Abstract
Background
High Density Lipoprotein (HDL) can be readily oxidized in inflammatory conditions and exhibit pro-inflammatory and dysfunctional (Dys-HDL) characteristics. We hypothesize that Dys-HDL may predict adverse outcomes and correlate with inflammatory cytokines in sepsis.
Methods
Emergency department (ED) patients with sepsis were enrolled. Blood was drawn at enrollment and after 48 hours. Dys-HDL, expressed as HDL inflammatory index (HII), and cytokines were measured. Multivariable logistic regression was used to determine the predictive ability of Dys-HDL for adverse outcomes (death, discharge to hospice or nursing home).
Results
Thirty five patients were included in the study. HII was not significantly different at baseline or 48 hours between patients with adverse outcomes versus those without. However, there was a significant difference in change in HII over the first 48 hours between those with adverse outcomes (+0.21, 95% CI −0.13 – 0.31) versus those without (−0.11, 95% CI −1 – 0.11) (p = .025). Logistic regression revealed increasing HII to be an independent predictor of adverse outcomes (OR 5.2, 95% CI 1.1–25.1 p = 0.040). Of the 24 patents with cytokine measurements at both time points, significant inverse correlations between change in HII and change in GRO (rs = −.52, p = .0088) and MCP-1 (rs = −.61, p = .0014) concentrations over 48 hours were observed.
Conclusion
Increasing Dys-HDL concentrations in the first 48 hours of sepsis are associated with an ongoing inflammatory response and adverse clinical outcomes. Early changes in HII may be a potential biomarker in ED patients admitted with sepsis.
Keywords: Sepsis, septic shock, cholesterol, high density lipoprotein
Introduction
Sepsis is a costly disease with a high mortality, resulting from a dysregulated host response to an infectious insult.1,2 This dysregulation involves early activation of both proinflammatory and immunosuppressive processes,3,4 as well as changes in several other biological systems which can lead to organ dysfunction. Furthermore, host and pathogen factors influence susceptibility to sepsis and disease severity which adds variability to the clinical phenotype of sepsis, making it challenging to diagnose.
Importantly, sepsis is a disease for which outcomes are largely predicted by early recognition and treatment, yet one of the greatest barriers to early sepsis management is the paucity of reliable, clinically-useful biomarkers. Cholesterol, and in particular high density lipoprotein cholesterol (HDL-C), is a potent antioxidant which plays a role in defense against infection, and cholesterol levels have been shown to rapidly decline in early sepsis, which implies potential utility as a sepsis biomarker. Chien et al. demonstrated that early HDL-C and apolipoprotein A-I (the main protein constituent of HDL) levels were significantly lower in patients who died of severe sepsis compared with survivors, and that there was an inverse correlation between HDL-C levels and interleukin-6 (IL-6) and TNFα.5 In severe community-acquired pneumonia, lower HDL-C and low density lipoprotein cholesterol (LDL-C) levels were present in intensive care unit (ICU) patients who died compared with survivors.6 Lagrost et al. demonstrated that lower baseline total cholesterol levels were present in patients who developed sepsis after cardiac surgery.7 Most recently, our group demonstrated that low LDL-C levels were predictive of long-term community acquired sepsis risk.8
The HDL particle potentially plays a protective role in sepsis via several mechanisms including direct binding and disposal of bacterial toxins,9,10 suppression of monocyte activation, prevention of macrophage and dendritic cell migration, prevention of the release of inflammatory cytokines,11,12 and inhibition of vascular and intercellular adhesion molecule expression.13 Interestingly, alteration of HDL surface proteins during sepsis may occur by myeloperoxidase (MPO - a pro-oxidant enzyme released by neutrophils),9,14 which can convert HDL to a proinflammatory, dysfunctional state (Dys-HDL) inhibiting its protective properties via direct action on Apo A-I.9,14–17 DiDonato et al. showed that MPO catalyzes the selective nitration and chlorination of tyrosine at the 166 position of Apo A-I under inflammation yielding Dys-HDL.18 Interestingly, displacement of Apo A-I by serum amyloid A (an acute phase protein) during sepsis was seen in up to 45% of patients at one day after admission for sepsis but was reduced to 5% during recovery.19 This phenomenon remains unexplained, but implies that the inflammatory changes of sepsis may alter HDL structure and function.
Therefore, the objectives of this pilot study were three-fold: 1) to determine for the first time if Dys-HDL is present in sepsis by measuring Dys-HDL in patients admitted from the emergency department (ED) with sepsis, 2) to determine if change in Dys-HDL is predictive of adverse outcomes, a composite defined as in-hospital death, discharge to hospice, or nursing home, and 3) to measure the correlation between changes in Dys-HDL and proinflammatory cytokine concentrations in the first 48 hours of sepsis. The choice of this composite adverse outcome is reflective of the fact that patients who initially survive their sepsis admission may experience poor outcomes other than in-hospital death, which may be characterized by physical dependence and reduced quality of life, or death soon after discharge from the hospital.20 We hypothesize that Dys-HDL will be present early after sepsis and that early changes in Dys-HDL will be predictive of adverse outcomes and potentially correlate with changes in cytokine levels. If these associations can be proven, we believe that a foundation for further investigations of Dys-HDL as a sepsis biomarker may be established.
Methods
Study Setting, Design and Patient Selection
This was a prospective, observational cohort study of adult patients (age ≥ 18 years) who presented to the University of Florida (UF) Health Jacksonville ED with sepsis or septic shock and were enrolled within 24 hours of sepsis recognition. The UF Health Jacksonville ED is a high acuity, academic, urban ED which treats approximately 95,000 patients per year. The research protocol was approved by the UF College of Medicine, Jacksonville Institutional Review Board (IRB).
Study inclusion criteria were: 1) infection with > 1 SIRS criteria, 2) lactate > 2 mmol/L, and 3) sequential organ failure assessment (SOFA) score ≥ 4, and 4) sepsis as the primary diagnosis for admission. Septic shock was defined as hypotension not responsive to at least 30 ml/kg intravenous fluids. Exclusion criteria were: 1) pregnancy, 2) lack of valid consent, 3) familial or genetic disorders of lipid metabolism, 4) active seizure, 5) cardiopulmonary resuscitation prior to enrollment. Patients meeting study criteria were approached for enrollment and consent was obtained as per IRB requirements.
Measurements and Interventions
Prospective data collection occurred at enrollment and 48 hours from enrollment. Enrollment data included age, sex, race, place of residence, comorbidities (diabetes mellitus, chronic obstructive pulmonary disease (COPD), end-stage renal disease (ESRD), Human Immunodeficiency Virus (HIV) status, active cancer, organ transplant), lactate levels, SOFA score, triage and enrollment vital signs, timing of antibiotics, volume of intravenous fluids administered in the first six and 24 hours, vasopressor use and duration, mechanical ventilation use, central venous pressures (CVP), urine output in the first six hours, suspected source of infection, familial disorders of lipid metabolism, statin use, admission disposition, hospital length of stay (LOS), and ICU LOS. At 48 hours, repeat clinical assessments were performed including repeat vital signs, hemodynamic and ventilator requirements, and SOFA score. Glasgow Coma Scale (GCS) scores were assessed prospectively at both time points. Chart reviews after enrollment were performed to confirm source of infection and sepsis diagnosis, culture results, ICU and hospital length of stay (LOS), and outcome and discharge disposition (death, nursing home, hospice, rehabilitation facility, or home).
Blood sampling occurred at baseline and 48 hours and included total cholesterol levels, HDL-C, LDL-C, triglycerides, laboratory tests for SOFA score calculation, serum for Dys-HDL testing, and plasma for inflammatory cytokines. At both time points serum and plasma samples were obtained, processed, and frozen at −80°C until further testing. The study protocol required blood to be drawn within four hours of planned collection times.
Dys-HDL was quantitated using a cell-free assay (CFA) and expressed as HDL Inflammatory Index (HII). The cell free assay for Dys-HDL requires HDL isolation from blood samples using dextran sulphate precipitation. LDL, necessary for the cell free assay, was prepared from a normal donor and aliquotted and cryo-preserved in sucrose. Dichlorofluorescein-diacetate was dissolved in fresh methanol at 2.0 mg/ml, incubated at room temperature with the control LDL and the sample HDL. Oxidation of LDL resulted in the release of dichlorofluorescein (DCFH) which was quantitated with an excitation wavelength of 485 nm, emission wavelength of 530 nm, and cutoff of 515 nm. The ability of sample HDL to protect LDL from oxidation was quantitated by the decline in fluorescence. The HII, used to quantitate Dys-HDL, was calculated by normalizing the CFA values obtained for LDL alone as 1.0. If addition of a test HDL along with LDL resulted in an HII of 1.0 or greater, the test HDL was classified as pro-inflammatory (Dys-HDL). Conversely, if addition of a test HDL to LDL resulted in a HII of less than 1.0, the test HDL was classified as anti-inflammatory. Values for intra-assay and inter-assay variability are 5.3±1.7% and 7.1±3.2%, respectively.21
The following plasma cytokine concentrations were measured using the Luminex®xMAP® multiplex assay: growth related oncogene (GRO), interleukin-6 (IL-6), interferon gamma-induced protein (IP-10), monocyte chemotactic protein-1 (MCP-1), IL-10, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ).
Outcomes and Data Analysis
Adverse outcome was the primary outcome of the study, as defined by in-hospital death, or discharge to a nursing home or hospice. Univariate analyses of multiple covariates were performed using Student’s T test for numerical, normally distributed data, Wilcoxon rank-sum for numerical, non-normally distributed data, and Fisher’s exact test for categorical data. These included patient demographics, physiological parameters, interventions, features of sepsis, and comorbidities and were used to derive a multivariable logistic regression model predictive of adverse outcome. Candidate variables were chosen from univariate analyses, and backwards elimination using p-values of 0.15 or less were used to inform the choice of predictor variables for the final regression model. To avoid over-fitting, given the relatively low number of primary outcomes (adverse outcomes or ICU stay ≥ 3 days) in the sample, we limited the number of independent variables to the two most significant.22 Spearman’s correlations were used to determine the correlation between change in HII (Dys-HDL) to change in each cytokine over 48 hours. Because this was based on preliminary pilot data from a larger study, a formal sample size justification and power calculation were not performed.
Results
The demographics, clinical features and outcomes of the 35 study patients are depicted in Table 1. Mean age was high at 64 years (SD 14); 57% were male and 60% were black. The majority (77%) were initially admitted to the ICU with median ICU stay of 4 days (IQR 1–8). Fifty one percent met the pre-specified designation of adverse outcome, and 49% did not. Comparisons between these groups are presented in Table 2. There was a trend toward increased age in the adverse outcome group, but there were no differences in other demographics, comorbidities, sources of sepsis, early physiologic parameters or initial SOFA score. Initial management was similar with the exception that significantly more adverse outcome patients required mechanical ventilation (p = .026).
Table 1.
Demographics, source of sepsis, comorbidities, early clinical features, and outcomes and disposition for study patients. SD = standard deviation. IQR = interquartile range.
| Variable | Value |
|---|---|
| Demographics | |
|
| |
| Age, mean (SD) | 64 years (14) |
| Male, % (N) | 57% (20/35) |
| White, % (N) | 40% (14/35) |
| Black, % (N) | 60% (21/35) |
|
| |
| Source of sepsis, n (%) | |
|
| |
| Pulmonary | 12 (34) |
| Urinary | 11 (31) |
| Intra-abdominal | 5 (14) |
| Indwelling catheter | 14 (40) |
|
| |
| Comorbidities | |
|
| |
| Any comorbidity | 69% (24/11) |
| Diabetes mellitus, % (N) | 34% (12/35) |
| Chronic obstructive pulmonary disease, % (N) | 9% (5/35) |
| End stage renal disease, % (N) | 14% (3/35) |
| Active cancer, % (N) | 11% (3/35) |
| Human Immunodeficiency Virus positive, % (N) | 9% (4/35) |
| Admitted from nursing home, % (N) | 17% (6/35) |
|
| |
| Early Clinical Features | |
|
| |
| Initial systolic blood pressure, mm Hg, mean (SD) | 113 (30) |
| Initial mean arterial pressure, mm Hg, mean (SD) | 82 (20) |
| Initial heart rate, beats/min, mean (SD) | 99 (1.8) |
| Initial temperature, °F, mean (SD) | 99.3 (1.9) |
| Initial oxygen saturation, mm Hg, mean (SD) | 98 (2.5) |
| Initial Lactate, mmol/L, mean (SD) | 3.6 (2) |
| SOFA score, median (IQR) | 6 (5–9) |
| Mechanical ventilation use, % (N) | 20% (7/35) |
| Vasopressor use, % (N) | 40% (14/35) |
| Duration of vasopressor use, hours, mean (SD) | 68 (67) |
|
| |
| Outcomes and Disposition | |
|
| |
| Mechanical ventilation, n (%) | 31% (11/35) |
| Initial ICU admission, % (N) | 77% (27/35) |
| ICU LOS, days, median (IQR) | 4 (1–8) |
| Hospital LOS, days, median (IQR) | 8 (5–10) |
| In-hospital mortality, % (N) | 11% (4/35) |
| Adverse outcome (death, discharge to nursing home or hospice), % (N) | 51% (18/35) |
Table 2.
Univariate comparisons of demographics, early physiologic parameters and sepsis severity, interventions, source of sepsis, and comorbidities by the composite adverse outcome. SD = standard deviation. IQR = interquartile range. Percentages are out of the entire study cohort of 35 patients.
| Variable | Death, Discharge to Hospice or Nursing Home (N= 18) | Discharge to Home or Rehab (N=17) | P-value* |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 68 (12) | 60 (SD 15) | 0.09 |
| Sex, male, n (%) | 11 | 9 | 0.63 |
| Black race, n (%) | 9 (26) | 12 (34) | 0.31 |
| Statin use | 7 (20) | 8 (23) | 0.74 |
| Early Physiologic parameters and Sepsis Severity | |||
| Triage SBP < 90, mm Hg, n (%) | 4 (11) | 4 (11) | >0.99 |
| Initial lactate, mmol/L, mean (SD) | 4.3 (2.2) | 4.0 (2.4) | 0.73 |
| Repeat lactate (within 6 hrs), mmol/L, mean (SD) | 4.1 (2.3) | 3.2 (1.8) | 0.29 |
| Change in Lactate, mean (SD), n = 27 | −.29 (1.4) | −1.6 (3.2) | 0.16 |
| SOFA score, median (IQR) | 7 (5–9) | 5 (4–7) | 0.15 |
| Blood culture positive, n (%) | 8 (23) | 9 (26) | >0.99 |
| Acute respiratory failure, n (%) | 6 (17) | 1 (3) | 0.052 |
| Acute kidney injury, n (%) | 14 (40) | 6 (17) | 0.056 |
| Septic shock, n (%) | 8 (23) | 6 (17) | 0.73 |
| Interventions | |||
| IVF at 6 hours, mL, mean (SD) | 3076 (1803) | 3625 (2256) | 0.44 |
| IVF at 24 hours, mL, mean (SD) | 5049 (2863) | 5616 (3221) | 0.59 |
| Vasopressor use, n (%) | 8 (23) | 6 (17) | 0.73 |
| Mechanical ventilation, n (%) | 9 (26) | 2 (6) | 0.026 |
| Time to antibiotics, minutes (SD) | 124 (74) | 178 (172) | 0.23 |
| Vasopressors at enrollment, n (%) | 5 (14%) | 5 (14%) | 0.92 |
| Source of sepsis, n (%) | |||
| Pulmonary | 7 (20) | 5 (14) | 0.73 |
| Urinary | 7 (20) | 4 (11) | 0.47 |
| Intra-abdominal | 3 (9) | 2 (6) | >0.99 |
| Indwelling venous catheter | 5 (14) | 9 (26) | 0.18 |
| Comorbidities, n (%) | |||
| Any comorbidity | 13 (37) | 11 (31) | 0.73 |
| Diabetes Mellitus | 6 (17) | 6 (17) | >0.99 |
| End Stage Renal Disease | 1 (3) | 4 (11) | 0.18 |
| COPD | 0 | 3 (9) | 0.10 |
| Human Immunodeficiency Virus | 0 | 3 (9) | 0.10 |
| Active Cancer | 4 (11) | 0 | 0.10 |
Student’s T test was used for numerical, normally distributed data, Wilcoxon rank-sum for numeric non-normally distributed data, and Fisher’s exact test for categorical data.
Table 3 depicts Dys-HDL expressed as HDL inflammatory index (HII) as well as HDL and LDL levels at enrollment and at 48 hours by outcomes. There was no significant difference in HII values at baseline (p = 0.70) versus 48 hours (p = 0.09). There was, however, a significant difference in the change in HII over the first 48 hours between patients with adverse outcomes (Z=.38, p = .025) compared with the rest of the cohort. Patients experiencing adverse outcomes had an overall increase in median HII levels over the first 48 hours of admission (+0.21, IQR −0.13 to +0.31) in comparison with those not experiencing the adverse outcome (−0.11, IQR −1.0 to +0.11). Finally, statin use did not influence change in HII over 48 hours (p = 0.74).
Table 3.
Dysfunctional high density lipoprotein (Dys-HDL) expressed as HDL inflammatory index (HII), HDL, and LDL levels at enrollment and at 48 hours by composite adverse outcome. Changes reflect 48 hour values – enrollment values. SD = standard deviation. IQR = interquartile range.
| Variable | Total (N=35) | Death, Discharge to Hospice or Nursing Home (N= 18) | Discharge to Home or Rehab (N=17) | P-value* |
|---|---|---|---|---|
| Dysfunctional HDL (HII), median (IQR) | ||||
|
| ||||
| Enrollment HII | 2.3 (1.8–3.0) | 2.3 (1.8–2.7) | 2.4 (1.9–3.5) | 0.70 |
| 48 hour HII | 2.2 (1.8–2.6) | 2.4 (2.1–2.8) | 2.1 (1.5–2.3) | 0.09 |
| Change in HII | 0.11 (−0.47 – 0.29) | 0.21 (−0.13 – 0.31) | −0.11 (−1 – 0.11) | 0.025 |
| HII Increase in first 48 hours | 20/35 | 13/18 | 7/17 | 0.09 |
|
| ||||
| High Density Lipoprotein (HDL), mean in mg/dL (SD) | ||||
|
| ||||
| Enrollment HDL | 29 (3.7) | 28 (4.1) | 31 (6.3) | 0.71 |
| 48 hour HDL | 18 (2.5) | 16 (4.9) | 19 (4.2) | 0.53 |
| Change in HDL | −11 (2.3) | −13 (3.3) | −9 (3.2) | 0.31 |
|
| ||||
| Low Density Lipoprotein (LDL), mean in mg/dL (SD) | ||||
|
| ||||
| Enrollment LDL | 48 (4.8) | 53 (6.5) | 41 (7.1) | 0.21 |
| 48 hour LDL | 52 (5.0) | 48 (4.3) | 56 (9.8) | 0.40 |
| Change in LDL | 2 (4.1) | −6 (4.2) | 13 (7.2) | 0.027 |
HII parameters were analyzed using Wilcoxon rank-sum test for numerical data and Fisher’s exact test for categorical data; HDL and LDL values were analyzed using student’s t test.
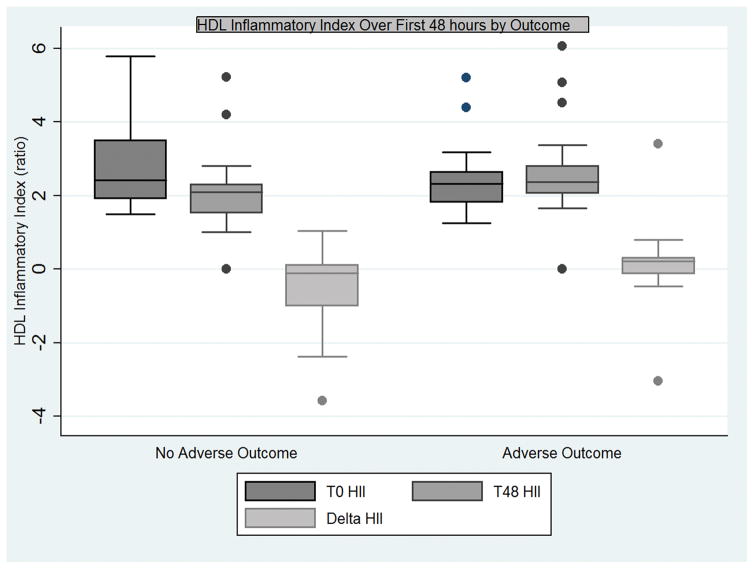
A multivariable logistic regression model revealed change in HII to be an independent predictor of adverse outcome (OR 5.2, 95% CI 1.1–25.1 p = 0.040), though age was not predictive of outcome (OR 1.06, 95% CI 0.99 – 1.12, p = 0.054). The relationship between baseline, 48 hour and change in HII with adverse outcome is displayed graphically in Figure 1. Values for HDL and LDL cholesterol were also analyzed. Interestingly, patients experiencing an adverse outcome had a significant drop in LDL (mean −6.0, SD 4.2) at 48 hours compared with the rest of the cohort (mean 13.0, SD 7.2, p = 0.027).
Figure 1.
Boxplot of interquartile ranges (25th–75th percentiles) of HDL Inflammatory Index (HII) of study patients at enrollment (T0 HII), 48 hours after enrollment (T48), and the change in HII (delta HII) for patients with and without adverse outcomes.
There were 24 patents with results for both Dys-HDL and inflammatory cytokines at both time points. There were significant inverse correlations between the change in HII and plasma GRO (rs = −0.52, p = .0088), as well as change in MCP-1 (rs = −0.61, p = .0014) concentrations over the 48 hours of the study period. There were no other significant correlations between change in HII and inflammatory cytokines. (Table 4)
Table 4.
Correlation Matrix displaying Spearman’s correlations between changes in HDL inflammatory index and changes in inflammatory cytokines and chemokines over the first 48 hours of admission. Dysfunctional high density lipoprotein (Dys-HDL) expressed as HDL inflammatory index (HII). GRO = growth related oncogene, interleukin-6 = IL-6, interferon gamma-induced protein = IP-10, MCP-1 = monocyte chemotactic protein-1, IL-10 = interleukin-10, TNF-α = tumor necrosis factor alpha, IFN-γ = interferon gamma. Significant P-values in bold.
| Change in Marker | HII | GRO | IL6 | IP10 | MCP1 | IL10 | TNF-α | IFN-γ |
|---|---|---|---|---|---|---|---|---|
| HII | 1.0000 | |||||||
|
GRO P-value |
−0.5226 0.0088 |
1.0000 | ||||||
|
IL-6 P-value |
−0.3400 0.1040 |
0.7113 0.0001 |
1.0000 | |||||
|
IP-10 P-value |
−0.2583 0.2230 |
0.1583 0.4601 |
0.4513 0.0269 |
1.0000 | ||||
|
MCP-1 P-value |
−0.6139 0.0014 |
0.4843 0.0165 |
0.6704 0.0003 |
0.5330 0.0073 |
1.0000 | |||
|
IL-10 P-value |
−0.0278 0.8973 |
0.2417 0.2551 |
0.6304 0.0010 |
0.5635 0.0041 |
0.3339 0.1108 |
1.0000 | ||
|
TNF-α P-value |
−0.2296 0.2805 |
0.1939 0.3639 |
0.5435 0.0061 |
0.8157 0.0000 |
0.4843 0.0165 |
0.6817 0.0002 |
1.0000 | |
|
IFN-γ P-value |
−0.2623 0.2156 |
0.4595 0.0239 |
0.4534 0.0261 |
0.4868 0.0158 |
0.3116 0.1382 |
0.2192 0.3034 |
0.2535 0.2319 |
1.0000 |
Discussion
In this pilot study of adult patients with sepsis, Dys-HDL was not only present sepsis but increasing Dys-HDL over the first 48 hours was predictive of adverse outcomes. Change in HII over the first 48 hours was also significantly inversely correlated with changes in inflammatory cytokines GRO and MCP-1. These findings indicate that Dys-HDL may play a role in predicting clinical outcomes in patients with sepsis.
The pathophysiologic significance of changes in cholesterol which occur during the early phase of sepsis are not fully understood. Rapid drops in both HDL and LDL cholesterol have been reported in previous studies,5–7 and our group recently demonstrated that low LDL is a risk factor for long-term community-acquired sepsis.8 Similarly, in this study, patients with adverse outcomes experienced more severe drops in LDL. There may be several reasons for early drops in lipid concentrations during sepsis. First, both LDL and HDL are central to clearance of bacterial toxins from the blood stream. Thus, increased LDL transport to the liver via HDL in a physiologic effort to clear bacterial toxins may result in an acute reduction in both HDL and LDL levels. HDL also facilitates transfer of cholesterol esters to the adrenal glands for the production of endogenous corticosteroids under stress (sepsis) via scavenger receptor BI (SR-BI).23,24 Both of these processes may result in acute reductions in serum lipid levels. Therefore the severity of the drop in HDL or LDL may be indicative of sepsis disease severity, which may explain why patients with lower baseline levels are predisposed to sepsis as their capacity to recover from sepsis may be impaired.
Our central hypothesis is that structural changes in HDL which occur under inflammatory stress may lead to impaired HDL function, resulting in pro-inflammation and loss of HDL’s protective functions. Our hypothesis is overall supported by this study, which is the first to demonstrate that Dys-HDL is not only present in sepsis but is associated with adverse outcomes when levels increase during early sepsis.
The results of this study may have important implications for future potential sepsis therapies. Apolipoprotein mimetic peptides (AMPs) are short synthetic peptides that are often based on Apo A-I, the main protein constituent of HDL. One of the most studied AMPs, the 4-F peptide, derived from an 18 amino acid peptide has been tested in clinical trials, but not specifically for sepsis.25–27 Kwon and colleagues demonstrated that L-4F improved survival in septic mice with endotoxemia and attenuated histologic damage in lung tissue.28 This improvement was accompanied by higher levels of HDL cholesterol and lower lung myeloperoxidase enzyme activity. Myeloperoxidase has been shown to be the possible culprit for the alteration of Apo A-I structure which causes Dys-HDL, however importantly, L-4F is resistant to alteration by myeloperoxidase.15,29 Sharifov and colleagues also demonstrated that L-4F inhibited lipopolysaccharide toxin activity in white blood cells from human patients with sepsis-induced acute respiratory distress syndrome.30 They also showed that L-4F administered intravenously significantly reduced lung and liver injury as well as mortality in a rat model of sepsis.31 Other HDL-based therapies include reconstituted HDL which have been shown to result in atherosclerosis regression in cardiac patients32 and reduction in monocyte CD11b expression, neutrophil adhesion, intercellular and vascular adhesion molecule-1 (ICAM-1 and VCAM-1) expression in endothelial cells.33
The study of the relationship between HII and inflammatory cytokines in this study was informative and warrants additional study. Interestingly, inverse correlations between Dys-HDL and GRO and MCP-1 were observed over time. This finding may be explained by the possibility that Dys-HDL is a more accurate prognosticator of ongoing inflammation, and that persistent Dys-HDL elevation indicates that the state of inflammation has not yet resolved despite reductions in cytokine levels. It may also indicate a transition from acute to chronic inflammation, and that Dys-HDL may play a role in ongoing inflammation that leads to long-term organ dysfunction and chronic critical illness. Therefore, Dys-HDL may have promise in predicting outcomes after sepsis or in identification of patients susceptible to treatment with novel lipid-based therapies. Future studies are needed to elucidate the role of Dys-HDL as either a novel biomarker or indicator of potential response to novel therapies.
This study had several limitations. First, it is a small pilot study, and it is likely that potential associations were not observed which may have been evident in a larger study. Second, as there were only four patients who died, the study was underpowered to assess death as the primary outcome. While this is an important outcome which will be investigated in future work, it is our belief that sepsis trials should have a change of focus from 28 day mortality to longer-term outcomes including long-term death (90 days or greater) and physical dependence after discharge.
Conclusion
In this study, we have demonstrated that Dys-HDL is present in sepsis and may be predictive of adverse outcomes from sepsis. This study has important implications for novel lipid-based therapies for sepsis. Future work should focus on elucidating the mechanisms by which Dys-HDL may potentiate adverse outcomes in sepsis and clinical trials of novel lipid-based therapies.
Supplementary Material
Acknowledgments
Funding: Funding was provided by a Society of Critical Care Medicine Vision Grant and the National Institute for General Medical Sciences (P50GM111152 and K23 GM115690-01A1).
References
- 1.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med. 2013;41:580–637. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman C, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walton A, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, Pachot A, Brooks TL, Deych E, Shannon WD, et al. Reactivation of multiple viruses in patients with sepsis. PLoS One. 2014;9:e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien J-Y, Jerng J-S, Yu C-J, Yang P-C. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit Care Med. 2005;33:1688–93. doi: 10.1097/01.ccm.0000171183.79525.6b. [DOI] [PubMed] [Google Scholar]
- 6.Chien Y-F, Chen C-Y, Hsu C-L, Chen K-Y, Yu C-J. Decreased serum level of lipoprotein cholesterol is a poor prognostic factor for patients with severe community-acquired pneumonia that required intensive care unit admission. J Crit Care. 2015;30:506–10. doi: 10.1016/j.jcrc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Lagrost L, Girard C, Grosjean S, Masson D, Deckert V, Gautier T, Debomy F, Vinault S, Jeannin A, Labbé J, et al. Low preoperative cholesterol level is a risk factor of sepsis and poor clinical outcome in patients undergoing cardiac surgery with cardiopulmonary bypass. Crit Care Med. 2014;42:1065–73. doi: 10.1097/CCM.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 8.Guirgis FW, Donnelly JP, Dodani S, Howard G, Safford MM, Levitan EB, Wang HE. Cholesterol levels and long-term rates of community-acquired sepsis. Crit Care. 2016;20:408. doi: 10.1186/s13054-016-1579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innate and adaptive immunity. Cardiovasc Res. 2014 doi: 10.1093/cvr/cvu150. cvu150 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Murphy A, Woollard K, Hoang A, Mukhamedova N, Stirzaker R, McCormick S, Remaley A, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 12.Murphy A, Woollard K, Suhartoyo A, Stirzaker R, Shaw J, Sviridov D, Chin-Dusting J. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1333–1341. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- 13.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 14.Madahian S, Navab K, Pourtabatabaei N, Seyedali S, Safar S, Vazirian S, Hough G. Inflammation, high density lipoprotein and endothelium. Curr Med Chem. 2014;21:2902–9. doi: 10.2174/0929867321666140414105530. [DOI] [PubMed] [Google Scholar]
- 15.Haraguchi Y, Toh R, Hasokawa M, Nakajima H, Honjo T, Otsui K, Mori K, Miyamoto-Sasaki M, Shinohara M, Nishimura K, et al. Serum myeloperoxidase/paraoxonase 1 ratio as potential indicator of dysfunctional high-density lipoprotein and risk stratification in coronary artery disease. Atherosclerosis. 2014;234:288–94. doi: 10.1016/j.atherosclerosis.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Yunoki K, Naruko T, Inaba M, Inoue T, Nakagawa M, Sugioka K, Ohsawa M, Iwasa Y, Komatsu R, Itoh A, et al. Gender-specific correlation between plasma myeloperoxidase levels and serum high-density lipoprotein-associated paraoxonase-1 levels in patients with stable and unstable coronary artery disease. Atherosclerosis. 2013;231:308–14. doi: 10.1016/j.atherosclerosis.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 17.Shao B, Tang C, Sinha A, Mayer P, Davenport G, Brot N, Oda M, Zhao X, Heinecke J. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114:1733–42. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiDonato J, Aulak K, Huang Y, Wagner M, Gerstenecker G, Topbas C, Gogonea V, DiDonato A, Tang W, Mehl R, et al. Site-specific nitration of apolipoprotein A-I at tyrosine 166 is both abundant within human atherosclerotic plaque and dysfunctional. J Biol Chem. 2014;289:10276–92. doi: 10.1074/jbc.M114.556506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Leeuwen H, Heezius E, Dallinga G, van Strijp J, Verhoef J, van Kessel K. Lipoprotein metabolism in patients with severe sepsis. Crit Care Med. 2003;31:1359–1366. doi: 10.1097/01.CCM.0000059724.08290.51. [DOI] [PubMed] [Google Scholar]
- 20.Guirgis FW, Brakenridge S, Sutchu S, Khadpe JD, Robinson T, Westenbarger R, Topp T, Kalynych CJ, Reynolds J, Dodani S, et al. The long-term burden of severe sepsis and septic shock: Sepsis recidivism and organ dysfunction. J Trauma Acute Care Surg. 2016 doi: 10.1097/TA.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 21.Navab M, Hama S, Hough G, Subbanagounder G, Reddy S, Fogelman A. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res. 2001;42:1308–1317. [PubMed] [Google Scholar]
- 22.Harrell JF, Lee K, Mark D. Tutorial in Biostatistics Multivariable Prognostic Models : Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Guo L, Zheng Z, Ai J, Howatt D, Mittelstadt P, Thacker S, Daugherty A, Ashwell J, Remaley A, Li X. Scavenger receptor BI and high-density lipoprotein regulate thymocyte apoptosis in sepsis. Arterioscler Thromb Vasc Biol. 2014;34:966–75. doi: 10.1161/ATVBAHA.113.302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo L, Ai J, Zheng Z, Howatt D, Daugherty A, Huang B, Li X. High density lipoprotein protects against polymicrobe-induced sepsis in mice. J Biol Chem. 2013;288:17947–17953. doi: 10.1074/jbc.M112.442699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson C, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, Navab M, Hama S, Hough G, Reddy S. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011;52:361–73. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloedon L, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot B, Movva R, Navab M, Fogelman A, Rader D. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffy D, Rader DJ. Drugs in development: targeting high-density lipoprotein metabolism and reverse cholesterol transport. Curr Opin Cardiol. 2005;20:301–6. doi: 10.1097/01.hco.0000168532.69342.26. [DOI] [PubMed] [Google Scholar]
- 28.Kwon WY, Suh GJ, Kim KS, Kwak YH, Kim K. 4F, apolipoprotein AI mimetic peptide, attenuates acute lung injury and improves survival in endotoxemic rats. J Trauma Acute Care Surg. 2012;72:1576–83. doi: 10.1097/TA.0b013e3182493ab4. [DOI] [PubMed] [Google Scholar]
- 29.Hewing B, Parathath S, Barrett T, Chung W, Astudillo Y, Hamada T, Ramkhelawon B, Tallant T, Yusufishaq M, Didonato JA, et al. Effects of native and myeloperoxidase-modified apolipoprotein a-I on reverse cholesterol transport and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2014;34:779–89. doi: 10.1161/ATVBAHA.113.303044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharifov O, Xu X, Gaggar A, Grizzle W, Mishra V, Honavar J, Litovsky S, Palgunachari M, White C, Anantharamaiah GM, et al. Anti-inflammatory mechanisms of apolipoprotein A-I mimetic peptide in acute respiratory distress syndrome secondary to sepsis. PLoS One. 2013;8:e64486. doi: 10.1371/journal.pone.0064486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharifov O, Nayyar G, Ternovoy V, Palgunachari M, Garber D, Anantharamaiah G, Gupta H. Comparison of anti-endotoxin activity of apoE and apoA mimetic derivatives of a model amphipathic peptide 18A. Innate Immun. 2013 doi: 10.1177/1753425913514621. [DOI] [PubMed] [Google Scholar]
- 32.Krause BR, Remaley AT. Reconstituted HDL for the acute treatment of acute coronary syndrome. Curr Opin Lipidol. 2013;24:480–486. doi: 10.1097/MOL.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 33.Patel S, Drew B, Nakhla S, Duffy S, Murphy A, Barter P, Rye K, Chin-Dusting J, Hoang A, Sviridov D, et al. Reconstituted High-Density Lipoprotein Increases Plasma High-Density Lipoprotein Anti-Inflammatory Properties and Cholesterol Efflux Capacity in Patients With Type 2 Diabetes. J Am Coll Cardiol. 2009;53:962–971. doi: 10.1016/j.jacc.2008.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.