Abstract
People generally prefer rewards sooner rather than later. This phenomenon, temporal discounting, underlies many societal problems, including addiction and obesity. One way to reduce temporal discounting is to imagine positive future experiences. Since there is overlap in the neural circuitry associated with imagining future experiences and remembering past events, here we investigate whether recalling positive memories can also promote more patient choice. We found that participants were more patient after retrieving positive autobiographical memories, but not when they recalled negative memories. Moreover, individuals were more impulsive after imagining novel positive scenes that were not related to their memories, showing that positive imagery alone does not drive this effect. Activity in the striatum and temporo parietal junction during memory retrieval predicted more patient choice, suggesting that to the extent that memory recall is rewarding and involves perspective-taking, it influences decision-making. Furthermore, representational similarity in the ventromedial prefrontal cortex between memory recall and decision phases correlated with the behavioral effect across participants. Thus, we have identified a novel manipulation for reducing temporal discounting—remembering the positive past—and have begun to characterize the psychological and neural mechanisms behind it.
Keywords: temporal discounting, intertemporal choice, autobiographical memory, nostalgia, positive prospection
Introduction
We often face decisions in which we trade off smaller, immediate gains against larger long-term benefits (e.g. choosing between saving money and spending it). In these intertemporal choices (Strotz, 1956), individuals tend to prefer immediate rewards to delayed rewards, sometimes even if the delayed reward is larger (a tendency known as temporal discounting). While most people exhibit some temporal discounting, the rate at which people discount future rewards varies widely (Peters and Büchel, 2011). Excessive temporal discounting is evident in a range of psychiatric disorders, including addiction and ADHD (Reynolds, 2006; Demurie et al., 2012). The latest research shows that intertemporal choices can be manipulated in the laboratory (Lempert and Phelps, 2016), providing the opportunity to identify easily implementable interventions to reduce temporal discounting. Here we introduce a novel technique for reducing temporal discounting—remembering positive autobiographical memories—and explore its neural mechanisms.
The hypothesis that positive autobiographical memory retrieval reduces temporal discounting was inspired by research showing that positive prospection reduces temporal discounting. When people imagine specific positive future events during or prior to intertemporal decision-making, they make more patient choices (Peters and Büchel, 2010; Benoit et al., 2011). This effect of prospection is supported by the integration of signals from an episodic memory and prospection network (i.e. medial temporal lobe, precuneus, dorsomedial prefrontal cortex, etc.) and valuation network (e.g. ventromedial prefrontal cortex (vmPFC), striatum; Peters and Büchel, 2010; Benoit et al., 2011). There is some evidence that the ability to construct vivid and coherent future scenarios is related to the strength of this effect (Peters and Büchel, 2010). In particular, older adults and amnesic individuals (two populations marked by episodic memory decline) do not show an effect of prospection on temporal discounting (Palombo et al., 2015; Sasse et al., 2017), even though their temporal discounting rates are no different from those of healthy young adults (Samanez-Larkin et al., 2011; Kwan et al., 2012). Positive affect also appears to play a crucial role, since imagining negative future events (Liu et al., 2013) actually leads to increased temporal discounting. Given that autobiographical memory retrieval relies on the same neural circuitry as imagining the future, and simulating the future involves the flexible recombination of episodic memories (Schacter and Addis, 2007; Schacter et al., 2007), we expected that positive memory retrieval might also reduce temporal discounting. If recalling positive memories reduces discounting, this suggests that thinking positively about oneself in a different time is sufficient to promote patient choice. If not, then manipulations to reduce temporal discounting should be specifically tailored toward episodic thinking about the future.
In Experiment 1, participants recalled positive autobiographical memories prior to making intertemporal choices. We expected people to show reduced temporal discounting rates in trials that followed positive memory retrieval compared to control trials. To examine if this effect was specific for positive memories, we conducted Experiment 2, in which participants recalled negative past events before making intertemporal choices. Here we predicted that we would observe no difference in temporal discounting between negative memory and control conditions. Finally, since positive autobiographical memory retrieval has been shown to increase positive affect (Speer et al., 2014), we tested whether induction of positive affect by itself would also lead to more patient choice, or if the memory component was critical. In Experiment 3, participants imagined novel positive scenes that were unrelated to their memories before making intertemporal choices. We hypothesized that this manipulation would not lead to more patient choice.
Finally, in Experiment 4, participants performed a temporal discounting task with positive memory retrieval in the MRI scanner so we could explore neural mechanisms underlying the influence of memory retrieval on discounting. We predicted that, consistent with studies of prospection, activity in regions related to valuation and episodic retrieval would mediate the influence of memory retrieval on intertemporal choice. In addition, we investigated whether similarity in BOLD signal patterns between the time of memory recall and the time of choice in a region that is critical for valuation (vmPFC) would predict the extent to which participants became more patient following memory recall.
Materials and methods
Experiment 1
Participants
For general information about participants and eligibility criteria, see Supplementary Methods. Forty-six participants completed Experiment 1. One subject was excluded for being above the age cutoff, one was excluded because of a technical error, and nine were excluded because their discount rates could not be computed in one or both experimental conditions. Of these nine, four chose all delayed rewards, three chose all immediate rewards and two chose too inconsistently for a discount rate to be meaningfully fit. Thus, 35 participants were included in final analyses.
Procedure
Participants wrote about memories prompted by each of 30 life event cues (e.g. family vacation). The cues were a compilation of cues from prior studies (Sharot et al., 2007; Speer et al., 2014; see Supplementary Methods for cue lists), and were designed to probe for neutral or positive memories. For each cue, participants selected a memory in which they had been personally involved and that had occurred at a specific place and time. For each memory, participants reported a brief description, location and date. They also gave subjective ratings for valence (1 = neutral; 2 = positive), emotional intensity (1–4: 1 = not intense, 4 = very intense) and feeling (i.e. how they felt when recalling the memory; 1–4: 1 = neutral, 4 = very good). Participants were instructed to select memories that were positive (e.g. visiting Disneyland) or neutral (e.g. packing for a trip), but not negative (e.g. lost luggage).
In preparation for the second session, 10 of each participant’s positive memories were selected. These 10 had been rated as positive (i.e. valence = 2), and had the highest combined intensity and feeling ratings. They were summarized in subject-specific event cues that the participants reviewed at the beginning of the second session, to ensure that they could identify the memory associated with each cue.
Participants returned for the second session three days later to perform an intertemporal choice task. On each trial of this task, they were presented with a screen showing two options: ‘$10 today’ and a monetary reward of larger magnitude available after a delay (e.g. ‘$20 in 30 days’; amounts varied from $11 to $40; delays from 4 to 180 days. All delayed reward amounts were paired with all delays). They made a button press, indicating which option they preferred. The order of the trials was randomized, and the immediate and delayed reward options switched sides of the screen randomly. After participants responded, they were shown the option they had just chosen for 1 s. After a 2-s inter-trial interval, the next choice screen appeared. There were 60 distinct trial types, shown 2× for a total of 120 trials.
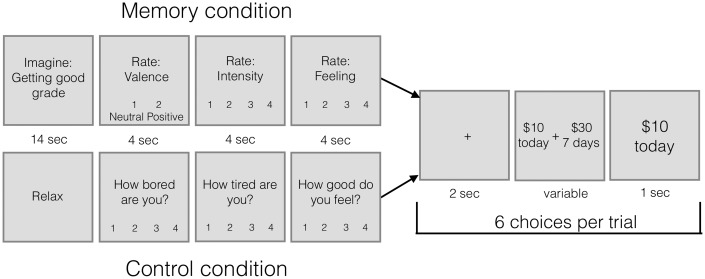
Participants made these choices in blocks (‘Memory’ and ‘Control’ blocks). In Memory blocks, participants re-accessed the ten positive memories triggered by cues from their questionnaire on Day 1 before making choices. At the beginning of each memory trial, a fixation point appeared for 3 s. Then, a memory cue was displayed for 14 s. Participants were asked to recall the memory described by this cue and to elaborate on it for as long as they could or until 14 s were up. After a 3-s inter-stimulus interval, participants rated the memory on valence, emotional intensity and feeling (allotted 4 s for each). Following this, participants made six intertemporal choices before the next memory cue appeared on the screen. One memory block consisted of 5 memories and 30 intertemporal choices (Figure 1).
Fig. 1.
Task layout for Experiment 1 (Positive memories). There were five memory trials per memory block, and five control trials per control block. Each trial contained six intertemporal choices. Each memory trial began with a memory cue, describing an autobiographical memory specific to the participant. The participant was asked to think about that positive memory for 14 s. Then they rated the valence (1 = neutral; 2 = positive), intensity (1–4; 1 = not intense; 4 = very intense) and feeling (1–4; 1 = neutral; 4 = very good) of the memory. Finally, they made six choices between $10 today and a larger amount of money in the future. The participant made a button press while the options were on the screen, and then was shown what they chose for 1 s before the next trial began. In the Control trials, participants were told to ‘Relax’ for 14 s and then to answer questions about how bored and tired they were, and how good they felt (1–4 scale for each). They then made the same intertemporal choices in this condition.
In control blocks, participants first saw the word ‘Relax’ on the screen for 14 s. They were instructed to rest during this time. Then, they rated how tired they were (1–4; 1 = very awake; 4 = very tired), how bored they were (1–4; 1 = not bored; 4 = very bored) and how good they felt (1–4; 1 = neither good nor bad; 4 = very good; 4 s for each rating). Following this, they made 6 intertemporal choices before the next ‘relax’ screen appeared. Each Control block consisted of 5 ‘relax’ screens and 30 intertemporal choices. There were two control blocks and two memory blocks, and the order was counterbalanced across subjects. The same choices were presented in both conditions.
Participants were told at the outset that one of the trials would be randomly selected and they would receive the amount they chose on that trial, at the delay specified. If they chose the immediate reward on that trial, they would receive the money in cash that day. If they chose the delayed reward, they would receive the money in their personal checking account via Paypal (www.paypal.com) after the delay had elapsed. This task was programmed using E-Prime 2.0 Stimulus Presentation Software (Psychology Software Tools).
See Supplementary Methods for full methodological details for Experiments 2, 3 and 4.
Analyses
Behavior
To quantify each individual’s temporal discounting rate, we fit their choices to the hyperbolic model (Green and Myerson, 2004; Kable and Glimcher, 2007) separately for choices in the Memory blocks (Experiments 1, 2 and 4; Imagination blocks for Experiment 3) and in the Control blocks. We determined the best-fitting discount parameter k in each condition:
Where SVdel is the subjective value of the delayed reward, A is the amount of the delayed reward, D is the delay and k is the parameter that represents the participant’s discount rate (higher k values correspond to more impatience). The discount rate parameters were log-transformed before statistical analyses were performed, since they are non-normally distributed. We conducted two-tailed paired t-tests to compare discount rates between conditions for each participant, for each experiment. For the replication experiments (Experiment 1 replication and Experiment 4), where there was a clear directional hypothesis, t-tests were one-tailed.
Whole-brain analyses (Experiment 4)
We constructed three general linear models (GLMs). In each, we convolved a boxcar (from stimulus onset to offset; 7 TR for Memory Recall and Control Cue and 3 TR for Memory and Control Choices) with a canonical HRF. Memory and Control ratings trials (8 TR each), choice trials with no response and six motion regressors were included as regressors of no interest.
In the first GLM, memory recall, control cue, memory choice (i.e. choices in memory blocks) and control choice (i.e. choices in control blocks) were the relevant regressors, and contrasts performed were memory recall > control cue, and memory choice > control choice.
In the second GLM, we investigated in which regions BOLD signal predicted the subjective value (SV) of the delayed reward on each trial, and if these regions differed in the memory and control conditions. Using discount rates fit separately to the control and memory choices, we computed the SV of the delayed reward for each trial for each subject, and parametrically modulated memory choice and control choice by these values. SVs were estimated by plugging the estimated discount rate k into the hyperbolic equation (Kable and Glimcher, 2007, 2010), and were z-scored within-subject. Contrasts performed were memory choice SV + control choice SV > baseline and memory choice SV > control choice SV. We also performed a conjunction analysis, in which we recovered only voxels that survived threshold (z > 2.3) in both control choice SV > baseline and memory choice SV > baseline contrasts. Finally, we performed the memory choice SV > control choice SV contrast with difference in discount rate between conditions as a between-subject covariate, to see if individual differences in SV coding between conditions at the time of choice could explain the extent of the behavioral effect.
In the third GLM, we were interested in which regions during the memory recall or control/rest phase predicted the size of the behavioral effect. Specifically, we calculated the extent to which the average SV of the delayed rewards following the cue differed from what would be expected if there were no effect of condition. First, we computed the SV of the delayed reward on each trial using the discount rate k derived from all choices in the experiment. Then for each trial, we subtracted this value from the SV computed using two different discount rates for the two conditions, yielding a ‘SV increase’ variable. We parametrically modulated the memory recall and control cue regressors by the average SV increase of the seven choices that followed each cue. The contrast of interest was memory recall SV increase + control cue SV increase > baseline. Note that the SV increase regressor is positive for memory trials and negative for control trials in individuals who showed the behavioral effect, and the opposite is true for participants who did not show the effect. Thus, this analysis reveals activity that accounts for individual differences in the size of the effect.
Representational similarity analysis in vmPFC (Experiment 4)
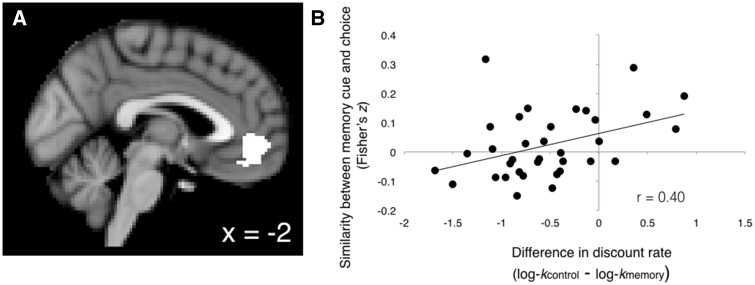
Since the vmPFC is critical for valuation in decision tasks (Kable and Glimcher, 2007, 2010; Bartra et al., 2013), we were particularly interested in how vmPFC activity when thinking about the past might relate to vmPFC activity during intertemporal choice. To this end, we conducted a representational similarity analysis (RSA) to compare activation patterns in vmPFC between the time when individuals were recalling memories and when they were making choices. We selected an independent vmPFC ROI from a quantitative meta-analysis of studies that report value-related neural signals during decision-making (Bartra et al., 2013). This ROI was defined from an analysis of 206 studies reporting SV effects (i.e., increased BOLD signal for increasingly valuable rewards; 609 voxels at 3 × 3 × 3 mm, centered on MNI coordinates x = −2, y = 40 and z = −8).
We extracted t-statistic maps for this ROI for the memory recall > baseline and memory choice > baseline contrasts from the first GLM for each participant. Then, for each participant, we ran a correlation across all voxels in this region to see how similar the pattern of activation was during the memory recall and memory choice phases. Correlation coefficients were transformed into Fisher’s z scores. We ran a correlation between Fisher’s z for each participant and their difference in discount rate between memory and control conditions: log(k)control – log(k)memory. We repeated this procedure for t-statistic maps from the control cue > baseline and control choice > baseline contrasts, to see if the effect was specific to the positive memory blocks. To test for spatial specificity of this effect, we conducted these analyses in four ROIs where activity should be unrelated to this task: two motor cortex ROIs (Right M1, Left M1) and two visual cortex ROIs (Right V1, Left V1). These were created based on the Juelich Histological Atlas in FSL. Only voxels that exceeded a 10% probability of being labeled as within those regions were included.
Results
Experiment 1: positive autobiographical memory retrieval reduces temporal discounting
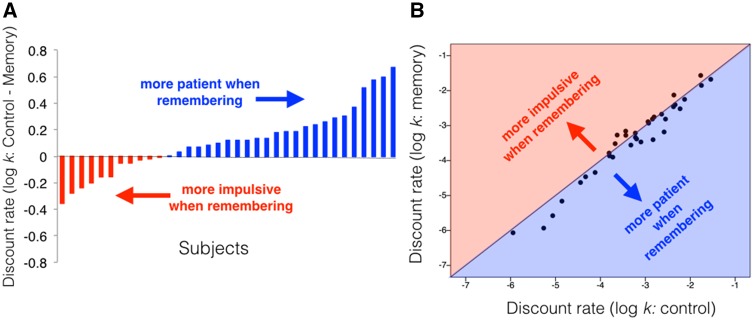
In Experiment 1, participants (N = 35; 22 F; mean age = 20.86; SD = 2.9) made a series of intertemporal choices either after retrieving positive autobiographical memories for 14 s (Memory blocks) or after relaxing for the same amount of time (Control blocks). Although they faced the same choices in the two conditions, they were significantly more patient in Memory blocks (t34 = 2.81; P = 0.008; Cohen’s d = 0.48; mean difference in log-k = 0.12; 95% CI = [0.03, 0.20]; Figure 2) than in Control blocks. Thus, positive autobiographical memory retrieval diminished impulsivity.
Fig. 2.
Positive memory retrieval reduces temporal discounting. (A) Difference between log-transformed discount rate in control condition and positive memory condition plotted for each subject. Positive difference (blue) indicates more patience in positive memory condition. Negative difference (red) indicates more impulsivity in positive memory condition. (B) Correlation between log-transformed discount rate in positive memory condition and control condition. Data points below the diagonal indicate more patience in the positive memory condition.
We selected only positive memories (i.e. valence rating of 2 on Day 1) for participants to remember during the choice task. Nevertheless, occasionally (∼6.3% of all memories), participants rated memories as neutral on Day 2. When we re-computed discount rates for all memory blocks excluding choice trials that followed memories marked as neutral, we obtained qualitatively similar results (t34 = 2.67; P = 0.01; Cohen’s d = 0.45; mean difference in log-k = 0.11; CI = [0.03, 0.19]). We replicated this result in an independent sample of participants (t34 =1.72; P = 0.047; Cohen’s d = 0.29; mean difference in log-k = 0.12; see Supplementary Results).
Experiment 2: negative memory retrieval does not influence intertemporal choice
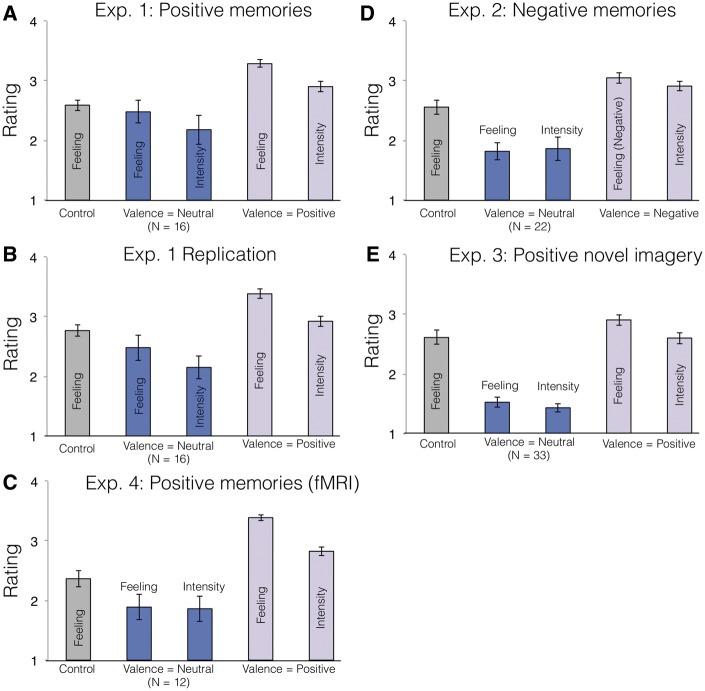
Consistent with past research (Speer et al., 2014), participants endorsed more positive feelings in memory blocks than in control blocks in Experiment 1 (t34 = 6.10; P < 0.001; Cohen’s d = 1.03; mean difference in rating = 0.61; CI = [0.41, 0.82]; Figure 3, Supplementary Table S1). To test whether positive affect was essential to our manipulation, or whether memory retrieval by itself would be sufficient, we conducted Experiment 2. In memory blocks of Experiment 2, participants recalled negative autobiographical memories prior to making intertemporal choices (N = 35; 24 F; mean age = 22; SD = 2.95). We found no significant differences between discount rate in memory blocks and in control blocks (t33 = −0.36; P = 0.73; Cohen’s d = 0.06; mean difference in log-k = −0.02; CI = [−0.13, 0.09]; Supplementary Fig. 4; Supplementary Results). We tested for an interaction between memory valence (positive/negative) and condition (memory/control) by collapsing across Experiments 1 and 2, and running a repeated-measures ANOVA with Experiment (1 or 2) as a between-subjects factor. The valence x condition interaction was significant (F(1,67) = 4.04; P = 0.049). The interaction remained significant when we included the replication of Experiment 1 in the ANOVA as well (F(1,102) = 3.99; P = 0.048). This suggests that retrieving autobiographical memories is insufficient to decrease discount rate, and that memories need to have a positive (or at least non-negative) valence.
Fig. 3.
Average feeling and intensity ratings across participants for (A) Exp. 1, (B) Exp. 1 replication, (C) Exp. 4, (D) Exp. 2 and (E) Exp. 3, separately for control blocks, and for memories rated as neutral (valence = 1) and positive (valence = 2) on Day 2. Not all participants labeled any memories as neutral; number of participants included in average for neutral memories is indicated.
Experiment 3: positive non-mnemonic imagery increases temporal discounting
Finally, from Experiments 1 and 2, it is unclear whether positive affect induced by mental imagery would lead to more patient decisions, or whether there is something unique about the retrieval of positive memories. To test this, in Experiment 3, participants imagined novel positive scenes before making intertemporal choices (N = 35; 28 F; mean age = 21.49; SD = 3.27).
We found no significant differences between discount rates in the imagination blocks and the control blocks (t34 = −1.18; P = 0.25; Cohen’s d = 0.20; mean difference in log-k = −0.06; CI = [−0.18, 0.05]). However, participants rated many novel scenes as neutral. Since our aim was to look specifically at positive imagery, we excluded all trials following the imagination of neutral scenes and re-computed discount rates. We excluded six subjects who had either no or too few (<12) positive trials for this analysis. After excluding trials following images rated as neutral, participants were significantly more impulsive in positive imagination blocks than in control blocks (t28 = −2.83; P = 0.009; Cohen’s d = 0.52; mean difference in log-k = −0.13; CI = [−0.23, −0.04]; Supplementary Figure S5).
As a manipulation check, we compared the ‘feeling’ ratings in the positive imagination blocks to those in the control blocks and found that participants felt significantly more positive in the imagination blocks than in the control blocks (t28 = 3.15; P = 0.004; Cohen’s d = 0.59; mean difference in rating = 0.34; CI = [0.12, 0.56]). Moreover, this difference was analogous to that in Experiment 1, when comparing positive memory blocks to control blocks (t62 = 1.87; P = 0.07; mean difference = 0.28; CI = [−0.01, 0.56]).
Experiment 4: neuroimaging study
Behavioral results
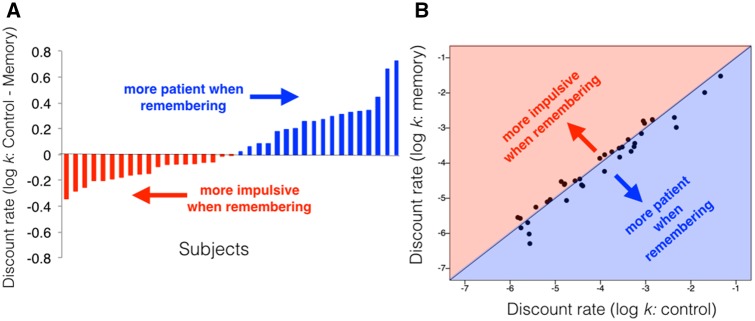
We were then interested in examining the neural mechanisms associated with reduced temporal discounting after positive memory recollection using fMRI. In Experiment 4, participants performed the same paradigm as in Experiment 1, but on Day 2, they did the choice task in the MRI scanner. Once again, participants were more patient in Memory blocks than in Control blocks, although this pattern was trending toward significance (t36 =1.54; P = 0.068; for positive memories only: t36 = 1.66; P = 0.053, 5.6% of memories dropped to neutral on Day 2; Figure 4). Because there were individual differences in the degree to which participants showed the positive memory effect (mean difference in log-k = 0.03; range = [−0.15, 0.32]), we leveraged such individual differences in our neuroimaging analyses.
Fig. 4.
Positive memory retrieval reduces temporal discounting (results from fMRI experiment). (A) Difference between log-transformed discount rate in control condition and positive memory condition plotted for each subject. Positive difference (blue) indicates more patience in positive memory condition. Negative difference (red) indicates more impulsivity in positive memory condition. (B) Correlation between log-transformed discount rate in positive memory condition and control condition. Data points below the diagonal indicate more patience in the positive memory condition.
Participants also endorsed having more positive feelings in memory blocks than in control blocks (t36 = 7.58; P < 0.001; Cohen’s d = 1.25; mean difference in rating = 0.94; CI: [−0.54, 2.42]). In an exploratory analysis collapsing across Experiment 1, the replication of Experiment 1 and Experiment 4, we found that average positive affect ratings across subjects did not predict temporal discounting, or the difference in temporal discounting between conditions (see Supplementary Results).
Neuroimaging results
In our first GLM, we modeled the onsets of memory recall, control cue, memory choice and control choice trials, to see which regions in the whole brain showed increased BOLD signal when participants recalled memories (memory recall) compared to when they rested for the same amount of time (control cue). This contrast revealed widespread activation in regions involved in memory retrieval and prospection (Supplementary Figure S6; Table 1), including medial prefrontal cortex, posterior cingulate cortex, angular gyrus, temporo-parietal junction, superior temporal sulcus and medial temporal lobe (hippocampus and amygdala). This suggests that participants were engaging in autobiographical memory retrieval when prompted to. No regions survived thresholding for the memory choice > control choice contrast.
Table 1.
Regions showing differences between memory recall and control cue phases
| X | Y | Z | z-stat | |
|---|---|---|---|---|
| Memory recall > control cue | ||||
| L Posterior cingulate cortex | −4 | −48 | 34 | 7.18 |
| −4 | −60 | 26 | 7.15 | |
| −6 | −56 | 14 | 7.04 | |
| −2 | −52 | 18 | 6.93 | |
| L Parietal lobe/angular gyrus | −44 | −68 | 36 | 7.03 |
| R Parietal lobe/angular gyrus | 50 | −72 | 34 | 5.28 |
| 48 | −64 | 30 | 4.75 | |
| 40 | −60 | 28 | 3.68 | |
| R Temporoparietal junction | 50 | −62 | 22 | 4.48 |
| R Medial temporal lobe | 66 | −44 | −10 | 3.69 |
| R Ventromedial prefrontal cortex | 2 | 60 | −2 | 6.8 |
| R Superior temporal sulcus | 54 | −44 | 2 | 3.36 |
| Control cue > memory recall | ||||
| R Postcentral gyrus | 62 | −28 | 36 | 4.66 |
| 58 | −30 | 42 | 4.36 | |
| 64 | −20 | 28 | 4.35 | |
| 56 | −36 | 36 | 4.14 | |
| 48 | −32 | 26 | 3.64 | |
| 58 | −12 | 12 | 3.04 | |
| L Postcentral gyrus | −62 | −30 | 18 | 4.3 |
| −66 | −32 | 30 | 4.09 | |
| L Supramarginal gyrus | −64 | −36 | 42 | 4.12 |
| −64 | −36 | 34 | 3.9 | |
| −58 | −30 | 42 | 3.71 | |
| −66 | −16 | 16 | 3.51 | |
| R Precuneus | 12 | −72 | 40 | 4.22 |
| 18 | −60 | 38 | 3.88 | |
| 18 | −64 | 38 | 3.84 | |
| 22 | −66 | 34 | 3.81 | |
| R Occipital lobe | 16 | −86 | 40 | 3.62 |
| 22 | −88 | 38 | 3.27 | |
| 54 | −74 | −2 | 3.52 | |
| 50 | −60 | −4 | 3.51 | |
| 52 | −70 | −2 | 3.36 | |
| 40 | −86 | 18 | 2.91 | |
| 48 | −74 | 8 | 2.91 | |
| R Fusiform gyrus | 44 | −60 | −4 | 3.09 |
Note: Table lists peak and local maxima. L, left; R, right.
We then examined where activity increased as a function of subjective value (SV) at the time of choice in both memory and control conditions. As expected, regions typically implicated in valuation (i.e. the ventromedial prefrontal cortex, ventral striatum and posterior cingulate cortex) tracked the SV of delayed rewards in both conditions (Table 2). Only one cluster in the ventral striatum, extending into the vmPFC, survived the more stringent SV conjunction analysis (Supplementary Figure S7; Table 2). No regions survived thresholding for the memory choice SV > control choice SV contrast. When entering difference in discount rate between conditions as a between-subjects covariate, no regions additionally accounted for differences in SV coding between the memory and control conditions.
Table 2.
Regions associated with subjective value at choice
| X | Y | Z | z-stat | |
|---|---|---|---|---|
| Memory choice SV + control choice SV | ||||
| L Caudate nucleus | −4 | 16 | 2 | 4.83 |
| R Caudate nucleus | 4 | 14 | 2 | 4.61 |
| R Ventral striatum/nucleus accumbens | 6 | 14 | −4 | 4.09 |
| R Medial prefrontal cortex | 20 | 30 | 2 | 4.1 |
| 20 | 34 | 10 | 3.95 | |
| L Medial prefrontal cortex | −8 | 54 | 0 | 4 |
| L Medial temporal lobe | −60 | −40 | −4 | 4.1 |
| −58 | −14 | −12 | 4.04 | |
| R Medial temporal lobe | 70 | −36 | −2 | 3.59 |
| L Temporo parietal junction | −56 | −54 | 20 | 3.75 |
| −58 | −62 | 20 | 3.9 | |
| L Superior temporal sulcus | −50 | −66 | 14 | 3.66 |
| R Superior temporal sulcus | 64 | −38 | −4 | 3.98 |
| L Posterior cingulate cortex | −4 | −48 | 36 | 4.8 |
| −4 | −58 | 30 | 2.95 | |
| R Posterior cingulate cortex | 8 | −54 | 32 | 3.17 |
| 12 | −48 | 42 | 3.16 | |
| R Inferior temporal gyrus | 42 | −54 | −2 | 3.13 |
| R Fusiform gyrus | 34 | −58 | 10 | 3.09 |
| R Corpus callosum | 32 | −46 | 18 | 4 |
| 36 | −56 | 4 | 3.47 | |
| L Precuneus | −14 | −52 | 36 | 4.13 |
| R Precuneus | 4 | −54 | 34 | 3.1 |
| L Occipital lobe | −44 | −66 | 12 | 3.86 |
| Memory choice SV + control choice SV (conjunction) | ||||
| L Caudate nucleus | −6 | 18 | 4 | 4.04 |
| R Caudate nucleus | 4 | 14 | 2 | 3.65 |
| 8 | 20 | 2 | 3.09 | |
| L Ventromedial prefrontal cortex | −10 | 26 | −4 | 3.23 |
| −6 | 44 | −6 | 3.11 | |
| L Ventral striatum/nucleus accumbens | −4 | 10 | −2 | 2.94 |
Notes: Table lists peak and local maxima. L, left; R, right.
In the third GLM, we entered the average “SV increase” of the delayed rewards in each choice block as a parametric modulator for the Memory Recall and Control Cue regressors. This analysis probes for where BOLD signal at the time of memory recall or rest is associated with more patient behavior in the choices directly following memory recall or rest, relative to what would be expected if there were no effect of condition (i.e. how much the SV increased because of the manipulation beforehand). Thus, this analysis reveals neural activity that accounts for between-subject variance in effect size. Here we found three clusters: one in the striatum, extending to the anterior insula and orbitofrontal cortex; one in the right temporo parietal junction (TPJ) extending into the superior temporal sulcus, and one in the cerebellum (Figure 5; Table 3).
Fig. 5.
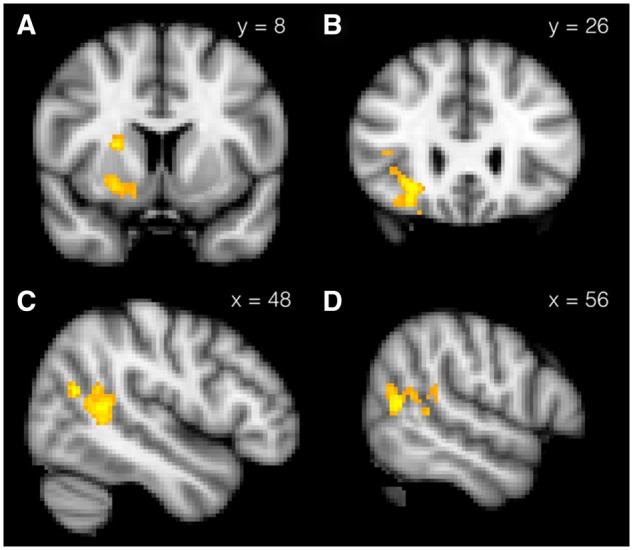
Neural activity during positive memory retrieval or control cue predicting increase in subjective value of delayed rewards following cue. Two coronal views of cluster containing (A) ventral striatum and (B) orbitofrontal cortex and two sagittal views (C and D) of cluster containing right temporoparietal junction and superior temporal sulcus. All Z > 2.3, cluster threshold corrected to P < 0.05.
Table 3.
Regions at cue predicting size of behavioral effect (SV increase in choices after cue)
| X | Y | Z | z-stat | |
|---|---|---|---|---|
| Memory recall SV increase + control cue SV increase | ||||
| R Anterior insula | 28 | 24 | −10 | 3.7 |
| R Putamen | 24 | 16 | 4 | 3.56 |
| 26 | 2 | 0 | 3.45 | |
| 24 | 10 | −6 | 3.16 | |
| R Caudate | 22 | 10 | 12 | 3.41 |
| R Orbitofrontal cortex | 28 | 26 | −16 | 3.33 |
| R Temporoparietal junction | 56 | −56 | 10 | 3.98 |
| 50 | −48 | 20 | 3.56 | |
| 48 | −60 | 20 | 3.48 | |
| 52 | −60 | 18 | 3.17 | |
| R Superior temporal sulcus | 46 | −46 | 4 | 3.66 |
| 48 | −44 | 10 | 3.55 | |
| L Cerebellum | −26 | −82 | −30 | 3.72 |
| −20 | −64 | −30 | 3.5 | |
| −6 | −72 | −20 | 3.25 | |
| −36 | −68 | −26 | 3.19 | |
| −14 | −66 | −22 | 3.11 | |
| −38 | −82 | −32 | 2.83 | |
Notes: Table lists peak and local maxima. L, left; R, right.
Finally, we used RSA to compare activation patterns in vmPFC between the time of memory recall and the time of choice following memory recall. We selected a vmPFC ROI from a quantitative meta-analysis of studies that reported SV signals during decision-making (Bartra et al., 2013). We found that individuals who became more patient after recalling positive memories showed more similar patterns of activation in vmPFC between memory recall and the choices that followed it (r = 0.40; P = 0.016; Figure 6). This effect was specific to the Memory condition and selective to the vmPFC (although correlation coefficients did not significantly differ from each other; see Supplementary Results).
Fig. 6.
Greater representational similarity in vmPFC between memory recall and memory choice is associated with reduced temporal discounting. (A) vmPFC ROI taken from Bartra et al. (2013) meta-analysis of subjective value in the brain. T-statistic maps for memory recall and memory choice were extracted from this ROI for the representational similarity analysis. (B) Similarity in BOLD signal pattern between the memory recall and memory choice phases predicted behavioral effect across subjects (r = 0.40; P = 0.01). Specifically, participants who were more likely to choose delayed rewards in the memory condition (lower discount rates in memory condition compared to control condition) had more similarity in vmPFC activation patterns between the memory retrieval and memory choice phases.
Discussion
Here we found that positive autobiographical memory retrieval prior to intertemporal decision-making reduced temporal discounting. This effect was specific for positive, and not negative, memories, since negative memory retrieval did not lead to any significant change in decision-making. Our result also cannot be explained by positive affect alone, since positive non-mnemonic imagery increased temporal discounting. The neuroimaging study results suggest that a likely neural mechanism by which positive memory retrieval reduces temporal discounting is through increased activity in the striatum and temporo parietal junction at the time of memory retrieval. Increased pattern similarity in vmPFC between the time of memory retrieval and the time of choice also predicted the size of the behavioral effect across participants.
Whereas many studies have shown that thinking positively about the future can lead to more future-oriented choice (Peters and Büchel, 2010; Benoit et al., 2011; Daniel et al., 2015; Palombo et al., 2015; Sasse et al., 2015), this is the first to show that thinking positively about the past can also reduce temporal discounting. Thus, it is not necessary that the temporal focus of this mental imagery be on the future. Yet, both positive affect and episodic memory appear to be critical, since neither negative memory retrieval nor novel positive imagery decreased temporal discounting.
These results are consistent with a line of research on nostalgia, or a sentimental longing for one's past (Sedikides et al., 2015). Nostalgia, often induced in the lab by cueing positive memory retrieval, has been shown to make individuals more future-oriented (Sedikides and Wildschut, 2016), by increasing optimism (Cheung et al., 2013) and prosocial behavior (Zhou et al., 2012), and by fostering self-continuity (Sedikides et al., 2016). Self-continuity (Bartels and Urminsky, 2011; Hershfield, 2011) has been linked to lower temporal discounting, suggesting that evoking nostalgia may decrease temporal discounting as well. The current study was not designed to study nostalgia specifically, however. Whereas some of the memories here might have been nostalgic, future studies will be needed to determine if the level of nostalgia in these memories is what led to our behavioral effect, or if positive autobiographical memories of any kind will have the same effect.
Our neuroimaging results revealed that, at the time of the memory recall or control cue, activity in three clusters predicted more patient choice in the memory condition. First, activity in the striatum, extending into the orbitofrontal cortex and anterior insula predicted a larger behavioral effect. Increased activation in these reward regions is consistent with previous studies of positive memory retrieval (Speer et al., 2014; Speer and Delgado, 2017), and is in line with the finding that recalling these memories is intrinsically rewarding (Speer et al., 2014). This result suggests that to the extent that memories are processed as rewarding, they lead to a reduction in discounting. This idea is further supported by our result that a region of the vmPFC known to be associated with valuation processes showed more similar activity patterns during the time of memory recall and the time of choice for participants who showed the behavioral effect more strongly. Since negative memory retrieval does not lead to a decrease in discount rate, these neural results provide additional evidence that a positive view toward the past is critical to alter choice. However, it is not the only crucial element, since average positive affect ratings across subjects did not predict temporal discounting, and novel imagery that also elicited positive affect actually increased temporal discounting. Moreover, since this vmPFC region is also active during processes like affective regulation and self-reflection (Denny et al., 2012; Delgado et al., 2016), we cannot conclude that similarity in valuation processes specifically contributes to the effect of memory retrieval on choice.
BOLD signal in right TPJ extending into superior temporal sulcus was also associated with the size of the behavioral effect. These regions have been consistently implicated in social cognition and perspective-taking (Saxe and Kanwisher, 2003). While it seems counterintuitive that the TPJ would be involved in temporal discounting, a recent study using transcranial magnetic stimulation to TPJ found that this region plays a causal role in patient decision-making (Soutschek et al., 2016). The role of the TPJ in temporal discounting may be to facilitate perspective-taking of the “future self,” to overcome the bias to choose the immediate reward (Ersner-Hershfield et al., 2009; Mitchell et al., 2011). In this study, one possibility is that during memory retrieval, people who take the perspective of the ‘past-self’ are those that show the effect of memory retrieval on choice. Another possibility is that participants who take the perspective of others while recalling memories show more future-oriented behavior. Nostalgic memories have been shown to increase self-continuity by increasing social connectedness (Sedikides et al., 2016), so perhaps recall of positive social memories reduces temporal discounting.
Finally, cerebellar activation also predicted behavioral effect size. The precise role of the cerebellum in cognition is still unclear (Rapoport et al., 2000), although it is noteworthy that nostalgic cues have been found to elicit cerebellar activity (Oba et al., 2016).
The finding that positive memory retrieval reduces temporal discounting adds to a growing literature on the benefits of engaging in positive memory retrieval. A recent study reported that recalling positive memories dampens cortisol responses and negative affect following acute stress (Speer and Delgado, 2017). Recalling nostalgic memories increases approach motivation (Stephan et al., 2014), optimism (Cheung et al., 2013), prosocial behavior (Zhou et al., 2012), creativity (van Tilburg et al., 2015) and inspiration (Stephan et al., 2015). Finally, recalling times for which one feels grateful has been shown to reduce temporal discounting (DeSteno et al., 2014) and increase prosocial behavior (Bartlett et al., 2012). Future research will be needed to examine if these benefits will extend to other choices, such as decisions about spending, or risk and ambiguity.
A few limitations of the study warrant mention. First, we did not investigate the effects of neutral memory retrieval on intertemporal choice, since neutral memories are more semantic, less vivid and less engaging than positive memories (Talarico et al., 2004; Schaefer and Philippot, 2005; Speer et al., 2014). They are also difficult to generate; even in response to neutral cues, people tend to recall memories that have an affective valence (Sharot et al., 2007). Next, our control condition did not involve an active task. While this gave us less experimental control, it provided a stringent test of the manipulation that we have identified here, since participants were likely engaging default mode network regions in both the control and memory conditions (Spreng et al., 2009; Andrews-Hanna et al., 2014). Thus, this is a potential strength, because it allows us to conclude that mind-wandering on its own does not reduce temporal discounting, as some studies might suggest (Smallwood et al., 2013).
In conclusion, just as positive prospection leads to more patient choice, positive retrospection has a similar effect. This finding contributes to the emerging literature on the integral role of the episodic memory system in economic decision-making. The manipulation identified here may prove useful for reducing temporal discounting in certain disorders, such as addiction (Reynolds, 2006; Demurie et al., 2012). By identifying ways to modulate valuation during this decision process, we can provide people with opportunities for flexibly altering choice.
Supplementary Material
Acknowledgements
We thank Shivani Patel and Julia Honoroff for help with data collection.
Funding
This research was supported by: funding from the National Institutes of Health (R01AG039283) awarded to E.A.P., a National Science Foundation Graduate Research Fellowship awarded to K.M.L., and the New York University Center for Brain Imaging. M.R.D. and M.E.S. were supported by funding from the National Institutes of Health to M.R.D. (DA027764).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D.M., Urminsky O. (2011). On Intertemporal Selfishness: How the Perceived Instability of Identity Underlies Impatient Consumption. Journal of Consumer Research, 38, 182–98. [Google Scholar]
- Bartlett M.Y., Condon P., Cruz J., Baumann J., Desteno D. (2012). Gratitude: prompting behaviours that build relationships. Cognition & Emotion, 26, 2–13. [DOI] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit R.G., Gilbert S.J., Burgess P.W. (2011). A neural mechanism mediating the impact of episodic prospection on farsighted decisions. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31, 6771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W.-Y., Wildschut T., Sedikides C., Hepper E.G., Arndt J., Vingerhoets A.J.J.M. (2013). Back to the future: nostalgia increases optimism. Personality & Social Psychology Bulletin, 39, 1484–96. [DOI] [PubMed] [Google Scholar]
- Daniel T.O., Said M., Stanton C.M., Epstein L.H. (2015). Episodic future thinking reduces delay discounting and energy intake in children. Eating Behaviors, 18, 20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Beer J.S., Fellows L.K., et al. (2016). Viewpoints: dialogues on the functional role of the ventromedial prefrontal cortex. Nature Neuroscience, 19, 1545–52. [DOI] [PubMed] [Google Scholar]
- Demurie E., Roeyers H., Baeyens D., Sonuga-Barke E. (2012). Temporal discounting of monetary rewards in children and adolescents with ADHD and autism spectrum disorders. Developmental Science, 15, 791–800. [DOI] [PubMed] [Google Scholar]
- Denny B.T., Kober H., Wager T.D., Ochsner K.N. (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24, 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSteno D., Li Y., Dickens L., Lerner J.S. (2014). Gratitude: a tool for reducing economic impatience. Psychological Science, 25, 1262–7. [DOI] [PubMed] [Google Scholar]
- Ersner-Hershfield H., Garton M.T., Ballard K., Samanez-Larkin G.R., Knutson B. (2009). Don’t stop thinking about tomorrow: Individual differences in future self-continuity account for saving. Judgment and Decision Making, 4, 280–6. [PMC free article] [PubMed] [Google Scholar]
- Green L., Myerson J. (2004). A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin, 130, 769–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield H.E. (2011). Future self-continuity: how conceptions of the future self transform intertemporal choice. Annals of the New York Academy of Sciences, 1235, 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience, 10, 1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. (2010). An “as soon as possible” effect in human intertemporal decision making: behavioral evidence and neural mechanisms. Journal of Neurophysiology, 103, 2513–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D., Craver C.F., Green L., Myerson J., Boyer P., Rosenbaum R.S. (2012). Future decision-making without episodic mental time travel. Hippocampus, 22, 1215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert K.M., Phelps E.A. (2016). The malleability of intertemporal choice. Trends in Cognitive Sciences, 20, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Feng T., Chen J., Li H. (2013). The value of emotion: How does episodic prospection modulate delay discounting? PLoS One, 8, e81717.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.P., Schirmer J., Ames D.L., Gilbert D.T. (2011). Medial prefrontal cortex predicts intertemporal choice. Journal of Cognitive Neuroscience, 23, 857–66. [DOI] [PubMed] [Google Scholar]
- Oba K., Noriuchi M., Atomi T., Moriguchi Y., Kikuchi Y. (2016). Memory and reward systems coproduce “nostalgic” experiences in the brain. Social Cognitive and Affective Neuroscience, 11, 1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo D.J., Keane M.M., Verfaellie M. (2015). The medial temporal lobes are critical for reward-based decision making under conditions that promote episodic future thinking. Hippocampus, 25, 345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Büchel C. (2010). Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron, 66, 138–48. [DOI] [PubMed] [Google Scholar]
- Peters J., Büchel C. (2011). The neural mechanisms of inter-temporal decision-making: understanding variability. Trends in Cognitive Sciences, 15, 227–39. [DOI] [PubMed] [Google Scholar]
- Rapoport M., van Reekum R., Mayberg H. (2000). The role of the cerebellum in cognition and behavior. A Selective Review. The Journal of Neuropsychiatry and Clinical Neurosciences, 12, 193–8. [DOI] [PubMed] [Google Scholar]
- Reynolds B. (2006). A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural Pharmacology, 17, 651–67. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Mata R., Radu P.T., Ballard I.C., Carstensen L.L., McClure S.M. (2011). Age differences in striatal delay sensitivity during intertemporal choice in healthy adults. Frontiers in Neuroscience, 5, 126.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse L.K., Peters J., Brassen S. (2017). Cognitive control modulates effects of episodic simulation on delay discounting in aging. Frontiers in Aging Neuroscience, 9, 58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse L.K., Peters J., Büchel C., Brassen S. (2015). Effects of prospective thinking on intertemporal choice: the role of familiarity. Human Brain Mapping, 36, 4210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. NeuroImage, 19, 1835–42. [DOI] [PubMed] [Google Scholar]
- Schacter D.L., Addis D.R. (2007). The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London Series Biological Sciences, 362, 773–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D.L., Addis D.R., Buckner R.L. (2007). Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience, 8, 657–61. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Philippot P. (2005). Selective effects of emotion on the phenomenal characteristics of autobiographical memories. Memory (Hove, England), 13, 148–60. [DOI] [PubMed] [Google Scholar]
- Sedikides C., Wildschut T. (2016). Past forward: nostalgia as a motivational force. Trends in Cognitive Sciences, 20, 319–21. [DOI] [PubMed] [Google Scholar]
- Sedikides C., Wildschut T., Cheung W.-Y., et al. (2016). Nostalgia fosters self-continuity: uncovering the mechanism (social connectedness) and consequence (eudaimonic well-being). Emotion, 16, 524–39. [DOI] [PubMed] [Google Scholar]
- Sedikides C., Wildschut T., Routledge C., Arndt J., Hepper E.G., Zhou X. (2015). To nostalgize: mixing memory with affect and desire. Advances in Experimental Social Psychology, 51, 189–273. [Google Scholar]
- Sharot T., Riccardi A.M., Raio C.M., Phelps E.A. (2007). Neural mechanisms mediating optimism bias. Nature, 450, 102–5. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Ruby F.J.M., Singer T. (2013). Letting go of the present: mind-wandering is associated with reduced delay discounting. Consciousness and Cognition, 22, 1–7. [DOI] [PubMed] [Google Scholar]
- Soutschek A., Ruff C.C., Strombach T., Kalenscher T., Tobler P.N. (2016). Brain stimulation reveals crucial role of overcoming self-centeredness in self-control. Science Advances, 2, e1600992.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer M.E., Bhanji J.P., Delgado M.R. (2014). Savoring the past: positive memories evoke value representations in the striatum. Neuron, 84, 847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer M.E., Delgado M.R. (2017). Reminiscing about positive memories buffers acute stress responses. Nature Human Behaviour, 1, 93.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S.N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience, 21, 489–510. [DOI] [PubMed] [Google Scholar]
- Stephan E., Sedikides C., Wildschut T., Cheung W.-Y., Routledge C., Arndt J. (2015). Nostalgia-evoked inspiration: mediating mechanisms and Motivational Implications. Personality & Social Psychology Bulletin, 41, 1395–410. [DOI] [PubMed] [Google Scholar]
- Stephan E., Wildschut T., Sedikides C., et al. (2014). The mnemonic mover: nostalgia regulates avoidance and approach motivation. Emotion, 14, 545–61. [DOI] [PubMed] [Google Scholar]
- Strotz R.H. (1956). Myopia and inconsistency in dynamic utility maximization. Review of Economic Studies, 23, 165–80. [Google Scholar]
- Talarico J.M., LaBar K.S., Rubin D.C. (2004). Emotional intensity predicts autobiographical memory experience. Memory & Cognition, 32, 1118–32. [DOI] [PubMed] [Google Scholar]
- van Tilburg W.A.P., Sedikides C., Wildschut T. (2015). The mnemonic muse: nostalgia fosters creativity through openness to experience. Journal of Experimental Social Psychology, 59, 1–7. [Google Scholar]
- Zhou X., Wildschut T., Sedikides C., Shi K., Feng C. (2012). Nostalgia: the gift that keeps on giving. Journal of Consumer Research, 39, 39–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.