Colorectal cancer (CRC) incidence and mortality in the United States have changed strikingly in recent decades. Overall, CRC incidence decreased by >30% from 1975 (59.5 per 100,000) to 2013 (37.9 per 100,000).1 CRC mortality similarly declined from 28.1 per 100,000 in 1975 to 14.5 per 100,000 in 2013—nearly a 50% decrease.1 Screen-eligible populations, particularly those over age 65, have experienced the largest declines in incidence and mortality.2,3
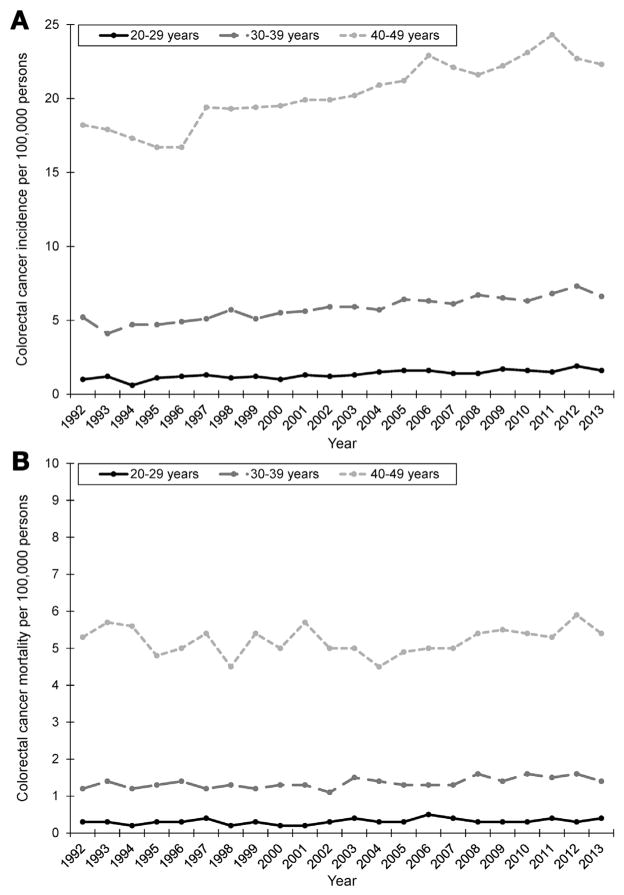
In marked contrast with populations >50 years of age, the CRC incidence is increasing in younger adults.4,5 Starting in the early 1990s, incidence rates have increased in this population (ages 20–49 years), from 8.5 per 100,000 in 1992 to 10.7 per 100,000 in 2013, a 26% increase.1 The largest absolute increases have occurred in the 40- to 49-year age group, from 18.2 per 100,000 in 1992 to 22.3 per 100,000 in 2013 (Figure 1A). Mortality rates have remained stable during the same period (around 2.4 per 100,000), ranging from 2.1 per 100,000 in 1998 to 2.7 per 100,000 in 2012 (Figure 1B).
Figure 1.
Age-adjusted (2000 US standard population) incidence (A) and mortality (B) of young-onset colorectal cancer by 10-year age group, Surveillance, Epidemiology, and End Results (SEER) 13, 1992–2013. Colorectal cancer (CRC) incidence and mortality were derived from the National Cancer Institute’s SEER program during 1992–2013. SEER 13 registries include Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia, and Alaska Native Tumor Registry. Age-adjusted incidence and mortality (by using the 2000 US standard population) were obtained by using SEER*Stat version 8.3.2 as rates per 100,000 persons.
The increasing incidence of young-onset CRC has prompted discussion of the relative contribution of risk factors (eg, obesity, dietary patterns) and diagnostic factors (eg, screening, case ascertainment, practice patterns) to these observed patterns. Screening can influence incidence trends.6 For example, as evidence accumulated supporting the effectiveness of stool-based screening tests and sigmoidoscopy,7,8 the overall CRC incidence increased from 59.3 per 100,000 in 1975 to 66.3 per 100,000 in 1985 and subsequently decreased through 2013.1 CRC screening in the United States has gradually increased after it was first formally recommended9 in the late 1980s, particularly screening with colonoscopy.10 Although guidelines recommend average-risk CRC screening starting at age 50,11 younger adults may undergo colonoscopy for many reasons, including diagnostic evaluation (ie, for bleeding), family history of CRC and/or polyps, and inflammatory bowel disease. Colonoscopy in this setting may facilitate earlier detection of small tumors that increase incidence and survival without changing mortality.
Trends in young-onset CRC and diffusion of colonoscopy as a screening test raise the question of whether increasing incidence rates are an artifact of earlier diagnoses or increasing disease burden. To clarify the extent to which colonoscopy is performed in younger populations, we examined patterns of colonoscopy use in a commercially insured population of adults younger than age 50 and variation in use by age, sex, and geographic region.
Patterns of Colonoscopy Use
Using MarketScan Commercial Claims and Encounters data (Truven Health Analytics, Ann Arbor, MI), we examined patterns of colonoscopy use in younger adults (age <50 years) from 2001 to 2014. MarketScan is a large, employer-based claims database that includes 77 contributing employers and 12 contributing health plans, with 126 unique carriers and 8 Medicaid states. We summed the total number of months individuals aged 18–49 years were enrolled in their insurance plan in each calendar year as standardized denominators of “enrollee-time.” We then described time trends in colonoscopy by calculating a rate of colonoscopy per 1000 enrollee-years in each calendar year, assuming constant rates within each calendar year. We also examined colonoscopy rates by sex (male vs female), age group (20–29, 30–39, and 40–49 years), and geographic region (northeast, north central, west, south).
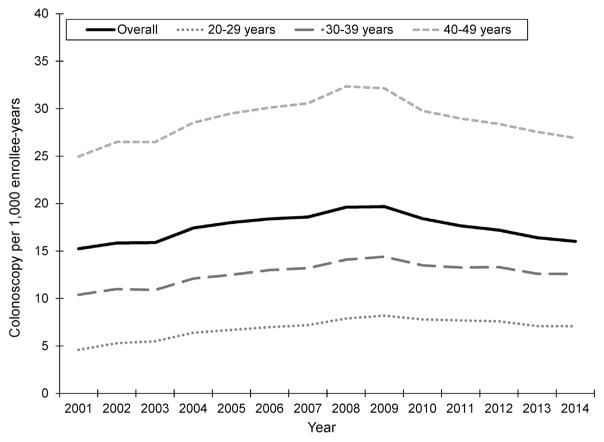
A total of 3,216,798 colonoscopies were performed in 181,665,189 enrollee-years from 2001 to 2014. Overall colonoscopy rate increased from 15.2 per 1000 in 2001 to 19.7 per 1000 in 2009 and subsequently decreased to 16.0 per 1000 in 2014 (Figure 2). Detailed colonoscopy rates by age group, sex, and geographic region are available in Supplementary Table 1.
Figure 2.
Colonoscopies performed per 1000 enrollee-years, overall and by 10-year age group, MarketScan Commercial Claims and Encounters Data, 2001–2014.
Colonoscopy rates were highest in the 40- to 49-year age group and lowest in the 20- to 29-year age group in all calendar years (Figure 2). Within each age group, rates generally increased from 2001 to 2009, with slight decreases through 2014 (Figure 2). For example, among 40- to 49-year-olds, the colonoscopy rate increased from 25.0 per 1000 in 2001 to 32.1 per 1000 in 2009 and decreased to 26.9 per 1000 in 2014. Women more frequently received colonoscopy in all years compared with men (Supplementary Figure 1). At its peak in 2009, the colonoscopy rate among women was 21.6 per 1000 and 17.6 per 1000 among men, with smaller decreases among women after 2010 than men. Colonoscopy rates were similar in the north central and southern regions but remained consistently higher in the northeast and lower in the west (Supplementary Figure 2).
Colonoscopy Rates in Younger Adults Parallel Increases in CRC Incidence
Colonoscopy rates in younger adults increased by 30% from 2001 (15.2 per 1000) to 2009 (19.7 per 1000), which parallels increases in young-onset CRC adults during the same period (Figure 1A). Although it is unclear whether colonoscopy at younger ages represents overuse, symptomatic assessment, or high-risk screening, this increase occurred consistently across age group, sex, and geographic region. Many ambulatory surgery centers opened in the late 1990s and early 2000s after colonoscopy was endorsed as a preferred screening strategy,12 and Medicare expanded reimbursement to include screening colonoscopy for average-risk persons,13 increasing endoscopy capacity and efficiency.14 This diffusion of colonoscopy into clinical practice may have had “spillover” effects, lowering thresholds for performing colonoscopy in younger patients.
Colonoscopies were most frequently performed in this commercially insured population among the oldest age group (40–49 years), where absolute increases in CRC incidence were also greatest (Figure 1A). Roughly 1 in every 30 adults in this age group underwent colonoscopy, suggesting colonoscopy close to age 50, when guidelines recommend average-risk screening, has become increasingly common.15 Providers may be more apt to recommend colonoscopy in clinically nuanced situations, anticipating need for CRC screening in coming years. Or they may offer patients colonoscopy as part of preventive care, based on the small body of evidence suggesting similar prevalence of colorectal neoplasia in average-risk 40- to 49-year-olds and 50- to 59-year-olds.16 Some organizations have also recommended screening African Americans starting at age 45.17
Greater awareness of family or personal history of CRC and/or polyps may also explain colonoscopy rates in the 40- to 49-year age group. Consensus guidelines generally recommend CRC screening for those with a family history (first-degree relative) begin at age 40 or 10 years before the youngest relative was diagnosed with CRC.18 Guidelines also recommend earlier screening for first-degree relatives of patients with adenomas. Widespread colonoscopy over age 50 likely detects more adenomas, leading to more colonoscopies in younger relatives as a consequence.
Screening is recommended even earlier in individuals with hereditary syndromes (eg, familial adenomatous polyposis, the Lynch syndrome), which may account for some colonoscopies in the youngest age group (20–29 years). Although mutations in known susceptibility genes only account for a small proportion (3%–5%) of CRCs,19 recent research efforts incorporate several strategies into clinical care that identify and screen high-risk individuals.20 These include family history screening tools,21,22 outreach to high-risk family members,23 increased recognition of Lynch syndrome families in the population,24 and testing all CRC cases, regardless of age at diagnosis, for microsatellite instability.25 Penetrance of genetic mutations is unlikely to have changed over time,26 but more conscientious adherence to these guidelines in higher risk populations could contribute to greater use of colonoscopy.
Declining Colonoscopy Rates After the Economic Recession
Starting in 2010, colonoscopy rates in younger adults consistently decreased through 2014 (Figure 2), although the CRC incidence in this population has continued to increase or remain stable (Figure 1A). Decreasing colonoscopy rates may reflect economic downturn after the 2008 financial crisis. Many Americans used less health care during the recession, and those who remained continuously insured curtailed use of costly and/or elective health care services.27 This trend was particularly true among younger or employed adults who lost or had reduced insurance benefits during the recession as employer-sponsored coverage declined.28 Indeed, 500,000 fewer screening colonoscopies were performed among adults aged 50–64 years during the recession than would have been expected based on trends before the recession.29 Downstream economic effects of the recession may have also affected patients’ willingness to see providers and ability to afford (expensive) colonoscopy copayments. Around the same time, health care reform emphasized high-value care and penalized unnecessary medical tests and procedures. Both the economic impact of the recession and changes in health care policy may have contributed to lower colonoscopy rates in younger adults, where routine use is not recommended.
Implications for Young-Onset CRC
Considering clinical and diagnostic practices is important when monitoring incidence trends of screen-detected cancers. Colonoscopy use generally increased among younger adults, although differences between Market-Scan and population-based cancer registry data make it difficult to draw conclusions regarding the impact on young-onset CRC. MarketScan includes persons receiving employer-based insurance and may only partially overlap with Surveillance, Epidemiology, and End Results registry populations. Colonoscopy rates in commercially insured younger adults paralleled population trends in CRC incidence from 2001 to 2009. Mortality rates remained relatively unchanged (Figure 1B), suggesting increases in incidence may reflect earlier detection via screening (ie, lead-time bias). This was most apparent in the 40- to 49-year-age group, where the majority of colonoscopies were performed (Figure 2), and absolute incidence increased the most (Figure 1A). However, colonoscopy rates decreased through 2014, although the incidence continued to increase, albeit slightly. Declining colonoscopy rates in more recent years may indicate other factors—beyond those related to detection and diagnosis—also contribute to young-onset CRC.
Overall, CRC incidence and mortality rates have declined drastically, likely, in part, because of increased screening among individuals ≥50 years of age.3 These decreases have not occurred in younger (<50 years) adults. CRC incidence rates have increased in younger populations, whereas mortality has remained stagnant. Increasing cancer incidence rates with stable mortality rates are often seen when greater use of screening detects cancers earlier. Given increased concerns about the rising incidence of young-onset CRC, etiologic studies identifying the mechanisms that explain increases in CRC incidence should carefully consider the influence of shifts in colonoscopy use over time.
Supplementary Material
Acknowledgments
Funding
National Institutes of Health grants T32DK007634 and K12CA120780 (JL Lund)
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2017.04.030.
Conflicts of interest
The authors disclose the following: Dr Lund received a Research Starter Award from the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation. The remaining authors disclose no conflicts.
Contributor Information
CAITLIN C. MURPHY, Division of Epidemiology, Department of Clinical Sciences, University of Texas Southwestern, Medical Center, Dallas, Texas
JENNIFER L. LUND, Department of Epidemiology, University of North Carolina at Chapel Hill
ROBERT S. SANDLER, Center for Gastrointestinal Biology and Disease, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program. Available at: www.seer.cancer.gov. Released April 2016, based on the November 2015 submission.
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Murphy CC, Sandler RS, Sanoff HK, et al. Decrease in incidence of colorectal cancer among individuals 50 years or older after recommendations for population-based screening. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.08.037. Epub 2016 Sept 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1695–1698. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 6.Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: lessons from history and study design issues. Semin Oncol. 2010;37:202–215. doi: 10.1053/j.seminoncol.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardcastle JD, Armitage NC, Chamberlain J, et al. Fecal occult blood screening for colorectal cancer in the general population. Results of a controlled trial Cancer. 1986;58:397–403. doi: 10.1002/1097-0142(19860715)58:2<397::aid-cncr2820580235>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Hardcastle JD, Farrands PA, Balfour TW, et al. Controlled trial of faecal occult blood testing in the detection of colorectal cancer. Lancet. 1983;2:1–4. doi: 10.1016/s0140-6736(83)90001-6. [DOI] [PubMed] [Google Scholar]
- 9.Working guidelines for early cancer detection rationale and supporting evidence to decrease mortality. Washington, DC: National Cancer Institute; 1987. [Google Scholar]
- 10.Vernon SW, Murphy CC, McQueen A, et al. Colorectal cancer screening. In: Holland JC, Breitbart WS, Jacobsen PB, et al., editors. Psycho-Oncology. 3. New York: Oxford University Press; 2015. [Google Scholar]
- 11.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 12.Rex DK, Johnson DA, Lieberman DA, et al. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. Am J Gastroenterol. 2000;95:868–877. doi: 10.1111/j.1572-0241.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 13.Shih YC, Zhao L, Elting LS. Does Medicare coverage of colonoscopy reduce racial/ethnic disparities in cancer screening among the elderly? Health Aff (Millwood) 2006;25:1153–1162. doi: 10.1377/hlthaff.25.4.1153. [DOI] [PubMed] [Google Scholar]
- 14.Vicari JJ. The future value of ambulatory endoscopy centers in the United States: challenges and opportunities. Gastrointest Endosc. 2012;76:400–405. doi: 10.1016/j.gie.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Rutter CM, Greenlee RT, Johnson E, et al. Prevalence of colonoscopy before age 50. Prev Med. 2015;72:126–129. doi: 10.1016/j.ypmed.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rundle AG, Lebwohl B, Vogel R, et al. Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology. 2008;134:1311–1315. doi: 10.1053/j.gastro.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol. 2005;100:515–523. doi: 10.1111/j.1572-0241.2005.41829.x. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 19.Win AK, Jenkins MA, Dowty JG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:404–412. doi: 10.1158/1055-9965.EPI-16-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel SG, Lowery JT, Gatof D, et al. Practical opportunities to improve early detection and prevention of colorectal cancer (CRC) in members of high-risk families. Dig Dis Sci. 2015;60:748–761. doi: 10.1007/s10620-015-3567-2. [DOI] [PubMed] [Google Scholar]
- 21.Kastrinos F, Allen JI, Stockwell DH, et al. Development and validation of a colon cancer risk assessment tool for patients undergoing colonoscopy. Am J Gastroenterol. 2009;104:1508–1518. doi: 10.1038/ajg.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skinner CS, Gupta S, Bishop WP, et al. Tailored information increases patient/physician discussion of colon cancer risk and testing: the Cancer Risk Intake System trial. Prev Med Rep. 2016;4:6–10. doi: 10.1016/j.pmedr.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowery JT, Horick N, Kinney AY, et al. A randomized trial to increase colonoscopy screening in members of high-risk families in the colorectal cancer family registry and cancer genetics network. Cancer Epidemiol Biomarkers Prev. 2014;23:601–610. doi: 10.1158/1055-9965.EPI-13-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel SG, Ahnen DJ, Kinney AY, et al. Knowledge and uptake of genetic counseling and colonoscopic screening among individuals at increased risk for Lynch syndrome and their endoscopists from the family health promotion project. Am J Gastroenterol. 2016;111:285–293. doi: 10.1038/ajg.2015.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissman SM, Burt R, Church J, et al. Identification of individuals at risk for Lynch syndrome using targeted evaluations and genetic testing: National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Colorectal Cancer joint practice guideline. J Genet Couns. 2012;21:484–493. doi: 10.1007/s10897-011-9465-7. [DOI] [PubMed] [Google Scholar]
- 26.Limburg PJ, Harmsen WS, Chen HH, et al. Prevalence of alterations in DNA mismatch repair genes in patients with young-onset colorectal cancer. Clin Gastroenterol Hepatol. 2011;9:497–502. doi: 10.1016/j.cgh.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartman M, Martin A, Nuccio O, et al. Health spending growth at a historic low in 2008. Health Aff (Millwood) 2010;29:147–155. doi: 10.1377/hlthaff.2009.0839. [DOI] [PubMed] [Google Scholar]
- 28.Holahan J. The 2007–09 recession and health insurance coverage. Health Affairs. 2011;30:145–152. doi: 10.1377/hlthaff.2010.1003. [DOI] [PubMed] [Google Scholar]
- 29.Dorn SD, Wei D, Farley JF, et al. Impact of the 2008–2009 economic recession on screening colonoscopy utilization among the insured. Clin Gastroenterol Hepatol. 2012;10:278–284. doi: 10.1016/j.cgh.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.