Abstract
Poverty and exposure to adversity have been linked with decreased educational success. Various environmental and neurobiological pathways have been proposed for these associations, however, existing models have several clear drawbacks. Here we outline existing models, and propose an alternate model linking exposure to adverse experiences in childhood to education success. Specifically, we propose that measured dimensions of experience (e.g., decreased cognitive enrichment or increased exposure to violence), instead of named exposures (e.g., poverty) impact neurobiology through neurodevelopmental processes of neuroplasticity. Our model results in testable hypotheses and clear intervention strategies. We predict that exposure to trauma will have a distinct neurobiological impact from exposure to a lack of cognitive stimulation and that these distinct exposures will benefit from different interventions. Specificity in this arena is thus likely to better support educational achievement for disadvantaged children.
Extensive evidence links childhood poverty to decreased educational success 1. Children raised in poverty have lower school achievement, greater academic problems, and are less likely to graduate from high school than children who never experience poverty 2. These associations have been observed consistently for decades and have generated considerable interest in developing strategies for reducing socio-economic disparities in educational outcomes. More recently, a wider range of adverse childhood experiences (ACEs)—including child abuse, community violence exposure, parental psychopathology and loss of a parent—has also been associated with educational outcomes (Bethell, Newacheck, Hawes, & Halfon, 2014; Jimenez, Wade, Lin, Morrow, & Reichman, 2016). Together, these findings indicate clearly that adverse social and environmental experiences early in life exert a lasting influence on educational success. In this paper, we explore neurobiological pathways that might explain these relationships.
We first review two leading conceptual models that posit specific pathways through which early experience influences educational outcomes. The literature linking poverty with educational success has developed largely independently from the literature linking ACEs with developmental outcomes. As a result, the hypothesized pathways through which early experience are conceptualized to influence child development are different in each of these models. Next, we present an alternative and integrated view of how diverse environmental experiences may come to shape educational success. This integrated approach brings together and extends the poverty and ACEs models, providing testable hypotheses about the neurobiological pathways impacted by different early experiences. In relaying this model, we hope to provide novel targets for interventions aimed at reducing educational disparities.
Poverty, Learning Experiences, and Education
The link between poverty and educational outcomes has often been attributed to differential exposure to cognitively-stimulating experiences and opportunities for learning as a function of socio-economic status (SES). Children born to wealthier parents with more education are likely to have better formal and informal educational opportunities beginning at an early age than children from families with less education and fewer resources. Children from high-SES families live in houses with more books where parents speak more often and in more complex ways to their children, and when they enter school they are more likely to experience an enriched educational environment than children in low-SES families. Children from high-SES homes are more likely to visit museums, engage in extracurricular activities, and spend greater time in the company of an invested adult than their lower-SES peers 2,3. Variation in these types of early experiences is thought to influence neurocognitive development, including language, memory, attention, and both implicit and explicit learning processes 4,5. Lack of learning opportunities is thought to directly drive atypical neurocognitive development; for example, low linguistic complexity in parental speech predicts poor child language development 6. Disruptions in these domains of neurocognitive development might explain why children from low-SES families enter school at a disadvantage compared to their higher-SES peers 7. Further, the impact of these early learning opportunities may further impact a child’s ability to learn in school once they enter formal education 5.
Adversity, Stress, and Education
Leading conceptual models of childhood adversity argue that negative child development outcomes are the result of exposure to stress rather than a lack of exposure to cognitively-stimulating experiences 8. The most prominent model of adversity, hereafter referred to as the ACEs model, focuses on the number of adverse childhood experiences: as the number of ACEs increases, risk for negative outcomes, including poor educational success, increases 9,10. Within this model, poverty is either controlled for as a confounder or is just one of a number of adversities that can influence developmental outcomes. Importantly, childhood adversity is associated with detrimental outcomes across virtually every indicator of healthy development, ranging from mental and physical health to labor market success (Dube, et al., 2001; Edwards, Holden, Felitti, & Anda, 2003; Green et al., 2010; Johnson, Crosnoe, & Elder, 2011; Johnson & Schoeni, 2011; McLaughlin et al., 2010; Wickrama, Simons, & Baltimore, 2012). The broad nature of the impact of adversity on child development is thought to result, at least in part, from neurobiological mechanisms or biological embedding of environmental experience (Hertzman & Boyce, 2009). The most commonly hypothesized pathway asserts exposure to environmental adversity chronically activates neurobiological stress response systems. Greater exposure to these adversities results in “wear and tear” on the neurobiological systems supporting the stress response. This wear and tear is termed allostatic load 11 The processes and consequences of allostatic load have been widely studied in animal models 12 and attempts have been made to replicate many of these findings in humans (with mixed success, see 13).
Poverty model vs. ACEs model
These models of the impact of adversity on educational success have different strengths. First, the ACEs model encompasses a variety of adverse experiences that influence educational success, acknowledging that it is not just a lack of resources that hinders child development, but also exposure to violence, parental psychopathology, and parental absence. Second, the ACEs model acknowledges that these adversities are highly co-occurring, whereby the presence of one adversity increases the likelihood that a second will exist, and that this co-occurrence yields greater risk than the presence of a single adversity 10,14. In addition, specifying a neurobiological mechanism affords a precise description of how adversity impacts child development that can be assessed across a wide variety of populations. For example, in our research we have used identical tasks and physiological measurements of stress response system function to assess the impact of diverse adversities, including poverty maltreatment, and institutionalization13,15,16. Similarly, we and others have used neuroimaging to assess neural structure across widely different adversities and settings17,18. These assessments overcome the numerous problems inherent in child or parent-report survey measures. Despite these attractive aspects of the ACEs model, there are several shortcomings. The proposed neurobiological pathways of the ACEs model are precise and suggest that prevention of ACEs would have substantial downstream impacts on health and education. However, while the ACEs model is useful for identifying children in need of intervention, it does not yield a specific intervention strategy to prevent downstream developmental consequences of stress dysregulation for children exposed to adversity beyond stress reduction programs, such as mindfulness-based meditation interventions. While there is some evidence that such therapies can be useful, and they are increasingly widely implemented, they are understudied in non-clinical populations of children and youth or with groups exposed to adversity 21. Finally, the assumption of the ACEs model that all exposures contribute equally to developmental outcomes through a single neurobiological mechanism is questionable and ignores the importance of adversity type, timing, or chronicity on child development, including educational outcomes.
The poverty model addresses several of the gaps in the ACEs model. Primarily, the poverty model focuses on a specific pathway through which poverty impacts education success: insufficient learning experiences 7. This pathway lends itself admirably to very specific intervention strategies in which children are provided enhanced access to learning opportunities through early educational programs, increased access to learning materials such as books, and greater parent-child interactions. Indeed, several randomized control trials have conclusively demonstrated that these interventions have positive long-term educational effects for children in poverty 22–25, providing experimental support for the learning pathway linking poverty and educational success. However, the poverty model falls short in several ways. First, it ignores the wide variety of adverse experiences that influence child development and educational success. Living in poverty clearly increases the probability that a child will experience other adversities. In a population-representative sample of U.S. youths, 75% who experienced economic hardship were also exposed to at least one other ACE 26. However, the kinds of co-occurring adversities varied, and ranged from parental divorce to sexual abuse. Relatedly, in a different population-representative sample, parental SES was associated with use of physical discipline, but the correlation was small (r = .10) after accounting for covariates 27. Thus, it is clear that while SES is associated with greater exposure to other forms of adversity, it is not so completely overlapping with other ACEs that it can be assumed that the mechanisms underlying their impact on child development are identical. Second, the resource pathway, while specific, is limited in scope. For example, a lack of educational resources cannot account for the breadth of impact of poverty on child development. Poverty in childhood is associated with a range of negative outcomes including increased mental and physical health problems that are not an obvious consequence of lack of educational resources 28,29. Finally, the lack of a neurobiological pathway through which lack of resources may impact child development makes this pathway less precisely defined than the ACEs pathway and not measureable across diverse settings and adversity types. Perhaps most importantly, the lack of a neurobiological pathway is inconsistent with the mounting evidence of an association between poverty and neural structure and function 17,20,30–32.
Deprivation and Threat
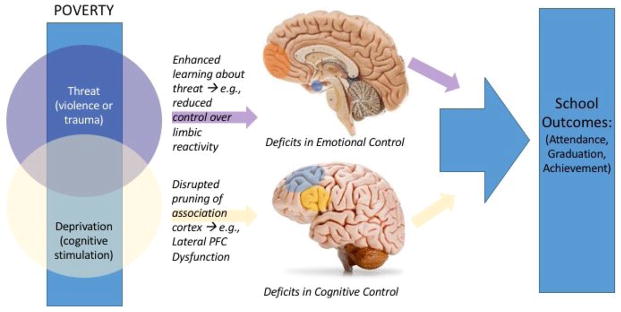
We propose an alternate approach to investigating the impact of adversity on educational success and child developmental outcomes more generally 33,34. This alternative is based on two principles. First, across the range of adverse childhood experiences (e.g., maltreatment, community violence, lack of educational resources) it is possible to extract dimensions of adversity that encompass numerous types of exposures. Two initial dimensions of exposures are proposed: the absence of cognitive and social stimulation, termed deprivation, and the presence of experiences involving harm or threat of harm, termed threat. Conceptually, these dimensions cut across numerous exposures. For example, threat is a core feature of sexual abuse, physical abuse, and community violence and deprivation is a core feature of poverty, neglect, and institutionalization. Second, unique emotional, cognitive, and neurobiological pathways underlie the association of these dimensions of experience with developmental outcomes. Specifically, the absence of cognitive enrichment—deprivation—will influence the development of higher-order cognitive processes such as linguistic processing and executive function. We expect that exposure to threat in childhood provides a specific type of learning experience that will influence mechanisms involved in the acquisition and extinction of fear, with downstream consequences on emotion processing. These hypotheses are derived from animal models of early threat and deprivation exposure 35,36,37,38. See Figure 1 for an overview of our model.
Figure 1.
Within adversity exposure, we hypothesize that dimensions of experience can be measured which will differentially impact neurodevelopment leading reductions in academic success. First panel: While exposure to cognitive enrichment (Deprivation dimension) is likely to be reduced as a function of poverty, it may not be, thus the deprivation circle is shown as not completely overlapping with poverty. Similarly, exposure to violence or trauma is likely to be, but is not always, increased for children living in poverty. These two circles are shown as overlapping because children are sometimes exposed to both deprivation and threat. Second panel: Next we show a cartoon of the hypothesized cognitive, emotional, and neurobiological pathways through which these kinds of exposures may impact school outcomes. Third panel: In the final part of the figure we show that school outcomes are an equifinal outcome for both dimensions of exposure. Thus, examining the neurobiology is central to distinguishing the impacts of various dimensions of exposure.
Specifically, we hypothesize that the effects of deprivation and threat impact neurodevelopment through the typical developmental processes of neuroplasticity. In the case of deprivation, rodent and human studies suggest that a lack of environmental stimulation leads to dramatic increases in synaptic pruning; when rodents are raised in low enrichment environments, global decreases in cortical volume are observed 38. Similarly, when cognitive enrichment is low during early human development, for example among children raised in institutions with limited caregiver contact, cortical volume and thickness are reduced throughout the cortex 19,18. In cases where cognitive deprivation is more mild, as in poverty, we would expect the effects to similar but attenuated; recent evidence is consistent with this prediction 17,39. General reductions in cortical volume are not isolated phenomena but are likely to yield deficits in higher-order cognitive functions, such as language and executive functions, because they require coordinated function of multiple areas of association cortex and rely on late-developing areas of the brain such as the prefrontal cortex. This model thus expands upon the learning mechanisms outlined in the poverty model to encompass a neurobiological pathway underlying deprivation-related deficits in neurocognitive function that, in turn, will decrease educational success. Exposure to threat during periods of developmental plasticity, in contrast, shifts development of cortical and subcortical structures involved in coordinating fear responses and processing other negative emotions. Specifically, the presence of learning experiences involving high degrees of threat will bias these systems towards early detection of other environmental threats. While this initial prediction is a functional one, such functional neural changes must be instantiated structurally to some degree; thus in the case of threat we may expect specific structural changes only in cortical and subcortical structures which support emotional control. With regard to educational outcomes, children exposed to threat will be hindered in their ability to control their response to negative emotions in the classroom resulting in disruptive behavior and negatively impacting their learning opportunities.
The influence of threat and deprivation on developmental outcomes are mediated through basic neurodevelopmental processes involved in pruning and potentiation of synapses. Pruning is the mechanism underlying the impact of many childhood environmental experiences on neural development (e.g., phonemic retention in the context of multiple language exposure, visual cortex organization) 40–42. Long-term potentiation is the basic neurobiological mechanism through which learning occurs throughout development 43,44. The hypothesized pathways described here are in contrast to the stress pathway proposed in the ACEs model which relies on the existence of developmentally atypical processes of synaptic re-organization through abnormal dendritic changes for their hypothesized effect on a myriad of outcomes. The fact that synaptic pruning and potentiation, which we propose here, are the mechanisms responsible for typical acquisition of skills and abilities during development make them likely pathways through which neural development is shaped by adverse experiences.
This alternate conceptual model addresses many of the limitations in the ACEs and poverty models when considering the impact of adversity on educational outcomes. First, our model encompasses a variety of adverse experiences. Second, we propose neurobiological mechanisms that are specific to particular kinds of experiences at a testable level of specificity. Although adverse experiences are co-occurring, they can and should be measured separately to determine whether specificity exists in the cognitive, emotional, and neurobiological processes that they influence. Importantly, although threat and deprivation each impact educational success, they are likely to do so through distinct cognitive, emotional, and neurobiological pathways. Thus, the neurobiological impact of deprivation can be assessed while statistically controlling for violence exposure, and vice versa. Third, this model has clear implications for interventions to improve the education experiences of children who have experienced adversity. Given our hypothesized differences linking deprivation and threat to educational outcomes, we likewise expect that children exposed to different types of adversity might benefit from distinct interventions. For example, a child with emotion regulation difficulties following exposure to traumatic violence and a child with executive function deficits following a lack of cognitive stimulation at home both may display disruptive behavior in the classroom. However, interventions for these two hypothetical children could be very different. In the former case, the child may benefit from a trauma focused therapy designed to increasing self-regulatory capacity via emotional awareness whereas in the latter case the child may benefit from increased scaffolded learning opportunities in the classroom.
Importantly, the deprivation and threat model builds on existing models of the impact of adversity on child development. The concept of deprivation is derived from the poverty literature identifying decreased cognitive stimulation as one pathway through which poverty influences education, and the concept of threat is related to stress exposure postulated in the ACEs model. Even the neurobiological pathways we propose to account for the impact of these dimensions on child development have been articulated by previous authors 45. Our model brings together multiple dimensions of adversity and pathways through which these adversities could be embedded to yield novel testable hypotheses. This approach is an alternate to existing models of the impact of adversity on education that promises to generate novel and more targeted intervention strategies which have the potential to enhance education opportunities for the most disadvantaged children.
Highlights.
Poverty and childhood adversity are associated with decrements in educational success
Current neurobiological models for these effects are limited
We provide an alternate model for the impact of adversity on academic performance.
This model emphasizes developmental neurobiological pathways
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How much does childhood poverty affect the life chances of children? Am Sociol Rev. 1998:406–423. [Google Scholar]
- 2**.Duncan GJ, Brooks-Gunn J. Consequences of Growing Up Poor. Russell Sage Foundation; 1999. [Google Scholar]
- 3.Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 4.Farah MJ, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 5.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoff E. The Specificity of Environmental Influence: Socioeconomic Status Affects Early Vocabulary Development Via Maternal Speech. Child Dev. 2003;74:1368–78. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- 7*.Yeung WJ, Linver MR, Brooks-Gunn J. How money matters for young children’s development: parental investment and family processes. Child Dev. 2002;73:1861–1879. doi: 10.1111/1467-8624.t01-1-00511. [DOI] [PubMed] [Google Scholar]
- 8.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA J Am Med Assoc. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 9.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 10.Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychol Bull. 2013;139:1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- 11*.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 13*.McLaughlin KA, et al. Causal effects of the early caregiving environment on development of stress response systems in children. Proc Natl Acad Sci U S A. 2015;112:5637–5642. doi: 10.1073/pnas.1423363112. (13) This paper is one of the only examples of assessing the effect of adversity on stress reactivity where causal inference can be drawn because adversity in early childhood was randomly assigned. This paper concludes that exposure to profound deprivation and threat (early institutionalization) results in a blunted stress response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong M, et al. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl. 2004;28:771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin KA, Sheridan MA, Alves S, Mendes WB. Child maltreatment and autonomic nervous system reactivity: identifying dysregulated stress reactivity patterns by using the biopsychosocial model of challenge and threat. Psychosom Med. 2014;76:538–546. doi: 10.1097/PSY.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Dev Sci. 2013 doi: 10.1111/desc.12087. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Noble KG, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. (17) This paper used a large (~1000 children) publically available data set to examine the impact of SES on neural structure. Nobel and colleagues observed global differences in surface area and thickness by SES even controlling for some aspects of genetic variation. It is one of the best demonstrations of this association to date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA., 3rd Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci U S A. 2012;109:12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin KA, et al. Widespread Reductions in Cortical Thickness Following Severe Early-Life Deprivation: A Neurodevelopmental Pathway to Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 22**.Campbell F, et al. Early Childhood Investments Substantially Boost Adult Health. Science. 2014;343:1478–1485. doi: 10.1126/science.1248429. (22) In this and (23) results of a longitudinal follow up of the Abecedarian Project (ABC) are reported on. The Abecedarian project is an evaluation of a high-quality early childhood program. This program addressed many aspects of early childhood experiences and the results have been equally broad. Random assignment to the treatment group results in long-term differences in health and well-being across a variety of measures. This article reports on heart and metabolic health. The results of this study, like reference 13 indicate that early adverse exposures causally impact child development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muennig P, Schweinhart L, Montie J, Neidell M. Effects of a prekindergarten educational intervention on adult health: 37-year follow-up results of a randomized controlled trial. Am J Public Health. 2009;99:1431–1437. doi: 10.2105/AJPH.2008.148353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds AJ, Temple JA, Robertson DL, Mann EA. Long-term effects of an early childhood intervention on educational achievement and juvenile arrest: A 15-year follow-up of low-income children in public schools. JAMA J Am Med Assoc. 2001;285:2339–2346. doi: 10.1001/jama.285.18.2339. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds AJ. Effects of a preschool plus follow-on intervention for children at risk. Dev Psychol. 1994;30:787–804. [Google Scholar]
- 26**.McLaughlin KA, et al. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch Gen Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson S, et al. Predicting abuse-prone parental attitudes and discipline practices in a nationally representative sample. Child Abuse Negl. 1999;23:15–29. doi: 10.1016/s0145-2134(98)00108-2. [DOI] [PubMed] [Google Scholar]
- 28.Adler NE, et al. Socioeconomic status and health: The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 29.Bøe T, Overland S, Lundervold AJ, Hysing M. Socioeconomic status and children’s mental health: results from the Bergen Child Study. Soc Psychiatry Psychiatr Epidemiol. 2011 doi: 10.1007/s00127-011-0462-9. [DOI] [PubMed] [Google Scholar]
- 30.Hanson JL, et al. Family poverty affects the rate of human infant brain growth. PloS One. 2013;8:e80954. doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PloS One. 2011;6:e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheridan MA, Sarsour K, Jutte D, D’Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PloS One. 2012;7:e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Sheridan M, McLaughlin K. Dimensions of Early Experience and Neural Development: Deprivation and Threat. Trends Cogn Sci. 2014;18:580–585. doi: 10.1016/j.tics.2014.09.001. (34) In this and (33) the arguments and predictions described in the current review are laid out in more detail focusing on specific hypothesized neurobiological outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci Off J Soc Neurosci. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 37.Diamond MC, et al. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J Comp Neurol. 1966;128:117–126. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- 38**.Diamond MC, Rosenzweig MR, Bennett EL, Lindner B, Lyon L. Effects of environmental enrichment and impoverishment on rat cerebral cortex. J Neurobiol. 1972;3:47–64. doi: 10.1002/neu.480030105. [DOI] [PubMed] [Google Scholar]
- 39*.Mackey AP, et al. Neuroanatomical correlates of the income-achievement gap. Psychol Sci. 2015;26:925–933. doi: 10.1177/0956797615572233. (39) In this paper, Mackey and colleagues directly assess the possibility that the income-achievement gap is related to neural structure. They observe, consistent with the predictions in this article concerning deprivation or cognitive stimulation, that family income is related to cortical thickness (but not surface area or white mater volume) and that these associations mediate the impact that family income has on achievement test performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol. 2005;69:215–237. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- 41.Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 42**.Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- 43.Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci U S A. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittenger C, Kandel ER. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2003;358:757–763. doi: 10.1098/rstb.2002.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Weiss MJ, Wagner SH. What explains the negative consequences of adverse childhood experiences on adult health? Insights from cognitive and neuroscience research. Am J Prev Med. 1998;14:356–360. doi: 10.1016/s0749-3797(98)00011-7. [DOI] [PubMed] [Google Scholar]