Abstract
Recently, growing evidence has revealed a significant association between Parkinson’s disease (PD) and cancer. However, controversy still exists concerning the association between PD and prostate cancer. A comprehensive article search for relevant published studies was performed using the online databases PubMed, Web of Science and Embase up to January 1, 2017. The pooled risk ratios (RRs) and their 95% confidence intervals (CIs) were calculated using the method of inverse variance with a random-effects model. Fifteen studies comprising 346,153 PD patients were included in this study. The results of the present study showed that PD was significantly associated with a decreased risk of prostate cancer in the Western population (RR: 0.83, 95% CI: 0.72–0.95, P < 0.01), while an increased risk of prostate cancer was shown in the Asian population (RR: 1.80, 95% CI: 1.52–2.13, P < 0.001). In the subgroup analysis, the reduced risk of prostate cancer in PD patients from Western populations was consistent regardless of study design or study quality. In conclusion, PD was significantly associated with a reduced risk of prostate cancer in the Western population. The relationship between those conditions in the Asian population needs to be confirmed by future studies.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the world, with a prevalence of 1% in the population over 60 years old1. Resting tremor, rigidity, hypokinesia, and postural instability are considered the four cardinal motor symptoms of PD, resulting from the loss of dopaminergic neurons in the substantia nigra pars compacta2. However, the pathogenesis of PD is not yet fully understood.
In recent years, accumulating epidemiological and clinical studies have reported the relationship between PD and cancer, lighting the way to explore the potential common pathogenic pathway involved in both diseases3. It has been noted that cancer rates are lower in patients with Parkinson’s disease than in the general population, and PD has different relationships with the risk of different cancers4. For instance, Wang et al.5 reported a lack of association between PD and prostate cancer in a previous meta-analysis. However, its conclusions were not very convincing owing to the relatively small sample size, limited number of studies and duplicate population of some included studies. Controversy still exists regarding the relationship between PD and prostate cancer. Lately, some articles have reported that patients with PD have a decreased risk of prostate cancer6,7, while some have found no significant negative association between them8,9, and some even hold the opposite opinion and indicate that an increased risk of prostate cancer could be observed among people with PD10. On the other hand, there is some potential evidence to link PD to prostate cancer. The most commonly used medications for Parkinson’s disease are levodopa, dopamine agonists and anticholinergics11, which all affect neurotransmitter activity, and it is possible that these treatments may affect tumor occurrence.
Indeed, differences in characteristics such as ethnicity, study design, PD diagnosis time and PD treatment have led to discrepancies in estimates of the association between Parkinson’s disease and risk of prostate cancer. Therefore, we conducted this meta-analysis to provide a quantitative assessment of current epidemiological evidence on the association between PD and risk of prostate cancer in different subgroups and explore the potential factors which can affect the association.
Results
Eligible studies
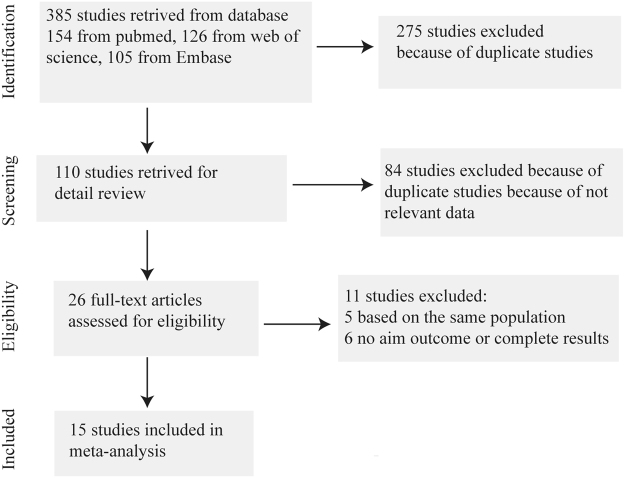
A total of 385 potentially relevant studies were identified in the database search. After exclusion of 275 duplicated studies, 110 studies remained. After title and abstract review, 84 studies were excluded. After full-text review of the remaining 26 studies, 5 duplicated studies that were performed in the same population and 6 studies without relevant outcomes of interest or without complete results were excluded. Ultimately, 15 studies were included in our meta-analysis6–10,12–21 (Fig. 1).
Figure 1.
Flow diagram of study selection procedure.
These studies were published between 2002 and 2016 and included 346,153 patients with PD. Among these 15 studies, 1 study were performed in an Asian population, while 14 studies were conducted in Western populations (6 in the USA, 3 in the UK, 1 in Canada, 2 in Denmark, 1 in Israel and 1 in Sweden). The baseline characteristics of each study are shown in Table 1.
Table 1.
Baseline characteristics of included studies in different populations.
| Author | Year | Country/Area | Study design | Number of case, source | Number of control, source | Tumor identification | Adjustment | OI | SQ | Ethnicity |
|---|---|---|---|---|---|---|---|---|---|---|
| Elbaz | 2002 | USA | Case-control | 196, Rochester Epidemiology Project | 196, general population | Before | Age, sex | OR | 7 | Western population |
| Guttman | 2004 | Canada | Cohort | 15306, OHIP, ODB RPDB | 30612, general population | After | Age, sex | RR | 6 | Western population |
| Powers | 2006 | USA | Case-control | 352, clinics of Group Health Cooperative | 484, enrollees of Group Health Cooperative | Before | Age, sex, year of enrollment, geographical location. | OR | 6 | Western population |
| Driver | 2007 | USA | Cohort | 572, Physician’s Health Study | 487, Physician’s Health Study | After | Age | RR | 6 | Western population |
| Fois | 2010 | UK | Cohort | 4355, UK National Health Service hospitals | NR, UK National Health Service hospitals | Before/after | Age, sex, calendar year of first recorded admission | RR | 7 | Western population |
| Lo | 2010 | USA | Cohort | 692, Kaiser Permanente Northern California Medical Care Plan | 761, Kaiser Permanente Northern California Medical Care Plan | Before/after | Age, sex, cigarette smoking, alcohol consumption, body mass index | RR | 7 | Western population |
| Becker | 2010 | UK | Cohort | 2993, UK-based General Practice Research Database | 3003, UK-based General Practice Research Database | After | Age, sex, general practice, diagnosis date, years of history | IRR | 6 | Western population |
| Rugbjerg | 2012 | Denmark | Cohort | 20343 Danish Hospital Register | NR, general population | After | Age, sex, calendar period | SIR | 7 | Western population |
| Wirdefeldt | 2013 | Sweden | Cohort | 11786, Swedish Patient Register | 58930, Swedish Patient Register | Before/after | Age. sex | HR | 7 | Western population |
| Ong | 2014 | UK | Cohort | 219194, English national Hospital Episode Statistics | 9015614, English national Hospital Episode Statistics | After | Age, sex, calendar year, region of residence, quintile of patients | RR | 8 | Western population |
| Lin | 2015 | Taiwan | Cohort | 62023, National Health Insurance | 124046, National Health Insurance | After | Age, sex | HR | 7 | Asian population |
| Peretz | 2016 | Israel | Cohort | 7125, Maccabi Health Services | NR, Maccabi Health Services | After | Age, sex, chronological year | SIR | 7 | Western population |
| Tacik | 2016 | USA | Case-control | 971, Mayo Clinic | 478, Mayo Clinic | Before | Age, sex | OR | 5 | Western population |
| Freedman | 2016 | USA | Case-control | NR, SEER-Medicare | NR, SEER-Medicare | After | Age, sex, selection year | OR | 5 | Western population |
| Jespersen | 2016 | Denmark | Case-control | 245, Danish Civil Registration System | 1656, Danish Civil Registration System | After | Age, sex. index date | OR | 7 | Western population |
HR: hazard ratio; IRR: incidence rate ratio; NR: not reported; ODB: Ontario Drug Benefit; OHIP: Ontario Health Insurance Plan; OR: odds ratio; OI: outcome of interest; RPDB: Registered Persons Database; RR: relative risk; SEER: Surveillance, Epidemiology, and End Results; SIR: standardized incidence ratio; SQ: score of study quality. Study quality was judged based on the Newcastle-Ottawa Scale.
PD and risk of prostate cancer
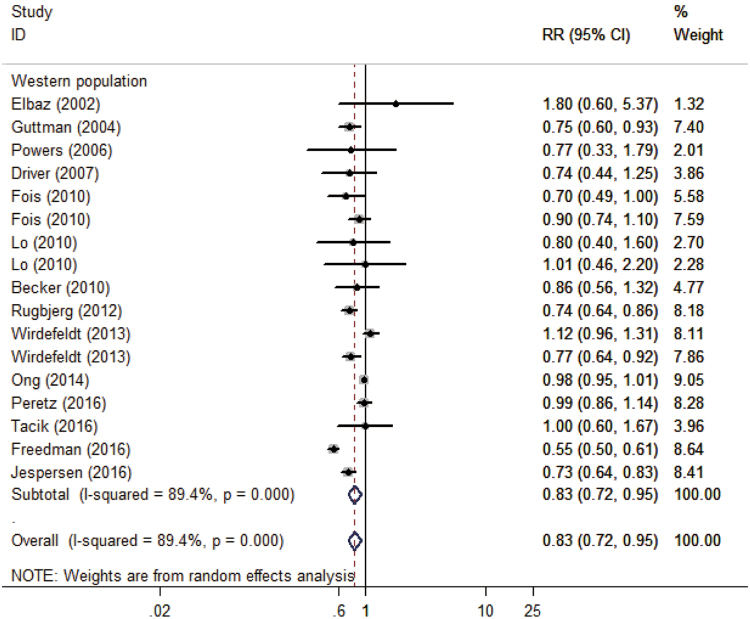
The result of 10 cohort studies and 5 case-control studies indicated that the pooled RR of prostate cancer in PD patients versus control patients was 0.88 (95% CI: 0.76–1.02, P = 0.082, I2 = 92%, Fig. 2), thus showing that PD patients had no significant risk of prostate cancer compared with the general population.
Figure 2.
Forest plot of risk ratio for the association between Parkinson’s disease and risk of prostate cancer in different populations. OR, odds ratio; CI, confidence interval.
In addition, we excluded one study that was conducted in an Asian population10 and performed further analyses in Western populations. The pooled result showed that PD was significantly associated with a decreased risk of prostate cancer in Western populations (RR: 0.83, 95% CI: 0.72–0.95, P < 0.01, I2 = 85%, Table 2). Furthermore, subgroup analyses revealed that the significance of the inverse association between PD and risk of prostate cancer in Western populations was not affected by study design and study quality (Table 2).
Table 2.
Subgroup analyses for association between Parkinson’s disease and the risk of prostate cancer in Western population.
| Categories | N | Pooled RR | 95% CI | P value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 (%) | P′ | |||||
| Overall effect | 14 | 0.83 | (0.72, 0.95) | 0.007 | 89.4% | <0.001 |
| Study design | ||||||
| Cohort | 9 | 0.88 | (0.80, 0.97) | 0.008 | 66.7% | <0.001 |
| Case-control | 5 | 0.73 | (0.56, 0.94) | 0.016 | 78.4% | <0.001 |
| Study quality | ||||||
| >6 | 8 | 0.88 | (0.78, 0.98) | 0.019 | 78.0% | <0.001 |
| <6 | 6 | 0.72 | (0.58, 0.91) | 0.005 | 64.9% | 0.014 |
| The diagnosis time of Prostate cancer | ||||||
| Before PD | 6 | 1.03 | (0.92, 1.16) | 0.624 | 0.0% | 0.506 |
| After PD | 11 | 0.77 | (0.65, 0.92) | 0.003 | 93.0% | <0.001 |
CI: confidence interval; N: number of studies; RR: risk ratio; PD: Parkinson’s disease. P’: p value of Q test for heterogeneity.
Furthermore, according to the diagnosis time of prostate cancer, we divided the studies into “before PD” and “after PD” groups to perform subgroup analysis in the Western population. The result indicated that a significant inverse relationship between PD and the risk of prostate cancer could be found in the “after PD group” (RR: 0.77, 95% CI: 0.65–0.92, P < 0.01), but it could not be found in the “before PD” group (RR: 1.03, 95% CI: 0.92–1.16, P = 0.62, Table 2).
Sensitivity analysis
In our meta-analysis, sensitivity analysis was used to assess the stability of the results. The significant inverse association between PD and risk of prostate cancer in the Western population did not change in the sensitivity analysis, which was conducted by removing each study in turn (Supplementary Figure S1).
Cumulative meta-analysis
We used cumulative meta-analysis to evaluate the association between PD and prostate cancer risk in relation to publication year. Our result indicated that from 2007 to present, the significant inverse relationship between PD and risk of prostate cancer in the Western population remained consistent (Supplementary Figure S2).
Publication bias analysis
In our meta-analysis, we used Begg’s and Egger’s tests to evaluate the effect of publication bias. There was no obvious evidence of publication bias as revealed by Begg’s funnel plots (Begg, P = 0.232, Supplementary Figure S3A). However, publication bias was detected by Egger’s regression test (Egger, P = 0.007, Supplementary Figure S3B).
Discussion
PD is characterized by the significant loss of dopaminergic neurons in the substantia nigra pars compacta and the presence of intraneuronal proteinaceous cytoplasmic inclusions termed Lewy bodies22, but the etiology of the disease is still poorly understood. Although PD and cancer seem to drive the cells to different outcomes, that is, either degeneration or overproliferation, the association between PD and cancer has been supported by plenty of epidemiologic studies, which have shown that the incidence rates of most cancers are lower in PD patients than in controls4,23.
In this study, the pooled result in all populations indicated that PD patients had no significant risk of developing prostate cancer. However, PD was significantly associated with a decreased risk of prostate cancer in the Western population, while an increased risk of prostate cancer was indicated in the Asian population. The significance of the inverse association between PD and risk of prostate cancer in the Western population was unaffected by the factors of study design and study quality. The results of sensitivity analysis and cumulative meta-analysis also confirmed the robustness of the relationship between them. On the other hand, the results from the Asian population showed almost the opposite findings compared with the studies performed in Western populations. This discrepancy may be explained by different genetic backgrounds24,25 and different environmental exposures26,27, which play important roles in a diverse array of disease pathogenetic processes. Furthermore, only one study10 was performed in an Asian population, and this seemed far more likely to represent the combined effect of publication and reporting bias than an actual underlying association in the Asian population. More studies are warranted to investigate the association between Parkinson’s disease and prostate cancer in the Asian population in the future.
Possible mechanisms of the significant negative association between PD and prostate cancer in the Western population are as follows. First, the different essential characteristics of these two diseases may be one explanation. PD is a neurodegenerative disorder characterized by dopaminergic neuronal death, whereas prostate cancer is a disease characterized by unlimited cell proliferation and lack of apoptosis. Cells in PD patients may be more likely to undergo apoptosis to fight against the progression of cancer9. The second explanation for this discrepancy could be consequences of the pharmacological treatment of Parkinson’s disease, which affects neurotransmitter activity. The main goal of medical treatment of Parkinson’s disease is to increase the amount of dopamine in the central nervous system; this dopamine can ultimately be converted to adrenalin, which stimulates the sympathetic nervous system28. Additionally, anticholinergic drugs, used in the treatment of Parkinson’s disease, can stimulate the parasympathetic nervous system29. Further research showed that stimulation of newly formed sympathetic nerves and parasympathetic nerve fibers in the autonomic nervous system can promote early stages of tumor genesis and then promote cancer dissemination30. Third, previous studies reported that smoking conferred a decreased risk of PD13 and a modestly increased risk of prostate cancer31. Thus, smoking may also partly explain the significant inverse association between PD and the risk of prostate cancer.
This study has several strengths. We involved more eligible evidences and carefully assessed the quality of evidence, which made the results much more reliable, and we also excluded duplicate studies that were based on the same population. Moreover, the results of this study in the Western population are robust, as shown by further subgroup analysis, sensitivity analysis and cumulative meta-analysis. Several limitations should be considered in the interpretation of our results. First, some of the included studies in our analysis were retrospective cohort studies or case-control studies. Second, subgroup analyses were not performed according to factors such as gender, age, smoking and alcohol consumption because insufficient data were extracted from the primary articles. Third, publication bias and other forms of bias may have existed in our results due to limitations in the inclusion criteria.
Methods
Literature search strategy
A comprehensive article search for relevant published studies was performed using the following online databases: PubMed, Web of Science and Embase.
The main search terms “(Parkinson* OR parkinson’s disease OR Parkinsons disease OR Parkinson disease) AND (prostate cancer or prostate cancers or prostate neoplasm or prostate neoplasms or prostate carcinoma or prostate carcinomas)” were used to search for relevant studies published up to January 1, 2017. Moreover, the references of relevant reviews and included articles were also carefully reviewed to identify additional studies that might be suitable for inclusion.
Study selection and data extraction
The eligible studies were included in our meta-analysis on the basis of the following criteria: (1) the study was a cohort and/or case-control study evaluating the relationship between PD and risk of prostate cancer; (2) an estimate of association [e.g., incidence rate ratio, odds ratio, risk ratio (RR), hazard ratio or standardized incidence ratio] with measures of variation (i.e., confidence intervals, CI) was provided; (3) the study was published in English. When duplicated studies (based on the same population32–36) were identified, only the most informative study was included10,20,21. Case reports and abstracts from meetings were excluded.
Data from each study were extracted independently by two authors (Chunli Chen and Haiping Zheng) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines37, and any disagreements were resolved by discussion or involvement of a third reviewer if necessary. The following data were extracted from each included study: the first author, population country, study design (cohort or case-control), publication year, patient information (i.e., sample size, source, age and sex), ethnicity, follow-up time in years and outcome of interest. The Newcastle-Ottawa scale (NOS) was used to assess the quality of the included studies38. In addition, studies with NOS scores >6 were considered high-quality studies (Table S1).
Statistical analysis
The pooled RRs and their 95% confidence intervals (CI) were calculated using the method of inverse variance with a random-effects model. Statistical heterogeneity was evaluated using Cochran’s Q test and I2 statistic39. Begg’s and Egger’s tests were used to evaluate the effect of publication bias40,41. Cumulative meta-analysis was used to assess the evolution of the combined RR in relation to the year of publication42. STATA software (version 13.0; Stata Corporation, College Station, TX, USA) was used to perform the data analyses in this study. A result was considered statistically significant when its P value was less than 0.05.
Conclusions
In summary, the result of this study indicated that PD was significantly associated with a reduced risk of prostate cancer in the Western population. Future studies are warranted to confirm the association between those two conditions in the Asian population.
Electronic supplementary material
Supplementary Information for Association between Parkinson's disease and risk of prostate cancer in different populations: An updated meta-analysis
Acknowledgements
This work was supported by funding from The National Natural Science Foundation of China (Grant nos. 81471335) and Hunan Provincial Innovation Foundation for Postgraduate (CX2017B070).
Author Contributions
Chunli Chen and Haiping Zheng wrote the main manuscript text and prepared Figs 1 and 2 and Tables 1 and 2. Zhiping Hu revised the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-13834-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. Jama. 2014;311:1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 2.Postuma RB, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 3.D’Amelio M, Ragonese P, Sconzo G, Aridon P, Savettieri G. Parkinson’s disease and cancer: insights for pathogenesis from epidemiology. Annals of the New York Academy of Sciences. 2009;1155:324–334. doi: 10.1111/j.1749-6632.2008.03681.x. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj A, Driver JA, Schernhammer ES. Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer causes & control: CCC. 2010;21:697–707. doi: 10.1007/s10552-009-9497-6. [DOI] [PubMed] [Google Scholar]
- 5.Wang T. The link between Parkinson’s disease and breast and prostate cancers: A meta-analysis. The International journal of neuroscience. 2015;125:895–903. doi: 10.3109/00207454.2014.986265. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DM, et al. Associations between cancer and Parkinson’s disease in U.S. elderly adults. International journal of epidemiology. 2016;45:741–751. doi: 10.1093/ije/dyw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jespersen CG, Norgaard M, Borre M. Parkinson’s disease and risk of prostate cancer: A Danish population-based case-control study, 1995-2010. Cancer epidemiology. 2016;45:157–161. doi: 10.1016/j.canep.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Peretz C, et al. Cancer incidence among Parkinson’s disease patients in a 10-yrs time-window around disease onset: A large-scale cohort study. Parkinsonism & related disorders. 2016;28:68–72. doi: 10.1016/j.parkreldis.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Ong EL, Goldacre R, Goldacre M. Differential risks of cancer types in people with Parkinson’s disease: a national record-linkage study. European journal of cancer (Oxford, England: 1990) 2014;50:2456–2462. doi: 10.1016/j.ejca.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Lin PY, et al. Association Between Parkinson Disease and Risk of Cancer in Taiwan. JAMA oncology. 2015;1:633–640. doi: 10.1001/jamaoncol.2015.1752. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira JJ, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. European journal of neurology. 2013;20:5–15. doi: 10.1111/j.1468-1331.2012.03866.x. [DOI] [PubMed] [Google Scholar]
- 12.Guttman M, Slaughter PM, Theriault ME, DeBoer DP, Naylor CD. Parkinsonism in Ontario: comorbidity associated with hospitalization in a large cohort. Movement disorders: official journal of the Movement Disorder Society. 2004;19:49–53. doi: 10.1002/mds.10648. [DOI] [PubMed] [Google Scholar]
- 13.Powers KM, et al. Diabetes, smoking, and other medical conditions in relation to Parkinson’s disease risk. Parkinsonism & related disorders. 2006;12:185–189. doi: 10.1016/j.parkreldis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Driver JA, Logroscino G, Buring JE, Gaziano JM, Kurth T. A prospective cohort study of cancer incidence following the diagnosis of Parkinson’s disease. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1260–1265. doi: 10.1158/1055-9965.EPI-07-0038. [DOI] [PubMed] [Google Scholar]
- 15.Fois AF, Wotton CJ, Yeates D, Turner MR, Goldacre MJ. Cancer in patients with motor neuron disease, multiple sclerosis and Parkinson’s disease: record linkage studies. Journal of neurology, neurosurgery, and psychiatry. 2010;81:215–221. doi: 10.1136/jnnp.2009.175463. [DOI] [PubMed] [Google Scholar]
- 16.Lo RY, et al. Comorbid cancer in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2010;25:1809–1817. doi: 10.1002/mds.23246. [DOI] [PubMed] [Google Scholar]
- 17.Becker C, Brobert GP, Johansson S, Jick SS, Meier CR. Cancer risk in association with Parkinson disease: a population-based study. Parkinsonism & related disorders. 2010;16:186–190. doi: 10.1016/j.parkreldis.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Wirdefeldt K, et al. Parkinson’s disease and cancer: A register-based family study. American journal of epidemiology. 2014;179:85–94. doi: 10.1093/aje/kwt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tacik P, et al. Cancer in Parkinson’s disease. Parkinsonism & related disorders. 2016;31:28–33. doi: 10.1016/j.parkreldis.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elbaz A, et al. Nonfatal cancer preceding Parkinson’s disease: a case-control study. Epidemiology (Cambridge, Mass.) 2002;13:157–164. doi: 10.1097/00001648-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Rugbjerg K, Friis S, Lassen CF, Ritz B, Olsen JH. Malignant melanoma, breast cancer and other cancers in patients with Parkinson’s disease. International journal of cancer. 2012;131:1904–1911. doi: 10.1002/ijc.27443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 23.Catala-Lopez F, et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychotherapy and psychosomatics. 2014;83:89–105. doi: 10.1159/000356498. [DOI] [PubMed] [Google Scholar]
- 24.Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harbor perspectives in medicine. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts SA, Gordenin DA. Hypermutation in human cancer genomes: footprints and mechanisms. Nature reviews. Cancer. 2014;14:786–800. doi: 10.1038/nrc3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frigerio R, et al. Chemical exposures and Parkinson’s disease: a population-based case-control study. Movement disorders: official journal of the Movement Disorder Society. 2006;21:1688–1692. doi: 10.1002/mds.21009. [DOI] [PubMed] [Google Scholar]
- 27.Barnhill LM, Bronstein JM. Pesticides and Parkinson’s disease: is it in your genes? Neurodegenerative disease management. 2014;4:197–200. doi: 10.2217/nmt.14.18. [DOI] [PubMed] [Google Scholar]
- 28.Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet (London, England) 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 29.Chang A, Fox SH. Psychosis in Parkinson’s Disease: Epidemiology, Pathophysiology, and Management. Drugs. 2016;76:1093–1118. doi: 10.1007/s40265-016-0600-5. [DOI] [PubMed] [Google Scholar]
- 30.Magnon C, et al. Autonomic nerve development contributes to prostate cancer progression. Science (New York, N.Y.) 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 31.Islami F, Moreira DM, Boffetta P, Freedland SJ. A systematic review and meta-analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies. European urology. 2014;66:1054–1064. doi: 10.1016/j.eururo.2014.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moller H, Mellemkjaer L, McLaughlin JK, Olsen JH. Occurrence of different cancers in patients with Parkinson’s disease. BMJ (Clinical research ed.) 1995;310:1500–1501. doi: 10.1136/bmj.310.6993.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen JH, Friis S, Frederiksen K. Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology (Cambridge, Mass.) 2006;17:582–587. doi: 10.1097/01.ede.0000229445.90471.5e. [DOI] [PubMed] [Google Scholar]
- 34.Elbaz A, et al. Risk of cancer after the diagnosis of Parkinson’s disease: a historical cohort study. Movement disorders: official journal of the Movement Disorder Society. 2005;20:719–725. doi: 10.1002/mds.20401. [DOI] [PubMed] [Google Scholar]
- 35.Sun LM, et al. Analysis of Parkinson’s disease and subsequent cancer risk in Taiwan: a nationwide population-based cohort study. Neuroepidemiology. 2011;37:114–119. doi: 10.1159/000331489. [DOI] [PubMed] [Google Scholar]
- 36.Olsen JH, et al. Atypical cancer pattern in patients with Parkinson’s disease. British journal of cancer. 2005;92:201–205. doi: 10.1038/sj.bjc.6602279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine151, 264–269, w264 (2009). [DOI] [PubMed]
- 38.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 42.Lau, J., Schmid, C. H. & Chalmers, T. C. Cumulative meta-analysis of clinical trials builds evidence for exemplary medical care. Journal of clinical epidemiology48, 45–57; discussion 59–60 (1995). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information for Association between Parkinson's disease and risk of prostate cancer in different populations: An updated meta-analysis