Abstract
The SGLT2 inhibitors (SGLTi) and glucagon-like-1 receptor agonists (GLP-1 RAs) effectively reduce HbA1c, but via very different mechanisms, making them an effective duet for combination therapy. Recently, drugs in both of these antidiabetic classes have been shown to reduce cardiovascular events, most likely by different mechanisms. SGLT2i appear to exert their CV protective actions by hemodynamic effects, while GLP-1 RAs work via anti-atherogenic/anti-inflammatory mechanisms, raising the possibility that combined therapy with these two classes may produce additive CV benefits. The SGLT2i and GLP-1 RAs also reduced macroalbuminuria, decreased the time for doubling of serum creatinine, and slowed the time to end stage renal disease. In this perspective, we review the potential benefit of combination SGLT2i/GLP-1 RA therapy on metabolic-cardiovascular-renal disease in patients with type 2 diabetes mellitus.
Keywords: Type 2 diabetes mellitus, GLP-1 receptor agonist, SGLT2i inhibitor, Glycemic control, Cardiovascular disease
PATHOPHYSIOLOGY OF TYPE 2 DIABETES MELLITUS (T2DM)
The Triumvirate
In 1987 our understanding of the pathophysiology of type 2 diabetes mellitus (T2DM) was relatively simple and referred to as the TRIUMVIRATE (1). Individuals destined to develop T2DM are characterized by moderate-severe muscle and hepatic insulin resistance (2–4), but glucose tolerance remains normal because the beta cells are able to increase insulin secretion to offset the defect in insulin action (Figure 1). With time the beta cells begin to fail and hepatic insulin resistance becomes manifest as an overproduction of glucose throughout the sleeping hours, resulting in fasting hyperglycemia (5). Following meal ingestion insulin secretion is stimulated and the rise in plasma insulin concentration stimulates muscle and liver glucose uptake and suppresses hepatic glucose production (HGP) (6). Insulin resistance in muscle, combined with impaired suppression of HGP and impaired insulin secretion, are the primary abnormalities responsible for postprandial hyperglycemia.
Figure 1.
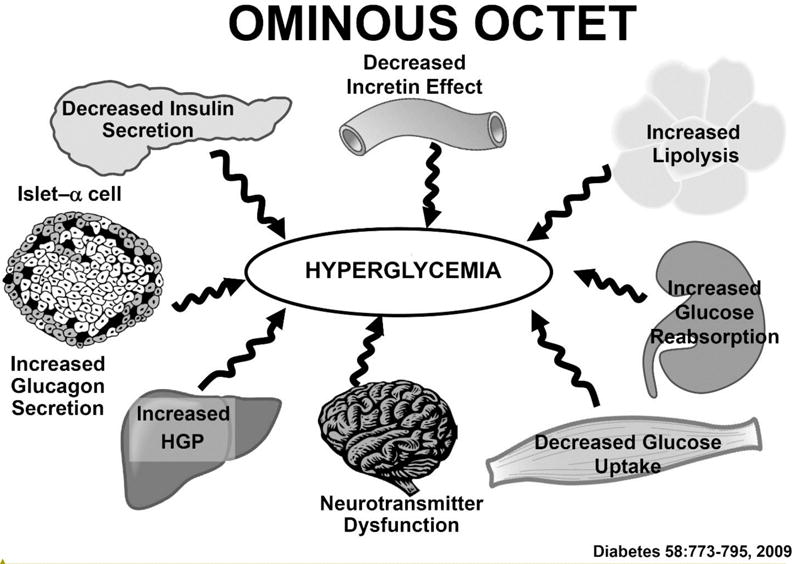
The Ominous Octet. The pathophysiology of type 2 diabetes mellitus involves at least 8 distinct disturbances that collectively contribute to fasting and postprandial hyperglycemia (see text for a more detailed discussion). From reference #2.
The Ominous Octet
Over the last 30 years our understanding of the pathophysiology of T2DM has revealed that the disease is more complicated than previously appreciated with at least 8 distinct pathogenic abnormalities which collectively comprise the OMINOUS OCTET (2) (Figure 1).
The adipocyte is the primary tissue responsible for fat storage. As long as the fat remains in the adipocytes, it cannot exert a deleterious effect on glucose metabolism. However, as the fat cell enlarges it becomes resistant to insulin (3), the antilipolytic effect of insulin becomes impaired, triglyceride breakdown is accelerated, and fatty acid release is increased (Figure 1). The increase in plasma FFA aggravates the insulin resistance in liver and muscle (7), inhibits insulin secretion (8), and promotes beta cell apoptosis (9).
The gastrointestinal tract is a giant endocrine organ that secretes two incretin hormones: (i) glucagon-like peptide-1 (GLP-1) by L-cells (located in the distal small intestine and large intestine) and glucose-dependent insulinotrophic peptide (GIP) by K cells (located in the early small intestine) (10,11). GLP-1 and GIP account for 60–70% of insulin secreted in response to a typical mixed meal (12). In T2DM beta cells are resistant to both GLP-1 and GIP (13) (Figure 1); GLP-1 and GIP secretion are normal or only moderately reduced (14). Beta cell resistance to GLP-1 and GIP plays a major role in the defect in insulin secretion and glucose intolerance. GLP-1, but not GIP, also inhibits glucagon secretion and suppresses appetite (15). GLP-1 resistance contributes to hyperglucagonemia and excessive HGP, as well as weight gain.
Excess glucagon secretion by the alpha cells plays an important role in the pathophysiology of T2DM (16). Diabetic patients have elevated fasting plasma glucagon levels that fail to suppress normally after a meal (17) (Figure 1). This contributes to both fasting and postprandial hyperglycemia. The liver also is hypersensitive to the stimulatory effect of glucagon on HGP, especially in the presence of impaired insulin secretion (18).
The kidney contributes to the pathogenesis of T2DM via two mechanisms (19,20) (Figure 1). Following an overnight fast, the kidney contributes ~15–20% of endogenous glucose production and this is under the control of insulin (19,20). Because the kidney is resistant to insulin (19,20), renal glucose production is increased and contributes to the increase in fasting plasma glucose (FPG) concentration. With a normal glomerular filtration rate (GFR) (180 L/day) and mean day-long plasma glucose (PG) concentration of ~100 mg/day, the kidney filters ~180 grams of glucose/day and all of the filtered glucose is reabsorbed by the sodium glucose transporter 2 (SGLT2) (80–90%) and SGLT1 transporter (10–20%). In T2DM the maximum renal reabsorptive capacity (TmG) and threshold for glucose spillage into the urine are markedly increased (19–21), contributing to the maintenance of hyperglycemia. Importantly, the increase in threshold already is present in well controlled (A1c = 6.5%) T2DM patients and increases progressively with increasing A1c.
The brain is the eighth member of the Ominous Octet (22) (Figure 1). The current epidemic of diabetes is being driven by the obesity epidemic. Obesity is an insulin resistant state (1,2,23) and, when superimposed on the insulin resistance of diabetes, the beta cell cannot secrete sufficient amounts of insulin to offset the insulin resistance (Figure 1). Insulin, like amylin which is cosecreted with insulin, is an anorectic molecule and works in the hypothalamus to suppress the appetite (24). Brain resistance to insulin leads to increased energy intake and weight gain. In animal models of diabetes the brain insulin resistance also has been shown to directly contribute to muscle and hepatic insulin resistance (25).
Given the complexity of the pathogenesis of T2DM, it is not surprising that monotherapy, especially with agents, i.e. sulfonylureas and metformin, that do not reverse the underlying pathophysiologic defects present in T2DM, fail to produce a sustained reduction in A1c and this is best exemplified by the UKPDS (26) and ADOPT (27) studies. In contrast, agents that ameliorate the insulin resistance (i.e., thiazolidinediones) (28) and improve and maintain the improvement in insulin secretion (i.e., GLP-1 RAs, SGLT2i, pioglitazone) (29–31) cause a sustained reduction in A1c (32,33). Thus, the modern day approach to the treatment of T2DM has seen a shift from monotherapy to combination therapy when instituting therapy in newly diagnosed diabetic patients (2) and this approach is best exemplified by the AACE algorithm, which recommends starting antihyperglyemic therapy with 2–3 antidiabetic agents in diabetic patients with an A1c > 7.5% (34).
T2DM is a Metabolic-Cardiovascular Disease
Microvascular complications (retinopathy, nephropathy, neuropathy) in T2DM are related to two factors: (i) the severity of hyperglycemia, as quantified by the A1c and (ii) the duration of hyperglycemia. However, the major (~ 70–80%) cause of death in T2DM patients is related to cardiovascular disease (35,36). Thus, T2DM is two distinct diseases: (i) one of the microvascular (eye, kidney, nerve) and (ii) one of the macrovasculature (myocardial infarction [MI], stroke, peripheral vascular disease [PVD]). Thus, comprehensive treatment of the T2DM patient requires correction of the risk factors for both the microvascular (i.e., hyperglycemia) and macrovascular (blood pressure, lipids, insulin resistance) complications.
The results of recently published cardiovascular outcomes trials (37–39) have opened a new era for the treatment of patients with T2DM. Thus, clinicians have the opportunity to intervene with newer agents that: (i) correct both the pathophysiologic disturbances present in T2DM, leading to a sustained reduction of A1c, and (ii) reduce cardiovascular risk and mortality. Moreover, these newer agents also hold promise for prevention of diabetic nephropathy, independent of their effect to reduce glycemia (40,41).
EMPA-REG OUTCOME STUDY
The EMPA-REG OUTCOME Study (37) randomized 7022 T2DM patients with a prior cardiovascular (CV) event to treatment with the SGLT2 inhibitor empagliflozin or placebo. After a median follow up of 3.1 years the MACE (Major Adverse Cardiovascular Events) endpoint was reduced by 14% (HR = 0.86, P=0.04 for superiority) (Figure 2). CV mortality was decreased by 38% (HR = 0.62, P<0.001), while myocardial and stroke did not change significantly. Hospitalization for heart failure was reduced by 35% (HR = 0.65, P=0.002). Based upon the results of EMPA REG OUTCOME Study the US FDA approved empagliflozin “to reduce the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.” Similar statements have been generated by the Canadian Diabetes Association and the European Union. The mechanism(s) responsible for the decrease in CV mortality were not defined, but were observed within the first 3 months after the initiation of empagliflozin therapy, making improved glycemic control an unlikely explanation. Moreover, hyperglycemia is a weak risk factor for the development of CV disease (CVD) (26), intensive glycemic control in populations at similarly increased risk (42–45) failed to show any benefit on CV outcomes, and the decrement in A1c in EMPA REG OUTCOME was modest (ΔA1c = 0.24% at study end). It is most likely that the CV benefits observed in EMPA REG OUTCOME were related to the hemodynamic benefits of the drug including simultaneous: (i) preload reduction (due to increased salt/water excretion and intravascular volume depletion), (ii) postload reduction (due to decrease in blood pressure), and (iii) decreased aortic stiffness (45). Other potential mechanisms are reviewed in reference #46. It should be emphasized that the mechanism(s) contributing to the progressive, late separation of the Kaplan Meier curves for CV mortality (i.e. weight loss, improved insulin sensitivity, etc) (Figure 2) in empagliflozin-treated versus placebo-treated groups could be quite different from those responsible for the early separation.
Figure 2.
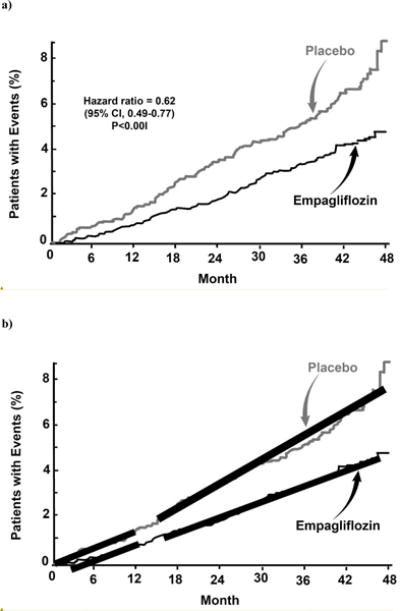
Kaplan Meier curves for the time to cardiovascular mortality in the EMPA REG OUTCOME Study (left panel) (from reference #37). The right panel emphasizes that the slopes of the lines for early versus late cardiovascular mortality are very different (constructed by the author).
At the present it is unclear whether other SGLT2 inhibitors (SGLT2i), i.e. canagliflozin and dapagliflozin, will exert the same cardiovascular benefit or whether the CV benefit of empagliflozin will be observed in T2DM patients earlier in the natural history of the disease and who have not experienced a CV event. With respect to the former, interim analysis of 9,632 T2DM patients with a prior CV event who were treated with canagliflozin in CANVAS (n=6,305) and non-CANVAS (n=3,327) studies demonstrated a hazard ratio for 3-point MACE (HR = 0.91; p value not provided) and hazard ratio for the individuals components of MACE that were very similar to those with empagliflozin (47). A metaanalysis of all phase 2B and phase 3 studies with dapagliflozin demonstrated a hazard ratio for 3-point MACE of 0.77 (P<0.10) (48). A definitive statement about class effect will await the final outcome of CANVAS (canagliflozin; due in 2017) and DECLARE (dapagliflozin; due in 2019). Whether the results of EMPA REG OUTCOME study are generalizable to T2DM patients who are earlier in the natural history of the disease and who have not had a prior CV event and who do not manifest clinical CV disease remains to be determined. However, we believe that this is unlikely to be the case. More likely, within the very high risk (prior CV event) T2DM population of EMPA REG OUTCOME, there was an even higher risk subpopulation (i.e., with severe LV dysfunction, myocardial ischemia, ventricular irritability) who was at imminent risk of experiencing a CV event and the simultaneous, acute reduction in both preload and after load reduced myocardial oxygen consumption, decreased myocardial stretch and ventricular irritability, and enhanced myocardial energetics, thereby rapidly reducing their CV mortality risk. However, proof of this hypothesis cannot be provided by the limited hemodynamic data provided in EMPA REG OUTOME Study.
GLP-1 RECEPTOR AGONISTS
Two studies, LEADER (38) and SUSTAIN-6 (39) have provided evidence that GLP-1 RAs can reduce cardiovascular events in high risk T2DM individuals. In LEADER, 9,340 high risk T2DM patients (81.3% with a prior CV event and 18.7% with multiple CV risk factors) were randomized to liraglutide, 1.2 mg/day (which subsequently was uptitrated to 1.8 mg/day in the majority of subjects), or placebo. After a median follow up of 3.8 years, 3-point MACE in LEADER was reduced by 13% (HR =0.87, P=0.01 for superiority) (Figure 3) and, unlike the EMPA REG OUTCOME study, all three individual components of MACE (MI, stroke, CV mortality) contributed to the cardiovascular benefit including reduced CV mortality. Further, a significant increase in vascular procedures in the placebo-treated group may have reduced the actual CV benefit of liraglutide on mortality. Also, unlike the EMPA REG OUTCOME study separation in the Kaplan Meier curves for 3-point MACE did not become evident until year one, suggesting that different mechanisms were responsible for the CV benefit. Since the reductions in A1c and blood pressure were modest, these factors are unlikely to explain the CV benefit of liraglutide. Although modest weight loss was observed in LEADER, a greater weight loss in diet-treated patients for 9.5 years in LOOK AHEAD had no CV benefit (49).
Figure 3.
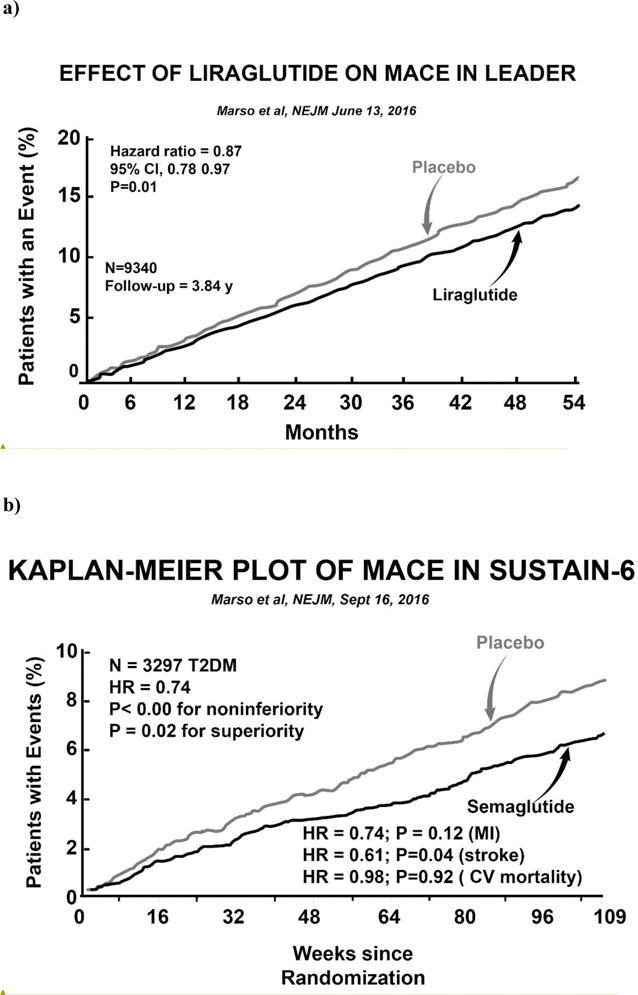
Kaplan Meier curves for the time to 3-point MACE in the LEADER Study (from reference #38) (left panel) and in SUSTAIN-6 (from reference #39) (right panel).
In SUSTAIN-6 (39) 3,297 high risk T2DM patients (83% had established cardiovascular disease, chronic kidney disease, or both) were randomized to semaglutide (0.5 or 1.0 mg once weekly) or placebo for a median of 2.1 years. Semaglutide is liraglutide whose structure has been altered to prolong its half life and protect against degradation by dipeptidylpeptidase 4. Semaglutide reduced 3-point MACE by 26% (HR = 0.74 for noninferiority, P<0.001) and, like LEADER, separation in the Kaplan Meier curves did not become apparent until year one (Figure 3). All three MACE endpoints (nonfatal, MI, nonfatal stroke, CV mortality) contributed to the CV benefit. The decrement in A1c (Δ = 0.7%) was more robust than in LEADER but, as previously discussed, similar A1c reductions in ACCORD, ADVANCE and VADT (42–44) failed to reduce CV events. The modest reductions in blood pressure and body weight also are unlikely to explain the CV benefit of semaglutide.
In summary, we now have two large prospective, double blind, placebo-controlled studies demonstrating that two different GLP-1 receptor agonists can reduce cardiovascular events in T2DM patients at high risk for CV disease. Unlike the EMPA REG OUTCOME Study, where the primary benefit was on CV mortality and was observed within 3 months, in both GLP-1 RA studies (LEADER and SUSTAIN-6) the CV benefit was not observed until one year and MI, stroke, and CV death all contributed to the reduction in 3-point MACE. This later pattern of CV benefit is more consistent with an anti-atherogenic, rather than a hemodynamic, mechanism. The anti-atherogenic, anti-inflammatory action of the GLP-1 RAs have recently been reviewed (50). Because the GLP-1 RAs and SGLT2 inhibitors (empagliflozin) appear to work via different mechanisms, this raises the interesting possibility that these two classes of antidiabetic medications, when used in combination, might produce an additive cardiovascular benefit.
Combination GLP-1 RA/SGLT2i Therapy (Figure 4)
Figure 4.
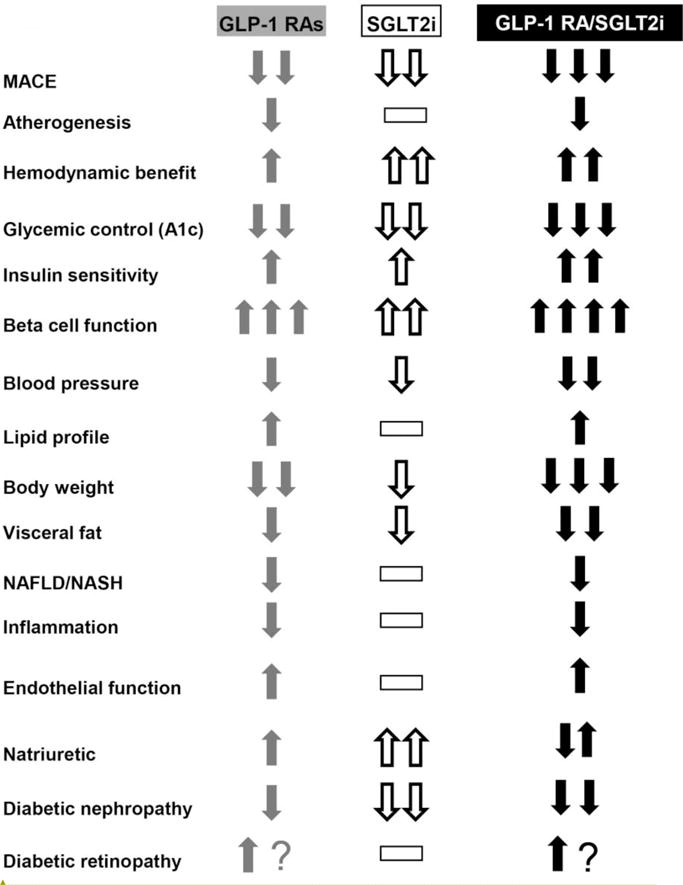
Effect of GLP-1 RAs (column 1) and SGLT2 inhibitors (column 2) on metabolic/cardiovascular/renal parameters. The potential of combined GLP-1 RA/SGLT2i therapy to produce an additive effect is shown in column 3. The number of arrows (one [modest]), two [strong], three [very strong]) represents the relative potency of the effect. Dashed line (-) indicates neutrality.
The GLP-1 RAs and SGLT2i share a number of metabolic, cardiovascular, and renal actions that make them an ideal duet for combination therapy (Figure 4).
Mechanism of Action
The GLP-1 RAs correct 6 of the 8 components of the Ominous Octet (2): (i) they enhance insulin secretion (15,28,29) and (ii) inhibit glucagon secretion (15) leading to (iii) a reduction in hepatic glucose production (51); (iv) they correct the gastrointestinal incretin defect by providing a pharmacologic dose of GLP-1 (2); (v) they exert a central effect on the brain to suppress appetite and promote weight loss (52); (vi) indirectly, the weight loss enhances both muscle and hepatic sensitivity to insulin (53). The GLP-1 RA do not directly improve tissue sensitivity to insulin.
The primary mechanism of action of the SGLT2i is to inhibit glucose reabsorption in the proximal tubule leading to glucosuria which is in the range of 60–80 grams per day (19,20). The resultant decline in plasma glucose concentration ameliorates glucotoxicity (54) leading to a 25–30% improvement in insulin sensitivity (55) and a 2-fold increase in beta cell function (31).
In summary, when used in combination, the GLP-1 RAs and SGLT2i correct 7 of the 8 defects that comprise the Ominous Octet; only the adipocyte insulin resistance is not ameliorated.
Glycemic Control
When used as monotherapy, the GLP-1 RAs (56) and SGLT2i (57) reduce the HbA1c by ~1.2–1.4% and ~0.8–1.0%, respectively, in T2DM individuals with a baseline HbA1c in the 8.0–8.2% range (Figure 4). Greater HbA1c reductions are observed in T2DM patients with higher baseline HbA1c (58). Because the GLP-1 RAs and SGLT2i work by different mechanisms, one might expect them to provide an additive effect to reduce HbA1c when used in combination. Surprisingly, few studies have examined this combination. In CANVAS, 95 T2DM participants were receiving a GLP-1 RA and had canagliflozin added to their regimen (59). The starting HbA1c (8.1%) declined by 0.86% after 18 weeks compared to a 0.17% rise in the placebo-treated group (p<0.001).
In a well designed, prospective, placebo-controlled study 695 T2DM patients inadequately controlled on maximum doses of metformin randomly were assigned to receive dapagliflozin alone (n=233), exenatide alone (n=231), or dapagliflozin plus exenatide (n=231) for 28 weeks (60) (Table 1). The baseline A1c (9.3%) declined by 1.4%, 1.6%, and 2.0% in the dapagliflozin, exenatide, and dapagliflozin/exenatide groups, respectively. In absolute terms, the decrement in A1c in the combination exenatide/dapagliflozin group was less than the additive effects of dapagliflozin alone plus exenatide alone. Two points are noteworthy. First, the decrement in A1c with all antidiabetic therapies is highly dependent on the starting A1c (58). Since monotherapy with both dapagliflozin (ΔA1c = −1.4%) and exenatide (ΔA1c = −1.6%) caused a large reduction in A1c, if the drugs were given in sequential order the second drug never would cause the same A1c reduction as that observed when the baseline A1c was 9.3%. Thus, when two antidiabetic drugs are given in combination, it is difficult to determine whether the decrement in A1c is truly additive. Second, from Table 1, it is clear that the higher is the baseline A1c, the more likely are the two drugs (when administered as monotherapy) to produce an additive decrement in A1c that is greater than when the two drugs are administered as combined therapy. This simply could represent the continuum of the preceding explanation which would be expected to result in an increasingly greater difference in the A1c reduction as the baseline A1c increases. Alternatively, we (55) and others (61) have shown that SGLTi cause a “paradoxical” rise in hepatic glucose production (HGP) that offsets by up to 50% the amount of glucose excreted in the urine. Further, the magnitude of increase in HGP correlates strongly with the magnitude of glucosuria (55). Thus, the higher is the baseline A1c, the greater is the glucosuria and the greater is the stimulation of HGP. It is possible that the ability of the GLP-1 RAs to inhibit the rise in HGP becomes progressively impaired with increasing A1c levels. Whatever the explanation, it is clear that combination therapy with exenatide plus dapagliflozin produced a robust reduction in A1c (Table 1) with 44% of individuals with a starting A1c of 9.3% reaching the ADA goal of < 7.0% and 30% achieving an A1c of < 6.5%.
Table 1.
Effect of exenatide and dapagliflozin used as monotherapy and in combination on the decrement in HbA1c in DURATION-8 (constructed from the results in reference #60).
| DECREMENT IN HbA1c IN DURATION-8 | ||||
|---|---|---|---|---|
| EXENatide
|
DAPAgliflozin
|
EXEN/DAPA (observed decrement)
|
EXEN Monotherapy + DAPA Monotherapy*
|
|
| Baseline A1c = 9.3% (all subjects) |
−1.6 | −1.4 | −2.0 | −3.0 |
| Baseline A1c < 8% | −0.7 | −1.0 | −1.6 | −1.7 |
| Baseline A1c ≥ 8 to < 9% | −1.3 | −1.0 | −1.7 | −2.3 |
| Baseline A1c ≥ 9% | −1.9 | −1.6 | −2.2 | −3.5 |
These data are not real data; they represent the sum of the two monotherapies.
Insulin Secretion & Insulin Sensitivity
GLP-1 RAs exert a potent direct effect on the beta cell to augment beta cell function (2,28,29) (Figure 4). This effect is mediated via the GLP-1 receptor, is observed within 8 hours (29), and persists for at least 3 years (28). The SGLT2i also augment beta cell function (31), but their effect is mediated indirectly via reversal of glucotoxicity (54). Therefore, one might expect an additive or somewhat additive effect to augment beta cell function when these two agents are used in combination.
The SGLT2i cause a modest improvement in insulin sensitivity via two indirect mechanisms: reversal of glucotoxicity and weight loss (19,20,54,55). The GLP-1 RAs also cause a modest increase in insulin sensitivity indirectly by promoting weight loss and ameliorating glucotoxicity (54). Since the effects of GLP-1 RAs and SGLT2i on weight loss are additive (59,60) (see below), when used in combination, one might expect to observe an additive effect of the two medications to enhance peripheral tissue (muscle) sensitivity to insulin. One also might expect the GLP-1 RAs, by enhancing insulin secretion and inhibiting glucagon secretion, to block, at least in part, the stimulation of HGP caused by the SGLT2i. Unfortunately, no studies have examined the effect of combined SGLT2i/GLP-1 RA to correct the muscle/hepatic insulin resistance in T2DM patients.
Body Weight
SGLT2i cause weight loss by increasing the excretion of calories in the urine (1 gram of glucose = 4 calories). However, the weight loss consistently is less than that calculated from the amount of glucosuria because of an increase in food intake (62). Because the GLP-1 RAs inhibit the appetite, one might expect combination therapy with a GLP-1 RA and SGLT2i to be especially effective in promoting weight loss (Figure 4).
Two retrospective studies involving small numbers of subjects have shown that canagliflozin and dapagliflozin, when added to poorly controlled T2DM treated with a GLP-1 RA, reduced body weight by 4.6 kg (63) and 3.1 kg (64) over a mean follow up of 30 and 4.6 months, respectively. These reductions in body weight are similar to those observed in placebo-controlled studies in which an SGLT2i is added to drug naïve or metformin-treated T2DM patients (57). In CANVAS (59) addition of canagliflozin to a GLP-1 RA agonist (n=75) reduced body weight by 3.6 kg. In DURATION-8 (60) the weight loss with exenatide monotherapy (n=227) and dapagliflozin monotherapy (n=230) was 1.54 kg and 2.19 kg, respectively, and this was only slightly lower than the weight loss observed in the exenatide/dapagliflozin combination therapy group (3.41 kg). Thus, even though the decrements in A1c with exenatide and dapagliflozin were less than additive, the effect of the two antidiabetic agents to reduce body weight was similar, indicating that different mechanisms may be responsible for the two actions of the drugs.
Both GLP-1 RAs (65) and SGLT2i (66) reduce visceral fat and the decrease is disproportionate to the decrease in total body fat. GLP-1 RAs also have been shown to decrease hepatic fat in T2DM patients with nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) (67,68). Thus, combination therapy with an SGLT2i plus GLP-1 RA might be particularly effective in reducing hepatic fat content and treating NAFLD/NASH.
Blood Pressure
SGLT2i induce a natriuresis and the reduction in intravascular volume and/or alteration in intrarenal hemodynamics is associated with modest reductions in systolic/diastolic blood pressure (57,69). GLP-1 RA therapy also is associated with a modest decrease in blood pressure, which variably has been attributed to vasodilation and mild natriuresis (70,71). Because the drugs appear to reduce blood pressure via different mechanisms, one might expect an additive effect of GLP-1 RA and SGLT2i when used in combination (Figure 4). In CANVAS (59) addition of canagliflozin to GLP-1 RA produced an impressive 7.1/2.6 mmHg decrease in systolic/diastolic blood pressure. In DURATION-8 (60) the decrement in systolic blood pressure with combined exenatide/dapagliflozin therapy (4.1 mm Hg) was slightly greater than the additive effects with exenatide monotherapy (1.3 mmHg) and dapagliflozin monotherapy (1.8 mmHg), again suggesting that different mechanisms may be responsible for the two actions of the drugs.
Lipid Profile
The effect of SGLT2i on the plasma lipid profile is very modest with small increases in LDL cholesterol (~3.5%) and small decreases in plasma triglycerides (5–10%) being reported (72). GLP-1 RAs reduce intestinal chylomicron production/secretion and are associated with decreased postprandial triglycerides and apo B48, as well as reduced fasting plasma triglyceride levels (73). In DURATION-8 no significant difference in plasma lipid levels, including triglycerides, was observed between dapagliflozin monotherapy, exenatide monotherapy, and combined dapagliflozin/exenatide treated groups.
Inflammation
Multiple preclinical and clinical studies in animals and humans have demonstrated that GLP-1 RAs exert potent anti-inflammatory and anti-atherogenic effects on the CV system (reviewed in ref #50). These effects include reduced plasma levels of IL-6, ICAM-1, markers of oxidative stress, nitrotyrosine, 8-iso prostaglandin F2α, MCP-1, TNFα, and inflammatory markers within monocytes. Increased GLP-1 receptor signaling has been demonstrated to produce robust cardioprotection (decreased infarct size, preservation of ventricular function, improved survival, and reduced levels of creatinine kinase-MB and heparin I) following coronary artery occlusion in animals, after angioplasty in humans, and after ST-segment elevation myocardial infarction (STEMI) in humans (reviewed in ref #50). Reduced thrombosis and platelet aggregation also has been demonstrated in preclinical and clinical studies (50). The effect of native GLP-1 and GLP-1 RAs on endothelial function in humans is controversial with many, but not all, studies demonstrating a vasodilatory effect (reviewed in reference #50). Collectively, the results of these animals and human studies provide an anti-inflammatory/anti-atherogenic basis for the decrease in MI, stroke, and cardiovascular mortality observed in LEADER (38) and SUSTAIN-6 (39).
Preclinical and clinical studies examining the effect of SGLT2i on anti-inflammatory and anti-atherogenic mechanisms are lacking. However, SGLT2 receptors are primarily localized in the kidney and are not found in the vasculature or myocardium, making a direct anti-atherogenic effect unlikely to explain the benefit of empagliflozin on 3-point MACE in the EMPA REG OUTCOME Study (46). Because of (i) the lack of beneficial effect of empagliflozin on nonfatal stroke and nonfatal MI, (ii) absence of reduction in unstable angina, and (iii) the rapid onset of decrease in CV mortality and hospitalization for heart failure, it is highly unlikely that the beneficial effect of empagliflozin in EMPA REG OUTCOME Study resulted from a slowing of the atherogenic process. More likely, the CV protection afforded by empagliflozin resulted from its beneficial hemodynamic effects including reduced blood pressure (after load reduction), decreased intravascular volume (preload reduction), and diminished aortic stiffness (after load reduction) (46). Other potential mechanisms are reviewed by DeFronzo et al in reference #46. Because the cardiovascular benefit of empagliflozin most likely is related to the drug’s hemodynamic benefit, while those of the GLP-1 RAs are related to their anti-atherogenic/anti-inflammatory actions, the two classes of antidiabetic medications may produce an additive cardiovascular benefit (Figure 4).
Natriuresis
Both the SGLT2i (19,20,72) and GLP-1 RAs (70,71) augment renal sodium excretion. In the proximal tubule the SGLT2 transporter facilitates sodium and glucose reabsorption in a 1:1 ratio (19,20). Thus, SGLT2 inhibition results in increased excretion of both glucose and sodium, leading to a reduction in intravascular volume and arterial smooth muscle sodium content, both of which would be expected to reduce the blood pressure. The mechanism(s) via which GLP-1 RAs increase renal sodium excretion are poorly studied but include vasodilation and possibly a direct effect on the renal tubule. Since the mechanisms responsible for the increase in renal sodium excretion with SGLT2i and GLP-1 RAs are different, one would expect combined therapy to produce a greater reduction in systolic/diastolic blood pressure and this, indeed, was observed in DURATION-8 (60) (see prior discussion about blood pressure) (Figure 4).
Protection Against Diabetic Nephropathy
In the EMPA REG OUTCOME Study empagliflozin reduced the composite endpoint for renal disease (doubling of serum creatinine with eGFR < 45 ml/min•1.73 m2; progression to macroalbuminuria; renal replacement therapy) by 39% (p<0.001) (41), while in LEADER liraglutide reduced the composite endpoint (doubling of serum creatinine, development of macroalbuminuria, dialysis/transplantation by 31% (HR = 0.69, p<0.001) (40) (Figure 4). In poorly controlled T2DM patients, the filtered load of glucose is increased and glucose, along with sodium, reabsorption in the proximal tubule is enhanced. This leads to decreased sodium delivery to the juxtaglomerular apparatus (JGA), making it appear that the kidney is being under-perfused. This generates a signal to increase afferent renal arteriolar plasma flow, leading to an elevation in intraglomerular pressure and GFR, i.e. hyperfiltration (74). On a long term basis the increase in intraglomerular pressure results in diabetic glomerulosclerosis. SGLT2i, by blocking glucose and with it sodium reabsorption in the proximal tubule, increases sodium delivery to the JGA and turns off the signal responsible for increased afferent arteriolar plasma flow, thereby decreasing the elevated intraglomerular.
The mechanism(s) responsible for the renal protective effect of liraglutide are less well defined but could include anti-inflammatory effects (decreased TNFα, IL-6, IL-1, TLR-4, JNK), decreased oxidative stress, altered intrarenal hemodynamics secondary to inhibition of sodium reabsorption, renal vasodilation with reduced anoxia, increased nitric oxide, activation of as-of-yet unknown neurohumoral mechanisms, weight loss, decreased protein intake, and reduced blood pressure. In hypertensive-prone rats, a 14-day infusion of GLP-1 prevents hypertension, improves endothelial function, and reduces renal damage (75). Similarly, in obese humans, a 3-hour infusion of GLP-1 enhances sodium excretion and decreased hyperfiltration (174). Because the mechanisms responsible for renal protective effects of SGLT2i and GLP-1 RAs are likely to differ, combination therapy with agents from these two classes may exert an additive effect to prevent diabetic glomerulosclerosis (Figure 4).
Hypoglycemia
Hypoglycemia is a major deterrent to the achievement of good glycemic control. Although the GLP-1 RAs augment insulin and inhibit glucagon secretion, these actions are glucose-dependent (77). Thus, as the plasma glucose concentration returns to normal, the effects on insulin and glucagon secretion dissipate, thus preventing hypoglycemia. The SGLT2i also improve beta cell function, but they do so by reversal of glucotoxicity (31). Thus, they can return insulin secretion toward, but not in excess of, normal. The SGLT2i also stimulate HGP (55). Although the increase in HGP offsets, in part, the glucosuric effect to reduce the plasma glucose concentration, it also serves to prevent hypoglycemia. Thus, combined SGLT2i/GLP-1 RA therapy is unlikely to cause hypoglycemia unless used in combination with an insulin secretagogue or insulin (Figure 4). The incidence of hypoglycemia in diabetic patients treated with liraglutide (LEADER) (38), semaglutide (SUSTAIN-6) (39), and empagliflozin (EMPA-REG OUTCOME) (37) was similar to or less than that in the placebo-treated groups.
Diabetic Retinopathy
In SUSTAIN-6 (39) semaglutide was associated with a worsening of diabetic retinopathy including vitreous hemorrhage, blindness, retinal complications requiring an intravitreal agent or photocoagulation) (HR = 1.76, p=0.02), while in LEADER the hazard for diabetic retinopathy was increased (HR = 1.15, P = NS), although not significantly. The reason(s) for the worsening diabetic retinopathy in SUSTAIN-6 are unclear but could be related to rapid reduction in A1c. No adverse effects on diabetic retinopathy were reported in the EMPA REG OUTCOME Study. Therefore, there is no reason to anticipate a negative, or beneficial, interaction and diabetic eye disease when these two antidiabetic agents are used in combination (Figure 4).
Adverse Events
The most common side effects observed with the GLP-1 RAs are related to the gastrointestinal tract: nausea (~15–20%), vomiting (~5%), constipation (~5%), and diarrhea (~5%). These side effects are generally mild to moderate in intensity and resolve spontaneously within 4–6 weeks of initiating therapy. Pancreatitis rarely may occur with the GLP-1 RAs. Vaginal mycotic infections in females (~6–8%) and penile mycotic infections, primarily in uncircumcised males, (~2–3%) are the most common side effects encountered with SGLT2 inhibitors. Symptoms related to mild intravascular volume depletion and increased urination have been reported in ~5% of individuals treated with an SGLT2 inhibitor. Elderly diabetic patients and patients treated with loop diuretics should be evaluated for orthostatic hypotension before starting therapy with an SGLT2 inhibitor. An increased incidence of urinary tract infection has been reported with SGLT2i in some, but not all, studies. Hypoglycemia is uncommon with both SGLT2i and GLP1 RAs unless they are used in combination with an insulin secretagogue or insulin. Because the side effect profile with the SGLT2 inhibitors differs from that of the GLP-1 RAs, one would not expect to observe any additive negative interaction when the two classes of antidiabetic medications are used in combination. Lastly, expense always is a consideration with the newer antidiabetic medications.
SUMMARY
With the recent completion of large, prospective, double-blind, placebo-controlled cardiovascular outcome studies, we have moved into a new era of diabetes treatment in which antidiabetic medications that both reduce glycemia, thereby preventing microvascular complications, and prevent macrovascular complications (MI, stroke, CV mortality) should be considered as first line therapy for the treatment of T2DM patients at high cardiovascular risk. It is the authors’ personal belief that such antidiabetic medications should be used earlier in the natural history of T2DM in less high risk individuals although randomized controlled clinical trials to substantiate this approach are not yet available and never may be available because of the length of time and cost to complete such studies. Antidiabetic medications that correct multiple components of the Ominous Octet, cause a durable reduction in A1c, and can be used in combination to produce additive or synergistic effects to reduce macrovascular and microvascular complications, improve CV risk factors, and prevent diabetic nephropathy should be given preference. Based upon their different mechanisms of action, combination therapy with an SGLT2i plus GLP-1 RA would be expected to fulfill these criteria. Therefore, well designed, large, prospective, placebo-controlled studies should be initiated to evaluate the cardiovascular benefits of combined SGLT1i/GLP-1 RA therapy in T2DM patients.
Acknowledgments
This work was supported by NIH grant DK24092-33. The ideas described herein are those of the author. The manuscript was written by RAD based upon his review of the literature.
References
- 1.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA. From the triumvirate to the Ominous Octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989;38:387–395. doi: 10.1016/0026-0495(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Ferrannini E. Regulation of intermediary metabolism. In: James JL, DeGroot LJ, editors. Endocrinology. 6th. Sanders/Elsevier; Philadelphia, PA: 2014. pp. 673–698. [Google Scholar]
- 7.Abdul-Ghani M, Mujahid O, Mujahid A, DeFronzo RA, Zirie M, Jayyousi A. Efficacy of exenatide in combination with pioglitazone versus basal/bolus insulin in T2DM patients with very high glycated hemoglobin: results from the QATAR study. JCEM. (in press) [Google Scholar]
- 8.Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–2474. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- 9.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 12.Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 13.Hojberg PV, Vilsboll T, Rabol R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 14.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54:10–18. doi: 10.1007/s00125-010-1896-4. [DOI] [PubMed] [Google Scholar]
- 15.Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95:513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- 16.Unger RH, Aguilar-Parada E, Muller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49:837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36:274–283. doi: 10.2337/diab.36.3.274. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda M, DeFronzo RA, Glass L, Consoli A, Giordano M, Bressler P, Del Prato S. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism. 2002;51:1111–1119. doi: 10.1053/meta.2002.34700. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. American journal of physiology Renal physiology. 2015;309:F889–900. doi: 10.1152/ajprenal.00267.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Hompesch M, Kasichayanula S, Liu X, Hong Y, Pfister M, Morrow LA, Leslie BR, Boulton DW, Ching A, LaCreta FP, Griffen SC. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013;36:3169–3176. doi: 10.2337/dc13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Haring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016;96:1169–1209. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Soman V, Sherwin RS, Hendler R, Felig P. Insulin binding to monocytes and insulin action in human obesity, starvation, and refeeding. J Clin Invest. 1978;62:204–213. doi: 10.1172/JCI109108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 25.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 26.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 27.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 28.Bunck MC, Corner A, Eliasson B, Heine RJ, Shaginian RM, Taskinen MR, Smith U, Yki-Jarvinen H, Diamant M. Effects of exenatide on measures of beta-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2041–204731. doi: 10.2337/dc11-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang AM, Jakobsen G, Sturis J, Smith MJ, Bloem CJ, An B, Galecki A, Halter JB. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52:1786–1791. doi: 10.2337/diabetes.52.7.1786. [DOI] [PubMed] [Google Scholar]
- 30.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007;292:E871–883. doi: 10.1152/ajpendo.00551.2006. [DOI] [PubMed] [Google Scholar]
- 31.Merovci A, Mari A, Solis C, Xiong J, Daniele G, Chavez-Velazquez A, Tripathy D, Urban McCarthy S, Abdul-Ghani M, DeFronzo RA. Dapagliflozin lowers plasma glucose concentration and improves beta-cell function. J Clin Endocrinol Metab. 2015;100:1927–1932. doi: 10.1210/jc.2014-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdul-Ghani MA, Puckett C, Triplitt C, Maggs D, Adams J, Cersosimo E, DeFronzo RA. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab. 2015;17:268–275. doi: 10.1111/dom.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdul-Ghani M, Mujahid O, Mujahid A, DeFronzo RA, Zirie M, Jayyousi A. efficacy of exenatide plus pioglitazone versus basal/bolus insulin in T2DM patients with very high HbA1c. JCEM. doi: 10.1210/jc.2016-3423. (in press) [DOI] [PubMed] [Google Scholar]
- 34.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2017 Executive Summary. Endocr Pract. 2017 Jan 17; doi: 10.4158/EP161682.CS. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Emerging Risk Factors C. Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, O’Keeffe LM, Gao P, Wood AM, Burgess S, Freitag DF, Pennells L, Peters SA, Hart CL, Haheim LL, Gillum RF, Nordestgaard BG, Psaty BM, Yeap BB, Knuiman MW, Nietert PJ, Kauhanen J, Salonen JT, Kuller LH, Simons LA, van der Schouw YT, Barrett-Connor E, Selmer R, Crespo CJ, Rodriguez B, Verschuren WM, Salomaa V, Svardsudd K, van der Harst P, Bjorkelund C, Wilhelmsen L, Wallace RB, Brenner H, Amouyel P, Barr EL, Iso H, Onat A, Trevisan M, D’Agostino RB, Sr, Cooper C, Kavousi M, Welin L, Roussel R, Hu FB, Sato S, Davidson KW, Howard BV, Leening MJ, Rosengren A, Dorr M, Deeg DJ, Kiechl S, Stehouwer CD, Nissinen A, Giampaoli S, Donfrancesco C, Kromhout D, Price JF, Peters A, Meade TW, Casiglia E, Lawlor DA, Gallacher J, Nagel D, Franco OH, Assmann G, Dagenais GR, Jukema JW, Sundstrom J, Woodward M, Brunner EJ, Khaw KT, Wareham NJ, Whitsel EA, Njolstad I, Hedblad B, Wassertheil-Smoller S, Engstrom G, Rosamond WD, Selvin E, Sattar N, Thompson SG, Danesh J. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 37.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 38.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, LEADER Steering Committee. LEADER Trial Investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, VisBoll T, SUSTAIN-6 investigator Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 40.Mann JF, Frandsen KB, Daniels G, Kristensen P, Nauck M, Nissen S, Pocock S, Poulter N, Rasmussen S, Steinberg W, Stockner M, Zinman B, Baeres F, Bergenstal R, Marso S, Buse J. Liraglutide and renal outcomes in type 2 diabetes: Results of the LEADER Trial. J Am Soc Nephrol (Abstract supplement) 2016;27:1B. [Google Scholar]
- 41.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B, Investigators E-RO Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 42.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 44.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, Investigators V Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 45.Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, Woerle HJ, von Eynatten M, Broedl UC. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: Lessons learned from the EMPA-REG OUTCOME Study. Diabetes Care. 2016;39:717–725. doi: 10.2337/dc16-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000SumR.pdf
- 48.Sonesson C, Johansson PA, Johnsson E, Gause-Nilsson I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta-analysis. Cardiovasc Diabetol. 2016 Feb 19;15:37. doi: 10.1186/s12933-016-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LOOK AHEAD RESEARCH GROUP. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Cervera A, Wajcberg E, Sriwijitkamol A, Fernandez M, Zuo P, Triplitt C, Musi N, DeFronzo RA, Cersosimo E. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab. 2008;294:E846–852. doi: 10.1152/ajpendo.00030.2008. [DOI] [PubMed] [Google Scholar]
- 52.van Bloemendaal L, RG IJ, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, Veltman DJ, Diamant M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–4196. doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 53.Sarkar G, Alattar M, Brown RJ, Quon MJ, Harlan DM, Rother KI. Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care. 2014;37:666–670. doi: 10.2337/dc13-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 55.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists–available efficacy and safety data and perspectives for the future. Diabetes Obes Metab. 2011;13:394–407. doi: 10.1111/j.1463-1326.2011.01357.x. [DOI] [PubMed] [Google Scholar]
- 57.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 58.DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trials. Diabet Med. 2010;27:309–317. doi: 10.1111/j.1464-5491.2010.02941.x. [DOI] [PubMed] [Google Scholar]
- 59.Fulcher G, Matthews DR, Perkovic V, de Zeeuw D, Mahaffey KW, Mathieu C, Woo V, Wysham C, Capuano G, Desai M, Shaw W, Vercruysse F, Meininger G, Neal B, group Ctc Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:82–91. doi: 10.1111/dom.12589. [DOI] [PubMed] [Google Scholar]
- 60.Frias JP, Guja C, Hardy E, Ahmed A, Dong F, Ohman P, Jabbour SA. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–1016. doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 61.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730–1735. doi: 10.2337/dc15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saroka RM, Kane MP, Busch RS, Watsky J, Hamilton RA. SGLT-2 inhibitor therapy added to GLP-1 agonist therapy in the management of T2DM. Endocr Pract. 2015;21:1315–1322. doi: 10.4158/EP15877.OR. [DOI] [PubMed] [Google Scholar]
- 64.Deol H, Lekkakou L, Viswanath AK, Pappachan JM. Combination therapy with GLP-1 analogues and SGLT-2 inhibitors in the management of diabesity: the real world experience. Endocrine. 2017;55:173–178. doi: 10.1007/s12020-016-1125-0. [DOI] [PubMed] [Google Scholar]
- 65.Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, Düring M, Zdravkovic M, Strauss BJ, Garber AJ, LEAD-2 and LEAD-3 Study Groups Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diab Obe Metab. 2009;11:1163–1172. doi: 10.1111/j.1463-1326.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 66.Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 67.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, Garelli P, Casini A, Manco M, Mingrone G, Risaliti A, Frega GN, Benedetti A, Gastaldelli A. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–1297. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 68.Carbone LJ, Angus PW, Yeomans ND. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:23–31. doi: 10.1111/jgh.13026. [DOI] [PubMed] [Google Scholar]
- 69.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koska J, Sands M, Burciu C, D’Souza KM, Raravikar K, Liu I, Truran S, Franco DA, Schwartz EA, Schwenke DC, D’Alessio D, Migrino RQ, Reaven PD. Exenatide protects against glucose- and lipid-induced endothelial dysfunction: Evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes. 2015;64:624–2635. doi: 10.2337/db14-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tonneijck L, Smits MM, Muskiet MH, Hoekstra T, Kramer MH, Danser AH, Diamant M, Joles JA, van Raalte DH. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2016;59:1412–1421. doi: 10.1007/s00125-016-3938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomized, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–50. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 73.Hermansen K, Bækdal TA, Düring M, Pietraszek A, Mortensen LS, Jørgensen H, Flint A. Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat-rich meal in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, cross-over trial. Diabetes Obes Metab. 2013;15:1040–8. doi: 10.1111/dom.12133. [DOI] [PubMed] [Google Scholar]
- 74.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 75.Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–35. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbueh M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89:3055–61. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 77.Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. The lancet Diabetes & endocrinology. 2016;4:525–536. doi: 10.1016/S2213-8587(15)00482-9. [DOI] [PubMed] [Google Scholar]


