Abstract
Objective:
To characterize pretreatment behavioral problems and differential effects of initial therapy in children with childhood absence epilepsy (CAE).
Methods:
The Child Behavior Checklist (CBCL) was administered at baseline, week 16–20, and month 12 visits of a randomized double-blind trial of ethosuximide, lamotrigine, and valproate. Total problems score was the primary outcome measure.
Results:
A total of 382 participants at baseline, 310 participants at the week 16–20 visit, and 168 participants at the month 12 visit had CBCL data. At baseline, 8% (95% confidence interval [CI] 6%–11%) of children with CAE had elevated total problems scores (mean 52.9 ± 10.91). At week 16–20, participants taking valproic acid had significantly higher total problems (51.7 [98.3% CI 48.6–54.7]), externalizing problems (51.4 [98.3% CI 48.5–54.3]), attention problems (57.8 [98.3% CI 55.6–60.0]), and attention-deficit/hyperactivity problems (55.8 [98.3% CI 54.1–57.6]) scores compared to participants taking ethosuximide (46.5 [98.3% CI 43.4–49.6]; 45.8 [98.3% CI 42.9–48.7]; 54.6 [98.3% CI 52.4–56.9]; 53.0 [98.3% CI 51.3–54.8]). Lack of seizure freedom and elevated week 16–20 Conner Continuous Performance Test confidence index were associated with worse total problems scores. At month 12, participants taking valproic acid had significantly higher attention problems scores (57.9 [98.3% CI 55.6–60.3]) compared to participants taking ethosuximide (54.5 [95% CI 52.1–56.9]).
Conclusions:
Pretreatment and ongoing behavioral problems exist in CAE. Valproic acid is associated with worse behavioral outcomes than ethosuximide or lamotrigine, further reinforcing ethosuximide as the preferred initial therapy for CAE.
Clinicaltrials.gov identifier:
Classification of evidence:
This study provides Class II evidence that for children with CAE, valproic acid is associated with worse behavioral outcomes than ethosuximide or lamotrigine.
Childhood absence epilepsy (CAE) is the most common childhood-onset epilepsy syndrome.1,2 There is increasing evidence that a noteworthy proportion of these children have persistent problems in attention, academic performance, and behavior.3–15 Recent data suggest that these problems may persist even when seizures are fully controlled.15–17
An NIH-funded double-blind, randomized trial examined the comparative efficacy and tolerability of ethosuximide, lamotrigine, and valproic acid in children with newly diagnosed CAE. At week 16–20, ethosuximide and valproic acid exhibited better efficacy at controlling seizures than lamotrigine (primary outcome), while patients taking valproic acid had more attentional problems compared to patients taking ethosuximide (secondary outcome).15–17 This report characterizes this trial's pretreatment behavior findings and subsequent differential medication behavioral effects (predefined tertiary outcome).
METHODS
Primary research question.
What are the differential behavioral effects of initial monotherapy medication in children with CAE?
Patient population.
Details of the inclusion/exclusion criteria have been published previously.16,17 Briefly, children 2.5–13 years old with newly diagnosed CAE and EEG evidence of 2.7–3.5 Hz generalized spike-wave discharges, a normal background, and ≥1 burst lasting ≥3 seconds were enrolled. Children previously treated with antiepileptic drugs for >7 days or with a history of major psychiatric disease or autism were excluded.16,17
Study design.
The study was a parallel, randomized, double-blinded comparative effectiveness study (randomized controlled trial [RCT]), with partial crossover to open label (at treatment failure only) with subsequent long-term follow-up.16–18 Participants had a pretreatment baseline visit including a detailed history and examination, a video EEG, age-specific battery of neuropsychological tests,15 and parental questionnaires about behavior utilizing the Child Behavior Checklist (CBCL).19,20 Participants were randomized within age strata (younger or older than 6 years) equally to ethosuximide, lamotrigine, or valproic acid and continued receiving double-blind study medication as long as they did not meet any treatment failure criteria.16,17
Measure of behavior.
The CBCL,19,20 the primary measure of behavior, was administered at baseline, week 16–20, and month 12 visits along with any discontinuation visit prior to month 12. The CBCL, an empirically based scale, is validated from age 1½ years through adulthood. For this study, the 1½- to 5-year-old and 6- to 18-year-old scales were used. All scales are normalized to a mean of 50 and SD of 10. Scores ≥70 are considered clinically significant. In the general pediatric population, approximately 2% of children are expected to have scores ≥70.19,20 The CBCL was centrally scored by one author (R.C.S.), who was blinded to study arm and treatment outcome.
Outcome measures.
The primary behavior outcome was the CBCL total problems score. Four other CBCL scales were secondary behavior outcomes: internalizing problems, externalizing problems, syndrome scale for attention problems, and DSM-oriented scale for attention-deficit/hyperactivity problems.
Cognitive data.
The trial's cognitive testing methodology and results have been described previously.15 The primary objective attention measure was the confidence index from the Conner Continuous Performance Test (CPT-II) and Kiddie CPT. The CPT confidence index value indicates the probability that the child has a clinically significant attention deficit. A confidence index ≥0.60 value indicates a ≥60% probability and is considered clinically significant. Commission and omission scores were also examined.15,21 Wisconsin Card Sorting Test perseverative response measured executive function.15–17
Seizure freedom.
Seizure freedom determination included both clinical and EEG assessments and has been described previously.16,17,22 Participants were considered seizure-free if they were both clinically and electrographically seizure-free.
Statistical analysis.
Baseline, week 16–20, and month 12 CBCL outcome data were summarized as group means and percentage of individual scale scores ≥70. Week 16–20 CBCL scores included that visit's scores and scores from any earlier discontinuation visit after the week 4 visit. Similarly, month 12 CBCL scores included scores from earlier discontinuation visits after the week 16–20 visit. CPT confidence index values used a dichotomous grouping approach (≥0.60 vs <0.60).
Baseline demographics and EEG features22 were compared using Fisher exact test, exact χ2 tests, t tests, or analysis of variance (ANOVA) as appropriate. Linear regression was used to determine whether clinical, demographic, EEG, and cognitive features, including CPT variables, were predictive of baseline CBCL outcome scores. CBCL scale scores at week 16–20 and month 12 were compared using exact χ2 test for percentages of scores ≥70 and ANOVA and analyses of covariance (ANCOVAs), correcting for baseline CBCL scores, for means, as prespecified in the protocol. Change scores were analyzed similarly. ANOVA/ANCOVA were also used to explore treatment by seizure freedom interaction in explaining CBCL outcomes. These analyses were post hoc and descriptive. All pairwise comparisons used a Bonferroni correction (p = 0.017 for 3 pairwise comparisons between treatments).
Although the study's sample size of 446 was determined by the parent clinical trial,16,17 it was prespecified that a sample size of 112 patients per treatment group would be sufficient to detect a difference of 2 points on the overall CBCL total problems, attention problems, both syndrome and DSM, and externalizing and internalizing problems at the week 16–20 visit from the best treatment to the second and 3 points from the second to the worst with 80% power, while controlling for α = 0.01 (5 outcome scales, Bonferroni correction).
All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) and StatXact version 8.0 (Cytel Inc., Cambridge, MA). A p value of <0.05 was considered significant except as noted otherwise above.
Standard protocol approvals, registrations, and patient consents.
All 32 site institutional review boards approved the study. Written parental informed consent and, when appropriate, child assent was obtained from all participants. The trial was conducted under a Food and Drug Administration–approved Investigational New Drug application and is listed at clinicaltrials.gov/ under NCT00088452.
RESULTS
Patient population.
Baseline CBCL data were available from 382 (86%) of 446 RCT participants. The average age at study entry was 7.6 ± 2.2 years, 57% were female, 75.4% were white, 17.3% were African American, and 23% were Hispanic. The cohort's baseline IQ was 95.15 ± 15.5 (n = 343, 95% CI 93.5–96.8). There were no significant differences in age, sex, ethnicity, treatment group assignment, or shortest seizure duration on baseline EEG between the 382 participants with baseline CBCL data and 64 participants without. A higher proportion of black participants did not have a baseline CBCL (31% vs 17.3%, p = 0.012). There were no differences in baseline characteristics among the 3 treatment subgroups.
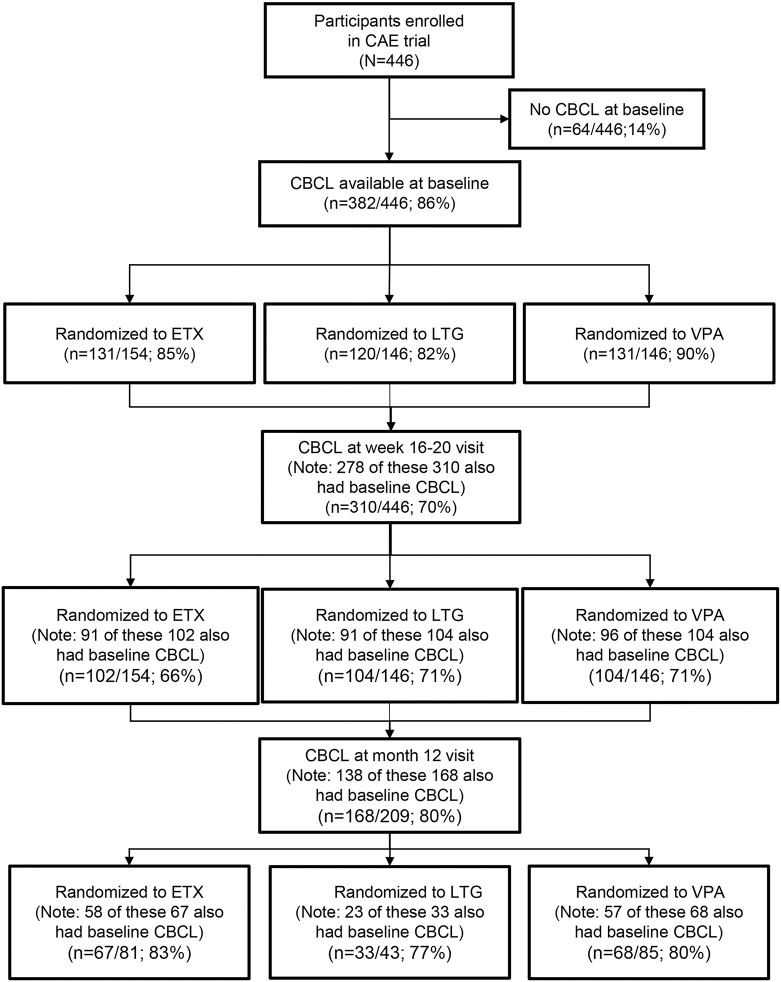
At week 16–20, completed CBCL data were available for 310 participants. At month 12, completed CBCL data were available for 168 participants (80% of 209 participants with freedom from failure at week 16–20 visit). There were no significant differences in age, sex, race, or ethnicity between the 310 participants with CBCL data at the week 16–20 visit and the 136 participants without, or between the 168 participants with CBCL data at the month 12 visit and the 278 participants without. There were substantially fewer participants initially assigned to lamotrigine still in the study at the month 12 visit,17 so a higher percentage of lamotrigine participants did not have month 12 CBCL data compared to those in the other 2 treatment groups (figure 1).
Figure 1. Child Behavior Checklist (CBCL) testing in participants during the initial 12 months of double-blind treatment.
CAE = childhood absence epilepsy; ETX = ethosuximide; LTG = lamotrigine; VPA = valproic acid.
Baseline CBCL scores.
At baseline, 8% of participants had total problem scores ≥70 (95% CI 6%–11%), 5% had internalizing problems ≥70 (95% CI 3%–8%), 6% had externalizing problems ≥70 (95% CI 4%–9%), 15% had attention problems ≥70 (95% CI 12%–19%), and 7% had attention-deficit/hyperactivity problems score ≥70 (95% CI 4.5%–10%). Attention problems also had the highest mean score (60.1 ± 9.78) (table 1).
Table 1.
Baseline Child Behavior Checklist (CBCL) results overall and by age
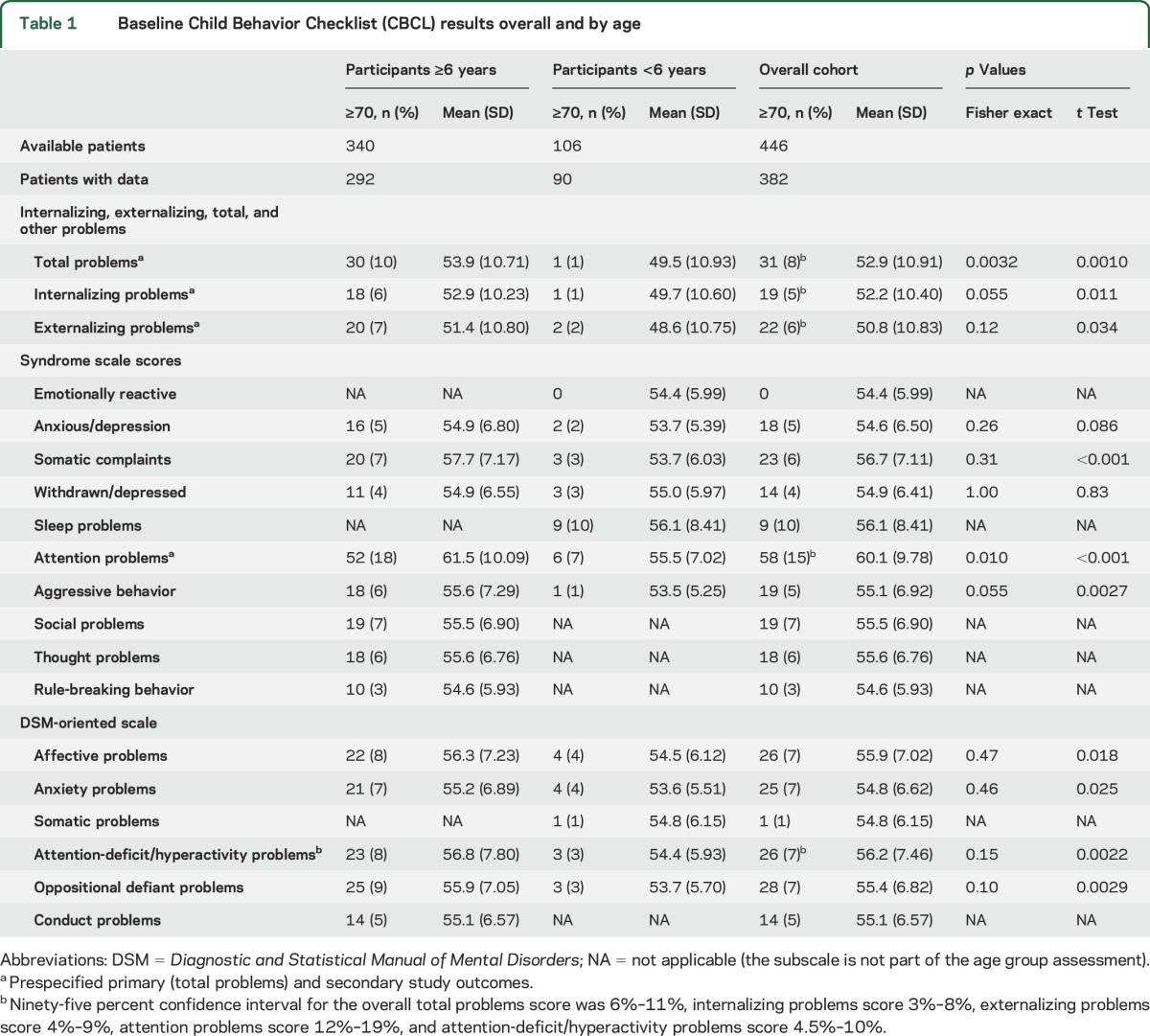
Parents were more likely to identify behavioral issues in children ≥6 years old (table 1). Both mean total problems score and percentage of children with scores ≥70 were higher in the ≥6-year-old group compared to the younger group (53.9 ± 10.7 vs 49.5 ± 10.9, p = 0.0010; 10% vs 1%, p = 0.0032). Similarly, worse scores were noted for children ≥6 years for attention problems, internalizing, and externalizing scores (table 1).
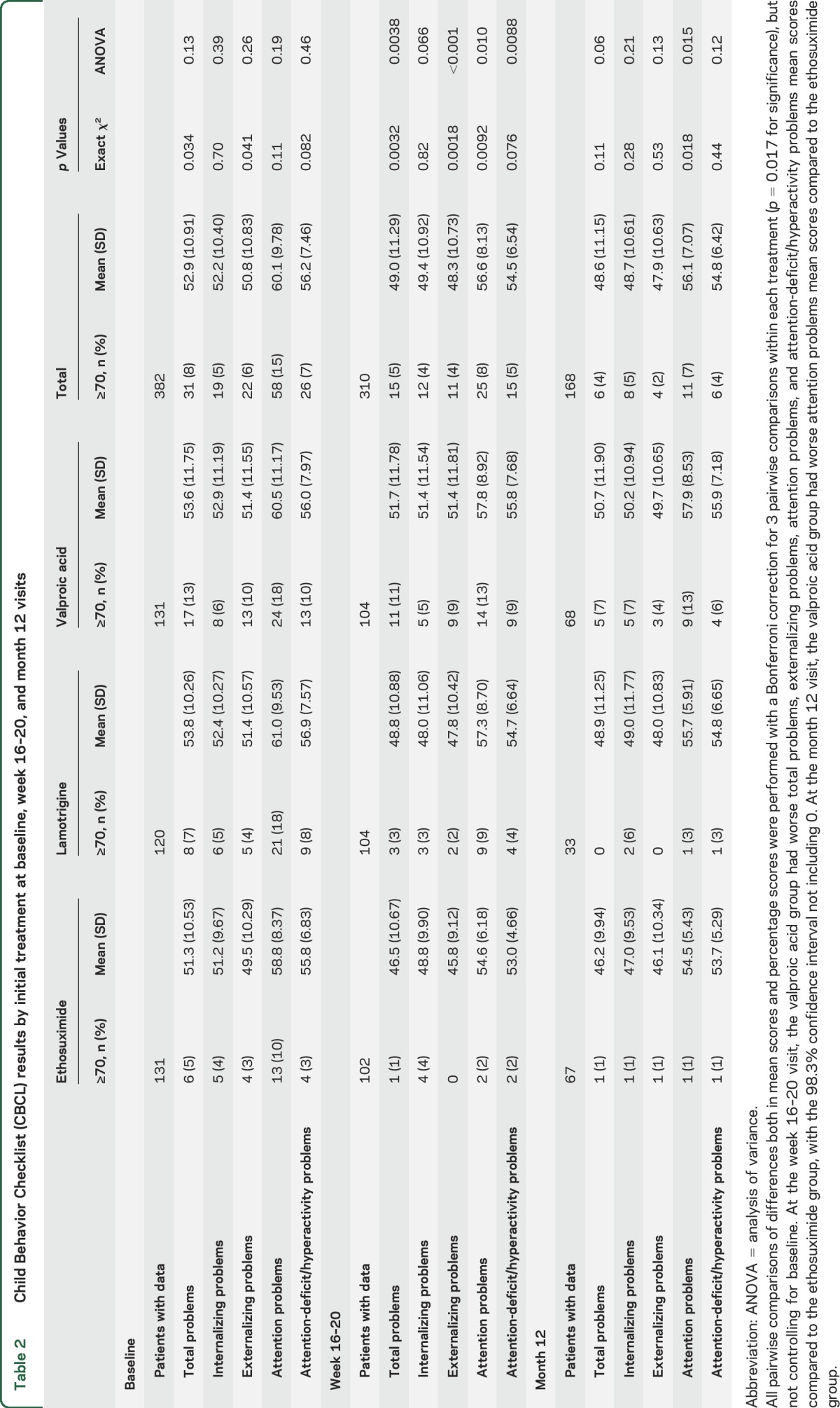
There were no significant differences in baseline mean scores across the 3 treatment subgroups for the total problems score or the 4 subscales used (table 2). However, more patients taking valproate had pretreatment total problems scores ≥70 (valproate 13%, ethosuximide 5%, lamotrigine 7%; p = 0.034) and externalizing problems score ≥70 (valproate 10%, ethosuximide 3%, lamotrigine 4%; p = 0.041) (table 2).
Table 2.
Child Behavior Checklist (CBCL) results by initial treatment at baseline, week 16–20, and month 12 visits
Factors associated with higher (worse) baseline total problems scores in a linear regression were age ≥6 years (p = 0.0007), higher CPT confidence index (p = 0.0001), higher CPT omission scores (p = 0.0072), lower full-scale IQ (p < 0.0001), and lower verbal IQ (p = 0.029). Each factor accounted for only 2%–6% of the variability in total problems score, and combined, for 10% variability. These factors along with higher perseverative response on Wisconsin Card Sorting Test scores were associated with worse externalizing problems and attention problems scores, accounting for 1%–10% of the variability individually, and 10%–20% combined. Factors associated with higher internalizing problems scores were age ≥6 years (p = 0.0056), Hispanic ethnicity (p = 0.018), shortest duration of seizure on EEG (p = 0.044), higher CPT confidence index (p = 0.016), and lower full-scale IQ (p = 0.036), each accounting for 1%–2% of variability, and 5% when combined.
There was a correlation between baseline CPT confidence index scores and the CBCL attention score (p ≤ 0.0001). A higher CPT confidence index was associated with worse CBCL attention scores (figure e-1 at Neurology.org).
Week 16–20 CBCL scores.
At the week 16–20 visit, participants taking valproic acid had higher mean total problems score (51.7 [98.3% CI 48.6–54.7]) and a higher percentage of total problems scores ≥70 (10.6% [98.3% CI 4.6%–19.9%]) compared to participants taking ethosuximide (46.5 [98.3% CI 43.4–49.5]; 1% [98.3% CI 0.0%–6.5%]) and participants taking lamotrigine (48.8 [98.3% CI 45.8–51.9]; 2.9% [98.3% CI 0.4%–9.5%]) (p = 0.0038 and p = 0.0032 for the overall comparisons by χ2 and ANOVA, respectively) (table 2). These findings persisted when corrected for differences in baseline scores (ANCOVA p = 0.003 overall, p = 0.0017 pairwise to ethosuximide) (table e-1).
There were differences by treatment in week 16–20 results in externalizing problems, attention problems, and attention-deficit/hyperactivity problems scores (table 2) in an ANOVA with treatment as a factor. Pairwise comparisons using a Bonferroni correction but not controlling for baseline scores showed the valproic acid group had worse total problems (mean difference 5.2 [98.3% CI 0.9–9.5]), externalizing problems (mean difference 5.6 [98.3% CI 1.5–9.7]), attention problems (mean difference 3.2 [98.3% CI 0.05–6.3]), and attention-deficit/hyperactivity problems (mean difference 2.8 [98.3% CI 0.3–5.3]) compared to the ethosuximide group (table 2).
When controlling for baseline scores, all pairwise differences remained significant. In addition, patients taking valproic acid had significantly worse total problems, internalizing problems, externalizing problems, and attention-deficit/hyperactivity problems mean scores compared to patients taking lamotrigine (table e-1).
At week 16–20, there were no differences in CBCL outcomes between those with freedom from failure (n = 174) and those without (n = 136) or those who attained seizure freedom (n = 200) and those who did not (n = 110).
At the week 16–20 visit, participants with baseline CPT confidence index ≥0.60 (n = 96) had significantly worse total problems, externalizing problems, attention problems, attention-deficit/hyperactivity problems, aggressive behavior, and oppositional defiant behavioral scores than those with confidence index scores <0.60 (n = 184).
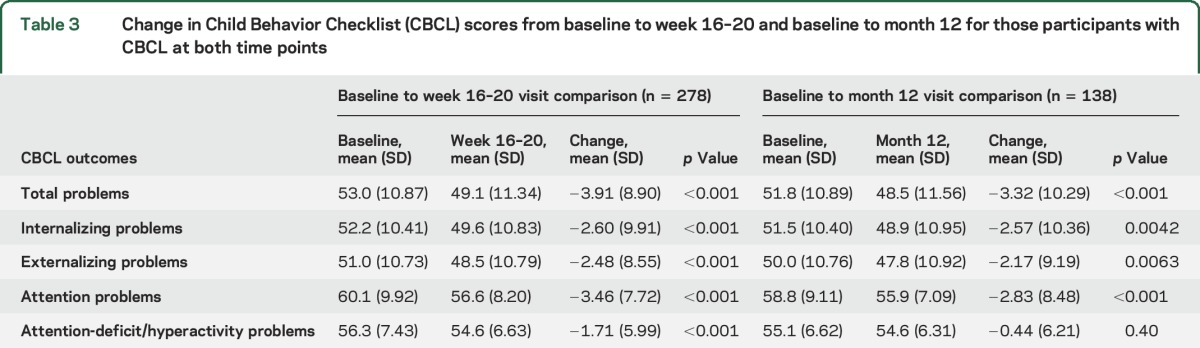
For the 278 participants who had a CBCL at baseline and at week 16–20, there was modest improvement (p < 0.001) between visits in total problems score and the 4 secondary behavioral outcomes (table 3). Within these 278 participants, when correcting for baseline CBCL scores, participants achieving freedom from failure (n = 153) had better total problems scores (48.1 ± 11.97 vs 50.4 ± 10.44, p = 0.030), externalizing problems scores (47.9 ± 11.30 vs 49.2 ± 10.14, p = 0.043), and attention problems scores (55.7 ± 7.32 vs 57.7 ± 9.08, p = 0.032) compared to those experiencing treatment failure (n = 125).
Table 3.
Change in Child Behavior Checklist (CBCL) scores from baseline to week 16–20 and baseline to month 12 for those participants with CBCL at both time points
Among these 278 participants, there were no significant differences in any of the 5 study outcomes between those reaching seizure freedom at week 16–20 (n = 177) and those who did not (n = 101). However, post hoc group comparisons controlling for baseline scores showed the negative effects of valproic acid relative to ethosuximide and lamotrigine treatment, beneficial effects of seizure freedom, and the influence of baseline CBCL score for the week 16–20 visit total problems, internalizing problems, and externalizing problems scores (tables 4, e-2, and e-3).
Table 4.
Child Behavior Checklist (CBCL) summary at week 16–20 by seizure status and within initial treatment
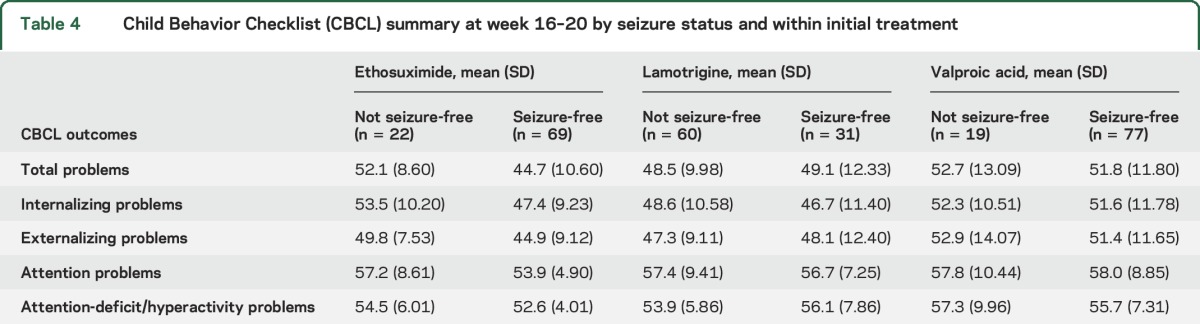
At week 16–20, all 5 study behavioral outcomes were significantly worse in participants with week 16–20 visit CPT confidence index scores ≥0.60 (n = 82) compared to those participants with week 16–20 visit CPT confidence index scores <0.60 (n = 149) when the analysis controlled for baseline CBCL scores. When analyzed by both treatment and week 16–20 confidence index, both a treatment effect and CPT confidence index effect were noted. Valproic acid use and CPT confidence index ≥0.60 were associated with worsening total problems scores.
Month 12 CBCL scores.
For the 168 participants who had a CBCL at month 12, there were no differences in total problems, internalizing problems, externalizing problems, or attention-deficit/hyperactivity problems scores between treatment groups. However, patients taking valproic acid had a higher mean attention problems score and a higher percentage of attention problems scores ≥70 compared to patients taking ethosuximide and patients taking lamotrigine (table 2).
Pairwise comparison within the ANOVA using a Bonferroni correction but not controlling for baseline revealed the valproic acid group had worse attention problems scores (57.9 [98.3% CI 55.6–60.3]) compared to the ethosuximide group (54.5 [98.3% CI 52.1–56.9]). When controlling for baseline scores, the valproic acid–ethosuximide difference remained significant (table e-1).
Controlling for baseline CBCL scores, there was no difference in month 12 behavioral outcomes between those who remained seizure-free (n = 123) and those who did not (n = 15) except for slightly worse attention problems scores in those experiencing treatment failure (58.9 ± 10.55 vs 55.6 ± 6.51; p = 0.032). Total, externalizing, and attention problems were worse in participants with month 12 CPT confidence index scores ≥0.60 (n = 47) than in those with month 12 confidence index scores <0.60 (n = 78) controlling for baseline CBCL scores.
For the 138 participants with a CBCL both at baseline and at month 12, there was a modest but significant improvement between visits in total problems score and all secondary outcomes except attention-deficit/hyperactivity problems (table 3). There were no significant differences in the 5 study behavioral outcomes by treatment between week 16–20 and month 12.
DISCUSSION
This study identified preexisting behavioral problems in children with CAE. The subsequent behavioral assessments identified differential negative effects of valproic acid compared to ethosuximide and lamotrigine as well as negative effects of ongoing seizures and pretreatment behavior problems.
Although this RCT did not include a control group, the percentage of participants with total problems scores ≥70 (8%; 95% CI 6%–11%) is higher than would be expected of a healthy control group (2.1%) since all scores are normalized to a mean of 50 with a standard deviation of 10. CBCL subscales demonstrated this same elevation relative to an expected healthy control group (table 1).
Recently, there has been increased recognition of the comorbidities of cognition and behavior in children with epilepsy.3–15,23 Studies of large cohorts of children with newly diagnosed epilepsy have shown that these comorbidities are often present at baseline and likely predate the onset of clinical seizures.4,6,23 Austin and colleagues4 examined a cohort of school-aged children with newly diagnosed epilepsy including both generalized and localization-related syndromes using the CBCL. They found an increased risk of total problems, as well as internalizing problems, attention problems, thought problems, and somatic complaints, in children with new-onset seizures compared to their siblings without seizures. Hermann and colleagues6 found that the inattentive form of attention-deficit/hyperactivity disorder was significantly more prevalent in their group of children (8–18 years) with newly diagnosed idiopathic epilepsy than in healthy controls and that in the majority it occurred prior to the diagnosis of epilepsy. This is consistent with our findings15,16 and may explain why parental report of attention, which is often based on hyperactivity, does not correlate well with formal measurements of attention such as the CPT15 in this study. However, there is still an increase in other behavior problems as well and this is consistent with other studies.3,4,8–10,12–14 More recent literature suggests that behavior problems as well as issues with cognition are present in even the most benign forms of epilepsy at diagnosis regardless of whether medications are initiated.24–27
Psychiatric comorbidities and behavior problems are also increased in children with epilepsy. They occur in children with both localization-related and generalized epilepsy including CAE.3,4,6–14 Studies focusing on CAE have reported that, compared to normal controls, children with CAE have higher rates of attention-deficit, anxiety, and affective disorders as well as of suicidal ideation.3,8–10 Our study of newly diagnosed CAE demonstrates that these are present at baseline and allows us to examine medication effects.
Some studies suggest that while behavioral problems exist at baseline in children with epilepsy, they improve over time,4 and we did find modest improvement in the first year in those participants remaining in the study. Not surprisingly, family variables have an important effect on behavioral outcomes.4 However, long-term outcomes of CAE show a high rate of behavioral issues and poor academic performance.3 The current CAE study is examining long-term outcomes and will ultimately be able to correlate them with baseline behavior scores.
It is not surprising that increased behavioral problems correlate with poorer performance on measures of cognition including attention, executive function, and intelligence.4,23 The worse reported behavioral outcomes in the children ≥6 years may partly be a caregiver recognition issue. Attention issues and aggressive behavior are more noticeable and less acceptable to parents of older children. The long-term follow-up will determine if there is a difference once the children are older. But while older age at onset has a higher rate of developing generalized tonic-clonic seizures,28 this report identifies that older age at onset is also associated with more behavioral comorbidities in CAE.
Both treatment assignment and seizure outcomes affected behavioral outcomes. Those assigned to valproic acid did substantially worse than those assigned to ethosuximide or lamotrigine. Behavioral outcomes for lamotrigine are not better than those for ethosuximide. These data reinforce the compelling result that ethosuximide is the preferred first-line treatment for CAE absence15–17,28 as it has superior efficacy to lamotrigine and fewer cognitive and behavioral adverse effects than valproic acid. An analysis of a prospective observational cohort also reported better long-term seizure outcomes in those initially treated with ethosuximide than those treated with valproic acid.29
This RCT, with longitudinal behavioral assessments, identified an elevated rate of pretreatment behavioral issues in children with CAE, the relationships between negative behaviors and ongoing seizures and attention problems, and that ethosuximide is the preferred initial monotherapy for children with CAE.
Supplementary Material
ACKNOWLEDGMENT
A list of contributors can be found in appendix e-1 at Neurology.org.
GLOSSARY
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- CAE
childhood absence epilepsy
- CBCL
Child Behavior Checklist
- CI
confidence interval
- CPT
Continuous Performance Test
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- RCT
randomized controlled trial
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Childhood Absence Epilepsy Study Group, Harry Abram, Ellen Albers, Samar J. Bahna, Karen Ballaban-Gil, Jose Barrera, Tekolla Belaineh, Mary Bertrand, Hal Blumenfeld, Sarah Borror, Charlie Borzy, Susan Brantz, Candace Cardoza, Kevin Chapman, Harry T. Chugani, Robert R. Clancy, Peggy O. Clark, Yong Collins, Terrie Conklin, Joan A. Conry, Patricia K. Crumrine, Jason Czachor, M. Susan Dean, Sandra Dewar, Michael Duchowny, Deborah Dye, Tammy Eaton, Mary Jo Elgie, Michelle Ellis, Roy Elterman, Daniella Escobedo, Andrew Francis, L. Matthew Frank, Tracy A. Glauser, Hilary Gray, La June Grayson, May L. Griebel, Laurie Guidry, Samantha Hagopian, Jennifer Haky, Amelia Halac, Angel Hernandez, Lee Howard, Krisa Hoyle Elgin, Pong Kankirawatana, Kent R. Kelley, Juli Kidd, Divya Khurana, Paul M. Levisohn, Donna Lowery, Ricardo Luzondo, Mary Lou Maher, Angela Martinez, Karen McEwen, Sarah J. McVey, Mary Miceli, Daniel K. Miles, Jennifer Monahan, Elena Morales, Dianne Morus, Cyndi Mott, JoAnn Narus, Mark Nespeca, Edward Novotny, Jr, Suzanne Oken, Juliann Paolicchi, Bryan Philbrook, Tami Quintero, Amber Reese-Porter, Jong M. Rho, Tracee Ridley-Pryor, Angela Riggs, Colin Roberts, Kathy Romine, Veronique Ruppe, Russell P. Saneto, Raman Sankar, Mark S. Scher, Rebecca Schultz, Michael Schwabe, Dina Schwam, Sagar Shah, Gregory Sharp, Rolla Shbarou, Ruth C. Shinnar, Shlomo Shinnar, Marcio Sotero de Menezes, Donovan Stock, Christina Lopez Talley, Susanna Taylor, Mamello Tekateka, Doris A. Trauner, Edwin Trevathan, William R. Turk, Colin B. Van Orman, GeorgAnn Vanderjagt, Mary Warde, Jorge Vidaurre, Arie Weinstock, Nanastasia Welnick, Rhonda Werner, James Wheless, Angus A. Wilfong, Korwyn Willliams, Shelley Williams, Teresa Williams, Jennifer Williamson, Khaled Zamel, and Mary Zupanc
AUTHOR CONTRIBUTIONS
Study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision: R.C. Shinnar, S. Shinnar, A. Cnaan, P. Clark, D. Dlugos, D.G. Hirtz, F. Hu, C. Liu, D. Masur, E.F. Weiss, T.A. Glauser. Statistical analysis: A. Cnaan, F. Hu, C. Liu. R.C. Shinnar and S. Shinnar wrote the first and final draft of the manuscript. S. Shinnar, A. Cnaan, P. Clark, D. Dlugos, D.G. Hirtz, D. Masur, E.F. Weiss, and T.A. Glauser along with R.C. Shinnar, F. Hu, and C. Liu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
STUDY FUNDING
Supported by NIH (U01-NS045911 and U01-NS045803).
DISCLOSURE
R.C. Shinnar is funded by NIH grants 2R37NS043209 and 2U01-NS045911 and by a grant from Rett Syndrome Research Trust. S. Shinnar is funded by NIH grants 2R37-NS043209, 2U01-NS045911, U10NS077308, 1U01NS088034, and 1R01NS094257; serves on the editorial board of Pediatric Neurology; serves on 2 DSMBs for UCB Pharma and a DSMB for Eisai; has received personal compensation for consulting for Malinckrodt, Neurelis, Upsher-Smith, and Xeris; has received royalties from Elsevier for coediting Febrile Seizures; serves as an expert consultant for the US Department of Justice; and has received compensation for work as an expert on medico-legal cases. A. Cnaan was funded by NIH grants 2U01-NS045911, UL1RR031988, P30HD040677, P50AR060836, R01AR061875, and R01HD058567, Department of Defense grants W81XWH-09-1-0592 and W81XWH-12-1-0417, and Department of Education grant H133B090001. P. Clark is funded by NIH grants 2U01-NS045911 and U10-NS077311 and has received consulting and speaking fees from Eisai and Supernus. D. Dlugos is funded by NIH grants 1R01NS053998, 2U01NS045911, 1R01LM011124, and U01NS077276, by the Epilepsy Study Consortium, and by prestudy protocol development agreements with Insys Therapeutics and Bio-Pharm Solutions, and has given expert testimony in medico-legal cases. D. Hirtz reports no disclosures relevant to the manuscript. F. Hu was funded by NIH grants 2U01-NS045911, P30HD040677, P50AR060836, R01AR061875, and R01HD058567, Department of Defense grants W81XWH-09-1-0592 and W81XWH-12-1-0417, and Department of Education grant H133B090001. C. Liu reports no disclosures relevant to the manuscript. D. Masur is funded by NIH grants 2R37NS043209 and 2U01-NS045911 and has given expert testimony in medico-legal cases. E. Weiss is funded by NIH grants 2R37NS043209, 2U01-NS045911, and R01-AG046949. T. Glauser is funded by NIH grants 2U01-NS045911, U10-NS077311, R01-NS053998, R01-NS062756, R01-NS043209, R01-LM011124, and R01-NS065840; has received consulting fees from Supernus, Sunovion, Eisai, UCB, Lundbeck, and Questcor; serves as an expert consultant for the US Department of Justice; has received compensation for work as an expert on medico-legal cases; and receives royalties from a patent license. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B. How well can epilepsy syndromes be identified at diagnosis? A reassessment 2 years after initial diagnosis. Epilepsia 2000;41:1269–1275. [DOI] [PubMed] [Google Scholar]
- 2.Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study: Coordination Active du Reseau Observatoire Longitudinal de l' Epilepsie. Epilepsia 2001;42:464–475. [DOI] [PubMed] [Google Scholar]
- 3.Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy: sometimes a wolf in sheeps' clothing. Arch Pediatr Adolesc Med 1997;151:152–158. [DOI] [PubMed] [Google Scholar]
- 4.Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics 2001;107:115–122. [DOI] [PubMed] [Google Scholar]
- 5.Wilson SJ, Baxendale S. The new approach to classification: rethinking cognition and behavior in epilepsy. Epilepsy Behav 2014;41:307–310. [DOI] [PubMed] [Google Scholar]
- 6.Hermann B, Jones J, Dabbs K, et al. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain 2007;130:3135–3148. [DOI] [PubMed] [Google Scholar]
- 7.Hermann BP, Jones JE, Sheth R, et al. Growing up with epilepsy: a two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia 2008;49:1847–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan R, Siddarth P, Stahl L, et al. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia 2008;49:1838–1846. [DOI] [PubMed] [Google Scholar]
- 9.Caplan R, Siddarth P, Gurbani S, Hanson R, Sankar R, Shields WD. Depression and anxiety disorders in pediatric epilepsy. Epilepsia 2005;46:720–730. [DOI] [PubMed] [Google Scholar]
- 10.Caplan R, Sagun J, Siddarth P, et al. Social competence in pediatric epilepsy: insights into underlying mechanisms. Epilepsy Behav 2005;6:218–228. [DOI] [PubMed] [Google Scholar]
- 11.Chaix Y, Daquin G, Monteiro F, Villeneuve N, Laguitton V, Genton P. Absence epilepsy with onset before age three years: a heterogeneous and often severe condition. Epilepsia 2003;44:944–949. [DOI] [PubMed] [Google Scholar]
- 12.Jones JE, Siddarth P, Gurbani S, Shields WD, Caplan R. Cognition, academic achievement, language, and psychopathology in pediatric chronic epilepsy: short-term outcomes. Epilepsy Behav 2010;18:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vega C, Guo J, Killory B, et al. Symptoms of anxiety and depression in childhood absence epilepsy. Epilepsia 2011;52:e70–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg AT, Vickrey BG, Testa FM, Levy SR, Shinnar S, DiMario F. Behavior and social competency in idiopathic and cryptogenic childhood epilepsy. Dev Med child Neurol 2007;49:487–492. [DOI] [PubMed] [Google Scholar]
- 15.Masur D, Shinnar S, Cnaan A, et al. Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy. Neurology 2013;81:1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. New Engl J Med 2010;362:790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia 2013;54:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cnaan A, Shinnar S, Arya R, et al. Second monotherapy in childhood absence epilepsy. Neurology 2017;88:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington: University of Vermont, Research Center for Children, Youth & Families; 2000. [Google Scholar]
- 20.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington: University of Vermont, Research Center for Children, Youth & Families; 2001. [Google Scholar]
- 21.Conners CK. Conners' Continuous Performance Test II: Technical Guide and Software Manual North Tonawanda. New York: Multi-Health Systems; 2002. [Google Scholar]
- 22.Dlugos D, Shinnar S, Cnaan A, et al. Pretreatment EEG in childhood absence epilepsy: associations with attention and treatment outcome. Neurology 2013;81:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg AT, Smith SN, Frobish D, et al. Special education needs of children with newly diagnosed epilepsy. Dev Med child Neurol 2005;47:749–753. [DOI] [PubMed] [Google Scholar]
- 24.Almane D, Jones JE, Jackson DC, Seidenberg M, Hermann BP. The social competence and behavioral problem substrate of new- and recent-onset childhood epilepsy. Epilepsy Behav 2014;31:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vannest J, Tenney JR, Gelineau-Morel R, Maloney T, Glauser TA. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2015;45:85–91. [DOI] [PubMed] [Google Scholar]
- 26.Samaitiene R, Norkuniene J, Jurkeviciene G, Grikiniene J. Behavioral problems in children with benign childhood epilepsy with centrotemporal spikes treated and untreated with antiepileptic drugs. Medicina 2012;48:338–344. [PubMed] [Google Scholar]
- 27.Oostrom KJ, Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Behavioral problems in children with newly diagnosed idiopathic or cryptogenic epilepsy attending normal schools are in majority not persistent. Epilepsia 2003;44:97–106. [DOI] [PubMed] [Google Scholar]
- 28.Shinnar S, Cnaan A, Hu F, et al. Long-term outcomes of generalized tonic-clonic seizures in a childhood absence epilepsy trial. Neurology 2015;85:1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg AT, Levy SR, Testa FM, Blumenfeld H. Long-term seizure remission in childhood absence epilepsy: might initial treatment matter? Epilepsia 2014;55:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.