Abstract
Purpose of Review
Activated fibroblasts are critically implicated in repair and remodeling of the injured heart. This manuscript discusses recent progress in the cell biology of fibroblasts in the infarcted and remodeling myocardium, highlighting advances in understanding the origin, function and mechanisms of activation of these cells.
Recent Findings
Following myocardial injury, fibroblasts undergo activation and myofibroblast transdifferentiation. Recently published studies have suggested that most activated myofibroblasts in the infarcted and pressure-overloaded hearts are derived from resident fibroblast populations. In the healing infarct, fibroblasts undergo dynamic phenotypic alterations in response to changes in the cytokine milieu and in the composition of the extracellular matrix. Fibroblasts do not simply serve as matrix-producing cells, but may also regulate inflammation, modulate cardiomyocyte survival and function, mediate angiogenesis, and contribute to phagocytosis of dead cells.
Summary
In the injured myocardium, fibroblasts are derived predominantly from resident populations and serve a wide range of functions.
Keywords: fibroblast, myofibroblast, myocardial infarction, cardiac remodeling, cytokine
Introduction
Heart failure is a major cause of morbidity and mortality in western societies [1]. Despite extensive research in the field, prognosis for patients with heart failure remains poor, reflecting our limited understanding of the pathophysiology of the disease and the challenges in development and implementation of new therapeutic strategies [2]. Cardiac fibrosis is one of the major pathophysiologic underpinnings of heart failure [3]. Expansion of the cardiac interstitium and deposition of extracellular matrix proteins are consistently noted in experimental models of heart failure and in human patients with cardiomyopathic conditions, regardless of etiology. In the injured heart, fibrosis is an important part of the reparative response. Cardiomyocytes have very limited regenerative capacity; as a result, sudden loss of significant amounts of cardiac muscle in myocardial infarction activates a fibrotic response that preserves the structural integrity of the heart, preventing catastrophic events, such as cardiac rupture. The reparative function of cardiac fibrosis is dependent on timely activation and suppression of signals that mediate matrix deposition. Excessive or prolonged fibrogenic activation following myocardial injury increases chamber stiffness causing diastolic dysfunction. Moreover, perturbations of the myocardial architecture in fibrotic hearts can also trigger systolic dysfunction [4].
Fibroblasts are the central cellular effectors of fibrosis. Following myocardial injury, fibroblasts undergo dramatic phenotypic changes in response to microenvironmental alterations in the cytokine milieu and in the composition of the extracellular matrix [5],[6],[7],[8]. Traditional concepts paint a unidimensional picture of cardiac fibroblasts, as the main cellular source of extracellular matrix proteins in the injured myocardium [9]. However, a growing body of evidence suggests that fibroblasts are functionally and phenotypically heterogeneous, and may play diverse roles in cardiac homeostasis and disease [10],[11],[12],[13]. The current review manuscript discusses recent advances in our understanding of the biology of fibroblasts in cardiac remodeling. We will focus on the cellular origin and function of activated fibroblasts in infarcted and remodeling hearts, and we will discuss key molecular signals implicated in fibroblast activation.
Fibroblasts in normal myocardium
Extensive experimental evidence suggests that the adult mammalian heart contains a large population of interstitial cells; many of these cells exhibit fibroblast-like characteristics. Early reports using transmission electron microscopy suggested that fibroblasts may be the most abundant myocardial cells [14]. More recent studies using combinations of markers for cell labeling suggested that in adult mouse hearts less than 20% of non-cardiomyocytes can be identified as fibroblasts [15]. The relative abundance of myocardial fibroblasts reported in different studies varies depending on the species, gender and age of experimental subjects, and on the markers used for cell identification. The absence of specific markers is a major limitation for definitive identification of fibroblast populations in both normal and injured hearts.
The role of fibroblasts in cardiac homeostasis remains poorly understood. In vitro studies have suggested that embryonic cardiac fibroblasts stimulate cardiomyocyte proliferation, whereas adult cells promote hypertrophy [16]. In the absence of injury, resident cardiac fibroblasts may serve to maintain the cardiac extracellular matrix network. Because of their abundance and their close interactions with cardiomyocytes and vascular cells, fibroblasts may also play an important role in regulating baseline cardiac function. However, in vivo experiments testing this intriguing hypothesis have not been performed.
Fibroblasts in the infarcted myocardium
The adult mammalian heart has limited regenerative capacity; as a result, sudden death of a large number of cardiomyocytes following infarction triggers a reparative response, forming a collagen-based scar that preserves the structural integrity of the ventricle [17]. Cardiac repair following myocardial infarction can be divided in three distinct but overlapping phases: the inflammatory phase, the proliferative phase, and the maturation phase [18]. In response to the dramatic changes in the cytokine milieu and to the alterations in composition of the surrounding extracellular matrix following infarction, cardiac fibroblasts exhibit dynamic phenotypic changes during the 3 phases of cardiac repair [5],[19],[20].
The fibroblasts during the inflammatory phase of infarct healing
In the infarcted myocardium, necrosis of cardiomyocytes activates innate immune signaling pathways triggering an intense inflammatory reaction [21],[22], associated with marked upregulation of pro-inflammatory cytokines and chemokines [23]. Upon stimulation with interleukin (IL)-1 or tumor necrosis factor (TNF)-α, cardiac fibroblasts are capable of secreting large amounts of pro-inflammatory mediators and proteases [24],[25],[13]. Considering their relative abundance and their strategic location in close proximity to vessels and cardiomyocytes, fibroblasts may be important cellular effectors of the post-infarction inflammatory response. Although in vivo experiments testing this hypothesis have not been performed, a growing body of evidence suggests that resident cardiac fibroblasts may promote early post-ischemic dysfunction, at least in part, through activation of a pro-inflammatory program [12],[11].
The fibroblasts during the proliferative phase: myofibroblast transdifferentiation
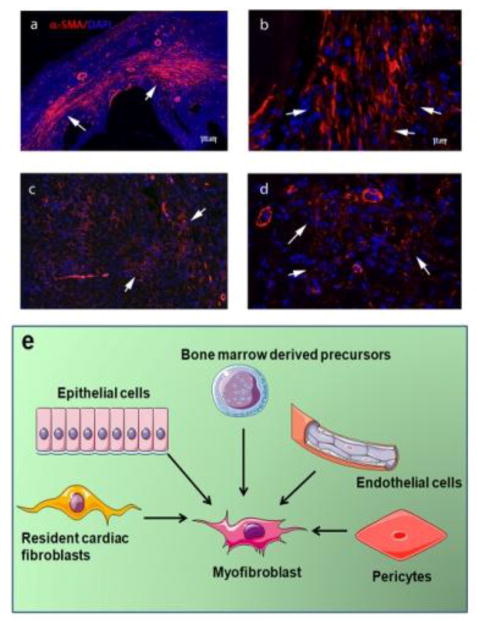
Activation of endogenous pathways that inhibit innate immune signaling and suppress pro-inflammatory activation [26] marks the transition from the inflammatory to the proliferative phase of infarct healing. As the neutrophil infiltrate is cleared by macrophages, fibroblasts expand and undergo myofibroblast transdifferentiation [13], expressing contractile proteins, such as α-smooth muscle actin (αSMA) (Figure 1), and secreting large amounts of extracellular matrix proteins [27],[28]. Activated fibroblasts play a critical role in preservation of the structural integrity of the infarcted ventricle [10]; however excessive or prolonged activation of fibroblast populations may reduce ventricular compliance, promote adverse remodeling and precipitate heart failure [29]. In addition to their established role in matrix synthesis, injury-associated myofibroblasts (or specific subsets of these cells) may serve a wide range of additional roles. In the infarcted myocardium, activated fibroblasts have been implicated in phagocytosis of dead cells [30]. Moreover, activated fibroblasts may modulate cardiomyocyte survival, hypertrophy and function under conditions of stress [31]. Recent evidence has suggested that following injury, myocardial fibroblasts exhibit remarkable phenotypic plasticity and may generate endothelial cells contributing to neovascularization [32].
Figure 1. Myofibroblasts in the infarcted and remodeling myocardium.
αSMA immunofluorescence identifies abundant myofibroblasts (arrows) in infarcted mouse hearts (after 7 days of coronary occlusion) (a, b) and in pressure overloaded hearts after 7 days of transverse aortic constriction (c, d). (e) Myofibroblasts in injured hearts may originate from a variety of sources, including epicardial epithelial cells, endothelium (through EndMT), vascular pericytes, bone marrow derived precursors, and resident cardiac fibroblasts. Recently published studies in mouse models using lineage tracing approaches suggest that resident cardiac fibroblast populations may be the most important source of activated myofibroblasts in infarcted and pressure-overloaded hearts.
Although the heart contains abundant resident cardiac fibroblasts that can respond to activating signals, several other potential cellular sources have been proposed to explain the expanding myofibroblast population in the infarcted and remodeling myocardium. Endothelial cells, hematopoietic fibroblast progenitors, pericytes and vascular smooth muscle cells, epicardial epithelial cells have been proposed as important contributors to myocardial fibrotic responses (Figure 1e) [33]. Over the last 10 years several investigative groups have combined bone marrow transplantation experiments, parabiosis and lineage tracing strategies to investigate the cellular origin of fibroblasts in the infarcted and remodeling myocardium [34],[35],[36],[37],[38],[10]. Interpretation of the findings is inherently challenging because of the functional and phenotypic heterogeneity of fibroblast populations and the lack of specific molecular markers to identify fibroblasts [39]. Moreover, it should be emphasized that the relative contributions of various cell types may depend on the type of myocardial injury. In pathophysiologic conditions associated with extensive cardiomyocyte necrosis (such as myocardial infarction), intense upregulation of chemokines may drive recruitment of non-resident populations that may significantly contribute to the activated fibroblast populations. Table 1 provides an overview of recently-published investigations examining the cellular origin of activated fibroblasts in infarcted and pressure-overloaded hearts. Although earlier studies have suggested important contributions of endothelial cells [34],[35] and hematopoietic progenitors [36], recent investigations combining lineage tracing approaches with several distinct Cre drivers suggested that subpopulations of resident cardiac fibroblasts are the main source for activated myofibroblasts in infarcted and remodeling hearts [37],[38],[10].
Table 1.
Overview of the studies identifying the cellular sources of activated fibroblasts in the infarcted and remodeling myocardium
| Reference | Cellular Source(s) of activated fibroblasts | Species | Model of Cardiac Remodeling | Strategies used to identify the cellular origins of fibroblasts | Markers used for fibroblast labeling |
|---|---|---|---|---|---|
| Kanisicak et al. [10] | Activated fibroblasts in infarcted and remodeling hearts are derived from Tcf21+ tissue-resident fibroblasts. Endothelial cells, myeloid cells and smooth muscle cells do not significantly contribute to the activated fibroblast population. | Mouse | Myocardial infarction. Pressure overload induced through Transverse aortic constriction (TAC) | Lineage tracing using PeriostinMCM, Tcf21MCM (to label resident fibroblasts), LysM-Cre (to label myeloid cells), Cdh5Cre (for endothelial cells) and Myh11CreERT2 (for smooth muscle cells). | Vimentin, PDGFRα, αSMA |
| Ruiz- Villalba et al. [63] | Epicardial-derived resident mesenchymal cells (Wt1Cre− eYFP+/CD31− /CD90+/αSMA low/−) (Major contribution) and bone marrow-derived cells (Lin/cKit+/Sca1+/Flk2+/ CD34) (Minor contribution), mobilized in response to hemotactic stromal cell–derived factor (SDF)-1α gradient | Mouse | Myocardial infarction Pressure overload induced through angiotensin infusion. | Permanent genetic tracing of epicardium- derived cell and bone marrow–derived blood cell lineages using Wt1/IRES/GFP-Cre (Wt1Cre) mice crossed with Rosa26R-eYFP activating permanent reporter enhanced yellow fluorescent protein (eYFP+) expression in the Wt1+ cell lineage (Wt1Cre-YFP+). | Collagen I, FSP1, DDR2, CD90, αSMA |
| Aisaghboni et al. [34] | A significant proportion of activated fibroblasts (35–40%) is derived from endothelial cells. | Mouse | Myocardial infarction | Cell lineage tracing using TOPGAL reporter transgenic mouse line, which carries the lacZ gene under the control of three tandem β-catenin-responsive consensus TCF/LEF- binding motifs upstream of a minimal fos promoter and double transgenic line carrying the inducible Cre recombinase under the endothelial- specific enhancer of the stem cell leukemia (SCL) gene and the R26RstoplacZ locus | αSMA, Snail, FSP1, Vimentin and Collagen I |
| Zhou et al. [64] | Epicardium-derived cells differentiated into fibroblasts in the infarcted myocardium. | Mouse | Myocardial infarction | Genetic lineage tracing strategy using tamoxifen induced Cre allele, Wt1CreERT2/+, with epicardium- restricted cardiac activity, crossed with Rosa26mTmG/+ reporter line, which switches from mRFP to mGFP expression following Cre catalysed recombination | FSP1, procollagen I, collagen III, fibronectin, α-SMA |
| Van Amerongen et al. [65] | Bone marrow-derived cells contributed to the myofibroblast population in the infarcted myocardium (approximately 24% of myofibroblasts were bone marrow-derived). | Mouse | Myocardial infarction | MI induced in C57BL/6 mice reconstituted with BM transgenic for EGFP, as a reporter molecule, or with BM cells that express two reporter genes (luciferase and β-galactosidase) under the control of the promoter and enhancer elements of the collagen I (α2 chain) gene | α-SMA+ cells with spindle shaped morphology |
| Fujita et al. [66] | Blood-derived cells contributed to the myofibroblast population. | Mouse | Myocardial infarction | Whole Bone marrow or single hematopoietic cell transplantation from GFP-transgenic mice | CD45low/− elongated cells expressing Vimentin and αSMA |
| Mollmann et al. [36] | A large population of infarct fibroblasts is derived from bone marrow cells (57% on day 7 after infarction, 32% on day 21) | Mouse | Myocardial infarction | Bone marrow transplantation from enhanced green fluorescent protein (eGFP)-transgenic mice | vimentin, αSMA, SMemb |
| Yano et al. [67] | Circulating bone marrow cells did not contribute to the myofibroblast population. | Rat | Myocardial infarction | Bone marrow transplantation from green fluorescent protein (GFP)+transgenic mice into nude rats | vimentin, αSMA |
| Ali et al. [37] | The majority of cardiac fibroblasts in the pressure-overloaded myocardium are derived from epicardial populations, a minority from endothelial cells, and a small fraction from Pax3-expressing cells. | Mouse | Pressure overload through TAC | Fate-mapping models using Pax3Cre/+, Tie2Cre/+, Wt1CreERT2/+, Myh11cre/+-GFP, Vav1Cre/+, Tbx18Cre transgenic mice, Myh6-GFP and R26RmT/mG mice, bone marrow transplantation and parabiosis, global- and fibroblast-specific gene expression analysis | Collagen I, DDR2, PDGFRα, Vimentin, αSMA, CD90 exclusion criteria for hematopoietic cells, macrophages and endothelial cells. |
| Moore- Morris et al. [38] | Activated fibroblasts in the pressure-overloaded myocardium are derived from 2 resident fibroblast populations, and not from hematopoietic cells, endothelial cells or epithelial cells | Mouse | Pressure overload through TAC | Genetic lineage tracing using transgenic GFP reporter mouse line driven by a collagen1a1 enhancer crossed with Wt1-Cre, Tie2-Cre, Vav-Cre, VE-cadherin-CreERT2, Tbx18-Cre, Wt1- CreERT2, Nfatc1-Cre and Rosa-tdT-Cre | Vimentin, PDGFRα, Thy1, DDR2 |
| Zeisberg et al. [35] | Activated fibroblasts in the pressure-overloaded myocardium are derived from endothelial cells through endothelial- mesenchymal transition (EndMT) (27–35% of all fibroblasts) either FSP1+ or αSMA+), and from bone marrow-derived cells (13.4% of FSP1+ cells and 21.1% of α-SMA+ cells) | Mouse | Pressure overload through TAC | Lineage tracing using Tie1Cre;R26Rstoplac Z mice, in which cells of endothelial origin are irrevocably marked by lacZ expression, and FSP1-GFP transgenic mice, in which green fluorescent protein (GFP) is expressed under the control of the promoter of fibroblast-specific protein 1 (FSP1), bone marrow transplantation of WT mice with Tie1Cre;R26Rstoplac Z bone marrow | FSP-1, αSMA, DDR2, type I collagen α1 |
| Krammann et al. [68] | Gli-1+ pericytes contribute to the myofibroblast population in the remodeling pressure-overloaded myocardium (approximately 60% of activated fibroblasts are derived from Gli1+ cells) | Mouse | Pressure overload induced through angiotensin infusion or ascending aortic constriction | Lineage tracing using Gli1CreERT2 mice. | Collagen I, PDGFRα, αSMA |
Signals mediating myofibroblast activation in the remodeling myocardium
Myofibroblast activation in the infarcted and remodeling myocardium requires the co-operation of growth factors and specialized matrix proteins, which signal through cell surface receptors to activate transcription of extracellular matrix proteins. Macrophages, mast cells and lymphocytes infiltrating the remodeling heart play an important role in fibroblast activation by secreting a wide range of bioactive mediators, including cytokines (such as Transforming Growth Factor (TGF)-β and IL-10) and matricellular proteins [40],[41],[42],[43],[44]. Stimulated cardiomyocytes and vascular cells in the area of injury may also activate molecular cascades that modulate fibroblast behavior [45].
Activation of the renin-angiotensin-aldosterone system signaling plays an important role in fibroblast proliferation and activation in the infarcted and remodeling myocardium. Experimental studies have demonstrated that angiotensin type 1 receptor (AT1) and aldosterone signaling activate fibroblasts in healing myocardial infarcts [46],[47]. Clinical studies in human patients with acute infarction support this concept demonstrating that administration of an aldosterone antagonist reduces the levels of circulating markers of collagen synthesis [48]. Moreover in patients with hypertensive heart disease, AT1 blockade significantly reduced indicators associated with myocardial fibrosis [49].
The pleiotropic mediator TGF-β also plays a crucial role in activation of fibroblasts in the remodeling myocardium. TGF-β isoforms are markedly upregulated in the infarcted and remodeling myocardium and are secreted by macrophages, fibroblasts, platelets, vascular cells and cardiomyocytes in a latent form [50],[51]. Activation of TGF-β in the cardiac interstitium requires protease actions and an interaction with the matricellular protein thrombospondin-1 [52],[53]. Following activation, the TGF-β dimer binds to a heterodimeric complex of TGFβ receptor I and II activating canonical signaling cascades that involve the intracellular effectors Smad2 and Smad3, and triggering Smad-independent pathways. Smad3 signaling appears to play an important role in fibroblast-mediated matrix synthesis and αSMA expression [54],[55]. Although effects of Smad-independent pathways have been documented in hypertrophic remodeling and dysfunction of cardiomyocytes in the pressure-overloaded myocardium [56], the in vivo role of non-Smad signaling in cardiac fibroblast function has not been documented.
Recent studies have revealed that profibrotic mediators, such as angiotensin II or TGF-β act by activating the Transient Receptor Potential (TRP) Channel- calcineurin axis. In fibroblasts, TRPC6 is induced through TGF-β-mediated Smad-independent signaling and is implicated in cardiac myofibroblast transdifferentiation by activating a calcineurin-Nuclear Factor of Activated T cells (NFAT) cascade [57],[58]. Experiments in atrial fibroblasts suggested that TRPM7 is implicated in TGF-βinduced calcium signaling and in myofibroblast transdifferentiation [59] TRPV4 is also involved in cardiac myofibroblast activation by integrating signals from secreted growth factors (such as TGF–β) and mechanosensitive stimuli [60].
Fibroblast de-activation, quiescence and apoptosis in the infarcted and remodeling myocardium
As the healing scar matures, myofibroblasts become quiescent, reducing synthesis of extracellular matrix proteins. Many myofibroblasts in the infarct border zone may undergo apoptosis. Despite their potential importance in protecting the infarcted and remodeling myocardium from overactive fibrosis and dysfunction, the inhibitory signals responsible for myofibroblast de-activation in the healing scar are poorly understood. Our experimental work has suggested that at all stages of repair, fibroblasts are exposed to inhibitory mediators, such as the CXC chemokine Interferon-γ inducible protein (IP)-10/CXCL10 that may serve to prevent excessive fibrosis [61],[62]. However, the role of specific endogenous inhibitory pathways in negative regulation of TGF-β and angiotensin-mediated responses following infarction has not been investigated.
Conclusions and future directions
Cardiac fibroblasts play a crucial role in repair of the infarcted myocardium, but are also implicated in the pathogenesis of adverse remodeling and heart failure following cardiac injury. Despite the recent expansion of our knowledge on the cellular origins of fibroblasts in infarcted and remodeling hearts, our understanding of the molecular signals implicated in fibroblast activation following myocardial injury remains limited. Future research needs to focus on in vivo experiments to identify functionally distinct fibroblast subsets in injured and remodeling hearts, and on studies dissecting the molecular pathways mediating specific fibroblast responses. Moreover, study of endogenous inhibitory signals that inhibit fibroblast activity is crucial in order to design novel strategies protecting from adverse remodeling and heart failure.
Acknowledgments
SOURCES OF FUNDING: Supported by grants from the National Institutes of Health (R01 HL76246 and R01 HL85440 to N.G.F.), the Department of Defense (PR151134 and PR151029 to N.G.F.) and a post-doctoral award by the American Heart Association Founders’ affiliate (to A.V.S.).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Arti Shinde and Nikolaos Frangogiannis declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
Recently published papers of particular interest have been highlighted as:
*of high importance
**of outstanding importance
- 1.Christiansen MN, Kober L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, et al. Age-specific Trends in Incidence, Mortality and Comorbidities of Heart Failure in Denmark 1995–2012. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.025941. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Hsieh AF, Dharmarajan K, Masoudi FA, Krumholz HM. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998–2010. Circulation. 2013;128(24):2577–84. doi: 10.1161/CIRCULATIONAHA.113.003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–74. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117(3):568–75. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol. 2014;70C:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105(12):1164–76. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner NA, Porter KE. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis & tissue repair. 2013;6(1):5. doi: 10.1186/1755-1536-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobaczewski M, de Haan JJ, Frangogiannis NG. The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium. J Cardiovasc Transl Res. 2012;5(6):837–47. doi: 10.1007/s12265-012-9406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147(2):325–38. [PMC free article] [PubMed] [Google Scholar]
- 10**.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature communications. 2016;7:12260. doi: 10.1038/ncomms12260. This study used a wide range of lineage tracing experiments to investigate the cellular origin of activated myofibroblasts in healing myocardial infarction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frangogiannis NG. The Functional Pluralism of Fibroblasts in the Infarcted Myocardium. Circ Res. 2016;119(10):1049–51. doi: 10.1161/CIRCRESAHA.116.309926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodall MC, Woodall BP, Gao E, Yuan A, Koch WJ. Cardiac Fibroblast GRK2 Deletion Enhances Contractility and Remodeling Following Ischemia/Reperfusion Injury. Circ Res. 2016;119(10):1116–27. doi: 10.1161/CIRCRESAHA.116.309538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, et al. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J Immunol. 2013;191(9):4838–48. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28(109):41–61. [PubMed] [Google Scholar]
- 15*.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, et al. Revisiting Cardiac Cellular Composition. Circ Res. 2016;118(3):400–9. doi: 10.1161/CIRCRESAHA.115.307778. A robust and systematic characterization of the cellular composition of the adult mouse heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16(2):233–44. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangogiannis NG. Pathophysiology of Myocardial Infarction. Compr Physiol. 2015;5(4):1841–75. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 18.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110(1):159–73. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta. 2013;1833(4):945–53. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frangogiannis NG. Fibroblast-Extracellular Matrix Interactions in Tissue Fibrosis. Curr Pathobiol Rep. 2016;4(1):11–8. doi: 10.1007/s40139-016-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119(1):91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15(2):117–29. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96(8):881–9. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 24.van Nieuwenhoven FA, Hemmings KE, Porter KE, Turner NA. Combined effects of interleukin-1alpha and transforming growth factor-beta1 on modulation of human cardiac fibroblast function. Matrix Biol. 2013;32(7–8):399–406. doi: 10.1016/j.matbio.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Zymek P, Nah DY, Bujak M, Ren G, Koerting A, Leucker T, et al. Interleukin-10 is not a critical regulator of infarct healing and left ventricular remodeling. Cardiovasc Res. 2007;74(2):313–22. doi: 10.1016/j.cardiores.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, et al. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol. 2012;32(11):2598–608. doi: 10.1161/ATVBAHA.112.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinde AV, Humeres C, Frangogiannis NG. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta. 2017;1863(1):298–309. doi: 10.1016/j.bbadis.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovasc Res. 2000;48(1):89–100. doi: 10.1016/s0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 29.Kaur H, Takefuji M, Ngai CY, Carvalho J, Bayer J, Wietelmann A, et al. Targeted Ablation of Periostin-Expressing Activated Fibroblasts Prevents Adverse Cardiac Remodeling in Mice. Circ Res. 2016;118(12):1906–17. doi: 10.1161/CIRCRESAHA.116.308643. [DOI] [PubMed] [Google Scholar]
- 30.Nakaya M, Watari K, Tajima M, Nakaya T, Matsuda S, Ohara H, et al. Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction. J Clin Invest. 2017;127(1):383–401. doi: 10.1172/JCI83822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124(5):2136–46. doi: 10.1172/JCI70577. The study documents effects of fibroblasts in regulation of cardiomyocyte hypertrophy, mediated through secretion of miRNA-rich exosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514(7524):585–90. doi: 10.1038/nature13839. This study suggests that fibroblasts exhibit remarkable plasticity and may generate endothelial cells contributing to neovessel formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225(3):631–7. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4(4):469–83. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 36.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, et al. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71(4):661–71. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 37**.Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, et al. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res. 2014;115(7):625–35. doi: 10.1161/CIRCRESAHA.115.303794. A systematic and well-documented investigation on the cellular origins of activated fibroblasts in remodeling pressure-overloaded hearts. [DOI] [PubMed] [Google Scholar]
- 38**.Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124(7):2921–34. doi: 10.1172/JCI74783. This study identified resident fibroblast populations as the main cellularb source of activated fibroblasts in the remodeling pressure-overloaded myocardium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2013;305(9):H1363–72. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson S, Helterline D, Asbe L, Dupras S, Minami E, Farris S, et al. Cardiac macrophages adopt profibrotic/M2 phenotype in infarcted hearts: Role of urokinase plasminogen activator. J Mol Cell Cardiol. 2016 doi: 10.1016/j.yjmcc.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velazquez F, Aronovitz M, et al. Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure. Circ Heart Fail. 2015;8(4):776–87. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol. 2014;307(8):H1233–42. doi: 10.1152/ajpheart.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016;126(6):2151–66. doi: 10.1172/JCI85782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92(2):635–88. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sassi Y, Ahles A, Truong DJ, Baqi Y, Lee SY, Husse B, et al. Cardiac myocyte-secreted cAMP exerts paracrine action via adenosine receptor activation. J Clin Invest. 2014;124(12):5385–97. doi: 10.1172/JCI74349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ju H, Zhao S, Jassal DS, Dixon IM. Effect of AT1 receptor blockade on cardiac collagen remodeling after myocardial infarction. Cardiovasc Res. 1997;35(2):223–32. doi: 10.1016/s0008-6363(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 47.van den Borne SW, Isobe S, Zandbergen HR, Li P, Petrov A, Wong ND, et al. Molecular imaging for efficacy of pharmacologic intervention in myocardial remodeling. JACC Cardiovasc Imaging. 2009;2(2):187–98. doi: 10.1016/j.jcmg.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi M, Tsutamoto T, Wada A, Tsutsui T, Ishii C, Ohno K, et al. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post-infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation. 2003;107(20):2559–65. doi: 10.1161/01.CIR.0000068340.96506.0F. [DOI] [PubMed] [Google Scholar]
- 49.Ciulla MM, Paliotti R, Esposito A, Diez J, Lopez B, Dahlof B, et al. Different effects of antihypertensive therapies based on losartan or atenolol on ultrasound and biochemical markers of myocardial fibrosis: results of a randomized trial. Circulation. 2004;110(5):552–7. doi: 10.1161/01.CIR.0000137118.47943.5C. [DOI] [PubMed] [Google Scholar]
- 50.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51(4):600–6. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, et al. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164(2):665–77. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, et al. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58(5):902–11. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, et al. The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111(22):2935–42. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 54.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, et al. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–38. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 55.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107(3):418–28. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, et al. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121(6):2301–12. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishida M, Onohara N, Sato Y, Suda R, Ogushi M, Tanabe S, et al. Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem. 2007;282(32):23117–28. doi: 10.1074/jbc.M611780200. [DOI] [PubMed] [Google Scholar]
- 58**.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23(4):705–15. doi: 10.1016/j.devcel.2012.08.017. This study provides the first in vivo demonstration of an important role for TRPC6 in myofibroblast activation in healing wounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, et al. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106(5):992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adapala RK, Thoppil RJ, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, et al. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol. 2013;54:45–52. doi: 10.1016/j.yjmcc.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saxena A, Bujak M, Frunza O, Dobaczewski M, Gonzalez-Quesada C, Lu B, et al. CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res. 2014;103(2):217–27. doi: 10.1093/cvr/cvu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, et al. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105(10):973–83. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruiz-Villalba A, Simon AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, et al. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol. 2015;65(19):2057–66. doi: 10.1016/j.jacc.2015.03.520. [DOI] [PubMed] [Google Scholar]
- 64.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121(5):1894–904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, et al. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214(3):377–86. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 66.Fujita J, Mori M, Kawada H, Ieda Y, Tsuma M, Matsuzaki Y, et al. Administration of granulocyte colony-stimulating factor after myocardial infarction enhances the recruitment of hematopoietic stem cell-derived myofibroblasts and contributes to cardiac repair. Stem Cells. 2007;25(11):2750–9. doi: 10.1634/stemcells.2007-0275. [DOI] [PubMed] [Google Scholar]
- 67.Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, et al. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol. 2005;14(5):241–6. doi: 10.1016/j.carpath.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 68*.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16(1):51–66. doi: 10.1016/j.stem.2014.11.004. This study documents the contribution of Gli1+ pericytes as a source of activated myofibroblasts in the injured myocardium and in other fibrotic tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]