Abstract
Throughout history and into the modern era, human groups have been continually subjected to a wide range of societal threats, from natural disasters to pandemics to terrorism. Yet despite this fundamental aspect of human existence, there has been little research on how societal threat affects social coordination at both the neural and the behavioral level. Here, we show for the first time that individuals are better able to coordinate under high societal threat as compared to low or no threat (Experiment 1). Using a method of hyperscanning electroencephalography (EEG), which simultaneously measures brain activity among interacting subjects, we further illustrate that interbrain synchrony of gamma band oscillations is enhanced when people are under high threat, and increased gamma interbrain synchrony is associated with lower dyadic interpersonal time lag (i.e. higher coordination) (Experiment 2). To our knowledge, the current work provides some of the first empirical evidence that gamma interbrain synchrony is associated with social coordination when humans are under threat.
Keywords: threat, coordination, brain synchrony, EEG, hyperscanning, gamma
Introduction
In the course of our 200,000-year history, humans have been subjected to numerous threats to our survival, including ecological threats such as natural disasters, resource scarcity and pathogens, as well as human-made threats like territorial invasions. In the 21st century, threats to human groups, from climate change to pandemics to terrorism, continue unabated. Yet surprisingly, there has been little research on the behavioral or neural mechanisms through which humans coordinate under high societal threat. From an evolutionary point of view, the ability of humans to effectively synchronize their actions under threat would presumably confer an important survival advantage (Roos et al., 2015).
To address this question, we combine state of the art hyperscanning techniques with exposure to real-world threat. Hyperscanning techniques, which record multiple brains’ neural activity simultaneously with great precision as humans interact over time (Montague, 2002; Dumas et al., 2011; Burgess, 2013), are perfectly situated to elucidate the interbrain mechanisms underlying social coordination under high societal threat. Accumulating hyperscanning eletroenthephalograph (EEG) studies have indeed shown that interbrain synchrony plays a critical role in various forms of human coordination, such as the ability to synchronize body movements (Dumas et al, 2010) and speech rhythms (Kawasaki et al., 2013) and to perform duets (Sänger et al., 2013).
We complement previous research by examining the role interbrain synchrony plays in coordination when humans are under threat. Using a coordination game validated in previous research (Mu et al., 2016), in Experiment 1, we examined whether dyads exposed to ingroup threat (IGT) would exhibit greater coordination as compared to dyads exposed to outgroup threat (OGT) or no threat control conditions (IGC).
In Experiment 2, we combined hyperscanning EEG with the same threat manipulation (i.e. IGT, OGT and IGC) and the same coordination game employed in Experiment 1 to investigate whether interbrain synchrony would help humans coordinate under conditions of high societal threat. Using a dual-EEG setup, we tested how societal threat influences interbrain synchrony while participants attempted to coordinate. Previous hyperscanning EEG studies have shown that alpha interbrain synchrony is activated in a variety of social coordination tasks, including interactional synchrony (Dumas et al., 2010), coordinated teamwork (Astolfi et al., 2012) and synchronized counting (Mu et al., 2016). Thus, we examined whether alpha interbrain synchrony would be recruited to support social coordination in an unexplored context, namely that of societal threat.
We also examined other bands of interbrain synchrony which may be particularly relevant to social coordination under threat—most notably gamma band, a high frequency band (>28 Hz) that is a threat-sensitive neural marker. In particular, single brain analyses have shown that gamma band oscillations contribute to threat detection, reflecting the involvement of a quick subcortical route to the amygdala(Luo et al., 2007), which plays a central role in processing threat-related stimuli, such as fearful images (Adolphs et al., 1994; Coccaro et al., 2007) and threat-related words (Isenberg et al., 1999). Gamma activity is also higher in anxiety disorder patients who experience chronic fear (Oathes et al., 2008). Thus, if threat affects interpersonal coordination by modulating interbrain synchrony linked to threat processing, we would expect that gamma band synchrony may be associated with human coordination under threat.
Experiment 1
Method
Participants
Ninety graduate and undergraduate college students at a Chinese university (mean age = 23.2 years, range: 18–31 years; 44 males) were recruited online for a laboratory study and were paid for their participation. Same-gender dyads were formed in the lab and were assigned to one of three threat conditions: ingroup threat, outgroup threat and ingroup control (15 dyads per condition, see below for more details on the threat manipulations). According to previous research (Mu et al., 2016), the sample size (N = 90) was adequate for testing the effects of threat on social coordination. To control for individual differences, we assessed participants’ political ideology on a scale from 1 (Very Liberal) to 7 (Very Conservative). Subjective socioeconomic status was also assessed using McArthur’s Self-Anchoring Scale (Adler et al., 2000). No differences in age, political ideology, or socioeconomic status were found between groups (all P values > 0.05, Supplementary Table S1). Participants were right-handed and had normal or corrected-to-normal vision. All individuals gave their written informed consent before starting the experiment.
Societal threat manipulation
An experimenter informed participants that they would be completing two separate and brief experimental tasks. They were then given a handout which stated: ‘For the next task, you will read an article posted recently and we will ask you to give us your general opinions on this article.’ Each dyad was randomly assigned to read one of the three articles that represented different conditions: the ingroup threat (IGT), the outgroup threat (OGT) and the ingroup no threat control (IGC). In the IGT condition, participants read an article reporting that their own country (China) was facing a serious external threat from its neighbor (Japan) (excerpts include ‘The report points out that China is facing serious external threats from its neighbors…Japan has an increase of 2% on military expenditures this year. This is 1.6 times greater than China totally…People in China have to be concerned about external threats on their territory.’
In the OGT condition, participants read an identical article in which threat was also activated, but not on their own country’s soil. It described another country (Ethiopia) that was facing serious external threats from its neighbors (Eritrea) (excerpts include ‘The report points out that Ethiopia is facing serious external threats from its neighbors… Eritrea has an increase of 2% on military expenditures this year… People in Ethiopia have to be concerned about external threats on their territory.’).
In the IGC condition, participants read an article which described non-threatening events that were occurring on their own soil (e.g. ‘The report points out that China is predicted to become a market receptive to electric vehicles in the next five years’). This condition was included as a no-threat baseline condition to ensure that any effects of the ingroup threat were not simply due to references to the ingroup (China) but rather were due to exposure to high ingroup threat specifically. The articles used in the three conditions were matched in terms of overall length and the construction of sentences, and were piloted before their use (see Supplementary Material for the articles and pilot test). Once participants finished reading the article, they were asked to summarize the article and list 3–4 representative keywords for the article to ensure that they had read it carefully and understood it correctly.
Coordination game
To test the effect of threat on coordination, we conducted a real-time coordination game after the social threat manipulation. This paradigm has been validated in previous studies on mental coordination (Mu et al., 2016). During the game, the two participants were seated in a sound-shielded room, separated by two monitors. They were then given a second handout that stated: ‘You have completed the article assessment task. The second study is investigating individuals’ ability to count time in their minds without a clock or a watch.’ In this game, each participant was asked to synchronize with his/her partner (coordination task) or with a computer (control task) by attempting to finish counting at the same time in a within-subject design. The computer control condition, which was identical to the coordination task in terms of stimuli and feedback, was included to rule out the possibility that the effects of threat on coordination were due to any other unrelated psychological factors (e.g. arousal level, motor preparation, feedback learning).
Participants completed 20 practice trials before beginning the actual task. The task consisted of four sessions, each of which included two 10-trial blocks. Each block started with a 3-s instruction for the coordination or control tasks. The order of the coordination and control tasks was counterbalanced over sessions so that half sessions started with the coordination task and half with the control task.
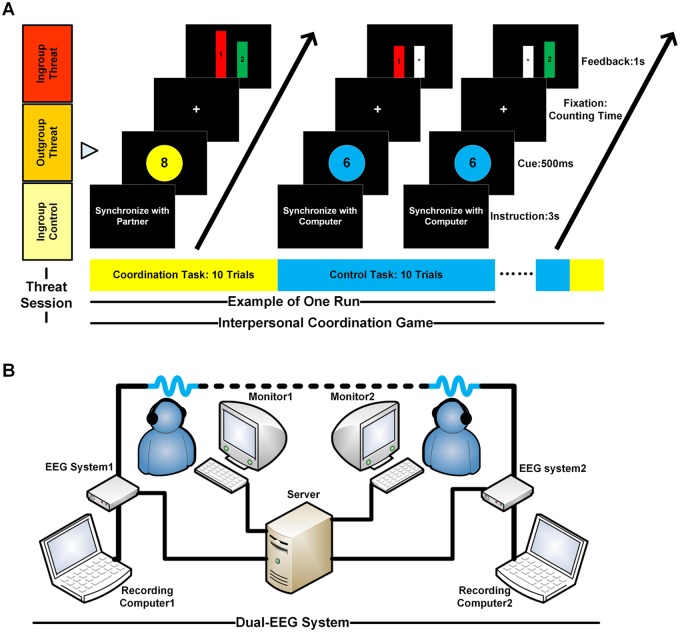
During each trial, the computer monitor displayed an integer (from 6 to 10) for 500 ms that indicated the time in seconds that the dyad should silently count to. The color (yellow or blue) of a disk on which the integers were displayed indicated whether participants were supposed to synchronize with another human or synchronize with a computer. Participants then began to count silently while looking at a fixation point, and they pressed a key on the keyboard to indicate that they had finished counting. After a delay of 1.5–2.5 s, feedback bars were then presented for 1 s (see Figure 1A). A red and a green feedback bar labeled with the participants’ IDs (e.g. ‘1’ or ‘2’) represented the counting time of each participant relative to his/her partner. A white bar labeled with an asterisk represented the counting time of the computer relative to each participant in the control task. Higher feedback bars indicated longer counting times. The assignments of different colors to instructions, feedback bars and response buttons were counterbalanced across participants. The target counting time (from 6 to 10 s) was also balanced across trials for the two conditions. All stimuli and feedback used in the computer control task were identical to those used in the coordination task, with the only difference being the target (person or computer) with whom participants needed to synchronize. All the stimuli were created and displayed using the Matlab PsychToolbox (Brainard, 1997; Pelli, 1997).
Fig. 1.
Interpersonal coordination game and dual-EEG system. (A) The interpersonal coordination paradigm. Each dyad was randomly assigned to one of the three threat conditions: ingroup threat, outgroup threat and ingroup no-threat control. After the threat manipulation, each dyad was given an interpersonal coordination game during which their EEG signals were recorded. Participants engaged in 8 sessions (4 sessions in Experiment 1), within which there were two blocks of 10 trials each. For each session, participants engaged in one block of the coordination task (synchronizing with partner) and one block of the control task (synchronizing with computer). During the coordination task, participants were asked to count the length of time in their heads (e.g. 8-s) and to finish counting at the same time as each other. During the control task, participants were asked to count the same length of time in their heads (e.g. 6-s) and to finish counting at the same time as the computer clock. The order of the two tasks was randomly assigned for each session across subjects. Each block started with a 3-s task instruction. During each trial, a number (e.g. 8) was presented on the monitors for a duration of 500 ms. This number represents the time in seconds for participants to count silently in their own heads. Participants started counting when the fixation appeared on the screen. Once they completed counting, participants pressed a key to indicate that they had finished. Feedback bars representing the time lags between participants, or between a participant and the computer, were then presented for a duration of 1 s. In the coordination task, a red and a green bar labeled with subjects’ ID (e.g. ‘1’, ‘2’) illustrated the counting time of each participant relative to his/her partner. A white bar with ‘*’ illustrated the counting time of the computer relative to each participant in the control task. The higher the feedback bars, the longer the counting took. (B) The dual-EEG setup in Experiment 2. Behavioral responses and EEG signals of each dyad were recorded simultaneously and continuously using two 32-channel EEG systems. Stimuli and feedback bars were simultaneously presented to two individuals of a dyad on their respective monitors, which were connected to the same server.
On each trial, to give dyads feedback regarding their relative synchronized performance, we developed a computer program that first calculated the time lags between each participant and his/her partner and then automatically rated the time lags (Subject 1’s counting time to Subject 2’s counting time) on a 9-point scale from 1 (Extremely Slow) to 9 (Extremely Fast) (Please see Supplementary Table S2 for more details on the scale rating). As shown in Figure 1A, subjects were given a picture of two bars (without units) after each trial that illustrated their relative performance based on this rating. Relative to the absolute/numerical feedbacks, such as bars with absolute units or numbers showing actual time lags or speeds, our relative/qualitative feedback—two bars next to one another without numerical information—visually shows the relative speed discrepancy between individuals which is analogous to the kind of feedback that we receive from others in daily social coordination. For example, when we are walking together with our friends and trying to synchronize with them, we might have little idea about our exact speeds or the absolute differences between us, but we have an intuition of our relative speed (who is faster or slower), that can help us successfully coordinate our next steps.
To motivate participants to synchronize their counting, they were given a point for each successful synchronous response (i.e. an interval <150 ms between the responses within the dyad or between the response of a participant and a computer) and their payments for participation were calculated based on the point they obtained. Each point earned 0.5 RMB (∼7 cents). The participants didn’t know the conversion rate in advance but were told this when they got paid at the end. In the control task, the counting time of the computer was calculated by its internal clock. Participants were not informed any knowledge about the accuracy and strategy of the computer’s counting.
Analysis of behavioral performance
Behavioral responses of each participant during the coordination and control tasks were recorded simultaneously. To quantify the interpersonal coordination performance of each dyad, we first calculated the ith trial interpersonal time lag, as shown below:
where RTi,sub_1 and RTi,sub_2 are reaction times of two individuals of a dyad on the ith trial. A smaller δinter_i reflects higher synchronization of a dyad’s responses. The algorithm of the interpersonal time lag allowed us to remove the effect of dyads’ differences in counting time as well as variance in the amount of target time required in different trials from this behavioral index. Then we calculated the mean interpersonal time lag by averaging the interpersonal time lags across all valid trials (which excluded the trials with counting times over three standard deviations above the mean). To assess the threat effects on behavioral coordination, we conducted 2 (Task: coordination and control) × 3 (Threat: IGT, OGT and IGC) repeated measures analyses of variance (ANOVAs) on the mean interpersonal time lag with Task as a within-subjects independent variable and Threat as a between-subjects variable.
To further illustrate the probability distributions and confidence intervals of the enhanced behavioral coordination under each threat condition, we applied a bootstrapping approach on the differential interpersonal time lags of the coordination vs control tasks. The differential time lag (the contrast between the coordination vs control tasks) is the most appropriate way to compare the effects of the threat manipulations, as it enabled us to examine the effect of threat on social coordination without being contaminated by individual differences in counting speed (i.e. counting fast or slow). The bootstrapping procedure involved choosing random samples with replacement from original dyads under each condition. The number of elements in each bootstrap sample equals the number of elements in the original sample set (N = 15 per threat condition). This procedure was repeated 5000 times to generate a new bootstrap sample. The 95% bootstrapping confidence interval and the probability distribution of the bootstrap sample were estimated by the MATLAB bootstrap toolbox (Efron and Tibshirani, 1993; Zoubir and Iskander, 1998).
Results
Interpersonal time lag
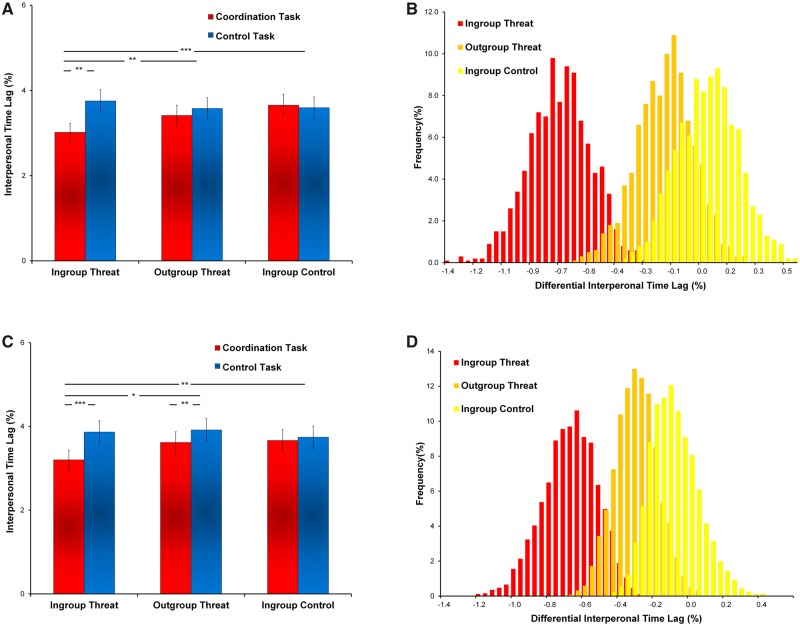
We conducted a 3 (Threat as between-subject factor: IGT, OGT or IGC) × 2 (Task as within-subject factor: coordination or control) ANOVA on the interpersonal time lags (with smaller time lags indicating greater coordination, see Method). The results identified a task effect, showing smaller interpersonal time lag in the coordination task than the control task (F(1,42) = 7.57, P < 0.01, ηp2 = 0.15, Figure 2A). We also found support for a Threat × Task interaction (F(2,42) = 5.38, P < 0.01, ηp2 = 0.20). Analyses revealed that lower interpersonal time lag of the coordination vs control tasks was found in the IGT condition than in the OGT (F(1,28) = 5.19, P < 0.05, ηp2 = 0.15) and IGC conditions (F(1,28) = 9.61, P < 0.01, ηp2 = 0.26), indicating that the dyads under high threat exhibited greater interpersonal coordination than those under low or no threat. Using bootstrapping techniques, we confirmed that a robust decrease in interpersonal time lag of the coordination vs control tasks was shown in the IGT condition vs the other two conditions (Figure 2B).
Fig. 2.
Behavioral coordination. (A) Interpersonal time lag in Experiment 1. The bar chart shows the mean values of the interpersonal time lags of the coordination and control tasks under the ingroup threat (IGT), outgroup threat (OGT) and ingroup control (IGC) conditions, respectively. A smaller mean value of the interpersonal time lags is observed in the coordination (vs control) task under the IGT than the other two conditions, suggesting high societal threats enhance interpersonal synchrony. (B) The bootstrap sampling distributions in Experiment 1. The histogram illustrates the bootstrap sampling (N = 5000) distribution of the differential interpersonal time lags of the coordination (vs control) task under the IGT, OGT and IGC conditions (N = 5000), showing a more negative shift (i.e. more coordination) for the IGT than the other two conditions (95% Confidence Intervals (CI): [−1.12,−0.40] for IGT, [−0.47,0.15] for OGT, [−0.25,0.37] for IGC. (C) Interpersonal time lag in Experiment 2. The bar chart shows the mean values of the interpersonal time lags of the coordination and control tasks under the IGT, OGT and IGC conditions, respectively. (D) The bootstrap sampling distributions in Experiment 2. The histogram illustrates the bootstrap sampling distribution of the differential interpersonal time lags of the coordination (vs control) task under the IGT, OGT and IGC conditions, showing a more negative shift (i.e. more synchrony) for the IGT than the other two conditions (95% Confidence Intervals (CI): [−1.05,−0.44] for IGT, [−0.54,−0.08] for OGT, [−0.31,0.07] for IGC (*P < 0.1; **P < 0.05; ***P < 0.001).
Discussion
From the Boston bombing to the SARs epidemic to Tsunamis, human groups are continually subject to a wide range of societal threats. Recent evolutionary game theoretic models have shown that groups that face a high degree of threat develop more coordination and cooperation for survival relative to those that face little threat (Roos et al., 2015). By combining the societal threat manipulation with the coordination game in Experiment 1, we provide some of the first empirical evidence that human dyads exhibit greater coordination when they are subjected to societal threat. This expands upon the findings of computer-based computational models by measuring real human behaviors (Roos et al., 2015). Our second goal of this research was to uncover whether and which interbrain mechanisms would support social coordination among dyads under high societal threat. To test this hypothesis, we combined the same threat manipulation and the same coordination game with hyperscanning EEG in Experiment 2.
Experiment 2
Method
Participants
Ninety healthy participants (mean age = 22.5 years; 44 male) were recruited and paid for their participation in Experiment 2 (100 RMB, which is ∼14 dollars, plus any bonus they earned). Fifteen same-gender dyads were randomly assigned to one of the three conditions: in-group threat, outgroup threat and in-group control. According to previous hyperscanning EEG studies (e.g. Dumas et al., 2010; Yun et al., 2012; Mu et al., 2016), the sample size (N = 90) was adequate for testing the neural mechanisms of the threat effects on social coordination. The sample used in Experiment 2 was independent from the one recruited in Experiment 1. Similar to Experiment 1, we measured participants’ political ideology and subjective socioeconomic statuses to control for individual differences. No differences in age, political ideology, or socioeconomic status were found between groups (all P values > 0.05, Supplementary Table S3). All participants were right-handed and had normal or corrected-to-normal vision. All individuals gave their written informed consent before starting experiment
Societal threat manipulation
In Experiment 2, we performed the societal threat manipulation (which was identical to Experiment 1) 30 min before EEG recording started.
Coordination game
In Experiment 2, participants were asked to perform the same coordination game as in Experiment 1, only this time their EEG signals were recorded simultaneously after the threat manipulation. To acquire better and more reliable EEG signals for further analysis of interbrain synchrony, participants in the EEG study completed 8 sessions of 20 trials (twice as many as those in Experiment 1). After they completed these trials, we asked them to recall information from the article they were given prior to the EEG recording. All participants passed the manipulation check. Finally, we assessed participants’ current emotional status across six domains (pleasant, sad, calm, nervous, happy and relaxed) with 5-point Likert scales from 1 (Not At All) to 5 (Very Much).
Dual-EEG setup and data acquisition
In Experiment 2, EEG signals as well as the behavioral responses of each dyad during the coordination and computer control tasks were recorded simultaneously and continuously using two 32-channel Neuroscan portable EEG systems. The dual-EEG system received synchronizing triggers from a parallel port of a server computer (Figure 1B). Stimuli and feedback bars were simultaneously presented to two individuals of a dyad on their respective monitors, which were connected to the same server. EEG signals were recorded from 30 electrodes arranged according to the international 10/20 system and referenced to the electrode at the right mastoid. The electrode impedance was kept to <5 kohms. Eye blinks and vertical eye movements were monitored using two electrodes located above and below the left eye. The horizontal electro-oculogram was recorded from two electrodes placed 1.5 cm lateral to the left and right external canthi. EEG was amplified (band pass 0.01–100 Hz), digitized at a sampling rate of 250 Hz and stored for off-line analysis. Similar to previous research (Mu et al., 2016), the current dual-EEG setup allowed us to acquire behavioral and EEG signals from each subject without delays and enabled precise analyses of interbrain activity.
Analysis of behavioral performance
To test the replicability of the behavioral results found in Experiment 1, we analyzed the interpersonal time lags by using the same ANOVA analyses and bootstrap resampling method in Experiment 2.
Analysis of interbrain synchrony
We used an interbrain phase-locking-value index to estimate the interbrain phase synchrony between two interacting individuals. This interbrain phase synchrony index has been developed to measure whether the signals from the two interacting individuals are phase locked across time (Lachaux et al., 1999; Dumas et al., 2010).
To assess interbrain synchrony, the acquired EEG signals were first preprocessed to reduce external noises and artifacts. First, EEG signals were treated with band-pass filtering (0.1–45 Hz). Then, filtered EEG signals were re-referenced to the algebraic average of the electrodes at the left and right mastoids. Next, a regression-based approach was used for ocular artifact rejection (Gasser and Möcks, 1982; Woestenburg et al., 1983). The ocular channel was used to estimate the parameters of ocular artifacts which were removed from each participant’s continuous EEG signal. Finally, the artifact-free EEG signal from each trial was segmented from 200 ms before to 6000 ms after the onset of the number presentation. The cut off for segmentation was set to 6000 ms because the range of the target counting time (6–10 s) was equal to or longer than 6 s. Accordingly, the EEG data beyond 6000 ms was excluded from further analysis.
Next, the segmented EEG signals were subjected to time-frequency analysis to measure dynamic phase changes in both time and frequency domains. This was done using a Morlet wavelet transform method, as in previous EEG studies (Lachaux et al., 1999; Gross et al., 2004; Lutz et al., 2004; Doesburg et al., 2008). The Morlet wavelet w (t, f0) (Kronland-Martinet et al., 1987) showed a Gaussian shape in time (SD σt) and frequency (SD σf) domains around its central frequency f0 in the following way:
with σf = 1/2πσt. Wavelets were normalized so that their total energy was 1. The normalization factor A was equal to: . Here, to acquire high temporal and frequency resolution, we used a slowly ascending number of wavelet cycles between 2 and 40 Hz in 1 Hz steps. This method provides better temporal resolution at low frequencies and better frequency resolution at high frequencies(Delorme and Makeig, 2004; Mu and Han, 2013).
Third, in line with previous hyperscanning research (Dumas et al., 2010; Mu et al., 2016), the interbrain phase-locking-value (PLV) at a given time t and frequency f was calculated as the absolute value of the sum of the phase ϕ differences of two electrodes (j, k) from two individuals of a dyad across N epochs:
where 0 indicates randomly dispersed phases and 1 indicates fully phase locked oscillations between the two participants’ brain activity in both the coordination and computer control tasks. In the computer control task, we also simultaneously collected the two subjects’ brain activity even though they were not interacting with each other, but rather were interacting with a computer doing the same counting task. Thus, both brains’ EEG signals, not just one, were used for the calculation in the computer condition. The interbrain PLV in the control task was used as a control, reflecting interbrain phase synchronization between two participants when they were asked to count in time with a computer. The interbrain PLV was calculated at the representative electrodes of the four brain areas (frontal, central, parietal and occipital). The averaged interbrain PLV in the following frequency bands was calculated for further statistical analysis: delta (2–4 Hz), theta (5–7 Hz), alpha (8–13 Hz), beta (14–28 Hz) and gamma (28–40 Hz). These five bands have been identified as typical frequency ranges in previous EEG research (Luo et al., 2007; Dumas et al., 2010; Mu and Han, 2013).
The modulations of interbrain PLV on each region were assessed using 2 (Task: Coordination vs Control) × 3 (Threat: IGT, OGT and IGC) ANOVAs, with Task as a within-subject variable and Threat as a between-subject variable. The Greenhouse and Geisser correction was applied to the ANOVAs with more than one degree of freedom, and a significance level of alpha = 0.05 was used for all comparisons (Greenhouse and Geisser, 1959). In addition, to avoid multiple comparison issues, all the EEG results reported in our paper were corrected using cluster-based correction. Clusters were defined by any three successive 100-ms time windows and any 3 adjacent electrodes pairs. To control the family wise error (FWE), we performed bootstrapping resampling and a permutation approach (N = 5000) on the cluster statistics, as demonstrated in previous research (Pantazis et al. 2005). We only reported the results of the electrode pairs from the clusters that exceeded the corrected threshold (alpha = 0.05). Pearson correlation coefficients were calculated to assess the relationship between the differential interpersonal time lag (coordination vs control tasks) and the mean differential interbrain neural activity (coordination vs control tasks). The differential interpersonal time lag, which was defined by the contrast of the coordination vs control tasks, was chosen as the behavioral index because it enabled us to connect participants’ increased neural activity in the coordination vs control task with the enhanced behavioral synchrony of the coordination vs control task. A lower differential interpersonal time lag was indicative of more synchronous counting in the coordination vs control tasks. The confidence interval of correlation analysis was estimated by the bootstrap resampling method (N = 5000). Mediation analysis was conducted using the PROCESS macro for SPSS (Hayes, 2013), with threat (IGT vs OGT/IGC) as the independent variable, the differential gamma interbrain synchrony of the coordination vs control tasks as the mediator, and the differential interpersonal time lag of the coordination vs control tasks as the dependent variable.
Results
Interpersonal time lag
A 2 (Task: coordination and control tasks) × 3 (Threat: IGT, OGT and IGC) repeated measures ANOVA on the interpersonal time lag confirmed a robust task effect (F(1,42) = 17.18, P < 0.0002, ηp2 = 0.29) and an interaction between threat and task (F(2,42) = 4.19, P < 0.05, η2 = 0.17, Figure 2C and D), replicating the findings of Experiment 1. Post hoc analyses revealed that a smaller interpersonal time lag of the coordination vs control tasks was found in the IGT condition relative to the other two threat conditions (IGT vs OGT: F(1,28) = 3.20, P = 0.08, ηp2 = 0.10; IGT vs IGC: F(1,28) = 7.33, P < 0.02, ηp2 = 0.21, Figure 2C). No differences in the interpersonal time lags were found between the OGT and IGC conditions (F(1,28) = 1.31, P = 0.26, ηp2 = 0.04). The estimation of the confidence intervals of the bootstrap distribution consistently validated the noteworthy decrease in the interpersonal time lags in the coordination vs control tasks in the threat conditions (see Figure 2D).
To clarify which aspect of the dyad’s interpersonal time lag statistic is driving the overall effect of the interpersonal time lag, we conducted two follow-up ANOVAs on the numerator of the interpersonal time lag statistic (difference in counting time) and on the denominator of the interpersonal time lag statistic (the sum of dyad’s counting time), respectively (see Supplementary Results). The ANOVA on the numerator (difference in counting time) showed a similar Threat × Task interaction (P < 0.05). This interaction effect was not observed on the denominator analysis (P > 0.05). This finding further supports the notion that societal threat decreases the actual time lag between participants while coordinating with each other, rather than simply making the participants slower.
Interbrain synchrony
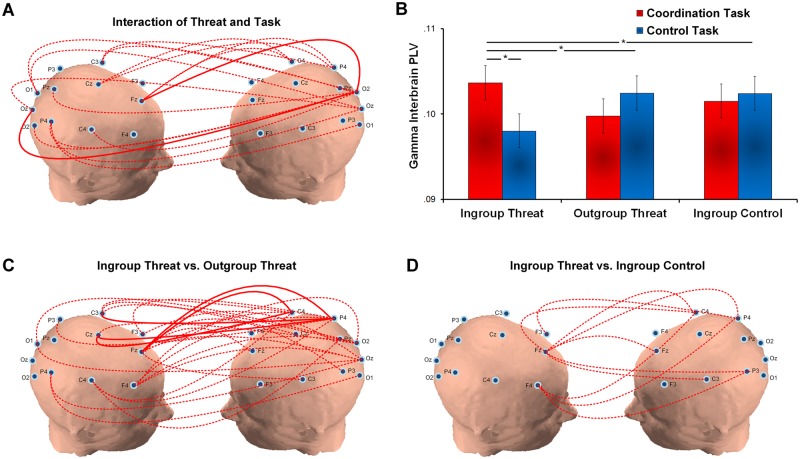
To estimate the interbrain synchrony between the two interacting individuals under threat, we calculated the interbrain phase-locking-value (PLV) (see Method) which has been developed to measure whether the signals from the two interacting individuals are perfectly phase locked across time (Lachaux et al., 1999; Dumas et al., 2010). First, we ran a 2 × 3 ANOVA on the interbrain phase synchrony in the gamma band. The results revealed a significant Threat × Task interaction at 2000–3000 ms for widespread regions (F(2,42) = 6.94, corrected P < 0.05, Figure 3A and B). Neither the main effect of task nor the main effect of threat was significant (all P values > 0.05). A post-hoc 2 × 2 ANOVA further revealed that greater gamma interbrain synchrony (2000–3000 ms) of the coordination (vs computer control) task was observed in the dyads under the IGT as compared to those under the OGT (F(1,28) = 17.17, corrected P < 0.05, Figure 3C) and IGC threat conditions (F(1,28) = 7.85, corrected P < 0.05, Figure 3D), indicating that people under high societal threat show greater interbrain synchrony in gamma oscillations. This greater gamma interbrain synchrony of the coordination (vs control) task was negatively correlated with the differential interpersonal time lag of the coordination (vs control) task across all threat conditions (r(45) = −0.37, P < 0.02, 95% Confidence Interval: [−0.63, −0.05]). Further mediation analyses confirmed that gamma interbrain synchrony mediated the effect of ingroup threat on interpersonal coordination (see Supplementary Results), supporting the hypothesis that high societal threat facilitates interpersonal coordination by means of coupling two individuals’ brain oscillations. Such threat effects on coordination were not shown in any other bands, suggesting social threat selectively modulates gamma band activity (all P values > 0.05, see Supplementary Results).
Fig. 3.
Gamma band interbrain synchrony. (A) The 2 (Task: coordination vs control) × 3 (Threat: ingroup threat (IGT), outgroup threat (OGT) and ingroup no-threat control (IGC)) interaction is significant for widespread regions at 2000–3000 ms. Higher gamma interbrain synchrony of the coordination (vs control) task is observed under the IGT than the other two conditions. (B) The bar chart shows increased gamma interbrain synchrony of the coordination (vs control) task under the IGT and not the OGT and IGC conditions. (C) The 2 (Task: coordination vs control) × 2 (Threat: IGT, OGT) interaction is significant for widespread regions. Gamma interbrain synchrony of the coordination (vs control) task is greater under the IGT than the OGT condition. (D) The 2 (Task: coordination vs control) × 2 (Threat: IGT, IGC) interaction is significant between the frontal and posterior regions. Gamma interbrain synchrony of the coordination (vs control) task is greater under the IGT than the IGC condition (*P < 0.05; **P < 0.01; ***P < 0.001; red dotted line: corrected P < 0.05; red solid line: corrected P < 0.01).
Next, we conducted a 2 (Task: coordination and control tasks) × 3 (Threat: IGT, OGT and IGC) ANOVA on the alpha interbrain PLV. No significant task effect or Task × Threat interaction were observed on the alpha interbrain synchrony (all P values > 0.05, see Supplementary Table S4). A main effect of threat was observed, showing lower alpha interbrain synchrony between the frontal/central electrodes from one participant and the frontal/central electrodes from his/her partner at 2000–3000 ms under the IGT than the other two threat conditions (F(1,42) = 4.33, corrected P < 0.05, see Supplementary Figure S1).
Discussion
Across human history, humans have encountered a broad array of ecological (i.e. natural disasters) and man-made threats (i.e. invasions). The ability to coordinate under threat would presumably confer an important evolutionary advantage, which has been supported by recent evolutionary game theoretic models (Roos et al., 2015). Yet the neurobiological mechanisms supporting social coordination when humans are under threat have yet to be examined empirically. Using the hyperscanning method, the current EEG research not only replicated the behavioral findings of Experiment 1 but more importantly offered an underlying neural testimony that societal threat facilitates mental coordination among humans via synchronizing two brains’ high-frequency gamma oscillations. Our findings expand upon single brain research on the role of gamma activity in processing threat, and provide evidence that interbrain gamma synchronization is associated with social coordination when humans are under societal threat.
Previous hyperscanning EEG studies have shown that social coordination involves interbrain synchrony of the oscillatory activities in multiple bands, such as theta in speech coordination (Kawasaki et al., 2013), alpha in mental coordination (Mu et al., 2016) and beta in hand movement synchronization (Dumas et al., 2010). However, previous research on social coordination has rarely examined how interbrain activity might change as a function of the social context in which coordination occurs. Our current work illustrated that the social context, societal threat, in particular, invoked specific threat-related interbrain neural representation, namely gamma band activity. Interestingly, we also found a main effect for task, such that coordinating with human partner (vs a computer) across all conditions recruited theta and beta band activities but not gamma band activities. This opens up, we believe, a new way of thinking about research in this area—namely that certain features of the tasks and context affect specific aspects of brain synchrony. In this regard, we speculate that there may be two levels of neural systems supporting coordination among humans. Under non-threatening or routine contexts, social coordination may recruit first level neural systems, namely lower frequency band activities (e.g. theta, alpha) to regulate and monitor behavior. But under conditions of high threat or novel contexts, our brains may trigger second level neural systems-high frequency activities (i.e. gamma), fostering a shared emotional representation among individuals which enables them to coordinate better under societal threat.
The current results also provide insight into when our brains begin to synchronize neural oscillatory activities in support of social coordination under societal threat. Specifically, gamma activity occurred in an early window (e.g. 2000–3000 ms) when compared to previous research on alpha activity, which occurred in a later window (e.g. 4000–5000 ms) (Mu et al., 2016). We speculate that this might be due to the fact that the two bands serve distinct functional roles in social coordination. Alpha interbrain synchrony is highly proximal to behavioral responses, suggesting that it may modulate motor preparation and control processing to facilitate behavioral responses. The gamma effect, occurring much earlier, may be helpful during stages where dyads perform more mental adjustment to cope with perceived threat rather than helping with the regulation of motor responses.
We note that while our results did not find any evidence for theta activity in response to societal threat, other modalities of threat stimuli at the perceptual level (i.e. fearful faces and fear conditioned tones) have been associated with theta band and theta-gamma coupling (Maratos et al., 2009; Stujenske et al., 2014). By contrast, our manipulation of threat was based on newspaper articles depicting real-world events (e.g. potential invasions) and not perceptual level fearful stimuli. Nevertheless, to the extent that people both read about potential threats and witness fearful stimuli associated with them (e.g. dead bodies), they might engender both theta and gamma brain oscillations.
This research is not without limitations. Hyperscanning EEG is well-suited to examine synchrony between two brains in temporal and frequency domains, but it has inherent limitations with respect to spatial resolution, and thus is unable to address precisely which brain areas account for the gamma interbrain synchronization observed in this study. Previous research using magnetoencephalography (MEG) has shown that the generators of gamma oscillations have been localized in the amygdala (Luo et al., 2007), which selectively codes the presence of threat and stimuli that convey fear (Adolphs et al., 1994; Whalen, 1998; Etkin et al., 2004). An interesting direction for future research therefore would be to use advanced hyperscanning MEG to go beyond single-brain analyses to examine whether gamma band synchrony is localized in the amygdala regions (among other possible regions, see Başar-Eroglu et al., 1996; Muthukumaraswamy and Johnson, 2004). This research also opens up interesting questions regarding the genetic factors that modulate interbrain synchrony when humans experience societal threat. Prior research has revealed that short-allele carriers (5-HTTLPR) exhibit a hypersensitivity of neural circuits relevant to threat (Klumpers et al., 2015). Moreover, countries with a long history of ecological and human-made threats tend to have a higher frequency of short-allele carriers (Mrazek et al., 2013). From a culture-gene coevolution perspective, it is possible that gamma interbrain activity has been evolved to a greater degree in short-allele carriers to help them survive ecological and societal threats.
It is also worth noting that the meaning of neural co-activations in our study and other hyperscanning studies more generally, needs to be explored further in future research. For example, we believe that co-occuring brain activations among two individuals performing the same task without any explicit or implicit interpersonal communication are distinct from interbrain synchrony that arises as participants work together to reach a joint goal. Put differently, in our view, people who are merely viewing the same stimuli but not interacting in a joint task would be not be attempting to understand and/or share the mental and emotional states of their partners in the service of a common goal. Accordingly, we would not suggest that neural activation in this context reflects ‘interbrain synchrony’. Indeed, extant empirical evidence is suggestive of this account. For example, in our previous study (Mu et al., 2016), interbrain synchrony selectively occurred between two individuals who were interacting for a shared goal but not between two individuals who were randomly paired but didn’t actually interact with each other. In the current study, likewise, we compared people who were interacting with each other with people who were interacting with a computer performing the exact same counting tasks. We again found that enhanced interbrain synchrony was only observed in the former and not the latter. In all, we believe that the increased interbrain synchrony in our study reflects a unique interbrain mechanism of the two interacting subjects attempting to coordinate under high societal threat, which cannot be simply identified as ‘similar brain activities’ among two individuals who are performing the same task.
To our knowledge, this is the first study to illustrate the role of shared neural representations or brain synchrony in social coordination under societal threat. The findings support what the father of Sociology, Emile Durkheim, argued decades ago: that groups are made powerful by sharing thought and action processes, what he termed ‘collective effervescence’ (Durkheim, 1965). The ability to foster collective action is particularly critical in times of societal threat, and this research shows that the sharing of gamma activity at the neural level plays a role in this collective ability among human groups. The neural findings of synchronized interbrain networks are potentially relevant to wide array of collective phenomena, whether it is why rituals have had such powerful binding effects for human groups across the centuries, how humans respond and coordinate to terrorist threats in the modern era, and why extreme events (e.g. being hurt in an earthquake) lead to collective action among humans.
Finally, this research represents an important frontier in social neuroscience, namely moving beyond single-brain analysis to study the collective brain processes of human groups. Indeed, single brain indices are generally not highly predictive of dyad level phenomena (Mu et al., 2016). Hyperscanning EEG and fMRI can now be used to study many group processes, including group decision-making, negotiation and leadership among many other important social-organizational phenomena that urgently await neuroscience perspectives.
Funding
This work was funded by an Annaliese Maier Research Award from Alexander von Humboldt Foundation.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Supplementary Material
References
- Adler N.E., Epel E.S., Castellazzo G., Ickovics J.R. (2000). Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy, White women. Health Psychology, 19(6), 586. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Tranel D., Damasio H., Damasio A. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372(6507), 669–72. [DOI] [PubMed] [Google Scholar]
- Astolfi L., Toppi J., Borghini G., et al. (2012). Cortical activity and functional hyperconnectivity by simultaneous EEG recordings from interacting couples of professional pilots. In: Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE. IEEE, 4752–5. [DOI] [PubMed]
- Başar-Eroglu C., Strüber D., Schürmann M., Stadler M., Başar E. (1996). Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. International Journal of Psychophysiology, 24(1), 101–12. [DOI] [PubMed] [Google Scholar]
- Brainard D.H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–6.9176952 [Google Scholar]
- Burgess A.P. (2013). On the interpretation of synchronization in EEG hyperscanning studies: a cautionary note. Frontiers in Human Neuroscience, 7, 881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro E.F., McCloskey M.S., Fitzgerald D.A., Phan K.L. (2007). Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry, 62(2), 168–78. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Doesburg S.M., Roggeveen A.B., Kitajo K., Ward L.M. (2008). Large-scale gamma-band phase synchronization and selective attention. Cerebral Cortex, 18, 386–96. [DOI] [PubMed] [Google Scholar]
- Dumas G., Lachat F., Martinerie J., Nadel J., George N. (2011). From social behaviour to brain synchronization : review and perspectives in hyperscanning. IRBM, 32(1), 48–53. [Google Scholar]
- Dumas G., Nadel J., Soussignan R., Martinerie J., Garnero L. (2010). Inter-brain synchronization during social interaction. PLoS One, 5, doi:10.1371/journal.pone.0012166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkheim E. (1965). The Elementary Forms of Religious Life. New York: Free Press. [Google Scholar]
- Efron B., Tibshirani R.J. (1993). An introduction to the bootstrap. Boca Raton, FL: Chapman & Hall. [Google Scholar]
- Etkin A., Klemenhagen K.C., Dudman J.T., et al. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44(6), 1043–55. [DOI] [PubMed] [Google Scholar]
- Gasser T., Möcks J. (1982). Correction of EOG artifacts in event-related potentials of the EEG: aspects of reliability and validity. Psychophysiology, 19(4), 472–80. [DOI] [PubMed] [Google Scholar]
- Greenhouse S.W., Geisser S. (1959). On methods in the analysis of profile data. Psychometrika, 24, 95–112. [Google Scholar]
- Gross J., Schmitz F., Schnitzler I., et al. (2004). Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proceedings of the National Academy of Sciences of the United States of America, 101, 13050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press. [Google Scholar]
- Isenberg N., Silbersweig D., Engelien A., et al. (1999). Linguistic threat activates the human amygdala. Proceedings of the National Academy of Sciences United States of America, 96(18), 10456–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M., Yamada Y., Ushiku Y., Miyauchi E., Yamaguchi Y. (2013). Inter-brain synchronization during coordination of speech rhythm in human-to-human social interaction. Scientific Reports, 3, 1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F., Kroes M.C., Heitland I., et al. (2015). Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biological Psychiatry, 78(8), 582–9. [DOI] [PubMed] [Google Scholar]
- Kronland-Martinet R., Morlet J., Grossmann A. (1987). Analysis of sound patterns through wavelet transforms. International Journal of Pattern Recognition and Artificial Intelligence, 1, 273–302. [Google Scholar]
- Lachaux J.P., Rodriguez E., Martinerie J., Varela F.J. (1999). Measuring phase synchrony in brain signals. Human Brain Mapping, 8, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q., Holroyd T., Jones M., Hendler T., Blair J. (2007). Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage, 34(2), 839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A., Greischar L.L., Rawlings N.B., Ricard M., Davidson R.J. (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences of the United States of America, 101, 16369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratos F.A., Mogg K., Bradley B.P., Rippon G., Senior C. (2009). Coarse threat images reveal theta oscillations in the amygdala: a magnetoencephalography study. Cognitive, Affective, & Behavioral Neuroscience, 9(2), 133–43. [DOI] [PubMed] [Google Scholar]
- Montague P. (2002). Hyperscanning: simultaneous fMRI during linked social interactions. NeuroImage, 16(4), 1159–64. [DOI] [PubMed] [Google Scholar]
- Mrazek A.J., Chiao J.Y., Blizinsky K.D., Lun J., Gelfand M.J. (2013). The role of culture–gene coevolution in morality judgment: examining the interplay between tightness–looseness and allelic variation of the serotonin transporter gene. Culture and Brain, 1(2–4), 100–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y., Guo C., Han S. (2016). Oxytocin enhances inter-brain synchrony during social coordination in male adults. Social Cognitive and Affective Neuroscience, 11, 1882–93.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y., Han S. (2013). Neural oscillations dissociate between self-related attentional orientation versus evaluation. Neuroimage, 67, 247–56. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Johnson B.W. (2004). Primary motor cortex activation during action observation revealed by wavelet analysis of the EEG. Clinical Neurophysiology, 115(8), 1760–6. [DOI] [PubMed] [Google Scholar]
- Oathes D.J., Ray W.J., Yamasaki A.S., et al. (2008). Worry, generalized anxiety disorder, and emotion: evidence from the EEG gamma band. Biological Psychology, 79(2), 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis D., Nichols T.E., Baillet S., Leahy R.M. (2005). A comparison of random field theory and permutation methods for the statistical analysis of MEG data. NeuroImage, 25(2), 383–94. [DOI] [PubMed] [Google Scholar]
- Pelli D.G. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision, 10(4), 437–42.9176953 [Google Scholar]
- Roos P., Gelfand M.J., Nau D., Lun J. (2015). Societal threat and cultural variation in the strength of social norms: an evolutionary basis. Organizational Behavior and Human Decision Processes, 129, 14–23. [Google Scholar]
- Sänger J., Müller V., Lindenberger U. (2013). Directionality in hyperbrain networks discriminates between leaders and followers in guitar duets. Frontiers in Human Neuroscience, 7, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stujenske J.M., Likhtik E., Topiwala M.A., Gordon J.A. (2014). Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron, 83(4), 919–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen P.J. (1998). Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7(6), 177–88. [Google Scholar]
- Woestenburg J.C., Verbaten M.N., Slangen J.L. (1983). The removal of the eye-movement artifact from the EEG by regression analysis in the frequency domain. Biological Psychology, 16(1), 127–47. [DOI] [PubMed] [Google Scholar]
- Yun K., Watanabe K., Shimojo S. (2012). Interpersonal body and neural synchronization as a marker of implicit social interaction. Scientific Reports, 2, 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubir A.M., Iskander D.R. (1998). Bootstrap Matlab toolbox Version 2.0. Software Reference Manual, Brisbane: Queensland University of Technology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.