Abstract
The effectiveness of plant growth – promoting bacteria is variable under different biotic and abiotic conditions. Abiotic factors may negatively affect the beneficial properties and efficiency of the introduced PGPR inoculants. The aim of this study was to evaluate the effect of plant growth – promoting rhizobacteria on plant growth and on the control of foot and root rot of tomatoes caused by Fusarium solani under different soil salinity conditions. Among the five tested strains, only Pseudomonas chlororaphis TSAU13, and Pseudomonas extremorientalis TSAU20 were able to stimulate plant growth and act as biological controls of foot and root rot disease of tomato. The soil salinity did not negatively affect the beneficial impacts of these strains, as they were able to colonize and survive on the roots of tomato plants under both saline and non-saline soil conditions. The improved plant height and fruit yield of tomato was also observed for plants inoculated with P. extremorientalis TSAU20. Our results indicated that, saline condition is not crucial factor in obtaining good performance with respect to the plant growth stimulating and biocontrol abilities of PGPR strains. The bacterial inoculant also enhanced antioxidant enzymes activities thereby preventing ROS induced oxidative damage in plants, and the proline concentrations in plant tissue that play an important role in plant stress tolerance.
Keywords: Rhizobacteria, Biocontrol, Soil salinity, Plant growth, Nutrient uptake
1. Introduction
The increase in harsh abiotic stresses such as drought, salinity and abrupt changes in temperature are part of the main consequences of climate change. These stresses have led to loss of soil organic matter and other forms of soil degradation that negatively affect agricultural productivity (Ahmad et al., 2015). Another important consequence of climate change and abiotic stresses is the increased infection/infestation of plants by pathogens and pests (Chakraborty, 2013). Intensive research attempts are underway to improve plant growth, and tolerance to various abiotic stresses, and to protect plants from soil borne pathogens using plant growth promoting rhizobacteria (PGPR) which have great potential for sustainable crop production (Lugtenberg and Kamilova, 2009, Berg and Martinez, 2015, Egamberdieva et al., 2015a, Egamberdieva et al., 2015b, Egamberdieva et al., 2016).
Root associated microbes, including endophytes, closely cooperate with each other and can mediate important physiological processes, especially nutrient acquisition and plant fitness to abiotic stresses (Berg et al., 2013, Abd_Allah et al., 2015). Plants inoculated with PGPR produce more root hairs and take up mineral and microelements more efficiently from the soil. The growth of several plants is enhanced by PGPR treatment, e.g. lentil (Lens esculenta) (Faisal, 2013), pea (Pisum sativum L.) (Meena et al., 2015), cucumber (Cucumis sativus), (Egamberdieva et al., 2011), rice (Oryza sativa) (Yadav et al., 2014) and soybean (Glycine max) (Egamberdieva et al., 2015b). PGPR also induces systemic tolerance to various abiotic stresses in plants such as salinity, drought and heavy metals through alteration of plant physiology (Wang et al., 2012). The beneficial traits of plant growth promoting bacteria include the ability to synthesize biological active compounds such as plant growth stimulators (Parray et al., 2016; Egamberdieva et al., 2017), osmolytes (Berg et al., 2013), antifungal compounds (Landa et al., 2004), and 1-aminocyclopropane-1-carboxylate (ACC) deaminase enzyme (Ali et al., 2014). The strain Pseudomonas was able to suppress soybean root disease caused by fungal pathogens (Susilowati et al., 2010) and showed antagonistic activity against several fungal pathogens, such as Fusarium oxysporum, and Rhizoctonia solani. In another study, P. agglomerans and Bacillus sp. reduced the charcoal root rot of soybean caused by Macrophomina phaseolina under greenhouse conditions (Vasebi et al., 2013). An induced systemic resistance in plants against foliar pathogens by PGPR was also reported (Choudhary et al., 2007a, Choudhary et al., 2007b). In addition some reports have suggested that some PGPR induces systemic tolerance (IST) in plants through elevated antioxidant responses at the levels of enzyme activity and metabolite accumulation (Hashem et al., 2015, Hashem et al., 2016; Jha et al., 2011). The antioxidant defence system plays a major role in plant adaptation to salinity stress that allow the scavenging of reactive oxygen species (ROS) (Ahanger et al., 2014).
The variability in the effectiveness of biologicals is of concern when used under different conditions or cropping systems. Hostile environmental conditions are deleterious for the root associated microbiome and effective functioning of the introduced PGPR inoculants (Landa et al., 2004). In earlier studies Landa et al. (2001) observed an effect of temperature on plant growth and biological control ability of PGPR Pseudomonas fluorescens. In their study fusarium wilt of chickpea was suppressed by these rhizobacteria at 30 °C, but not at 25 °C at which temperature disease potential was high. Interestingly this suppression was related to the production of extracellular metabolites that inhibit F. oxysporum, and the root colonization and plant growth stimulating abilities of rhizobacteria, which was higher at 30 °C (Landa et al., 2004). In this study, we evaluated the effect of plant growth promoting rhizobacteria on plant growth and on the control foot and root rot disease of tomato caused by Fusarium solani under different conditions of soil salinity.
2. Materials and methods
2.1. Soil, seeds and bacterial strains
The soil used for pot experiments was selected from deep tillage (0–40 cm) irrigated agricultural fields in the Tashkent (non-saline, EC 1.3 dS m−1) and Syr-Darya Provinces (affected by salinity, EC 7.1 dS m−1) of Uzbekistan. Soils with an EC of greater than 4.0 dS m−1 soil were considered saline. The characterisation of the experimental soil used in the current study are described in Table 1. Tomato seeds (Lycopersicon esculentum cv. Bella) were obtained from Enza Zaden, the Netherlands. The bacterial strains Pseudomonas putida TSAU1, P. extremorientalis TSAU6, P. chlororaphis TSAU13, P. extremorientalis TSAU20, and P. aurantiaca TSAU22 were obtained from the culture collection of the Faculty of Biology, National University of Uzbekistan. These strains were previously isolated from the rhizosphere of wheat grown in salinated soil (Egamberdieva and Kucharova, 2009). All Pseudomonas strains were grown on King’s B agar medium (KB; Difco Laboratories, Detroit, MI, USA) at 28 °C. The fungal pathogen Fusarium solani was obtained from the National University of Uzbekistan and was grown on potato dextrose agar plates (PDA; Difco Laboratories, Detroit, MI, USA).
Table 1.
Soil characterization.
| Soila | EC dS m−1 |
K+ |
Ca+2 |
Mg+2 |
CO32− |
N |
P |
COrg |
Na+ |
Cl− |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg g−1 soil) | (µg g−1 soil) | |||||||||
| None-saline | 2.3 | 5.92 | 53.4 | 23.7 | 16.1 | 1.06 | 1.30 | 8.69 | 600.2 | 52.0 |
| Saline | 7.1 | 6.58 | 67.4 | 24.6 | 17.6 | 0.95 | 1.23 | 7.19 | 813.1 | 94.2 |
EC = Electrical Conductivity. K+ = exchangeable potassium. Ca+2 = exchangeable calcium. Mg+2 = exchangeable magnesium. Na+ = exchangeable sodium. CO32− = carbonate and Cl− = chloride. CT = total C. COrg = total organic C. N = total nitrogen. and P = total phosphorus.
Non-saline soil was collected from Tashkent province. Saline soil from Syrdarya province of Uzbekistan.
2.2. Fungal isolate
The fungal pathogen Fusarium solani was previously isolated from diseased tomato plants grown in salinated Uzbek soil that showed typical Fusarium foot and root rot symptoms. The procedure for the isolation and identification of pathogen from tomato root was described in previous work by Egamberdieva et al. (2011). Briefly, a small piece of tissue from the diseased plant was plated on potato dextrose agar (PDA) and incubated at 28 °C in the dark for 5 days. A single microconidial culture was prepared from isolate. The pathogenicity test was carried out on tomato under controlled growth chamber conditions. A randomized complete block design experiment with five replicates was carried out to examine the pathogenicity of the isolated phytopathogen (F. solani) to fulfil Koch’s postulates. Healthy tomato seedlings that had developed their second set of true leaves were transplanted into plastic pot (500 ml capacity) containing sand:peat-moss:vermiculite mixture (1:1:1, w/w/w) which infested with the phytopathogen (F. solani) as 2 ml of spore suspension (4 × 107 spore/ml). Control pots (without fungal pathogen) were used as references. The pots were incubated in growth chamber for five weeks at 27 ± 2 °C, then symptoms of root rot disease were evaluated using the following formula.
After 5 weeks, a piece of diseased root from a sick plant was removed and plated on a PDA plate followed by incubation for 5 days to isolate the pathogen. Prior to identification, fungus was grown on sterile filter paper placed on PDA agar. The filter paper containing the fungal hyphae was collected and ground in liquid nitrogen. DNA was isolated from pulverized fungal biomass using the Nucleon Phytopure kit (Amersham Biosciences GmbH, Freiburg, Germany). To identify the fungal isolates, the mtSSU rDNA sequences of two strains were analyzed. The mtSSU rDNA fragments were amplified using MS1 and MS2 primers (Zeng et al., 2003) and sequenced by ServiceXS (Leiden, the Netherlands). The sequences of the fragments were compared with those in GenBank using the BLAST program. The sequences of the two analyzed strains showed 99% similarity with the mtSSU rDNA sequence from Fusarium solani f. sp. glycines isolate 1-potato (GenBank Acc. N. AF125026). Therefore the isolates can be referred to as Fusarium solani on the basis of their mtSSU rDNA fragment sequences.
2.3. Salt tolerance of tomato plants
The tomato seeds were sterilized by immersion in 70% ethanol for 5 min followed by an immersion in 0.1% HgCl2 for 1 min, after which they were washed several times with sterile water. Germination tests were carried out in Petri dishes (Ø 85 mm × 15 mm) containing 1% water agar. Salinity conditions were generated by the addition of NaCl to final concentrations of 75, 100 and 125 mM NaCl. Twenty healthy and surface-sterilized tomato seeds were placed in each Petri dish and were arranged in a randomized complete block design with three replications. Eventually, the Petri dishes were covered with a polyethylene sheet to avoid the loss of the moisture through evaporation and were kept in the plant growth chamber at 28 °C. The seeds were observed daily and the percent of germination was recorded after three days of incubation. Seeds were considered to have germinated when the emerging radicals were over 0.2 cm long. The lengths of roots and shoots of germinated seedlings were measured and recorded.
2.4. Plant growth promotion
The effect of inoculation with selected bacterial strains on the growth of tomato was studied under non saline and saline soil condition. Bacterial inoculants were prepared and the tomato seeds were sterilized as described in Egamberdieva et al. (2011). Briefly, Pseudomonas strains were grown overnight in KB broth, and cell suspension was centrifuged. The cell pellets were suspended with phosphate-buffered saline (PBS; 20 mM sodium phosphate, 150 mM NaCl, pH 7.4). Tomato seeds were coated with bacteria by dipping the seeds in bacterial suspensions (cell densities of 107–108 cells/ml).
Inoculated seedlings were sown in the plastic pots (7 cm diameter; 10 cm deep) and were set-up in a randomized design with ten replications. The treatments were: (i) un-inoculated seeds, and (ii) seeds inoculated with bacterial strains. The tomato plants were grown under non saline and saline soil for six weeks under open natural conditions with temperatures ranged between 26 °C and 28 °C during the day and between 12 °C and 14 °C at night. At harvest, the shoot lengths were measured and whole plants were dried to constant weight at 100 °C and were weighed.
2.5. Biological control of tomato root rot by bacterial strains
Seeds were germinated and the seedling were sown into a plastic pot (capacity 500 ml) filled with non-saline and saline soil. The treatments were as follows: (i) control, without pathogen and bacteria, (ii), control with pathogen, and (iii) pathogen and bacteria. To prepare the fungal inoculant Erlenmeyer flasks (250 ml capacity) containing Potato-dextrose broth (Difco Laboratories, Detroit, MI, USA) were inoculated with 0.5 cm agar disc cut from 7 days old culture of the pathogenic fungi, F. solani. The flasks were incubated at 28 ± 2 °C under shaking status (110 rpm) for ten days. The spores were removed using sterile glass wool and were adjusted to a concentration of 2 × 107 spores/ml. Randomized complete block designs were used for treatments with four replications. Each treatment contained 24 replicate pots. The plants were grown for five weeks under open natural conditions with temperatures ranging between 26 °C and 28 °C during the day and between 12 °C and 14 °C at night and were watered as needed. At harvest, plants were removed from the soil, and roots were washed and examined for the symptoms of root rot.
2.6. Survival of bacterial strains on plant roots
Antibiotic resistant strains were used to determine the effect of salinity on the survival of inoculated strains. Rifampicin resistant mutants of Pseudomonas putida TSAU1, P. extremorientalis TSAU6, P. chlororaphis TSAU13, P. extremorientalis TSAU20, and P. aurantiaca TSAU22 were obtained by plating the parental strain onto KB agar plates containing 200 µg/ml rifampicin. After incubating for 5 days at 28 °C, antibiotic resistant colonies having colony morphology and growth rate similar to the the parental strain were selected and transferred onto KB agar plates containing rifampicin. The plant seeds were sterilized, germinated and coated with rifampicin resistant mutants as described above. Plants were grown in plastic pots filled with potting soil for four weeks under greenhouse condition as described above. The plants were watered when necessary and salinity condition was established by addition of 75 mM NaCl. At harvest, the adhering soil was removed from the plant roots and 1 g of roots was shaken in 9 ml of sterile phosphate-buffered saline (PBS; 20 mM sodium phosphate, 150 mM NaCl, pH 7.4). The number of bacteria in the resulting suspensions was determined as colony forming units (CFU) using the dilution-plate method and KB agar containing 200 µg/ml of rifampicin. After incubation for 2–3 days at 28 °C, the number of rifampicin resistant colonies was counted.
2.7. Greenhouse experiments
The strains which showed the best performance with respect to stimulating plant growth and controlling root rot of tomato plants were evaluated for their effect on tomato growth and yield under greenhouse conditions. The greenhouse experiment was carried out in the Tashkent province of Uzbekistan using a calcareous sierozem soil with 2.4% organic matter, N 0.1%, P 1.34%, K 7.1% and soil EC value of 5.6 dSm and pH 7.8. The bacterial inoculants were prepared as described by Egamberdieva et al. (2011). The coated seed with bacterial inoculant and uncoated seeds were sown in pots (one seed per pot), containing a mixture of soil and biohumus (2:1, v/v). Seedlings were initially kept in a small greenhouse under semi-controlled environmental conditions (temperature fluctuating between 18 and 22 °C). When seedlings reached the 2–4 leaf stage, they were transferred to an un-heated greenhouse and transplanted in six rows in each experimental plot (3 m × 2.5 m). Intra-row spacing was 50 cm and rows were 60 cm apart. Four replicate plots per treatment were used (total 12 of treatments), and the experiment was set up in a randomized complete block design. The temperature in the greenhouse ranged between 24 and 27 °C during the day and between 14 and 17 °C at night. At harvest the plant height and total fruit yield were determined. The N, P, and K acquisition of plants grown under field conditions were determined. The shoots were separated from the roots and dried in an oven at 75 °C for 48 h and then were powdered. Total nitrogen (Nt) was determined after dry combustion using a CNS elemental analyzer (LECO Corporation, St. Joseph, MI) according to DIN ISO 15178 (2001). The P, K, and Mg contents were analyzed according to DIN 38414-S (1983).
2.8. Antioxidant enzyme activity
The plant extract used to measure enzymes activities was prepared as described by Ahmad et al. (2015). Briefly, fresh leaves (10 g) were crushed in 50 volumes of 100 mM Tris-HCl (pH 7.5) containing 5 mM DTT (Dithiothreitol), 10 mM MgCl2, 1 mM EDTA (Ethylene diaminetetra acetic acid), 5 mM magnesium acetate, 1.5% PVP-40 (Polyvinylpyrrolidone), 1 mM PMSF (phenylmethanesulfonyl fluoride) and 1 μg ml−1 aproptinin. The homogenate was filtered using a cheese cloth and centrifuged for 15 min at 10,000 rpm. The enzyme-containing supernatant was collected after centrifugation. For the analysis of APX activity, tissues were separately homogenized with 2 mM AsA. All experiments were performed at 4 °C. Activity of superoxide dismutase (SOD) (EC 1.15.1.1) was estimated according to Van Rossum et al. (1997) following the photoreduction of nitrobluetetrazolium (NBT). The activity of SOD was expressed as enzyme unit (EU) mg−1 protein. One unit of SOD was defined as the amount of protein causing 50% decrease in the SOD-inhibitable NBT reduction. The method of Nakano and Asada (1981) was followed to determine the ascorbate peroxidase (APX) activity, with the absorbance read at 290 nm. The APX activity was expressed as EU mg−l protein. Catalase (CAT) (EC 1.11.1.6) activity was determined according to Luck (1974). The activity of CAT was calculated using the extinction co-efficient of 36 × 103 mM−l cm−l and expressed as EU mg−1 protein. The activity of glutathione reductase (GR) (EC 1.6.4.2) was determined according to Carlberg and Mannervik (1985) with the absorbance read at 340 nm for 2 min. The activity of GR was calculated using the extinction co-efficient of NADPH of 6.2 mM−1 cm−1 and expressed as EU mg−l protein.
2.9. Statistical methods
The statistical analyses were performed using the analysis of variance package included in Microsoft Excel 2007. Mean comparisons were conducted using a least significant difference (LSD) test (P = 0.05), student’s t-test.
3. Results
3.1. Germination of seeds and seedling growth under salt stress
The effects of salinity on seed germination of tomato were evaluated in Petri plates using NaCl concentrations of 75, 100 and 125 mM. Seed germination was decreased slightly with increasing salt concentrations (from 75 to 125 mM NaCl), compared to the control seeds (water only). The observed seed germination was 89 ± 4.6% with distilled water and 76 ± 3.5% at 75 mM NaCl, 61 ± 4.9% at 100 mM NaCl and 47 ± 3.9% at 125 mM NaCl concentration. The heighest salinity assayed (125 mM) inhibited the root and shoot length of tomato seedling grown in pots for 10 days. NaCl concentrations of 100 mM reduced the dry weight of tomato by 27% ± 1.0 and 125 mM NaCl by 33% ± 0.6 compared to unstressed plants.
3.2. Plant growth in pots
The bacterial strains Pseudomonas putida TSAU1, Pseudomonas sp. TSAU5, P. extremorientalis TSAU6, P. chlororaphis TSAU13, P. extremorientalis TSAU20, and P. aurantiaca TSAU22 were selected to asses their plant growth stimulation properties in pot experiments. Three strains Pseudomonas sp. TSAU5, P. chlororaphis TSAU13, and P. extremorientalis TSAU20 significantly (P < 0.05) increased plant length by 13, 26, and 19% compared to the untreated control, respectively (Table 2). The dry weight of tomato was significantly increased only by strain P. extremorientalis TSAU20. Saline soil had a negative effect on the stimulation of tomato plant growth by several strains, but not P. extremorientalis TSAU20. The bacterial strain TSAU20 increased the shoot length and dry weight of tomato plants by 28%, and 27% in a significant way compared with the untreated control (Table 2).
Table 2.
Effect of selected plant growth promoting bacteria on shoot length and dry weight of tomato growing in non-saline (EC value 2.3 dS/m) and saline soil (EC value 7.1 dS/m).
| Bacterial strains | Non-saline |
Saline |
||||
|---|---|---|---|---|---|---|
| Shoot lengtha | Root lengtha | Dry weightb | Shoot lengtha | Root lengtha | Dry weightb | |
| Control | 8.2 | 5.7 | 0.151 | 7.6 | 5.6 | 0.140 |
| Pseudomonas putida TSAU1 | 7.4 | 4.6 | 0.134 | 7.3 | 5.0 | 0.140 |
| 7.2 | 4.9 | 0.125* | 7.4 | 5.5 | 0.126 | |
| 7.8 | 4.9 | 0.137 | 6.9 | 4.6 | 0.122 | |
| P. extremorientalis TSAU6 | 6.9 | 5.0 | 0.128 | 7.2 | 5.2 | 0.140 |
| 7.3 | 5.4 | 0.190 | 7.2 | 5.2 | 0.132 | |
| 8.0 | 5.4 | 0.151 | 6.3 | 4.6 | 0.126 | |
| P. chlororaphis TSAU13 | 10.3* | 8.0* | 0.156 | 8.0 | 6.5 | 0.127 |
| 9.4* | 7.5* | 0.127 | 8.3 | 6.6 | 0.124 | |
| 8.9 | 6.8 | 0.132 | 7.1 | 6.1 | 0.113 | |
| P. extremorientalis TSAU20 | 9.7* | 6.8* | 0.171* | 8.8* | 6.9* | 0.163* |
| 9.8* | 7.4* | 0.188* | 9.7* | 7.3* | 0.178* | |
| 9.6* | 6.9* | 0.209* | 9.9* | 6.9* | 0.156* | |
| P. aurantiaca TSAU22 | 9.1* | 7.0 | 0.169 | 8.3* | 6.9 | 0.154 |
| 8.7 | 7.5* | 0.160 | 7.6 | 6.4 | 0.138 | |
| 8.5 | 6.5 | 0.157 | 7.5 | 6.0 | 0.138 | |
Expressed as cm per plant.
Expressed as gram per plant.
Significantly different from the control at P < 0.05.
3.3. Biological control of tomato root rot
The assayed strains were also evaluated for their biological control potential against tomato root rot caused by Fusarium solani under non-saline and saline soil conditions. The results showed that in non-saline soil that lacked F. solani, the percentage of diseased plants was 18%, while disease symptoms increased to 36% in saline soil (Table 3). All tested bacterial strains were able to control tomato root rot caused by F. solani compared to the pathogen infected control plants without bacteria (Table 3). Two strains P. chlororaphis TSAU13 and P. extremorientalis TSAU20 reduced the incidence of disease in plants by 23, and 14% under non saline conditions and by 42, 25% in saline soil compared to the Fusarium-infected control plants, respectively.
Table 3.
Biological control of tomato root rot by PGPR bacterial strains.
| Treatments | Diseased plants (%) |
|
|---|---|---|
| Non-saline soil | Saline soil | |
| Control | 40.6 ± 8.1 | 71.9 ± 8.1 |
| Pseudomonas putida TSAU1 | 38.1 ± 9.9 | 48.2 ± 6.1 |
| P. extremorientalis TSAU6 | 30.2 ± 4.9 | 50.3 ± 9.4 |
| P. chlororaphis TSAU13 | 23.4 ± 9.4* | 42.2 ± 7.9* |
| P. extremorientalis TSAU20 | 14.1 ± 7.9* | 25.0 ± 5.1* |
| P. aurantiaca TSAU22 | 36.3 ± 8.2 | 40.9 ± 8.3* |
Bacteria were coated on pre-germinated tomato seeds, plants were grown under open natural conditions in pots containing non-saline (EC value 2.3 dS/m) and saline soil (EC value 7.1 dS/m) infested with F. solani spores. ±SD: Standard deviation.
Significantly different from the negative control at P < 0.05.
3.4. Survival of bacterial strains in the tomato root
The survival of bacterial strains in the rhizosphere of tomato grown under two different soil conditions was determined using rifampicin resistant mutants of Pseudomonas putida TSAU1, P. extremorientalis TSAU6, P. chlororaphis TSAU13, P. extremorientalis TSAU20, and P. aurantiaca TSAU22. The results showed that all five bacterial strains were able to colonize and survive on the roots of tomato plants (Table 4). However, their colonization was partly inhibited under salt stress (75 mM NaCl). Among these bacterial strains the rhizosphere colonization by P. chlororaphis TSAU13, and P. extremorientalis TSAU20 was higher under both soil conditions.
Table 4.
The survival (log10 (CFU/g root) of rifampicin resistant mutants of bacterial inoculants in the rhizosphere of *tomato grown under non saline and saline conditions.
| Treatments | Survival (log10 (CFU/g root) of rifampicin resistant mutants bacterial inoculants |
|
|---|---|---|
| Non-saline | Saline | |
| Pseudomonas putida TSAU1 | 3.45 ± 0.15 | 2.15 ± 0.18 |
| P. extremorientalis TSAU6 | 3.58 ± 0.11 | 2.75 ± 0.17 |
| P. chlororaphis TSAU13 | 3.61 ± 0.19 | 2.83 ± 0.18 |
| P. extremorientalis TSAU20 | 3.65 ± 0.22 | 2.81 ± 0.28 |
| P. aurantiaca TSAU22 | 3.29 ± 0.21 | 2. 43 ± 0.29 |
±SD: Standard deviation.
Tomato plants were grown in a greenhouse conditions in potting soil for 4 weeks under non saline (0.5 dS/m) and saline (7.5 dS/m) soil condition.
3.5. Greenhouse trials
The best selected bacterial strains P. chlororaphis TSAU13, and P. extremorientalis TSAU20 which showed growth stimulation in non salinated and salinated soil condition were chosen to test their effect on plant growth and fruit yield of tomato under greenhouse conditions. The treatment of tomato plants with P. chlororaphis TSAU13 increased the plant height of tomato by 16% and fruit yield by 14% compared to the uninoculated control plants. However, the effect was not significant, and only the effect of P. extremorientalis TSAU20 was statistically significant. The height and fruit yields of tomato inoculated with selected bacterial strains were significantly higher by 27% and 22% compared to un-inoculated plants respectively. The nitrogen, phosphorus and potassium content of plants inoculated with the both strains contained 20% and 33% higher nitrogen contents (Table 5). Higher phosphorus content was observed in plant tissues treated with P. extremorientalis TSAU20 (23%) compared to un-inoculated control. However, the potassium uptake of plants was not affected by bacterial inoculation, with exception of the P. extremorientalis TSAU20 strain, which produce a slightly increased K uptake.
Table 5.
Effect of Pseudomonas strains (TSAU13 and TSAU20) on tomato growth and fruit yield in greenhouse experiments.
| Treatment | Plant height (cm) | Fruit yield (kg/m2) | Elements uptake (%) |
||
|---|---|---|---|---|---|
| Nitrogen | Phosphorus | Potassium | |||
| None | 125 ± 3.7 | 13.9 ± 0.9 | 2.4 ± 0.2 | 0.42 ± 0.02 | 1.91 ± 0.06 |
| TSAU13 | 145 ± 5.1 | 15.9 ± 1.4 | 2.9 ± 0.3 | 0.49 ± 0.03 | 1.89 ± 0.08 |
| TSAU20 | 157* ± 4.2 | 17.0* ± 0.7 | 3.2* ± 0.1 | 0.52* ± 0.01 | 2.10 ± 0.10 |
Tomato plants were grown in greenhouse for four months. the temperature range was: 22–24 °C at day and 12–14 °C at night.
Significantly different from the negative control at P < 0.05.
3.6. Accumulation of antioxidant compounds and antioxidant enzyme activity
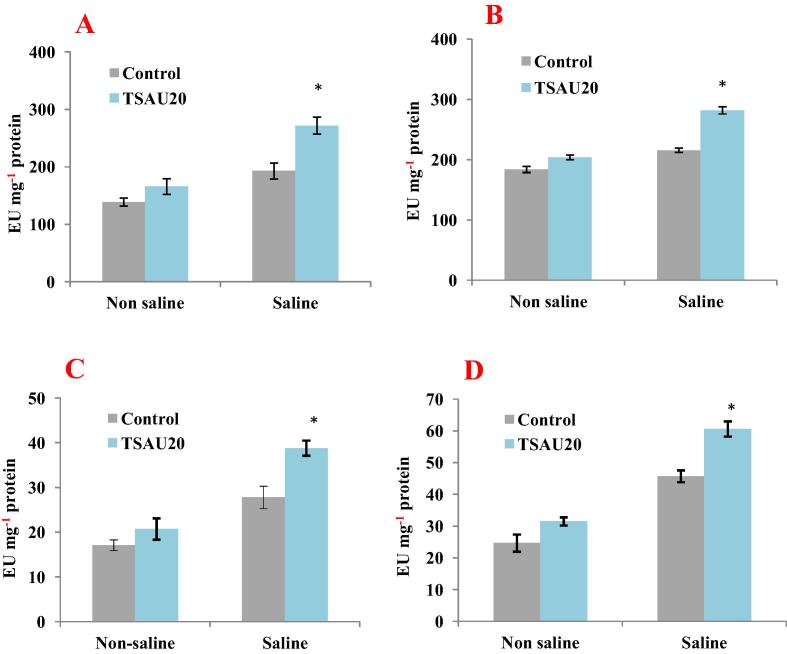
We studied the effect of selected bacterial strain P. extremorientalis TSAU20, which showed plant growth stimulation and biocontrol under saline soil condition (75 mM NaCl) on the some plant physiological parameters involved in defence systems against oxidative stress. Salt stress inhibited the plant fresh weight, whereas P. extremorientalis TSAU20 increased the plant biomass by 5% under non saline and 31% under saline soil condition (Table 6). Tomato plants grown under saline condition contained higher level of hydrogen peroxide in their leaves compared to control plants. Plants inoculated with P. extremorientalis TSAU20 showed a reduced level of H2O2 by 47% under saline soil condition. The concentration of proline in leaves of tomato was also increased by the bacterial inoculation. The antioxidant enzymes in the plant tissue were also affected by bacterial inoculation under salt stress. A reduction in ascorbate peroxidase (APX) and glutathione reductase (GR) activities in leaves was 30% and 40% over the control plant grown under salt stress, respectively (Fig. 1). The inoculation of plants with strain TSAU20 led to a 39 and 32% increase in SOD and CAT activitties compared to the control plants grown under salt stress respectively. Thus, the PGPR treatment led to an overall increase in antioxidant enzyme activity compared to the control.
Table 6.
Effect of P. extremorientalis TSAU20 on hydrogen peroxide (H2O2) glutathione and chlorophyll contents in tomato plants.
| Soil | Treatment | Plant weight g/plant | Hydrogen peroxidea | Prolineb |
|---|---|---|---|---|
| Non-saline | Control | 0.78 ± 0.07 | 70.25 ± 1.31 | 0.36 ± 0.03 |
| TSAU20 | 0.82 ± 0.02 | 50.43 ± 1.96 | 0.82 ± 0.04* | |
| Saline | Control | 0.29 ± 0.03 | 210.9 ± 2.07 | 1.46 ± 0.16 |
| TSAU20 | 0.38 ± 0.05* | 112.8 ± 1.54* | 3.61 ± 0.06* | |
nM/g fresh weight.
mM/g fresh weight.
Significantly different from the negative control at P < 0.05.
Fig. 1.
Effects of P. extremorientalis TSAU20 inoculation on antioxidant enzyme activities. A, glutathione reductase (GR); B, ascorbate peroxidase (APX); C, superoxide dismutase (SOD) and D, catalase (CAT). Tomato plants were grown in a greenhouse for six weeks in potting soil under non saline and saline soil condition. Columns represent means for four plants (N = 4) with error bars showing standard deviation.
4. Discussion
The plant beneficial microbes improve plant growth, nutrient acquisition, and stress tolerance to various abiotic stresses such as drought and salinity, and control plant fungal disease (Egamberdiyeva and Hoflich, 2003, Berg et al., 2013, Hashem et al., 2016, Vardharajula et al., 2011). The alleviation of salt stress in plants by PGPR inoculants has been shown on various crops and vegetables, including licorice (Egamberdieva et al., 2016), and chili pepper (Park et al., 2013). In our study, four PGPR strains stimulated plant growth of tomato, but the activity of some bacterial inoculants was reduced under saline soil condition. It has been reported that the root colonization and plant growth stimulating traits of plant growth promoting bacteria affected by biotic and abiotic factors, such as indigenous microorganisms, temperature, drought, salinity, and soil type (Compant et al., 2010). Salt tolerant and root colonizing bacteria which are physiologically adaptated to abiotic stress could survive in such harsh environment and help plant to tolerate salt stress (Ahmad et al., 2013, Cho et al., 2015, Egamberdieva et al., 2015a, Egamberdieva et al., 2016). The strain P. extremorientalis is a salt tolerant and potential root colonizing bacteria (Egamberdieva and Kucharova, 2009) that showed the best performance in plant growth stimulation of tomato under non saline and saline soil condition. Yao et al. (2010) reported that Pseudomonas putida strain isolated from alkaline soil could increase salt tolerance of cotton and growth in saline soil. We have also observed plant growth stimulation and biological control of cucumber by salt tolerant P. extremorientalis TSAU20 and P. fluorescens PCL1751 strains under saline soil condition (Egamberdieva et al., 2011).
Previous studies showed that higher saline conditions increased the susceptibility of plants to plant pathogens (Sanogo, 2004, Triky-Dotan et al., 2005), e.g. severity of Phytophthora root and crown rot in tomato was higher in salt affected soil (Swieckil and MacDonald, 1991). We have also observed a higher incidence of tomato root rot in salinated soil. The inoculation of tomato with PGPR strains reduced tomato root rot caused by F. solani compared to the pathogen infected control plants under both non-saline and saline soil conditions. Rangarajan et al. (2003) also reported that Pseudomonas strains exhibiting antibiosis activity suppressed both bacterial leaf blight and sheath blight diseases in rice under both natural and saline soil conditions.
The survival of introduced bacteria in the plant root is crucial to generate significant effect and is dependent on the physiological adaptation of the introduced cells and, biotic and abiotic factors (Rekha et al., 2007, Cho et al., 2015). In earlier studies the negative effect of salt stress on the colonization of bacteria introduced into the rhizosphere was observed (Sato and Jiang, 1996). It has been found that salt tolerant bacteria that are able to survive in the rhizosphere of plants grown under harsh environmental condition through their persistence and proliferation in semi-arid soils (Paul and Nair, 2008, Egamberdieva et al., 2013). The bacterial strains were salt tolerant (up to 3% NaCl) and showed potential root colonising abilities for wheat (Egamberdieva and Kucharova, 2009) and common bean (Egamberdieva et al., 2011). In our current study the colonization potential of P. chlororaphis TSAU13 and P. extremorientalis TSAU20 strains was not inhibited by salt stress since they were able to colonize tomato root under saline soil conditions. The potential colonization ability of introduced strains is reported as one of the mechanism in the beneficial effects of introduced strains (Lugtenberg et al., 2001) and this ability of strains in our study was not inhibited by salinity. The involvement of auxin synthesized by bacterial inoculants in stimulation of root system of plants was reported previously (Remans et al., 2008, Egamberdieva, 2009). The bacterial strains P. chlororaphis TSAU13 and P. extremorientalis TSAU20 which were able to growth in medium containing up to 4% NaCl, were able to produce indole 3-acetic acid in medium containing up to 3% NaCl and showed antagonistic activity against plant fungal pathogens (Egamberdieva et al., 2009). The salinity did not have a negative effect on their plant beneficial traits. The best selected strains increased plant growth and fruit yield of tomato under greenhouse condition. A similar observation was reported by Almaghrabi et al. (2013), where an increased shoot dry weight, plant height and fruit yield was observed by inoculating tomato plants with P. putida and P. fluorescens. An improved root growth by bacterial inoculants facilitated plants to have better access to soil minerals such as nitrogen (N), phosphorus (P) and potassium (K). Abiotic stress is known to inhibit root systems, thus reducing plant ability to acquire nutrient resources in soil (Hashem et al., 2014). In our study, tomato plants inoculated with TSAU 13 and TSAU20 strains showed better N and P uptake compared to un-inoculated plants.
Salt stress leads to enhanced cell membrane leakage due to an increased peroxidation of membrane lipids resulting in the loss of membrane integrity (Ahmad et al., 2015). It has been demonstrated that root associated microbes may reduce the peroxidation of membrane lipids through an enhancement in free radical scavenging mechanisms, thereby strengthening the ability of membranes to withstand abiotic stress. (Alqarawi et al., 2014, Abd_Allah et al., 2015). The accumulation of compatible osmolytes in plant tissues is increased under abiotic stress by their active role in osmotic adjustment, which helps plants to survive during hostile condition (Ahanger et al., 2014). We have also observed an enhanced concentration of proline in tomato leaves inoculated with P. extremorientalis TSAU20. Similar result have been reported by Hashem et al. (2016), where Bacillus subtilis inoculation of Acacia gerrardii induced synthesis of proline in plant tissue resulting in stress adaptation through the maintenance of tissue water balance in plants. It has been reported that plant associated microbes enhance antioxidant enzyme activities as systemic resistance tools against salt stress (Abd_Allah et al., 2015, Hashem et al., 2016). We have observed an increased activity of antioxidant enzymes such as SOD, APX and CAT which help to strengthen the antioxidant defence system. Heidari et al. (2011) and Heidari and Golpayegani (2012) reported on the upregulation of antioxidant enzyme activities in Osmium basilicum by PGPR inoculants. Increased activity of SOD and CAT alleviates stress damage by the removal of H2O2 (Wu et al., 2014). The stimulation of antioxidant enzyme activities in plant tissue reduces the chances of hydroxyl (OH—) radical formation thereby facilitating normal membrane functioning. The efficient detoxifications of ROS help to maintain normal physiological processes in plants under abiotic stress (Ahmad et al., 2015).
In conclusion, our study suggests that salinity stress is not crucial for good performance in the plant growth stimulation and biocontrol abilities of PGPR strains. The salt tolerant and root associated Pseudomonas strains have a notable flexibility with respect to performing under different soil conditions. They are able to colonize root system, stimulate plant growth and increase the fruit yield of tomato under unfavourable condition. The bacterial inoculant also enhanced antioxidant enzymes activities thereby preventing ROS induced oxidative damage in plants, while also increasing proline concentrations in plant tissues, which is known to play an important role in plant stress tolerance.
Acknowledgements
This research was supported by an EU-INTAS project, Georg Forster Research Fellowship (HERMES), Alexander von Humboldt Foundation for DE. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Research group NO (RG-1435-014).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Dilfuza Egamberdieva, Email: dilfuza.egamberdieva@zalf.de.
Elsayed Fathi Abd_Allah, Email: eabdallah@ksu.edu.sa.
References
- Abd_Allah E.F., Hashem A., Alqarawi A.A., Bahkali A.H., Alwhibi M.S. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2015;22:274–283. doi: 10.1016/j.sjbs.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanger M.A., Hashem A., Abd_Allah E.F., Ahmad P. Arbuscular mycorrhiza in crop improvement under environmental stress. In: Ahmad P., Rasool S., editors. vol. 2. Academic Press; USA: 2014. pp. 69–95. (Emerging Technologies and Management of Crop Stress Tolerance). [Google Scholar]
- Ahmad P., Hashem A., Abd_Allah E.F., Alqarawi A.A., John R., Egamberdieva D., Gucel S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 2015;6:868. doi: 10.3389/fpls.2015.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., Zahir Z.A., Khalid M., Nazli F., Arshad M. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer's fields. Plant Phys. Biochem. 2013;63:170–176. doi: 10.1016/j.plaphy.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Ali S., Charles T.C., Glick B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014;80:160–167. doi: 10.1016/j.plaphy.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Almaghrabi O.A., Massoud S.I., Abdelmoneim T.S. Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J. Biol. Sci. 2013;20:57–61. doi: 10.1016/j.sjbs.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqarawi A.A., Abd_Allah E.F., Hashem A. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J. Plant Interact. 2014;9(1):802–810. [Google Scholar]
- Berg, G., Alavi, M., Schmidt, C.S., Zachow, C., Egamberdieva, D., Kamilova, F., Lugtenberg, B., 2013. Biocontrol and osmoprotection for plants under saline conditions. In: de Bruijn, Frans J. (Ed.), Molecular Microbial Ecology of the Rhizosphere. Wiley-Blackwell, USA, http://dx.doi.org/10.1002/9781118297674.ch55.
- Berg G., Martinez J.L. Friends or foes: can we make a distinction between beneficial and harmful strains of the Stenotrophomonas maltophilia complex? Front. Microbiol. 2015;6:241. doi: 10.3389/fmicb.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Chakraborty S. Migrate or evolve: options for plant pathogens under climate change. Glob. Change Biol. 2013;19(7):1985–2000. doi: 10.1111/gcb.12205. [DOI] [PubMed] [Google Scholar]
- Choudhary D.K., Prakash A., Johri B.N. Induced systemic resistance (ISR) in plants: mechanism of action. Indian J. Microbiol. 2007;47:289–297. doi: 10.1007/s12088-007-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.T., Chang H.H., Egamberdieva D., Kamilova F., Lugtenberg B., Kuo C.H. Genome analysis of Pseudomonas fluorescens PCL1751: a rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS ONE. 2015 doi: 10.1371/journal.pone.0140231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary D.K., Prakash A., Johri B.N. Induced systemic resistance (ISR) in plants: mechanism of action. Indian J. Microbiol. 2007;47:289–297. doi: 10.1007/s12088-007-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S., Clément C., Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010;42:669–678. [Google Scholar]
- Egamberdieva D., Wirth S., Jabborova D., Räsänen L.A., Berg G., Liao H. Coordination between Bradyrhizobium and root colonizing Pseudomonas alleviates salt stress in soybean (Glycine max L.) through altering root system architecture and improving nodulation. J. Plant Inter. 2017;12(1):100–107. [Google Scholar]
- Egamberdieva D., Li Li., Lindström K., Räsänen L. A synergistic interaction between salt tolerant Pseudomonas and Mezorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis Fish.) under salt stress. Appl. Microbiol. Biotechnol. 2016;100(6):2829–2841. doi: 10.1007/s00253-015-7147-3. [DOI] [PubMed] [Google Scholar]
- Egamberdieva D., Jabborova D., Hashem A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi J. Biol. 2015;22(6):773–779. doi: 10.1016/j.sjbs.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva D., Jabborova D., Berg G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, nodulation and nutrition of soybean under salt stress. Plant Soil. 2015;405:35. [Google Scholar]
- Egamberdieva D., Berg G., Lindström K., Räsänen L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat's rue) by co-inoculation of rhizobium with root colonising Pseudomonas. Plant Soil. 2013;369(1):453–465. [Google Scholar]
- Egamberdieva D., Kucharova Z., Davranov K., Berg G., Makarova N., Azarova T., Chebotar V., Tikhonovich I., Kamilova F., Validov S., Lugtenberg B. Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol. Fertil. Soils. 2011;47:197–205. [Google Scholar]
- Egamberdieva D., Kucharova Z. Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol. Fertil. Soils. 2009;45:561–573. [Google Scholar]
- Egamberdieva D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009;31:861–864. [Google Scholar]
- Egamberdiyeva D., Hoflich G. Influence of growth promoting bacteria on the growth of wheat at different soils and temperatures. Soil Biol. Biochem. 2003;35:973–978. [Google Scholar]
- Faisal M. Inoculation of plant growth promoting bacteria Ochrobactrum intermedium, Brevibacterium sp. and Bacillus cereus induce plant growth parameters. J. Appl. Biotech. 2013;1:45–53. [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A., Al-Huqail A.A., Wirth S., Egamberdieva D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Plant Sci. 2016;2016(7):1089. doi: 10.3389/fmicb.2016.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Aldebasi A., Egamberdieva D. Arbuscular mycorrhizal fungi enhance salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Inter. 2015;10:230–242. [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Al Huqail A.A., Egamberdieva D. Alleviation of abiotic salt stress in Ochradenus baccatus (Del.) by Trichoderma hamatum (Bonord.) Bainier. J. Plant Inter. 2014;9:857–868. [Google Scholar]
- Heidari M., Mousavinik S.M., Golpayegani A. Plant growth promoting rhizobacteria (PGPR) effect on physiological parameters and mineral uptake in basil (Ociumum basilicum L.) under water stress. J. Agri. Biol. Sci. 2011;6:6–11. [Google Scholar]
- Heidari M., Golpayegani A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.) J. Saudi Soc. Agri. Sci. 2012;11:57–61. [Google Scholar]
- Jha Y., Subramanian R.B., Patel S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 2011;33:797–802. [Google Scholar]
- Landa B.B., Navas-Cortés J.A., Jiménez-Díaza R.M. Influence of temperature on plant–rhizobacteria interactions related to biocontrol potential for suppression of Fusarium wilt of chickpea. Plant Pathol. 2004;53:341–352. [Google Scholar]
- Landa B.B., Navas-Cortés J.A., Hervás A., Jiménez-Díaz R.M. Influence of temperature and inoculum density of Fusarium oxysporum f. sp. ciceris on suppression of Fusarium wilt of chickpea by rhizosphere bacteria. Phytopathology. 2001;91:807–816. doi: 10.1094/PHYTO.2001.91.8.807. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Kamilova F. Plant-growth-promoting rhizobacteria. Ann. Rev. Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B.J.J., Dekkers L., Bloemberg G.V. Molecular determinants of rhizosphere colonization by Pseudomanas. Annu. Rev. Phytopath. 2001;39:461–490. doi: 10.1146/annurev.phyto.39.1.461. [DOI] [PubMed] [Google Scholar]
- Luck, H., 1974. Methods in Enzymatic Analysis II (ed.) Bergmeyer. (Publ.) Academic Press, New York. 885.
- Meena V.S., Maurya B.R., Verma J.P., Aeron A., Kumar A., Kim K., Bajpai V.K. Potassium solubilizing rhizobacteria (KSR): isolation, identification, and K-release dynamics from waste mica. Ecol. Eng. 2015;81:340–347. [Google Scholar]
- Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Park J.W., Balaraju K., Kim J.W., Lee S.W., Park K. Systemic resistance and growth promotion of chili pepper induced by an antibiotic producing Bacillus vallismortis strain BS07. Biol. Control. 2013;65:246–257. [Google Scholar]
- Parray A.P., Jan S., Kamili A.N., Qadri R.A., Egamberdieva D., Ahmad P. Current perspectives on plant growth promoting rhizobacteria. Plant Growth Regul. 2016;35(3):877–902. [Google Scholar]
- Paul D., Nair S. Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J. Basic Microbiol. 2008;48(5):378–384. doi: 10.1002/jobm.200700365. [DOI] [PubMed] [Google Scholar]
- Rekha P.D., Lai W.A., Arun A.B., Young C.C. Effect of free and encapsulated Pseudomonas putida CC-FR2-4 and Bacillus subtilis CC-pg104on plant growth under gnotobiotic condition. Bio. Res. Technol. 2007;98:447–451. doi: 10.1016/j.biortech.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Remans R., Ramaekers L., Schelkens S., Hernandez G., Garcia A., Reyes J.L., Mendez N., Toscano V., Mulling M., Galvez L., Vanderleyden J. Effect of Rhizobium-Azospirillum coinoculation on nitrogen fixation and yield of two contrasting Phaseolus vulgaris L. genotypes cultivated across different environments in Cuba. Plant Soil. 2008;312:25–37. [Google Scholar]
- Sanogo S. Response of chile pepper to Phytophthora capsici in relation to soil salinity. Plant Dis. 2004;88:205–209. doi: 10.1094/PDIS.2004.88.2.205. [DOI] [PubMed] [Google Scholar]
- Sato K., Jiang J.Y. Gram-negative bacterial flora on the root surface of wheat (Triticum aestivum) grown under different soil conditions. Biol. Fert. Soils. 1996;23:273–281. [Google Scholar]
- Rangarajan S., Saleena L.M., Vasudevan P., Nair S. Biological suppression of rice diseases by Pseudomonas spp. under saline soil conditions. Plant Soil. 2003;251(1):73–82. [Google Scholar]
- Susilowati A., Wahyudi A.T., Lestari Y., Wiyono S., Suwanto A. Genetic diversity of antifungi-producing rhizobacteria of Pseudomonas sp. isolated from rhizosphere of soybean plant. Microbiol. Indones. 2010;4:33–38. [Google Scholar]
- Swieckil T.J., MacDonald J.D. Soil salinity enhances phytophthora root rot of tomato but hinders asexual reproduction by Phytophthora parasitic. J. Amer. Soc. Hort. Sci. 1991;116(3):471–477. http://journal.ashspublications.org/content/116/3/471.full.pdf+html [Google Scholar]
- Triky-Dotan S., Yermiyahu U., Katan J., Gamliel A. Development of crown and root rot disease of tomato under irrigation with saline water. Phytopathology. 2005;95:1438–1444. doi: 10.1094/PHYTO-95-1438. [DOI] [PubMed] [Google Scholar]
- Van Rossum M.W.P.C., Alberda M., Van der Plas L.H.W. Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci. 1997;130:207–216. [Google Scholar]
- Vardharajula S., Ali S.Z., Grover M., Reddy G., Bandi V. Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Inter. 2011;6:1–14. [Google Scholar]
- Vasebi Y., Safaie N., Alizadeh A. Biological control of soybean charcoal root rot disease using bacterial and fungal antagonists in vitro and greenhouse condition. J. Crop Prot. 2013;2:139–150. [Google Scholar]
- Wang C.J., Yang W., Wang C., Gu C., Niu D.D., Liu H.X., Wang Y.P., Guo J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE. 2012;7:e52565. doi: 10.1371/journal.pone.0052565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Q.S., Z. Ying-Ning and E.F. Abd-Allah. 2014. Mycorrhizal Association and ROS in Plants. In: Ahmad, P. (Ed.), Oxidative Damage to Plants. http://dx.doi.org/10.1016/B978-0-12-799963-0.00015.
- Yadav J., Verma J.P., Jaiswal D.K., Kumar A. Evaluation of PGPR and different concentra-tion of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa) Ecol. Eng. 2014;62:123–128. [Google Scholar]
- Yao L., Wu Z., Zheng Y., Kaleem I., Li C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010;46:49–54. [Google Scholar]
- Zeng Q.Y., Wang X.R., Blomquist G. Development of mitochondrial ssu rDNA-based oligonucleotide probes for specific detection of common airborne fungi. Mol. Cell. Probes. 2003;17:281–288. doi: 10.1016/s0890-8508(03)00067-7. [DOI] [PubMed] [Google Scholar]