Abstract
Objective
Low inhibitory control and strong hedonic response towards food are considered to contribute to overeating and obesity. Based on previous research, the present study aimed at examining the potentially crucial interplay between these two factors in terms of long-term weight loss in people with obesity.
Methods
BMI, inhibitory control towards food, and food liking were assessed in obese adults prior to a weight reduction programme (OPTIFAST® 52). After the weight reduction phase (week 13) and the weight loss maintenance phase (week 52), participants' BMI was re-assessed.
Results
Baseline BMI, inhibitory control and food liking alone did not predict weight loss. As hypothesised, however, inhibitory control and food liking interactively predicted weight loss from baseline to week 13 and to week 52 (albeit the latter effect was less robust). Participants with low inhibitory control and marked food liking were less successful in weight reduction.
Conclusion
These findings underscore the relevance of the interplay between cognitive control and food reward valuation in the maintenance of obesity.
Key Words: Response inhibition, Go/no-go, Food liking, Overweight, Weight reduction
Introduction
The prevalence of obesity in Western countries has increased enormously during the last two decades and is considered one of the major public health concerns [1,2,3,4]. In most cases, obesity is a chronic condition that predisposes to a range of somatic diseases such as diabetes, cardiovascular disease and cancer [5,6,7]. A major factor contributing to the obesity epidemic is overeating, which is facilitated by the oversupply and promotion of highly palatable foods in Western societies [8,9]. However, not all people who are exposed to this ‘obesogenic environment’ show excessive food intake. One factor that is considered to contribute to excessive food intake refers to an altered hedonic processing of food cues [10]. People differ in their hedonic response to food, and those who experience greater pleasure in response to food are assumed to be more susceptible to external food cues. In support of this assumption, individuals with overweight/obesity reported greater liking of food than normal-weight individuals [11]. In addition, explicit ratings of food liking were positively associated with implicit wanting (i.e. the desire) for fatty and sweet food, and with binge eating [12,13]. Furthermore, neuroimaging studies found that increased activity in brain reward networks in response to food cues was associated with overweight and predicted weight gain [14,15] although findings are not completely consistent in this regard [16].
However, increased liking of food alone may not simply translate into excessive food intake as long as an individual is able to utilise inhibitory control to resist the temptation to eat excessively. Accordingly, the basic cognitive function of inhibitory control is considered to play just as important a role in the resistance to overeat [17,18]. The construct of inhibitory control refers to the ability to suppress, interrupt or delay pre-potent (automatic) behavioural responses towards external cues including food [19,20]. Thus, individuals with low inhibitory control are thought to be more vulnerable to the ubiquitous cues of highly palatable food in Western countries that trigger the urge to binge eat [17,21,22]. In line with this assumption, obesity was found to be associated with impulsive behaviour [23,24,25], and a recent meta-analysis found robust evidence for impaired inhibitory control in bulimic-type eating disorders, particularly in response to disorder-related stimuli such as food [26]. Similarly, individuals with obesity feature lower inhibitory control than normal-weight controls [27,28,29]. Furthermore, the idea that low inhibitory control contributes to overeating has received empirical support from experimental studies that examined actual food intake: Both spontaneously occurring and experimentally induced low inhibitory control was associated with greater amounts of food eaten during a subsequent taste test in the laboratory [30,31]. These studies strongly suggest that low inhibitory control contributes to overeating and thereby to obesity. However, long-term observations of the potential effects of low inhibitory control on actual weight change are rather scarce. Nederkoorn et al. [32] found that low inhibitory control at baseline was associated with less weight reduction in 19 children who completed an 8- to10-week outpatient weight reduction programme. Similarly, Kulendran et al. [33] found that increases in inhibitory control were associated with decreases in body weight in a larger sample of adolescents with obesity who attended a 2- to 8-week lifestyle and physical activity programme. Likewise, Pauli-Pott al. [34] found that those children and adolescents with overweight/obesity who succeeded in a 1-year weight reduction programme featured better inhibitory control than those who did not succeed. These studies clearly underscore the relevance of inhibitory control for overeating and obesity.
Taken together, there is considerable evidence that both increased hedonic response and decreased inhibitory control towards food contribute to overeating and thereby to the obesity epidemic. When viewed as a whole, one may further assume that these two factors may not act independently. Rather a combination of strong inhibitory control and low hedonic response towards food can be assumed to be most advantageous for people who aim to lose weight. In support of this notion, Nederkoorn et al. [18] have shown that the opposite combination of low inhibitory control and a strong preference for snack food (rather than both factors alone) predicted weight gain in female undergraduate students over a 1-year follow-up period.
The previous studies that examined the predictive value of inhibitory control for long-term weight change uniformly used the stop signal task to measure inhibitory control [19]. The stimuli used in this task are typically geometrical shapes and are thus not food-related. Thus, the task measures inhibitory control as a general (non-specific) cognitive function. It could be speculated that inhibitory control towards food cues is even more predictive for actual food intake [35,36]. In line with this assumption, inhibitory control in bulimic-type eating disorders is particularly low towards food-related stimuli [26]. Therefore, we applied an inhibitory control task containing food cues instead of geometrical shapes in the present study. In addition, the mentioned studies have almost exclusively examined children and adolescents [32,33,34]. Only one study examined adults but this one was restricted to predominantly normal-weight female students. Since prevalence rates for obesity are by far higher in adults than in children and adolescents [37], and because weight reduction appears most urgent in those who are already affected by obesity, it is important to increase our knowledge about the potential effects of cognitive food processing on the success of weight reduction programmes in adults with obesity. Therefore, the present study examined these factors in a sample of men and women with severe obesity who attended a physician-guided weight reduction programme. Given the empirical evidence outlined above, we hypothesized that participants featuring both relatively strong inhibitory control and low hedonic response towards food will be most successful in reducing their body weight during the weight reduction programme.
Material and Methods
Participants
The study sample consisted of 20 adult women (75s%) and men (25s%) with obesity, aged between 20 and 62 years (mean = 41.45 years, SD = 12.25 years) who took part in the OPTIFAST® 52 programme at a university hospital. The following main somatic diseases had been reported by the participants: hypertension (55s%), cardiac disease (5s%), diabetes type 1 (5s%), diabetes type 2 (20s%) and cancer (5s%). Mean BMI at the beginning of the programme was 43.84 ± 7.55 kg/m2. 20s% of the participants had class I obesity, another 20s% had class II obesity, and 60s% had class III obesity. Inclusion criteria were: obesity according to WHO criteria (BMI > 30 kg/m2), participation in the OPTIFAST 52 programme and a minimum age of 18 years. Exclusion criteria were: current psychotropic medication other than SSRI or current psychotherapeutic treatment, acute medical (e.g. electrolyte abnormalities) or psychiatric (e.g. acute suicidality) instability and a history of a brain lesion. The OPTIFAST 52 weight reduction programme is a 52-week lifestyle intervention including weekly group sessions and supervision by physicians, nutritional advisers, psychologists and physiotherapists. The programme is divided up into four phases: i) a 1-week introduction; ii) a 12-week active weight loss period with low-calorie formula diet; iii) a 8-week transition period focusing on the step-by-step substitution of the formula diet by self-prepared low-calorie food, and iv) a 31-week long-term weight management phase in which participants are guided regarding their optimal calorie intake (put together the latter two phases can be regarded as an overall weight loss maintenance phase). Three participants were lost at 13-week follow-up, and further 4 participants at 52-week follow-up. Thus, the findings for the 13-week and the 52-week follow-up assessments are based on the data from 17 and13 completers, respectively. All participants provided written informed consent and received financial compensation. The study protocol was approved by the local ethics committee.
Measures
Food-related inhibitory control was measured by a go/no-go paradigm [38]. In this task, participants were required to respond to frequently occurring go stimuli (non-food images) by pressing a response key and to withhold their response towards infrequently occurring (20s%) no-go stimuli (images of high caloric food such as chocolate, cake, crisps and pizza). Before the experiment, participants were asked to select those eight food pictures from a set of 85 custom-made pictures showing high-caloric sweet and savoury foods that best matched with their most favourite foods. Non-food images (household and office items, matched for colour and visual complexity) were the same for all participants. The experiment was comprised of eight blocks. In each block, 40 stimuli were presented in pseudo-randomised order. The first stimulus in each block was a go stimulus, and no more than two no-go stimuli appeared in a row. The time between two no-go stimuli varied between 1,500 and 31,500 ms (mean = 6,000 ms). Before each block, participants were visually instructed about the go stimulus in the following block (2,000 ms). Each block ended with a rest period of 13,000 ms during which participants viewed a fixation cross on the screen. Each trial lasted 1,500 ms, including 500 ms for stimuli presentation. During the inter-trial interval of 1,000 ms, a fixation cross was presented. One no-go block lasted 75,000 ms. A high mean percentage of false reactions to no-go stimuli (i.e. commission errors) was used as an indicator of low food-related inhibitory control.
Food liking was assessed by subjective ratings on a Likert scale of how much participants liked the eight food items they had chosen for the go/no-go paradigm (‘How much do you like eating this food?’; score range: 1–10). The mean rating score was used as an indicator of explicit hedonic response towards food cues.
Body weight and height were determined objectively by staff members. Participants were weighed in underwear using a digital scale.
Procedure
Participants were assessed for body weight and height, inhibitory control and hedonic response towards food before entering the weight reduction programme. Baseline assessment took place in the morning hours after an overnight fast. Upon arrival, participants received a standardised, light breakfast in the outpatient department (1 white bread roll, butter, either cheese or sausage, 1 cup of tea or coffee). This was done to rule out potential confounding effects of energy imbalance. Thereafter, they underwent the experimental paradigm. After the weight reduction phase (at week 13) and weight loss maintenance phase (at week 52), participants were re-assessed for their body weights.
Statistical Analyses
Percent BMI reduction was used as the primary outcome variable. Statistical analyses were performed using IBM SPSS Statistics version 21 (SPSS Inc., IBM Corporation, Armonk, New York, NY, USA), the statistical platform R 2.14.0 [39] and the PROCESS macro for SPSS [40]. The significance level for all analyses was set at α = 0.05. In order to test our hypothesis that inhibitory control and hedonic response towards food interactively predict weight loss, we first conducted two separate hierarchical linear regression analyses with s% BMI reduction from baseline to the end of the weight reduction phase (first regression), and from baseline to the end of the weight loss maintenance phase (second regression) as outcome variable. In the first step of both regressions, baseline BMI was entered as a predictor to control for baseline differences in body weight. In the second step, inhibitory control and food liking were entered as separate variables. Finally, in the third step, the interaction term of inhibitory control and food liking was entered. All predictor variables were mean centred. Due to the small sample size, 95s% bias-corrected and accelerated (BCa) Bootstrap confidence intervals (CIs), based on 1,000 re-samples, were calculated in addition to examine the robustness (i.e., the stability) of effects [41,42]. The interpretation of the CIs is straightforward: if it does not contain zero, the effect can be considered as significant and robust at the given significance level.
Results
BMI, go/no-go and food liking data are summarised in table 1. Treatment completers did not differ from drop-outs in terms of baseline BMI, food liking and food-related inhibitory control (all p > 0.098). Average s% BMI reduction reached 21.7s% (SD = 6.6s%) from baseline to the end of the weight reduction phase, and 23.8s% (SD = 8.5s%) from baseline to the end of the weight loss maintenance phase. Food-related inhibitory control and food liking were uncorrelated, r = −0.186, p = 0.433. In the first regression analysis, baseline BMI was no significant predictor of percent BMI reduction from baseline to the end of the weight reduction phase (B = 0.002, SE B = 0.002, β = 0.254, p = 0.325). Likewise, neither food-related inhibitory control (B = −0.003, SE B = 0.002, β = −0.452, p = 0.099) nor food liking alone (B = −0.001, SE B = 0.007, β = −0.033, p = 0.908) predicted weight reduction during the weight reduction phase of the programme. However, the interaction term of food-related inhibitory control and food liking emerged as a significant predictor of weight reduction (B = 0.001, SE B = 0.000, β = 0.608, p = 0.020). The proportion of the total variance in s% BMI reduction uniquely attributable to the interaction of food-related inhibitory control and food liking was 28s%. The overall model including baseline BMI, food-related inhibitory control, food liking and the interaction term of food-related inhibitory control and food liking explained 53s% of the variance in s% BMI reduction.
Table 1.
Descriptive statistics
| Variable | M (SD) |
|---|---|
| BMI at baseline, kg/m2 | 43.84 (7.55) |
| BMI at end of weight reduction phase, kg/m2 | 34.85 (5.97) |
| BMI at end of weight loss maintenance phase, kg/m2 | 34.55 (5.17) |
| Food liking (possible score range 1–10) | 6.54 (2.51) |
| Inhibitory control | 15.47 (9.82) |
Food liking = subjective rating of food liking; Inhibitory control = Percent false reactions to go/no-go stimuli (food images) in the no-go task.
Visual inspection of histograms and plots as well as the Shapiro Wilk test (p = 0.860) showed that standardised residuals were normally distributed. Furthermore, both the Breusch-Pagan (χ2(1) = 2.990, p = 0.559) and the Koenker test (χ2(1) = 2.204, p = 0.698) confirmed homoscedasticity. In addition, all cases were within the boundary of three times the average leverage (k s+ 1 / n = 0.294 × 3 = 0.882). However, we found two cases that had an undue influence on the parameters of the regression model (i.e., multivariate outliers). Both cases had Cook's distance and standardised DFBeta values above 1. Performing the regression analysis again with these two cases excluded, however, resulted in the same pattern of findings (table 2). Likewise, a robust regression based on M estimation with bi-square weighting using iteratively re-weighted least squares [43] and approximate p values [44] revealed the same pattern of findings with non-significant effects for baseline BMI (coefficient = 0.000, SE = 0.002, t(12) = −0.034, (approximate) p = 0.973), food-related inhibitory control (coefficient = −0.002, SE = 0.001, t(12) = −1.071, (approximate) p = 0.284), and food liking (coefficient = −0.001, SE = 0.005, t(12) = −0.187, (approximate) p = 0.852), but a significant effect for the interaction of food liking and food-related inhibitory control (coefficient = 0.001, SE = 0.000, t(12) = 2.695, (approximate) p = 0.007).
Table 2.
Prediction of s% BMI reduction during the weight reduction phase
| B | SE B | β | 95s% BCa Bootstrap CIs |
||
|---|---|---|---|---|---|
| LL | UL | ||||
| Step 1 | |||||
| BMI | 0.001 | 0.002 | 0.133 | −0.0031 | 0.0066 |
| Step 2 | |||||
| Inhibitory control | −0.004 | 0.002 | −0.658* | −0.0081 | 0.0079 |
| Food liking | 0.001 | 0.007 | 0.026 | −0.0109 | 0.0263 |
| Step 3 | |||||
| Interaction inhibitory control food liking | 0.002 | 0.001 | 1.098* | 0.0003 | 0.0041 |
Inhibitory control = percentage of false reactions towards go/no-go stimuli (food cues); food liking = subjective rating of food liking; 95s% BCa Bootstrap CIs = 95s% bias-corrected and accelerated Bootstrap confidence intervals, based on 1,000 re-samples; LL = lower limit; UL = upper limit; R2 = 0.018 for step 1 (ns); ΔR2 = 0.359 for step 2 (ns); ΔR2 = .415 for step 3 (p = 0.001)
p < 0.05.
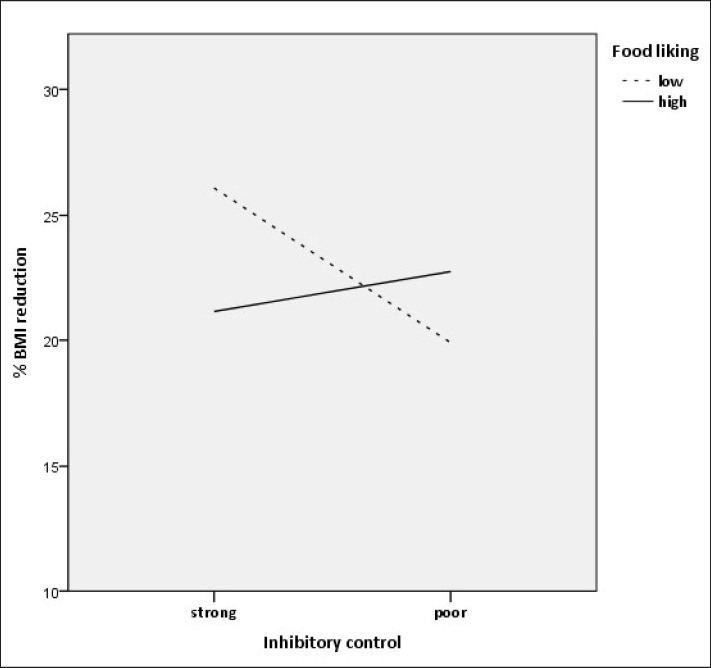
As the regression analysis yielded a significant and robust interaction effect, it was followed up by an examination of simple slopes in order to further examine the direction of the interaction. To this end, food-related inhibitory control was used as the predictor and food liking as the moderator variable [18]. The 10th, 25th, 50th, 75th, and 90th percentiles of the moderator were used to estimate the conditional effects of the predictor on the outcome (s% BMI reduction) on a fine-grained level. As can be seen in table 3, among those participants who showed low and very low food liking (i.e., within the 10th and 25th percentile), those showed larger weight reduction who featured stronger food-related inhibitory control (as indicated by a low error percentage in response to no-go stimuli in the go/no-go task). However, among those participants who showed moderate, high, and very high levels of food liking, food-related inhibitory control had no significant effect on weight reduction (for a better illustration see also fig. 1).
Table 3.
Conditional effects of food-related inhibitory control on percent BMI reduction during the weight reduction phase at values of food liking
| Food liking | Effect | SE | t | p | 95s% BCa Bootstrap CIs |
|
|---|---|---|---|---|---|---|
| LL | UL | |||||
| −3.885 | −0.006 | 0.002 | −3.687 | 0.003 | −0.010 | −0.003 |
| −2.760 | −0.005 | 0.001 | −3.428 | 0.005 | −0.008 | −0.002 |
| 0.740 | −0.000 | 0.002 | −0.187 | 0.855 | −0.004 | 0.003 |
| 1.990 | 0.001 | 0.002 | 0.630 | 0.539 | −0.003 | 0.006 |
| 3.365 | 0.003 | 0.003 | 1.179 | 0.260 | −0.003 | 0.009 |
Inhibitory control = percentage of false reactions towards non-targets (food cues); Food liking = subjective rating of food liking; 95s% BCa Bootstrap CIs = 95s% bias-corrected and accelerated Bootstrap confidence intervals, based on 1,000 re-samples; LL = lower limit; UL = upper limit.
Fig. 1.
s% BMI reduction as a function of strong and poor inhibitory control (1 SD below and 1 SD above the mean error percentage in response to no-go stimuli in the go/no-go task) and low versus high food liking (1 SD below and 1 SD above the mean rating score).
In the second regression analysis, none of the independent variables could significantly predict s% BMI reduction from baseline to the end of the weight loss maintenance phase (R2 = 0.114 (ns), ΔR2 = 0.191 for step 2 (ns); ΔR2 = 0.215 for step 3 (p = 0.095)). However, the effect of the interaction term of food-related inhibitory control and food liking reached marginal significance (B = 0.002, SE B = 0.001, β = 0.481, p = 0.095). As in the first regression analysis, standardised residuals were normally distributed (Shapiro Wilk, p = 0.167), and homoscedasticity was given (Breusch-Pagan, χ2(1) = 2.728, p = 0.604; Koenker, χ2(1) = 2.235, p = 0.693). Also, all cases were within the boundary of three times the average leverage (k s+ 1 / n = 0.307 × 3 = 0.923). However, the same two cases as in the first regression analysis emerged as outliers with Cook's distance and standardised DFBeta values above 1. Thus, we also conducted this regression analysis a second time, with these two cases excluded. As can be seen in table 4, baseline BMI, food-related inhibitory control and food liking alone did not predict s% BMI reduction from baseline to the end of the weight loss maintenance phase. In contrast, the interaction of food liking and food-related inhibitory control reached significance. However, as apparent from the Bootstrap CIs this effect was less robust. In a robust regression (that aims to remediate the undue influence of outliers), inhibitory control and food liking alone failed to reach significance (baseline BMI: coefficient = 0.010, SE = 0.005, t(8) = 2.09, (approximate) p = 0.037; food-related inhibitory control: coefficient = 0.000, SE = 0.003, t(8) = −0.121, (approximate) p = 0.904; food liking: coefficient = −0.017, SE = 0.012, t(8) = −1.445, (approximate) p = 0.148), whereas the interaction of food liking and food-related inhibitory control reached marginal significance (coefficient = 0.002, SE = 0.001, t(8) = 1.856, approximate p = 0.063). Given the marginal significance and instability of this effect, the analysis was not followed up by an examination of simple slopes.
Table 4.
Prediction of s% BMI reduction from baseline to the end of the weight loss maintenance phase
| B | SE B | β | 95s% BCa Bootstrap CIs |
|||
|---|---|---|---|---|---|---|
| LL | UL | |||||
| Step 1 | ||||||
| BMI | 0.000 | 0.004 | −0.015 | −0.0079 | 0.0047 | |
|
| ||||||
| Step 2 | ||||||
| Inhibitory control | −0.005 | 0.002 | 0.004 | 0.016 | −0.502 | 0.081 |
| Food liking | −0.0047 | −0.0560 | 0.0354 | 0.0401 | ||
|
| ||||||
| Step 3 | ||||||
| Interaction inhibitory control food liking | 0.004 | 0.002 | 1.125* | −0.0036 | 0.0111 | |
Inhibitory control = percentage of false reactions towards go/no-go stimuli (food cues); food liking = subjective rating of food liking; 95s% BCa Bootstrap CIs = 95s% bias-corrected and accelerated Bootstrap confidence intervals, based on 1,000 re-samples; LL = lower limit; UL = upper limit R2 = 0.000 for Step 1 (ns); ΔR2 = 0.205 for Step 2 (ns); ΔR2 = .439 for Step 3 (p = 0.035);
p < 0.05.
Discussion
The present study is the first that examined the interplay of food-related inhibitory control and hedonic response towards food in predicting weight reduction during a weight reduction programme for adults with obesity. Results confirmed our hypothesis in that the interaction of food-related inhibitory control and hedonic response towards food would be a stronger predictor of weight reduction than the both factors alone. Participants with relatively strong food-related inhibitory control and low hedonic response towards food were most successful in losing weight during the weight reduction phase of the OPTIFAST 52 programme. This finding is in line with previous studies that have demonstrated both increased food liking [11,12,13] and decreased inhibitory control in people with overweight/obesity [26,27,28,30,31]. Moreover, the present finding is in keeping with previous studies that found a positive relation between inhibitory control and successful weight reduction in guided treatment programmes [32,33,34], and particularly with a study showing that inhibitory control and snack preference interactively predict long-term weight change [18]. In the latter study, individuals with a high implicit preference for snack food and poor response inhibition gained more weight during a year after testing. This finding mirrors the results of the present study in that people with the opposite combination of low food liking and strong response inhibition towards food were most successful in reducing their body weights. However, the findings from the Nederkoorn et al. [18] study could also be interpreted in a fashion that an individual's level of response inhibition only matters if they feature strong hedonic responses towards food as in this study response inhibition had no significant influence on weight gain for participants with low preference for snack food. This interpretation would contrast slightly with the findings of the present study which do not suggest that response inhibition only matters in the presence of strong hedonic responses towards food but rather that a combination of low hedonic response and strong response inhibition towards food is the most advantageous one for people who aim to lose weight.
The present study extends these previous studies by specifically assessing food-related instead of general inhibitory control and in examining adult men and women with obesity instead of children/adolescents or normal-weight women. Although successful long-term weight reduction (from baseline to the end of the weight loss maintenance phase) could also be predicted by the interaction of food liking and food-related inhibitory control, the effect was less robust. This might, at least in part, result from the decreased sample size at 52-week follow-up. Thus, future studies with larger samples should examine the predictive value of food-related inhibitory control and hedonic response towards food for long-term weight loss maintenance. Additionally, it is of great interest to further investigate in a larger sample whether food liking and food-related inhibitory control change in response to treatment and whether these changes are associated with changes in body weight. This will improve the understanding of therapeutic processes associated with weight-reduction programmes.
The present study has several limitations. First of all, the sample size was very small, and the findings should thus be regarded as preliminary up until replication in larger samples. Due to this small sample size, the statistical power might have been also too low to detect small effects of the single predictors. Furthermore, the majority of participants were women with severe obesity. Thus, the findings may not be simply transferrable to other populations with less extreme body weights. The go/no-go paradigm in this study used only food pictures as no-go stimuli. Thus, no conclusions can be drawn whether it was actually food-specific or general inhibitory control that contributed to weight reduction in combination with hedonic responses towards food. In addition, attentional biases towards food cues may have contributed to altered food-related inhibitory control [45]. Future studies within larger samples should also take a range of potential confounders such as age, gender, subjective levels of hunger (particularly in combination with food-related inhibitory control) and medication into account. Future studies may also use more sophisticated and informative measures of food liking, e.g. in combination with a taste test or in the form of a neuropsychological task [46]. Moreover, future research in this area may also benefit from assessing not only explicit food liking but also craving, implicit wanting, and automatic approach tendencies towards food as well as general cognitive abilities such as intelligence, attention, memory and executive functions [47,48,49] in order to promote a more comprehensive understanding of cognitive processes that may affect the ability to resist food intake and thereby the success in weight reduction programmes.
Finally, the present finding provides an avenue for innovative treatment approaches such as computer-based inhibitory control training that may be particularly suitable for those individuals who experience strong hedonic response towards food but are striving to lose body weight. Such trainings may provide useful add-ons to existing weight reduction programmes. Indeed, there is preliminary evidence that fostering food-specific inhibitory control by means of computer-based training can reduce subsequent food intake and (to a limited degree) also body weight [35,36,50,51,52].
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgements
The study was funded by the German Diabetes Foundation (Deutsche Diabetes Stiftung).
References
- 1.World Health Organization Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization Technical Report Series. 2000;894:1–253. [PubMed] [Google Scholar]
- 2.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ. Prevalence of obesity in the United States. JAMA. 2014;312:188–189. doi: 10.1001/jama.2014.6225. [DOI] [PubMed] [Google Scholar]
- 4.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser J. Cancer. Cholesterol forges link between obesity and breast cancer. Science. 2013;342:1028. doi: 10.1126/science.342.6162.1028. [DOI] [PubMed] [Google Scholar]
- 8.O'Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57:2905–2910. doi: 10.2337/db08-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raman J, Smith E, Hay P. The clinical obesity maintenance model: an integration of psychological constructs including mood, emotional regulation, disordered overeating, habitual cluster behaviours, health literacy and cognitive function. J Obes. 2013;2013:240128. doi: 10.1155/2013/240128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlayson G, King N, Blundell JE. Liking vs. wanting food: importance for human appetite control and weight regulation. Neurosci Biobehav Rev. 2007;31:987–1002. doi: 10.1016/j.neubiorev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Dressler H, Smith C. Food choice, eating behavior, and food liking differs between lean/normal and overweight/obese, low-income women. Appetite. 2013;65:145–152. doi: 10.1016/j.appet.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Finlayson G, Arlotti A, Dalton M, King N, Blundell JE. Implicit wanting and explicit liking are markers for trait binge eating. A susceptible phenotype for overeating. Appetite. 2011;57:722–728. doi: 10.1016/j.appet.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Dalton M, Finlayson G. Psychobiological examination of liking and wanting for fat and sweet taste in trait binge eating females. Physiol Behav. 2014;136:128–134. doi: 10.1016/j.physbeh.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring) 2014;22:2544–2551. doi: 10.1002/oby.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García‐García I, Narberhaus A, Marqués‐Iturria I, Garolera M, Rădoi A, Segura B, Pueyo R, Ariza M, Jurado MA. Neural responses to visual food cues: insights from functional magnetic resonance imaging. Eur Eat Disord Rev. 2013;21:89–98. doi: 10.1002/erv.2216. [DOI] [PubMed] [Google Scholar]
- 16.Stice E, Yokum S, Burger KS. Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biol Psychiatry. 2013;73:869–876. doi: 10.1016/j.biopsych.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity. Obesity (Silver Spring) 2009;17:640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- 18.Nederkoorn C, Houben K, Hofmann W, Roefs A, Jansen A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010;29:389–393. doi: 10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- 19.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci. 1997;8:60–64. [Google Scholar]
- 20.Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- 21.Jasinska AJ, Yasuda M, Burant CF, Gregor N, Khatri S, Sweet M, Falk EB. Impulsivity and inhibitory control deficits are associated with unhealthy eating in young adults. Appetite. 2012;59:738–747. doi: 10.1016/j.appet.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van den Eynde F, Guillaume S, Broadbent H, Stahl D, Campbell IC, Schmidt U, Tchanturia K. Neurocognition in bulimic eating disorders: a systematic review. Acta Psychiatr Scand. 2011;124:120–140. doi: 10.1111/j.1600-0447.2011.01701.x. [DOI] [PubMed] [Google Scholar]
- 23.Müller A, Claes L, Wilderjans TF, de Zwaan M. Temperament subtypes in treatment seeking obese individuals: a latent profile analysis. Eur Eat Disord Rev. 2014;22:260–266. doi: 10.1002/erv.2294. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt F, Körber S, Zwaan M, Müller A. Impulse control disorders in obese patients. Eur Eat Disord Rev. 2012;20:e144–e147. doi: 10.1002/erv.2162. [DOI] [PubMed] [Google Scholar]
- 25.Meule A, Hermann T, Kübler A. Food addiction in overweight and obese adolescents seeking weight‐loss treatment. Eur Eati Disord Rev. 2015;23:193–198. doi: 10.1002/erv.2355. [DOI] [PubMed] [Google Scholar]
- 26.Wu M, Hartmann M, Skunde M, Herzog W, Friederich HC. Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PLoS One. 2013;8:e83412. doi: 10.1371/journal.pone.0083412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: the role of impulsivity. Eat Behav. 2006;7:315–322. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Nederkoorn C, Guerrieri R, Havermans RC, Roefs A, Jansen A. The interactive effect of hunger and impulsivity on food intake and purchase in a virtual supermarket. Int J Obes (Lond) 2009;33:905–912. doi: 10.1038/ijo.2009.98. [DOI] [PubMed] [Google Scholar]
- 29.Houben K, Nederkoorn C, Jansen A. Eating on impulse: the relation between overweight and food-specific inhibitory control. Obesity (Silver Spring) 2014;22:E6–E8. doi: 10.1002/oby.20670. [DOI] [PubMed] [Google Scholar]
- 30.Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, Jansen A. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007;49:66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Guerrieri R, Nederkoorn C, Schrooten M, Martijn C, Jansen A. Inducing impulsivity leads high and low restrained eaters into overeating, whereas current dieters stick to their diet. Appetite. 2009;53:93–100. doi: 10.1016/j.appet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behav Res Ther. 2007;45:1071–1075. doi: 10.1016/j.brat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Kulendran M, Vlaev I, Sugden C, King D, Ashrafian H, Gately P, Darzi A. Neuropsychological assessment as a predictor of weight loss in obese adolescents. Int J Obes (Lond) 2014;38:507–512. doi: 10.1038/ijo.2013.198. [DOI] [PubMed] [Google Scholar]
- 34.Pauli-Pott U, Albayrak O, Hebebrand J, Pott W. Does inhibitory control capacity in overweight and obese children and adolescents predict success in a weight-reduction program? Eur Child Adolesc Psychiatry. 2010;19:135–141. doi: 10.1007/s00787-009-0049-0. [DOI] [PubMed] [Google Scholar]
- 35.Houben K, Jansen A. Training inhibitory control. A recipe for resisting sweet temptations. Appetite. 2011;56:345–349. doi: 10.1016/j.appet.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Houben K, Jansen A. Chocolate equals stop. Chocolate-specific inhibition training reduces chocolate intake and go associations with chocolate. Appetite. 2015;87:318–323. doi: 10.1016/j.appet.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 38.Walther S, Goya-Maldonado R, Stippich C, Weisbrod M, Kaiser S. A supramodal network for response inhibition. Neuroreport. 2010;21:191–195. doi: 10.1097/WNR.0b013e328335640f. [DOI] [PubMed] [Google Scholar]
- 39. RCoreTeam R: A Language and Environment for Statistical Computing. www.gbif.org/resource/81287 (last accessed September 19, 2016)
- 40.Hayes AF. An Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press; 2013. [Google Scholar]
- 41.Beasley WH, Rodgers JL. Bootstrapping and Monte Carlo methods. In: Cooper H, Camic PM, Long DL, Panter AT, Rindskopf D, Sher KJ, editors. APA Handbook of Research Methods in Psychology Research Designs: Quantitative, Qualitative, Neuropsychological, and Biological. Washington, DC: American Psychological Association; 2012. pp. 407–425. [Google Scholar]
- 42.Adèr HJ, Mellenbergh GJ, Hand DJ. Advising on Research Methods: A Consultant's Companion. Huizen: Johannes van Kessel Publishing; 2008. [Google Scholar]
- 43.Ryan TP. Modern Regression Methods. New York: Wiley; 2008. [Google Scholar]
- 44. Bellio R, Ventura L. An Introduction to Robust Estimation with R Functions. www.academia.edu/25811981/An_introduction_to_robust_estimation_with_R_functions (last accessed September 19, 2016).
- 45.Lattimore P, Mead BR. See it, grab it, or stop! Relationships between trait impulsivity, attentional bias for pictorial food cues and associated response inhibition following in-vivo food cue exposure. Appetite. 2015;90:248–253. doi: 10.1016/j.appet.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Simon JJ, Skunde M, Hamze Sinno M, Brockmeyer T, Herpertz SC, Bendszus M, Herzog W, Friederich HC. Impaired cross-talk between mesolimbic food reward processing and metabolic signaling predicts body mass index. Front Behav Neurosci. 2014;8:359. doi: 10.3389/fnbeh.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fitzpatrick S, Gilbert S, Serpell L. Systematic review: are overweight and obese individuals impaired on behavioural tasks of executive functioning? Neuropsychol Rev. 2013;23:138–156. doi: 10.1007/s11065-013-9224-7. [DOI] [PubMed] [Google Scholar]
- 48.Reinert KR, Po'e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: A systematic literature review. J Obes. 2013;2013:820956. doi: 10.1155/2013/820956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12:740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 50.Veling H, van Koningsbruggen GM, Aarts H, Stroebe W. Targeting impulsive processes of eating behavior via the internet. Effects on body weight. Appetite. 2014;78:102–109. doi: 10.1016/j.appet.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Lawrence NS, O'Sullivan J, Parslow D, Javaid M, Adams RC, Chambers CD, Kos K, Verbruggen F. Training response inhibition to food is associated with weight loss and reduced energy intake. Appetite. 2015;95:17–28. doi: 10.1016/j.appet.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Treasure J, Cardi V, Leppanen J, Turton R. New treatment approaches for severe and enduring eating disorders. Physiol Behav. 2015;152:456–465. doi: 10.1016/j.physbeh.2015.06.007. [DOI] [PubMed] [Google Scholar]