Abstract
Objective
To investigate whether or not berberine could improve metabolic status of high-fat-fed rats through modulation of microbiota-gut-brain axis.
Methods
Berberine was administered on high-fat-fed Sprague-Dawley rats. Brain-gut hormones were detected, and changes of gut microbiota were analyzed by 16S rRNA gene sequencing.
Results
Berberine could reduce weight gain and lipolysis in the high-fat diet-fed group. Moreover, trends of ameliorated insulin resistance and decreased endogenous glucose production were observed. In addition, the microbiota-gut-brain axis was found to be modulated, including structural and diversity changes of microbiota, elevated serum glucagon-like peptide-1 and neuropeptide Y level, decreased orexin A level, up-regulated glucagon-like peptide-1 receptor mRNA level as well as ultra-structural improvement of the hypothalamus.
Conclusion
Taken together, our findings suggest that berberine improved metabolic disorders induced by high-fat diet through modulation of the microbiota-gut-brain axis.
Key Words: Berberine, Microbiota-gut-brain axis, Obesity, Gut hormones
Introduction
The prevalence of obesity and its related metabolic diseases, such as type 2 diabetes and cardiovascular diseases, has been increasing in both developed and developing countries [1]. During the recent two decades, the combined prevalence of overweight and obesity in China rose from 14.6% [2] to 32.3% [3]. Due to the rising prevalence of metabolic diseases and their significant impact on health, global research interests have been attracted to their etiologies, preventions, and therapies.
Recently, disorders of the microbiota-gut-brain axis have been found to be closely associated with metabolic diseases, including obesity, metabolic syndrome, and diabetes [4]. Human gut microbiota is considered to be an important internal environment factor that regulates energy balance and storage [5], which is associated with obesity and related disorders [6]. Moreover, gut microbial dysbiosis may play a causative role in obesity because intestinal microbiota transferred from obese mice could result in significantly greater adiposity in germ-free recipients [7]. The hormonal signaling pathway is one part of the complex network of communication between the gut microbiota and the brain [4]. Gut hormones, such as ghrelin, orexin, glucagon-like peptide-1 (GLP-1) and leptin, modulate feeding behavior, energy homeostasis, circadian rhythm etc. [8,9]. GLP-1 receptor agonists have also been used in the treatment of diabetes and obesity [10]. Therefore, the microbiota-gut-brain axis is a potential target for metabolic disease treatment.
As an isoquinoline alkaloid, berberine is the major pharmacological component of the Chinese herb called Coptidis rhizome (huanglian in Chinese) [11]. It has been mainly used for intestinal bacteria-related diarrhea for thousands of years [11]. Recently it has been found that berberine is clinically effective in the treatment of diabetes and obesity [12]. However, its clinical effectiveness is hard to explain because of its extremely low oral bioavailability. Only a maximum concentration of 0.4 ng/ml in plasma was detected after a single oral dosage of 400 mg of berberine in humans [13]. Several studies have shown that berberine could modulate the gut microbiota through enriching short-chain fatty acid(SCFA)-producing bacteria and reducing microbial diversity, which inhibits dietary polysaccharide degradation and decreases additional calorie intake in the gut, which may have beneficial effects to the host's metabolic status [14,15,16].
However, there are only few studies exploring the effects of berberine on the hormonal pathway of the microbiota-gut-brain axis. We hypothesized that berberine could ameliorate lipid and glucose metabolism by modulating the microbiota-gut-brain axis, e.g., the composition of the gut microbiota and serum brain-gut hormones as well as the activity of the hypothalamus. In order to understand the exact mechanisms, we used a high-fat diet-fed rat model to investigate these changes.
Material and Methods
Animal Experiments
18 male 12-week-old Sprague-Dawley (SD) rats were purchased from SLAC Laboratories, SIBS, Shanghai, China. Animals were housed at an ambient temperature of 22 ± 2 °C, maintained under a normal 12-hour light/dark cycle, and allowed with access to food and water ad libitum. After 2 weeks of acclimatization, rats were randomly assigned to 3 groups (n = 6 per group), with one conventionally fed with normal chow diets (NCD; containing 10% fat) and the other two with high-fat diets (HFD; containing 40% fat). Four months later, one of the HFD groups were orally co-administrated with 150 mg/kg/day berberine chloride (BBR; Sigma-Aldrich, St. Louis, MO, USA) while the other group were continued with solely HFD for another 4 months. BBR was suspended in normal saline (NS) before use. The rats without drug interventions were treated with an equal volume of NS. Animal treatment lasted for 8 months; body weight and fasting blood glucose (FBG) were measured every 2 weeks at the overnight fasting condition. Blood glucose levels were measured by an electronic glucometer (Terumo, Tokyo, Japan). All animal procedures were performed in accordance with the ethical principles in animal research adopted by the Department of Laboratory Animal Science, JiaoTong University School of Medicine, Shanghai, China.
Measurement of Insulin, Biochemical Indexes, and Brain-Gut Peptide Levels
Tail blood was separately collected from the caudal vein following overnight fasting at months 0, 4, and 8 for detection of insulin, lipid profiles, and brain-gut peptides. Triglycerides (TG), total cholesterol (TC), free fatty acid (FFA) and low-density lipoprotein cholesterol (LDL-C) levels were detected with Siemens Dimension MAX (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA); Fasting insulin (FINS) was assessed with ELISA kits (Shibayaji, Ishihara, Japan); the homeostasis assessment of insulin resistance (HOMA-IR) index was calculated according to the formula: HOMA-IR = FBG × FINS / 22.5. The Eppendorf tubes were immediately added with dipeptidyl dipeptidase IV inhibitor (Merck Millipore, Darmstadt, Germany) for active GLP-1 assessment and were immediately added with aprotinin (Sigma-Aldrich) for detection of neuropeptide Y (NPY) and orexin A. All of the brain-gut peptides were measured at room temperature with ELISA kits (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA).
Isotope Tracing Experiment
At the end of month 8, all rats passed through isotope tracing for quantitation of steady-state glucose and glycerol metabolism after an overnight fast. Two kinds of stable isotope tracers ((6,6-2D)-glucose and (U-13 C)-glycerol) were infused into the catheterized lateral tail vein by an infusion pump (Harvard Apparatus, Holliston, MA, USA), and the catheterized artery was for blood sampling, animals were conscious and relaxed throughout the experiments. (6, 6-2D)-glucose (2 μmol/kg/min) and (U-13 C)-glycerol (0.84 μmol/kg/min) were intravenously infused constantly for 90 min without priming with arterial blood samples (0.5 ml each) collected at 5-min intervals during the last 10 min (80-90 min). Plasma samples were run on gas chromatography (GC) / mass spectrometry (MS) (Agilent 5975C; Agilent Technologies, Basel, Switzerland) to obtain m/z: 321/319 (6, 6-2D) glucose and m/z: 221/218 (U-13C)-glycerol after derivatization by methoxyamine-HCl and BSTFA. GC operating condition was programmed initially at 70 °C for 4 min, increased to 240 °C at 10 °C/min and then to 300 °C at 20 °C/min, and sustained at 300 °C for 11 min. The MS was operated in the selected ion monitoring (SIM) mode monitoring fragments at mass to charge ratios (m/z) 319, 321 for unlabeled and (6, 6- 2H2) glucose. Measurement of GC/MS and calculation were done as previously described [17].
Transmission Electron Microscopy
Hypothalami were dissected and immediately placed in 2% glutaraldehyde in phosphate buffer (pH 7.2) and maintained at 4 °C for 2 h. Samples were then post-fixed in 1% osmium tetroxide for 2 h, dehydrated in increasing concentrations of alcohol (30% − 50% − 70%), immersed in propylene oxide, and embedded in Araldite 502 resin at 60 °C. Thick tissue sections were made and analyzed by light microscopy to confirm the anatomical location of hypothalamus. Ultrathin sections were placed on grids and stained with lead citrate, and then observed under a transmission electron microscope (Philip, CM-120, Philips, Amsterdam, The Netherlands).
Determination of GLP-1R in Hypothalamus with Quantitative Real-Time PCR Analysis
Hypothalamus was separated as mentioned above. Total RNA was isolated using a TRIzol reagent (TIANGEN, Shanghai, China) according to the manufacturer's instructions. RNA quantity and purity were evaluated by a model ND-2000 apparatus (NanoDrop 2000; Thermo Fisher Scientific, Waltham, MA, USA). The integrity of the RNA was confirmed by agarose-formaldehyde gel electrophoresis. First, strand cDNA was synthesized from individual samples from 2,000 ng of total RNA with a cDNA Reverse Transcription Kit (Promega Corporation, Madison, WI, USA) following the manufacturer's instructions. The real-time PCR was conducted by LightCycler 96 (Roche Applied Science, Penzberg, Germany) employing SYBR Green I as the dsDNA-specific binding dye for continuous fluorescence monitoring. The PCR protocol comprised 5 min at 95 °C; 45 cycles of 15 s at 95 °C, 15 s at 60 °C and 15 s at 72 °C. The sequences of the GLP-1R primers were forward 5′-AGT AGT GTG CTC CAA GGG CAT-3′, reverse 5′-AAG AAA GTG CGT ACC CCA CCG-3′, and those of β-actin forward 5′-GCC CCT CTG AAC CCT AAG-3′, reverse 5′-CAT CAC AAT GCC AGT GGT A-3′. The genes of GLP-1 receptor were normalized to β-actin expression.
Fecal DNA Extraction and 16S rRNA Gene Sequencing
Fresh stool sample were collected in month 8 and immediately stored at −80 °C for subsequent analysis. The sample sizes were 5 in control group,5 in HFD group, and 4 in HFD + BBR group. There is no difference in sampling between the groups studied. Genomic DNA of microbiota was extracted from fecal sample by TIANamp stool DNA kits (TIANGEN). DNA was quantified by the Nanodrop 2000. The extracted DNA from each sample was used as the template to amplify the V3 and V4 hypervariable regions of ribosomal 16S rRNA genes. Briefly, the purified 1 μg of genomic DNA were fragmented to an average size of 300-400 bp and ligated with adapters. The PCR was performed using a primer cocktail that anneals to the ends of the adapters to enrich DNA fragments that have adapter molecules on both ends and followed by clean up and quantification. Sequencing was performed using a 300-bp paired-end sequencing protocol on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) at Oebiotech Company, Shanghai, China. Raw paired-end reads were subjected to quality filtering using Trimmomatic software before paired-end read assembling with FLASH software. All chimeras of assembling sequences were eliminated to reach high-quality sequences.
Statistical Analysis
All microbiota sequences were assigned to Operational Taxonomic Units (OTUs) using the UCLUST algorithm in CD-HIT with 97% threshold of pairwise identity, and the most abundant sequence of each OTU was selected as the representative sequence and subjected to RDP classifier for taxonomical assignment with a bootstrap cutoff of 50%. The rarefaction estimates and Shannon-Wiener index were calculated using QIIME. The representative sequences of OTUs were used to generate a phylogenetic tree using FasTree. The phylogenetic tree was then used for unweighted UniFrac principal coordinates analysis (PCoA). The relative abundances of gut microbiota in each sample and other measurement data were expressed as mean ± SEM and evaluated with one-way analysis of variance (ANOVA) with Fisher's least significant difference (LSD) post hoc test. Statistical significance was accepted as p ℋ 0.05 (two-sided significance testing). All statistical analyses were performed using SPSS 22.0 statistical software (Chicago, IL, USA).
Results
Berberine Attenuated the High-Fat Diet-Induced Obesity and Insulin Resistance
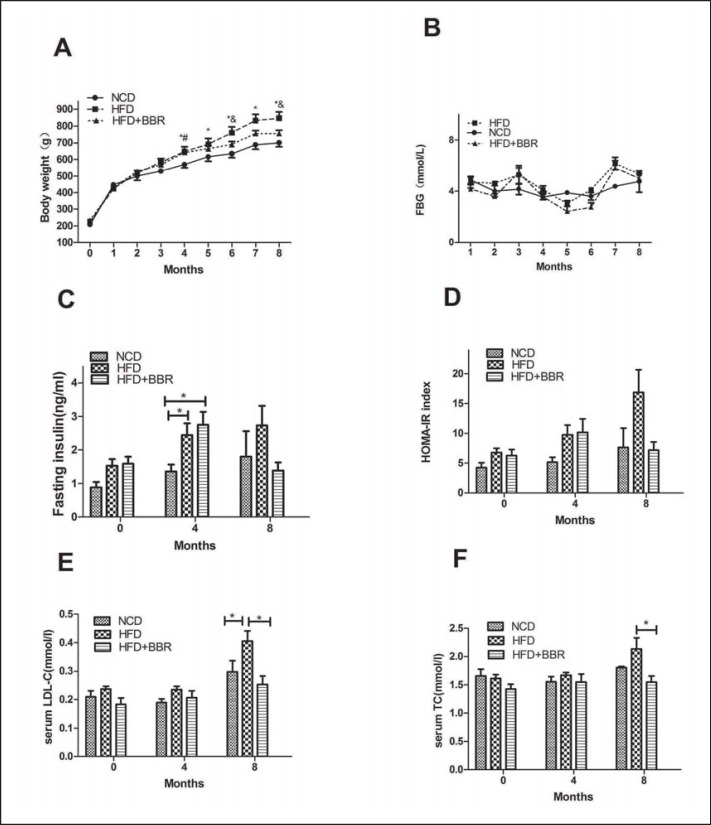
Compared with NCD-fed rats, HFD feeding over 4 months induced a significant body weight increase (p ℋ 0.05) (fig. 1A), whereas the rats were obviously protected from weight gain by the co-administration of BBR over 4 months at a dosage of 150 mg/kg/day in HFD + BBR group. which displayed significant differences in body weight when compared with the continuously HFD-fed groupMoreover, the plasma lipid profiles revealed that BBR could effectively decrease the elevated LDL level as well as the plasma TC level in HFD-fed rats (p ℋ 0.05) (fig. 1E). However, the TG and FFA levels were comparable between the HFD and the BBR group (data not shown).
Fig. 1.
Berberine attenuates body weight gain, insulin resistance and serum LDL-C level and TC level of HFD-fed rats. A Body weight gain; B fasting blood glucose concentration; C fasting serum insulin concentration of the 0th,4th, and 8th month separately; D HOMA-IR index of the 0th,4th, and 8th month separately; E serum LDL-C concentration of the 0th,4th, and 8th month separately; F serum TC concentration of the 0th, 4th, and 8th month separately. Values are expressed as mean ± SEM. Difference were assessed by ANOVA and denoted as following: *p ℋ 0.05, NCD versus HFD; #p ℋ 0.05, HFD versus HFD + BBR; &p ℋ 0.05, HFD versus HFD+BBR.
Although there was no difference in FBG levels between the NCD and HFD groups during the entire trails, HFD-fed rats exhibited elevated FINS and HOMA-IR index levels. BBR administration reversed the increase of FINS (p = 0.224) (fig. 1C) as well as that of HOMA-IR (p = 0.076) (fig. 1D) of HFD groups to a level similar to that of the NCD group, with only marginal differences.
Berberine Decreased the Endogenous Glucose Production and Lipolysis of HFD-Fed Rats
The isotope tracing was conducted before the rats were sacrificed to assess the glucose and glycerol metabolism of steady states. The figures showed that the rate of glycerol appearance (Ra of glycerol) was greater in the HFD group than in the NCD and HFD + BBR groups (p ℋ 0.05) (fig. 2B). The Ra of glucose showed the same trend (p = 0.094) (fig. 2A), which indicated that BBR could reduce the elevated hepatic glucose production as well as the lipolysis resulting from HFD feeding.
Fig. 2.
Berberine reduces the Ra of glucose and glycerol of HFD-fed rats. A Ra of glucose; B Ra of glycerol. Values are expressed as mean ± SEM. Difference were assessed by ANOVA and denoted as follows: *p ℋ 0.05.
Structural Changes of Gut Microbiota Modulated by Berberine in HFD-Fed Rats
A total of 421,846 usable reads (with 99.6% distributed in 400-500 bp length) were obtained from 14 samples and were delineated into 4,031 OTUs at similarity level cutoff of 97% using CD-HIT. The OTUs were assigned to defined phyla by RDP classifier with a bootstrap cutoff of 50%. Rarefaction and Shannon diversity curves revealed that most of the diversity had already been captured (fig. 3A, B), although new rare phenotypes would be expected with additional sequencing. The Shannon-Wiener index revealed that the microbiota diversity of NCD and HFD groups were comparable; however, co-administration of BBR with HFD resulted in a significant decrease in species diversity (p ℋ 0.01) and richness of the microbiota confirmed by rarefaction estimates (fig. 3C, D).
Fig. 3.
Overall structural changes of gut microbiota. A The rarefaction curves; B the Shannon curves; C rarefaction estimates, calculated after rarefying to an equal number of sequence reads for all samples; D Shannon-Wiener index, calculated after rarefying to an equal number of sequence reads for all samples. Values are expressed as mean ± SEM. Difference were assessed by ANOVA and denoted as *p ℋ 0.05.
As revealed by taxon-based analysis, Firmicutes, Bacteroidetes, and Proteobacteria remained the major phyla of fecal microbiota in all three groups (supplementary table 1, available at http://content.karger.com/ProdukteDB/produkte.asp?doi=4449507; fig. 4A). Although there was no difference in the relative abundances of main phyla among three groups, co-administration of HFD with BBR led to a decline in Firmicutes abundance (35.80 ± 10.45% vs. 54.19 ± 2.64%) and a moderate increase in Bacteroidetes ratios (60.29 ± 10.99% vs. 41.72 ± 2.45%) compared to merely HFD. This resulted in a higher Bacteroidetes-to-Firmicutes (B/F) ratio in the HFD + BBR group than in the HFD group (fig. 4B), even though with only reached a marginal statistical significance (p = 0.09). At the class level (supplementary table 1, available at http://content.karger.com/ProdukteDB/produkte.asp?doi=4449507), HFD resulted in a higher proportion of Coriobacteriia (Actinobacteria phylum), Erysipelotrichi (Firmicutes phylum) and Gammaproteobacteria (Proteobacteria), all of which were decreased by BBR treatment (p ℋ 0.05). Markedly enriching effects of BBR on Bacteroidaceae and Rikenellaceae families (both belonging to the Bacteroidetes phylum) were observed when compared to the HFD only group (p ℋ 0.05). In contrast, the elevated abundances of Christensenellaceae, Dehalobacteriaceae, Erysipelotrichaceae and Peptococcaceae (all belonging to the Firmicutes phylum) in the HFD group were obviously declined when HFD was combined with BBR administration. Furthermore, Alcaligenaceae (Proteobacteria Phylum) showed a greater proportion in the HFD + BBR group compared to both the HFD and the NCD groups.
Fig. 4.
BBR regulates the intestinal composition of HFD-fed rats. A The bar charts represent average relative abundances of bacterial phylum. B B/F ratio. C The heat map reflects the bacterial abundances at the genus level. D PCoA were performed based on unweighted UniFrac metrics. Values are expressed as mean ± SEM. Difference were assessed by ANOVA and denoted as *p ℋ 0.05.
As observed at the genus level (supplementary table 1, available at http://content.karger.com/ProdukteDB/produkte.asp?doi=4449507; fig. 4C), BBR led to marked changes of microbiota composition in majority genus, with a significant enriching effects on Bacteriodes (Bacteriodetes phylum) (p ℋ 0.05). In addition, the levels of most genera from phylum Firmicutes, such as Dorea (p ℋ 0.05), rc4-4 (p ℋ 0.05), Roseburia (p = 0.05) and Blautia (p for ANOVA = 0.098), showed an increase with HFD feeding and were reverted by co-administration of BBR. In contrast, the abundance of the genus Anaerofilum was increased in the BBR-treated group (p ℋ 0.05). Furthermore, genera such as Sutterella (p ℋ 0.05), Bilophila (p ℋ 0.05) and Desulfovibrio (p = 0.07) from phylum Proteobacteria revealed a higher proportion after BBR administration. Among the taxa mentioned above, Bacteriodes, Bilophila were confirmed as SCFA-producing bacteria [15].
Unweighted UniFrac PCoA revealed striking shifts in microbiota in response to BBR administration, which was mainly reflected on the PC1 axis (29.41%) (fig. 4D).
Effects of Berberine on Brain-Gut Peptide and GLP-1R of Hypothalamus in HFD-Fed Rats
The fasting GLP-1 level was decreased in HFD-fed rats compared with NCD-fed rats, which could be elevated by berberine intervention, although the difference did not reach significance (p = 0.053) (fig. 5A). The NPY values were slightly elevated with HFD feeding when compared to those of the NCD group; this increase was prevented in the HFD + BBR group (p ℋ 0.05) (fig. 5B). The concentration of orexin A was much lower in HFD-fed animals than in NCD-fed ones and were found to be elevated to a new level after BBR administration (p ℋ 0.05) (fig. 5C). In addition, the expression of GLP-1R mRNA in the hypothalamus of the HFD + BBR group was increased compared with that of the NCD and HFD groups (p = 0.127) (fig. 5D).
Fig. 5.
Berberine results in the changes of gut-brain peptide and GLP-1 receptor of hypothalamus. A Serum GLP-1 concentration; B serum NPY concentration; C serum orexin A concentration; D expression of GLP-1R of hypothalamus. Values are expressed as mean ± SEM. Difference were assessed by ANOVA and denoted as *p ℋ 0.05.
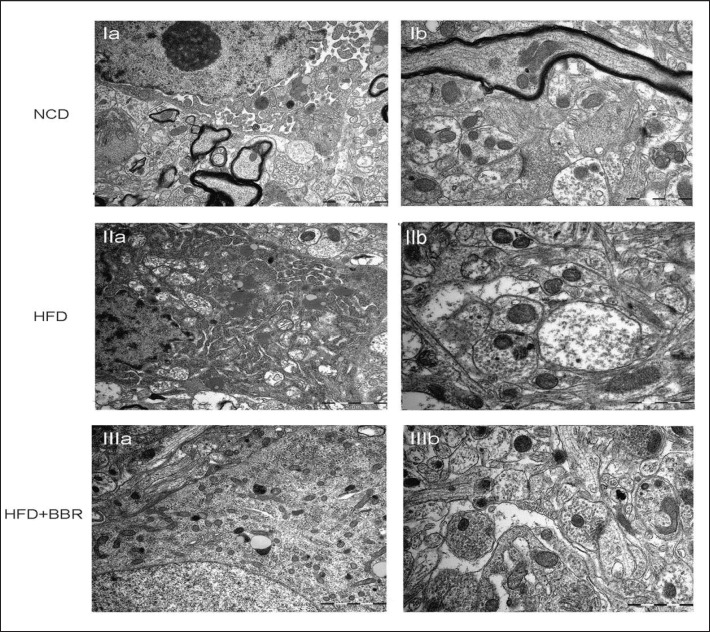
Ultrastructural Analysis of the Hypothalamus
Ultrastructural features suggested that HFD feeding caused distortion and swelling of the mitochondria accompanied by unclear internal composition as well as dilatation of Golgi complex and rough endothelial reticulum fragments (fig. 6IIa). BBR intervention could reverse the structural distortion and cytoplasm swelling of the cellular body (fig. 6IIIa). In addition, HFD feeding resulted in swollen axonal synaptic button with vacuolated and swollen mitochondria and high dispersion of synaptic vesicles. Narrow and fuzzy synaptic cleft could be observed as well (fig. 6IIb), which were ameliorated by BBR administration (fig. 6IIIb).
Fig. 6.
The ultrastructural changes of hypothalamus revealed by transmission electron telescope. Ia NCD: the nucleus and organelles. Ib NCD: the neuropil and axonal synaptic bouton. IIa HFD: the nucleus and organelles; the shrinked nuclear membrane, scattered nucleoli and swollen mitochondria. IIb HFD: the swollen axonal bouton and fuzzy synaptic cleft. IIIa HFD+BBR: the structural destruction of nucleus and organelles were alleviated. IIIb HFD+BBR: the structure of axonal bouton and synaptic cleft were ameliorated by BBR administration. Scale bars in Ia, IIa, IIIa : 2 µm; Ib, IIb, IIIb: 1 µm.
Discussion
In the present study, we found that BBR administration at a dosage of 150 mg/kg body weight in rats effectively decreased weight, plasma lipid levels such as those of LDL-C and TC as well as lipolysis. BBR treatment could lead to a decrease in hepatic glucose production as well as to amelioration of insulin resistance induced by long-term HFD treatment, although these differences did not reach significance. These metabolic changes may be due to the modulation of the microbiota-gut-brain axis. BBR increased the B/F ratio, further elevated plasma GLP-1 and orexin A levels, and decreased NPY levels. It also raised GLP-1 receptor expression in the hypothalamus.
The beneficial effects of berberine on the metabolic status found by us were consistent with previous animal studies and the human trials [12,14,15,18]. Zhang et al. [12] confirmed that oral administration of berberine (1.0 g/day for 3 months) effectively and safely ameliorated the plasma glucose, HbA1c and lipid profile in patients with diabetes and dyslipidemia. However, in our study, even in the early stage of metabolic dysregulation when there was weight gain, insulin resistance and high LDL-C but normal plasma glucose, berberine still could decrease insulin resistance and the hepatic glucose production, which is similar to the anti-diabetic mechanism of metformin. This indicates that it might be effective to prevent individuals with prediabetes from progressing into diabetes. However, further animal studies and human trials are needed to confirm this.
We also found that berberine could modulate gut microbiota through up-regulation of B/F abundance ratio and reduction of the gut microbiota diversity. However, some other studies were not able to show an increase of the B/F ratio after berberine treatment [14,15]. However, Turnbaugh et al. [19] demonstrated that adiposity was associated with the down-regulated intestinal B/F ratio that might increase the capacity to harvest energy from the diet. Moreover, berberine could increase the proportion of SCFA-producing bacteria such as Bacteriodes and Bilophila [15]. Previous studies showed that SCFA-producing bacteria could alleviate inflammatory responses, supply energy for colonocytes [20], and suppress colonic epithelial permeability against pathogens [21].
What are the potential mechanisms linking gut microbiota with GLP-1? SCFA may be the principal one. About two decades ago, Longo et al. [22] already found that SCFA could stimulate gut hormone production in isolated rabbit colon and linked microbial activity with gut L-cells. Also, rats fed with prebiotics such as oligofructose and inulin-type fructans [23] showed increased plasma and intestinal GLP-1 levels [24]. In a cohort study, it was first confirmed that the gut microbiota fermentation of inulin-type fructans has been correlated with higher plasma GLP-1 levels [25]. SCFAs could bind to the G-protein-coupled receptors GPR41 and GPR43 and then trigger GLP-1 secretion by the L-cells [26]. GPR119 ligands such as oleoylethanolamide and 2-oleoylglycerol could also increase GLP-1 secretion [26]. Moreover, berberine may also alleviate metabolism disorder through the gonadotropin-releasing hormone-GLP-1 and MAPK pathways in the intestine [27].
Not only plasma GLP-1 but also GLP-1R in the hypothalamus and neuropeptide secretion may be modulated by berberine. The rodent, nonhuman primate, and human hypothalamus show clear GLP-1R expression [28,29,30]. Diabetic patients taking GLP-1R agonists had reduced appetite and food intake contributing to weight loss, which indicates that these effects are partly mediated through central GLP-1R [31]. GLP-1R mRNA was also decreased in diabetic patients [32]. More importantly, central injection of GLP-1R agonist in mice stimulated weight loss, brown adipose tissue thermogenesis, and adipocyte browning. Moreover, neuropeptides such as orexin A and NPY were also involved. Orexin A is a critical homeostatic mediator of central control of energy metabolism and maintenance of sleep/wake cycle [33], and mice with ablation of orexin neurons have late-onset obesity [34]. NPY is a potent orexigenic peptide and found at various levels of the brain-gut axis. It may affect the microbiota-gut-brain axis through influencing the vitality of certain gut bacteria, modifying gut motility, regulating food intake and energy homeostasis etc. [35]. Therefore, NPY Y1 and Y5 receptor antagonists are being considered as anti-obesity drugs [36].
Our study offers some novel insights. First, this study measured three gut-brain hormones simultaneously. Second, besides neuropeptides, we also detected ultrastructural abnormalities in the hypothalamus of HFD-treated rats, and berberine could partly ameliorate these abnormalities. Third, we found that berberine may decrease hepatic glucose production in the early stage of metabolic disorders, indicating that it may be a drug to prevent diabetes. However, there are also some limitations. First, the dosages of berberine were not gradient, so we could not observe gradient changes of metabolic status. Second, food intake was not studied, so we could not make sure whether or not the weight loss was due to reduced food intake or more energy expenditure after berberine administration. In addition, we had no other measurement of body fat changes besides weight. We did not conduct an accurate measurement such as an insulin tolerance test or hyperinsulinemic clamp measurement to confirm ameliorated insulin resistance of HFD-fed rat after berberine treatment. Third, microbiota, neuropeptides, and metabolic parameters were not measured on month 4 but only once on month 8, so we were not able to say which one changed first and whether or not the berberine treatment caused of these changes.
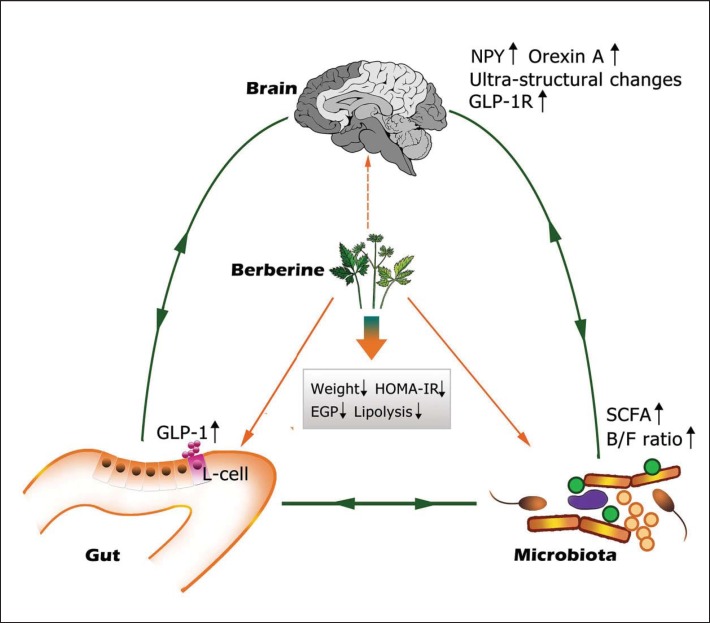
In conclusion, our study provides evidence that a marked berberine-induced modulation of the microbiota-gut-brain axis, including inhibition of the microbiota diversity, elevation of B/F ratio, plasma GLP-1 and orexin-A, and up-regulation of GLP-1 receptor expression in hypothalamus, contributes to the beneficial effects of berberine against insulin resistance, obesity, and metabolic syndrome (fig. 7). This study provided information for further investigation of the mechanisms by which berberine ameliorates the metabolic status. Moreover, these results may be useful in devising therapeutic strategies for metabolic disorders such as obesity, hyperlipidemia, diabetes, and even prediabetes. Further studies should be performed to confirm these results.
Fig. 7.
The microbiota-gut-brain loop modulated by berberine in high-fat diet induced obesity. Administration of berberine could increase the B/F ratio and the ratios of SCFA-producing bacteria, which promoted an elevated level of GLP-1 expression in gut L cells. The expression of GLP-1R as well as hormones such as NPY and Orexin A in brain are upregulated. Ultra-structural changes could also be observed in hypothalamus. All of the above changes results in decreased weight gain, ameliorated insulin resistance, reduced endogenous glucose production and lipolysis.
Contribution Statement
HS, NW and ZC designed and performed research, analyzed data, and wrote the manuscript. CZ, XN and LZ contributed reagents, provided intellectual input, and revised the manuscript. FX, HZ and YL designed research, analyzed data, provided intellectual input, and revised the manuscript. All authors approved the final version. YL is the guarantor of this work.
Disclosure Statement
The authors declare that there is no duality of interest associated with this manuscript.
Acknowledgements
This study was supported by National Natural Science Foundation of China (81270885 and 81300653); Clinical Potential Subject Construction of Shanghai Jiaotong University School of Medicine (2014); Ministry of Science and Technology in China (2012CB524906); Science and Technology Commission of Shanghai Municipality (14495810700, 16410723200).
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31:177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 3.Li XY, Jiang Y, Hu N, Li YC, Zhang M, Huang ZJ, Zhao WH. Prevalence and characteristic of overweight and obesity among adults in China, 2010 (in Chinese) Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46:683–686. [PubMed] [Google Scholar]
- 4.Burokas A, Moloney RD, Dinan TG, Cryan JF. Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol. 2015;91:1–62. doi: 10.1016/bs.aambs.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 7.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron J, Doucet E. Getting to the bottom of feeding behaviour: who's on top? Appl Physiol Nutr Metab. 2007;32:177–189. doi: 10.1139/h06-072. [DOI] [PubMed] [Google Scholar]
- 9.De Silva A, Bloom SR. Gut hormones and appetite control: a focus on PYY and GLP-1 as therapeutic targets in obesity. Gut Liver. 2012;6:10–20. doi: 10.5009/gnl.2012.6.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan BM, Rudser KD, Abuzzahab MJ, Fox CK, Coombes BJ, Bomberg EM, Kelly AS. Predictors of weight-loss response with glucagon-like peptide-1 receptor agonist treatment among adolescents with severe obesity. Clin Obes. 2016;6:73–78. doi: 10.1111/cob.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J Ethnopharmacol. 2009;126:5–17. doi: 10.1016/j.jep.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, Wu P, Ren G, Ning G. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 13.Hua W, Ding L, Chen Y, Gong B, He J, Xu G. Determination of berberine in human plasma by liquid chromatography-electrospray ionization-mass spectrometry. J Pharm Biomed Anal. 2007;44:931–937. doi: 10.1016/j.jpba.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, Li M, Zhang C, Zhang Z, Zhang Y, Li X, Ning G, Zhao L. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PloS One. 2012;7:e42529. doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, Zhang X, Zhao L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie W, Gu D, Li J, Cui K, Zhang Y. Effects and action mechanisms of berberine and rhizoma coptidis on gut microbes and obesity in high-fat diet-fed c57bl/6j mice. PloS One. 2011;6:e24520. doi: 10.1371/journal.pone.0024520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia F, Xu X, Zhai H, Meng Y, Zhang H, Du S, Xu H, Wu H, Lu Y. Castration-induced testosterone deficiency increases fasting glucose associated with hepatic and extra-hepatic insulin resistance in adult male rats. Reprod Biol Endocrinol. 2013;11:106. doi: 10.1186/1477-7827-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 20.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 22.Longo WE, Ballantyne GH, Savoca PE, Adrian TE, Bilchik AJ, Modlin IM. Short-chain fatty acid release of peptide YY in the isolated rabbit distal colon. Scand J Gastroenterol. 1991;26:442–448. doi: 10.3109/00365529108996507. [DOI] [PubMed] [Google Scholar]
- 23.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Leotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104((suppl 2)):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 24.Kok NN, Morgan LM, Williams CM, Roberfroid MB, Thissen JP, Delzenne NM. Insulin, glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide and insulin-like growth factor I as putative mediators of the hypolipidemic effect of oligofructose in rats. J Nutr. 1998;128:1099–1103. doi: 10.1093/jn/128.7.1099. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 26.Everard A, Cani PD. Gut microbiota and GLP-1. Rev Endocr Metab Disord. 2014;15:189–196. doi: 10.1007/s11154-014-9288-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Xiao X, Li M, Li W, Yu M, Zhang H, Ping F, Wang Z, Zheng J. Berberine moderates glucose metabolism through the GNRH-GLP-1 and MAPK pathways in the intestine. BMC Complement Altern Med. 2014;14:188. doi: 10.1186/1472-6882-14-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, Knudsen LB, Vrang N, Grove KL. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology. 2015;156:255–267. doi: 10.1210/en.2014-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ten Kulve JS, van Bloemendaal L, Balesar R, RG IJ, Swaab DF, Diamant M, la Fleur SE, Alkemade A. Decreased hypothalamic glucagon-like peptide-1 receptor expression in type 2 diabetes patients. J Clin Endocrinol Metab. 2016;101:2122–2129. doi: 10.1210/jc.2015-3291. [DOI] [PubMed] [Google Scholar]
- 30.Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab. 2015;4:718–731. doi: 10.1016/j.molmet.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Ferno J, Salvador J, Escalada J, Dieguez C, Lopez M, Fruhbeck G, Nogueiras R. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 33.Nixon JP, Mavanji V, Butterick TA, Billington CJ, Kotz CM, Teske JA. Sleep disorders, obesity, and aging: the role of orexin. Ageing Res Rev. 2015;20:63–73. doi: 10.1016/j.arr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 35.Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:195–219. doi: 10.1007/978-1-4939-0897-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacNeil DJ. NPY Y1 and Y5 receptor selective antagonists as anti-obesity drugs. Curr Top Med Chem. 2007;7:1721–1733. doi: 10.2174/156802607782341028. [DOI] [PubMed] [Google Scholar]