultaneous, controlled delivery of anti-inhibitory molecules, growth factors, and stem cell-derived neurons is feasible, but still requires refinement to improve overall efficacy (Karimi-Abdolrezaee et al. 2010, Wilems et al. 2015).
5.3 Transgenic and Gene Delivery Approaches
While biomaterials and viral vectors have been designed to exogenously deliver therapeutic factors (Tuinstra et al. 2012, Tuinstra et al. 2014, Thomas et al. 2015) (Figure 3), cells can also be engineered as self-renewing drug delivery vehicles if appropriately modified. This presents an opportunity for sustained local delivery of growth factors or anti-inhibitory molecules at the site of the injury without the need for synthetic particles (Blesch et al. 2002, Thuret et al. 2006, Walthers and Seidlits 2015). The inclusion of tetracycline-inducible systems can help control long-term gene expression and thereby mitigate issues associated with gene overexpression, including the “candy store effect” which limits axon extension out of the graft (Blesch et al. 2002). Transducing stem cells in vitro prevents complications from direct in vivo gene manipulations, but can result in limited cell survival post-transplantation (Walthers and Seidlits 2015).
Gene delivery can also be used to direct differentiation of transplanted stem cells or endogenous adult NSCs and reactive astrocytes into more pro-regenerative glia or neurons (Tang and Low 2007, Corti et al. 2012, Guo et al. 2014). Transgenic modifications can help to enrich specific PSC-derived neuronal populations of interest and/or eliminate undifferentiated PSCs that could generate teratomas. We have developed several transgenic ESC lines to obtain specific ventral spinal populations that are difficult to isolate from primary tissues, but may also suffer from low differentiation efficiencies (McCreedy et al. 2012, McCreedy et al. 2014a, Xu et al. 2015, Iyer et al. 2016).
6. Conclusions, Challenges, and Future Outlook
The potential for stem cell therapies has never been greater. Ethical impediments caused by stem cell research have largely been mitigated by advancements in iPSC and direct reprogramming technologies. Human stem cells can be sourced from autologous patient tissue, permitting personalized strategies that reduce the risk of immune rejection as well as enabling disease modeling. In vitro methods to obtain specific spinal cell types have been made possible by the significant strides in research parsing spinal cord development and the genetic events that contribute to cell fate determination. After transplantation, stem cell-derived populations remyelinate host axons, administer trophic support, and contribute to relay circuits that promote functional recovery. Combinatorial transplantation strategies are increasingly comprehensive as biomedical engineers design scaffolding systems capable of supporting stem cell-derived populations while simultaneously mediating drug delivery and providing mechanical and chemical cues to promote regeneration.
Challenges persist. Reprogramming somatic cells into iPSCs has historically required viral overexpression of oncogenic transcription factors that compound the issue of stem cell tumorgenicity. Non-viral methods that minimize the tumorgenic potential have been developed, but reprogramming efficiencies are insufficient for clinical use, and the time needed to isolate and expand these populations remains unfeasibly high. There is also a risk that reprogrammed cells may revert to a pluripotent state. A reliance on animal products and feeder cells in either stem cell culture media or biomaterials matrices also reduces the potential clinical utility.
Differentiation methods face similar issues; neuronal induction efficiencies are lower than desirable, human cells can require weeks to months to mature in culture, and the yields can be variable, especially between in vitro and in vivo studies. Such inconsistencies can result in large histological and behavioral differences in SCI models, which ultimately harm the advancement of stem cell therapies. Our ability to refine induction methods is limited in part because the impact of various experimental conditions on differentiation and subtype specification is poorly understood. These may include the cell media composition, the types of morphogens and growth factors being applied, the material substrate and format, the environmental and atmospheric conditions, the cellular heterogeneity and density, etc.—slight changes may dramatically alter induction efficiencies in ways that are currently unknown. Furthermore, the signaling cascades responsible for spinal diversification and maturation are still being determined. More markers need to be available to identify unique neuronal and glial subtypes, as well as research suggesting how these populations may contribute to motor function and regeneration.
Active engagement between translational researchers and basic scientists will be important for the field to move forward. High throughput gene expression methods, single-cell sequencing, proteomics, and similar techniques will allow for a more comprehensive investigation and comparison of cells generated during development and in the dish. The biology ought to inform both differentiation and therapeutic strategies. The complex interplay between transplanted cells, materials, pharmacological agents, and the in vivo environment will require combinatorial systems where the components complement, rather than detract from one another, as can so often be the case. Creative design is necessary to get these novel strategies from the bench top and into the clinic.
References
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance 2014 [Google Scholar]
- Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct Reprogramming of Adult Human Fibroblasts to Functional Neurons under Defined Conditions. Cell Stem Cell. 2011;9(2):113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S. Motor Circuits in Action: Specification, Connectivity, and Function. Neuron. 2012;74(6):975–989. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Ashton RS, Banerjee A, Punyani S, Schaffer DV, Kane RS. Scaffolds Based on Degradable Alginate Hydrogels and Poly(Lactide-Co-Glycolide) Microspheres for Stem Cell Culture. Biomaterials. 2007;28(36):5518–5525. doi: 10.1016/j.biomaterials.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Asterias B. Asterias Biotherapeutics Announces Positive New Long-Term Follow-up Results for Ast-Opc1. 2016 from http://asteriasbiotherapeutics.com/asterias-biotherapeutics-announces-positive-new-long-term-follow-up-results-for-ast-opc1/
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic Stem Cells Express Neuronal Properties in Vitro. Dev Biol. 1995;168(2):342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Bakshi A, Fisher O, Dagci T, Himes BT, Fischer I, Lowman A. Mechanically Engineered Hydrogel Scaffolds for Axonal Growth and Angiogenesis after Transplantation in Spinal Cord Injury. J Neurosurg Spine. 2004;1(3):322–329. doi: 10.3171/spi.2004.1.3.0322. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The Injured Spinal Cord Spontaneously Forms a New Intraspinal Circuit in Adult Rats. Nat Neurosci. 2004;7(3):269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of New Glial Cells in Intact and Injured Adult Spinal Cord. Cell Stem Cell. 2010;7(4):470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Benveniste RJ, Keller G, Germano I. Embryonic Stem Cell-Derived Astrocytes Expressing Drug-Inducible Transgenes: Differentiation and Transplantion into the Mouse Brain. J Neurosurg. 2005;103(1):115–123. doi: 10.3171/jns.2005.103.1.0115. [DOI] [PubMed] [Google Scholar]
- Blanchard JW, Eade KT, Szucs A, Lo Sardo V, Tsunemoto RK, Williams D, Sanna PP, Baldwin KK. Selective Conversion of Fibroblasts into Peripheral Sensory Neurons. Nat Neurosci. 2015;18(1):25–35. doi: 10.1038/nn.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Lu P, Tuszynski MH. Neurotrophic Factors, Gene Therapy, and Neural Stem Cells for Spinal Cord Repair. Brain Res Bull. 2002;57(6):833–838. doi: 10.1016/s0361-9230(01)00774-2. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB. Spinal Cord Repair Strategies: Why Do They Work? Nat Rev Neurosci. 2006;7(8):644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and Culture of Adult Neurons and Neurospheres. Nat Protoc. 2007;2(6):1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. Specification of Neuronal Fates in the Ventral Neural Tube. Curr Opin Neurobiol. 2001;11(1):43–49. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Brown CR, Butts JC, McCreedy DA, Sakiyama-Elbert SE. Generation of V2a Interneurons from Mouse Embryonic Stem Cells. Stem Cells Dev. 2014;23(15):1765–1776. doi: 10.1089/scd.2013.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic Stem Cell-Derived Glial Precursors: A Source of Myelinating Transplants. Science. 1999;285(5428):754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM, Brownstone RM. Circuits for Grasping: Spinal Di3 Interneurons Mediate Cutaneous Control of Motor Behavior. Neuron. 2013;78(1):191–204. doi: 10.1016/j.neuron.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo MM, Dell’Anno T, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct Generation of Functional Dopaminergic Neurons from Mouse and Human Fibroblasts. Nature. 2011;476(7359):224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Caiazzo M, Giannelli S, Valente P, Lignani G, Carissimo A, Sessa A, Colasante G, Bartolomeo R, Massimino L, Ferroni S, Settembre C, Benfenati F, Broccoli V. Direct Conversion of Fibroblasts into Functional Astrocytes by Defined Transcription Factors. Stem Cell Reports. 2015;4(1):25–36. doi: 10.1016/j.stemcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent Stem Cells Engrafted into the Normal or Lesioned Adult Rat Spinal Cord Are Restricted to a Glial Lineage. Exp Neurol. 2001;167(1):48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly Efficient Neural Conversion of Human Es and Ips Cells by Dual Inhibition of Smad Signaling. Nat Biotechnol. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi SH, Studer L. Combined Small-Molecule Inhibition Accelerates Developmental Timing and Converts Human Pluripotent Stem Cells into Nociceptors. Nat Biotechnol. 2012;30(7):715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liu H, Liu J, Qi J, Wei B, Yang J, Liang H, Chen Y, Chen J, Wu Y, Guo L, Zhu J, Zhao X, Peng T, Zhang Y, Chen S, Li X, Li D, Wang T, Pei D. H3k9 Methylation Is a Barrier During Somatic Cell Reprogramming into Ipscs. Nat Genet. 2013;45(1):34–42. doi: 10.1038/ng.2491. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging Roles of Astrocytes in Neural Circuit Development. Nat Rev Neurosci. 2013;14(5):311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conta AC, Stelzner DJ. Differential Vulnerability of Propriospinal Tract Neurons to Spinal Cord Contusion Injury. J Comp Neurol. 2004;479(4):347–359. doi: 10.1002/cne.20319. [DOI] [PubMed] [Google Scholar]
- Corti S, Nizzardo M, Simone C, Falcone M, Donadoni C, Salani S, Rizzo F, Nardini M, Riboldi G, Magri F, Zanetta C, Faravelli I, Bresolin N, Comi GP. Direct Reprogramming of Human Astrocytes into Neural Stem Cells and Neurons. Exp Cell Res. 2012;318(13):1528–1541. doi: 10.1016/j.yexcr.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal Regeneration and Functional Recovery after Complete Spinal Cord Transection in Rats by Delayed Treatment with Transplants and Neurotrophins. J Neurosci. 2001;21(23):9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of Nonfunctional Spinal Circuits into Functional States after the Loss of Brain Input. Nat Neurosci. 2009;12(10):1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of Supraspinal Control of Stepping Via Indirect Propriospinal Relay Connections after Spinal Cord Injury. Nat Med. 2008;14(1):69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional Regeneration Beyond the Glial Scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Lopez-Vales R, Wee Yong V. Harmful and Beneficial Effects of Inflammation after Spinal Cord Injury: Potential Therapeutic Implications. Handb Clin Neurol. 2012;109:485–502. doi: 10.1016/B978-0-444-52137-8.00030-9. [DOI] [PubMed] [Google Scholar]
- Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJ. Transplanted Astrocytes Derived from Bmp- or Cntf-Treated Glial-Restricted Precursors Have Opposite Effects on Recovery and Allodynia after Spinal Cord Injury. J Biol. 2008;7(7):24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Dusenbery BN, Williams LA, Klim JR, Eggan K. How to Make Spinal Motor Neurons. Development. 2014;141(3):491–501. doi: 10.1242/dev.097410. [DOI] [PubMed] [Google Scholar]
- Del Barrio MG, Taveira-Marques R, Muroyama Y, Yuk DI, Li S, Wines-Samuelson M, Shen J, Smith HK, Xiang M, Rowitch D, Richardson WD. A Regulatory Network Involving Foxn4, Mash1 and Delta-Like 4/Notch1 Generates V2a and V2b Spinal Interneurons from a Common Progenitor Pool. Development. 2007;134(19):3427–3436. doi: 10.1242/dev.005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E, Ribes V, Balaskas N, Yang LL, Pierani A, Kicheva A, Novitch BG, Briscoe J, Sasai N. Dynamic Assignment and Maintenance of Positional Identity in the Ventral Neural Tube by the Morphogen Sonic Hedgehog. PLoS Biol. 2010;8(6):e1000382. doi: 10.1371/journal.pbio.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVivo M, Chen Y, Mennemeyer S, Deutsch A. Costs of Care Following Psinal Cord Injury. Topics in Spinal Cord Injury Rehabilitation: Spring 2011. 2011;16(4):1–9. [Google Scholar]
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing Fgf and Retinoid Pathways Control Ventral Neural Pattern, Neuronal Differentiation, and Segmentation During Body Axis Extension. Neuron. 2003;40(1):65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and Its Role in Neuroprotection, Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Exp Neurol. 2008;209(2):378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras P, Fossati V. Generation and Isolation of Oligodendrocyte Progenitor Cells from Human Pluripotent Stem Cells. Nat Protoc. 2015;10(8):1143–1154. doi: 10.1038/nprot.2015.075. [DOI] [PubMed] [Google Scholar]
- Durston AJ, van der Wees J, Pijnappel WW, Godsave SF. Retinoids and Related Signals in Early Development of the Vertebrate Central Nervous System. Curr Top Dev Biol. 1998;40:111–175. doi: 10.1016/s0070-2153(08)60366-x. [DOI] [PubMed] [Google Scholar]
- Dyck J, Lanuza GM, Gosgnach S. Functional Characterization of Di6 Interneurons in the Neonatal Mouse Spinal Cord. J Neurophysiol. 2012;107(12):3256–3266. doi: 10.1152/jn.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, D’Souza SL, Kothari HP, Qadeer ZA, Germano IM. Efficient Differentiation of Human Embryonic and Induced Pluripotent Stem Cells into Functional Astrocytes. Stem Cells Dev. 2012;21(3):404–410. doi: 10.1089/scd.2010.0560. [DOI] [PubMed] [Google Scholar]
- Erceg S, Ronaghi M, Oria M, Rosello MG, Arago MA, Lopez MG, Radojevic I, Moreno-Manzano V, Rodriguez-Jimenez FJ, Bhattacharya SS, Cordoba J, Stojkovic M. Transplanted Oligodendrocytes and Motoneuron Progenitors Generated from Human Embryonic Stem Cells Promote Locomotor Recovery after Spinal Cord Transection. Stem Cells. 2010;28(9):1541–1549. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in Culture of Pluripotential Cells from Mouse Embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Falnikar A, Li K, Lepore AC. Therapeutically Targeting Astrocytes with Stem and Progenitor Cell Transplantation Following Traumatic Spinal Cord Injury. Brain Res. 2015;1619:91–103. doi: 10.1016/j.brainres.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner J, Keirstead HS. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitors for the Treatment of Spinal Cord Injury. Transpl Immunol. 2005;15(2):131–142. doi: 10.1016/j.trim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The Glial Scar and Central Nervous System Repair. Brain Res Bull. 1999;49(6):377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Filli L, Schwab ME. Structural and Functional Reorganization of Propriospinal Connections Promotes Functional Recovery after Spinal Cord Injury. Neural Regen Res. 2015;10(4):509–513. doi: 10.4103/1673-5374.155425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JR, Graham BA, Galea MP, Callister RJ. The Role of Propriospinal Interneurons in Recovery from Spinal Cord Injury. Neuropharmacology. 2011;60(5):809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Fuhrmann T, Obermeyer J, Tator CH, Shoichet MS. Click-Crosslinked Injectable Hyaluronic Acid Hydrogel Is Safe and Biocompatible in the Intrathecal Space for Ultimate Use in Regenerative Strategies of the Injured Spinal Cord. Methods. 2015;84:60–69. doi: 10.1016/j.ymeth.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Gao M, Lu P, Bednark B, Lynam D, Conner JM, Sakamoto J, Tuszynski MH. Templated Agarose Scaffolds for the Support of Motor Axon Regeneration into Sites of Complete Spinal Cord Transection. Biomaterials. 2013;34(5):1529–1536. doi: 10.1016/j.biomaterials.2012.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous Progenitors Remyelinate Demyelinated Axons in the Adult Cns. Neuron. 1997;19(1):197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Kuypers NJ. How to Make an Oligodendrocyte. Development. 2015;142(23):3983–3995. doi: 10.1242/dev.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther MI, Weidner N, Muller R, Blesch A. Cell-Seeded Alginate Hydrogel Scaffolds Promote Directed Linear Axonal Regeneration in the Injured Rat Spinal Cord. Acta Biomater. 2015;27:140–150. doi: 10.1016/j.actbio.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer’s Disease Model. Cell Stem Cell. 2014;14(2):188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D, Tator CH, Shoichet MS. Fast-Gelling Injectable Blend of Hyaluronan and Methylcellulose for Intrathecal, Localized Delivery to the Injured Spinal Cord. Biomaterials. 2006;27(11):2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, Greber B, Yang JH, Lee HT, Schwamborn JC, Storch A, Scholer HR. Direct Reprogramming of Fibroblasts into Neural Stem Cells by Defined Factors. Cell Stem Cell. 2012;10(4):465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong J, Wu J, Liang W, Chen L, Zhao Y, Chen B, Dai J. The Promotion of Neural Regeneration in an Extreme Rat Spinal Cord Injury Model Using a Collagen Scaffold Containing a Collagen Binding Neuroprotective Protein and an Egfr Neutralizing Antibody. Biomaterials. 2010;31(35):9212–9220. doi: 10.1016/j.biomaterials.2010.08.040. [DOI] [PubMed] [Google Scholar]
- Hatami M, Mehrjardi NZ, Kiani S, Hemmesi K, Azizi H, Shahverdi A, Baharvand H. Human Embryonic Stem Cell-Derived Neural Precursor Transplants in Collagen Scaffolds Promote Recovery in Injured Rat Spinal Cord. Cytotherapy. 2009;11(5):618–630. doi: 10.1080/14653240903005802. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential Mrna Expression for Immediate Early Genes, Cytokines, and Neurotrophins in Spinal Cord Injury. J Neurotrauma. 2000;17(3):203–218. doi: 10.1089/neu.2000.17.203. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Specification of Dorsal Spinal Cord Interneurons. Curr Opin Neurobiol. 2003;13(1):42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Hester ME, Murtha MJ, Song S, Rao M, Miranda CJ, Meyer K, Tian J, Boulting G, Schaffer DV, Zhu MX, Pfaff SL, Gage FH, Kaspar BK. Rapid and Efficient Generation of Functional Motor Neurons from Human Pluripotent Stem Cells Using Gene Delivered Transcription Factor Codes. Mol Ther. 2011;19(10):1905–1912. doi: 10.1038/mt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi S, Tropepe V, Ekker M, van der Kooy D. Neural Stem Cell Lineages Are Regionally Specified, but Not Committed, within Distinct Compartments of the Developing Brain. Development. 2002;129(1):233–244. doi: 10.1242/dev.129.1.233. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow Stromal Cells Form Guiding Strands in the Injured Spinal Cord and Promote Recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER., 2nd Axon Guidance Molecules and Neural Circuit Remodeling after Spinal Cord Injury. Neurotherapeutics. 2015 doi: 10.1007/s13311-015-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, Tapia N, Lim KT, Kim J, Ahn HS, Ko K, Shin CY, Lee HT, Scholer HR, Hyun JK, Han DW. Therapeutic Potential of Induced Neural Stem Cells for Spinal Cord Injury. J Biol Chem. 2014;289(47):32512–32525. doi: 10.1074/jbc.M114.588871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn EM, Beaumont M, Shu XZ, Harvey A, Prestwich GD, Horn KM, Gibson AR, Preul MC, Panitch A. Influence of Cross-Linked Hyaluronic Acid Hydrogels on Neurite Outgrowth and Recovery from Spinal Cord Injury. J Neurosurg Spine. 2007;6(2):133–140. doi: 10.3171/spi.2007.6.2.133. [DOI] [PubMed] [Google Scholar]
- Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural Differentiation of Human Induced Pluripotent Stem Cells Follows Developmental Principles but with Variable Potency. Proc Natl Acad Sci U S A. 2010;107(9):4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W, Gao L, Shen L, Huang Y, Xie G, Zhao H, Jin Y, Tang B, Yu Y, Zhao J, Pei G. Direct Conversion of Normal and Alzheimer’s Disease Human Fibroblasts into Neuronal Cells by Small Molecules. Cell Stem Cell. 2015;17(2):204–212. doi: 10.1016/j.stem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of Pluripotent Stem Cells by Defined Factors Is Greatly Improved by Small-Molecule Compounds. Nat Biotechnol. 2008;26(7):795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Koo BK. Modeling Mouse and Human Development Using Organoid Cultures. Development. 2015;142(18):3113–3125. doi: 10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- Husch A, Van Patten GN, Hong DN, Scaperotti MM, Cramer N, Harris-Warrick RM. Spinal Cord Injury Induces Serotonin Supersensitivity without Increasing Intrinsic Excitability of Mouse V2a Interneurons. J Neurosci. 2012;32(38):13145–13154. doi: 10.1523/JNEUROSCI.2995-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Nishimura Y. Plasticity for Recovery after Partial Spinal Cord Injury - Hierarchical Organization. Neurosci Res. 2014;78:3–8. doi: 10.1016/j.neures.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Itosaka H, Kuroda S, Shichinohe H, Yasuda H, Yano S, Kamei S, Kawamura R, Hida K, Iwasaki Y. Fibrin Matrix Provides a Suitable Scaffold for Bone Marrow Stromal Cells Transplanted into Injured Spinal Cord: A Novel Material for Cns Tissue Engineering. Neuropathology. 2009;29(3):248–257. doi: 10.1111/j.1440-1789.2008.00971.x. [DOI] [PubMed] [Google Scholar]
- Iyer NR, Huettner JE, Butts JC, Brown CR, Sakiyama-Elbert SE. Generation of Highly Enriched V2a Interneurons from Mouse Embryonic Stem Cells. Exp Neurol. 2016 doi: 10.1016/j.expneurol.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal Specification in the Spinal Cord: Inductive Signals and Transcriptional Codes. Nat Rev Genet. 2000;1(1):20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Joannides AJ, Fiore-Heriche C, Battersby AA, Athauda-Arachchi P, Bouhon IA, Williams L, Westmore K, Kemp PJ, Compston A, Allen ND, Chandran S. A Scaleable and Defined System for Generating Neural Stem Cells from Human Embryonic Stem Cells. Stem Cells. 2007;25(3):731–737. doi: 10.1634/stemcells.2006-0562. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a Neural Stem Cell in the Adult Mammalian Central Nervous System. Cell. 1999;96(1):25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Parker SR, Sakiyama-Elbert SE. Controlled Release of Neurotrophin-3 from Fibrin-Based Tissue Engineering Scaffolds Enhances Neural Fiber Sprouting Following Subacute Spinal Cord Injury. Biotechnol Bioeng. 2009;104(6):1207–1214. doi: 10.1002/bit.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Parker SR, Sakiyama-Elbert SE. Fibrin-Based Tissue Engineering Scaffolds Enhance Neural Fiber Sprouting and Delay the Accumulation of Reactive Astrocytes at the Lesion in a Subacute Model of Spinal Cord Injury. J Biomed Mater Res A. 2010a;92(1):152–163. doi: 10.1002/jbm.a.32343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama-Elbert SE. Tissue-Engineered Fibrin Scaffolds Containing Neural Progenitors Enhance Functional Recovery in a Subacute Model of Sci. Soft Matter. 2010b;6(20):5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE. Controlled Release of Neurotrophin-3 and Platelet-Derived Growth Factor from Fibrin Scaffolds Containing Neural Progenitor Cells Enhances Survival and Differentiation into Neurons in a Subacute Model of Sci. Cell Transplant. 2010c;19(1):89–101. doi: 10.3727/096368909X477273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH. Chronic Intrathecal Infusions after Spinal Cord Injury Cause Scarring and Compression. Microsc Res Tech. 2001;54(5):317–324. doi: 10.1002/jemt.1144. [DOI] [PubMed] [Google Scholar]
- Kabu S, Gao Y, Kwon BK, Labhasetwar V. Drug Delivery, Cell-Based Therapies, and Tissue Engineering Approaches for Spinal Cord Injury. J Control Release. 2015;219:141–154. doi: 10.1016/j.jconrel.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakulas BA. The Applied Neuropathology of Human Spinal Cord Injury. Spinal Cord. 1999;37(2):79–88. doi: 10.1038/sj.sc.3100807. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury. J Neurosci. 2006;26(13):3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic Effects of Transplanted Adult Neural Stem/Progenitor Cells, Chondroitinase, and Growth Factors Promote Functional Repair and Plasticity of the Chronically Injured Spinal Cord. J Neurosci. 2010;30(5):1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaja MD, Gage FH. Reactive Astrocytes Are Substrates for the Growth of Adult Cns Axons in the Presence of Elevated Levels of Nerve Growth Factor. Neuron. 1991;7(6):1019–1030. doi: 10.1016/0896-6273(91)90346-2. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cell Transplants Remyelinate and Restore Locomotion after Spinal Cord Injury. J Neurosci. 2005;25(19):4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE. High Molecular Weight Hyaluronic Acid Limits Astrocyte Activation and Scar Formation after Spinal Cord Injury. J Neural Eng. 2011;8(4):046033. doi: 10.1088/1741-2560/8/4/046033. [DOI] [PubMed] [Google Scholar]
- Khazaei M, Siddiqui AM, Fehlings MG. The Potential for Ips-Derived Stem Cells as a Therapeutic Strategy for Spinal Cord Injury: Opportunities and Challenges. J Clin Med. 2014;4(1):37–65. doi: 10.3390/jcm4010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct Reprogramming of Mouse Fibroblasts to Neural Progenitors. Proc Natl Acad Sci U S A. 2011;108(19):7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Habiba A, Doherty JM, Mills JC, Mercer RW, Huettner JE. Regulation of Mouse Embryonic Stem Cell Neural Differentiation by Retinoic Acid. Dev Biol. 2009;328(2):456–471. doi: 10.1016/j.ydbio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Park SR, Choi BH. Biomaterial Scaffolds Used for the Regeneration of Spinal Cord Injury (Sci) Histol Histopathol. 2014;29(11):1395–1408. doi: 10.14670/HH-29.1395. [DOI] [PubMed] [Google Scholar]
- Kim MS, Ahn HH, Shin YN, Cho MH, Khang G, Lee HB. An in Vivo Study of the Host Tissue Response to Subcutaneous Implantation of Plga- and/or Porcine Small Intestinal Submucosa-Based Scaffolds. Biomaterials. 2007;28(34):5137–5143. doi: 10.1016/j.biomaterials.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Knight GT, Sha J, Ashton RS. Micropatterned, Clickable Culture Substrates Enable in Situ Spatiotemporal Control of Human Psc-Derived Neural Tissue Morphology. Chem Commun (Camb) 2015;51(25):5238–5241. doi: 10.1039/c4cc08665a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K, Tsuji O, Toyama Y, Yamanaka S, Nakamura M, Okano H. Pre-Evaluated Safe Human Ipsc-Derived Neural Stem Cells Promote Functional Recovery after Spinal Cord Injury in Common Marmoset without Tumorigenicity. PLoS One. 2012;7(12):e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Zhang SC. Directed Differentiation of Functional Astroglial Subtypes from Human Pluripotent Stem Cells. Nat Protoc. 2011;6(11):1710–1717. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinova S, Horak D, Hejcl A, Plichta Z, Kotek J, Proks V, Forostyak S, Sykova E. Sikvav-Modified Highly Superporous Phema Scaffolds with Oriented Pores for Spinal Cord Injury Repair. J Tissue Eng Regen Med. 2015;9(11):1298–1309. doi: 10.1002/term.1694. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science. 2014;345(6194):1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- Lang C, Guo X, Kerschensteiner M, Bareyre FM. Single Collateral Reconstructions Reveal Distinct Phases of Corticospinal Remodeling after Spinal Cord Injury. PLoS One. 2012;7(1):e30461. doi: 10.1371/journal.pone.0030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV. Sustained Delivery of Thermostabilized Chabc Enhances Axonal Sprouting and Functional Recovery after Spinal Cord Injury. Proc Natl Acad Sci U S A. 2010;107(8):3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Dietrich P, Jessell TM. Genetic Ablation Reveals That the Roof Plate Is Essential for Dorsal Interneuron Specification. Nature. 2000;403(6771):734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The Specification of Dorsal Cell Fates in the Vertebrate Central Nervous System. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- Li HY, Fuhrmann T, Zhou Y, Dalton PD. Host Reaction to Poly(2-Hydroxyethyl Methacrylate) Scaffolds in a Small Spinal Cord Injury Model. J Mater Sci Mater Med. 2013a;24(8):2001–2011. doi: 10.1007/s10856-013-4956-8. [DOI] [PubMed] [Google Scholar]
- Li X, Liu X, Cui L, Brunson C, Zhao W, Bhat NR, Zhang N, Wen X. Engineering an in Situ Crosslinkable Hydrogel for Enhanced Remyelination. FASEB J. 2013b;27(3):1127–1136. doi: 10.1096/fj.12-211151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D, Zhu J, Du X, Xiong L, Du Y, Xu J, Xiao X, Wang J, Chai Z, Zhao Y, Deng H. Small-Molecule-Driven Direct Reprogramming of Mouse Fibroblasts into Functional Neurons. Cell Stem Cell. 2015;17(2):195–203. doi: 10.1016/j.stem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of Motoneurons from Human Embryonic Stem Cells. Nat Biotechnol. 2005;23(2):215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Liddelow S, Hoyer D. Astrocytes: Adhesion Molecules and Immunomodulation. Curr Drug Targets. 2016 doi: 10.2174/1389450117666160101120703. [DOI] [PubMed] [Google Scholar]
- Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, Ding S. A Chemical Platform for Improved Induction of Human Ipscs. Nat Methods. 2009;6(11):805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann ES, Williams CE, Ruhl DA, Estevez-Silva MC, Chapman ER, Coon JJ, Ashton RS. Deterministic Hox Patterning in Human Pluripotent Stem Cell-Derived Neuroectoderm. Stem Cell Reports. 2015;4(4):632–644. doi: 10.1016/j.stemcr.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LS, Tuszynski MH. Long-Distance Axonal Growth from Human Induced Pluripotent Stem Cells after Spinal Cord Injury. Neuron. 2014;83(4):789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct Conversion of Mouse Fibroblasts to Self-Renewing, Tripotent Neural Precursor Cells. Proc Natl Acad Sci U S A. 2012;109(7):2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Zurita L, Bruneau BG. Chromatin Modulators as Facilitating Factors in Cellular Reprogramming. Curr Opin Genet Dev. 2013;23(5):556–561. doi: 10.1016/j.gde.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shoichet MS. A Photolabile Hydrogel for Guided Three-Dimensional Cell Growth and Migration. Nat Mater. 2004;3(4):249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic Acid in the Development, Regeneration and Maintenance of the Nervous System. Nat Rev Neurosci. 2007;8(10):755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Marro S, Yang N. Transdifferentiation of Mouse Fibroblasts and Hepatocytes to Functional Neurons. Methods Mol Biol. 2014;1150:237–246. doi: 10.1007/978-1-4939-0512-6_16. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Mahony S, Closser M, Morrison CA, Nedelec S, Williams DJ, An D, Gifford DK, Wichterle H. Synergistic Binding of Transcription Factors to Cell-Specific Enhancers Programs Motor Neuron Identity. Nat Neurosci. 2013;16(9):1219–1227. doi: 10.1038/nn.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreedy DA, Brown CR, Butts JC, Xu H, Huettner JE, Sakiyama-Elbert SE. A New Method for Generating High Purity Motoneurons from Mouse Embryonic Stem Cells. Biotechnol Bioeng. 2014a;111(10):2041–2055. doi: 10.1002/bit.25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreedy DA, Rieger CR, Gottlieb DI, Sakiyama-Elbert SE. Transgenic Enrichment of Mouse Embryonic Stem Cell-Derived Progenitor Motor Neurons. Stem Cell Res. 2012;8(3):368–378. doi: 10.1016/j.scr.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreedy DA, Wilems TS, Xu H, Butts JC, Brown CR, Smith AW, Sakiyama-Elbert SE. Survival, Differentiation, and Migration of High-Purity Mouse Embryonic Stem Cell-Derived Progenitor Motor Neurons in Fibrin Scaffolds after Sub-Acute Spinal Cord Injury. Biomater Sci. 2014b;2(11):1672–1682. doi: 10.1039/c4bm00106k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted Embryonic Stem Cells Survive, Differentiate and Promote Recovery in Injured Rat Spinal Cord. Nat Med. 1999;5(12):1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of Ng2-Positive Cells and Altered Oligodendrocyte Numbers in the Contused Rat Spinal Cord. J Neurosci. 2001;21(10):3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Brauninger M, Stec A, Schackert G, Lutolf M, Tanaka EM. 3d Reconstitution of the Patterned Neural Tube from Embryonic Stem Cells. Stem Cell Reports. 2014;3(6):987–999. doi: 10.1016/j.stemcr.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N, Shupliakov O, Frisen J. Spinal Cord Injury Reveals Multilineage Differentiation of Ependymal Cells. PLoS Biol. 2008;6(7):e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RR, Szabo E, Benoit YD, Case DT, Mechael R, Alamilla J, Lee JH, Fiebig-Comyn A, Gillespie DC, Bhatia M. Activation of Neural Cell Fate Programs toward Direct Conversion of Adult Human Fibroblasts into Tri-Potent Neural Progenitors Using Oct-4. Stem Cells Dev. 2014;23(16):1937–1946. doi: 10.1089/scd.2014.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 Control Inhibitory and Excitatory Cell Fate in Spinal Sensory Interneurons. Nat Neurosci. 2006;9(6):770–778. doi: 10.1038/nn1706. [DOI] [PubMed] [Google Scholar]
- Mohtaram NK, Montgomery A, Willerth SM. Biomaterial-Based Drug Delivery Systems for the Controlled Release of Neurotrophic Factors. Biomed Mater. 2013;8(2):022001. doi: 10.1088/1748-6041/8/2/022001. [DOI] [PubMed] [Google Scholar]
- Moreno-Manzano V, Rodriguez-Jimenez FJ, Garcia-Rosello M, Lainez S, Erceg S, Calvo MT, Ronaghi M, Lloret M, Planells-Cases R, Sanchez-Puelles JM, Stojkovic M. Activated Spinal Cord Ependymal Stem Cells Rescue Neurological Function. Stem Cells. 2009;27(3):733–743. doi: 10.1002/stem.24. [DOI] [PubMed] [Google Scholar]
- Muhr J, Graziano E, Wilson S, Jessell TM, Edlund T. Convergent Inductive Signals Specify Midbrain, Hindbrain, and Spinal Cord Identity in Gastrula Stage Chick Embryos. Neuron. 1999;23(4):689–702. doi: 10.1016/s0896-6273(01)80028-3. [DOI] [PubMed] [Google Scholar]
- Muller T, Brohmann H, Pierani A, Heppenstall PA, Lewin GR, Jessell TM, Birchmeier C. The Homeodomain Factor Lbx1 Distinguishes Two Major Programs of Neuronal Differentiation in the Dorsal Spinal Cord. Neuron. 2002;34(4):551–562. doi: 10.1016/s0896-6273(02)00689-x. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou AH. Neural Induction, the Default Model and Embryonic Stem Cells. Nat Rev Neurosci. 2002;3(4):271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Murashov AK, Pak ES, Hendricks WA, Owensby JP, Sierpinski PL, Tatko LM, Fletcher PL. Directed Differentiation of Embryonic Stem Cells into Dorsal Interneurons. FASEB J. 2005;19(2):252–254. doi: 10.1096/fj.04-2251fje. [DOI] [PubMed] [Google Scholar]
- Murphy SV, Atala A. 3d Bioprinting of Tissues and Organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- Najm FJ, Lager AM, Zaremba A, Wyatt K, Caprariello AV, Factor DC, Karl RT, Maeda T, Miller RH, Tesar PJ. Transcription Factor-Mediated Reprogramming of Fibroblasts to Expandable, Myelinogenic Oligodendrocyte Progenitor Cells. Nat Biotechnol. 2013;31(5):426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, Mitrecic D, Falnikar A, Lepore AC. Transplantation of Stem Cell-Derived Astrocytes for the Treatment of Amyotrophic Lateral Sclerosis and Spinal Cord Injury. World J Stem Cells. 2015;7(2):380–398. doi: 10.4252/wjsc.v7.i2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human Embryonic Stem Cells Differentiate into Oligodendrocytes in High Purity and Myelinate after Spinal Cord Transplantation. Glia. 2005;49(3):385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Nomura H, Katayama Y, Shoichet MS, Tator CH. Complete Spinal Cord Transection Treated by Implantation of a Reinforced Synthetic Hydrogel Channel Results in Syringomyelia and Caudal Migration of the Rostral Stump. Neurosurgery. 2006;59(1):183–192. doi: 10.1227/01.NEU.0000219859.35349.EF. discussion 183–192. [DOI] [PubMed] [Google Scholar]
- Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted Human-Induced Pluripotent Stem-Cell-Derived Neurospheres Promote Motor Functional Recovery after Spinal Cord Injury in Mice. Proc Natl Acad Sci U S A. 2011;108(40):16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A Requirement for Retinoic Acid-Mediated Transcriptional Activation in Ventral Neural Patterning and Motor Neuron Specification. Neuron. 2003;40(1):81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Nutt SE, Chang EA, Suhr ST, Schlosser LO, Mondello SE, Moritz CT, Cibelli JB, Horner PJ. Caudalized Human Ipsc-Derived Neural Progenitor Cells Produce Neurons and Glia but Fail to Restore Function in an Early Chronic Spinal Cord Injury Model. Exp Neurol. 2013;248:491–503. doi: 10.1016/j.expneurol.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh TH, Markelonis GJ, Von Visger JR, Baik B, Shipley MT. Acidic Ph Rapidly Increases Immunoreactivity of Glial Fibrillary Acidic Protein in Cultured Astrocytes. Glia. 1995;13(4):319–322. doi: 10.1002/glia.440130408. [DOI] [PubMed] [Google Scholar]
- Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD. Development of Neuronal Precursor Cells and Functional Postmitotic Neurons from Embryonic Stem Cells in Vitro. Mech Dev. 1996;59(1):89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- Okada Y, Shimazaki T, Sobue G, Okano H. Retinoic-Acid-Concentration-Dependent Acquisition of Neural Cell Identity During in Vitro Differentiation of Mouse Embryonic Stem Cells. Dev Biol. 2004;275(1):124–142. doi: 10.1016/j.ydbio.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Olson HE, Rooney GE, Gross L, Nesbitt JJ, Galvin KE, Knight A, Chen B, Yaszemski MJ, Windebank AJ. Neural Stem Cell- and Schwann Cell-Loaded Biodegradable Polymer Scaffolds Support Axonal Regeneration in the Transected Spinal Cord. Tissue Eng Part A. 2009;15(7):1797–1805. doi: 10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lim E, Back S, Na H, Park Y, Sun K. Nerve Regeneration Following Spinal Cord Injury Using Matrix Metalloproteinase-Sensitive, Hyaluronic Acid-Based Biomimetic Hydrogel Scaffold Containing Brain-Derived Neurotrophic Factor. J Biomed Mater Res A. 2010;93(3):1091–1099. doi: 10.1002/jbm.a.32519. [DOI] [PubMed] [Google Scholar]
- Parr AM, Kulbatski I, Zahir T, Wang X, Yue C, Keating A, Tator CH. Transplanted Adult Spinal Cord-Derived Neural Stem/Progenitor Cells Promote Early Functional Recovery after Rat Spinal Cord Injury. Neuroscience. 2008;155(3):760–770. doi: 10.1016/j.neuroscience.2008.05.042. [DOI] [PubMed] [Google Scholar]
- Parr AM, Tator CH, Keating A. Bone Marrow-Derived Mesenchymal Stromal Cells for the Repair of Central Nervous System Injury. Bone Marrow Transplant. 2007;40(7):609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- Patani R, Compston A, Puddifoot CA, Wyllie DJ, Hardingham GE, Allen ND, Chandran S. Activin/Nodal Inhibition Alone Accelerates Highly Efficient Neural Conversion from Human Embryonic Stem Cells and Imposes a Caudal Positional Identity. PLoS One. 2009;4(10):e7327. doi: 10.1371/journal.pone.0007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patani R, Hollins AJ, Wishart TM, Puddifoot CA, Alvarez S, de Lera AR, Wyllie DJ, Compston DA, Pedersen RA, Gillingwater TH, Hardingham GE, Allen ND, Chandran S. Retinoid-Independent Motor Neurogenesis from Human Embryonic Stem Cells Reveals a Medial Columnar Ground State. Nat Commun. 2011;2:214. doi: 10.1038/ncomms1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Joseph G, Patel A, Patel S, Bustin D, Mawson D, Tuesta LM, Puentes R, Ghosh M, Pearse DD. Suspension Matrices for Improved Schwann-Cell Survival after Implantation into the Injured Rat Spinal Cord. J Neurotrauma. 2010;27(5):789–801. doi: 10.1089/neu.2008.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peljto M, Dasen JS, Mazzoni EO, Jessell TM, Wichterle H. Functional Diversity of Esc-Derived Motor Neuron Subtypes Revealed through Intraspinal Transplantation. Cell Stem Cell. 2010;7(3):355–366. doi: 10.1016/j.stem.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidou P, Dasen JS. Hox Genes: Choreographers in Neural Development, Architects of Circuit Organization. Neuron. 2013;80(1):12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeshchik Y, Puttonen KA, Kidin I, Ruponen M, Lehtonen S, Malm T, Akesson E, Hovatta O, Koistinaho J. Transplanted Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Do Not Promote Functional Recovery of Pharmacologically Immunosuppressed Mice with Contusion Spinal Cord Injury. Cell Transplant. 2015;24(9):1799–1812. doi: 10.3727/096368914X684079. [DOI] [PubMed] [Google Scholar]
- Raspa A, Pugliese R, Maleki M, Gelain F. Recent Therapeutic Approaches for Spinal Cord Injury. Biotechnol Bioeng. 2016;113(2):253–259. doi: 10.1002/bit.25689. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural Progenitors from Human Embryonic Stem Cells. Nat Biotechnol. 2001;19(12):1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct Reprogramming of Mouse and Human Fibroblasts into Multipotent Neural Stem Cells with a Single Factor. Cell Stem Cell. 2012;11(1):100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, Schwartz M. The Bright Side of the Glial Scar in Cns Repair. Nat Rev Neurosci. 2009;10(3):235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- Romanyuk N, Amemori T, Turnovcova K, Prochazka P, Onteniente B, Sykova E, Jendelova P. Beneficial Effect of Human Induced Pluripotent Stem Cell-Derived Neural Precursors in Spinal Cord Injury Repair. Cell Transplant. 2015;24(9):1781–1797. doi: 10.3727/096368914X684042. [DOI] [PubMed] [Google Scholar]
- Rossi SL, Nistor G, Wyatt T, Yin HZ, Poole AJ, Weiss JH, Gardener MJ, Dijkstra S, Fischer DF, Keirstead HS. Histological and Functional Benefit Following Transplantation of Motor Neuron Progenitors to the Injured Rat Spinal Cord. PLoS One. 2010;5(7):e11852. doi: 10.1371/journal.pone.0011852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybon L, Lamas NJ, Garcia-Diaz A, Yang EJ, Sattler R, Jackson-Lewis V, Kim YA, Kachel CA, Rothstein JD, Przedborski S, Wichterle H, Henderson CE. Human Stem Cell-Derived Spinal Cord Astrocytes with Defined Mature or Reactive Phenotypes. Cell Rep. 2013;4(5):1035–1048. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungarunlert S, Techakumphu M, Pirity MK, Dinnyes A. Embryoid Body Formation from Embryonic and Induced Pluripotent Stem Cells: Benefits of Bioreactors. World J Stem Cells. 2009;1(1):11–21. doi: 10.4252/wjsc.v1.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saric T, Frenzel LP, Hescheler J. Immunological Barriers to Embryonic Stem Cell-Derived Therapies. Cells Tissues Organs. 2008;188(1–2):78–90. doi: 10.1159/000118784. [DOI] [PubMed] [Google Scholar]
- Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K. Up-Regulation of Glial Cell Line-Derived Neurotrophic Factor (Gdnf) Following Traumatic Spinal Cord Injury. Neuroreport. 2000;11(17):3877–3881. doi: 10.1097/00001756-200011270-00054. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and Regeneration of Axons in the Lesioned Spinal Cord. Physiol Rev. 1996;76(2):319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cell Transplants Improve Recovery after Cervical Spinal Cord Injury. Stem Cells. 2010;28(1):152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabuddin LS, Ray J, Gage FH. Fgf-2 Is Sufficient to Isolate Progenitors Found in the Adult Mammalian Spinal Cord. Exp Neurol. 1997;148(2):577–586. doi: 10.1006/exnr.1997.6697. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Coykendall K, Li Y, Moon A, Priyadarshani P, Yao L. Repair of Injured Spinal Cord Using Biomaterial Scaffolds and Stem Cells. Stem Cell Res Ther. 2014;5(4):91. doi: 10.1186/scrt480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NA, Sousa N, Reis RL, Salgado AJ. From Basics to Clinical: A Comprehensive Review on Spinal Cord Injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration Beyond the Glial Scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global Prevalence and Incidence of Traumatic Spinal Cord Injury. Clin Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of Pluripotential Embryonic Stem Cell Differentiation by Purified Polypeptides. Nature. 1988;336(6200):688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal Signaling Promotes Specification of Human Embryonic Stem Cells into Neuroectoderm. Dev Biol. 2008;313(1):107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of Mouse and Human Fibroblasts into Functional Spinal Motor Neurons. Cell Stem Cell. 2011;9(3):205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokols S, Tuszynski MH. Freeze-Dried Agarose Scaffolds with Uniaxial Channels Stimulate and Guide Linear Axonal Growth Following Spinal Cord Injury. Biomaterials. 2006;27(3):443–451. doi: 10.1016/j.biomaterials.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Straley KS, Foo CW, Heilshorn SC. Biomaterial Design Strategies for the Treatment of Spinal Cord Injuries. J Neurotrauma. 2010;27(1):1–19. doi: 10.1089/neu.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung HJ, Meredith C, Johnson C, Galis ZS. The Effect of Scaffold Degradation Rate on Three-Dimensional Cell Growth and Angiogenesis. Biomaterials. 2004;25(26):5735–5742. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tang BL, Low CB. Genetic Manipulation of Neural Stem Cells for Transplantation into the Injured Spinal Cord. Cell Mol Neurobiol. 2007;27(1):75–85. doi: 10.1007/s10571-006-9119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Liao X, Shi B, Qu Y, Huang Z, Lin Q, Guo X, Pei F. The Effects of Controlled Release of Neurotrophin-3 from Pcla Scaffolds on the Survival and Neuronal Differentiation of Transplanted Neural Stem Cells in a Rat Spinal Cord Injury Model. PLoS One. 2014;9(9):e107517. doi: 10.1371/journal.pone.0107517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tator CH, Fehlings MG. Review of the Secondary Injury Theory of Acute Spinal Cord Trauma with Emphasis on Vascular Mechanisms. J Neurosurg. 1991;75(1):15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of Neurotrophin-3 from Fibrin Enhances Neuronal Fiber Sprouting after Spinal Cord Injury. J Control Release. 2006;113(3):226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Sakiyama-Elbert SE. Effect of Controlled Delivery of Neurotrophin-3 from Fibrin on Spinal Cord Injury in a Long Term Model. J Control Release. 2006;116(2):204–210. doi: 10.1016/j.jconrel.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A Systematic Review of Cellular Transplantation Therapies for Spinal Cord Injury. J Neurotrauma. 2011;28(8):1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Palma JL, Shea LD. Sponge-Mediated Lentivirus Delivery to Acute and Chronic Spinal Cord Injuries. J Control Release. 2015;204:1–10. doi: 10.1016/j.jconrel.2015.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic Interventions after Spinal Cord Injury. Nat Rev Neurosci. 2006;7(8):628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Timmer JR, Wang C, Niswander L. Bmp Signaling Patterns the Dorsal and Intermediate Neural Tube Via Regulation of Homeobox and Helix-Loop-Helix Transcription Factors. Development. 2002;129(10):2459–2472. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- Tobias CA, Han SS, Shumsky JS, Kim D, Tumolo M, Dhoot NO, Wheatley MA, Fischer I, Tessler A, Murray M. Alginate Encapsulated Bdnf-Producing Fibroblast Grafts Permit Recovery of Function after Spinal Cord Injury in the Absence of Immune Suppression. J Neurotrauma. 2005;22(1):138–156. doi: 10.1089/neu.2005.22.138. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal Cord Injury Is Accompanied by Chronic Progressive Demyelination. J Comp Neurol. 2005;486(4):373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Tsai EC, Dalton PD, Shoichet MS, Tator CH. Matrix Inclusion within Synthetic Hydrogel Guidance Channels Improves Specific Supraspinal and Local Axonal Regeneration after Complete Spinal Cord Transection. Biomaterials. 2006;27(3):519–533. doi: 10.1016/j.biomaterials.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Tso D, McKinnon RD. Cell Replacement Therapy for Central Nervous System Diseases. Neural Regen Res. 2015;10(9):1356–1358. doi: 10.4103/1673-5374.165209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, Ko SY, Margul DJ, Bartels AK, Boehler RM, Cummings BJ, Anderson AJ, Shea LD. Multifunctional, Multichannel Bridges That Deliver Neurotrophin Encoding Lentivirus for Regeneration Following Spinal Cord Injury. Biomaterials. 2012;33(5):1618–1626. doi: 10.1016/j.biomaterials.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Margul DJ, Goodman AG, Boehler RM, Holland SJ, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD. Long-Term Characterization of Axon Regeneration and Matrix Changes Using Multiple Channel Bridges for Spinal Cord Regeneration. Tissue Eng Part A. 2014;20(5–6):1027–1037. doi: 10.1089/ten.tea.2013.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler JY, Xu XM, Cheng JX. Nanomedicine for Treating Spinal Cord Injury. Nanoscale. 2013;5(19):8821–8836. doi: 10.1039/c3nr00957b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct Isolation of Human Central Nervous System Stem Cells. Proc Natl Acad Sci U S A. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdzikova LM, Ruzicka J, LaBagnara M, Karova K, Kubinova S, Jirakova K, Murali R, Sykova E, Jhanwar-Uniyal M, Jendelova P. Human Mesenchymal Stem Cells Modulate Inflammatory Cytokines after Spinal Cord Injury in Rat. Int J Mol Sci. 2014;15(7):11275–11293. doi: 10.3390/ijms150711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct Conversion of Fibroblasts to Functional Neurons by Defined Factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walthers CM, Seidlits SK. Gene Delivery Strategies to Promote Spinal Cord Repair. Biomark Insights. 2015;10(Suppl 1):11–29. doi: 10.4137/BMI.S20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase Combined with Rehabilitation Promotes Recovery of Forelimb Function in Rats with Chronic Spinal Cord Injury. J Neurosci. 2011;31(25):9332–9344. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A Rock Inhibitor Permits Survival of Dissociated Human Embryonic Stem Cells. Nat Biotechnol. 2007;25(6):681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent Cns Stem Cells Are Present in the Adult Mammalian Spinal Cord and Ventricular Neuroaxis. J Neurosci. 1996;16(23):7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzer G. Embryonic Stem Cell-Derived Embryoid Bodies: An in Vitro Model of Eutherian Pregastrulation Development and Early Gastrulation. Handb Exp Pharmacol. 2006;174:21–51. [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed Differentiation of Embryonic Stem Cells into Motor Neurons. Cell. 2002;110(3):385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic Factors and Receptors in the Immature and Adult Spinal Cord after Mechanical Injury or Kainic Acid. J Neurosci. 2001;21(10):3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilems TS, Pardieck J, Iyer N, Sakiyama-Elbert SE. Combination Therapy of Stem Cell Derived Neural Progenitors and Drug Delivery of Anti-Inhibitory Molecules for Spinal Cord Injury. Acta Biomater. 2015;28:23–32. doi: 10.1016/j.actbio.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilems TS, Sakiyama-Elbert SE. Sustained Dual Drug Delivery of Anti-Inhibitory Molecules for Treatment of Spinal Cord Injury. J Control Release. 2015;213:103–111. doi: 10.1016/j.jconrel.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of Fibrin Scaffolds for Differentiation of Murine Embryonic Stem Cells into Neural Lineage Cells. Biomaterials. 2006;27(36):5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Faxel TE, Gottlieb DI, Sakiyama-Elbert SE. The Effects of Soluble Growth Factors on Embryonic Stem Cell Differentiation inside of Fibrin Scaffolds. Stem Cells. 2007;25(9):2235–2244. doi: 10.1634/stemcells.2007-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Rader A, Sakiyama-Elbert SE. The Effect of Controlled Growth Factor Delivery on Embryonic Stem Cell Differentiation inside Fibrin Scaffolds. Stem Cell Res. 2008;1(3):205–218. doi: 10.1016/j.scr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Sakiyama-Elbert SE. Approaches to Neural Tissue Engineering Using Scaffolds for Drug Delivery. Adv Drug Deliv Rev. 2007;59(4–5):325–338. doi: 10.1016/j.addr.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Sakiyama-Elbert SE. Combining Stem Cells and Biomaterial Scaffolds for Constructing Tissues and Cell Delivery. Stembook; Cambridge MA: 2008. [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid Leukaemia Inhibitory Factor Maintains the Developmental Potential of Embryonic Stem Cells. Nature. 1988;336(6200):684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Wilson L, Maden M. The Mechanisms of Dorsoventral Patterning in the Vertebrate Neural Tube. Dev Biol. 2005;282(1):1–13. doi: 10.1016/j.ydbio.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Edlund T. Neural Induction: Toward a Unifying Mechanism. Nat Neurosci. 2001;4(Suppl):1161–1168. doi: 10.1038/nn747. [DOI] [PubMed] [Google Scholar]
- Woerly S, Pinet E, de Robertis L, Van Diep D, Bousmina M. Spinal Cord Repair with Phpma Hydrogel Containing Rgd Peptides (Neurogel) Biomaterials. 2001;22(10):1095–1111. doi: 10.1016/s0142-9612(00)00354-9. [DOI] [PubMed] [Google Scholar]
- Xu H, Iyer N, Huettner JE, Sakiyama-Elbert SE. A Puromycin Selectable Cell Line for the Enrichment of Mouse Embryonic Stem Cell-Derived V3 Interneurons. Stem Cell Res Ther. 2015;6:220. doi: 10.1186/s13287-015-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Sakiyama-Elbert SE. Directed Differentiation of V3 Interneurons from Mouse Embryonic Stem Cells. Stem Cells Dev. 2015;24(22):2723–2732. doi: 10.1089/scd.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lu P, McKay HM, Bernot T, Keirstead H, Steward O, Gage FH, Edgerton VR, Tuszynski MH. Endogenous Neurogenesis Replaces Oligodendrocytes and Astrocytes after Primate Spinal Cord Injury. J Neurosci. 2006;26(8):2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, Hawkins JS, Geissler R, Barres BA, Wernig M. Generation of Oligodendroglial Cells by Direct Lineage Conversion. Nat Biotechnol. 2013;31(5):434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. Microrna-Mediated Conversion of Human Fibroblasts to Neurons. Nature. 2011;476(7359):228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii S, Oka M, Shima M, Taniguchi A, Taki Y, Akagi M. Restoration of Function after Spinal Cord Transection Using a Collagen Bridge. J Biomed Mater Res A. 2004;70(4):569–575. doi: 10.1002/jbm.a.30120. [DOI] [PubMed] [Google Scholar]
- Zeis T, Enz L, Schaeren-Wiemers N. The Immunomodulatory Oligodendrocyte. Brain Res. 2015 doi: 10.1016/j.brainres.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In Vitro Differentiation of Transplantable Neural Precursors from Human Embryonic Stem Cells. Nat Biotechnol. 2001;19(12):1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte Heterogeneity: An Underappreciated Topic in Neurobiology. Curr Opin Neurobiol. 2010;20(5):588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, Sudhof TC. Rapid Single-Step Induction of Functional Neurons from Human Pluripotent Stem Cells. Neuron. 2013;78(5):785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RR, Andrews MR, Wang D, Warren P, Gullo M, Schnell L, Schwab ME, Fawcett JW. Combination Treatment with Anti-Nogo-a and Chondroitinase Abc Is More Effective Than Single Treatments at Enhancing Functional Recovery after Spinal Cord Injury. Eur J Neurosci. 2013;38(6):2946–2961. doi: 10.1111/ejn.12276. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Thurmer F, Jork A, Weber M, Mimietz S, Hillgartner M, Brunnenmeier F, Zimmermann H, Westphal I, Fuhr G, Noth U, Haase A, Steinert A, Hendrich C. A Novel Class of Amitogenic Alginate Microcapsules for Long-Term Immunoisolated Transplantation. Ann N Y Acad Sci. 2001;944:199–215. doi: 10.1111/j.1749-6632.2001.tb03833.x. [DOI] [PubMed] [Google Scholar]


