Abstract
How do joint measures of premorbid cognitive ability and familial cognitive aptitude (FCA) reflect risk for a diversity of psychiatric and substance use disorders? To address this question, we examined, using Cox models, the predictive effects of school achievement (SA) measured at age 16 and FCA—assessed from SA in siblings and cousins, and educational attainment in parents—on risk for 12 major psychiatric syndromes in 1 140 608 Swedes born 1972–1990. Four developmental patterns emerged. In the first, risk was predicted jointly by low levels of SA and high levels of FCA—that is a level of SA lower than would be predicted from the FCA. This pattern was strongest in autism spectrum disorders and schizophrenia, and weakest in bipolar illness. In these disorders, a pathologic process seems to have caused cognitive functioning to fall substantially short of familial potential. In the second pattern, seen in the internalizing conditions of major depression and anxiety disorders, risk was associated with low SA but was unrelated to FCA. Externalizing disorders—drug abuse and alcohol use disorders—demonstrated the third pattern, in which risk was predicted jointly by low SA and low FCA. The fourth pattern, seen in eating disorders, was directly opposite of that observed in externalizing disorders with risk associated with high SA and high FCA. When measured together, adolescent cognitive ability and FCA identified four developmental patterns leading to diverse psychiatric disorders. The value of cognitive assessments in psychiatric research can be substantially increased by also evaluating familial cognitive potential.
INTRODUCTION
Poor cognitive performance in childhood and adolescence is a risk factor for a wide range of psychiatric disorders.1–7 We recently showed that examining the joint effects of school achievement (SA) and familial cognitive aptitude (FCA)—calculated from SA measures in siblings and cousins, and educational attainment in parents—provided potentially important insights into the nature of the cognitive vulnerability to schizophrenia and bipolar illness (BPI).8 Risk for schizophrenia was strongly indexed by the degree to which SA fell short of that expected from their FCA. Similar but much weaker effects were seen for BPI.
In this report, we applied these methods to risk prediction for a broader array of psychiatric disorders, 12 in total, including psychotic, mood, anxiety, substance use and neurodevelopmental disorders. Our hope was to identify distinct patterns of cognitive development that predispose to particular psychiatric disorders. We also sought to replicate our results using SA—assessed at age 16 in both sexes—with intelligence quotient (IQ) measured at ages 18–20 in males only.
MATERIALS AND METHODS
We collected information on individuals from Swedish population-based registers with national coverage. The registers were linked using each person’s unique identification number. We secured approval for this study from the Regional Ethical Review Board of Lund University.
The National School Registry contains SA (a grade point average) for all students at the end of grade nine (usually age 16) from 1988 to 2012. Students were incentivized to perform well that year as high grades facilitated admission to desirable secondary schools. For each year and gender, we standardized the grade score into a SA Z-score (mean of 0, s.d. of 1). From 1988 to 1997, the score was expressed on a 1–5 scale (mean 3.2), reflecting performance compared with Swedish peers with corrections applied to ensure equivalence across schools. From 1998, the score changed to vary from 10 to 320 (mean 207) utilizing a criterion referenced system. Scores were neither standardized across schools nor normally distributed.
A full IQ measurement constructed from four subtests (logical, spatial, verbal and technical abilities) was available from the Military Conscription Register. During the years of this study, the examination was required by law for men born in Sweden, exempting only those (~4.2%) with serious medical conditions or disabilities. The global IQ score was standardized to a Gaussian distributed score between one and nine. For each year, we standardized this score (mean of 0, s.d. of 1).
From the Swedish Hospital Discharge Register and the Swedish Outpatient Register, we identified all individuals registered for autism spectrum disorders (ASD), schizophrenia (SZ), other non-affective psychoses (ONAP), obsessive compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), BPI, major depression (MD), anxiety disorders (AD), anorexia nervosa, bulimia nervosa (BN), drug abuse (DA) and alcohol use disorder (AUD). We applied a lifetime hierarchical diagnostic approach from ASD to BN in the order listed above. AUD and DA, which were also ascertained from the criminal registry,9,10 were excluded from the hierarchy. For definitions of the disorders see Supplementary Appendix Table S1.
Sample
We began with all individuals born in Sweden 1972 to 1990 who had neither died nor migrated before age 16 (N = 1 861 934). For the main analysis, we included individuals with (i) SA scores at age 16 and (ii) at least one sibling and one cousin with measures of SA and both parents with educational status in the registers (n = 1 140 608). These biological relatives were identified through the Swedish multigenerational register. Educational status was categorized into five groups ((1) < 9 years, (2) 9 years, (3) 10–11 years, (4) 12 years and (5) > 12 years)), and then standardized. We performed a linear regression predicting SA in the proband from the mean SA in sibling(s) and cousin(s), and parental educational status. FCA was defined as the predicted value from that regression analysis. The mean (s. d.) number of siblings and cousins contributing to FCA scores were, respectively, 1.56 (0.8) and 5.68 (4.2). We then predicted individually the risk for 12 psychiatric disorders using Cox proportional hazards models with SA and FCA scores as predictor variables.
Robust standard errors were used to adjust the 95% confidence intervals, given multiple probands from the same family as one individual might be included as a proband, sibling and cousin. Furthermore, we weighted each individual based on the number of siblings/cousins who were included in the FCA with siblings and cousins weighted 0.5 and 0.125, respectively. Follow-up time in number of years was measured from year of the SA score until year of first registration (of the particular disorder), death, emigration or end of follow-up (2012), whichever came first. In all models, we investigated the proportionality assumption—that the hazard rate was constant over time. In situations where it was not fulfilled, we investigated the nature of the failure by examining three follow-up periods: 0–4, 5–14 and > 15 years.
All analyses were repeated using IQ. A new FCA was calculated for these analyses utilizing both the SA and IQ of the sibling(s) and cousin(s), and educational status in mother and father. In the main models, IQ and FCA were used as predictor variables. As the military conscript register only includes males, the study sample decreased (n = 113 927). Individuals with registration for the disorder prior to the SA (or IQ) measures were censored. Statistical analyses were performed using SAS 9.311 and no corrections were applied for multiple testing.
RESULTS
School achievement
Table 1 presents descriptive results for the 12 psychiatric disorders we here examine. The most common were DA and MD, and the rarest AN and SZ. Mean age at first registration for all disorders, except AN, was in the mid-20s. Cases were lost to analysis if (i) they had no SA measure, (ii) we could not calculate an FCA due to small families or missing parents or (iii) first registration prior to age 16—the age of SA assessment. ASD and ADHD had the highest percentage of cases missing SA and having a first registration < age 16.
Table 1.
The source of cases and age at first registration of 11 major psychiatric syndromes in our sample that had (i) school achievement measures at age 16, (ii) sufficient relatives to define familial cognitive aptitude and (iii) no prior registration for the disorder in question
| Disorder | Total cases | No. of measure of school achievement |
No. of measure of familial cognitive aptitude |
Age at first registration prior to SA |
Total N analyzed (%) |
Age at first registration |
|---|---|---|---|---|---|---|
| Autism spectrum disorders | 8674 | 3028 | 573 | 583 | 4490 (0.4) | 24.5 (5.5) |
| Schizophrenia | 3507 | 368 | 1574 | 72 | 1493 (0.1) | 26.0 (5.1) |
| Other non-affective psychosis | 8069 | 914 | 3454 | 92 | 3609 (0.3) | 25.3 (5.2) |
| OCD | 8017 | 384 | 3028 | 270 | 4335 (0.4) | 25.0 (5.2) |
| ADHD | 21 012 | 2942 | 8,092 | 1160 | 8818 (0.8) | 25.9 (5.8) |
| Bipolar illness | 9751 | 421 | 3818 | 92 | 5420 (0.5) | 26.8 (5.3) |
| Major depression | 64 130 | 3270 | 25 271 | 776 | 34 813 (3.1) | 25.3 (5.5) |
| Anxiety disorders | 19 581 | 1115 | 7975 | 275 | 10 216 (0.9) | 25.7 (5.2) |
| Anorexia nervosa | 4112 | 129 | 1188 | 715 | 2080 (0.2) | 20.3 (4.1) |
| Bulimia | 1631 | 36 | 471 | 72 | 1052 (0.1) | 23.7 (4.3) |
| Drug abuse | 83 073 | 8751 | 30 730 | 603 | 42 989 (3.8) | 23.0 (4.6) |
| Alcohol use disorder | 53 633 | 4649 | 23 015 | 2542 | 23 427 (2.1) | 24.1 (5.8) |
Abbreviations: ADHD, attention deficit hyperactivity disorder; OCD, obsessive compulsive disorder.
Table 2 depicts the results of individual Cox models predicting first registration for each disorder with SA and FCA as predictor variables and sex as a covariate. We focus initially on the 10 disorders (in italics in Table 2) where age at first registration plausibly indexes of age at onset. Based upon an examination of the joint effects of SA and FCA, we identified four patterns of cognitive development.
Table 2.
Prediction of first registration for 11 major psychiatric disorders by Cox regression models from school standardized achievement measured at age 16 (both sexes) or IQ measured at ages 18–20 (males only) and familial cognitive aptitude
| School achievement | IQ | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Disorder | Developmental pattern | School achievement (HR, 95% CIs) |
Familial cognitive aptitude (HR, 95% CIs) |
Males vs Femalesa (HR, 95% CIs) |
IQ (HR, 95% CIs) | Familial cognitive aptitude (HR, 95% CIs) |
| Autism spectrum disorder | 1 | 0.42 (0.41; 0.44) | 2.12 (1.96; 2.30) | 1.56 (1.45; 1.66) | 0.65 (0.55; 0.78) | 2.24 (1.64; 3.07) |
| Schizophrenia | 1 | 0.57 (0.53; 0.61) | 1.68 (1.47; 1.93) | 1.84 (1.63, 2.08) | 0.55 (0.46; 0.66) | 1.55 (1.08; 2.23) |
| Other non-affective psychosis | 1 | 0.67 (0.64; 0.71 | 1.37 (1.25; 1.58) | 1.10 (1.03, 1.19) | 0.60 (0.52; 0.68) | 1.86 (1.41; 2.44) |
| Obsessive compulsive disorder | 1 | 0.78 (0.74; 0.81) | 1.32 (1.22; 1.42) | 0.58 (0.54; 0.62) | 0.75 (0.64; 0.88) | 1.66 (1.20; 2.30) |
| ADHD | 1 | 0.37 (0.36; 0.38) | 1.18 (1.12; 1.25) | 1.23 (1.18; 1.29) | 0.52 (0.46; 0.58) | 1.03 (0.84; 1.25) |
| Bipolar illness | 1 | 0.77 (0.74; 0.80) | 1.14 (1.07; 1.22) | 0.45 (0.42; 0.48) | 0.84 (0.72; 0.97) | 1.21 (0.92; 1.58) |
| Major depression | 2 | 0.72 (0.71; 0.73) | 1.01 (0.98; 1.04) | 0.51 (0.50; 0.52) | 0.83 (0.78; 0.87) | 1.03 (0.92; 1.15) |
| Anxiety disorders | 2 | 0.66 (0.64; 0.67) | 0.98 (0.93; 1.03) | 0.61 (0.58; 0.63) | 0.78 (0.71; 0.85) | 0.94 (0.79; 1.13) |
| Anorexia nervosa | 3 | 1.40 (1.29; 1.53) | 1.40 (1.25; 1.57) | 0.03 (0.03; 0.04) | — | — |
| Bulimia nervosa | 3 | 1.18 (1.07, 1.30) | 1.26 (1.09; 1.47) | 0.01 (0.01; 0.02) | — | — |
| Drug abuse | 4 | 0.48 (0.47; 0.48) | 0.86 (0.84; 0.88) | 2.67 (2.61; 2.74) | 0.59 (0.57; 0.61) | 0.74 (0.69; 0.80) |
| Alcohol use disorder | 4 | 0.51 (0.50; 0.52) | 0.84 (0.82; 0.87) | 1.66 (1.61; 1.71) | 0.59 (0.56; 0.62) | 0.65 (0.59; 0.72) |
Abbreviation: ADHD, attention deficit hyperactivity disorder; CI, confidence interval; HR, hazard ratio; IQ, intelligence quotient.
HR> 1 indicated more common in males.
The first pattern we characterized by low SA and high FCA. That is, controlling for the level of SA, risk for these disorders were increased by higher levels of FCA. These results can be viewed in an alternative and particularly informative way—that risk results from the degree to which an individual’s SA falls short of that expected given their FCA.
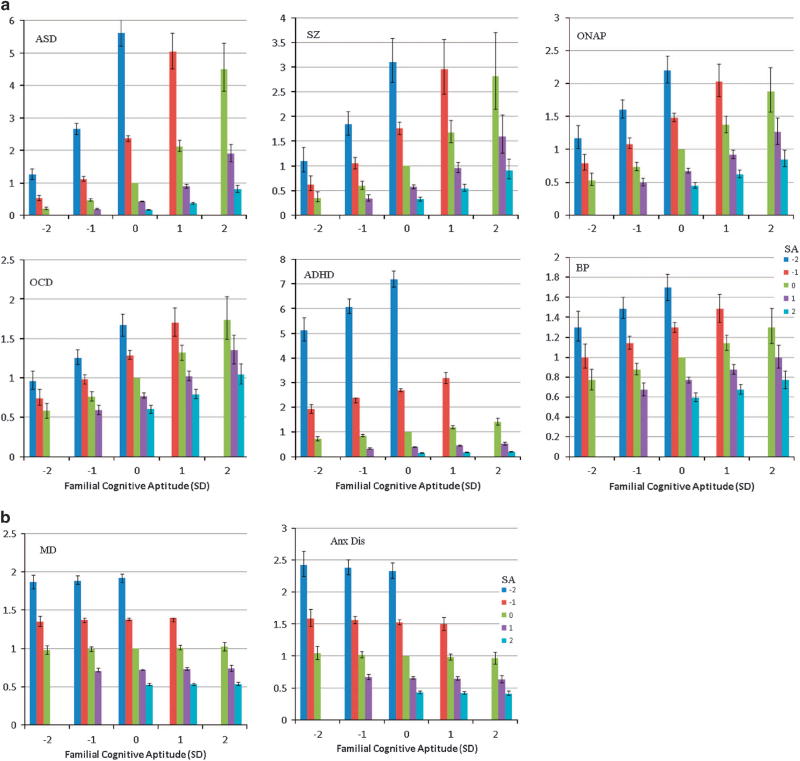
Controlling for SA, the four disorders with this developmental pattern could be ranked by the relative impact of FCA. This was strongest for schizophrenia, followed by ONAP, OCD and BPI. Figure 1a depicts these results for these four disorders. The impact of FCA controlling for SA can be seen by examining columns of the same color (which reflect SA) across different values of FCA. For example, for schizophrenia, an individual with average SA (green column) has HRs of 0.4, 0.6, 1.0, 1.7 and 2.8 when coming, respectively, from families with very low, low, average, high and very high FCA. The steepness of that regression line reflects the strength of the FCA effect. Thus in BPI, the parallel figures are 0.8, 0.9, 1.0, 1.1 and 1.3, indicating a much less potent impact of FCA.
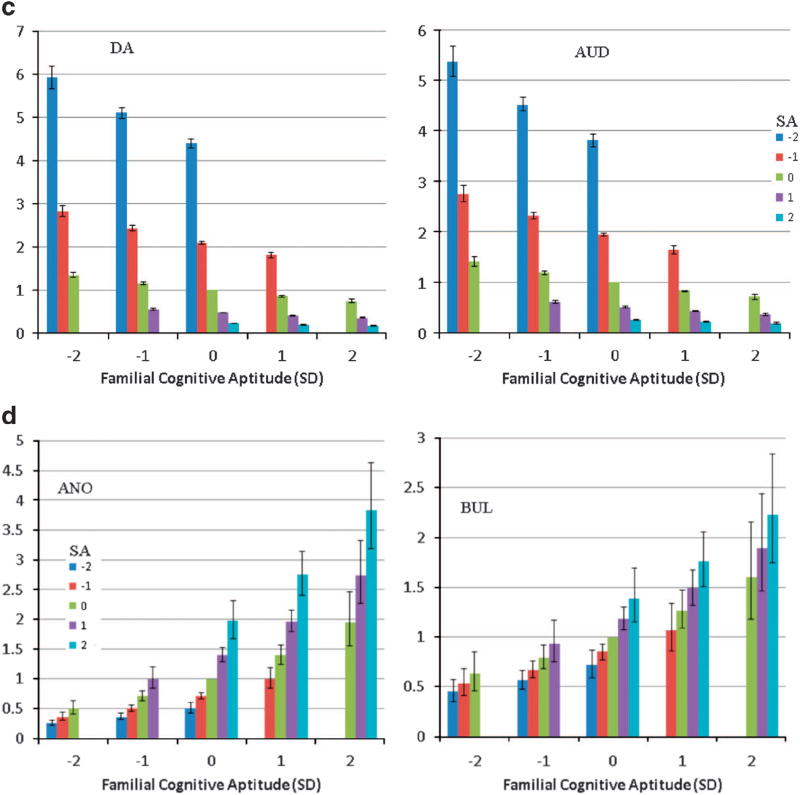
Figure 1.
(a) The hazard ratio (and 95% confidence intervals) for first registration for six neurodevelopmental psychiatric disorders (ASD, autism spectrum disorder; SZ, schizophrenia, ONAP, other non-affective psychoses; OCD, obsessive compulsive disorder; ADHD, attention deficit hyperactive disorder; BP, bipolar disorder) in 1 140 608 Swedish individuals born 1972–1990 as a function of their school achievement at age 16 and their familial cognitive aptitude calculated from school achievement in full siblings and cousins and educational status in parents. s.d.—standard deviation. The X-axis reflects the familial cognitive aptitude divided into five groups: very low (− 2 s.d. below the mean), low (−1 s.d. below the mean), average, high (+1 s.d. above the mean) and very high (+2 s.d. above the mean). The Y-axis reflects the school achievement levels, in s.d. units, of individuals within each family type. Not all possible combinations are presented because some potential groups (e.g. familial cognitive aptitude of +2 s.d. and school achievement of −1 or − 2 s.d.) were too rare to calculate with confidence. (b) The hazard ratio (and 95% confidence intervals) for first registration for two internalizing psychiatric disorders (MD—major depression and Anx Dis—anxiety disorders) in 1 140 608 Swedish individuals born 1972–1990 as a function of their school achievement at age 16 and their familial cognitive aptitude. For further details, see legend to (a). (c) The hazard ratio (and 95% confidence intervals) for first registration for two externalizing psychiatric disorders (DA—drug abuse and AUD—alcohol use disorders) in 1 140 608 Swedish individuals born 1972–1990 as a function of their school achievement at age 16 and their familial cognitive aptitude. For further details, see legend to (a). (d) The hazard ratio (and 95% confidence intervals) for first registration for two eating disorders (Ano—anorexia nervosa, Bul—bulimia nervosa) in 1 140 608 Swedish individuals born 1972–1990 as a function of their school achievement at age 16 and their familial cognitive aptitude. For further details, see legend to (a).
The second developmental pattern is seen in the two internalizing disorders—MD and AD. In these disorders, risk of illness is predicted by low SA but is not predicted by FCA. This pattern can be seen in Figure 1b where risk is nearly the same for individuals of varying SA, independent of their FCA scores.
In the third developmental pattern, risk is increased by both low SA and low FCA. This pattern is seen in our two externalizing syndromes of DA and AUDs as illustrated in Figure 1c where it can be seen most clearly for individuals with moderately low SA (red column). For AUD, such individuals when coming from families with very low, low, average and high FCA have HRs for AUD for 2.9, 2.4, 2.0 and 1.7. In the fourth developmental pattern, risk is predicted both by high SA and high FCA. This pattern is seen for the two eating disorders, AN and BN (Figure 1d).
Table 2 also presents results for two disorders, ASD and ADHD, where age at first registration is unlikely to accurately reflect age at onset as these conditions typically begin in childhood. We include these disorders here because of their relevance to our disorders display the first pattern in which risk is predicted by low SA and high FCA. Controlling for SA, the association between FCA and risk of illness is stronger for ASD than for any other disorders in this group. As seen in Figure 1a, the risk for ASD varies dramatically as a function of their FCA. ADHD was noteworthy for having the strongest main effect of SA and controlling for that, a moderate level of increasing risk with FCA that placed it in between OCD and BPI.
Intelligence quotient
Our male-only population cohort contained 113 927 individuals with IQ scores obtained at ages 18–20 (mean assessment age—18.2) and a sufficient number of relatives to calculate their FCA. Supplementary Appendix Table S2 provides the prevalence and mean age at first registration for 10 disorders in this cohort, AN and BN being too rare to analyze. Given that we censored onsets prior to the administration of the IQ test, our ages at first registration were later than seen in our SA cohort. With the exception of drug and alcohol abuse, prevalence rates for the disorders also tended to be lower in this sample.
Table 2 depicts the results of individual Cox models predicting first registration for each disorder from IQ and FCA. The pattern of results replicate those found with SA with the exception of ADHD which, in this sample, was not significantly predicted by FCA.
Examination of possible biases
We ascertained our disorders in a cohort born 1972–1990 from both the hospital discharge registry beginning in 1973 and from the outpatient registry begun in 2001. Biases could arise from the different kinds of cases ascertained in these two settings. Focusing on our better powered SA analyses, we examined the possibility of bias in two ways. First, we repeated our key analyses in the youngest portion of our cohort born 1985–1990. In this group, the oldest were 16 when ascertainment from outpatient treatment began. As seen in the left side of Table 3, the pattern of results is very similar to that seen for the entire cohort in Table 2.
Table 3.
Test of possible biases in our analyses by examining the predictive effect of standardized school achievement and familial cognitive aptitude in the youngest part of our cohort, utilizing only inpatient diagnoses and with no age-at-onset restrictions
| Born 1985–1990 | Inpatient diagnoses onlya | No age at onset restrictions | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Disorder | School achievement (HR, 95% CIs) |
Familial cognitive aptitude (HR, 95% CIs) |
M vs Fb (HR, 95% CIs) |
School achievement (HR, 95% CIs) |
Familial cognitive aptitude (HR, 95% CIs) |
M vs Fb HR, 95% CIs) |
School achievement (HR, 95% CIs) |
Familial cognitive aptitude (HR, 95% CIs) |
M vs Fb (HR, 95% CIs) |
| Autism spectrum disorder | 0.40 (0.38; 0.42) | 2.10 (1.88; 2.35) | 1.55 (1.41; 1.70) | 0.40 (0.36; 0.44) | 3.16 (2.57; 3.87) | 1.34 (1.15; 1.57) | 0.41 (0.39; 0.42) | 2.21 (2.05; 2.39) | 1.62 (1.52; 1.73) |
| Schizophrenia | 0.53 (0.47; 0.61) | 1.80 (1.32; 2.45) | 1.58 (1.20, 2.07) | 0.56 (0.51; 0.61) | 1.68 (1.42; 1.99) | 1.99 (1.73, 2.30) | 0.57 (0.53; 0.61) | 1.69 (1.47; 1.93) | 1.82 (1.62; 2.06) |
| Other non-affective psychosis | 0.66 (0.61; 0.72) | 1.44 (1.23; 1.68) | 1.19 (1.04; 1.35) | 0.69 (0.65; 0.72) | 1.49 (1.34; 1.64) | 1.14 (1.05; 1.24) | 0.67 (0.64; 0.70) | 1.38 (1.26; 1.51) | 1.09 (1.0; 1.17) |
| Obssesive compulsive disorder | 0.97 (0.74; 0.84) | 1.31 (1.16; 1.49) | 0.58 (0.52; 0.64) | 0.68 (0.60; 0.77) | 1.31 (1.06; 1.63) | 0.73 (0.61; 0.88) | 0.77 (0.73; 0.80) | 1.36 (1.27; 1.47) | 0.59 (0.55; 0.63) |
| ADHD | 0.39 (0.38; 0.41) | 1.16 (1.07; 1.25) | 1.21 (1.13; 1.29) | 0.35 (0.32; 0.37) | 1.10 (0.93; 1.30) | 1.19 (1.02; 1.40) | 0.37 (0.36; 0.38) | 1.17 (1.11; 1.23) | 1.39 (1.33; 1.46) |
| Bipolar illness | 0.76 (0.72; 0.81) | 1.15 (1.02; 1.29) | 0.37 (0.33; 0.42) | 0.76 (0.71; 0.81) | 1.28 (1.15; 1.44) | 0.55 (0.50; 0.61) | 0.77 (0.74; 0.80) | 1.14 (1.07; 1.22) | 0.45 (0.42; 0.48) |
| Major depression | 0.70 (0.69; 0.72) | 1.07 (1.02; 1.13) | 0.49 (0.47; 0.51) | 0.66 (0.64; 0.68) | 1.00 (0.95; 1.06) | 0.57 (0.54; 0.59) | 0.71 (0.70; 0.73) | 1.03 (1.00; 1.06) | 0.50 (0.49; 0.52) |
| Anxiety disorders | 0.65 (0.62; 0.67) | 0.98 (0.90; 1.06) | 0.55 (0.51; 0.59) | 0.56 (0.52; 0.60) | 1.03 (0.88; 1.20) | 0.61 (0.53; 0.69) | 0.66 (0.64; 0.67) | 0.98 (0.94; 1.03) | 0.61 (0.58; 0.63) |
| Anorexia | 1.62 (1.44; 1.83) | 1.61 (1.37; 1.89) | 0.04 (0.03; 0.05) | 1.28 (1.16; 1.42) | 1.26 (1.09; 1.45) | 0.03 (0.03; 0.05) | 1.45 (1.35; 1.57) | 1.32 (1.19; 1.46) | 0.05 (0.04; 0.06) |
| Bulimia nervosa | 1.27 (1.08; 1.49) | 1.45 (1.15; 1.83) | 0.02 (0.01; 0.04) | 1.11 (0.90; 1.38) | 0.93 (0.68; 1.27) | 0.01 (0.00; 0.03) | 1.17 (1.06; 1.29) | 1.27; 1.09; 1.47) | 0.01 (0.01; 0.02) |
| Drug abuse | 0.50 (0.49; 0.51) | 0.89 (0.85; 0.92) | 2.78 (2.69; 2.88) | — | — | — | 0.47 (0.47, 0.48) | 1.29 (1.15; 1.45) | 2.63 (2.57; 2.69) |
| Alcohol use disorder | 0.53 (0.52; 0.55) | 0.87 (0.82; 0.92) | 1.23 (1.17; 1.29) | — | — | — | 0.52 (0.51; 0.52) | 0.85 (0.82; 0.87) | 1.57 (1.53; 01.61) |
Abbreviations: ADHD, attention deficit hyperactivity disorder; CI, confidence interval; HR, hazard ratio.
Drug abuse and alcohol use disorder diagnoses arise from both medical, criminal and pharmacy registries and thus could not be meaningfully calculated only from inpatient data.
HR41 indicated more common in males.
Second, we repeated our analyses utilizing only in-patient diagnoses. As seen in the middle section of Table 3, the pattern of findings resembled those seen in our main analyses although the effect of high FCA was somewhat stronger especially for ASD and BPI.
Finally, our initial approach prospectively predicted onset of disorders from SA and FCA under the assumption that the onset of symptoms might confound these causal relationships. This was not feasible for ASD and ADHD. To examine this effect more broadly, we repeated our analysis examining all cases in our cohort regardless of age at first registration. As seen in the right side of Table 3, the pattern of results was similar to those seen in our main analyses, suggesting that onset prior to the SA may not substantially distort the pattern of observed associations, especially for disorders like ASD and ADHD.
DISCUSSION
We examined, in a large representative Swedish cohort, the joint predictive effects of SA measured in mid-adolescence and FCA on a wide range of psychiatric syndromes. Our goal was to discover several distinct patterns of cognitive development.
Our results were encouraging. We could readily identify four discrete patterns. The first, risk prediction by low SA and high FCA, was seen for 6 of our 12 examined disorders. As previously seen for schizophrenia,8 controlling for level of SA, liability to illness was strongly and positively associated with the average cognitive ability of their family. This arises, we suggest, because risk of illness was reflected by the degree to which an individual’s cognitive ability fell short of that expected given their familial potential. With our much larger set of disorders, we were able to define a continuum of neurodevelopmental impairment indexed by the effect size of FCA controlling for level of SA. At the severe end was ASD followed by schizophrenia and ONAP. Next came OCD and ADHD where the impact of FCA was more moderate. BPI was at the milder end of this developmental continuum.
The second developmental pattern, where risk of illness was modestly predicted by low SA but unrelated to FCA described our two internalizing disorders: MD and AD. In these disorders, poor school performance reflected liability to illness, but risk was not further associated with the degree to which their cognitive development differed from their familial potential.
In the third pattern, illness liability was strongly predicted by a combination of both low SA and low FCA. This pattern fitted our two externalizing disorders of DA and AUD. To test whether this pattern generalized to other externalizing behaviors, we examined, in our model, Swedish national data on criminal convictions.12 The pattern of prediction by SA and FCA was very similar to those found for DA and AUD (Supplementary Appendix Figure S1). Further research will be needed to fully understand these findings but they suggest that those most vulnerable to externalizing disorders are the most cognitively disadvantaged individuals from disadvantaged families.
The fourth developmental pattern, in which risk was predicted by a combination of high SA and high FCA, fitted our two eating disorders—AN and BN. These results are consistent with prior evidence13–15 that AN is positively associated with social class of origin, SA16 and intelligence.17 Parallel results for BN have been similar albeit less consistent.13,16,18 In every other disorder examined, within families, risk was inversely related to SA. However, for AN and BN, risk was positively associated with SA. Thus the highest risk for AN and BN was, to put it simply, in the smartest individuals from the smartest families. These results might be related to substantial prior evidence that the personality trait of perfectionism predicts risk for both AN19,20 and BN.21,22 While the underlying mechanisms are far from clear, our results indicate that the cognitive neurodevelopmental substratum of eating disorders differ substantially from the other disorders here examined and in particularly is exactly the opposite of that seen with externalizing disorders.
The findings from this study fit within the context of much prior research on cognitive impairment in psychiatric disorders, with the predictive value of FCA controlling for level of SA broadly paralleling the magnitude of demonstrated cognitive impairment. The disorders with the most severe impairment, ASD and schizophrenia,23–25 showed the strongest predictive effect of FCA controlling for level of SA. For those disorders demonstrating, on average, more moderate cognitive impairment—ADHD, OCD and BPI26–29—the impact of SA-controlled FCA on risk was more modest. For MD and AD where cognitive deficits are typically subtle,7,30–32 we saw no effect of FCA on risk, suggesting that these common syndromes are not fundamentally neurodevelopmental disorders. Our results are also consistent with the concept of a psychiatric ‘gradient of neurodevelopmental pathology’ and particularly with the hypothesis that the more severe neurodevelopmental syndromes (e.g. ASD and schizophrenia) are particularly enriched for de novo DNA structural and single-nucleotide variants which could, among a range of other possible mechanisms, explain the deviation of their cognitive potential from familial expectations.33–36
We do not claim that disorders with the same cognitive developmental pattern are necessarily closely etiologically related. Twin studies suggest substantial sharing of genetic risk between MD and AD37,38 and between AN and BN.39 However, modern molecular methods indicate that while SZ and BPI share a high proportion of their risk genes, the genetic correlation is much lower between SZ and both ADHD and ASD.40 The broad cognitive developmental patterns we have tentatively identified are likely etiologically heterogeneous, and could represent final common pathways of a diversity of genetic and environmental risk factors. Furthermore, our results are not entirely congruent with emerging analyses using polygenic risk scores showing that schizophrenia and ADHD are negatively genetically correlated with intelligence/SA while ASD and BPI are positively correlated.41–44
Given the novelty of these findings, it is important to consider their stability and freedom from bias. We addressed this in three ways. First, we replicated in males these findings from our SA analyses on our first three disorder groups using IQ obtained several years later. Second, concerned about artefactual results emerging from the addition of cases from outpatient contact, we replicated our results in the subset of our cohort ascertained in the last five years and thus eligible for both in- and outpatient ascertainment. Third, our main results were seen if we restricted our samples to cases ascertained through in-patient treatment. Finally, for 10 of the 12 disorders, the assessment of cognitive ability likely preceded onset of illness. We have previously shown that buffer periods between SA assessment and the counting of disease onsets up to 10 years do not alter the association between SA and risk for SZ, ONAP or BP.45 However, for two of the disorders —ASD and ADHD—onset was likely before SA assessment. Therefore, the impact of disease liability and the clinical effects of the disorder could be confounded and bias upward the impact of SA (and indirectly CSA) on risk for ASD and ADHD. An alternative hypothesis, supported by further analyses, is that for ASD and ADHD, ‘onset’ actually has little effect on SA, as the cognitive precursors of these syndromes are relatively stable.
Limitations
These results should be interpreted in the context of six potential methodological limitations. First, our analyses were based on clinical diagnoses not collected for research purposes. Therefore, we are studying treated prevalence not true population prevalence. Validation studies have been conducted of Swedish registries diagnoses of SZ and BPI and they performed well.46–49 Second, our sample was not fully representative of the Swedish population. We examine, in Supplementary Appendix Table S4, biases resulting from the most common exclusion from the sample (missing an FCA measure) or other reasons (missing an SA score or diagnosis before SA assessment). In the entire sample, the former was predicted modestly by older age while the latter by younger age, male sex and parents with low educational status. These patterns differed somewhat across specific disorders. Third, we sampled from the milder end of the ADHD and especially the ASD spectrum, as all individuals in our sample had to be ‘mainstreamed’ in standard secondary education and, for males, meet criteria to be called for the conscription examination.
Fourth, because SA was evaluated in two different ways in Sweden in 1988–1997 versus 1998–2012, we examined whether the predictive relationships between SA and our psychiatric outcomes differed for individuals assessed in the two time periods. No significant effect was found for six of our disorders and the effect sizes of the differences in the other six were too small to substantially influence our findings (Supplementary Appendix Table S5).
Fifth, with our very large sample sizes, we were well powered to detect violations in the proportionality assumptions of our Cox models. Indeed, for our SA analyses, we detected significant violations for all but two disorders (OCD and BPI). As seen in Supplementary Appendix Table S3, the most common violation was a decline in the impact of high FCA on risk with longer followup. This was likely the result of weaker FCA effects on cases with a later age of onset and was seen most prominently for ASD, ONAP and ADHD, but not in schizophrenia.
Finally, we presented our key results in Table 2 using a diagnostic hierarchy which could have distorted our findings. We therefore repeated these analyses without a hierarchy. As seen in Supplementary Appendix Table S6, the results changed little.
CONCLUSIONS
Using measures of cognitive functioning in adolescence and FCA —as assessed directly in cousins, siblings and parents—we identified four rather distinct cognitive developmental pathways to psychiatric illness each of which had a distinctive pattern of risk factors: (i) low SA and high FCA, (ii) low SA and no effect of FCFA, (iii) low SA and low FCA and (iv) high SA and high FCA. These four developmental patterns identified clinically coherent groups of disorders that could be described, respectively, as (i) neurodevelopmental, (ii) internalizing, (iii) externalizing and (iv) eating disorders.
Our results demonstrate the substantial value of adding, in a thorough assessment of the cognitive antecedents of psychiatric illness, measures of familial cognitive functioning. Prediction of risk for a number of psychiatric disorders appears to be substantially improved when individual cognitive performance is viewed in light of that which was expected given the family background.
Supplementary Material
Acknowledgments
This project was supported by the grants R01DA030005 and R01AA023534 from the National Institutes of Health, the Swedish Research Council (K2012-70X-15428-08-3), the Swedish Research Council for Health, Working Life and Welfare (In Swedish: Forte; Reg. nr: 2013-1836), the Swedish Research Council (2012-2378; 2014-10134) and FORTE (2014-0804) as well as ALF funding from Region Skåne awarded.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. We secured approval for this study from the Regional Ethical Review Board of Lund University.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, et al. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry. 2004;61:354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
- 2.Aylward E, Walker E, Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull. 1984;10:430–459. doi: 10.1093/schbul/10.3.430. [DOI] [PubMed] [Google Scholar]
- 3.Khandaker GM, Barnett JH, White IR, Jones PB. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res. 2011;132:220–227. doi: 10.1016/j.schres.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarty CA, Mason WA, Kosterman R, Hawkins JD, Lengua LJ, McCauley E. Adolescent school failure predicts later depression among girls. J Adolesc Health. 2008;43:180–187. doi: 10.1016/j.jadohealth.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- 6.Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendler KS, Ohlsson H, Mezuk B, Sundquist JO, Sundquist K. Observed cognitive performance and deviation from familial cognitive aptitude at age 16 years and ages 18 to 20 years and risk for schizophrenia and bipolar illness in a Swedish national sample. JAMA Psychiatry. 2016;73:465–471. doi: 10.1001/jamapsychiatry.2016.0053. [DOI] [PubMed] [Google Scholar]
- 9.Kendler KS, Sundquist K, Ohlsson H, Palmer K, Maes H, Winkleby MA, et al. Genetic and familial environmental influences on the risk for drug abuse: a national Swedish adoption study. Arch Gen Psychiatry. 2012;69:690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendler KS, Ji J, Edwards AC, Ohlsson H, Sundquist J, Sundquist K. An extended Swedish national adoption study of alcohol use disorder. JAMA Psychiatry. 2015;72:211–218. doi: 10.1001/jamapsychiatry.2014.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SAS Institute I. SAS/STAT User's Guide, Version 9.3. SAS Institute Inc; Cary, NC: 2011. [Google Scholar]
- 12.Kendler KS, Larsson Lönn S, Morris NA, Sundquist J, Långström N, Sundquist K. A Swedish National Adoption Study of Criminality. Psychol Med. 2014;44:1913–1925. doi: 10.1017/S0033291713002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Striegel-Moore RH, Bulik CM. Risk factors for eating disorders. Am Psychol. 2007;62:181–198. doi: 10.1037/0003-066X.62.3.181. [DOI] [PubMed] [Google Scholar]
- 14.McClelland L, Crisp A. Anorexia nervosa and social class. Int J Eat Disord. 2001;29:150–156. doi: 10.1002/1098-108x(200103)29:2<150::aid-eat1004>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 15.Lindberg L, Hjern A. Risk factors for anorexia nervosa: a national cohort study. Int J Eat Disord. 2003;34:397–408. doi: 10.1002/eat.10221. [DOI] [PubMed] [Google Scholar]
- 16.Sundquist J, Ohlsson H, Winkleby MA, Sundquist K, Crump C. School achievement and risk of eating disorders in a Swedish National Cohort. J Am Acad Child Adolesc Psychiatry. 2016;55:41–46. doi: 10.1016/j.jaac.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez C, Stahl D, Tchanturia K. Estimated intelligence quotient in anorexia nervosa: a systematic review and meta-analysis of the literature. Ann Gen Psychiatry. 2010;9:40. doi: 10.1186/1744-859X-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanz BJ, Detzner U, Lay B, Rose F, Schmidt MH. The intellectual functioning of adolescents with anorexia nervosa and bulimia nervosa. Eur Child Adolesc Psychiatry. 1997;6:129–135. doi: 10.1007/BF00538984. [DOI] [PubMed] [Google Scholar]
- 19.Bastiani AM, Rao R, Weltzin T, Kaye WH. Perfectionism in anorexia nervosa. Int J Eat Disord. 1995;17:147–152. doi: 10.1002/1098-108x(199503)17:2<147::aid-eat2260170207>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Halmi KA, Sunday SR, Strober M, Kaplan A, Woodside DB, Fichter M, et al. Perfectionism in anorexia nervosa: variation by clinical subtype, obsessionality, and pathological eating behavior. Am J Psychiatry. 2000;157:1799–1805. doi: 10.1176/appi.ajp.157.11.1799. [DOI] [PubMed] [Google Scholar]
- 21.Vohs KD, Bardone AM, Joiner TE, Jr, Abramson LY, Heatherton TF. Perfectionism, perceived weight status, and self-esteem interact to predict bulimic symptoms: a model of bulimic symptom development. J Abnorm Psychol. 1999;108:695–700. doi: 10.1037//0021-843x.108.4.695. [DOI] [PubMed] [Google Scholar]
- 22.Bulik CM, Tozzi F, Anderson C, Mazzeo SE, Aggen S, Sullivan PF. The relation between eating disorders and components of perfectionism. Am J Psychiatry. 2003;160:366–368. doi: 10.1176/appi.ajp.160.2.366. [DOI] [PubMed] [Google Scholar]
- 23.Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- 24.Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunsdon VE, Colvert E, Ames C, Garnett T, Gillan N, Hallett V, et al. Exploring the cognitive features in children with autism spectrum disorder, their co-twins, and typically developing children within a population-based sample. J Child Psychol Psychiatry. 2015;56:893–902. doi: 10.1111/jcpp.12362. [DOI] [PubMed] [Google Scholar]
- 26.Boonstra AM, Oosterlaan J, Sergeant JA, Buitelaar JK. Executive functioning in adult ADHD: a meta-analytic review. Psychol Med. 2005;35:1097–1108. doi: 10.1017/s003329170500499x. [DOI] [PubMed] [Google Scholar]
- 27.Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2014;44:1121–1130. doi: 10.1017/S0033291713001803. [DOI] [PubMed] [Google Scholar]
- 28.Keefe RS, Fox KH, Davis VG, Kennel C, Walker TM, Burdick KE, et al. The Brief Assessment of Cognition In Affective Disorders (BAC-A): performance of patients with bipolar depression and healthy controls. J Affect Disord. 2014;166:86–92. doi: 10.1016/j.jad.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170:1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 2010;24:9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- 31.O'Sullivan K, Newman EF. Neuropsychological impairments in panic disorder: a systematic review. J Affect Disord. 2014;167:268–284. doi: 10.1016/j.jad.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Scult MA, Paulli AR, Mazure ES, Moffitt TE, Hariri AR, Strauman TJ. The association between cognitive function and subsequent depression: a systematic review and meta-analysis. Psychol Med. 2017;47:1–17. doi: 10.1017/S0033291716002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craddock N, Owen MJ. The Kraepelinian dichotomy - going, going… but still not gone. Br J Psychiatry. 2010;196:92–95. doi: 10.1192/bjp.bp.109.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronemus M, Iossifov I, Levy D, Wigler M. The role of de novo mutations in the genetics of autism spectrum disorders. Nat Rev Genet. 2014;15:133–141. doi: 10.1038/nrg3585. [DOI] [PubMed] [Google Scholar]
- 35.Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hettema JM, Neale MC, Myers J, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry. 2006;163:857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- 38.Kendler KS, Aggen SH, Knudsen GP, Roysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. Am J Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulik CM, Thornton LM, Root TL, Pisetsky EM, Lichtenstein P, Pedersen NL. Understanding the relation between anorexia nervosa and bulimia nervosa in a Swedish national twin sample. Biol Psychiatry. 2010;67:71–77. doi: 10.1016/j.biopsych.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cross-Disorder Group of the Psychiatric Genomics Consortium (PGC-CDG) Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stergiakouli E, Martin J, Hamshere ML, Heron J, St PB, Timpson NJ, et al. Association between polygenic risk scores for attention-deficit hyperactivity disorder and educational and cognitive outcomes in the general population. Int J Epidemiol. 2016;dyw216:1–8. doi: 10.1093/ije/dyw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riglin L, Collishaw S, Richards A, Thapar AK, Maughan B, O'Donovan MC, et al. Schizophrenia risk alleles and neurodevelopmental outcomes in childhood: a population-based cohort study. Lancet Psychiatry. 2017;4:57–62. doi: 10.1016/S2215-0366(16)30406-0. [DOI] [PubMed] [Google Scholar]
- 43.Hill WD, Davies G, Liewald DC, McIntosh AM, Deary IJ. Age-dependent pleiotropy between general cognitive function and major psychiatric disorders. Biol Psychiatry. 2016;80:266–273. doi: 10.1016/j.biopsych.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith DJ, Anderson J, Zammit S, Meyer TD, Pell JP, Mackay D. Childhood IQ and risk of bipolar disorder in adulthood: prospective birth cohort study. BJPsych Open. 2015;1:74–80. doi: 10.1192/bjpo.bp.115.000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kendler KS, Ohlsson H, Mezuk B, Sundquist K, Sundquist J. A Swedish National Prospective and Co-relative Study of School Achievement at Age 16, and Risk for Schizophrenia, Other Nonaffective Psychosis, and Bipolar Illness. Schizophr Bull. 2015;42:77–86. doi: 10.1093/schbul/sbv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lichtenstein P, Bjork C, Hultman CM, Scolnick E, Sklar P, Sullivan PF. Recurrence risks for schizophrenia in a Swedish national cohort. Psychol Med. 2006;36:1417–1425. doi: 10.1017/S0033291706008385. [DOI] [PubMed] [Google Scholar]
- 47.Ekholm B, Ekholm A, Adolfsson R, Vares M, Osby U, Sedvall GC, et al. Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59:457–464. doi: 10.1080/08039480500360906. [DOI] [PubMed] [Google Scholar]
- 48.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 49.Sellgren C, Landen M, Lichtenstein P, Hultman CM, Langstrom N. Validity of bipolar disorder hospital discharge diagnoses: file review and multiple register linkage in Sweden. Acta Psychiatr Scand. 2011;124:447–453. doi: 10.1111/j.1600-0447.2011.01747.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.