Abstract
Background/purpose
The 21-gene recurrence score (RS) assay evaluates the likelihood of distant recurrence and benefit of chemotherapy in lymph node-negative, estrogen receptor (ER)-positive, HER2-negative breast cancer patients. The RS categories are associated with the risk of locoregional recurrence (LRR) in some, but not all studies.
Methods
We reviewed the institutional database to identify consecutive female patients with node-negative, ER+/HER2− breast carcinoma tested for the 21-gene RS assay and treated at our center from 2008 to 2013. We collected data on clinicopathologic features, treatment, and outcome. Statistical analysis was performed using SAS version 9.4 or R version 3.3.2.
Results
Of 2326 patients, 60% (1394) were in the low RS group, 33.4% (777) in the intermediate RS group, and 6.6% (155) in the high RS group. Median follow-up was 53 months. A total of 44 LRRs were observed, with a cumulative incidence of 0.17% at 12 months and 1.6% at 48 months. The cumulative incidence of LRR at 48 months was 0.84%, 2.72% and 2.80% for low, intermediate, and high RS groups, respectively (p<0.01). Univariate analysis showed that the risk of LRR was associated with the RS categories (p<0.01), T stage (p<0.01) and lymphovascular invasion (LVI) (p=0.009). There was no difference in LRR rates by initial local treatment (total mastectomy vs. breast-conserving surgery plus radiation therapy). The RS remained significantly associated with LRR after adjusting for LVI and T stage. Compared to patients with low RS, the risk of LRR was increased more than 4-fold (hazard ratio: 4.61, 95% CI 1.90–11.19, p<0.01), and 3-fold (hazard ratio: 2.81, 95% CI 1.41–5.56, p<0.01) for high and intermediate risk categories, respectively.
Conclusions
Our study confirms that RS is significantly associated with the risk of LRR in node-negative, ER+/HER2− breast cancer patients. Our findings suggest that in addition to its value for prognostic stage grouping and decision-making regarding adjuvant systemic therapy, the role of the RS in identifying patients not requiring radiotherapy should be studied.
Keywords: breast cancer, 21-gene recurrence score assay, recurrence score, locoregional recurrence
Introduction
Estrogen receptor (ER)-positive breast cancer accounts for approximately 80% of all invasive breast tumors[1]. Following endocrine therapy, this subtype generally has a favorable prognosis with 5-year locoregional recurrence (LRR) and distant recurrence rates of approximately 10%[2–6] and low rates of breast cancer specific mortality. Many clinicopathologic factors have been reported to be associated with an increased risk of LRR, including patient age, tumor size, lymphovascular invasion (LVI), nodal status, ER and progesterone receptor (PR) status, molecular subtypes (luminal A vs. luminal B) and the duration of endocrine therapy [7,2,5,6,4,8,9].
Multigene prognostic classifiers developed in the last two decades have been clinically used to identify patients at a higher risk of distant recurrence. The 21-gene recurrence score (RS) assay (Oncotype Dx™, Genomic Health, Redwood City, CA)[10] is recommended by the American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) for patients with early stage, ER+/HER2− invasive breast cancer[11,12], and has recently been included in the prognostic stage group in the 8th edition of American Joint Commission of Cancer (AJCC) staging manual[13]. Many prospective and retrospective studies have reported a strong, consistent association between the RS and the likelihood of distant recurrence, determining the magnitude of chemotherapy benefit[14–27]. Studies have investigated the relationship between the RS and the risk of LRR with conflicting results. The association between the RS and LRR was first suggested in large patient cohorts from National Surgical Adjuvant Breast and Bowel Project (NSABP) trials B-14 and B-20[28]. However, subsequent smaller studies, including retrospective analysis of a subset of patients in the E2197 prospective randomized trial by the Eastern Cooperative Oncology Group (ECOG), did not confirm this finding[29–31].
This study aimed to evaluate the value of the 21-gene RS assay for predicting the risk of LRR in a cohort of lymph node negative, ER+/HER2− breast cancer patients treated at a single institution.
Materials and methods
Patient cohort
Our retrospective cohort included unselected, consecutive female patients with lymph node negative (pN0 and pN0[i+])[32], ER+/HER2− invasive breast carcinoma with known 21-gene RS assay results treated at our center between September 2008 and August 2013. The exclusion criteria were male sex and failure of the assay for any technical reasons. Patients receiving neoadjuvant therapy were also excluded. At our institution, standard management of patients with early stage, lymph node negative, ER+/HER2− invasive breast cancer who are candidates for chemotherapy includes evaluation of tumor sample with the 21-gene RS assay. Carefully selected representative paraffin blocks from tumors measuring ≥0.5 cm are submitted to Genomic Health for testing. Occasional smaller tumors are also analyzed upon treating physician’s request.
The 21-gene RS assay is based on reverse transcriptase polymerase chain reaction (RT-PCR) using the RNA isolated from formalin-fixed paraffin-embedded tissue. It evaluates the expression of 16 cancer related genes normalized to the expression of five reference genes and yields a numeric variable of RS on a scale of 0 to 100[14,15,33]. Based on the RS values, patients were stratified into three risk categories: low risk (RS≤17), intermediate risk (RS 18–30), and high risk (RS≥31), as previously reported[14]. The results of the 21-gene RS assay were prospectively incorporated in the treatment plan as recommended[16–25]. Most low risk patients were treated with adjuvant endocrine therapy, whereas most high risk patients received a combination of endocrine therapy and chemotherapy. Treatment of patients with intermediate RS was variable depending on various clinicopathologic features and individual choices. RS was not routinely considered in the selection of locoregional therapy.
Clinicopathologic variables included patient age at breast cancer diagnosis, tumor size, histologic type of tumor, LVI, 21-gene RS result, local and systemic treatment, and clinical outcome. For multifocal/multicentric carcinomas, the size of the largest tumor and the highest RS result were recorded. For one patient with metachronous bilateral ER+/HER2− breast carcinomas with low RS, only the data pertaining to the first tumor were included. The institutional database and electronic medical records were reviewed to record date of last follow-up, date of death, date and type of LRR and distant recurrence. The Institutional Review Board approved the study.
Statistical analysis
Clinical and pathologic characteristics stratified by RS were summarized using frequency and percentage for categorical covariates, and the median and range for continuous factors. Categorical and continuous variables were compared using Fisher’s exact test and Wilcoxon rank-sum test, respectively. Overall survival was calculated from date of pathologic diagnosis of breast cancer to date of last follow-up, estimated using Kaplan-Meier methods and compared using the log-rank test. Cumulative incidence of LRR and distance recurrence were estimated using competing risk methods and compared between RS or between other clinical characteristics using Gray’s test[34]. LRR was defined as invasive breast cancer involving the ipsilateral breast parenchyma, axilla, regional lymph nodes, chest wall, or skin identified more than six months from the initial diagnosis of breast cancer[35]. The cut-off date for clinical follow-up was 9/1/2016.
Multivariable competing risk regression was used to examine the independent effect of RS on LRR, adjusting for other factors that were significantly associated with LRR from the univariate analysis[36]. Univariate association of RS score on LRR was also examined among the subset of women treated with endocrine therapy and chemotherapy using the methods described above. Statistical analyses were performed using SAS version 9.4 (SAS Institute, INC., Cary, NC, USA) or R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). P-values were two-sided and <0.05 were considered statistically significant.
Results
Patient cohort
We identified 2326 consecutive female patients with lymph node negative, ER+/HER2− breast carcinoma tested for RS and treated at our center during the study period (Table 1). The median age at the initial diagnosis of breast cancer was 57 years (range 22–90), and 6% (128/2326) of women were <40 years old. Most patients (60%; 1394/2326) were in the low RS group, 33.4% (777/2326) were in the intermediate RS group, and 6.6% (155/2326) were in the high RS group. The median tumor size was 1.2 cm (range 0.3–5.9), and 18% (420/2326) were multifocal/multicentric. Most tumors (79%; 1831/2326) were invasive ductal carcinoma not otherwise specified, 13% (305/2326) were invasive lobular carcinoma, 5% (113/2326) were mixed ductal and lobular, and 3% (77/2326) were special histologic subtypes. LVI was identified in 21% (486/2326) of patients.
Table 1.
Clinicopathologic characteristics of 2326 patients by RS results
| Total (n=2326) |
Low RS (n=1394) |
Intermediate RS (n=777) |
High RS (n=155) |
p-value | |
|---|---|---|---|---|---|
| Age at diagnosis, years | |||||
| <40 years | 128 (6%) | 63 (5%) | 47 (6%) | 18 (12%) | <0.01 |
| ≥40 years | 2198 (94%) | 1331 (95%) | 730 (94%) | 137 (88%) | |
| Median (range) | 57 (22–90) | 57.0 (22–90) | 57 (24–85) | 57 (27–83) | |
| Tumor size, median (range), cm | 1.2 (0.3–5.9) | 1.2 (0.3–5.8) | 1.2 (0.3–5.9) | 1.5 (0.35–5) | <0.01 |
| Multifocality/multicentricity | 420 (18%) | 297 (21%) | 105 (14%) | 18 (12%) | <0.01 |
| LVI | 486 (21%) | 265 (19%) | 176 (23%) | 45 (29%) | <0.01 |
| Local Treatment | |||||
| BCS | 89 (3.8%) | 48 (3.4%) | 33 (4.2%) | 8 (5.2%) | 0.73 |
| BCS + radiation | 1539 (66.2%) | 929 (66.6%) | 510 (65.6%) | 100 (64.5%) | |
| Total mastectomy | 682 (29.3%) | 417 (29.9%) | 220 (28.3%) | 45 (29%) | |
| BCS, radiation unknown | 16 (0.7%) | 0 | 14 (1.8%) | 2 (1.3%) | |
| Systemic therapy | |||||
| Endocrine therapy only | 1505 (64.7%) | 1182 (84.8%) | 309 (39.8%) | 14 (9%) | N/A* |
| Endocrine + chemotherapy | 699 (30.1%) | 168 (12.1%) | 398 (51.2%) | 133 (85.8%) | |
| Chemotherapy only | 22 (0.9%) | 1 (0.1%) | 19 (2.4%) | 2 (1.3%) | |
| None | 78 (3.4%) | 43 (3.1%) | 32 (4.1%) | 3 (1.9%) | |
| Unknown | 22 (0.9%) | 0 | 19 (2.4%) | 3 (1.9%) | |
BCS, breast-conserving surgery; LVI, lymphovascular invasion; RS, recurrence score;
Not provided as systemic treatment was guided by the RS results.
Overall, 66.2% (1539/2326) of patients were treated with breast-conserving surgery (BCS) and radiation therapy, 3.8% (89/2326) with BCS alone, and 29.3% (682/2326) with total mastectomy. Most patients (64.7%; 1505/2326) received endocrine therapy alone, and 30.1% (699/2326) were treated with chemotherapy plus endocrine therapy. Only 0.9% (22/2326) of women received chemotherapy alone, and 3.4% (78/2326) received no systemic therapy.
Association of RS with LRR
Median follow-up was 53 months (range 0.9–108). We observed a total of 44 LRRs (Table 2). Of these, 37 LRRs occurred as first recurrences, six occurred as simultaneous events within three months of distant metastases and one LRR developed seven months after the diagnosis of distant recurrence. LRR was seen in 1.8% (27/1539) of patients treated with BCS and radiation therapy, 3.4% (3/89) of those having BCS alone and 2.1% (14/682) of those undergoing total mastectomy. All patients with LRR had negative surgical resection margins at the initial surgery. The ipsilateral breast was the most common site of LRR (45%; 20/44), followed by the ipsilateral axilla (16%; 7/44), and the ipsilateral chest wall (16%; 7/44). Two patients recurred in the ipsilateral supraclavicular lymph nodes and one in the ipsilateral internal mammary nodes, while 16% (7/44) developed LRR in multiple sites. The cumulative incidence of LRR was 0.17% at 12 months and 1.6% at 48 months (Fig. 1). The cumulative incidence of LRR at 48 months was 0.84%, 2.7% and 2.8% for low, intermediate and high RS groups, respectively (Gray’s test p<0.01) (Fig. 2).
Table 2.
Clinicopathologic characteristics of 44 patients with LRR
| Total (n=44) |
Low RS (n=13) |
Intermediate RS (n=22) |
High RS (n=9) |
|
|---|---|---|---|---|
| Age at diagnosis, years | ||||
| <40 years | 39 (88.6%) | 11 (84.6%) | 2 (9.1%) | 1 (11.1%) |
| ≥40 years | 5 (11.4%) | 2 (15.4%) | 20 (90.9%) | 8 (88.9%) |
| Median (range) | 54 (34–79) | 54 (35–79) | 51 (38–75) | 57 (34–69) |
| Tumor size, median (range), cm | 1.6 (0.5–3.5) | 1.3 (0.5–3.5) | 1.7 (0.5–3.4) | 1.6 (0.6–2.6) |
| Multifocality/multicentricity | 9 (20.5%) | 6 (46.2%) | 3 (13.6%) | 0 |
| LVI | 17 (38.6%) | 4 (30.8%) | 11 (50%) | 2 (22.2%) |
| Local Treatment | ||||
| BCS | 3 (6.8%) | 2 (15.4%) | 0 | 1 (11.1%) |
| BCS + radiation | 27 (61.4%) | 7 (53.8%) | 14 (63.6%) | 6 (66.7%) |
| Total mastectomy | 14 (31.8%) | 4 (30.8%) | 8 (36.4%) | 2 (22.2%) |
| Systemic therapy | ||||
| Endocrine therapy only | 16 (36.4%) | 8 (61.5%) | 6 (27.3%) | 2 (22.2%) |
| Endocrine + chemotherapy | 25 (56.8%) | 4 (30.8%) | 15 (68.2%) | 6 (66.7%) |
| Chemotherapy only | 2 (4.5%) | 0 | 1 (4.5%) | 1 (11.1%) |
| None | 1 (2.3%) | 1 (7.7%) | 0 | 0 |
BCS, breast-conserving surgery; LVI, lymphovascular invasion; LRR, locoregional recurrence; RS, recurrence score.
Fig. 1.
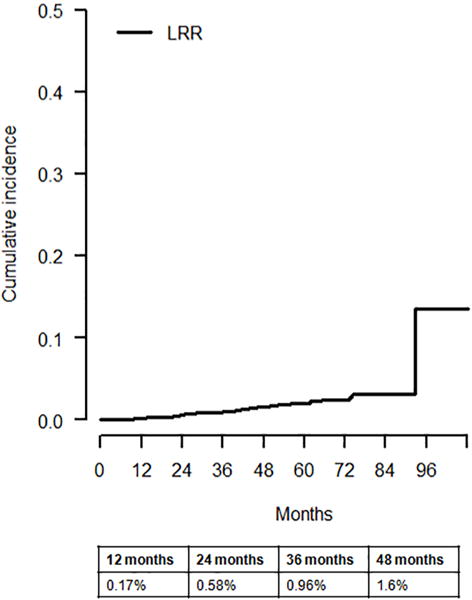
Cumulative incidence of locoregional recurrence (LRR)
Fig. 2.
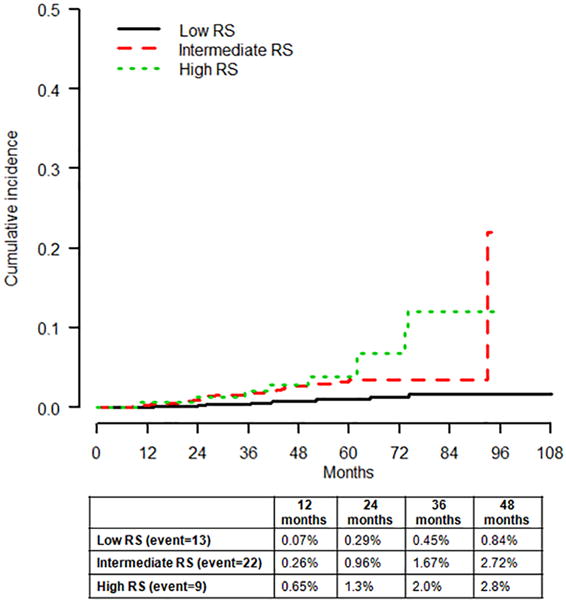
Cumulative incidence of locoregional recurrence (LRR) by recurrence score (RS) categories (Gray’s test p<0.01)
Univariate analysis showed that the risk of LRR was associated with RS categories (intermediate vs. low and high vs. low, both p<0.01), LVI (p=0.009), and T stage (p<0.01) (Table 3). The risk of LRR in the intermediate RS group was approximately half that in the high RS group (hazard ratio: 0.53, 95% CI: 0.25–1.16, p=0.11) but did not reach statistical significance, likely due to the small number of patients and events in the high risk category. Multifocality/multicentricity and age were not significantly associated with LRR in this population. The rates of LRR did not differ between patients treated with BCS plus radiation therapy vs. total mastectomy (p=0.781).
Table 3.
Association of clinicopathologic variables with LRR by univariate analysis
| HR | 95% CI | p-value | |
|---|---|---|---|
| RS score | |||
| Intermediate vs. low | 2.94 | (1.48–5.84) | <0.01 |
| High vs. low | 5.51 | (2.36–12.87) | <0.01 |
| T stage (T2-3 vs. T1) | 2.83 | (1.48–5.40) | <0.01 |
| LVI (yes vs. no) | 2.28 | (1.23–4.17) | 0.009 |
| Multifocal/multicentral (yes vs. no) | 1.16 | (0.56–2.42) | 0.693 |
| Age ≥40 vs. <40 years | 0.44 | (0.17–1.11) | 0.08 |
CI, confidence interval; HR, hazard ratio; LVI, lymphovascular invasion; LRR, locoregional recurrence; RS, recurrence score.
Of the 44 women with LRR, 16 were treated with endocrine therapy alone, 25 were treated with endocrine therapy plus chemotherapy, two were treated with chemotherapy alone, and one received no systemic therapy. Of the 25 LRRs observed in the 699 endocrine therapy plus chemotherapy treated patients, RS was low in four patients, intermediate in 15 patients, and high in six patients. There was no significant association between RS and the rates of LRR (p=0.63) among patients treated with endocrine therapy and chemotherapy. Subgroup analysis among patients treated with endocrine therapy alone was not possible due to low number of events (eight in the low RS group, six in the intermediate RS group and two in the high RS group).
Multivariable competing risk analysis
Multivariate competing risk analysis demonstrated that the RS categories were associated with the risk of LRR after adjusting for LVI and T stage (Table 4). There was a statistically significant association between LRR and high vs. low RS risk categories (hazard ratio: 4.61, 95% CI: 1.90–11.19, p<0.01), and intermediate vs. low RS risk categories (hazard ratio: 2.81, 95% CI: 1.41–5.56, p<0.01). However, the risk of LRR in intermediate vs. high RS risk categories did not vary significantly (hazard ratio: 0.61, 95% CI: 0.27–1.35, p=0.23).
Table 4.
Multivariable competing risk analysis of predictors of LRR in 2326 patients
| HR | 95%CI | p-value | |
|---|---|---|---|
| RS score | |||
| High vs. low | 4.61 | (1.90–11.19) | <0.01 |
| Intermediate vs. low | 2.81 | (1.41–5.56) | <0.01 |
| Intermediate vs. high | 0.61 | (0.27–1.35) | 0.23 |
| T stage (T2-3 vs T1) | 2.10 | (1.02–4.31) | 0.04 |
| LVI (yes vs. no) | 1.81 | (0.96–3.42) | 0.06 |
CI, confidence interval; HR, hazard ratio; LVI, lymphovascular invasion; LRR, locoregional recurrence; RS, recurrence score.
Association of RS with overall survival and distant metastases
During the follow-up period, 37 patients developed distant metastases and there were 36 deaths, eight due to breast cancer. Overall survival was significantly associated with the RS risk categories (log-rank p=0.01) (Fig. 3A). The cumulative incidence of distant recurrence at 48 months was 0.41%, 1.77% and 3.6% for low, intermediate and high RS risk categories, respectively (Gray’s test p<0.01) (Fig. 3B).
Fig. 3.
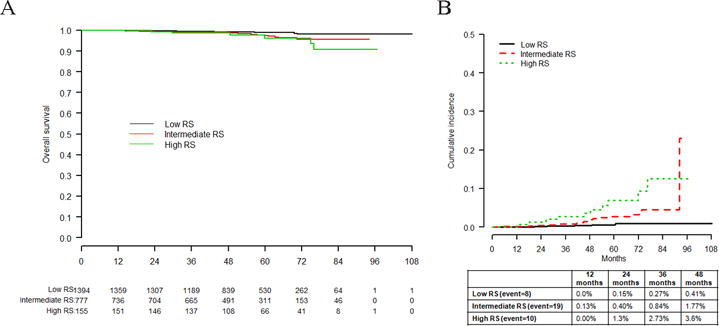
A Analysis of overall survival by recurrence score (RS) categories (log-rank p = 0.01). B Cumulative incidence of distance recurrence by RS categories (Gray’s test p value <0.01)
Discussion
Our study confirms that the 21-gene RS risk categories are significantly associated with the risk of LRR in a cohort of 2326 node negative, ER+/HER2− breast cancer patients by univariate and multivariate analyses. Mamounas et al. first reported this association in 895 tamoxifen treated patients from the NSABP B-14 and B-20 trials (p<0.001), 424 chemotherapy and tamoxifen treated patients from the B-20 trial (p=0.028), and 355 placebo patients from the B-14 trial (p=0.022)[28]. LRR was associated with RS>24 in patients treated with total mastectomy in another smaller study[29]. In contrast, a retrospective analysis of 388 patients treated with breast conservation, radiation and adjuvant systemic chemotherapy in the ECOG E2197 trial showed no association between LRR and RS. However, in the subset of ER+ and/or PR+/HER2− tumors for which the RS was designed to be used, the RS as a continuous variable was significantly associated with LRR (hazard ratio: 2.66; p=0.03)[30].
The overall LRR rates were very low in our study, with only 44 LRR events and a 1.6% cumulative incidence of LRR at 48 months, including 0.84%, 2.72% and 2.8% LRR rates for low, intermediate and high RS risk categories, respectively (p<0.01). Other studies examining RS as a predictor of LRR have reported higher rates of LRR. The 10-year Kaplan-Meier estimate of LRR was 4.3%, 7.2% and 15.8% in low, intermediate and high RS groups in tamoxifen treated patients in the NSABP B-14 and B-20 trials in the report by Mamounas et al [28]. However, in the B-14 study high RS patients did not receive chemotherapy as was done in our study, likely accounting for the higher rate of LRR. Data from the B-20 study shows that the use of chemotherapy plus endocrine therapy in high RS patients reduces, but does not eliminate the difference in LRR seen on the basis of RS[28]. The ECOG E2197 trial included both node negative and 1–3 node positive patients and reported the 10-year LRR rates of 3.8%, 5.1%, and 12 % for the low, intermediate, and high RS risk categories, respectively[30].
We found no significant difference in the LRR rates by patient age or the type of initial local treatment (total mastectomy vs. BCS with radiation therapy). Given the very low absolute risk of LRR in the high RS group and similar LRR rates irrespective of the surgical procedure, our study provides no evidence that total mastectomy is superior to BCS with radiation therapy with regard to reducing the risk of future LRR.
The distribution of the RS risk categories in our cohort was 60%, 33.4% and 6.6% in the low, intermediate and high RS groups, respectively. This is comparable with other studies of ER+/HER2− breast cancers reporting RS distributions of 47–54%, 21–37% and 15–27% for the three risk categories[37,14,15,30]. The differences in the distribution of the RS risk categories among institutions most likely reflect patient population and selection bias with variable testing thresholds exercised by clinicians[37]. Nevertheless, the 21-gene RS assay has become an integral part of standard management of node negative, ER+/HER2− breast cancer patients. With the recent implementation of the RS results in prognostic stage groupings of early stage (Tl-2 N0M0), ER+/HER2− invasive breast carcinomas by the AJCC[13], the 21-gene RS assay will likely continue to guide treatment decisions for the foreseeable future.
Strengths of this study include a large, unselected, consecutive population of breast cancer patients with clinical outcome whose RS results were prospectively included in the treatment planning and detailed knowledge of pathologic and treatment variables. Its main limitations are the retrospective study design, the low number of LRR events, and the follow-up time interval of less than five years in some patients. In addition, our tertiary academic institution predominantly treats women with screen-detected breast cancer and women from a specific geographic region. Nonetheless, our findings are in agreement with the results of other studies discussed above.
In conclusion, we report low LRR rates and a significant association between the RS risk categories (low, intermediate, high) and the risk of LRR in a cohort of 2326 lymph node negative, ER+/HER2− breast cancer patients by univariate and multivariate analyses. Our findings suggest that in addition to its value for prognostic stage grouping and decision making regarding adjuvant systemic therapy, the role of the RS in identifying patients not requiring radiotherapy should be studied.
Acknowledgments
This work was supported in part by a National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30CA008748). We thank Ms. Alicja Wiszowaty for her invaluable assistance with data collection.
Footnotes
Compliance with ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Institutional Review Board. For this type (retrospective) of study formal consent is not required.
Ethical Standards
The experiments (data analyses) comply with the current laws of the country in which they were performed (United States of America).
References
- 1.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 2.Millar EK, Graham PH, O’Toole SA, McNeil CM, Browne L, Morey AL, Eggleton S, Beretov J, Theocharous C, Capp A, Nasser E, Kearsley JH, Delaney G, Papadatos G, Fox C, Sutherland RL. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(28):4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 3.Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thurlimann B, Gianni L, Castiglione M, Gelber RD, Coates AS, Goldhirsch A. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(9):927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(10):1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, Bellon JR, Wong JS, Smith BL, Harris JR. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(14):2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 7.Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE, Jr, Jeong JH, Tan-Chiu E, Fisher B, Wolmark N. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(13):2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 8.Colleoni M, Rotmensz N, Maisonneuve P, Sonzogni A, Pruneri G, Casadio C, Luini A, Veronesi P, Intra M, Galimberti V, Torrisi R, Andrighetto S, Ghisini R, Goldhirsch A, Viale G. Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2007;18(10):1632–1640. doi: 10.1093/annonc/mdm268. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed RA, Martin SG, Mahmmod AM, Macmillan RD, Green AR, Paish EC, Ellis IO. Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: findings from a large case series with long-term follow-up. J Pathol. 2011;223(3):358–365. doi: 10.1002/path.2810. [DOI] [PubMed] [Google Scholar]
- 10.Gyorffy B, Hatzis C, Sanft T, Hofstatter E, Aktas B, Pusztai L. Multigene prognostic tests in breast cancer: past, present, future. Breast cancer research: BCR. 2015;17:11. doi: 10.1186/s13058-015-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC, Jr, American Society of Clinical O American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, Tunis S, Baker L, Crowley J, Deverka P, Veenstra D, Hortobagyi GN. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemporary clinical trials. 2013;34(1):1–9. doi: 10.1016/j.cct.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, Cancer.AJCo . AJCC cancer staging manual. 8th. Springer; New York; London: 2017. [Google Scholar]
- 14.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. The New England journal of medicine. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 15.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE, Jr, Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 16.Lo SS, Mumby PB, Norton J, Rychlik K, Smerage J, Kash J, Chew HK, Gaynor ER, Hayes DF, Epstein A, Albain KS. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(10):1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 17.Ademuyiwa FO, Miller A, O’Connor T, Edge SB, Thorat MA, Sledge GW, Levine E, Badve S. The effects of oncotype DX recurrence scores on chemotherapy utilization in a multi-institutional breast cancer cohort. Breast cancer research and treatment. 2011;126(3):797–802. doi: 10.1007/s10549-010-1329-6. [DOI] [PubMed] [Google Scholar]
- 18.Geffen DB, Abu-Ghanem S, Sion-Vardy N, Braunstein R, Tokar M, Ariad S, Delgado B, Bayme M, Koretz M. The impact of the 21-gene recurrence score assay on decision making about adjuvant chemotherapy in early-stage estrogen-receptor-positive breast cancer in an oncology practice with a unified treatment policy. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2011;22(11):2381–2386. doi: 10.1093/annonc/mdq769. [DOI] [PubMed] [Google Scholar]
- 19.Partin JF, Mamounas EP. Impact of the 21-gene recurrence score assay compared with standard clinicopathologic guidelines in adjuvant therapy selection for node-negative, estrogen receptor-positive breast cancer. Annals of surgical oncology. 2011;18(12):3399–3406. doi: 10.1245/s10434-011-1698-z. [DOI] [PubMed] [Google Scholar]
- 20.Albanell J, Gonzalez A, Ruiz-Borrego M, Alba E, Garcia-Saenz JA, Corominas JM, Burgues O, Furio V, Rojo A, Palacios J, Bermejo B, Martinez-Garcia M, Limon ML, Munoz AS, Martin M, Tusquets I, Rojo F, Colomer R, Faull I, Lluch A. Prospective transGEICAM study of the impact of the 21-gene Recurrence Score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2012;23(3):625–631. doi: 10.1093/annonc/mdr278. [DOI] [PubMed] [Google Scholar]
- 21.Joh JE, Esposito NN, Kiluk JV, Laronga C, Lee MC, Loftus L, Soliman H, Boughey JC, Reynolds C, Lawton TJ, Acs PI, Gordan L, Acs G. The effect of Oncotype DX recurrence score on treatment recommendations for patients with estrogen receptor-positive early stage breast cancer and correlation with estimation of recurrence risk by breast cancer specialists. The oncologist. 2011;16(11):1520–1526. doi: 10.1634/theoncologist.2011-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eiermann W, Rezai M, Kummel S, Kuhn T, Warm M, Friedrichs K, Schneeweiss A, Markmann S, Eggemann H, Hilfrich J, Jackisch C, Witzel I, Eidtmann H, Bachinger A, Hell S, Blohmer J. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2013;24(3):618–624. doi: 10.1093/annonc/mds512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast cancer research and treatment. 2013;141(1):13–22. doi: 10.1007/s10549-013-2666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinan MA, Mi X, Reed SD, Lyman GH, Curtis LH. Association Between Use of the 21-Gene Recurrence Score Assay and Receipt of Chemotherapy Among Medicare Beneficiaries With Early-Stage Breast Cancer, 2005–2009. JAMA oncology. 2015;1(8):1098–1109. doi: 10.1001/jamaoncol.2015.2722. [DOI] [PubMed] [Google Scholar]
- 25.Levine MN, Julian JA, Bedard PL, Eisen A, Trudeau ME, Higgins B, Bordeleau L, Pritchard KI. Prospective Evaluation of the 21-Gene Recurrence Score Assay for Breast Cancer Decision-Making in Ontario. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2015.62.8503. [DOI] [PubMed] [Google Scholar]
- 26.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE, Jr, Dees EC, Perez EA, Olson JA, Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. The New England journal of medicine. 2015 doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petkov V, Miller DP, Howlader N, Gliner N, Howe W, Schussler NC, Cronin K, Baehner FL, Penberthy L. Breast cancer specific mortality in patients with early-stage hormone receptor-positive invasive breast cancer and oncotype DX recurrence score results in the SEER database. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(7_suppl):176. [Google Scholar]
- 28.Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, Watson D, Geyer CE, Jr, Wickerham DL, Wolmark N. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(10):1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jegadeesh NK, Kim S, Prabhu RS, Oprea GM, Yu DS, Godette KG, Zelnak AB, Mister D, Switchenko JM, Torres MA. The 21-gene recurrence score and locoregional recurrence in breast cancer patients. Annals of surgical oncology. 2015;22(4):1088–1094. doi: 10.1245/s10434-014-4252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solin LJ, Gray R, Goldstein LJ, Recht A, Baehner FL, Shak S, Badve S, Perez EA, Shulman LN, Martino S, Davidson NE, Sledge GW, Jr, Sparano JA. Prognostic value of biologic subtype and the 21-gene recurrence score relative to local recurrence after breast conservation treatment with radiation for early stage breast carcinoma: results from the Eastern Cooperative Oncology Group E2197 study. Breast cancer research and treatment. 2012;134(2):683–692. doi: 10.1007/s10549-012-2072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaker NG, Hoffman KE, Stauder MC, Shaitelman SF, Strom EA, Tereffe W, Smith BD, Perkins GH, Huo L, Munsell MF, Pusztai L, Buchholz TA, Woodward WA. The 21-gene recurrence score complements IBTR! Estimates in early-stage, hormone receptor-positive, HER2-normal, lymph node-negative breast cancer. Springerplus. 2015;4:36. doi: 10.1186/s40064-015-0840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th. Springer; 2010. [Google Scholar]
- 33.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF, Breast Cancer Intergroup of North A Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. The Lancet Oncology. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16(3):1141–1154. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 35.Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA, Gelber RD, Zujewski JA. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 36.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.2307/2670170. [DOI] [Google Scholar]
- 37.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(5):721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]


