Abstract
Background
The Flo2 gene is a member of a conserved gene family in plants. This gene has been found to be related to thousand grain weight (TGW) in rice. Its orthologs in hexaploid wheat were cloned, and the haplotype variation in TaFlo2-A1 was tested for association with TGW.
Results
The cloned sequences of TaFlo2-A1, TaFlo2-B1 and TaFlo2-D1 contained 23, 23 and 24 exons, respectively. The deduced proteins of TaFlo2-A1 (1734 aa), TaFlo2-B1 (1698 aa) and TaFlo2-D1 (1682 aa) were highly similar (>94%) and exhibited >77% similarity with the rice FLO2 protein. Like the rice FLO2 protein, four tetratricopeptide repeat (TPR) motifs were observed in the deduced TaFLO2 protein. An 8-bp InDel (−10 to −17 bp) in the promoter region and five SNPs in first intron of TaFlo2-A1 together formed two haplotypes, TaFlo2-A1a and TaFlo2-A1b, in bread wheat. TaFlo2 was located on homeologous group 2 chromosomes. TaFlo2-A1 was inferred to be located on deletion bin ‘2AL1–0.85-1.00’. The TaFlo2-A1 haplotypes were characterized in the Chinese Micro Core Collection (MCC) and Pakistani wheat collection using the molecular marker TaFlo2-Indel8. TaFlo2-A1 was found to be associated with TGW but not with grain number per spike (GpS) in both the MCC and Pakistani wheat collections. The frequency of TaFlo2-A1b (positive haplotype) was low in commercial wheat cultivars; thus this haplotype can be selected to improve grain weight without negatively affecting GpS. The expression level of TaFlo2-A1 in developing grains at 5 DAF (days after flowering) was positively correlated with TGW in cultivars carrying the positive haplotype.
Conclusion
This study will likely lead to additional investigations to understand the regulatory mechanism of the Flo2 gene in hexaploid wheat. Furthermore, the newly developed molecular marker ‘TaFlo2-InDel8’ could be incorporated into the kit of wheat breeders for use in marker-assisted selection.
Electronic supplementary material
The online version of this article (10.1186/s12870-017-1114-3) contains supplementary material, which is available to authorized users.
Keywords: Floury endosperm, Haplotype variation, Gene cloning, TGW, Triticum aestivum
Background
Enhancing the grain yield potential of wheat is a key focus of wheat breeders. Grain yield is the product of various yield components. Wheat grain yield per unit area is the product of grain yield per spike (GYS) and the number of spikes per unit area. The latter depends on sowing density and is highly affected by environmental factors. The GYS is determined by grain number per spike (GpS) and thousand grain weight (TGW), which are variably correlated in different wheat collections/populations. A significant negative correlation between these two traits has been reported in bi-parental populations [1–3], but no significant correlation was observed between TGW and GpS in collections of Chinese landraces [4], French winter wheat cultivars [5] and CIMMYT-derived spring wheat collections [6]. On the other hand, a significant positive correlation between TGW and GpS was reported in modern Chinese cultivars [4].
TGW in wheat has been one of the target traits for selection during domestication and breeding [7, 8]. For example, in China, an increase in wheat yield potential from ~1 T ha−1 in 1992 to ~5.4 T ha−1 today is mainly due to the genetic increase in TGW from ~20 g to ~45 g, respectively [9]. However, genetic gain in TGW has not reached its limit and thus provides an opportunity to increase yield potential [9]. It is estimated that an increase in yield of 140–160 kg ha−1 can be obtained by just a 1-g increase in TGW [10]. However, genes and their roles in controlling TGW in wheat are still largely unknown. In wheat, TGW is a quantitative trait controlled by several genes/QTL distributed on all chromosomes [8, 11]. For example, Su et al. [12] discovered eight TGW-related QTL on chromosomes 2D, 4B, 5A, 7A and 7B, explaining up to 16.2% of the phenotypic variation. Similarly, four QTL for TGW on chromosomes 1D, 2A, 5D, and 6A explained 5.9 to 20.1% of phenotypic variation in different environments [13].
Nevertheless, from this plethora of QTLs, few loci/genes have been cloned by map-based cloning approaches mainly because of the large and complex hexaploid genome (~17 Gb) that consists of three homeologous genomes (A, B, D) and an abundance of repeat sequences (80%) [14]. Studies on comparative genomics have shown high synteny and collinearity among different grass genomes, such as those of wheat, barley, rice, millet, maize and sorghum. This pattern of genome organization in the members of the grass family provides a powerful approach for gene discovery in common wheat [15]. A large number of genes have been discovered in common wheat by synteny-based cloning, in which the gene sequences of model crops such as rice and barley have been used as references to identify orthologous genes in wheat. For example, the genes TaTGW6 [16, 17], TaCwi-A1 [18], TaSus2-2B [19], TaSus2-2A, TaSus1-7A [20], TaGW2-6A, 6B [9, 12, 21], TaCKX6-D1 [22], TaSAP1-A1 [23], TaGS1a [24], TaGS-D1 [25], and TaGASR-A1 [26] were discovered using rice-wheat synteny and using molecular markers in marker-assisted wheat breeding. Hence, the isolation and characterization of genes controlling grain size in common wheat will help breeders maximize yield potential by establishing gene-based breeding programs.
The FLOURY ENDOSPERM2 (Flo2) gene is a member of a conserved gene family in plants. In rice, this gene has been shown to have a tetratricopeptide repeat (TPR) motif consisting of 3–16 tandem repeats of 34 aa residues that mediate protein–protein interactions in the nucleus [27, 28]. The OsFlo2 gene was cloned in the indica cultivar ‘Kasalath’; this gene was found to have 23 exons and 22 introns and coded for a protein consisting of 1720 amino acid residues that had three TPR motifs in the middle [27]. The expression of Flo2 was constitutive in both vegetative tissues and developing seeds, and the expression was relatively high level in developing seeds. The flo2 mutants exhibit a significant reduction in amylose content and grain weight and exhibit altered expression of various starch synthesis-related genes, indicating its key role in regulating rice grain weight and starch quality [27, 28]. In this article, we report the rice-wheat synteny-based isolation of Flo2 orthologs in hexaploid wheat, the association of TaFlo2-A1 sequence polymorphisms with TGW and the comparison of temporal expression profiles of TaFlo2-A1 haplotypes in flag leaves and developing caryopses.
Methods
Plant materials
For cloning the TaFlo2 gene in hexaploid wheat, Chinese Spring (CS) and two sets of cultivars with lower and higher TGW were used; the set of cultivars with higher TGW included Dixiuzao (49.5 g), Enmai4 (49.2 g), Liying 5(49.4 g) and Laizhou 953 (52.2 g), and the set with lower TGW included Jinyang 60 (23.5 g), Baihuamai (24.1 g), Sanyuehuang (25.2 g) and Zipi (25.5 g). The Chinese Spring nulli-tetrasomic lines were used to assign TaFlo2 genes to wheat homeologous chromosomes. The Chinese Micro Core Collection (MCC, 262 accessions) and Pakistani wheat collection (130 accessions) were used to confirm the association between TaFlo2-A1 haplotypes and TGW. To avoid the effect of population structure, normalized MCC subpopulations were used for association analysis [19, 29]. The Pakistani collection was selected based on previous reports [30, 31] considering the effect of population structure on association analyses.
Cloning and characterization of TaFlo2 sequences
The genomic sequence of the rice OsFlo2 gene (NC_008397) was used as a query for BLAST searches against the wheat sequences database in the URGI (https://urgi.versailles.inra.fr/). All wheat scaffold sequences with high similarity to the rice OsFlo2 sequence were assembled to construct a putative TaFlo2 gene using DNAMAN (http://www.lynnon.com). Based on the scaffold sequences, six conserved primer pairs were used to specifically amplify TaFlo2 coding and promoter sequences from the three wheat sub-genomes: A, B and D (Table 1). The TaFlo2 mRNA of 4902 bp was cloned in Chinese Spring using three primer pairs designed from the predicted mRNA sequence (Table 1). Genomic DNA was extracted from young seedlings of each line using the CTAB method [32]. A 20-μl reaction volume comprising 0.5 μl (5 μM) of each primer, 2× Taq mix (GenStar, Beijing, China) and 100 ng of DNA was used for PCR amplification that consisted of a cycle profile of 5 min at 94 °C; 35 cycles of 30 s at 94 °C, 30 s at 60 °C and 4 min at 72 °C; and a final extension of 10 min at 72 °C. The PCR products were detected by electrophoresis in 1% agarose gels with nucleic acid dye (Tiangen, Beijing, China), and gel images were captured using a UV spectrometer (BioRad, Hercules, CA, USA). The targeted PCR products were obtained from the agarose gels and purified using the TIANgel MIDI Purification Kit (Tiangen, Beijing, China). The purified PCR products were then ligated into the pGEM-T Easy cloning vector (TransGen Biotech, Beijing, China). The ligation product was transformed to 50 μl of Trans1-T1 competent cells by the heat shock method (Tiangen, Beijing, China). Positive clones from each transformation were selected based on positive PCR tests and were sequenced (Beijing Genomics Institute). The sequences were analyzed using DNAMAN software (http://www.lynnon.com).
Table 1.
Primer sequences used in this study
| Primer name | Primer sequence (5′-3′) | Position on scaffold sequence | Annealing temperature (°C) | PCR product size | Function |
|---|---|---|---|---|---|
| Flo2-1F | TGTGCTGGAATCACCCACTC | 793–812 | 60 | 1061 | cloning TaFlo2 /polymorphism detection |
| Flo2-1R | GCGCGGCGAAAACTAATCAT | 1853–1844 | |||
| Flo2-2F | GTGCCGTCCATAATCGTTGC | 1546–1565 | 60 | 1781 | cloning TaFlo2 /polymorphism detection |
| Flo2-2R | CATGTGCGGCAAAAGACACA | 3326–3307 | |||
| Flo2-3F | AACGGGCATGTGTCTTTTGC | 3299–3318 | 60 | 3025 | cloning TaFlo2 /polymorphism detection |
| Flo2-3R | CGACGCAGCTCTGAAAATCG | 6332–6313 | |||
| Flo2-4F | CGCTTAGCAGTGGATTTGCC | 5719–5738 | 60 | 3948 | cloning TaFlo2 |
| Flo2-4R | ATCCAACAAACAGGTGCCCA | 9667–9647 | |||
| Flo2-5F | TTGCGGAAGCCCATCATTCT | 8387–8406 | 60 | 3836 | cloning TaFlo2 |
| Flo2-5R | TGACCTTCTGCGGATGCTTT | 1222–12,203 | |||
| Flo2-6F | CAGAACAGGGCCGGTACAAT | 11,368–11,387 | 60 | 2600 | cloning TaFlo2 |
| Flo2-6R | CGCTCATCTGGATAGGGCAA | 13,967–13,948 | |||
| TaFlo2-InDel8F | ACCCCTCCTCCGTTATCGTC | 1337–1356 | 60 | 145/153 | 8-bp InDel polymorphism in TaFlo2-A1 |
| TaFlo2-InDel8R | CCTCCTTCTTCTTGCGGTCG | 1470–1489 | |||
| Flo2-A1F | GTGCTCCGATCCGATGTGCAGTTAT | 5387–5411 | 58 | 587 | 2A specific |
| Flo2-A1R | GTGCACAACCAAGTAAAAGG | 5973–5954 | |||
| Flo2-B1F | GTC ATC ACTAGAGGA ATTTTCC | 6851–6872 | 58 | 902 | 2B specific |
| Flo2-B1R | CTCTCAGAACTGTGGAT | 7752–7736 | |||
| Flo2-D1F | CTGTATCTGTAATTTGTTCCG | 5378–5398 | 58 | 326 | 2D specific |
| Flo2-D1R | CTTCCGAAAAATGTGGGG | 5704–5687 | |||
| mFlo2-1F | TAACGGTGGTGCACTTGTGT | – | 58 | 1868 | Cloning mRNA |
| mFlo2-1R | TCAGCCGCAAGTTATGCTCA | – | |||
| mFlo2-2F | TGCGGACGAGATGGAAAACA | – | 58 | 1809 | Cloning mRNA |
| mFlo2-2R | AGCAGTCAGCCGATGGTATG | – | |||
| mFlo2-3F | ATGCGTACTCCCTAAGCGTG | – | 58 | 1889 | Cloning mRNA |
| mFlo2-3R | CACGAAGTGCTGCTTGCTTT | – | |||
| eTaFlo2F | CCATTCGGCTTTCGTGCAAA | – | 55 | 134 | Expression analysis |
| eTaFlo2R | TGTTTTCCATCTCGTCCGCA | – | |||
| ActinF | AGCCATACTGTGCCAATC | – | 55 | 134 | Internal control |
| ActinR | GCAGTGGTGGTGAAGGAGTAA | – |
Characterization of TaFlo2-A1 haplotypes and development of haplotype-specific markers
The 262 MCC and 130 Pakistani varieties were genotyped with the primer pair TaFlo2-InDel8, and PCR product was run on 8% polyacrylamide gels. Based on TaFlo2-InDel8 scoring, the MCC and Pakistani accessions sorted into two groups according to their haplotypes (TaFlo2-A1a or TaFlo2-A1b) for the TaFlo2-A1 gene. For MCC, the average values of TGW of the two haplotype groups were calculated using replicated data collected from 3 years (2002, 2005, 2006) of plants in Beijing [19]. For Pakistani varieties, the average values of TGW of the two haplotype groups were calculated using replicated data from 2 years (2009, 2010) of field trials at the University of Agriculture, Faisalabad. The resulting values were then compared and statistically analyzed using SPSS 13.0 for Windows (IBM, New York, USA).
Quantitative RT-PCR analysis of TaFlo2-A1 haplotypes
The Yangmai 19, Chinese Spring, Pubing3228, Shannong23 and Zhengmai9405 varieties were sown at the experimental station of the Institute of Genetics & Developmental Biology, CAS in Beijing, China in October 2014; three rows of each variety were planted. The length of each row was 2 m, and the row-to-row distance was 20 cm. The plants were managed in accordance with standard agronomic practices; irrigation and fertilizer were supplied for optimal growth. Twelve- day-old flag leaves of five plants from each variety were harvested and stored at −80 °C. Unfertilized grains were collected from each variety 1–2 days before flowering (DBF). Fertilized grains were collected from each variety at 5, 10, 15, 20 and 25 days after flowering (DAF). The flag leaf and developing grain samples were processed for the preparation of total RNA as described previously [33]. Three biological replicates that were collected from different plants were analyzed separately for each variety for quantitative RT-PCR evaluation. For TaFlo2-A1 transcripts analysis, the primer set eTaFlo2, which is specific for TaFlo2-A1 (Table 1), was designed and used. Quantitative RT-PCR was then carried out as described by Feng et al. [34]. The wheat actin gene was used as an internal control. The relative expression level of TaFlo2-A1 in each flag leaf and in each sample of developing grains was calculated using the data of three technical replicates as described previously [35]. Statistical comparisons of TaFlo2-A1 expression levels (presented as the mean ± SD) among different samples were made by ANOVA using SPSS 13.0.
Bioinformatics comparison of nucleotide and protein sequences
Nucleotide and protein identities among the compared sequences were calculated using DNAMAN (http://www.lynnon.com). Amino acid sequence alignment was accomplished using ClustalW2 in EMBLEBI (www.ebi.ac.uk/Tools/msa/clustalw2). Potential signal peptide sequences in the deduced proteins of TaFLO2 and its homologs were predicted using Softberry software (http://www.softberry.com/berry.phtml). The predicted TaFLO2 protein was BLASTed both in the NCBI smart blast system (http://blast.st-va.ncbi.nlm.nih.gov/smartblast) to search for homologous proteins and in the NCBI CD system (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) to search for conserved domains.
Results
Cloning and characterization of TaFlo2 genes
To select potential candidate TaFlo2 genes, the rice Flo2 sequence (NC_008397) was used as a query against the wheat genome sequences database in the URGI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Three bread wheat scaffolds (IWGSC_chr2AL_ab_k71_contigs_longerthan_200_6436403, IWGSC_chr2BL_ab_k71_contigs_longerthan_200_7959819, IWGSC_chr2DL_ab_k71_contigs_longerthan_200_9909583) with high similarity (E value = 0 and similarity >73%) were identified as potential orthologs to the rice Flo2 gene. The sequences of these scaffolds were downloaded and assembled with DNASTAR (http://www.dnastar.com/) to construct a putative TaFlo2 sequence.
To search TaFlo2 homologs and predict their deduced protein sequence and structure, Softberry (http://www.softberry.com/berry.phtml) and NCBI (https://www.ncbi.nlm.nih.gov/) tools were used. The deduced proteins of TaFlo2-A1 (1734 aa), TaFlo2-B1 (1698 aa) and TaFlo2-D1 (1682 aa) were highly similar (>94% identity among themselves) and exhibited >77% similarity with the rice FLO2 protein. Like in the rice FLO2 protein [27], four tetratricopeptide repeat (TPR) motifs were observed in the deduced TaFLO2 protein at the positions of 947–988, 1032–1072, 944–1017 and 1028–1106 amino acid residues. Furthermore, three mitochondrial CLU domains were also observed at 737–878, 50–162 and 357–401 amino acid residues (Fig. 1a). The TaFLO2 protein showed high similarity with Aegilops tauschii, Brachypodium distachyon and long-grain rice proteins (Fig. 1b).
Fig. 1.
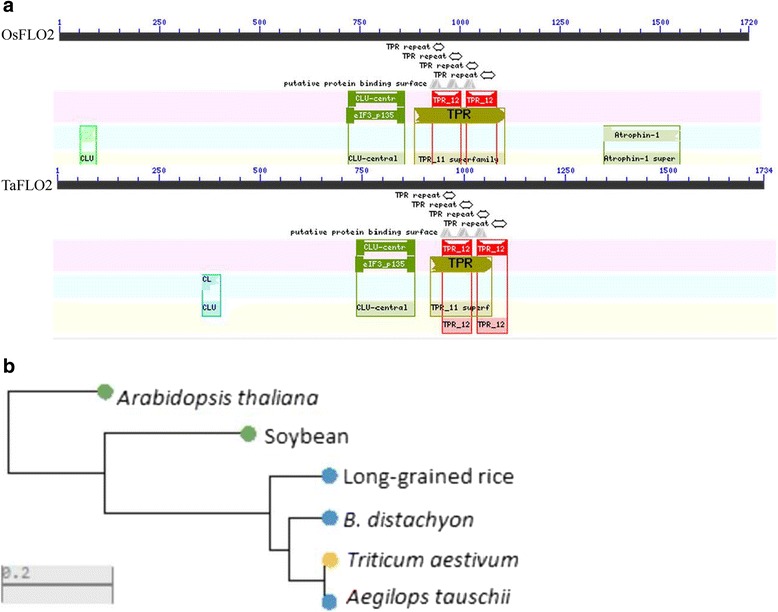
a Putative structure of the OsFLO2 and TaFLO2 proteins. Clu_N (mitochondrial function, CLU-N-term), CL (clustered mitochondria domain), CLU-center (an uncharacterized central domain of CLU mitochondrial proteins), TPR (tetratricopeptide repeat). b Similarity between the TaFLO2 protein and proteins from related plant species
To clone the full-length genomic sequence of TaFlo2 in Chinese Spring, six conserved primer pairs were used (Table 1). The assembly of sequences with the six conserved primer pairs yielded three fragments, 14,009, 14,078 and 13,814 bp. Based on alignment with wheat scaffolds in the database and genome-specific primers, the fragments were designated TaFlo2-A1, TaFlo2-B1 and TaFlo2-D1. The open reading frames of TaFlo2-A1, TaFlo2-B1 and TaFlo2-D1 were 12,183 bp, 12,270 bp and 12,022 bp in length, respectively. The TaFlo2 mRNA of 4902 bp was cloned with three primer pairs (Table 1). Based on the prediction and alignment with cloned mRNA, the cloned genomic sequences from 2AL, 2BL and 2DL consisted of 23, 23 and 24 exons, respectively (Fig. 2a). Among the three homoeologs, the sequence and size of the first four exons were conserved, whereas the size and sequence of the other exons varied.
Fig. 2.
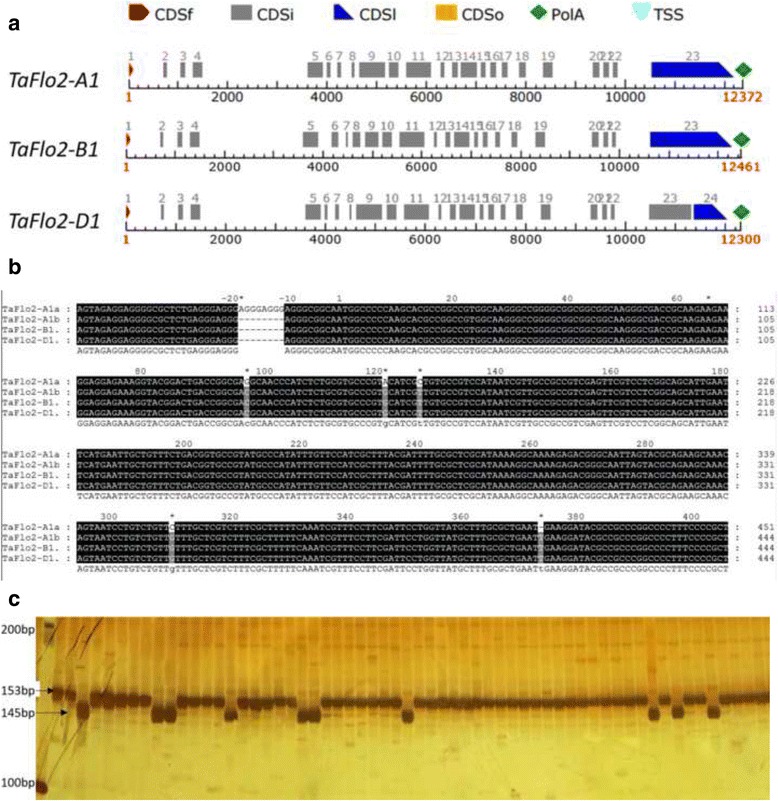
Polymorphism, molecular marker and ORF structure of TaFlo2 homoeologs. a Exon and intron pattern in the ORFs of TaFlo2-A1, TaFlo2-B1 and TaFlo2-D1. The length of each ORF (between the start and stop codons) is shown to the right of the graph. The number of nucleotides in each exon or intron is indicated. CDSf: First coding sequence, CDSi: Internal coding sequence, CDSl: Last coding sequence. b Alignment of the part of cloned TaFlo2 orthologs. Polymorphism both in the promoter and first intron is indicated by stars. c PCR product of molecular marker TaFlo2-InDel8 discriminated by PAGE in 60 MCC accessions
Polymorphism detection in TaFlo2-A1
To detect polymorphisms in the putative TaFlo2 sequences between high and low TGW accessions, three conserved primers that covered scaffold segments from 793 to 6332 bp were used (Table 1). Polymorphism in TaFlo2-A1 sequences between high and low TGW accessions was observed between 1396 to 1791 bp, while no sequence variation was observed between high and low TGW accessions in TaFlo2-B1 and TaFlo2-D1 (Fig. 2b; Additional file 1: Figure S1). The conserved sequences of TaFlo2-B1 and TaFlo2-D1 in all the higher and lower TGW accessions implicated non-functional nature of these genes. An 8-bp InDel was identified in TaFlo2-A1 sequences from 1396 to 1403 bp which was −17 to −10 bp upstream of the first coding sequence (ATG) at position 1417–1419 bp (Fig. 2b). Five SNPs (G/C, A/G, C/T, C/G and −/T) were observed at 1514, 1538, 1545, 1727 and 1791 bp. From the start codon (ATG), the positions of these five SNPs (G/C, A/G, C/T, C/G and −/T) were in the first intron at 98, 122, 128, 311 and 375 bp, respectively (Fig. 2b). The 8-bp InDel and the five SNPs together formed the two haplotypes designated TaFlo2-A1a and TaFlo2-A1b (Fig. 2b). From the position 1792 to 6332 bp, no polymorphism was observed in the TaFlo2-A1 sequence.
Molecular marker development and characterization of TaFlo2-A1 haplotypes
To characterize the observed TaFlo2-A1 haplotypes in large wheat populations, a molecular marker based on the 8-bp InDel observed in the promoter region was designed and named TaFlo2-Indel8 (Table 1). The forward and reverse primers of TaFlo2-Indel8 are located at −80 bp and 72 bp from the start codon, respectively. The PCR products of TaFlo2-Indel8 in the accessions with or without the 8-bp InDel have lengths of 153 bp and 145 bp, respectively. The bands of 153 bp and 145 bp were easily discriminated by polyacrylamide gel electrophoresis and represented the haplotypes TaFlo2-A1a and TaFlo2-A1b, respectively (Fig. 2c).
Chromosomal location of TaFlo2 genes
To assign chromosomal locations to TaFlo2 genes, genome-specific primers and a set of Chinese Spring nulli-tetrasomic lines were used. The TaFlo2 genes TaFlo2-A1, TaFlo2-B1 and TaFlo2-D1 were found to be located on chromosomes 2A, 2B and 2D (Fig. 3). The cloned sequences of TaFlo2-A1, TaFlo2-B1 and TaFlo2-D1 showed >99% similarity with 2AL, 2BL and 2DL scaffolds (IWGSC_chr2AL_ab_k71_contigs_longerthan_200_6436403, IWGSC_chr2BL_ab_k71_contigs_longerthan_200_7959819, IWGSC_chr2DL_ab_k71_contigs_longerthan_200_9909583). Further analysis revealed that the TaFlo2-A1 gene was located on deletion bin ‘2AL1–0.85-1.00’.
Fig. 3.
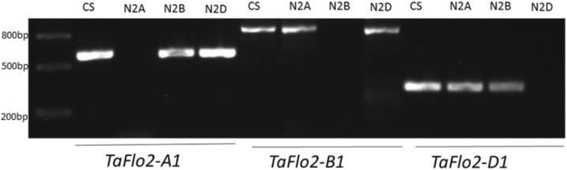
Assignment of TaFlo2-A1, TaFlo2-B1 and TaFlo2-D1 to wheat chromosomes 2A, 2B and 2D, respectively, by PCR mapping with the genomic DNA of Chinese Spring (CS) and derivative nulli-tetrasomic lines (N2AT2B, N2BT2A and N2DT2A). The size (kb) of DNA markers is shown to the left of the image
Association of TaFlo2-A1 with thousand grain weight
To associate TaFlo2-A1 with TGW, two natural populations, the Chinese Micro Core Collection (MCC) and the Pakistani collection, were used. In the MCC, the homozygous TaFlo2-A1a haplotype was found in 219 (83.5%) accessions, whereas the TaFlo2-A1b haplotype was found in 43 (16.5%) accessions. In the Pakistani wheat collection, the number of accessions carrying TaFlo2-A1a and TaFlo2-A1b were 85 (64.6%) and 45 (35.4%), respectively. Both in the MCC and Pakistani collections, the positive haplotype TaFlo2A-A1b had a lower frequency, which showed the scope of improving grain weight.
The difference in TGW between the haplotypes TaFlo2-A1a and TaFlo2-A1b was statistically significant in both populations (P < 0.05, Table 2). In the MCC, the mean difference in TGW between the groups of accessions having TaFlo2-A1a and TaFlo2-A1b was significant (P ≤ 0.05) across the 3 years of data. The mean differences in TGW between the two haplotypes in 2002, 2005 and 2006 were 7.00 ± 1.12 g, 7.80 ± 1.11 g and 8.40 ± 0.94 g, respectively. Consistent with the results of the MCC, the mean difference in TGW between groups of accessions having TaFlo2-A1a and TaFlo2-A1b was also significant (P ≤ 0.05) across both years of data in the Pakistani wheat collection. The values of the mean difference between the two haplotypes (TaFlo2-A1a and TaFlo2-A1b) in the Pakistani wheat population were 4.50 ± 0.71 g and 5.20 ± 0.72 g for 2009 and 2010, respectively. The phenotypic variance for TGW explained by TaFlo2-A1 haplotypes was 6.19% in 2002, 7.76% in 2005 and 8.37% in 2006 in the MCC. In the Pakistani collection, the phenotypic variance for TGW explained by TaFlo2-A1 haplotypes was 4.42% in 2009 and 5.11% in 2010 (Table 2). Moreover, to determine whether TaFlo2-A1 also affects grain number per spike (GpS), an association analysis was performed for GpS in both populations. However, the differences in GpS between the haplotypes TaFlo2-A1a and TaFlo2-A1b were not significant in either population (P < 0.05, Table 2).
Table 2.
Association of TGW and GpS with TaFlo2-A1 in the Chinese Micro Core Collection and Pakistani wheat collections
| Natural populations | Year (number of accessions) |
TaFlo2-A1a
Mean ± SEa (number of accessions) |
TaFlo2-A1b
Mean ± SE (number of accessions) |
Mean difference ± SE | PVE (%)b TGW | |||
|---|---|---|---|---|---|---|---|---|
| TGW | GpS | TGW | GpS | TGW | GpS | |||
| Chinese Micro Core Collection | 2002 (137) | 33.6 ± 0.54(98) | 50.8 ± 1.2(98) | 40.6 ± 1.2(39) | 49.7 ± 1.5(39) | 7.0 ± 1.1** | 1.06 ± 2.1ns | 6.19 |
| 2005 (169) | 30.7 ± 0.52(128) | 43.1 ± 0.8(128) | 38.5 ± 1.1(41) | 40.5 ± 1.1(41) | 7.8 ± 1.1** | 2.6 ± 1.5 ns | 7.76 | |
| 2006 (185) | 32.8 ± 0.43(141) | 51.4 ± 0.7(141) | 41.2 ± 0.9(44) | 48.6 ± 1.2(43) | 8.4 ± 0.9** | 2.7 ± 1.5 ns | 8.37 | |
| Pakistani collection | 2009 (130) | 40.6 ± 0.43(85) | 46.6 ± 1.1(85) | 45.1 ± 0.55(45) | 44.4–1.8(45) | 4.5 + 0.71** | 2.2 ± 1.9 ns | 4.42 |
| 2010 (130) | 40.5 ± 0.47(85) | 47.8 ± 1.2(85) | 45.7 ± 0.46(45) | 44.9 + 1.6(45) | 5.2 ± 0.72** | 2.9 ± 2.0 ns | 5.11 | |
**indicates significant differences, and ns indicates non-significant differences (P < 0.01; Student’s t-test) among groups carrying different haplotypes
aStandard error
bPercentage of phenotypic variance explained by association analysis
Collectively, our data demonstrated that TaFlo2-A1, like the OsFlo2 gene in rice, is associated with TGW in wheat. Furthermore, the lack of association of TaFlo2-A1 with GpS suggests that the high TGW of the examined genotypes is primarily due to the positive haplotype (TaFlo2-A1b) for high TGW instead of loci for low number of kernels per spike and/or low grain yield.
Expression of TaFlo2-A1 is positively related to TGW
To observe the contrasting effects of TaFlo2-A1a and TaFlo2-A1b on TGW at the gene expression level in flag leaves and developing grains, two polymorphic accessions were used. The expression level of TaFLO2 was positively correlated with TGW, which is consistent with previous results in rice [27]. The haplotype TaFlo2-A1a, which exhibits low expression levels, represented the group of accessions that have low average TGW, and the haplotype TaFlo2-A1b, which exhibits high expression levels, represented the group of accessions that have high average TGW in both Chinese and Pakistani wheat populations. Quantitative RT-PCR assays showed that for both types of haplotypes, the expression level was maximum in 12-day-old flag leaves followed by expression in developing grains sampled at 5 DAF. However, the expression of both types of haplotypes decreased rapidly in the fertilized caryopses collected at 10, 15, 20 and 25 DAF. The expression level of TaFlo2-A1b was higher than that of TaFlo2-A1a at all tested stages but significantly differed only in flag leaves and developing grains at 5 DAF (Fig. 4a). Furthermore, the expression level was positively correlated in Chinese Spring and three cultivars (Pubing3228, Shannong23, and Zhengmai9405) in developing grains sampled at 5 DAF. The expression level was lowest in Chinese Spring (TGW, 21.3 g) and highest in the cultivar Zhengmai9405 (TGW, 64.1 g) (Fig. 4b). All these cultivars contained the positive haplotype TaFlo2-A1b. Together, these results suggested that the relative expression level of TaFlo2-A1 was highest in flag leaves but started to decrease in developing grains. However, the expression in developing grains at 5 DAF was positively correlated with TGW in cultivars carrying the positive haplotype.
Fig. 4.
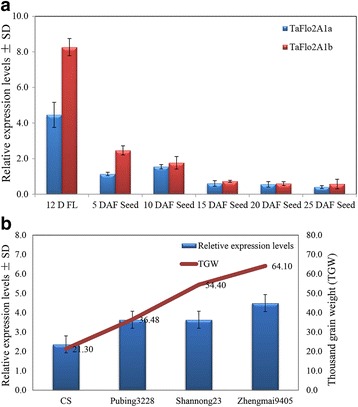
a Expression of TaFlo2-A1 in flag leaves and developing grains. b Expression level of TaFlo2-A1b in cultivars with different TGW values at 5 DAF
Discussion
Rice-wheat synteny-based gene cloning in wheat
The rice OSFLO2 orthologs TaFLO2-A1, TaFLO2-B1 and TaFLO2-D1 were cloned, characterized and found to be located on homeologous chromosome group 2 in wheat. Sequence polymorphism observed in the promoter region of TaFlo2-A1 was associated with TGW. Thus, TaFLO2-A1 is a yield-related gene, and its manipulation could be useful for improving the grain yield potential of bread wheat. Many genes related to TGW and grain yield have been isolated and characterized in wheat using rice-wheat synteny [15]. The success of rice-wheat orthology-based gene cloning in wheat is due to high nucleotide and amino acid similarity between the corresponding orthologous genes. For example, with their respective rice orthologs, TaTGW6 has 71% nucleotide and 68% amino acid similarity [16, 17]; TaGW2 has 98% nucleotide and ~ 87% amino acid similarity [12]; TaCKX6-D1 has 66% amino acid similarity [22]; TaGS-D1 has 75.5% cDNA and 72.2% amino acid similarity [24]; and TaGASR-A1 has 88% amino acid sequence similarity [26]. These data provide a genetic framework for marker-assisted selection (MAS) to pyramid positive alleles for TGW and yield during cultivar development. However, there are still many important genes that have been characterized in rice that are not being used as template for cloning their orthologs in wheat, e.g., OsTB1 [36], GW5 [37], GS5 [38], GW8 [39], GW7/GL7 [40, 41], and OsAGSW1 [42]. Thus, comparative genomics approaches between rice and wheat will remain useful in discovering orthologs of rice genes in wheat and will continue to enhance our understanding of the genetics of yield potential in wheat.
The TaFLO2-A1 gene is related to TGW in wheat
Map-based cloning using QTL mapping approaches is an important strategy to isolate loci and genes controlling genetic polymorphism [43]. However, progress on map-based cloning in wheat has been relatively slow compared to that in rice, and very few QTLs have been subjected to fine mapping in order to isolate candidate genes, mainly due to the complexity and large genome size of wheat.
In our study, TaFlo2-A1 was found to be associated with TGW and explained from 4.42% (in the Pakistani collection) to 8.37% (in the MCC) of phenotypic variation. The TGW-related QTL identified on 2AL includes ‘Xgwm339-Xbarc311’ in 139 RILs between two hard red spring wheat lines [44]; QTgw.ipk-2A (Xgwm372) in 111 BC2F3 lines derived from the cross ‘Flair × XX86’ [45]; QGwt.crc-2A (Xgwm558-Xgwm294) in a double-haploid population generated from the cross ‘RL4452 × AC Domain’ [46]; QTkw.sdau-2A (Xwmc181a-Xubc840c) in 131 RILs derived from ‘Chuan 35050’ × ‘Shannong 483’ [13]; QSZ.uaf-2A.1 (Xwmc455) in natural populations of 108 CIMMYT and Pakistani spring wheat accessions [47]; and QTkw.hwwgr-2AL (Xgwm312–IWA6090) in 127 RILs derived from ‘Ning7840’ × ‘Clark’ [42]. Furthermore, three QTL on 2AL that were stable across five trials were detected in the same MCC (262) used in present study [29].
These QTL on 2AL are located between Xgwm71.2/Xgwm558 and Xgwm294, with an interval of 22 cM according to the consensus map of Somers [48] or 16.1 cM according to the ITMI map (http://wheat.pw.usda.gov/ggpages/SSRclub/GeneticPhysical/). From this TGW-QTL-rich region, only one gene, TaCwi-A1, has been isolated thus far between the Xgwm 71.2 and Xbarc15 deletion bin ‘C-2AL1–0.85’, which is adjacent to the centromere [18]. By integrating the information from the ITMI (http://wheat.pw.usda.gov/ggpages/SSRclub/GeneticPhysical/) and ‘Yu 8679 × Jing 411’ SSR + SNP [49] maps, the location of TaFlo2-A1 was inferred on deletion bin ‘2AL1–0.85-1.00’. Hence, the TaFlo2-A1 is a TGW-related gene located on the distal deletion bin of chromosome 2AL, and the molecular marker ‘TaFlo2-InDel8’ is an addition to the kit of wheat breeders for marker-assisted selection.
Relationship between TGW and GpS
The relationship between the number of grains per spike (GpS) and the TGW was traditionally found as being negatively correlated [1–3]. However, the simultaneous selection of favored haplotypes for one plus neutral ones for the other or otherwise favored haplotypes for both traits has changed the correlations from negative to neutral or even positive [4]. Therefore, no significant correlation was observed between TGW and GpS in the collections of Chinese landraces [4], French winter wheat cultivars [5] or CIMMYT-derived spring cultivars and lines [6, 50], while significantly positive correlations were observed in Chinese modern cultivars [4].
In many genome-wide association studies (GWAS) for TGW and GpS, many loci were found to be associated with only one of the traits [4, 29, 50]. The favored haplotypes at these loci should increase the phenotypic value of the one trait without negatively affecting the phenotypic value of the others. Thus, selection of such QTL was likely a major factor in changing the relationship between TGW and GpS over time. Similarly, selection of the favored haplotype (TaFlo2-A1b) identified in this study would help to increase TGW without reducing the average GpS in wheat.
Effect and putative mechanism of TaFlo2-A1 in the determination of TGW
TaFlo2-A1, which is represented by two haplotypes in our study, was found to be significantly associated with TGW. Polymorphisms of an 8-bp InDel in the promoter and of five SNPs in the first intron were observed in TaFlo2-A1. The orthologs TaFlo2-B1 and TaFlo2-D1 lacked sequence variations associated with TGW (Fig. 2b). The association analysis of the Chinese Micro Core Collection (MCC) and Pakistani accessions indicated that TaFlo2-A1b was the superior haplotype for TGW. Nevertheless, some accessions that contained TaFlo2-A1a also had high TGW. This is mainly because the effect of TaFlo2-A1 is likely masked by other genes associated with grain size [20].
In wheat, TaFlo2-A1 consists of 23 exons that encode 1734 amino acids with four TPR motifs at the positions of 947 to 988, 1032 to 1072, 944 to 1017 and 1028 to 1106 amino acid residues. Furthermore, three mitochondrial CLU domains were also observed at 737–878, 50–162 and 357–401 amino acid residues (Fig. 1a). The rice OsFlo2 gene also consists of 23 exons and encodes 1720 amino acids with three TPR motifs at the positions of 933–966, 975–1008, and 1017–1050 amino acid residues [27]. However, no mitochondrial CLU was reported in rice FLO2 by She et al. [27]. To confirm the absence of mitochondrial CLU in rice FLO2, we BLASTed the rice FLO2 protein (accession: CAE03171) in an NCBI CD search. The results of the rice FLO2 protein (accession: CAE03171) query using the NCBI CD system showed the presence of two mitochondrial CLU domains at the intervals of 52–124 and 721–863 and four TPRs at the intervals of 932–973, 1017–1057, 929–1002 and 1013–1091 amino acid residues (Fig. 1a). Thus, the prediction of wheat and rice FLO2 protein structure using NCBI CD indicates high similarity between their structures.
Flo2 is considered to be a member of a conserved gene family in plants [27]. TaFlo2-A1 is abundantly expressed in flag leaves and in developing grains at 5 DAF stage, and the expression level of the positive haplotype (TaFlo2-A1b) was higher than that of the negative haplotype (TaFlo2-A1a). The phenomenon of higher expression of the positive TaFlo2 haplotype is consistent with the results for rice OsFlo2, in which the overexpression of the positive haplotype significantly increases grain size [27]. In rice, the flo2 mutation in the promoter and in the open reading frame hinders the expression of genes involved in the synthesis of starch and protein [27, 28]. In rice cultivars that have different genetic backgrounds, some flo2 mutations negatively affect grain quality attributes such as amylose content, grain appearance and physiochemical properties despite maintaining or increasing grain size [27, 28]. Based on these similarities between OsFlo2 and TaFlo2-A1 at the sequence, structure and expression levels, the 8-bp InDel mutation in the TaFlo2-A1 promoter likely regulates grain size by affecting the expression of genes involved in the synthesis of starch and protein in wheat grains. Therefore, the increased expression of TaFlo2-A1 has a positive effect on grain yield but may have a negative effect on some grain quality attributes in wheat, which shall need further investigations.
The newly developed molecular marker ‘TaFlo2-InDel8’ is an addition to the kit of wheat breeder for marker-assisted selection. This study likely lead to additional investigations to unveil the exact regulatory mechanism of the Flo2 gene in wheat.
Conclusions
The Flo2 orthologs in hexaploid wheat were cloned, and TaFlo2-A1 was found to be associated with TGW but not with grain number per spike (GpS) in both the MCC and Pakistani wheat collections. The frequency of TaFlo2-A1b (positive haplotype) was low in commercial wheat cultivars; thus this haplotype can be selected to improve grain weight. This study likely lead to additional investigations to understand the regulatory mechanism of the Flo2 gene in hexaploid wheat. The newly developed molecular marker ‘TaFlo2-InDel8’ could be incorporated into the kit of wheat breeders for use in marker-assisted selection.
Acknowledgments
We thank two anonymous reviewers for their critical review and suggestions.
Funding
This research was financially supported by National Natural Science Foundation of China (31571643) and the Chinese Academy of Sciences (XDA08010104).
Availability of data and materials
All the data supporting our findings is contained within the manuscript.
Abbreviations
- Aa
Amino acid
- ANOVA
Analysis of variance
- Bp
Base pair
- CIMMYT
the International Maize and Wheat Improvement Center
- CS
Chinese Spring
- CTAB
Cetyl trimethylammonium bromide
- DAF
Days after flowering
- Flo2
Floury endosperm2
- GpS
Grain number per spike
- GYS
Grain yield per spike
- InDel
Insertion and deletion
- ITMI
International Triticeae Mapping Initiative
- MCC
Micro Core Collection
- NCBI
National Center for Biotechnology Information
- QTL
Quantitative trait loci
- RIL
Recombinant inbred line
- TGW
Thousand grain weight
- TPR
Tetratricopeptide repeat
- URGI
Unité de Recherche Génomique Info
Additional file
a. Part of TaFlo2A1 sequence in eight accessions. The first four sequences are of accessions with low TGW and other four sequences are of accessions with high TGW. b. Part of TaFlo2-B1 sequence in eight accessions. The first four sequences are of accessions with low TGW and other four sequences are of accessions with high TGW. c. Part of TaFlo2-D1 sequence in eight accessions. The first four sequences are of accessions with low TGW and other four sequences are of accessions with high TGW. (PDF 377 kb)
Authors’ contributions
MS and XM carried out most of the experiments. MS wrote the manuscript. MS performed quantitative RT-PCR and analyzed the data. SHK and WY grew the plant samples, YS collected all the phenotypic data, and AM and DL conceptualized the experiments and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12870-017-1114-3) contains supplementary material, which is available to authorized users.
Contributor Information
Aimin Zhang, Phone: +86-10-64806618, Email: amzhang@genetics.ac.cn.
Dongcheng Liu, Phone: +86-10-64806618, Email: dcliu@genetics.ac.cn.
References
- 1.Kuchel H, Williams KJ, Langridge P, Eagles HA, Jefferies SP. Genetic dissection of grain yield in bread wheat. I. QTL analysis. Theor Appl Genet. 2007;115:1029–1041. doi: 10.1007/s00122-007-0629-7. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre CL, Mathews KL, Rattey A, Chapman SC, Drenth J, Ghaderi M, Reynolds M, Shorter R. Molecular detection of genomic regions associated with grain yield and yield-related components in an elite bread wheat cross evaluated under irrigated and rainfed conditions. Theor Appl Genet. 2010;120:527–541. doi: 10.1007/s00122-009-1173-4. [DOI] [PubMed] [Google Scholar]
- 3.Wu X, Chang X, Jing R. Genetic insight into yield-associated traits of wheat grown in multiple rain-fed environments. PLoS One. 2012;7:e31249. doi: 10.1371/journal.pone.0031249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang DL, Hao CY, Wang LF, Zhang XY. Identifying loci influencing grain number by microsatellite screening in bread wheat (Triticum aestivum L.) Planta. 2012;236:1507–1517. doi: 10.1007/s00425-012-1708-9. [DOI] [PubMed] [Google Scholar]
- 5.Brancourt-Hulme M, Doussinault G, Lecomte C, Berard P, Le Buanec B, Trottet M. Genetic improvement of agronomic traits of winter wheat cultivars released in France from 1946 to 1992. Crop Sci. 2003;43:37–45. doi: 10.2135/cropsci2003.3700. [DOI] [Google Scholar]
- 6.Maqbool R, Sajjad M, Khaliq I, Rehman A, Khan AS, Khan SH. Morphological diversity and traits association in bread wheat (Triticum Aestivum L.) Amer-Euras J Agric Environ Sci. 2010;8:216–224. [Google Scholar]
- 7.Botwright TL, Condon AG, Rebetzke GJ, Richards RA. Field evaluation of early vigour for genetic improvement of grain yield in wheat. Aust J Agric Res. 2002;53:1137–1146. doi: 10.1071/AR02007. [DOI] [Google Scholar]
- 8.Peng JH, Ronin YF, Fahima T, Ro¨der MS, Li YC, Nevo E, Korol A. Domestication quantitative trait loci in Triticum Dicoccoides, the progenitor of wheat. Proc Natl AcadSci USA. 2003;100:2489–2494. doi: 10.1073/pnas.252763199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin L, Hao C, Hou J, Wang Y, Li T, Wang L, Ma Z, Zhang X. Homologous haplotypes, expression, genetic effects and geographic distribution of the wheat yield gene TaGW2. BMC Plant Biol. 2014;14:107. doi: 10.1186/1471-2229-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian JC, Deng ZY, RB H, Wang YX. Yield components of super wheat cultivars with different types and the path coefficient analysis on grain yield. Acta Agron Sin. 2006;32:1699–1705. [Google Scholar]
- 11.QH W, Chen YX, Zhou SH, Fu L, Chen JJ, Xiao Y, et al. High-density genetic linkage map construction and QTL mapping of grain shape and size in the wheat population Yanda 1817 × Beinong 6. PLoS One. 2015;10:e0118144. doi: 10.1371/journal.pone.0118144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Z, Hao C, Wang L, Dong Y, Zhang X. Identification and development of a functional marker of TaGW2 associated with grain weight in bread wheat (Triticum aestivum L.) Theor Appl Genet. 2011;122:211–223. doi: 10.1007/s00122-010-1437-z. [DOI] [PubMed] [Google Scholar]
- 13.Sun XY, Wu K, Zhao Y, Kong FM, Han GZ, Jiang HM, Huang XJ, Li RJ, Wang HG, Li SS. QTL analysis of kernel shape and weight using recombinant inbred lines in wheat. Euphytica. 2009;165:615–624. doi: 10.1007/s10681-008-9794-2. [DOI] [Google Scholar]
- 14.IWGSC-International Wheat Genome Sequencing Consortium A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science. 2014;345:1251788. doi: 10.1126/science.1251788. [DOI] [PubMed] [Google Scholar]
- 15.Valluru R, Reynolds MP, Salse J. Genetic and molecular bases of yield associated traits: a translational biology approach between rice and wheat. Theor Appl Genet. 2014;127:1463–1489. doi: 10.1007/s00122-014-2332-9. [DOI] [PubMed] [Google Scholar]
- 16.Hanif M, Gao F, Liu J, Wen W, Zhang Y, Rasheed A, Xia X, He Z, Cao S. TaTGW6-A1, an ortholog of rice TGW6, is associated with grain weight and yield in bread wheat. Mol Breed. 2016;36:1. doi: 10.1007/s11032-015-0425-z. [DOI] [Google Scholar]
- 17.MJ H, Zhang HP, Cao JJ, Zhu XF, Wang SX, Jiang H, ZY W, Lu J, Chang C, Sun GL, Ma CX. Characterization of an IAA-glucose hydrolase gene TaTGW6 associated with grain weight in common wheat (Triticum aestivum L.) Mol Breed. 2016;36:25. doi: 10.1007/s11032-016-0449-z. [DOI] [Google Scholar]
- 18.Ma D, Yan J, He Z, Wu L, Xia X. Characterization of a cell wall invertase gene TaCwi-A1 on common wheat chromosome 2A and development of functional markers. Mol Breed. 2012;29:43–52. doi: 10.1007/s11032-010-9524-z. [DOI] [Google Scholar]
- 19.Jiang Q, Hou J, Hao C, Wang L, Ge H, Dong Y, Zhang X. The wheat (T. Aestivum) sucrose synthase 2 gene (TaSus2) active in endosperm development is associated with yield traits. Func Integr Genomics. 2011;11:49–61. doi: 10.1007/s10142-010-0188-x. [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Jiang Q, Hao C, Wang Y, Zhang H, Zhang X. Global selection on sucrose synthase haplotypes during a century of wheat breeding. Plant Physiol. 2014;164:1918–1929. doi: 10.1104/pp.113.232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z, Bai Z, Li X, Wang P, Wu Q, Yang L, Li L, Li X. SNP identification and allelic-specific PCR markers development for TaGW2, a gene linked to wheat kernel weight. Theor Appl Genet. 2012;125:1057–1068. doi: 10.1007/s00122-012-1895-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Zhao Y, Gao L, Zhao G, Zhou R, Zhang B, Jia J. TaCKX6-D1, the ortholog of rice OsCKX2, is associated with grain weight in hexaploid wheat. New Phytol. 2012;195:574–584. doi: 10.1111/j.1469-8137.2012.04194.x. [DOI] [PubMed] [Google Scholar]
- 23.Chang J, Zhang J, Mao X, Li A, Jia J, Jing R. Polymorphism of TaSAP1-A1 and its association with agronomic traits in wheat. Planta. 2013;237:1495–1508. doi: 10.1007/s00425-013-1860-x. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y, Sun J, Zhang G, Wang Y, Kong F, Zhao Y, Li S. Haplotype, molecular marker and phenotype effects associated with mineral nutrient and grain size traits of TaGS1a in wheat. Field Crops Res. 2013;154:119–125. doi: 10.1016/j.fcr.2013.07.012. [DOI] [Google Scholar]
- 25.Zhang Y, Liu J, Xia X, He Z. TaGS-D1, an ortholog of rice OsGS3, is associated with grain weight and grain length in common wheat. Mol Breed. 2014;34:1097–1107. doi: 10.1007/s11032-014-0102-7. [DOI] [Google Scholar]
- 26.Dong L, Wang F, Liu T, Dong Z, Li A, Jing R, Mao L, Li Y, Liu X, Zhang K, Wang D. Natural variation of TaGASR7-A1 affects grain length in common wheat under multiple cultivation conditions. Mol Breed. 2014;34:937–947. doi: 10.1007/s11032-014-0087-2. [DOI] [Google Scholar]
- 27.She KC, Kusano H, Koizumi K, et al. A novel factor FLOURY ENDOSPERM2 is involved in regulation of rice grain size and starch quality. Plant Cell. 2010;22:3280–3294. doi: 10.1105/tpc.109.070821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Pu C, Lin H, Huang H, Huang Y, Hong H, Chang M, Lin Y. Three novel alleles of FLOURY ENDOSPERM2 (FLO2) confer dull grains with low amylose content in rice. Plant Sci. 2014;233:44–52. doi: 10.1016/j.plantsci.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Ge H, Hao C, Dong Y, Zhang X. Identifying loci influencing 1,000-kernel weight in wheat by microsatellite screening for evidence of selection during breeding. PLoS One. 2012;7:e29432. doi: 10.1371/journal.pone.0029432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sajjad M, Khan SH, Qadir M, Rasheesd A, Kazi AM, Khan IA. Association mapping identifies QTLs on wheat chromosome 3A for yield related traits. Cer Res Commun. 2014;42:177–188. doi: 10.1556/CRC.2013.0061. [DOI] [Google Scholar]
- 31.Shahzad M, Khan SH, Khan AS, Sajjad M, Rehman A, Khan AI. Identification of QTLs on chromosome 1B for grain quality traits in bread wheat (Triticum aestivum L.) Cyt Genet. 2016;50:13–20. [PubMed] [Google Scholar]
- 32.Stein N, Herren G, Keller B. A new DNA extraction method for high-throughput marker analysis in a large genome species such as Triticum aestivum L. Plant Breed. 2001;120:354–356. doi: 10.1046/j.1439-0523.2001.00615.x. [DOI] [Google Scholar]
- 33.Dong L, Zhang X, Liu D, Fan H, Sun J, Zhang Z, Qin H, Li B, Hao S, Li Z, Wang D, Zhang A, Ling HQ. New insights into the organization, recombination, expression and functional mechanism of low molecular weight glutenin subunit genes in bread wheat. PLoS One. 2010;5:e13548. doi: 10.1371/journal.pone.0013548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng B, Dong Z, Xu Z, An X, Qin H, Wu N, Wang D, Wang T. Molecular analysis of lipoxygenase (LOX) genes incommon wheat and phylogenetic investigation of LOX proteins from model and crop plants. J Cereal Sci. 2010;52:387–394. doi: 10.1016/j.jcs.2010.06.019. [DOI] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Takeda T, Suwa Y, Suzuki M, Kitano H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M, Ueguchi C. The OsTB1 gene negatively regulates lateral branching in rice. Plant J. 2003;33:513–520. doi: 10.1046/j.1365-313X.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 37.Wan X, Weng J, Zhai H, Wang J, Lei C, Liu L, Guo T, Jiang L, Su N, Wan J. Quantitative trait loci (QTL) analysis for rice grain width and fine mapping of an identified QTL allele gw-5 in a recombination hotspot region on chromosome 5. Genetics. 2008;179:2239–2252. doi: 10.1534/genetics.108.089862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Fan C, Xing Y, Jiang Y, Luo L, Sun L, Shao D, Xu C, Li X, Xiao J, He Y, Zhang Q. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat Genet. 2011;43:1266–1269. doi: 10.1038/ng.977. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Wu K, Yuan Q, Liu X, Liu Z, Lin X, Zeng R, Zhu H, Dong G, Qian Q, Zhang G, Fu X. Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet. 2012;44:950–954. doi: 10.1038/ng.2327. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Li S, Liu Q, Wu K, Zhang J, Wang S, Wang Y, Chen X, Zhang Y, Gao C, Wang F, Huang H, Fu X. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat Genet. 2015;47:949–954. doi: 10.1038/ng.3352. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Xiong G, Hu J, Jiang L, Yu H, Xu J, Fang Y, Zeng L, et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat Genet. 2015;47:944–948. doi: 10.1038/ng.3346. [DOI] [PubMed] [Google Scholar]
- 42.Li C, Bai G, Carver BF, Chao S, Wang Z. Single nucleotide polymorphism markers linked to QTL for wheat yield traits. Euphytica. 2015;206:89–101. doi: 10.1007/s10681-015-1475-3. [DOI] [Google Scholar]
- 43.Xiao J, Li J, Yuan L, Tanksley SD. Identification of QTLs affecting traits of agronomic importance in a recombinant inbred population derived from sub specific rice cross. Theor Appl Genet. 1996;92:230–244. doi: 10.1007/BF00223380. [DOI] [PubMed] [Google Scholar]
- 44.Tsilo TJ, Hareland GA, Simsek S, Chao S, Anderson JA. Genome mapping of kernel characteristics in hard red spring wheat breeding lines. Theor Appl Genet. 2010;121:717–730. doi: 10.1007/s00122-010-1343-4. [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Kempf H, Ganal M, Röder M. Advanced backcross QTL analysis in progenies derived from a cross between a German elite winter wheat variety and a synthetic wheat (Triticum aestivum L.) Theor Appl Genet. 2004;109:933–943. doi: 10.1007/s00122-004-1708-7. [DOI] [PubMed] [Google Scholar]
- 46.McCartney CA, Somers DJ, Humphreys DJ, Lukow O. Mapping quantitative trait loci controlling agronomic traits in the spring wheat cross RL 4452 9 AC ‘domain. Genome. 2005;48:870–883. doi: 10.1139/g05-055. [DOI] [PubMed] [Google Scholar]
- 47.Ahmad MA, Khan SH, Khan AS, Kazi AM, Basra SMA. Identification of QTLs for drought tolerance traits on wheat chromosome 2A using association mapping. Int J Agric Biol. 2014;16:862–870. [Google Scholar]
- 48.Somers DJ, Isaac P, Edwards K. A high density microsatellite consensus map for bread wheat (Triticum Aestivum L.) Theor Appl Genet. 2004;109:1105–1114. doi: 10.1007/s00122-004-1740-7. [DOI] [PubMed] [Google Scholar]
- 49.Zhai HJ, Feng YZ, Liu XY, Cheng XJ, Peng HR, Yao YY, Sun QX, Ni ZF. A genetic linkage map with 178 SSR and 1901 SNP markers constructed using a RIL population in wheat (Triticum aestivum L.) J Integ Agric. 2015;14:1697–1705. doi: 10.1016/S2095-3119(14)60902-3. [DOI] [Google Scholar]
- 50.Ain Q, Rasheed A, Anwar A, Mahmood T, Imtiaz M, Mahmood T, Xia X, He Z, Quraishi UM. Genome-wide association for grain yield under rain fed conditions in historical wheat cultivars from Pakistan. Front Plant Sci. 2015;6:743. doi: 10.3389/fpls.2015.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting our findings is contained within the manuscript.


