Abstract
Background
Observational studies have reported a positive association between body mass index (BMI) and ovarian cancer risk. However, questions remain as to whether this represents a causal effect, or holds for all histological subtypes. The lack of association observed for serous cancers may, for instance, be due to disease-associated weight loss. Mendelian randomization (MR) uses genetic markers as proxies for risk factors to overcome limitations of observational studies. We used MR to elucidate the relationship between BMI and ovarian cancer, hypothesizing that genetically predicted BMI would be associated with increased risk of non-high grade serous ovarian cancers (non-HGSC) but not HGSC.
Methods
We pooled data from 39 studies (14 047 cases, 23 003 controls) in the Ovarian Cancer Association Consortium. We constructed a weighted genetic risk score (GRS, partial F-statistic = 172), summing alleles at 87 single nucleotide polymorphisms previously associated with BMI, weighting by their published strength of association with BMI. Applying two-stage predictor-substitution MR, we used logistic regression to estimate study-specific odds ratios (OR) and 95% confidence intervals (CI) for the association between genetically predicted BMI and risk, and pooled these using random-effects meta-analysis.
Results
Higher genetically predicted BMI was associated with increased risk of non-HGSC (pooled OR = 1.29, 95% CI 1.03-1.61 per 5 units BMI) but not HGSC (pooled OR = 1.06, 95% CI 0.88-1.27). Secondary analyses stratified by behaviour/subtype suggested that, consistent with observational data, the association was strongest for low-grade/borderline serous cancers (OR = 1.93, 95% CI 1.33-2.81).
Conclusions
Our data suggest that higher BMI increases risk of non-HGSC, but not the more common and aggressive HGSC subtype, confirming the observational evidence.
Keywords: Body mass index, obesity, ovarian neoplasms, Mendelian randomization analysis
Key Messages
Observational studies had reported a positive association between BMI and overall risk of ovarian cancer, but it was unclear whether the observed differences by subtype—no association for serous cancers but an association for the other subtypes—were meaningful or whether the observed associations represent a causal effect.
We used Mendelian randomization to clarify the relationship between BMI and risk of ovarian cancer.
Our study provides the clearest evidence to date that obesity increases risk of non-high grade serous ovarian cancer (non-HGSC) for women of European ancestry.
Our results also support the absence of a relationship between BMI and risk of the more aggressive high-grade serous ovarian cancers (HGSC), confirming evidence from previous observational studies.
This study confirms the clinical relevance of elevated BMI to risk of some subtypes of ovarian cancer; thus interventions to reduce obesity may alleviate the worldwide burden from non-HGSC.
Introduction
Observational studies, including two recent large pooled analyses, have reported a positive association between body mass index (BMI) and risk of ovarian cancer.1,2 In both, the association was observed only for non-serous cancers. However, although the subtype-specific estimates reported by the two pooled analyses were very similar,1,2 the authors reached different conclusions about whether the differences by subtype were meaningful. Potentially, the lack of association seen for invasive serous ovarian cancer, the most aggressive subtype accounting for 62% of adenocarcinomas,3 could result from reverse causality because of disease-associated weight loss before diagnosis. Furthermore, given the potential for biases and confounding in observational studies, the observed association with non-serous ovarian cancer might not reflect a causal effect. Mendelian randomization (MR) has the potential to overcome these limitations by using genetic markers as proxies [instrumental variables (IVs)] for conventionally measured traits in observational studies.4 We used MR to clarify the relationship between BMI and risk of ovarian cancer, using data from the international Ovarian Cancer Association Consortium (OCAC). Based on existing data and the current understanding that low- and high-grade serous ovarian cancers (HGSC) represent distinct entities,5 our a priori hypothesis was that genetically predicted BMI would be associated with increased risk of non-HGSC but not HGSC.
Methods
Study population and data available
We pooled data from 39 OCAC studies6 which included 14 047 cases and 23 003 controls, all of whom had > 90% European ancestry and were genotyped via the Collaborative Oncological Gene-Environment Study;7 22 studies were population-based and 17 were clinic- or family registry-based. Nine case-only studies were grouped with case-control studies in the same region (Table 1; Supplementary Table S1, available as Supplementary data at IJE online). Cases included women with primary ovarian, fallopian tube or peritoneal cancer. All studies provided demographic data and tumour characteristics (site, behaviour, grade, FIGO (Fédération Internationale de Gynécologie Obstétrique)/SEER (Surveillance, Epidemiology and End Results Program) stage and histology). A subset provided lifestyle data for > 50% of their participants, including usual weight 1 or 5 years before diagnosis (cases) or interview (controls), adult height, parity, oral contraceptive (OC) use, family history of cancer, education, smoking, menopausal status and hormone replacement therapy (HRT) use.
Table 1.
Characteristics of 39 OCAC studies and 37 050 participants of European ancestry included in the Mendelian randomization analysis
| Type of study | Study acronyma,b,c | Country | Diagnosis (years) | Median (range) age at diagnosis | Invasive HGSC (N) | Invasive non-HGSC (N)d | Borderline cases (N) | Median (interquartile range) BMIe |
|---|---|---|---|---|---|---|---|---|
| Population-based | AUS | Australia | 2002-06 | 58 (19-80) | 508 | 224 | 1 | 25.9 (22.7-29.7) |
| DOV | USA | 2002-09 | 57 (35-74) | 510 | 255 | 327 | 25.1 (22.2-29.5) | |
| GER | Germany | 1993-98 | 57 (21-75) | 81 | 62 | 24 | − | |
| HAW | USA | 1993-2008 | 56 (27-87) | 36 | 22 | 20 | 24.4 (22.0-28.8) | |
| HOC | Finland | 1975-99 | 46 (18-86) | 106 | 76 | 8 | − | |
| HOP | USA | 2003-09 | 58 (25-94) | 338 | 167 | 71 | 27.4 (23.6-32.2) | |
| MAL | Denmark | 1994-99 | 57 (31-80) | 197 | 204 | 138 | 23.6 (21.5-26.1) | |
| MCC | Australia | 1990-2008 | 65 (45-79) | 31 | 23 | 0 | 26.6 (23.2-29.0) | |
| NCO | USA | 1999-2008 | 57 (20-75) | 373 | 255 | 171 | 26.1 (22.8-30.5) | |
| NEC | USA | 1992-2003 | 52 (21-78) | 367 | 243 | 232 | 24.7 (22.0-28.6) | |
| NJO | USA | 2002-09 | 60 (25-88) | 92 | 62 | 0 | 25.9 (22.3-30.4) | |
| NOR | Norway | 2001-10 | 51 (18-86) | 123 | 64 | 12 | − | |
| NTH | Netherlands | 1997-2008 | 55 (18-83) | 94 | 139 | 3 | 24.5 (22.2-27.0) | |
| OVA | Canada | 2002-09 | 58 (19-80) | 344 | 186 | 161 | − | |
| POL | Poland | 2000-04 | 56 (24-74) | 101 | 69 | 0 | 23.8 (22.0-26.4) | |
| SEA | UK | 1998-2011 | 57 (19-78) | 643 | 599 | 76 | − | |
| SOC | UK | 1993-98 | 62 (22-92) | 91 | 116 | 20 | − | |
| SRO | Scotland | 1999-2001 | 59 (34-84) | 89 | 31 | 0 | − | |
| STA | USA | 1997-2002 | 50 (20-64) | 141 | 81 | 10 | − | |
| TOR | Canada | 1995-2007 | 58 (26-85) | 339 | 205 | 0 | 25.7 (23.1-29.1) | |
| UCI | USA | 1993-2005 | 56 (18-86) | 154 | 102 | 141 | 24.9 (21.9-29.1) | |
| USC | USA | 1992-2010 | 57 (22-82) | 418 | 187 | 152 | 24.2 (21.7-28.1) | |
| Clinic-based | BAV | Germany | 2002-08 | 58 (24-83) | 42 | 41 | 5 | 25.4 (22.7-28.7) |
| BEL | Belgium | 2007-10 | 46 (19-87) | 188 | 74 | 0 | − | |
| HJO | Germany | 2007-11 | 54 (18-88) | 136 | 43 | 13 | − | |
| HMO | Belarus | 2006-11 | 45 (22-76) | 50 | 20 | 0 | − | |
| HSK | Germany | 2000-07 | 58 (18-81) | 103 | 21 | 9 | − | |
| LAX | USA | 1989-2008 | 58 (31-88) | 213 | 43 | 0 | − | |
| MAY | USA | 2000-2010 | 61 (20-93) | 516 | 154 | 79 | 26.1 (23.0-30.3) | |
| MDA | USA | 1997-2009 | 62 (23-88) | 190 | 59 | 0 | − | |
| MSK | USA | 1997-2010 | 57 (18-89) | 354 | 50 | 0 | − | |
| ORE | USA | 2007-11 | 58 (22-86) | 40 | 11 | 9 | − | |
| POC | Poland | 1998-2008 | 55 (23-82) | 200 | 81 | 0 | − | |
| PVD | Denmark | 2004-09 | 63 (30-88) | 121 | 39 | 0 | − | |
| RMH | UK | 1993-96 | 52 (26-73) | 49 | 60 | 7 | − | |
| UKO | UK | 2006-10 | 63 (19-89) | 329 | 277 | 0 | − | |
| WOC | Poland | 1997-2010 | 44 (20-81) | 131 | 45 | 2 | − | |
| Familial registry | GRR | USA | 1981-2012 | 48 (21-83) | 72 | 33 | 0 | − |
| UKR | UK | 1991-2009 | 54 (24-77) | 23 | 11 | 0 | − |
aSee Supplementary Table S1 for study names and references. BMI, body mass index; HGSC, high-grade serous ovarian cancer; OCAC, Ovarian Cancer Association Consortium.
bFor analysis, we combined case-only with case-control sites: HSK combined with GER; GRR with HOP; PVD with MAL; RMH, SOC, SRO, UKR with SEA and UKO; ORE with DOV; LAX with UCI.
cNineteen studies (AUS, BAV, DOV, GER, HAW, HOP, MAL, MAY, NEC, NJO, NTH, POL, PVD, SEA, STA, TOR, UCI, UKO, USC) were used in menopausal/hormonal replacement therapy analyses as they provided these data for > 50% of participants.
dHistological subtypes other than serous, mucinous, endometrioid and clear cell carcinoma are not included.
eRecent BMI (1-5 years before diagnosis). BMI is summarized for 16 studies where > 50% participants had data available. These 16 studies were also used in conventional BMI analyses, as they provided data on potential confounders (parity, use of oral contraceptives and hormone replacement therapy, and family history of ovarian or breast cancer) for > 50% of participants. BMI, body mass index; HGSC, high-grade serous ovarian cancer; OCAC, Ovarian Cancer Association.
Outcome variables
For primary analysis, we classified cases as invasive HGSC, invasive non-HGSC and borderline (low malignant potential). The HGSC group (n = 7933) included all invasive serous cancers except low-grade (G1) (n = 469). We classified invasive serous cancers of unknown grade (n = 1452) and primary peritoneal cancers of unknown behaviour (n = 44) as HGSC because in both instances the majority would be HGSC. The non-HGSC-group (n = 4434) included G1 serous cancers and all invasive mucinous, endometrioid and clear cell cancers. The third group included borderline tumours (n = 1680) of any histology.
For secondary analysis by cancer site, we subdivided HGSC into ovarian/fallopian tube and primary peritoneal cancers. Two studies (AUS, SRO), where < 20% of women with peritoneal tumours were genotyped, were excluded from peritoneal analyses. For secondary analysis by histological subtype/behaviour, we divided the non-HGSC and borderline groups into four sub-categories: invasive low-grade and borderline serous cancers; invasive and borderline mucinous cancers; invasive endometrioid cancers; and invasive clear cell carcinomas.
Genetic risk score
Samples were genotyped using a custom-designed Illumina genotyping array (iCOGS) comprising over 200 000 single nucleotide polymorphisms (SNPs).7 Genotyped SNPs that were not in Hardy-Weinberg equilibrium, or with discordant duplicate samples or call rates < 95% or 99% [depending on SNP minor allele frequencies (MAF)], were excluded.7 Approximately 15 million additional SNPs were imputed from measured genotypes using 1000 Genome Project data.7,8
We used 87 of 97 loci reported to be associated with BMI in a meta-analysis of genome-wide association studies conducted by the Genetic Investigation of ANthropometric Traits (GIANT) Consortium (Supplementary Table S2, available as Supplementary data at IJE online).9 We excluded three loci which were associated with BMI only among men in the GIANT analysis, and seven loci where the GIANT SNP was not genotyped on iCOGS and was imputed with a quality score (estimated correlation between imputed and true genotype, r2) of < 0.6 in our data. Overall, 12 selected SNPs were genotyped and 75 imputed. We used imputed genotype probabilities where genotyped values were missing (< 0.7%, all genotyped SNPs). We constructed a weighted genetic risk score (GRS) for BMI by summing alleles associated with higher BMI across the 87 SNPs, assuming additive effects based on evidence from GIANT.9 We weighted alleles by β-coefficients for their association with BMI reported by GIANT investigators. All MAFs were > 5% in controls (except for two SNPs with MAFs of 4.7% and 2.8%), and were consistent with GIANT data.
Statistical analysis
We examined associations between the GRS and potential confounders of the BMI-ovarian cancer relationship using chi-square statistics or analysis of variance, stratified by study. In a two-stage predictor-substitution MR approach using individual-level data,10,11 we used multivariable logistic regression to model case-control status on BMI predicted by the GRS within each study. First, we predicted BMI from the GRS by using linear regression in 10 085 controls from 16 studies with BMI data available for > 50% of women. The model regressed BMI on the GRS, adjusting for age and the first five principal components from a principal-components analysis in European-ancestry OCAC participants.7 We applied the results of this model to predict BMI from the GRS for the whole study population (14 047 cases and 23 003 controls). In the second stage, we used logistic regression to determine the association between case-control status and this genetically predicted BMI, adjusted for age and the principal components. As MR is relatively new with multiple approaches proposed, we also tested alternative methods including the control function estimator (adjusting for residual variation in BMI not predicted by the GRS),10,12 the sub-sample estimator13 and inverse-variance weighted and likelihood-based MR (combining summary data across SNPs).14 The resulting odds ratios (OR) and 95% confidence intervals (CI) were very similar to those from our primary analysis, and so are not reported here. The robust standard errors obtained using seemingly unrelated regression and the delta method13 were identical to those estimated in our primary analysis.
For the primary analyses, study-specific IV-estimates per 5-unit increase in genetically predicted BMI were pooled to generate odds ratios (pOR) and 95% CI using random-effects meta-analysis.15,16 We also compared HGSC and non-HGSC directly in a single pooled model comparing HGSC vs non-HGSC cases, stratified by study. We examined inter-study heterogeneity of the association between the GRS and ovarian cancer risk by inspecting Cochran’s I2 and P-values for heterogeneity.17
We conducted sensitivity analyses including: removing two studies where MAFs for 27 or more SNPs (> 30%) exceeded two standard deviations from the mean; restricting the GRS to 56 SNPs with imputation quality scores ≥ 0.9; using a single-SNP instrument in the locus explaining most variation (FTO); and weighting the GRS using published β-coefficients for SNP associations with BMI in women.9 We also conducted MR-Egger regression18 to assess the robustness of our findings to pleiotropy.
Secondary analyses by tumour site and behaviour/histology were conducted using single models stratified by study, to maximize power. Similarly, we explored whether menopausal status or HRT use modified the relationship by conducting stratified models (women grouped as: pre-/peri-menopausal; postmenopausal without HRT; postmenopausal with HRT). Information on menopausal status and HRT use was available for 21 938 women (59.2%) from 19 studies. Among 16 studies with BMI and confounder data, we conducted traditional epidemiological analysis modelling case-control status on BMI, adjusted for age, parity, OC use, HRT use and family history of ovarian or breast cancer, stratified by study, for comparison with IV-estimates among the same women.
Analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC) and STATA 13.0 (StataCorp LP, College Station, TX) software. This analysis and each contributing study received approval from the appropriate institutional review board or equivalent committee. All participants provided written informed consent.
Results
Population characteristics
The 39 studies were conducted in Europe, North America and Australia (Table 1) and included 12 367 women with invasive cancer, 1680 with borderline tumours (from 20 studies), and 23 003 control women. The median diagnosis year was 2003, with 74.4% of cases diagnosed after 2000. Participants were aged between 18 and 92 (median 57) years. Median BMI ranged from 23.6 to 27.4 kg/m2 across 16 studies with these data, and was 25.0 (interquartile range 22.3–29.1) kg/m2 for controls and 25.4 (22.4–29.8) kg/m2 for cases (P < 0.001). Mean age varied by histological subtype: women with HGSC were older, and women with low-grade or borderline serous cancers younger, than controls (Supplementary Table S3, available as Supplementary data at IJE online). Compared with controls, a higher proportion of cases (all subtypes combined) were obese (BMI > 30kg/m2, P < 0.001).
Characteristics of the genetic risk score
The GRS was normally distributed among OCAC controls. GRS values ranged from 9.11 to 15.88 (median 12.62; interquartile range 12.01–13.23). Alone, the GRS explained 1.6% of variance in BMI among OCAC controls. After adjusting for age and principal components, the GRS explained 3.0% (partial R2 = 1.7%) (first-stage regression partial F-statistic = 172.0, P < 0.001). A 1-unit increase in GRS was associated with a 0.8 kg/m2 increase in BMI. Average BMI was 1.9 kg/m2 higher in the highest GRS quartile than the lowest.
There was no evidence of inter-study heterogeneity (I2 = 32%, P-heterogeneity = 0.11) in the relationship between the 87-SNP GRS and BMI among controls, nor for the simplified 56-SNP (I2 = 28%, P-heterogeneity = 0.14) GRS, or FTO (I2 = 21%, P-heterogeneity = 0.22) (Supplementary Figure S1, available as Supplementary data at IJE online). While BMI was associated with potential confounders of the BMI-ovarian cancer association (including parity, OC use and menopausal status, all P < 0.001), the GRS was not (all P > 0.10) (Supplementary Table S4, available as Supplementary data at IJE online). We also saw no substantial variation in GRS values by levels of potential confounders within individual studies.
The ORs (95% CI) for ovarian cancer per 1-unit increase in the GRS were 1.04 (1.01–1.08) for non-HGSC, 1.01 (0.98–1.04) for HGSC and 1.05 (0.99–1.11) for borderline tumours.
Association between genetically predicted BMI and primary outcomes
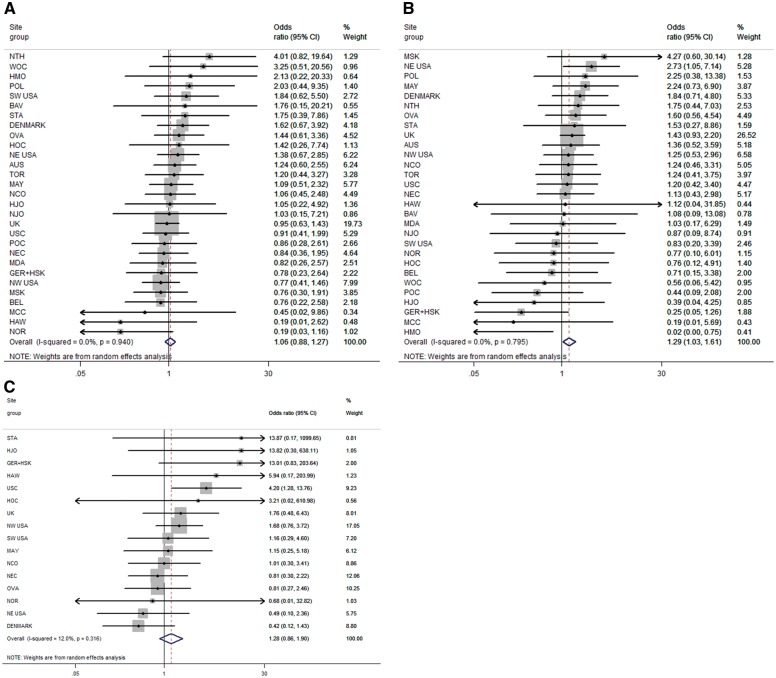
Higher genetically predicted BMI was associated with increased risk of non-HGSC (pOR = 1.29, 95% CI 1.03–1.61 per 5-unit predicted BMI increase) but not HGSC (pOR = 1.06, 95% CI 0.88–1.27) (Figure 1A, B;Table 2). The same pattern was seen for the simplified GRS comprising 56 SNPs (pOR = 1.33 vs 1.10 for non-HGSC and HGSC, respectively), and for FTO (pOR = 1.51 vs 0.88). Tests for heterogeneity between HGSC and non-HGSC gave P = 0.24 and P = 0.23 using the 87- and 56-SNP GRSs, respectively, and P = 0.046 when we predicted BMI from FTO alone. The pooled OR for borderline tumours was 1.28 (95% CI 0.86–1.90) (Figure 1C;Table 2).
Figure 1.
Association between increasing genetically predicted BMI and risks of high-grade serous, non-high grade serous, and borderline ovarian tumours. Increasing BMI per 5 kg/m2 predicted by weighted 87-locus genetic risk score among 39 studies. (A) Risk of high-grade serous cancers. (B) Risk of non-high grade serous cancers. (C) Risk of borderline ovarian tumours. Site groupings (case-only with case-control sites) are: GER+HSK (HSK with GER); NE USA (GRR with HOP); DENMARK (PVD with MAL); UK (RMH, SOC, SRO, UKR with SEA and UKO [for (A) and (B)]) or (RMH, SOC with SEA [for (C)]); NW USA (ORE with DOV); SW USA (LAX with UCI).
Table 2.
Association between increasing BMI (per 5 units)–predicted by a weighteda 87-locus genetic risk score–and risk of ovarian cancer by histological subtype, stratified by study
| Histological subtype | N studies | N controls | N cases | Odds ratios (95% CI)b |
|---|---|---|---|---|
| Primary outcomes | ||||
| High-grade serous | 39 | 23 003 | 7933 | 1.06 (0.88-1.27) |
| Non-high grade serous | 39 | 23 003 | 4434 | 1.29 (1.03-1.61) |
| Borderline | 20 | 16 463 | 1680 | 1.28 (0.86-1.90) |
| Secondary outcomes | ||||
| Serous | ||||
| High-grade ovary/tubal | 39 | 23 003 | 7466 | 1.06 (0.89-1.27) |
| High-grade peritoneal c | 37 | 22 026 | 447 | 1.77 (0.91-3.43) |
| Invasive low-grade and borderline | 39 | 23 003 | 1411 | 1.93 (1.33-2.81) |
| Mucinous (invasive and borderline) | 39 | 23 003 | 1563 | 1.18 (0.84-1.67) |
| Endometrioid | 39 | 23 003 | 2059 | 1.17 (0.87-1.59) |
| Clear cell | 39 | 23 003 | 962 | 1.27 (0.83-1.96) |
aWeights applied were β-coefficients for the relationship between each SNP and BMI as reported in a large meta-analysis of genome-wide association studies. BMI, body mass index; CI, confidence interval
bPooled odds ratios are reported for primary outcomes.
cExcludes two studies (AUS and SRO) where < 20% of women with primary peritoneal cancers were genotyped. BMI, body mass index; CI, confidence interval.
There was little evidence of inter-study heterogeneity in the association between genetically predicted BMI and ovarian cancer risk (Figure 1A, B, C). Results were similar when we used female-specific weights (β-coefficients) published by GIANT,9 when we removed two SNPs with MAF < 5% and when we excluded two studies (HMO, HOC) with extreme MAFs for ≥ 27 SNPs. The association between BMI and non-HGSC, but not HGSC, was seen when we excluded family registry-based studies or case-only studies. Excluding eight studies with tumour grade unknown for > 50% of invasive serous cases made little difference to HGSC results (pOR = 1.04, 95% CI 0.86–1.27). The results from an MR-Egger test suggested no bias from pleiotropy (P = 0.9 and P = 0.2 comparing traditional MR and MR-Egger results for HGSC and non-HGSC, respectively).
For women with GRS, BMI and confounder data, results of the conventional BMI analysis (Supplementary Table S5, available as Supplementary data at IJE online) and IV analysis (Table 2) were similar, although the association with non-HGSC was weaker (adjusted OR = 1.18, 95% CI 1.13–1.23 per 5 kg/m2) in the former, suggesting the true association might be stronger than that seen in conventional epidemiological analyses.
Secondary outcomes
Secondary analyses stratifying HGSC by cancer site and subtype suggested that the lack of association with BMI might hold only for HGSC of the ovary and fallopian tube (Table 2). The estimate for HGSC of the peritoneum was elevated, but the CI was wide and crossed null (OR = 1.77, 95% CI 0.91–3.43) (Table 2). For non-HGSC sub-categories, the strongest association was seen for invasive low-grade and borderline serous cancers (OR = 1.93, 95% CI 1.33–2.81) and the weakest for endometrioid (OR = 1.17, 95% CI 0.87–1.59) and mucinous (OR = 1.18, 95% CI 0.84–1.67) cancers (Table 2), but the relatively small numbers (in the MR context) led to wide and overlapping confidence intervals.
The associations with HGSC and non-HGSC did not vary substantially by menopausal status or combined menopausal status/HRT use. The association between genetically predicted BMI and non-HGSC was slightly stronger for premenopausal women (OR = 1.62, 95% CI 0.88–3.01) compared with postmenopausal HRT users (OR = 1.26, 95% CI 0.57–2.82) and non-users (OR = 1.17, 95% CI 0.61–2.24).
Discussion
Having established the GRS as an appropriate instrument for BMI in our sample, we used this to assess the relationship between BMI and ovarian cancer risk for women of European ancestry. Our data suggest a likely causal effect of BMI on risk of non-HGSC, but do not support an association with the more common HGSC subtype. Secondary analyses had limited power so CI were wide; however, they suggested that the association was strongest for low-grade/borderline serous cancers, that higher BMI might increase risk of HGSC of the peritoneum and that the association with non-HGSC might be stronger for premenopausal women.
Ovarian cancer is a heterogeneous disease: the separate histological subtypes display distinct molecular profiles and have different risk factors.19,20 Our primary findings for genetically predicted BMI are consistent with results of the two large pooled analyses (one including 11 OCAC studies)1,2 which investigated conventionally measured BMI and ovarian cancer risk by histological subtype, although our data suggest the association with non-HGSC may be somewhat stronger than previously reported. Overall, our results suggest that the previously reported relationship with non-HGSC is probably not due to bias or confounding, and the lack of association with HGSC is unlikely to arise from reverse causality. We observed a positive association with BMI and risk of endometrioid tumours of the same magnitude (pOR = 1.17 per 5 kg/m2) seen in the previous OCAC study,1 but the 95% CI around our estimate (0.87–1.59) is wide. Similarly, we observed odds ratios for borderline tumours and for low-grade/borderline serous cancers which were comparable to findings from the previous OCAC study.1 Few studies have investigated an association between BMI and primary peritoneal cancers, but if a causal effect exists, rising obesity prevalence would result in increasing incidence of these cancers, which has been observed.21
Obesity has been associated with increased cancer risk at multiple body sites.22 Mechanisms hypothesized to explain this involve lipid signalling, inflammatory and adipokine pathways and insulin-like growth factor influencing cell proliferation.23 If adiposity affects ovarian cancer risk via a disrupted endocrine environment,23,24 then hormonal levels may modify this risk. Results by menopausal status and HRT use from previous studies have been inconsistent. In one pooled analysis, the BMI association was restricted to non-users of HRT,2 whereas another reported that the association for non-serous invasive cancers did not differ by menopausal status or HRT use.1 Our findings do not resolve this controversy.
The advantage of MR is that it allows non-causal explanations that might affect epidemiological studies (bias, confounding and reverse causality) to be excluded, provided several underlying assumptions are met.25 We satisfied the first assumption by using SNPs most strongly associated with BMI in a large external study, and confirming the association between GRS and BMI in OCAC. The F-statistic also exceeded the threshold below which weak-instrument bias is likely.26 To support the second MR assumption,25 we confirmed that the GRS was not associated with potential confounders of the BMI-ovarian cancer association. Our analysis has a number of other strengths. The variance in BMI explained by the GRS was consistent with GIANT results,9 and only modest inter-study heterogeneity was observed in the association between the GRS and BMI. Our primary results were consistent across multiple GRS versions, different subgroups of the study population, various MR methods and when using female-specific weights. The weaker association with non-HGSC risk for conventional BMI than genetically predicted BMI may arise from measurement error or residual confounding in observational studies.
The chief concerns regarding the validity of MR studies are: an absence of appropriate variants, due, for example, to canalization (developmental compensation for the effects of the SNPs); population structure influencing both SNP frequency and risk; and pleiotropy or linkage disequilibrium whereby the IV might influence risk via a non-BMI pathway.4,12,25 Canalization can weaken the association between the IV and risk factor but this effect, if present in our sample, did not prevent the GRS from being an adequate instrument for BMI. Population structure and/or pleiotropy may violate the third MR assumption (that the IV influences the outcome only via the risk factor).25 A limitation of MR studies is that this assumption cannot be tested directly. However, our IV estimates are likely to represent BMI-outcome effects for the following reasons. We restricted our analysis to an ethnically homogeneous analysis sample and adjusted models for principal components of population substructure. Using multiple independent variants can minimize potential bias from pleiotropy,27 and the biological effect of this IV is becoming more fully understood. The SNPs do not show much evidence of pleiotropy in genome-wide association studies, and none has been identified as, or is in linkage disequilibrium with, an ovarian cancer susceptibility SNP. In addition, MR-Egger regression results suggested a lack of bias from pleiotropy.
The significance of this study lies in the clear evidence it provides that obesity increases risk of non-HGSC for women of European ancestry. Our results do not support an association between obesity and risk of the more common and more aggressive HGSC subtype. This study also provides reassurance that the results of the large pooled epidemiological studies were not seriously biased. As the fifth most common cancer and the sixth most common cause of cancer death for women in more developed regions, ovarian cancer is responsible for a substantial health burden.28 The major risk factors identified to date, low parity and non-use or short-duration use of OCs, have barriers to their modification, especially at older ages. Given the high and increasing prevalence of overweight and obesity,29 our findings suggest that intervening on obesity may reduce the worldwide burden from these subtypes of ovarian cancer. This study adds to the body of evidence suggesting that maintaining healthy weight is important. Continued efforts should be made to develop effective interventions to reduce BMI and to identify women who would benefit most from these. Our results also suggest that we should pursue other avenues for prevention of HGSC. Further work is required to replicate these findings, to investigate the effects of adipose tissue distribution and to explore the mechanisms underlying the different associations for non-HGSC and HGSC.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by: the National Cancer Institute at the U.S. National Institutes of Health [K07-CA095666, K07-CA80668, K07-CA143047, K22-CA138563, N01-CN025403, N01-CN55424, N01-PC67001, N01-PC67010, P01-CA17054, P30-CA072720, P30-CA008748, P30-CA14089, P30-CA15083, P50-CA105009, P50-CA136393, P50-CA159981, R01-CA058860, R01 CA063678, R01 CA063682, R01-CA092044, R01-CA095023, R01-CA16056, R01-CA54419, R01-CA58598, R01-CA61107, R01-CA61132, R01-CA76016, R01-CA83918, R01-CA87538, R01-CA112523, R01-CA122443, R03-CA113148, R03-CA115195, U01-CA69417, U01-CA71966 and Intramural Research funds]; the European Commission's Seventh Framework Programme [agreement number 223175 HEALTH F2 2009-223175]; Cancer Research UK [C490/A16561, C536/A13086, C536/A6689, C1287/A10118, C1287/A 10710, C12292/A11174, C1281/A12014, C5047/A8384, C5047/A15007, C5047/A10692, and C8197/A16565]; a National Institutes of Health (CA128978), Cancer Post-GWAS Initiative [1U19-CA148537, 1U19-CA148065 and 1U19-CA148112 – the Genetic Associations and Mechanisms in Oncology (GAME‐ON) initiative]; the U.S. Department of Defense [DAMD17-02-1-0669, W81XWH-07-0449, DAMD17-02-1-0666, W81XWH-10-1-0280 and W81XWH-10-1-0341]; the Canadian Institutes of Health Research (CIHR) [MOP-86727 and MSH-87734 to L.E.K.] and the CIHR Team in Familial Risks of Breast Cancer; the Komen Foundation for the Cure; the Breast Cancer Research Foundation; the Ovarian Cancer Research Fund (thanks to donations by the family and friends of Kathryn Sladek Smith); the U.S. Army Medical Research and Materiel Command [DAMD17-01-1-0729 and DAMD17-02-1-0669]; the National Health and Medical Research Council of Australia [199600, 400281, 209057, 251533, 396414, 504715, 1073898 and fellowships to G.C-T. and P.M.W.]; Cancer Australia [Multi-State Grant Application Numbers 191, 211 and 182]; Cancer Council Queensland; Cancer Council Victoria; Cancer Council New South Wales; Cancer Council South Australia; Cancer Council Tasmania; Cancer Foundation of Western Australia; the ELAN Program of the University of Erlangen-Nuremberg; the Nationaal Kankerplan of Belgium; the German Federal Ministry of Education and Research of Germany Programme of Clinical Biomedical Research [01 GB 9401]; the German Cancer Research Center; the Roswell Park Cancer Institute Alliance Foundation [P30 CA016056]; the Rudolf-Bartling Foundation; the Helsinki University Central Hospital Research Fund; the National Institutes of Health/National Center for Research Resources/General Clinical Research Center [M01-RR000056]; an American Cancer Society Early Detection Professorship [SIOP-06-258-01-COUN to B.Y.K.]; the National Center for Advancing Translational Sciences (NCATS) [UL1TR000124 to B.Y.K.]; the Danish Cancer Society [94-222-52]; the Mermaid I project; the Mayo Foundation; the Minnesota Ovarian Cancer Alliance; the Fred C. and Katherine B. Andersen Foundation; the Cancer Institute of New Jersey; Helse Vest; the Norwegian Cancer Society; the Research Council of Norway; Radboud University Medical Centre; the Oregon Health and Science University (OHSU) Foundation; Pomeranian Medical University; the UK National Institute for Health Research Biomedical Research Centres at the University of Cambridge, University College London Hospital and the Royal Marsden Hospital; the Imperial Experimental Cancer Research Centre [C1312/A15589]; the U.S. Public Health Service [PSA-042205]; the Lon V. Smith Foundation [LVS-39420]; the Eve Appeal; the Oak Foundation; the California Cancer Research Program [00-01389V-20170 and 2II0200]; the Polish Ministry of Science and Higher Education [4 PO5C 028 14 and 2 PO5A 068 27]; and the Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Supplementary Material
Acknowledgments
We thank all the individuals who took part in this study and all the researchers, clinicians and technical and administrative staff who have made possible the many studies contributing to this work. In particular, for their contribution to the design and conduct of the individual studies that contributed to the analysis, we thank: D. Bowtell, A. deFazio, D. Gertig, A. Green, P. Parsons, N. Hayward and D. Whiteman (AUS); G. Peuteman, T. Van Brussel and D. Smeets (BEL); U. Eilber (GER); S. Reckemeyer, A. Korotkaia and S. Reina-Campanon (HJO); C. Hilker, S. Windebank and J. Vollenweider (MAY); I. Orlow, L. Paddock and L. Rodriguez-Rodriguez (NJO); the SEARCH team, C. Luccarini, C. Baynes, and D. Conroy (SEA); the Scottish Gynaecological Clinical Trials group and SCOTROC1 investigators (SRO); I. Jacobs, M.Widschwendter, E. Wozniak, A. Ryan, J. Ford, N. Balogun and C. Karpinskyj (UKO); and C. Pye (UKR).
Conflict of interest: P.A.F. is currently conducting research sponsored by Amgen, Novartis, Pfizer and Celgene and has received honoraria from Novartis, Pfizer, GSK, Amgen, Roche, Teva and Genomic Health. U.M. has stock ownership and research funding from Abcodia Ltd, a UCL spin-out company with an interest in biomarkers and ovarian cancer screening. D.E. has received fees for consultancy from Astra Zeneca.
References
- 1. Olsen CM, Nagle CM, Whiteman DC. et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer 2013;20:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med 2012;9:e1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howlader N, Noone AM, Krapcho M. et al. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute, 2015. [Google Scholar]
- 4. Davey Smith G, Ebrahim S.. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 5. Salvador S, Gilks B, Kobel M, Huntsman D, Rosen B, Miller D.. The fallopian tube: primary site of most pelvic high-grade serous carcinomas. Int J Gynecol Cancer 2009;19:58–64. [DOI] [PubMed] [Google Scholar]
- 6. Gayther SA, Song H, Ramus SJ. et al. Tagging single nucleotide polymorphisms in cell cycle control genes and susceptibility to invasive epithelial ovarian cancer. Cancer Res 2007;67:3027–35. [DOI] [PubMed] [Google Scholar]
- 7. Pharoah PDP, Tsai YY, Ramus SJ. et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet 2013;45:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abecasis GR, Altshuler D, Auton A. et al. A map of human genome variation from population-scale sequencing. Nature 2010;467:1061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Locke AE, Kahali B, Berndt SI. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burgess S. Identifying the odds ratio estimated by a two-stage instrumental variable analysis with a logistic regression model. Stat Med 2013;32:4726–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Didelez V, Meng S, Sheehan NA.. Assumptions of IV methods for observational epidemiology. Stat Sci 2010;25:22–40. [Google Scholar]
- 12. Palmer TM, Sterne JA, Harbord RM. et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am J Epidemiol 2011;173:1392–403. [DOI] [PubMed] [Google Scholar]
- 13. Pierce BL, Burgess S.. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol 2013;178:1177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burgess S, Butterworth A, Thompson SG.. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stukel TA, Demidenko E, Dykes J, Karagas MR.. Two‐stage methods for the analysis of pooled data. Stat Med 2001;20:2115–30. [DOI] [PubMed] [Google Scholar]
- 16. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JPT, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 18. Bowden J, Davey Smith G, Burgess S.. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagle CM, Olsen CM, Webb PM, Jordan SJ, Whiteman DC, Green AC.. Endometrioid and clear cell ovarian cancers: a comparative analysis of risk factors. Eur J Cancer 2008;44:2477–84. [DOI] [PubMed] [Google Scholar]
- 20. Risch H, Marrett L, Jain M, Howe G.. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol 1996;144:363–72. [DOI] [PubMed] [Google Scholar]
- 21. Goodman MT, Shvetsov YB.. Rapidly increasing incidence of papillary serous carcinoma of the peritoneum in the United States: fact or artifact? Int J Cancer 2009;124:2231–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L.. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet 2014;384:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Louie SM, Roberts LS, Nomura DK.. Mechanisms linking obesity and cancer. Biochim Biophys Acta 2013;1831:1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olsen CM, Green AC, Nagle CM. et al. Epithelial ovarian cancer: testing the ‘androgens hypothesis’. Endocr Relat Cancer 2008;15:1061–68. [DOI] [PubMed] [Google Scholar]
- 25. Didelez V, Sheehan N.. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res 2007;16:309–30. [DOI] [PubMed] [Google Scholar]
- 26. Staiger DO. Instrumental variables regression with weak instruments. Econometrica 1997;65:557–86. [Google Scholar]
- 27. Davey Smith G, Hemani G.. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23:R89–R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Agency for Research on Cancer. GLOBOCAN 2012. 2014. http://globocan.iarc.fr/ (23 December 2015, date last accessed). [Google Scholar]
- 29. Ng M, Fleming T, Robinson M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.