Abstract
Aging is an important factor in disrupted homeostasis of many tissues. While an increased incidence of tendinopathy and tendon rupture are observed with aging, it is unclear whether this is due to progressive changes in tendon cell function and mechanics over time, or an impaired repair reaction from aged tendons in response to insult or injury. In the present study we examined changes in the mechanical properties of Flexor Digitorum Longus (FDL), Flexor Carpi Ulnaris (FCU), and tail fascicles in both male and female C57Bl/6 mice between 3-27 months of age to better understand the effects of sex and age on tendon homeostasis. No change in max load at failure was observed in any group over the course of aging, although there were significant decreases in toe and linear stiffness in female mice from 3-months to 15, and to 22-27-months. No changes in cell proliferation were observed with aging, although an observable decrease in cellularity occurred in 31-month old tendons. Given that aging did not dramatically alter tendon mechanical homeostasis we hypothesized that a disruption in tendon homeostasis, via acute injury would result in an impaired healing response. Significant decreases in max load, stiffness, and yield load were observed in repairs of 22-month old mice, relative to 4-month old mice. No changes in cell proliferation were observed between young and aged, however a dramatic loss of bridging collagen extracellular matrix was observed in aged repairs suggest that matrix production, but not cell proliferation leads to impaired tendon healing with aging.
Keywords: tendon, aging, tendon healing, tenocytes, biomechanics
Introduction
Tendons are densely packed connective tissues that transmit forces from muscle to bone. Mature tendons are mainly composed of type I collagen fibers, which are tightly packaged into parallel fascicles that glide on one another when force is applied, giving tendons high tensile strength [1]. When at rest, tendons display a crimping pattern at intervals throughout their length, giving them elasticity during loading. Tenocytes are the resident fibroblast-like tendon cells, and maintain the tendon extracellular matrix [2, 3].
Aging is a complex and varied process that involves accumulation of molecular changes throughout the cells of the body, leading to cellular senescence and ultimately pathological degeneration of normal function [4, 5]. As tendons mature post-natally, tenocytes slow proliferation to become largely quiescent, leading to extremely slow tissue turnover and the absence of atrophy with age that is generally seen in other tissues such as muscle [6-8]. Tendons appear to retain the majority of their range of motion throughout the lifespan, but age-related changes in vasculature and composition may predispose older tendons to tendinopathy [9, 10]. While there are clinical observations correlating age with an increasing incidence of tendon degeneration or rupture [8, 11], the mechanisms underlying these pathological changes are not well understood, and may be related to biological and mechanical changes in the tendon over time. Additionally, loss of cellularity and a diminished stem cell pool over time may reduce the capability of tendons to regenerate following injury [12, 13]. Expanding the understanding of how aging affects tendons and increases the incidence of tendinopathies may provide targets for better therapeutic intervention or even prevention of age-related tendon pathology.
In addition to aging, sex differences may impact baseline tendon function and healing capacity. Pardis et al., demonstrated that Achilles tendons from female rats have enhanced material properties and resistance to deformation relative to males, consistent with an increase incidence of AT ruptures in males [14]. However, it is unknown whether these sex based differences occur in other tendons, or how these differences are affected by aging.
The current study aims to evaluate age-related changes in biomechanical properties in three distinct tendons; energy storing tendons: flexor carpi ulnaris (FCU), and flexor digitorum longus (FDL) and positional tendons: tail tendon fascicles isolated from male and female C57BL/6 mice between 3-31 months of age. We tested the hypotheses that i) tendon mechanical properties progressively decrease with aging, and ii) aging impairs the tendon healing response resulting in decreased mechanical properties, relative to young repairs. To our knowledge, this is the first study to directly compare multiple tendons at homeostasis over the murine lifespan. Moreover, we have examined the effects of sex and age on cell proliferation and matrix organization, as well as the impact of aging on flexor tendon healing.
Methods
Ethics statement
All animal studies were approved by the University of Rochester Committee for Animal Resources.
Mice
C57BL/6 mice were used for all studies. Mice were obtained from Jackson Laboratories or the National Institute of Aging (NIA) Aged Rodent Colony. Assessment of the effects of sex and age on tendon homeostasis was conducted in male and female mice at 3, 6, 15, 22-27 and 31 months of age. To determine the effects of aging on tendon healing, 4-month (young) and 22 month (aged) old male mice were used. Animals were group-housed with up to five animals for cage in pathogen-free housing with ad libitum access to food and water. No animals were excluded from this study for health or technical reasons.
Mechanical testing on un-injured tendon
Mouse forelimbs, hindlimbs and tails designated for mechanical testing were stored at -20° C. At the time of testing, 1 flexor carpi ulnaris (FCU) tendon (n=4-6 per age group per gender), 1 flexor digitorum longus (FDL) tendon (n=6 per age per gender) and 2 tail fascicles (n=6-10 per age per gender) were carefully dissected from each mouse. For FCU tendon dissections, the distal end of the tendon was detached from the muscle while the pisiform bone tendon was carefully removed from the wrist such that the tendon-bone insertion remained intact. For FDL tendon dissections, the proximal and distal ends were cut and the tendon was removed from surrounding soft tissues. For tail fascicle dissections, single fascicles were pulled from the proximal end and teased from the tail using jeweler's forceps.
Tendons were mechanically tested in a microscope-mounted, semi-customized, commercial mechanical testing device (ADMET, Norwood, MA) that enables simultaneous application of controlled deformation, force measurement and imaging. Custom fixtures were used to grip the tendons at their proximal and distal ends with target gauge lengths (i.e., grip-to-grip distances) of 2 mm for FCU tendons, 5 mm for FDL tendons and 7 mm for tail fascicles. To maintain hydration, the tissue remained in a phosphate-buffered saline (PBS) bath throughout testing. Each tendon was subjected to the following uniaxial tensile testing protocol: (1) equilibration to 0.050 N (preload) for FCU and FDL tendons (0.005 N for tail fascicles); (2) ramp to failure at 0.50% strain/s. Gauge length and specimen width were measured using reflected light micrographs of the tendon under preload. Tendon displacement/deformation was taken to be the controlled displacement of the grip, and reflected light micrographs were acquired throughout each test to ensure that no observable specimen slippage occurred at the grips. In tests of injured tendons (see below) with reduced strength, preconditioning was avoided to prevent possible mechanical damage prior to testing. To ensure consistency with these tests, preconditioning was also not applied in mechanical tests on uninjured tendons.
To analyze acquired load-extension (i.e., force-displacement) curves and determine the non-linear mechanical properties of tested tendons, the ramp to failure portion of each mechanical test was fit to a piecewise bilinear function as described previously [15, 16]. The slope of the first portion of the bilinear fit was taken to be the toe stiffness of the specimens, a measure of its resistance to deformation at small extensions. The slope of the second portion of the bilinear fit was taken to be the linear stiffness of the specimen, a measure of its resistance to deformation at large extensions. The extension at the intersection of the two portions of the piecewise bilinear fit was defined as the transition extension. This parameter is reflective of the level of deformation at which the tendon begins to stiffen. Finally, the maximum load was computed as the peak measured force along the load-extension curve. For a given gender and tendon type, gauge length, width, toe stiffness, linear stiffness, transition extension and maximum load were all compared across age using a one-way analysis of variance (ANOVA) with Tukey's post-hoc analysis.
Murine Flexor Tendon Healing Surgical Model
To reproduce the FT healing response, the distal flexor digitorum longus (FDL) tendon in the hindpaw was surgically transected and repaired as previously described [17, 18]. Mice were anesthetized via intraperitoneal injection of 4 mg/kg xylazine and 60 mg/kg ketamine. Under a dissecting microscope, a 0.5 cm incision was made on the lateral plantar surface of the right hind foot, the FDL tendon exposed with forceps. The tendon was transected at approximately mid-foot, and a modified Kessler stitch used to repair the ends in apposition with 8-0 nylon sutures. The FDL tendon was also transected at its myotendinous junction to reduce strain on the repair and prevent ruptures. All incisions in the skin were closed with 5-0 suture, and mice were allowed free motion in their cages following recovery from anesthesia. Analgesics were administered during the procedure and post-surgery as needed. All healing specimens were harvested at day 14 post-surgery.
Histology and preparation for immunohistochemistry
Following euthanasia, the hindpaw including the distal tibia was harvested for histological analysis (n=4 per age/sex) as previously described [17]. Briefly, the skin was removed from the dorsal side of the paw and distal ends of the toes to allow for efficient fixation. Limbs were fixed in 10% neutral buffered formalin for 72 hours, decalcified for two-weeks in 14% EDTA, and then routinely processed and embedded in paraffin, preserving anatomical position. Three-micron sagittal sections were stained with Alcian Blue/Hematoxylin Orange G (to assess tissue morphology), or Picrosirius Red staining using polarized light (to examine collagen composition and organization).
BromodeoxyUridine (BrdU) dosing
For homeostasis studies BrdU (0.8mg/ml) was given to the mice in their drinking water for one week prior to sacrifice. BrdU water was changed twice over the course of seven days. For healing studies 2mg BrdU (in a 10mg/mL solution in PBS) was administered via intraperitoneal (i.p.) injection two hours prior to sacrifice.
Immunohistochemistry
Slides were prepared as above, and antigen retrieval was performed with citrate buffer (10mM Na citrate, pH 7) for 3 hours at 70°C. Sections were circled with a hydrophobic barrier pen (Vector Laboratories, Burlingame, CA) and blocked with an endogenous peroxidase / alkaline phosphatase inhibitor (Bloxall, Vector Laboratories). After washing with PBST (PBS + 0.1% Tween-20), they were incubated for 1 hour at RT in blocking solution (5% normal goat serum + PBST). To visualize cellular proliferation, sections were incubated with primary antibodies or IgG control overnight at 4°C: PCNA (#931143, Invitrogen, Carlsbad, CA), anti-BrdU (#933943, Invitrogen), anti-Ki67 at 1:100 (Abcam, Cambridge MA), Vectastain Elite ABC rabbit IgG kit (Vector Laboratories) and DAB Chromogen (Vector Laboratories).
Biomechanical testing of healing FDL tendons
Following sacrifice, the lower limb was severed at the knee joint and skin/extraneous muscle tissue removed to the ankle, maintaining integrity of the FDL tendon (n=7 young repairs, n=8 aged repairs). The tendon was identified then carefully freed from the tarsal tunnel via dissection medially along the bone through the flexor retinaculum. The calcaneus was then removed, freeing the proximal end of the tendon for direct gripping in the mechanical test as described [19]. Briefly, the proximal end of the tendon mounted between two square pieces of tape using a thin layer of superglue. The tape was secured between the jaws of the top grip of an Instron 8841 uniaxial testing system, and the distal foot positioned in the bottom grip (Instron Corporation, Norwood, MA). The tendon was loaded at a rate of 30 mm/minute until failure, and force-displacement curves were plotted to determine maximum tensile force and stiffness.
Results
Aging does not alter the biomechanical properties of the FDL, FCU and tail tendon
For both genders, measured specimen dimensions (gauge length and width) did not significantly change with age in FCU tendons, FDL tendons or tail fascicles (See Supplementary Material). Toe stiffness, linear stiffness, transition extension and maximum load were also unaffected by age in all groups except female FCU tendons, where toe and linear stiffness were significantly heightened only at 3 months.
Aging does not alter tenocyte quiescence in vivo
To determine structural or cellular changes in tendon during aging, we focused on the FDL tendon in more detail. Histologically, the tendon displays only minor differences between 3 and 31 months of age; histological analysis reveals a slight alteration in alignment of the collagen fibers following alcian blue hematoxylin staining, observed as small ‘score marks’ perpendicular to the collagen fibers (Yellow arrows, Figure 2A & D). These alterations in organization become more apparent with picrosirius red staining (Yellow arrows, Figure 2C & F). Picrosirius red staining also demonstrates that young tendons appear solidly green under polarized light, whereas aged tendons exhibit orange-red patches in the same conditions, suggesting that collagen composition may change with age in flexor tendons.
Figure 2. FDL tendons exhibit microstructural changes in composition without a change in cellular proliferation with age.
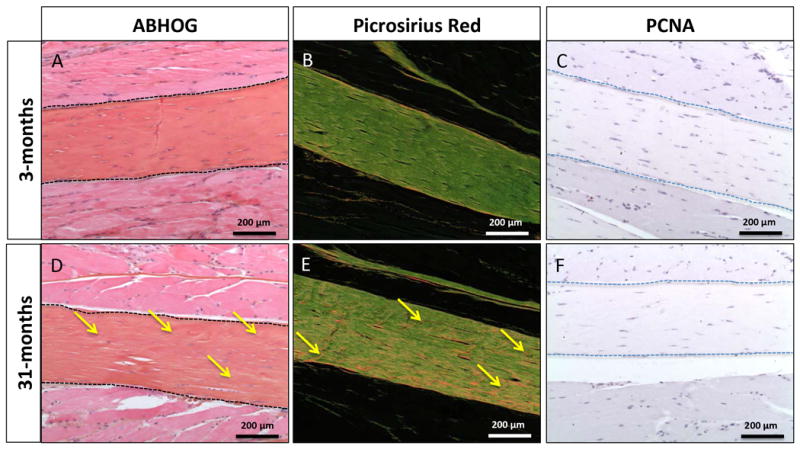
Yellow arrows indicate an observable collagen fiber re-alignment (perpendicular ‘score’ marks in the tendon) in the aged tendon seen with Alcian Blue/ Hematoxylin/ Orange G staining in 1D, and a change in composition with picrosirius red staining in 1E. No proliferating cells are seen with PCNA staining within the tendon body in young or aged FDL tendons (1C, F).
Immunostaining for three different markers of proliferation was performed - Ki67, BrdU, and PCNA. No PCNA expressing proliferating cells were observed in the tendon (Figure 2B & E). Furthermore, the nuclear counterstain hematoxylin demonstrates an observable decrease in cellularity in the aged tendons, and the cells are seen to be markedly thinner and elongated in comparison to young tendons (Figure 2B & E). BrdU & Ki67 immunostaining confirmed the PCNA results (data not shown). As an internal positive control abundant proliferative cells were observed in the skin of all mice (Figure S2).
Aged tendon exhibits impaired healing via loss of biomechanical strength following injury
Tendons have been shown to display an increased rate of rupture and impaired healing concurrent with advancing age in various models, and here we attempt to recapitulate this effect using our surgical model of tendon repair. The FDL tendon in the hind paws of young (4 months) and aged (22 months) C57BL/6J male mice was transected and surgically repaired. At 14 days post-surgery, there was a significant decrease in max load at failure in aged tendons (1.7 ± 0.2 N), relative to young tendons (2.3 ± 0.2 N, p=0.036) (Figure 3A). Stiffness of the aged tendon repairs, (3.4 ± 0.5 N/mm), was also significantly decreased compared to the young tendons (5.8 ± 0.9 N/mm, p=0.048) (Figure 3B). Yield load for the aged tendons (1.6 ± 0.2 N), also followed this pattern relative to young mice (2.2 ± 0.2 N, p=0.044) (Figure 3C). The energy to max was not significantly different between young (0.6 ± 0.1 N*mm) and aged tendon repairs (0.4 ± 0.1N*mm, p=0.1) (Figure 3D).
Figure 3. Aged tendons display reduced mechanical properties following injury.
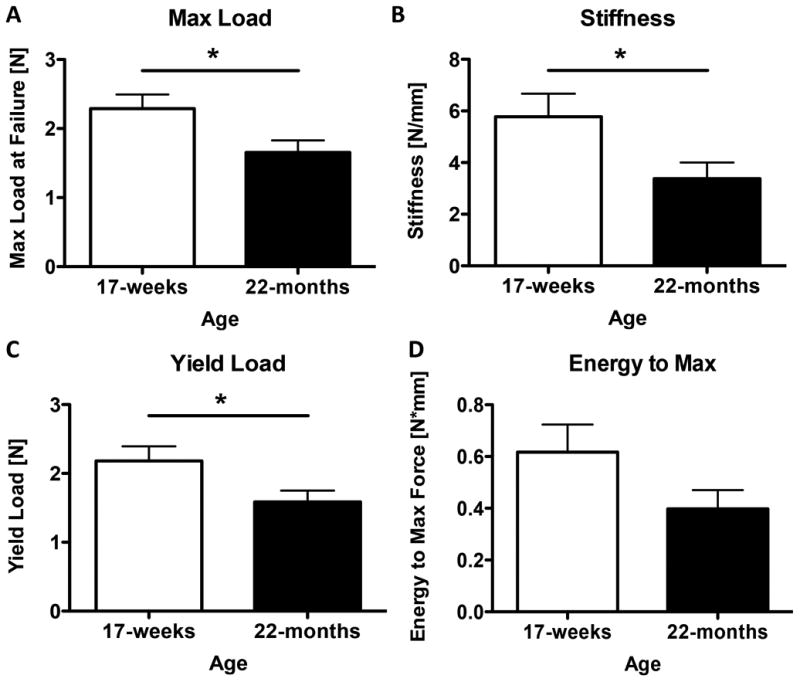
At 14 days post-surgery, max load, stiffness, and yield load were significantly decreased in the aged FDL tendon (3A, B, C) (p=0.036, p=0.048, and p = 0.044 respectively). The tested values for energy to max were not significantly different between young and aged tendon repairs, despite a slight observable decrease in the aged tendon (3D) (p=0.1).
Granulation tissue following injury is reduced and more disorganized in aged tendons, with no apparent change in proliferation
Histologically, young tendon healed with a more robust and cellular granulation tissue ‘callus’ at the repair site by day 14 post-surgery as compared to the aged tendon (Figure 4; outlined in light blue and marked with arrows, tendons are outlined in black and marked with a “T”). Consistent with this data, picrosirius red staining revealed dense collagen fibrils branching across the native tendon ends of the young repair, compared to the thinner disorganized fibers joining the tendon ends of aged tendon (Figure 5; marked by the white arrows, tendons shown outlined in red and marked with a “T”). Notably, all young repairs (n=4), healed with bridging collagen, while three of four aged repairs healed with very minimal or no bridging collagen, and one aged repair healed with bridging collagen more reflective of the young phenotype. PCNA staining showed no obvious difference in the number of proliferating cells within the granulation tissue between the young and aged repairs (Figure 6).
Figure 4. Aged tendons exhibit decreased granulation tissue following injury.
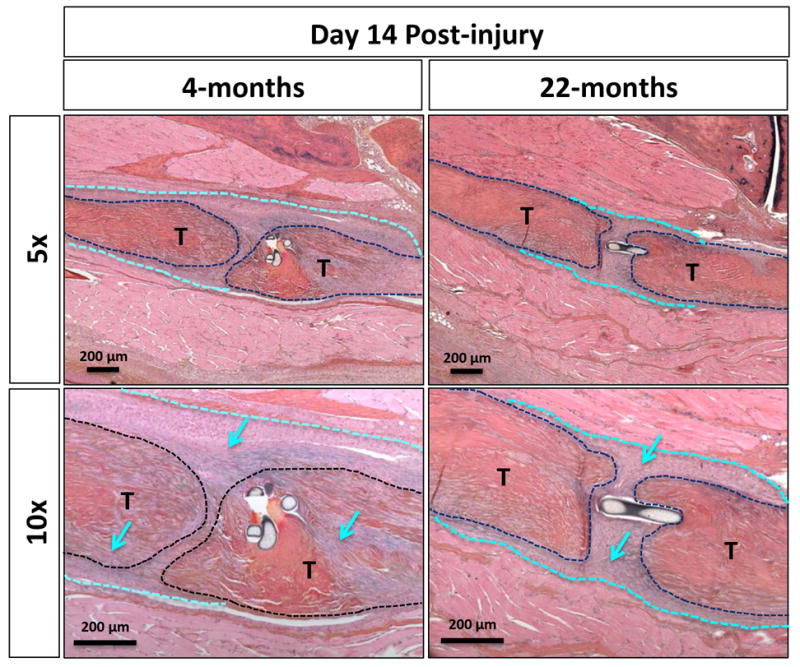
At D14 after surgical transection and repair, aged FDL noticeably deposit a reduced amount of granualation tissue surrounding the site of injury following ABHOG staining (dotted light blue line and arrows). Tendons are outlined in black and marked with a “T”.
Figure 5. Aged tendons heal with thinner, more disorganized collagen fibers bridging the ends.
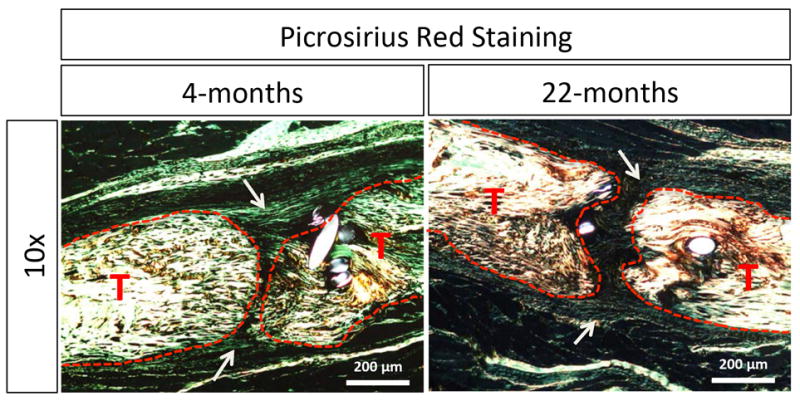
Picrosirius red staining reveals robust collagen deposition between tendon ends in the aged mice, as compared to the thinner fibers seen bridging the aged tendons (white arrows). Native tendon is outlined in red and marked with a “T”.
Figure 6. Comparable levels of proliferating cells are seen in the granulation tissue in young vs. aged FDL repairs.
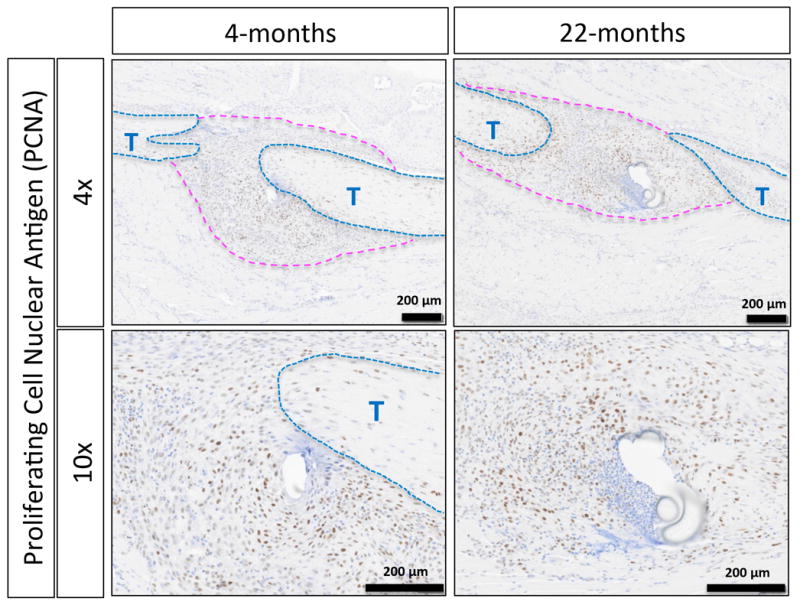
PCNA staining done at D14 following surgy on the FDL in young and aged mice does not indicate an appreciable difference in cellular proliferation surrounding the injury site at this time point. Granulation tissue is denoted by the pink dotted line, and native tendons with blue and marked by a “T”.
Discussion
The aim of this study was to investigate potential cellular or mechanical changes with age in tendons, which may be associated with the increased rate of rupture and impaired healing response seen clinically [8, 20]. We examined three different tendons at homeostasis taken from young and aged mice, the FCU, FDL, and tail tendon fascicles, and did not detect any major changes in mechanical properties with age, or as a function of sex. Our secondary focus was investigating the effects of aging on FDL tendon healing. Consistent with healing in other tendons, our data demonstrate that aging impairs restoration of mechanical properties and results in decreased matrix deposition [21, 22].
Tendon cell number decreases over the lifespan of mature tendons, as seen in mice patellar tendons [23], rat Achilles tendon [6, 24], and rat tail tendon [25], likely due to a dramatic reduction in cell proliferation post-natally [26-28]. Moreover, the remaining cells have an altered morphology, with rounder cells in the young tendon giving way to a thinner, more elongated phenotype in aged tenocytes, which is associated with a lower metabolic activity [6, 22]. This decrease in cellularity also corresponds with a decline in the pool of tendon/stem progenitor cells (TSPCs) [12, 13, 29]. Consistent with this, our 31-month old tendons demonstrate a decrease in cell density, relative to tendons from 3-month old mice, and as these cells age, many appear to lose their rounded morphology and take on a more elongated shape. Although it is unknown what proportion of these cells are TSPC, the lower cell count along with a more inactive phenotype may provide a biological explanation for an impaired tendon healing response with age.
The extracellular matrix of tendons changes with age, both in regards to the composition [23, 30-32] and altered matrix turnover and organization [12, 33, 34]. We examined alterations in matrix composition and deposition between young and aged tendons using picrosirius red staining, which allows investigation of collagen fiber structure and organization. Under polarized light, the majority of the young tendon appears green, while the aged tendon exhibits scattered patches of orange-red amongst green throughout the tendon body. This indicates that the collagen fibrils of the young tendon are largely homogenous and arranged in parallel, whereas the composition and alignment in the aged tendon are altered. Additionally, small “score marks” are visible throughout the aged tendon, compared to the “smooth” structure of the young tendon. This data suggests a potential matrix defect that becomes apparent in the tendon with age, and may be an example of the “microtrauma” that are postulated to contribute to the increased incidence of tendinopathy with age after repeated loading of the tendons[6, 22, 35].
The effect of aging on tendon mechanics remains a controversial subject, with a recent review concluding there is currently no consensus on how mechanical properties of tendons change over time, other than a slight reduction in strength [8]. One parameter investigated here, tendon stiffness, has been shown in the literature to have inconsistent age-related outcomes depending on the tendon examined. An increase in either stiffness or Young's modulus with age has been shown in the human Achilles [36], and mouse tibialis anterior [37] tendons, while a decrease in the mouse patellar tendon [23], in the human Achilles [38], and no change in the human patellar tendon has also been observed [39]. Taken together, it is clear that there is a differential response to aging between tendons, possibly based on location and function [2]. Our data shows few statistically significant changes in the mechanical properties of three distinct murine tendons (FDL, FCU, and tail) at homeostasis over time, contrary to our hypothesis. While there have been no previous studies investigating age-related changes in the mechanical properties of the FDL & FCU, our data regarding the tail tendon agrees with previous work by Goh et al [40]. Although our data do not necessarily agree with the general consensus that aged tendons display a general increase in stiffness and corresponding loss of strength, the studies reporting this data have been done in humans, and may be more readily observed due to humans' far longer lifespan compared to mice (78 yrs vs 3 yrs) during which defects and microtrauma accumulate.
The effect of sex differences on tendon homeostasis is not clear. Males are much more susceptible to Achilles tendon rupture, likely due to the decrease in tendon material properties relative to female [14]. However, chronic estrogen deficiency can decrease Achilles tendon strain [41], and estrogen replacement therapy enhances tendon collagen matrix synthesis [42]. In the present study we have used female mice that span the reproductive endocrinology spectrum from young, sexually mature mice (3-6 months), perimenopausal (∼9 months) to reproductive senescence and menopause (12 months and older) [43]. Despite this, mechanical properties of FDL, FCU and tail tendon fascicles do not decline with age, suggesting that decreased estrogen levels associated with menopause in female mice has no effect on bulk mechanical properties.
In this study, structural mechanical properties (toe stiffness, linear stiffness, transition extension and maximum load) were evaluated in uninjured tendons and compared across age instead of material properties (toe modulus, linear modulus, transition strain and tensile strength) that are reflective of the intrinsic mechanical response of tendon. The disadvantage of structural mechanical property assessment is that unlike material properties, these parameters are sensitive to geometric changes (e.g., changes in length or cross sectional area). However, we successfully controlled the gauge length of the tested specimens such that there were no differences between age groups. Furthermore, the width did not vary across age, strongly suggesting that the cross-sectional area of the tested tendons was also independent of age. Although tendon thickness was not assessed, no qualitative differences in thickness across age were observed. Hence, the structural mechanical properties that were measured in this study should be reflective of material properties and – based on our findings – it can be deduced that the material properties of uninjured FCU tendons, FDL tendons and tail tendon fascicles do not change with age.
Although the biomechanical tests performed here did not exhibit any statistically significant differences in these tendons at homeostasis, small-scale changes may still be taking place. It has been observed that measurements of mechanical property alone may not be sufficient to determine structural changes in tendons [44]. Age-related increases in advanced glycation endproducts (AGEs) increase collagen cross-linking, decreasing collagen fibril sliding, and may be a potential contributor to the changes in mechanical properties seen in older tendons [45]. Although not directly addressed in the present study, exploring the potential link between AGEs and changes in mechanical properties with age may be worth investigating in order to understand how they may affect tendons at homeostasis and after injury.
Despite the fact that mechanical properties of tendons we studied were unchanged over the lifespan, we observed an impaired healing phenotype in aged FDL tendons compared to young tendons, consistent with previous studies in the rotator cuff [46] and patellar tendon [21], and supporting our hypothesis of decreased mechanical properties relative to repairs from young mice. Potential biological causes have been described previously, including a loss of cellularity in the aged tendon and an alteration in the matrix composition. Consistent with this, we see a decrease in collagen bridging the tendon injury site in aged mice, relative to abundant collagen bridging in the young mice. Concurrent with this, we observe no change in cell proliferation during healing as a function of aging, suggesting that the amount of ECM produced per cell may be diminished with age, as demonstrated in other tissues [47]. In addition, the TSPC pool has also been shown to decline in both size and function with age, which could contribute to the increased incidence of rupture and decline in wound healing response [13]. A study by Zhang et al [48], performed a window defect in rat patellar tendons and saw an enhanced healing response following exercise, corresponding to an increase in the TSPC pool and tenocyte-related genes such as collagen I & III, suggesting that an impaired healing response with age may also be due to a decreased stem cell population and impaired matrix deposition. Interestingly the max load at failure between un-injured tendons from 3-month old male, and injured tendons from 17-week old were not significantly different. However, we believe the differences in mechanical tests that were performed these two groups may explain this. Un-injured FDL tendons were isolated free of all surrounding tissues and from the bone-tendon junction, resulting in a max load at failure representative of only the tendon. In contrast, injured tendons are tested in situ with the bone-tendon junction remaining intact, as well as any fibrous connections, or ‘adhesions’ between the healing tendon and surrounding tissue, likely resulting in a much greater max load at failure than if the tendon if the tendon had been tested in isolation. Consistent with this, un-injured tendons that are testing in situ typically fail at a nearly 10N [17].
While we have clearly demonstrated the effects of aging on tendon homeostasis and repair in a murine model, there are some limitations to this study that must be considered. In our testing protocols, the absence of preconditioning and the use of grip displacement (instead of optically-evaluated tissue displacement) to measure tendon deformation may have increased specimen-to-specimen variability and affected our measurements. However, we decided not to precondition tested specimens in order to avoid possible damage to injured tendons, and we acquired reflected micrographs throughout each mechanical test to ensure that specimen slippage at the grips did not occur. In addition, as described earlier, we measured structural properties, but did not directly assess material properties. Nevertheless, since specimen dimensions were consistent across age, it can be inferred that the effects of aging on tendon material and structural properties are similar. Our study of mechanical properties of tendons at homeostasis over time was limited to three tendons, the FCU, FDL, and tail. Since it has been shown that the location and function of the tendon influences its response to aging/healing [2, 49], examination of a wider range of tendons would give a more comprehensive picture of the effects of aging on tendons. Additionally, all FDL repairs were conducted with male mice only, limiting assessment of potential sex differences in the healing response, a potentially important factor given that estrogen deficiency decreases the maximum stress of healing Achilles tendons [50].
Here we demonstrate that mechanical properties of the FDL, FCU and tail tendon fascicles are not altered as a function of sex or age, however, alterations in ECM composition and structure occur with aging in the FDL. Moreover, flexor tendon healing is impaired in aged mice due to a decrease in matrix deposition, resulting in decreased mechanical properties. Investigating the effects of aging on tendon maintenance and repair from both a biological and biomechanical standpoint gives us the unique ability to tie these two aspects into a greater understanding of the processes behind tendon aging and degeneration. Our data have identified ECM production and organization as a potential mechanism of age-related tendinopathy and poor healing outcomes. This suggests the need for further study in to ECM production and remodeling as a potential approach to identify therapeutic targets to enhance healing of aged tendons.
Supplementary Material
Figure S1. Specimen geometry is consistent among age groups for a given tendon type. For each tendon type, the gauge length and width of mechanically tested tendons did not vary with age. Thus, the age-dependent structural mechanical properties assessed in this study (Figure 1) should be reflective of age-dependent material properties.
Figure S2. Proliferating cells in the skin of the hind paw demonstrate positive staining for BrdU, Ki67 and PCNA. Highly proliferative cells are observed in the epidermis of the hindpaw, resulting in positive staining for Ki67 and PCNA, as well as BrdU, following seven days of BrdU administration in the drinking water. The epidermis was used as an internal positive control for Ki67, PCNA, and BrdU staining of tendons during aging. Scale bars represent 50 microns.
Figure 1. Male and female uninjured tendons from multiple sites display few alterations in mechanical properties with age.
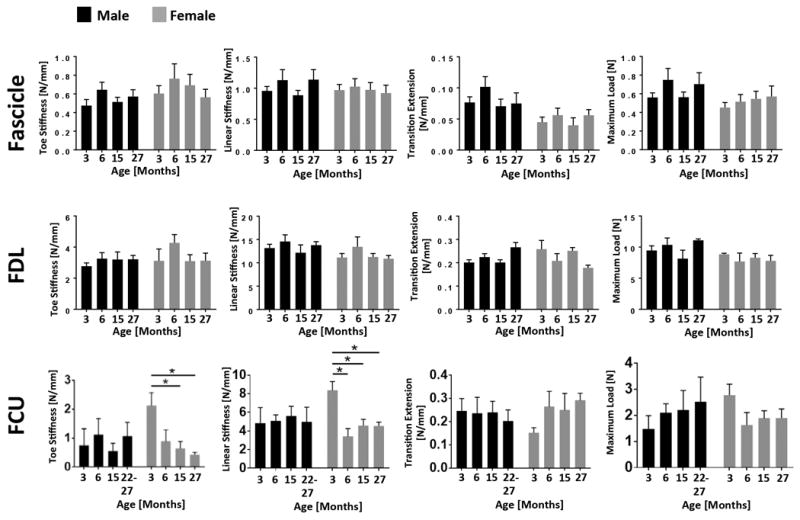
FCU, FDU and tail fascicles were mechanically tested at 3, 6, 15 and 27 months (22-27 months for male FCU) to identify age-related changes in toe stiffness, linear stiffness, transition extension and maximum load. Apart from an increased FCU toe stiffness at 3 months (p=0.027 versus 15 months and p=0.012 versus 27 months) and an increased linear stiffness at 3 months (p=0.0013 versus 9 months, p=0.012 versus 15 months and p=0.011 versus 27 months) in female mice, no age-associated changes in mechanical properties were found. (*) indicates p<0.05.
Acknowledgments
We would like to thank the Histology, Biochemistry and Molecular Imaging (HBMI) and the Biomechanics and Multimodal Tissue Imaging (BMTI) Cores for technical assistance. This work was supported by NIH/ NIAMS 1K01AR068386-01A1 (to AEL). The HBMI and BMTI Cores are supported by NIH/ NIAMS P30AR069655. Aged mice were provided by the National Institute of Aging Aged Rodent Colony (to JHJ).
Footnotes
Author contributions: Study conception and design: JHJ, AEL; Acquisition of data: JEA, IB; Analysis and interpretation of data: JEA, IB, MRB, AEL; Drafting of manuscript: JEA, IB, MRB, AEL; Revision and approval of manuscript: JEA, IB, JHJ, MRB, AEL.
References
- 1.Docheva D, et al. Biologics for tendon repair. Adv Drug Deliv Rev. 2015;84:222–39. doi: 10.1016/j.addr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Screen HR, et al. Tendon functional extracellular matrix. J Orthop Res. 2015;33(6):793–9. doi: 10.1002/jor.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley G. Tendinopathy--from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4(2):82–9. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 4.López-Otín C, et al. The Hallmarks of Aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia-Dey N, et al. Cellular Senescence as the Causal Nexus of Aging. Frontiers in Genetics. 2016;7:13. doi: 10.3389/fgene.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai WC, et al. Decreased proliferation of aging tenocytes is associated with down-regulation of cellular senescence-inhibited gene and up-regulation of p27. J Orthop Res. 2011;29(10):1598–603. doi: 10.1002/jor.21418. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe CT, et al. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J Biol Chem. 2010;285(21):15674–81. doi: 10.1074/jbc.M109.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svensson RB, et al. The effect of aging and exercise on the tendon. J Appl Physiol (1985) 2016 doi: 10.1152/japplphysiol.00328.2016. p jap.00328.2016. [DOI] [PubMed] [Google Scholar]
- 9.Swan MA, et al. The effect of age on rat rotator cuff muscle architecture. J Shoulder Elbow Surg. 2014;23(12):1786–91. doi: 10.1016/j.jse.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou B, Zhou Y, Tang K. An overview of structure, mechanical properties, and treatment for age-related tendinopathy. J Nutr Health Aging. 2014;18(4):441–8. doi: 10.1007/s12603-014-0026-2. [DOI] [PubMed] [Google Scholar]
- 11.Andarawis-Puri N, Flatow EL, Soslowsky LJ. Tendon basic science: Development, repair, regeneration, and healing. J Orthop Res. 2015;33(6):780–4. doi: 10.1002/jor.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9(5):911–5. doi: 10.1111/j.1474-9726.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler J, et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell. 2013;12(6):988–99. doi: 10.1111/acel.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardes AM, et al. Males have Inferior Achilles Tendon Material Properties Compared to Females in a Rodent Model. Ann Biomed Eng. 2016 doi: 10.1007/s10439-016-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bah I, et al. Mechanical changes in the Achilles tendon due to insertional Achilles tendinopathy. J Mech Behav Biomed Mater. 2016;53:320–8. doi: 10.1016/j.jmbbm.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lake SP, et al. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27(12):1596–602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loiselle AE, et al. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J Orthop Res. 2009;27(6):833–40. doi: 10.1002/jor.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackerman JE, Loiselle AE. Murine Flexor Tendon Injury and Repair Surgery. J Vis Exp. 2016:e54433. doi: 10.3791/54433. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasslund S, et al. Adhesions in a murine flexor tendon graft model: Autograft versus allograft reconstruction. J Orthop Res. 2008;26(6):824–33. doi: 10.1002/jor.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomopoulos S, et al. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832–9. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunkman AA, et al. The injury response of aged tendons in the absence of biglycan and decorin. Matrix Biol. 2014;35:232–8. doi: 10.1016/j.matbio.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorpe CT, et al. Ageing does not result in a decline in cell synthetic activity in an injury prone tendon. Scandinavian Journal of Medicine & Science in Sports. 2015 doi: 10.1111/sms.12500. p. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 23.Dunkman AA, et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32(1):3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, et al. Nanoparticle-mediated delivery of TGF-β1 miRNA plasmid for preventing flexor tendon adhesion formation. Biomaterials. 2013;34(33):8269–8278. doi: 10.1016/j.biomaterials.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 25.Lavagnino M, Gardner K, Arnoczky SP. Age-related changes in the cellular, mechanical, and contractile properties of rat tail tendons. Connect Tissue Res. 2013;54(1):70–5. doi: 10.3109/03008207.2012.744973. [DOI] [PubMed] [Google Scholar]
- 26.Dyment NA, et al. The paratenon contributes to scleraxis-expressing cells during patellar tendon healing. PLoS One. 2013;8(3):e59944. doi: 10.1371/journal.pone.0059944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Docheva D, et al. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25(2):699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruchti C, et al. Regional differences in renewal rates of fibroblasts in young adult female mice. Cell Tissue Res. 1983;232(3):625–36. doi: 10.1007/BF00216434. [DOI] [PubMed] [Google Scholar]
- 29.Klatte-Schulz F, et al. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur Cell Mater. 2012;24:74–89. doi: 10.22203/ecm.v024a06. [DOI] [PubMed] [Google Scholar]
- 30.Chang HN, et al. The effect of aging on migration, proliferation, and collagen expression of tenocytes in response to ciprofloxacin. J Orthop Res. 2012;30(5):764–8. doi: 10.1002/jor.21576. [DOI] [PubMed] [Google Scholar]
- 31.Kostrominova TY, Brooks SV. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age (Dordr) 2013;35(6):2203–14. doi: 10.1007/s11357-013-9514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couppe C, et al. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. Journal of applied physiology. 2009;107(3):880–886. doi: 10.1152/japplphysiol.00291.2009. [DOI] [PubMed] [Google Scholar]
- 33.Peffers MJ, et al. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J Biol Chem. 2014;289(37):25867–78. doi: 10.1074/jbc.M114.566554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sprenger CC, Plymate SR, Reed MJ. Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression. Int J Cancer. 2010;127 doi: 10.1002/ijc.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudhia J, et al. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell. 2007;6(4):547–556. doi: 10.1111/j.1474-9726.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 36.Turan A, et al. Sonoelastographic assessment of the age-related changes of the Achilles tendon. Med Ultrason. 2015;17(1):58–61. doi: 10.11152/mu.2013.2066.171.ayt. [DOI] [PubMed] [Google Scholar]
- 37.Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol (1985) 2011;111(4):999–1006. doi: 10.1152/japplphysiol.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stenroth L, et al. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol (1985) 2012;113(10):1537–44. doi: 10.1152/japplphysiol.00782.2012. [DOI] [PubMed] [Google Scholar]
- 39.Carroll CC, et al. Influence of aging on the in vivo properties of human patellar tendon. Journal of applied physiology. 2008;105(6):1907–1915. doi: 10.1152/japplphysiol.00059.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goh KL, et al. Ageing Changes in the Tensile Properties of Tendons: Influence of Collagen Fibril Volume Fraction. Journal of Biomechanical Engineering. 2008;130(2):021011–021011. doi: 10.1115/1.2898732. [DOI] [PubMed] [Google Scholar]
- 41.Bryant AL, et al. Effects of estrogen on the mechanical behavior of the human Achilles tendon in vivo. J Appl Physiol (1985) 2008;105(4):1035–43. doi: 10.1152/japplphysiol.01281.2007. [DOI] [PubMed] [Google Scholar]
- 42.Hansen M, et al. Effect of estrogen on tendon collagen synthesis, tendon structural characteristics, and biomechanical properties in postmenopausal women. J Appl Physiol (1985) 2009;106(4):1385–93. doi: 10.1152/japplphysiol.90935.2008. [DOI] [PubMed] [Google Scholar]
- 43.Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology. 2012;153(8):3571–8. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connizzo BK, et al. Effect of age and proteoglycan deficiency on collagen fiber re-alignment and mechanical properties in mouse supraspinatus tendon. J Biomech Eng. 2013;135(2):021019. doi: 10.1115/1.4023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fessel G, Gerber C, Snedeker JG. Potential of collagen cross-linking therapies to mediate tendon mechanical properties. J Shoulder Elbow Surg. 2012;21(2):209–17. doi: 10.1016/j.jse.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Teunis T, et al. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913–21. doi: 10.1016/j.jse.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Yukata K, et al. Aging Periosteal Progenitor Cells have Reduced Regenerative Responsiveness to Bone Injury and to the Anabolic Actions of PTH 1-34 Treatment. Bone. 2014;62:79–89. doi: 10.1016/j.bone.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Yuan T, Wang JH. Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats. Oncotarget. 2016;7(8):8498–512. doi: 10.18632/oncotarget.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shepherd JH, et al. Functionally distinct tendon fascicles exhibit different creep and stress relaxation behaviour. Proc Inst Mech Eng H. 2014;228(1):49–59. doi: 10.1177/0954411913509977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Circi E, et al. Biomechanical and histological comparison of the influence of oestrogen deficient state on tendon healing potential in rats. Int Orthop. 2009;33(5):1461–6. doi: 10.1007/s00264-009-0778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Specimen geometry is consistent among age groups for a given tendon type. For each tendon type, the gauge length and width of mechanically tested tendons did not vary with age. Thus, the age-dependent structural mechanical properties assessed in this study (Figure 1) should be reflective of age-dependent material properties.
Figure S2. Proliferating cells in the skin of the hind paw demonstrate positive staining for BrdU, Ki67 and PCNA. Highly proliferative cells are observed in the epidermis of the hindpaw, resulting in positive staining for Ki67 and PCNA, as well as BrdU, following seven days of BrdU administration in the drinking water. The epidermis was used as an internal positive control for Ki67, PCNA, and BrdU staining of tendons during aging. Scale bars represent 50 microns.


