Abstract
Aquaculture production is projected to expand from land-based operations to the open ocean as demand for seafood grows and competition increases for inputs to land-based aquaculture, such as freshwater and suitable land. In contrast to land-based production, open-ocean aquaculture is constrained by oceanographic factors, such as current speeds and seawater temperature, which are dynamic in time and space, and cannot easily be controlled. As such, the potential for offshore aquaculture to increase seafood production is tied to the physical state of the oceans. We employ a novel spatial model to estimate the potential of open-ocean finfish aquaculture globally, given physical, biological and technological constraints. Finfish growth potential for three common aquaculture species representing different thermal guilds—Atlantic salmon (Salmo salar), gilthead seabream (Sparus aurata) and cobia (Rachycentron canadum)—is compared across species and regions and with climate change, based on outputs of a high-resolution global climate model. Globally, there are ample areas that are physically suitable for fish growth and potential expansion of the nascent aquaculture industry. The effects of climate change are heterogeneous across species and regions, but areas with existing aquaculture industries are likely to see increases in growth rates. In areas where climate change results in reduced growth rates, adaptation measures, such as selective breeding, can probably offset potential production losses.
Keywords: aquaculture, open-ocean aquaculture, climate change adaptation, thermal performance curve, mariculture, offshore aquaculture
1. Introduction
Aquaculture is one of the fastest growing animal food production sectors [1], and production will probably continue to grow to meet rising demand for seafood [2]. Currently, most aquaculture takes place in land-based ponds or raceways or near the coast in cages and net-pens [3], but sustained growth in these sectors may be limited. For example, constraints to the expansion of traditional land-based systems include limited availability of freshwater, competition for suitable land and high rates of disease [4]. Similarly, coastal aquaculture is constrained by suitable space, competition with other sectors, such as wild capture fisheries, and anthropogenic and natural impacts on coastal ecosystems, such as pollution and hurricanes, respectively [5,6]. In contrast, open-ocean aquaculture, where production is located away from the coastal zone and in the open ocean (see [7,8] for discussions of technical definitions of open-ocean aquaculture), has been identified as one of several technologies that can help increase aquaculture production while avoiding these constraints [9–12]. However, the potential for open-ocean aquaculture to contribute to seafood production is poorly understood, especially in terms of changes in the state of key physical-ocean parameters, driven by climate change.
Engineering technology for farming seafood in the open ocean has improved from rudimentary cages to robotic pens that can be submerged to withstand or avoid challenging oceanic conditions [5,13]. However, control of ambient conditions (e.g. ocean currents and temperature), which strongly impact the growth of individual fish and the overall farm productivity, remains a challenge [14]. On land, control of ambient conditions is less problematic, as the culture environment is relatively easy to manipulate. For example, on-land aquaculturists modify and maintain water temperature, dissolved oxygen levels, water quality and other environmental parameters at desirable levels [13]. In open-ocean environments, aquaculturalists have minimal control over the environment, but instead rely on ocean currents to provide clean water and remove waste from farms [15]. As a consequence, fish growth and production are likely strongly constrained by the physical state of the ocean, including ambient seawater temperatures.
The relationship between temperature and somatic growth for cultured fish (and organisms in general) is commonly referred to as a thermal performance curve (TPC) for growth, and the shape varies across species, populations, and individuals and shifts with ontogeny and acclimation [16–18]. Many species exhibit a tent-like relationship, with growth rates increasing from zero-growth at a specific low temperature, increasing to a maximum at an intermediate temperature, and then decreasing back to zero-growth at a maximum temperature [19]. Given the relatively constrained environmental requirements of broodstock and early lifestage fish [20], offshore aquaculturists typically maintain broodstock and larviculture production on land, where temperatures are controlled to maximize health and production per unit of feed [21,22]. However, once fish reach juvenile stages (e.g. 5–100 g), they are transferred to open-ocean cages or pens for grow-out. During grow-out, the major constraint to growth and the overall production of the open-ocean farm is ambient water temperatures [19,22].
Surface ocean temperatures vary at a range of temporal scales, from sub-daily to seasonal to inter-annual scales; and, as a consequence, the growth of wild and farmed fish also varies. For example, El Niño is known to dramatically change the temperature profile of coastlines on both the western and eastern sides of the Pacific, with large consequences for fish population growth and wild capture fisheries landings [23]. In terms of climate change, the oceans are expected to warm in the coming decades as carbon emissions continue, following the A1B and representative concentration pathway +8.5 W m−2 scenarios (from the IPCC assessment report 4 and 5 respectively). Model projections suggest that global average surface warming will be in the range of 2–5°C by the end of the century [24]. Further, warming will be heterogeneous across space, with some locations warming more than others. Advances in model resolution have shed light on how coastal zones will change under high-carbon climate change scenarios [25]. New high-resolution models have identified that coarse-scale global models, previously used in the IPCC assessment reports 4 and 5 for example, underestimate the rate at which coastal areas will warm. For example, warming in the Northwest Atlantic, in high-resolution models, is twice that of the course global models. This warming is likely to impact the location of fish stocks (e.g. [26]), as well as the productivity of aquaculture operations.
Faced with increasing or decreasing ocean temperatures over the lifespan of a commercial operation, aquaculturists have several choices. Operations can be moved to a more favourable environment, although this can be economically costly, logistically difficult and politically challenging [14]. Another option is to culture a different species whose TPC for growth more closely aligns with the new environmental conditions. Switching species can require developing new expertise among staff, additional permitting approval and fees, and access to viable broodstock [27]. Finally, farmers can employ selective breeding programmes aimed at altering the TPC for growth of the culture species, in an attempt to overcome impacts of climate change in the operational area. All of these adaptations rely on knowing how temperatures and fish growth will change in the future.
Here, we build upon previous global open-ocean aquaculture studies (e.g. [14,28]) to quantify the dynamic growth potential of open-ocean finfish aquaculture, accounting for changes in ocean temperature and currents driven by a business-as-usual carbon emissions scenario. Oceanic changes are assessed by means of modelled data produced from an Earth System Model with high spatial resolution, allowing us to capture projected changes in both coastal and pelagic zones. Specifically, we focus on three commonly farmed marine finfish species—Atlantic salmon (Salmo salar), gilthead seabream (Sparus aurata) and cobia (Rachycentron canadum)—each varying in the shape and position of their TPCs for growth.
2. Methods
We use simple models of individual growth for three finfish species, each selected for their varying TPCs for growth, thermal guild, availability of temperature-dependent growth data, and role in current aquaculture production: (i) Atlantic salmon was selected as a well-studied, subpolar-temperate fish with a large role in the aquaculture sector; (ii) gilthead seabream was selected as a well-studied, temperate-subtropical fish with a moderate role in aquaculture; and (iii) cobia was selected as a well-studied, subtropical-tropical fish with an emerging role in the aquaculture sector. In 2013, Atlantic salmon, gilthead seabream and cobia made up 31.4%, 2.6% and 0.7%, respectively, of all brackish and marine finfish aquaculture production by tonnage [29].
(a). Modelling the growth of individual fish
For each species—salmon, seabream and cobia—the somatic growth of an individual fish (G; kg per month) is modelled as a temperature-dependent piecewise linear function:
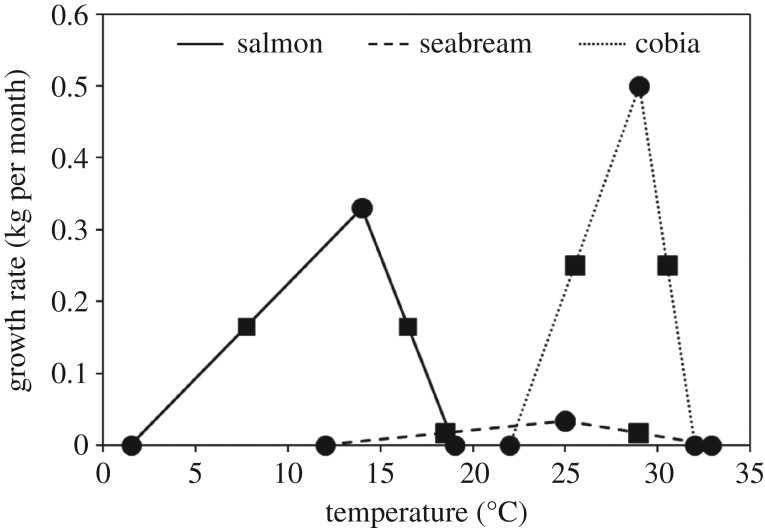
where T is sea surface temperature in °C, To is the optimal temperature for growth in °C, and a1 and a2 are slope parameters (kg per month per °C), and b1, and b2 are intercept parameters (kg per month). At temperatures below the minimum temperature for growth (Tmin, b1/a1) and above the maximum temperature for growth (Tmax, −b2/a2), G is forced to 0 to reflect the absence of growth. Where a2 is negative, To = (b1 + b2)/(a1 − a2). Values of these constants were determined based on temperature and growth studies from industry and academic literature (figure 1; electronic supplementary material, tables S1 and S2). Although TPCs are often more accurately described by nonlinear functions, for example exponentially modified Gaussian distributions [30], there are insufficient data to parameterize nonlinear performance curves across the grow-out period for most aquaculture-relevant species. As a result, we use a simplified piecewise linear function as it captures the main qualitative features of most thermal performance curves—an optimal growth temperature and two extremes, a minimum and maximum, where growth stops. Our thermal performance curves, while highly simplified, capture the main bounds to growth found in other studies [31]. Further, we constrain our analysis to only consider growth within the top 25% of each thermal performance curve, therefore avoiding sub-optimal growth and lethal temperature thresholds.
Figure 1.
Thermal performance curves (TPCs) for growth for three commercially important aquaculture species: Atlantic salmon, gilthead seabream and cobia. Circles indicate the minimum, optimal, and maximum temperatures and growth rate for each species, and squares indicate the minimum temperatures and growth rates for each species that are within the top 25% of the TPC.
(b). Modelled global ocean data
Present and future global sea-surface temperatures and horizontal ocean current data-fields were obtained from the Geophysical Fluid Dynamics Laboratory's Earth System Model CM2.6. The model runs with a grid spacing varying from 11 km at the equator to less than 4 km at high latitudes, and, as a consequence, simulates a highly realistic distribution of ocean features, from eddy-kinetic energy to regional-scale patterns of ocean currents and sea-surface temperature [32,33]. We obtained modelled sea-surface temperature and near-surface current speeds for the period 2016–2050 (electronic supplementary material, figure S1). CM2.6 projections were produced following a sensitivity experiment wherein carbon dioxide emissions were increased 1% per year until atmospheric concentrations were double that of preindustrial conditions. This scenario describes extremely fast warming, with atmospheric carbon dioxide concentrations doubling preindustrial conditions within 70 years. These data were obtained at a monthly resolution and used to force the biological growth model described above.
(c). Spatial constraints
Additional factors that can constrain open-ocean aquaculture production are included as static variables, where farming is presumed to not exist where the constraints are present. Physical constraints include unfavourably strong currents (more than 100 cm s−1 [28]) and depths beyond what is currently feasible for open-ocean operations (more than 2000 m). While areas with chronic or acute low current speeds can present operational challenges for marine aquaculture (e.g. poor nutrient dispersal, low dissolved oxygen levels and increased risks of disease outbreaks) [8,34], we do not constrain production by a minimum current due to emerging technological solutions (e.g. submergence, at-sea oxygenation and antifouling mesh) to overcome these challenges [35–38]. Additionally, past models have limited production to areas with depths of less than 50 m [28,39], but we use larger values due to the success of recent single-point mooring operations which can operate at greater depths [40]. Given the substantial investment required for development of open-ocean aquaculture enterprises, it is most likely that operations will be established in national waters, where there is likely to be less ambiguity concerning property rights and regulatory pathways [14]. As such, operations are also constrained to waters within national economic exclusion zones. Individual growth potential for each species is estimated and presented with and without spatial constraints.
(d). Adaptation measures
To investigate the possibility for adaptation to climate changes through selective breeding programmes, we calculate the changes in the shape of TPCs that would be required for each of the three species to maintain 2016 production levels through 2050. Two goals of selective breeding are explored. Where growth decreases due to unfavourable temperatures, farmers can select for increased growth rates across a range of temperatures or select for horizontally shifted TPCs, such that the optimal temperature for growth is higher or lower. The absolute change and rate of change that growth must increase or TPCs must shift (as determined by a linear regression of annual sums for each cell) are compared with the modifications that have been accomplished in historical selective breeding programmes [41,42].
3. Results
(a). Global growth potential for open-ocean aquaculture
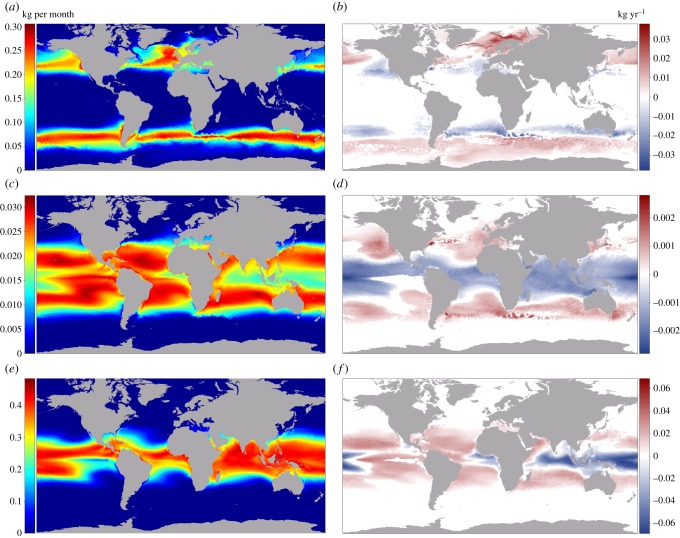
The three species in this study were chosen to represent three broad thermal zones. Salmon grow fastest in sub-polar and temperate waters, seabream in temperate and sub-tropical waters, and cobia in sub-tropical and tropical waters. Based on the average monthly growth potential between 2016 and 2020, the temperate species (seabream) has the greatest overall area available for farming in the top 25% of its TPC (195.6 million km−2 with no constraints and 19.8 million km−2 with all constraints applied), but has slower absolute growth than both the tropical (cobia) and sub-polar (salmon) species (figure 2a,c,e). Cobia has the second largest area (112.9 million km−2 with no constraints and 15.1 km−2 with all constraints), followed by salmon (50.1 million km−2 with no constraints and 5.6 million km−2 with all constraints; figure 2c,e). For all species, depth is the greatest constraint, followed by area within EEZs and areas of suitable currents (electronic supplementary material, figures S2–S4). Comparison of the top 25% of the TPCs in 5 year averages between 2046–2050 and 2016–2020 reveals only minor changes in global area available for fish growth (figure 3a–c). Unconstrained and fully constrained seabream area contracted (17.7 million km−2 or 9.1% and 3.0 million km−2 or 15.3%, respectively), while unconstrained and fully constrained cobia area expanded (10.8 million km−2 or 9.5% and 0.6 million km−2 or 4.3%, respectively). The suitable area of unconstrained salmon decreased (1.1 million km−2 or 2.2%), while fully constrained salmon area increased (0.3 million km−2 or 5.6%).
Figure 2.
Unconstrained mean monthly growth between 2016 and 2020 for (a) Atlantic salmon, (c) seabream and (e) cobia, and the linear annual rate of change in growth of (b) Atlantic salmon, (d) seabream and (f) cobia between 2016 and 2050. (Online version in colour.)
Figure 3.
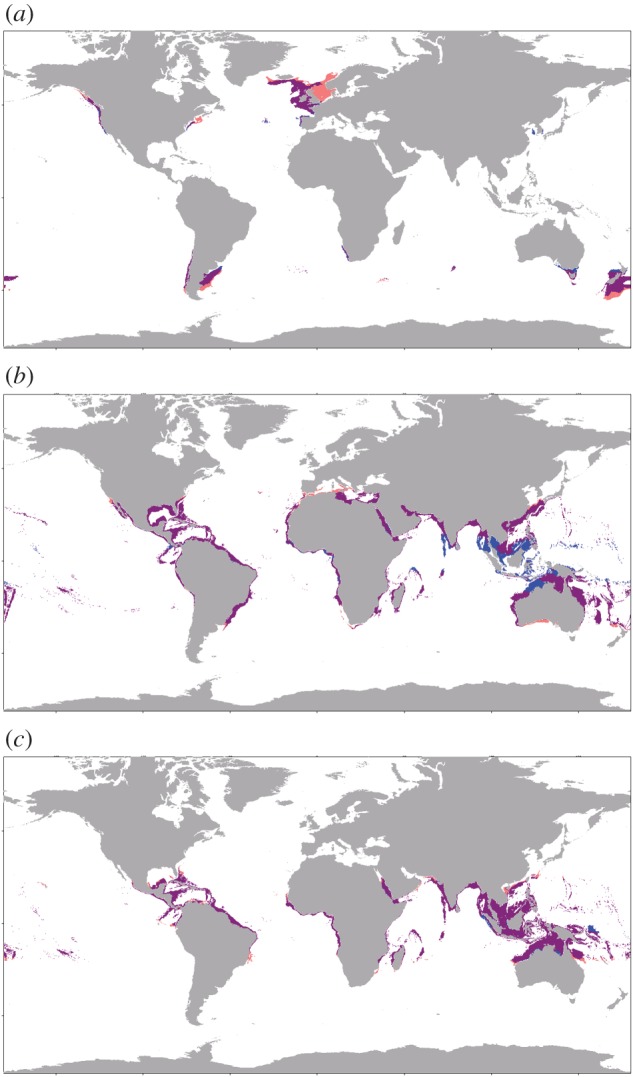
Constrained area available for growth within the top 25% of thermal performance curves for growth for (a) Atlantic salmon, (b) gilthead seabream and (c) cobia. The 2016–2020 mean (blue), 2046–2050 mean (red), and the overlap between the two time periods (purple), are constrained by depth, economic exclusion zones, and currents.
(b). Regional growth potential
At regional and national scales, there is substantial variability in growth potential and expected change over the 35 year time period, with locations seeing no change, increases, or decreases in the growth potential of each species. For example, the temperature increase along the western coast of the United States decreases the potential growth of salmon, increases seabream growth, but remains mostly too cold for cobia (figure 2b,d,f). In the warmer waters of Southeast Asia, increased water temperatures remain too warm for salmon, exceed the optimal for seabream and inhibit growth, and increase cobia growth (figure 2b,d,f). Countries near thermal gradients or with large EEZs, such as Australia, may also experience both decreases and increases of growth potential for individual species. For example, salmon growth rates decrease north of Tasmania in southeastern Australia but increase in offshore areas south of Tasmania. Seabream growth rates decrease off the northern half of the country and increase in the south. And cobia growth rates decrease in the north and increase off both central coasts (figure 2b,d,f). Other areas, such as central western Africa, may see decreased growth as water temperatures become too warm for salmon, seabream and cobia (figure 2b,d,f).
(c). Adaptation through selective breeding
The expected annual changes in individual fish growth in each location, as determined by a linear regression of annual production over the 35 year time horizon, are minimal. The means ± standard deviations for salmon, seabream and cobia, when all constraints are applied and where the p-value of the linear regression is <0.05, are 4 ± 7 g yr−1, 0.06 ± 0.4 g yr−1 and −0.08 ± 7 g yr−1, respectively. The minimum slope is −28 g yr−1 for salmon, −3 g yr−1 for seabream and −70 g yr−1 for cobia, indicating selective breeding programmes must alter growth at these rates to maintain productivity in the most extreme instances of climate change induced temperature changes. The maximum rate of increase was 38 g yr−1 for salmon, 3 g yr−1 for seabream and 40 g yr−1 for cobia. Over the 35 year period in areas at the extreme minimum slope, total annual growth would need to increase by 1.0 kg (25%) for salmon, 0.1 kg (25%) for seabream and 2.5 kg (41%) for cobia to compensate for climate-induced temperature changes.
4. Discussion
This analysis of growth potential for three species of finfish indicates that there are substantial areas that are physically suitable for fish growth and potential expansion of the nascent open-ocean aquaculture industry. Similar to previous analyses of open-ocean farming development, potential farm area is highly constrained by depth, currents, and area within economic exclusions zones [28]. Areas suitable for growth are globally distributed, meaning that, from a biological perspective, there are likely to be opportunities for open-ocean aquaculture development in a diversity of regions and countries. Operations could be sited in developing countries to increase food security through income generation or increased access to seafood or in developed countries to reduce seafood trade deficits [43,44]. Operations could also be sited near end markets to reduce costs and greenhouse gas emissions associated with shipping fresh seafood [45].
Areas of growth potential exhibit a shift away from the equator and towards the poles as ambient water temperatures increase as a result of climate change, similar to observations and models of range shifts in wild fish habitats [46]. In general, as ocean temperatures increase, the equatorial margins of a species growth area become too warm and growth performance is reduced, while polar margins generally see increased growth. For salmon and cobia, the total constrained area in the top 25% of their TPCs expands, indicating that the temperature increase at the polar margins creates more favourable growth area than temperature increases at the equatorial margins (figure 3a,c). Total constrained seabream area contracts, indicating that temperatures near the equator get too warm for seabream by a greater amount relative to the expansion of the species' farmed range at high latitudes (figure 3b).
Some current aquaculture locations are sub-optimal from a biological perspective (i.e. outside the top 25% of the TPC), but growth rates will increase as temperatures increase in those areas. For example, year-round water temperatures off Chile are closer to the thermal optimum for Atlantic salmon than waters off Norway, which have large seasonal temperature fluctuations. As a result, the model predicts greater annual growth potential in Chile than Norway, which aligns with current production reports where grow-out time is shorter in Chile than Norway [47]. Salmon growth potential is expected to increase in both areas due to climate change (figure 4a), which could further incentivize expansion of open-ocean salmon aquaculture. Similarly, there is substantial seabream aquaculture in the Mediterranean Sea and growth potential is expected to increase (figure 4b). Cobia production is currently dominated by China, and growth potential is expected to increase (figure 4c). Open-ocean aquaculture operations are sited based on numerous factors, not simply biological efficiency. If production remains geographically concentrated in its current location, growth is expected to increase slightly or remain the same across the EEZs of the top 4 producing countries for each species, as the mean slope of the linear regression of annual growth rate in each country is positive (figure 4).
Figure 4.
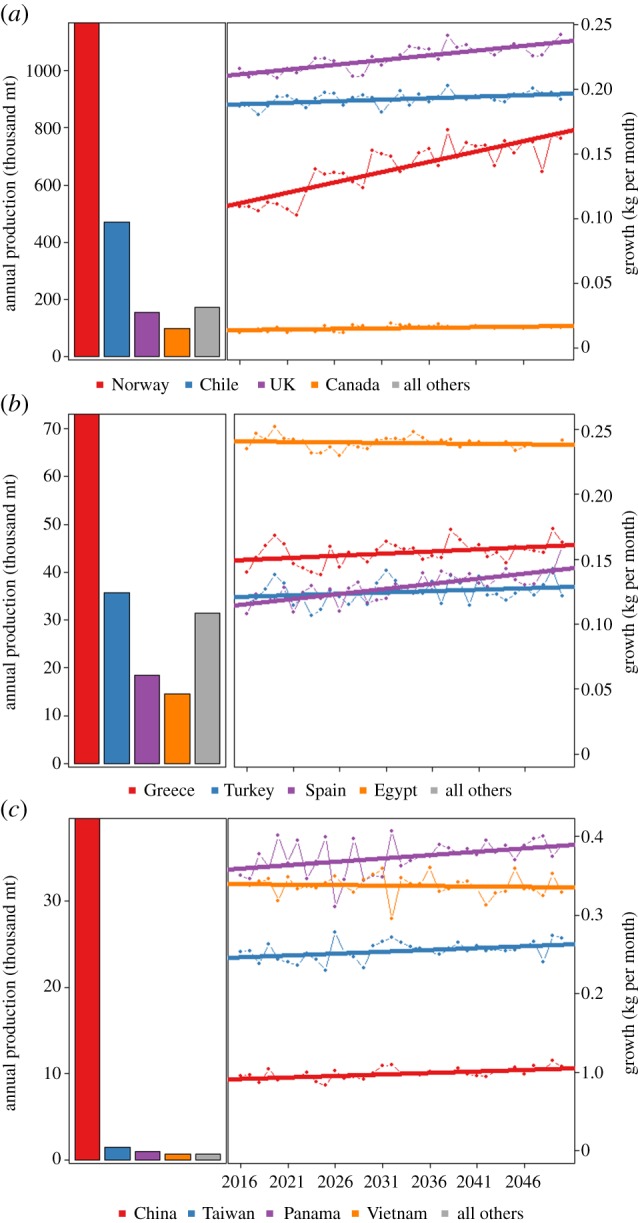
Annual aquaculture production of the top four largest producing countries in 2013 (left) and constrained average annual growth per month between 2016 and 2050 within each country's economic exclusions zone (right) for (a) Atlantic salmon, (b) seabream and (c) cobia. (Online version in colour.)
(a). Role of selective breeding
Selective breeding of culture organisms is a tool that aquaculturists can use to improve the profitability and resource efficiency of their operations [41,48]. Farmers can also use selective breeding to compensate for growth losses due to non-optimal temperatures, through selection for faster growth or horizontal shifts in TPCs.
Historically, most breeding programmes have selected for faster growth rates under a range of grow-out environments [41]. For some species, breeding programmes focused on increasing growth have altered both growth rates and total mass at the end of a grow-out cycle at levels beyond the maximum change to annual growth rates and total annual growth encountered over the 35 years evaluated in this study. For example, reviews of European breeding programmes found that selection for growth in Atlantic salmon increased harvest weight by 12% per generation and cumulative genetic gains in harvest weight over multiple generations of selection were around 200% [41,42]. Similarly, seabream harvest weight increased by 10–15% per generation with selection, resulting in a cumulative genetic gain in harvest weight over multiple generations of less than 100% [42]. Cobia would likely exhibit similar growth increases with selection, although results have not been reported. These values are greater than the 25–41% total increase in annual growth required to offset the most extreme climate-induced decreases and far exceed the means, indicating that selection for faster growth could offset the potential losses from climate change over the next 35 years.
In contrast to selection for faster growth across all temperatures, few breeding programmes have explicitly selected for shifts in the optimal temperature for growth or in the whole distribution of an organism's TPC, and evidence is mixed that future selection for horizontal shifts in TPCs alone will be adequate to adapt to changing ambient water temperatures. Genotype-by-environment interactions, where a genotype exhibits different growth performance in different environments (including different temperatures), can result in re-ranking in a breeding programme such that a selected individual or genotype performs well at one temperature but poorly in another [49]. For example, a review of genotype-by-environment interactions found that environment explained only a small portion of the variation in growth rates (e.g. 1–5%) when fish from different genotypes were exposed to a range of grow-out conditions [41], meaning there was little horizontal shift in TPCs. In contrast, another review of genotype-by-environment interactions found strong evidence of re-ranking in rainbow trout and seabream genotypes exposed to different temperatures, indicating that there may be strong genotype-by-environment interactions between growth and temperature and potential for horizontally shifting TPCs [49]. Further, studies of wild fish indicate that cold and warm adapted populations of the same species undergo both absolute changes in the growth rates (e.g. cold adapted populations with shorter growing seasons grow faster at all temperatures) and horizontal shifts in TPCs for growth [50].
Additional studies are required to better understand the capacity and rate of aquaculture-relevant species to horizontally shift TPCs for growth. Commercial selective breeding programmes often strive to improve fish performance across a range of environments while using juveniles from the same lineage to reduce costs [49,51]. Consequently, breeding programmes should be reevaluated to the extent that the benefits of employing environment specific breeding programmes (and the resulting horizontal shifts in TPCs for growth) are balanced against the added programmatic costs [52].
Despite the potential advantages of selective breeding, only 10% of global aquaculture is based on selectively bred stocks [51]. The use of selectively bred fish can pose risks to wild populations and ecosystems [53]. Cultured fish frequently escape from grow-out operations [54] and can interbreed with wild fish of the same species. Interbreeding can lead to reduced genetic variability and outbreeding depression in wild populations [55]. In some cases, governments have restricted the use of selectively bred fish out of concern for nearby wild ecosystems [56]. The production benefits of selective breeding should be evaluated against the potential ecological costs of escapes. In addition, there are limits to gains that can be achieved with selective breeding. Further studies are required to determine the limits to growth increases or modifications of TPCs, especially for species that have already undergone extensive selection for growth (e.g. Atlantic salmon). Genetic modification could also be employed to modify TPCs [57]. It is also important to consider that aquaculture enterprises in some countries may not have access to the capital required to develop effective selective breeding programmes, and extension and financial assistance may be necessary for aquaculturists to successfully adapt to changing environments.
(b). Data limitations
Temperature-dependent growth data are limited for most fish, and in order to project changes in other farmed species, further research is required to establish their TPCs for growth. See electronic supplementary material, §1.0 for additional discussion of data limitations on TPCs and physical oceanography and suggestions for improving future models.
(c). Estimating production in a dynamic industry and environment
This analysis of global growth potential with climate change is an important step towards understanding the likely potential of open-ocean aquaculture to help meet increasing demand for seafood now and in the future. Importantly, actual production is likely further constrained by additional physical, economic, regulatory, ecological and social factors (table 1). For example, production may be constrained by the scarcity and cost of feeds for culture organisms, fuel to service operations, and specialized equipment to build and maintain facilitates. The environmental impacts of increasing the scale and density of production in the ocean is uncertain, meaning the level of production that can be achieved without negative environmental impacts is unclear [11]. The permitting and regulation of open-ocean aquaculture at the national and international level is also likely to limit production potential, as rules are established to help internalize social costs that are external to the market (e.g. environmental impacts, restrictions on fishing and other uses, and navigation hazards) [14]. Production may be enhanced by synergistic factors such as co-development with other open-ocean industries (e.g. offshore wind structures, [58,59]) or development on existing offshore structures (e.g. mothballed oil platforms, [60]). Additional data and modelling efforts that incorporate robust economic and political factors would help determine the regional and global production potential of open-ocean aquaculture.
Table 1.
Factors that can affect siting and productivity of open-ocean finfish aquaculture operations.
| physical factors | references |
|---|---|
| temperature | [5,67] |
| dissolved oxygen | [5,67] |
| salinity | [5,67] |
| currents | [5,67] |
| wave climate | [5] |
| seabed characteristics | [5] |
| proximity to infrastructure (e.g. ports) | [5] |
| economic factors | |
| input costs (e.g. feed, fuel, labour) | [68] |
| output price | [68] |
| regulatory factors | |
| permitting | [10] |
| conflict with other stakeholders | [10] |
| marine spatial planning | [12,69] |
| ecological factors | |
| disease | [7] |
| harmful algal blooms | [5] |
| predators | [6] |
| areas with sensitive or vulnerable ecosystems | [6] |
| areas of ecological significance | [6] |
| social factors | |
| social licence to operate | [10,70] |
Global climate change can also result in secondary changes to the culture environment that can affect growth and production. Climate change has been linked to changes in the supply of feed inputs to aquaculture such as fishmeal, fish oil, and terrestrially derived ingredients and increased rates of eutrophication, harmful algal blooms, storminess, acidification, and disease [61,62]. Changing ambient water temperatures can result in the spread of existing pathogens, the increased virulence of existing pathogens, and the emergence of novel pathogens, all of which can reduce growth and diminish farm productivity [62,63]. Diseases play a large role in determining the profitability of aquaculture enterprises and the suitability of farming areas [64], but the signal and strength of these interactions are difficult to predict and incorporate into growth models [65]. Temperature is also linked to dissolved oxygen concentrations, and increases in temperature could also result in some areas being oxygen limited for certain species [66]. An improved understanding of the relationship between secondary functions associated with climate change and fish growth would improve estimates of growth potential and production risks.
5. Conclusion
Current and future ocean conditions will probably play a large role in determining which locations and species are used by the expanding open-ocean aquaculture sector, as ambient water temperature is a primary determinant of growth rates of ectothermic fish and therefore overall production efficiency of farms. This analysis shows that opportunities for open-ocean aquaculture are widely distributed and that although climate change may alter growth potential in many areas, adaptation measures, such as the use of selective breeding programmes, are probably adequate in the short term.
While these estimates of growth rates in open-ocean aquaculture are an important first step, future estimates of production potential and local, regional and global trade-offs and synergies could help aquaculture enterprises and managers site and develop open-ocean aquaculture in a manner that produces more seafood with fewer environmental impacts. This effort would be enhanced by an improved understanding of the relationship between growth and temperature (e.g. analyses based on population-specific TPCs) and other environmental factors in an open-ocean aquaculture context. Further, this effort reinforces that biophysical spatial models can be a powerful tool for understanding and optimizing the development of natural-resource based economic sectors in the face of current and future environmental conditions.
Supplementary Material
Data accessibility
The underlying dataset used in this study is the United States Department of Commerce, National Oceanic and Atmospheric Administration, Geophysical Fluid Dynamics Laboratory's (GFDL) Earth System Model CM2.6. Contact information at GFDL to obtain the large dataset is available here: https://www.gfdl.noaa.gov/high-resolution-climate-modeling/.
Authors' contributions
D.H.K., S.A.L. and J.R.W. conceived of the study, designed the study, coordinated the study, and helped draft the manuscript.
Competing interests
We have no competing interests.
Funding
This work is a deliverable of the project Green Growth Based on Marine Resources: Ecological and Socio-Economic Constraints (GreenMAR), which is funded by Nordforsk. We would also like to acknowledge the National Science Foundation grants nos. GEO-1211972 and OCE-1426746.
References
- 1.OECD-FAO. 2015. OECD-FAO agricultural outline 2015–2024. Paris, France: OECD Publishing. [Google Scholar]
- 2.World Bank. 2013. Fish to 2030: prospect for fisheries and aquaculture Washington, DC: World Bank. [Google Scholar]
- 3.FAO. 2014. The state of the world fisheries and aquaculture 2014. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- 4.Bostock J, et al. 2010. Aquaculture: global status and trends. Phil. Trans. R. Soc. B 365, 2897–2912. ( 10.1098/rstb.2010.0170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langan R. 2012. Ocean cage culture. In Aquaculture production systems (ed. Tidwell J.), pp. 135–157. New York, NY: John Wiley & Sons. [Google Scholar]
- 6.Dempster T, Sanchez-Jerez P.. 2008. Aquaculture and coastal space management in Europe: an ecological perspective. In Aquaculture in the ecosystem (ed. Holmer M.), pp. 87–117. Berlin, Germany: Springer. [Google Scholar]
- 7.Holmer M. 2010. Environmental issues of fish farming in offshore waters: perspectives, concerns and research needs. Aquac. Environ. Interact. 1, 57 ( 10.3354/aei00007) [DOI] [Google Scholar]
- 8.Froehlich HE, Smith A, Gentry RR, Halpern BS. 2017. Offshore aquaculture: I know it when I see it. Front. Mar. Sci. 4, 154 ( 10.3389/fmars.2017.00154) [DOI] [Google Scholar]
- 9.Wright J. 2011. Future lies with offshore aquaculture. Seafoodsource.com (4 October 2011).
- 10.Knapp G, Rubino MC. 2016. The political economics of marine aquaculture in the United States. Rev. Fish. Sci. Aquac. 24, 213–229. ( 10.1080/23308249.2015.1121202) [DOI] [Google Scholar]
- 11.Klinger DH, Naylor R. 2012. Searching for solutions in aquaculture: charting a sustainable course. Annu. Rev. Environ. Resour. 37, 247–276. ( 10.1146/annurev-environ-021111-161531) [DOI] [Google Scholar]
- 12.Gentry RR, Lester SE, Kappel CV, White C, Bell TW, Stevens J, Gaines SD. 2017. Offshore aquaculture: spatial planning principles for sustainable development. Ecol. Evol. 7, 733–743. ( 10.1002/ece3.2637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lekang O-I. 2013. Aquaculture engineering, 2nd edn Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 14.Lovatelli A, Aguilar-Manjarrez J, Soto D (eds). 2013. Expanding mariculture farther offshore: technical, environmental, spatial and governance challenges: FAO Technical Workshop, 22–25 March 2010, Orbetello, Italy.
- 15.Oppedal F, Dempster T, Stien LH. 2011. Environmental drivers of Atlantic salmon behaviour in sea-cages: a review. Aquaculture 311, 1–18. ( 10.1016/j.aquaculture.2010.11.020) [DOI] [Google Scholar]
- 16.Schulte PM. 2015. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 218, 1856–1866. ( 10.1242/jeb.118851) [DOI] [PubMed] [Google Scholar]
- 17.Dell AI, Pawar S, Savage VM. 2011. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl Acad. Sci. USA 108, 10 591–10 596. ( 10.1073/pnas.1015178108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Brett JR. 1979. Environmental factors and growth. In Fish physiology: bioenergetics and growth, vol. 8 (eds Hoar WS, Randall DJ, Brett JR), pp. 599–675. New York, NY: Academic Press. [Google Scholar]
- 20.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690–692. ( 10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 21.François NR Le, Lemieux H, Blier PU. 2002. Biological and technical evaluation of the potential of marine and anadromous fish species for cold water mariculture. Aquac. Res. 33, 95–108. ( 10.1046/j.1365-2109.2002.00652.x) [DOI] [Google Scholar]
- 22.Stickney RR. 1994. Principles of aquaculture. New York, NY: Wiley. [Google Scholar]
- 23.Ñiquen M, Bouchon M. 2004. Impact of El Niño events on pelagic fisheries in Peruvian waters. Deep Sea Res. Part II Top. Stud. Oceanogr. 51, 563–574. ( 10.1016/j.dsr2.2004.03.001) [DOI] [Google Scholar]
- 24.IPCC. 2014. Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds CB Field et al.). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 25.Saba VS, et al. 2016. Enhanced warming of the Northwest Atlantic Ocean under climate change. J. Geophys. Res. Oceans 121, 118–132. ( 10.1002/2015JC011346) [DOI] [Google Scholar]
- 26.Pinsky ML, Byler D. 2015. Fishing, fast growth and climate variability increase the risk of collapse. Proc. R. Soc. B 282, 20151053 ( 10.1098/rspb.2015.1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung P, Lee C-S, O'Bryen PJ (eds). 2007. Species and system selection for sustainable aquaculture. New York, NY: John Wiley & Sons. [Google Scholar]
- 28.Kapetsky JM, Aguilar-Manjarrez J, Jenness J. 2013. A global assessment of potential for offshore mariculture development from a spatial perspective Rome, Italy: FAO. [Google Scholar]
- 29.FAO. 2015. Fishstat J Rome, Italy: FAO. [Google Scholar]
- 30.Angilletta MJ. 2006. Estimating and comparing thermal performance curves. J. Therm. Biol. 31, 541–545. ( 10.1016/j.jtherbio.2006.06.002) [DOI] [Google Scholar]
- 31.Cheung WWL, Watson R, Pauly D. 2013. Signature of ocean warming in global fisheries catch. Nature 497, 365–368. ( 10.1038/nature12156) [DOI] [PubMed] [Google Scholar]
- 32.Delworth TL, et al. 2012. Simulated climate and climate change in the GFDL CM2.5 high-resolution coupled climate model. J. Clim. 25, 2755–2781. ( 10.1175/JCLI-D-11-00316.1) [DOI] [Google Scholar]
- 33.Winton M, Anderson WG, Delworth TL, Griffies SM, Hurlin WJ, Rosati A. 2014. Has coarse ocean resolution biased simulations of transient climate sensitivity? Geophys. Res. Lett. 41, 8522–8529. ( 10.1002/2014GL061523) [DOI] [Google Scholar]
- 34.Price C, Black KD, Hargrave BT, Morris JA Jr. 2015. Marine cage culture and the environment: effects on water quality and primary production. Aquac. Environ. Interact. 6, 151–174. ( 10.3354/aei00122) [DOI] [Google Scholar]
- 35.Bjelland HV, et al. 2015. Exposed aquaculture in Norway. In OCEANS 2015 MTS/IEEE Washington, 1–10. Piscataway, NJ: IEEE. [Google Scholar]
- 36.Endo A, Srithongouthai S, Nashiki H, Teshiba I, Iwasaki T, Hama D, Tsutsumi H. 2008. DO-increasing effects of a microscopic bubble generating system in a fish farm. Mar. Pollut. Bull.. 57, 78–85. ( 10.1016/j.marpolbul.2007.10.014) [DOI] [PubMed] [Google Scholar]
- 37.Dempster T, Korsøen Ø, Folkedal O, Juell J-E, Oppedal F. 2009. Submergence of Atlantic salmon (Salmo salar L.) in commercial scale sea-cages: a potential short-term solution to poor surface conditions. Aquaculture 288, 254–263. ( 10.1016/j.aquaculture.2008.12.003) [DOI] [Google Scholar]
- 38.Fitridge I, Dempster T, Guenther J, de Nys R. 2012. The impact and control of biofouling in marine aquaculture: a review. Biofouling 28, 649–669. ( 10.1080/08927014.2012.700478) [DOI] [PubMed] [Google Scholar]
- 39.Page S. 2012. Aquapod systems for sustainable ocean aquaculture. In Encyclopedia of sustainability science and technology (ed. Meters RA.), pp. 223–235. New York, NY: Springer. [Google Scholar]
- 40.Key G.2014. At the end of our rope: developing next-generation mariculture on a 4-km single point mooring. See www.was.org/meetings/ShowAbstract.aspx?Id=33364 .
- 41.Gjedrem T, Baranski M.. 2009. Selective breeding in aquaculture: an introduction. Berlin, Germany: Springer. [Google Scholar]
- 42.Janssen K, Chavanne H, Berentsen P, Komen H. 2016. Impact of selective breeding on European aquaculture. Aquaculture 472, 8–16. ( 10.1016/j.aquaculture.2016.03.012) [DOI] [Google Scholar]
- 43.Beveridge MCM, Thilsted SH, Phillips MJ, Metian M, Troell M, Hall SJ. 2013. Meeting the food and nutrition needs of the poor: the role of fish and the opportunities and challenges emerging from the rise of aquaculture. J. Fish Biol. 83, 1067–1084. ( 10.1111/jfb.12187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith MD, et al. 2010. Sustainability and global seafood. Science 327, 784–786. ( 10.1126/science.1185345) [DOI] [PubMed] [Google Scholar]
- 45.Ziegler F, Winther U, Hognes ES, Emanuelsson A, Sund V, Ellingsen H. 2013. The carbon footprint of Norwegian seafood products on the global seafood market. J. Ind. Ecol. 17, 103–116. ( 10.1111/j.1530-9290.2012.00485.x) [DOI] [Google Scholar]
- 46.Cheung WWL, Lam VWY, Sarmiento JL, Kearney K, Watson REG, Zeller D, Pauly D. 2010. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Change Biol. 16, 24–35. ( 10.1111/j.1365-2486.2009.01995.x) [DOI] [Google Scholar]
- 47.Marine Harvest. 2015. Salmon farming industry handbook 2015. Bergen, Norway: Marine Harvest. [Google Scholar]
- 48.Besson M, Aubin J, Komen H, Poelman M, Quillet E, Vandeputte M, van Arendonk JAM, de Boer IJM. 2016. Environmental impacts of genetic improvement of growth rate and feed conversion ratio in fish farming under rearing density and nitrogen output limitations. J. Clean. Prod. 116, 100–109. ( 10.1016/j.jclepro.2015.12.084) [DOI] [Google Scholar]
- 49.Sae-Lim P, Gjerde B, Nielsen HM, Mulder H, Kause A. 2015. A review of genotype-by-environment interaction and micro-environmental sensitivity in aquaculture species. Rev. Aquac. 7, 1–25. ( 10.1111/raq.12045) [DOI] [Google Scholar]
- 50.Yamahira K, Conover DO. 2002. Intra- vs. interspecific latitudinal variation in growth: adaptation to temperature or seasonality? Ecology 83, 1252–1262. ( 10.1890/0012-9658(2002)083%5B1252:IVILVI%5D2.0.CO;2) [DOI] [Google Scholar]
- 51.Gjedrem T, Robinson N, Rye M. 2012. The importance of selective breeding in aquaculture to meet future demands for animal protein: a review. Aquaculture 350–353, 117–129. ( 10.1016/j.aquaculture.2012.04.008) [DOI] [Google Scholar]
- 52.Ponzoni RW, Nguyen NH, Khaw HL, Ninh NH. 2008. Accounting for genotype by environment interaction in economic appraisal of genetic improvement programs in common carp Cyprinus carpio. Aquaculture 285, 47–55. ( 10.1016/j.aquaculture.2008.08.012) [DOI] [Google Scholar]
- 53.Lind CE, Ponzoni RW, Nguyen NH, Khaw HL. 2012. Selective breeding in fish and conservation of genetic resources for aquaculture. Reprod. Domest. Anim. 47, 255–263. ( 10.1111/j.1439-0531.2012.02084.x) [DOI] [PubMed] [Google Scholar]
- 54.Jensen Ø, Dempster T, Thorstad EB, Uglem I, Fredheim A. 2010. Escapes of fishes from Norwegian sea-cage aquaculture: causes, consequences and prevention. Aquac. Environ. Interact. 1, 71–83. ( 10.3354/aei00008) [DOI] [Google Scholar]
- 55.Hutchings JA, Fraser DJ. 2008. The nature of fisheries- and farming-induced evolution. Mol. Ecol. 17, 294–313. ( 10.1111/j.1365-294X.2007.03485.x) [DOI] [PubMed] [Google Scholar]
- 56.NOAA. 2016. Guidance and Procedures for Genetic Requirements for Gulf Aquaculture Permits. See http://seronmfsnoaagov/sustainable_fisheries/gulf_fisheries/aquaculture/documents/pdfs/genetic_requirements_guidancepdf.
- 57.Chen TT, Lin C-M, Chen MJ, Lo JH, Chiou PP, Gong H-Y, Wu J-L, Chen M H-C, Yarish C.. 2015. Transgenic technology in marine organisms. In Springer handbook of marine biotechnology (ed. Kim S-K.), pp. 387–412. Berlin, Germany: Springer. [Google Scholar]
- 58.Wever L, Krause G, Buck BH. 2015. Lessons from stakeholder dialogues on marine aquaculture in offshore wind farms: perceived potentials, constraints and research gaps. Mar. Policy 51, 251–259. ( 10.1016/j.marpol.2014.08.015) [DOI] [Google Scholar]
- 59.Buck BH, Krause G, Rosenthal H. 2004. Extensive open ocean aquaculture development within wind farms in Germany: the prospect of offshore co-management and legal constraints. Ocean Coast. Manag. 47, 95–122. ( 10.1016/j.ocecoaman.2004.04.002) [DOI] [Google Scholar]
- 60.Kaiser MJ, Yu Y, Snyder B. 2010. Economic feasibility of using offshore oil and gas structures in the Gulf of Mexico for platform-based aquaculture. Mar. Policy 34, 699–707. ( 10.1016/j.marpol.2010.01.002) [DOI] [Google Scholar]
- 61.Rosa R, Marques A, Nunes ML. 2012. Impact of climate change in Mediterranean aquaculture. Rev. Aquac. 4, 163–177. ( 10.1111/j.1753-5131.2012.01071.x) [DOI] [Google Scholar]
- 62.De Silva SS, Soto D. 2009. Climate change and aquaculture: potential impacts, adaptation and mitigation. In Climate change implications for fisheries and aquaculture: overview of current scientific knowledge (eds Cochrane K, Young CD, Soto D, Bahri T), pp. 151–212. Rome, Italy: FAO. [Google Scholar]
- 63.Weatherdon LV, Magnan AK, Rogers AD, Sumaila UR, Cheung WWL. 2016. Observed and projected impacts of climate change on marine fisheries, aquaculture, coastal tourism, and human health: an update. Front. Mar. Sci. 3, 473 ( 10.3389/fmars.2016.00048) [DOI] [Google Scholar]
- 64.Lafferty KD. et al. 2015. Infectious diseases affect marine fisheries and aquaculture economics. Annu. Rev. Mar. Sci. 7, 471–496. ( 10.1146/annurev-marine-010814-015646) [DOI] [PubMed] [Google Scholar]
- 65.Maynard J, et al. 2016. Improving marine disease surveillance through sea temperature monitoring, outlooks and projections. Phil. Trans. R. Soc. B 371, 20150208 ( 10.1098/rstb.2015.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pörtner HO, Peck MA. 2010. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J. Fish Biol. 77, 1745–1779. ( 10.1111/j.1095-8649.2010.02783.x) [DOI] [PubMed] [Google Scholar]
- 67.Beveridge MCM. 2004. Cage aquaculture. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 68.Knapp G. 2013. The development of offshore aquaculture: an economic perspective In Expanding mariculture farther offshore: technical, environmental, spatial and governance challenges – FAO Technical Workshop (eds A Lovatelli, J Aguilar-Manjarrez, D Soto), Orbetello, Italy, 22–25 March 2010, pp. 201–244. Rome, Italy: FAO. [Google Scholar]
- 69.Douvere F. 2008. The importance of marine spatial planning in advancing ecosystem-based sea use management. Mar. Policy 32, 762–771. ( 10.1016/j.marpol.2008.03.021) [DOI] [Google Scholar]
- 70.Leith P, Ogier E, Haward M. 2014. Science and social license: defining environmental sustainability of Atlantic salmon aquaculture in South-Eastern Tasmania, Australia. Soc. Epistemol. 28, 277–296. ( 10.1080/02691728.2014.922641) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The underlying dataset used in this study is the United States Department of Commerce, National Oceanic and Atmospheric Administration, Geophysical Fluid Dynamics Laboratory's (GFDL) Earth System Model CM2.6. Contact information at GFDL to obtain the large dataset is available here: https://www.gfdl.noaa.gov/high-resolution-climate-modeling/.