Abstract
Background
The prevalence of major depression in those with HIV/AIDS is substantially higher than in the general population. Mechanisms underlying this comorbidity are poorly understood. HIV-transactivator of transcription (Tat) protein, produced and excreted by HIV, could be involved. We determined whether conditional Tat protein expression in mice is sufficient to induce depression-like behaviors and oxidative stress. Further, as oxidative stress is associated with depression, we determined whether decreasing or increasing oxidative stress by administering methylsulfonylmethane (MSM) or diethylmaleate (DEM), respectively, altered depression-like behavior.
Methods
GT-tg bigenic mice received intraperitoneal saline or doxycycline (Dox, 25–100 mg/kg/day) to induce Tat expression. G-tg mice, which do not express Tat protein, also received Dox. Depression-like behavior was assessed with the tail suspension test (TST) and the two-bottle saccharin/water consumption task. Reactive oxygen/nitrogen species (ROS/RNS) were assessed ex vivo. Medial frontal cortex (MFC) oxidative stress and temperature were measured in vivo with 9.4-Tesla proton magnetic resonance spectroscopy (MRS).
Results
Tat expression increased TST immobility time in an exposure-dependent manner and reduced saccharin consumption. MSM decreased immobility time while DEM increased it in saline-treated GT-tg mice. Tat and MSM behavioral effects persisted for 28 days. Tat and DEM increased while MSM decreased ROS/RNS levels. Tat expression increased MFC glutathione levels and temperature.
Conclusions
Tat expression induced rapid and enduring depression-like behaviors and oxidative stress. Increasing/decreasing oxidative stress increased/decreased, respectively, depression-like behavior. Thus, Tat produced by HIV may contribute to the high depression prevalence among those with HIV. Further, mitigation of oxidative stress could reduce depression severity.
Keywords: HIV, glutathione, magnetic resonance spectroscopy, major depression, oxidative stress, Tat, transgenic mice
INTRODUCTION
Major depression is among the most common psychiatric comorbidities in those with HIV-1/AIDS, occurring in 30–50% of this population (1,2). This rate substantially exceeds the 11% depression prevalence in the general population (3). Major depression in HIV+ individuals is associated with poor antiretroviral therapy compliance (4–7), increased morbidity (8,9), and accelerated mortality (8–10). Accordingly, advancing our understanding of factors driving the high depression comorbidity among people with HIV may lead to treatments that reduce depression, improve treatment compliance, and reduce morbidity and mortality.
Mechanisms underlying the HIV-1/depression comorbidity have yet to be elucidated. However, virally-produced proteins, including HIV-transactivator of transcription (Tat) protein, may be involved. Tat protein is produced and excreted by HIV-1 virus, including that persisting in reservoirs during antiretroviral therapy. Tat protein or antibodies to newly-excreted Tat protein are detectable in cerebrospinal fluid or blood in 40–50% of patients (11–12), and, over the course of a year, in nearly 90% of antiretroviral therapy-treated patients (11). As infusion of Tat protein acutely increased depression-like behaviors in mice (13) and conditional Tat protein expression induced reward deficits in mice, possibly reflecting anhedonia, a core feature of depression (14), Tat protein could increase vulnerability for depression.
Tat triggers a number of biological effects but its capacity to increase oxidative stress (15–30) could catalyze symptoms of depression. Increased oxidative stress has been associated with behavioral depression in humans (31–37) and animals (38–42). Further, oxidative stress mitigation reduces depression severity in humans (33,43–45) and in animal models (38–42,46,47).
To further investigate whether Tat increases depression-like behavior and whether oxidative stress is involved, we used GT-tg bigenic mice, which contain a doxycycline (Dox)-inducible astrocyte-specific promoter enabling conditional Tat protein expression (48). These mice initially were constructed by crossing Teton-GFAP with TRE-Tat86 mice, to obtain GT-tg bigenic mice (48). Dox treatment induces Tat protein expression in these mice at concentrations of 0.1–0.9 ng/ml in vitro (48), comparable to cerebrospinal fluid Tat concentrations detected in antiretroviral therapy-treated HIV patients (12). Prior studies of Dox-induced Tat-expressing mice have reported cellular (48–55), brain structural (56), and behavioral or cognitive (14,49,50,52–55,57–60) abnormalities. However, to date, no studies have reported on whether controlled Tat protein expression induces depression-like behavior on tasks commonly used to assess such behavior in mice.
Accordingly, we used the tail suspension test (TST) and the two-bottle (saccharin/water) consumption test to determine whether Tat expression induces depression-like behavior. Both tests have been used extensively in mice to characterize depression-like behavior and antidepressant effects (13,38,39,42,46,47,61–64). We also tested whether pretreatment with the antioxidant methylsulfonylmethane (MSM), which mitigates Tat-induced oxidative stress in neuronal culture (30), or pretreatment with the pro-oxidant diethyl-maleate (DEM), which conjugates with and rapidly depletes brain and peripheral glutathione levels (65,66), altered TST behavior. We hypothesized that Tat expression would promote depression-like behaviors and that MSM and DEM would reduce and enhance, respectively, depression-like behavior.
To confirm that Tat protein expression, MSM, and DEM altered cerebral oxidative stress, we measured postmortem levels of whole brain reactive oxygen species/reactive nitrogen species (ROS/RNS). We hypothesized that Tat and DEM would increase ROS/RNS levels and that MSM would reduce ROS/RNS levels.
Lastly, we conducted 9.4 Tesla proton magnetic resonance spectroscopy (MRS) in separate groups of GT-tg bigenic mice to test whether Tat protein altered medial frontal cortex (MFC) glutathione (GSH) levels. MFC was selected for study because it is involved in major depression (67–69). GSH is the principal endogenous antioxidant molecule (70). GSH levels rise slowly with aging, possibly to counteract age-associated increases in cortical oxidative stress (71). However, MRS studies in people with major depression reported higher anterior cingulate cortex GSH levels and that GSH level was associated with depression symptom severity (32). Similarly, in people at risk for major depression, stabilization of thalamic GSH levels with fatty acid supplements was associated with lower depression severity than found in placebo-treated subjects, in whom GSH levels increased (33). While we are unaware of any MRS studies in HIV+ subjects or in animal models reporting on brain GSH levels, postmortem thalamic GSH levels were elevated in older HIV-1 transgenic rats (72), which exhibit depression-like behavior (64). Accordingly, we hypothesized that Tat protein expression would increase MFC GSH levels. We also characterized Tat’s effects on MFC temperature, which can be determined in proton spectra (73). Because Tat increases Tumor Necrosis Factor alpha levels (13,74–76), which increase expression of mitochondrial uncoupling proteins (77–81) that dissipate oxidative stress in the form of heat (82), we hypothesized that Tat protein expression in GT-tg mice would increase MFC temperature.
METHODS AND MATERIALS
Subjects
GT-tg bigenic mice (47) and G-tg mice, in which the Tat transgene was outbred from GT-tg mice, were produced at the University of Florida (UF). C57BL/6J mice (Jackson Laboratories), a parent strain of GT-tg mice, also were used in ROS/RNS studies. Male mice, 8–13 weeks of age, were studied. Subjects were maintained on a 12:12 light:dark cycle and had ad libitum food and water access. For imaging studies, GT-tg mice were transported from UF to McLean Hospital via commercial courier and acclimated to the McLean Hospital vivarium for at least one week prior to initiating study procedures. Animal procedures were approved by Institutional Animal Care and Use Committees at both institutions and conformed to National Research Council guidelines (83). Supplemental Information Table S1 summarizes studies that were conducted.
Drug Treatments
Tat protein expression was induced in GT-tg bigenic mice by intraperitoneal administration of Doxycycline hyclate (Dox, Sigma-Aldrich, St. Louis, MO). Dox was prepared fresh daily by dissolving in vehicle (sterile 0.9% saline) to the concentration enabling administration of 0.1 ml per 10 g body weight, and protected from light. Methylsulfonylmethane (MSM, Sigma-Aldrich), which has a half-life in rodents of about 12 hours (84), was administered intraperitoneally at 100 mg/kg/day, 20 minutes before GT-tg mice were administered saline/Dox, adapted from (85). Diethyl maleate (DEM, Sigma-Aldrich), which rapidly depletes GSH with a half-life of about 15 hours (64), was given intraperitoneally for one or two days. DEM was administered at a dose of 34.8 μM in 0.25 ml saline, and in pretreatment studies was given 30 minutes before saline or Dox based on prior studies in mice (65,86).
Tail Suspension Test (TST)
A TST protocol modified from (61) was conducted. Mice were supported by a cage lid and their tails were secured with laboratory tape to a horizontal surface 18 inches above the floor. Prior to taping, a small, clean plastic disc was placed over tails to prevent tail climbing, which confounds the assay. Then, cage tops were slowly removed to suspend mice for 5 minutes. Time spent immobile (the adoption of a stationary posture) was measured by treatment-blinded observers. TST tests were conducted in mice after 1 or 7 days of treatments, and again 7, 14, and 28 days after terminating drug treatments to assess for enduring treatment effects.
Saccharin Consumption Task
The saccharin consumption protocol was modified from (62). Individually-housed mice were habituated for 4 days to drink from two 100-mL bottles containing water. On day 5, the second bottle was filled with saccharin (0.2% w/v). To control for bottle position preference, bottle position was changed every 24 h at 1200. Body weight (g) and fluid intake (g and mL) were measured daily.
Magnetic Resonance Imaging (MRI) and Spectroscopy (MRS)
Imaging was performed at McLean Hospital in isoflurane-anesthetized (1.5–2%) GT-tg mice after 7 days of intraperitoneal saline or 100 mg/kg/day Dox injections. We used a 9.4 Tesla Direct Drive Scanner (Agilent, Inc.), a horizontal 60 mm inner diameter gradient insert with a strength of 100G/cm, and a custom-made volume coil for MRI and MRS. Vital signs were monitored (Small Animal Instruments, Inc.) and maintained by adjusting isoflurane concentration and warm air flow.
Fast-spin-echo scans were acquired coronally (TR (repetition time)=2.1 seconds, TE (echo time)=60 milliseconds, acquisition matrix=128×128) to enable MRS voxel (2.0×3.0×2.5 mm=15 μl) positioning over MFC (Figure 1). We used an ultrashort TE stimulated echo acquisition mode (STEAM) sequence (87) with TR/TE=4 seconds/3 milliseconds, 4096 complex points, 5000 Hz acquisition bandwidth, and 1 ms 90° excitation pulse. FASTMAP automated shimming (88) produced 10–13 Hz water linewidths. VAPOR water suppression (89) was used to acquire water-suppressed partial spectra as 5 blocks of 128 shots, taking 8.5 minutes each, to minimize effects of frequency drift. Traces from each block were added using the 2.0 ppm N-acetylaspartate (NAA) peak to synchronize blocks. Each complete MRS spectrum contained 640 shots (42.5 minutes). Water-unsuppressed spectra (4 shots) were acquired in tandem with each water-suppressed spectrum block using identical parameters, except for water suppression. Water-unsuppressed spectra were used to correct for phase and eddy current distortions, to derive tissue water concentration used to normalize metabolite levels, and to calculate water chemical shifts for MFC temperature measurement (see below).
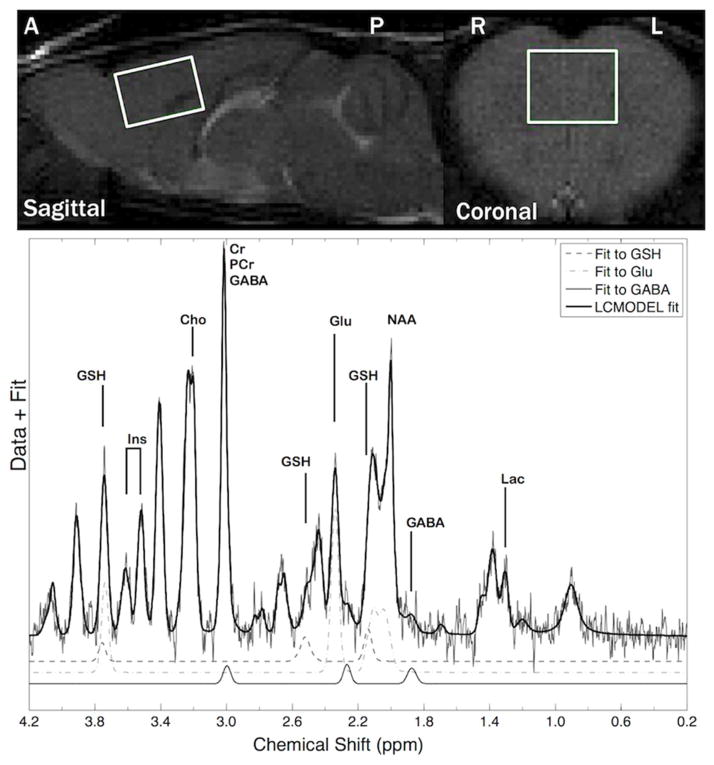
Figure 1.
(Top panel) Magnetic resonance images of a mouse frontal cortex MRS voxel. (A/P=anterior/posterior; R/L=right/left. (Bottom panel) Magnetic resonance spectroscopy (MRS) spectrum from a GT-tg bigenic mouse obtained on day 8 after 7 days of drug treatment. Shown are overall and GABA, glutamate (Glu) and glutathione (GSH) peak fits.
MRS Data analyses
Water-suppressed spectra were pre-processed using custom-written Matlab code (Mathworks, Inc., vR2012a). Spectra were visually inspected and apodized using a 4 Hz exponential filter, to remove high frequency noise, which improves spectrum quality without affecting metabolite ratios (90). Water-suppressed spectra were corrected for the phase and residual eddy currents (91). Pre-processed spectra were fitted with LC Model to determine metabolite levels and water peak frequencies (92) using a GAVA-simulated basis set (93). A sample fitted water-suppressed spectrum is shown (Figure 1).
Temperature Estimation
MFC temperature was estimated by calculating the chemical shift difference between each partial spectrum N-acetylaspartate (NAA) peak and its paired unsuppressed-water water peak (73). Spectra were processed with 2Hz exponential apodization and the auto peak picker function in MestRenova (v. 9.0) was used to identify water and NAA resonance frequencies. Temperature was estimated by comparing water (H2O) and NAA chemical shifts (δ) (73):
ROS/RNS Quantification
Whole-brain ROS/RNS were quantified ex vivo per manufacturer’s guidelines (OxiSelect fluorescence assay, STA-347, Cell Biolabs, Inc.) in brains from GT-tg mice administered saline/Dox for 1, 7 or 14 days, in mice pretreated with MSM (100 mg/kg i.p.), 10 minutes before saline/Dox for 7 or 14 days, and in C57BL/6J mice administered DEM (34.8 μM, 250 μL per 25g, 30% Solutol/Saline) for 1 or 2 days or vehicle (30% Solutol/Saline) for 1 or 5 days. After drug treatments, mice were anesthetized with isoflurane, euthanized by cervical dislocation, brains were extracted and flash frozen in liquid nitrogen, and stored at −80°C. Tissue was homogenized to 25 m g/mL concentration in phosphate buffered saline (pH 7.2) with a QSonica sonicator. Homogenate aliquots (1 mL) were centrifuged (5 minx6000 rpm, Corning® LSE™ Mini-Microcentrifuge) and supernatant was immediately assayed with a Synergy H1 Multi-mode reader (BioTek Instruments, Inc.).
Statistical analyses
Behavioral and ROS/RNS statistical analyses
Data are presented as mean±SEM. Behavioral data were analyzed via univariate ANOVA (Prism 7 GraphPad Software). A one-way ANOVA was used with the between-subject factor of Dox dose (0–100 mg/kg/d, 7 days) on TST immobility time in GT-tg mice. Additional group differences were examined by two-way ANOVAs with factors of duration (1 and 7 days) x treatment (saline or Dox, 100 mg/kg/d) and their interaction, or with factors of treatment (saline or Dox) x mouse strain and their interaction. Post hoc testing with Tukey’s HSD was used to compare all means. Saccharin consumption was analyzed by two-way ANOVA with factors time x treatment). Sidak’s Multiple Comparison post hoc tests were used to control family-wise error rates and assess group differences following main effects. A three-way ANOVA (SPSS software, IBM) was used to analyze effects of Tat (Dox or saline treatment), antioxidant treatment with each condition and post-treatment testing day as factors. Additional two-way ANOVAs were run to compare simple main effects of all interactions between factors, with Tukey’s HSD post hoc testing as appropriate. A one-way ANOVA was used to analyze effects of DEM exposure. One-way ANOVAs also were used to analyze ROS/RNS data with factors of time or mouse strain, and Tukey’s HSD post hoc test. Cohen’s d values were used to assess effect size magnitudes, where appropriate. Student-Neuman Keuls post hoc test was used in one case of ROS/RNS analysis (with C57BL/6J mice) to compensate for an uneven sample size. Analyses were considered significant when p≤0.05.
MRS statistical analyses
MRS metabolite differences were assessed using two-sample, two-tailed t-tests (Stata v12, StataCorp.). Temperature effects were assessed using repeated measures two-way ANOVA with treatment and partial acquisition as the between- and within-subjects factors, respectively, and with Bonferroni correction. GSH levels were compared to brain temperature via a Pearson correlation analysis, as detailed below. Brain temperature analyses were conducted with Prism 7 (GraphPad Software).
Data values two standard deviations from group means were excluded: 2 measurements were excluded from MRS, 1 from ROS/RNS measurements, 5 from brain temperature and 18 from TST measurements. Two of 16 Dox-treated mice died between days 13–14, and 3 died between days 15–16 of the saccharin consumption task, resulting in 14 missing data points from these later testing days.
RESULTS
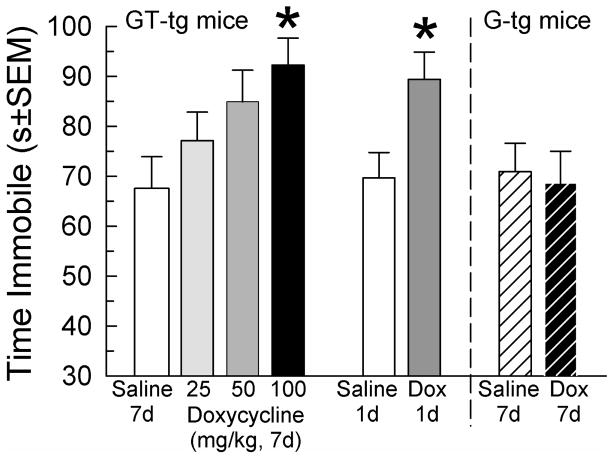
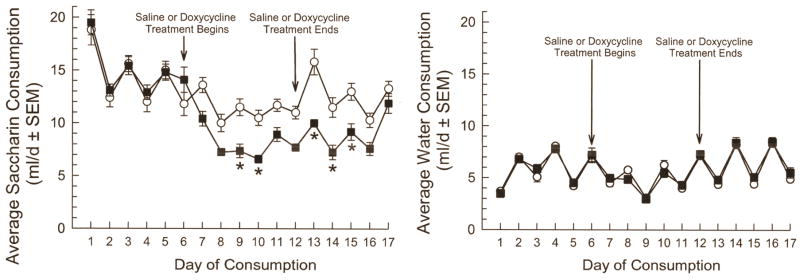
Tat expression in GT-tg bigenic mice for 7 days increased day 8 TST immobility time in a dose-related manner (F(3,66)=3.20, p=0.03; 1-way ANOVA with factor: dose Dox, Tukey’s HSD, Figure 2). Tat expression for only one day increased TST immobility time versus saline-treated GT-tg mice (F(1,80)=15.43, p=0.0002; 2-way ANOVA with factor: Dox treatment, with Tukey’s HSD; Figure 2), by a magnitude equivalent to that after 7 days of Dox administration (F(1,80)=0.005, p=0.95; 2-way ANOVA with factor: treatment days; p=0.73; Figure 2). By contrast, 7-day Dox treatment of G-tg mice did not increase day 8 immobility time over saline-treated G-tg mice (p=0.79, Student’s t-test; Figure 2). Effects of strain on day 8 immobility were significant (F(1,77)=4.76, p=0.03; 2-way ANOVA with factors: treatment x strain), indicating that Tat expression is required to observe immobility increases. Accordingly, subsequent behavioral experiments were conducted in GT-tg mice administered saline (controls) or induced with Dox (100 mg/kg/day, i.p.). Additional groups of GT-tg mice were administered Dox or saline for 7 days and tested for saccharin-flavored water consumption. Tat-expressing mice consumed less saccharin-flavored water (Figure 3). Group differences in saccharin preference became apparent after 1 day and significant after 3 days of Dox treatment (F(1,496)=4.31, p<0.0001; 2-way ANOVA, Sidak’s Test Figure 3). By contrast, Tat expression did not alter water consumption (F(1,496)=1.13, p=0.288; 2-way ANOVA, Figure 3).
Figure 2.
Tat protein increases immobility time in the tail-suspension test (TST) in a dose-related manner. GT-tg mice (open bars) were treated 7d with saline (N=16, white bar, far left) or Doxycycline (Dox, 25 (N=16), 50 (N=17), or 100 (N=18) mg/kg/d, i.p.) and tested on day 8. Additional GT-tg mice were treated 1d with saline (off white bar, center, N=25) or Dox (100 mg/kg, i.p.; dark grey bar, N=25). Mice lacking the Tat gene (G-tg mice, striped) were treated for 7d with saline (N=21) or Dox (100 mg/kg, i.p.; black striped bar, N=24) and then tested in the TST on day 8. *= p<0.05 vs. matching response of saline-treated (uninduced) GT-tg bigenic mice of same duration. Effect sizes (Cohen’s ds) were 0.86 and 1.03 for mice treated with Dox (100 mg/kg/day) for 1 and 7 days, respectively as compared to saline-treated mice.
Figure 3.
Tat protein exposure reduces saccharin, but not water consumption in a two-bottle assay. Daily consumption (ml/d) of saccharin (left panel) or water (right panel) shown 6d prior to drug administration, during daily saline (white circles, N=16) or Dox (100 mg/kg/d, i.p.; black squares, N=16) treatment for 7d, and for 5 days thereafter. *= p<0.05 vs. matching response of saline-treated (uninduced) mice of same day. Effect sizes (Cohen’s ds) for significantly different paired time points ranged from 1.18 to 1.72.
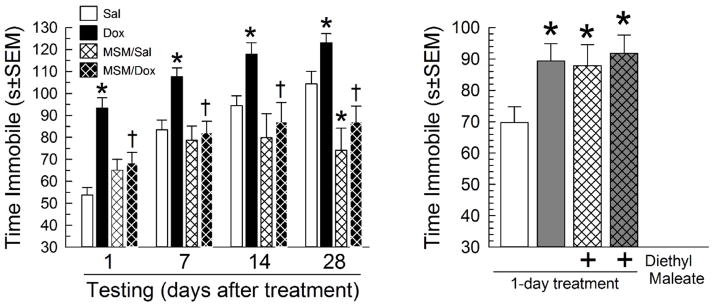
Next, we determined whether MSM pretreatment altered TST immobility time. There was an overall effect (F(15,360)=12.8, p<0.0001; 3-way ANOVA with factors of Tat exposure x drug treatment x testing time after treatment; Figure 4). Replicating and extending initial results, 7-day Dox treatment increased day 8 immobility time, an effect that persisted for up to 28 days after Tat induction was terminated, versus saline-treated controls (F(1,360)=28.5, p<0.001; 2-way ANOVA, factor: Tat exposure; Figure 4). MSM pretreatment had a globally significant effect on immobility time (F(1,360)=36.1, p<0.001; 2-way ANOVA, factor: drug treatment), reducing day 8 immobility time in Tat-expressing mice (F(1,360)=13.2, p<0.001; 2-way ANOVA, factors Tat exposure x drug treatment; Figure 4) and for up to 28 days later (p<0.01 each post hoc test; Figure 4). MSM treatment did not significantly alter day 8 immobility time in saline-treated controls (p=0.30; Figure 4a), but it reduced it in saline-treated mice tested 28 days after the last drug treatments (p<0.05, Tukey’s HSD post hoc test; Figure 4). By contrast, a single pretreatment with DEM before saline administration substantially increased day 2 TST immobility time (F(3,91)=3.21, p=0.03; 1-way ANOVA, Tukey’s HSD; Figure 4) by the same magnitude as in Dox-only treated mice (87.9±6.6 vs 89.4±5.4s, respectively). However, DEM pretreatment before Dox administration did not further increase immobility time in Tat-expressing mice (p=0.99; Figure 4).
Figure 4.
Left panel Antioxidant pretreatment mitigates Tat-induced increases in tail suspension test (TST) immobility time. GT-tg mice were treated daily for 7d with saline (white bars, N=30) or Dox (100 mg/kg/d, i.p.; black bars, N=30) alone, or were treated daily with saline (N=20) or Dox (N=22) 20 min after daily pretreatment with methylsulfonylmethane (MSM, 100 mg/kg/d, i.p.; thatched bars). Mice then were tested in the TST 1, 7, 14 and 28 days after drug treatments had concluded. *= p<0.05 vs. matching response of saline-treated (uninduced) GT-tg bigenic mice of same duration; †= p<0.05 vs. matching Dox-treated (Tat-exposed) GT-tg bigenic mice of same duration. Effect sizes (Cohen’s ds) for Dox alone vs saline across paired time points ranged from 1.72 (d1) to 0.71 (d28) and effect sizes of MSM plus Dox versus Dox alone ranged from 1.01 (d1) to 1.55 (d28). Right Panel: Diethyl maleate (DEM) increases immobility time in the tail-suspension test. GT-tg mice were treated 1-day with saline (N=25, white bars) or Dox (N=25, 100 mg/kg, i.p.; grey bars) alone or pretreated with DEM (34.8 μM/0.25 ml, i.p.; N=23 (with saline), N=22 (with Dox), thatched bars) and tested the next day. *= p<0.05 vs. matching response of saline-treated (uninduced) GT-tg bigenic mice. Effect sizes (Cohen’s ds) versus saline controls were 0.75 (Dox alone), 0.64 (DEM+saline) and 0.84 (DEM+Dox).
Saline treatment for 7 days resulted in statistically equivalent ROS/RNS levels in GT-tg and G-tg mice (N’s=12/group) and in C57BL/6J mice administered vehicle (N=8) for 5d (3940±402 vs. 3860±282, 3850±384, respectively; F(2,29)=0.02, p=0.98; 1-way ANOVA, Tukey’s HSD). Dox administration for 1, 7, or 14 days (N’s=10/group) increased ROS/RNS levels in GT-tg mice (4990±379, 4340±200 and 5010±292, respectively), an effect attaining significance at 14 days (p=0.04, Effect size (Cohen’s d) = 1.16). By contrast, G-tg mice administered Dox for these durations (N’s=10/group) exhibited no significant ROS/RNS increase (3980±460, 3980±318 and 4220±273, respectively). Separate groups of GT-tg mice were pretreated for 7 or 14 days with MSM 10 min before saline (N=10 for 7d and N=12 for 14d treatment groups) or Dox (N=12 for both 7 and 14d treatment groups). MSM decreased ROS/RNS in saline-treated animals at both time points (2900±199 and 2760±104, respectively; F(3,40)=6.57 p=0.001; 1-way ANOVA w/Student-Newman-Keuls post hoc Method) and abolished Tat-induced ROS/RNS increases at both time points (2680±101 and 2440±116, respectively; F(3,39)=50.1, p<0.001; 1-way ANOVA, Tukey’s HSD). In C57BL/6J mice, DEM treatment for 1 (N=7) or 2 (N=13) days increased ROS/RNS levels (4680±631 and 5520±376, respectively; F(3,30)=5.16, p=0.005; 1-way ANOVA, Tukey’s HSD) to a degree equivalent to those detected in GT-tg mice after prolonged Tat exposure.
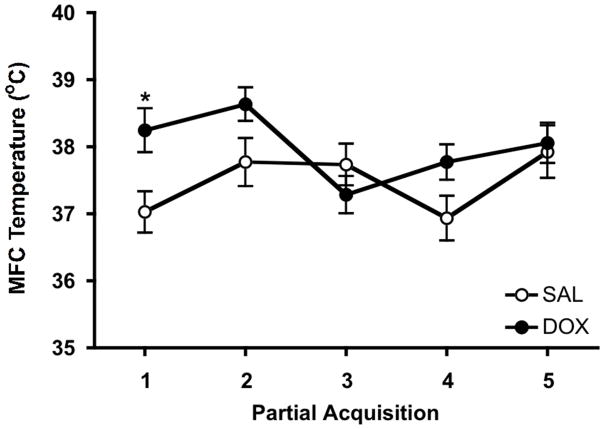
Dox administration to GT-tg mice for 7 days increased MFC GSH/water ratios, which averaged 130% of saline controls (t=2.98, p=0.006; Table 1). Voxel water content did not differ by treatment (Table 1). Thus, the GSH/water ratio difference is attributable to a group difference in GSH level. Dox-treated mice also exhibited higher MFC temperature than saline controls (F(1, 22)=5.42; p=0.030; Figure 5) and there was an effect of time, e.g., partial acquisition order (F(4, 88)=2.65; p=0.038). Post-hoc testing revealed that temperature was elevated only during the first MRS partial acquisition block (p=0.036). Accordingly, we tested for an association between first partial acquisition temperatures and GSH/H2O ratios and found a positive correlation between these measures (r=0.47, p=0.024; Figure S1).
Table 1.
Medial frontal cortex MRS measures on day 8 in GT-tg mice administered doxycycline or saline (control).
| MRS Measure | Sala (Ns) | Doxa (Ns) | p-valuesb | (t-statistic, df) |
|---|---|---|---|---|
| GABA/H2O | 15.5 ± 3.4 (14) | 14.3 ± 6.9 (14) | 0.57 | (−0.58, 26) |
| Gln/H2O | 19.9 ± 5.7 (12) | 21.1 ± 8.1 (11) | 0.68 | (0.42, 21) |
| Glu/H2O | 93.3 ± 12.3 (14) | 87.0 ± 18.8 (14) | 0.30 | (−1.06, 26) |
| Gly/H2O | 18.2 ± 6.4 (11) | 17.7 ± 9.1 (12) | 0.87 | (−0.17, 21) |
| GSH/H2O | 15.1 ± 3.5 (14) | 19.0 ± 3.3 (14) | 0.006 | (2.98, 26)c |
| Ins/H2O | 45.9 ± 6.7 (15) | 47.5 ± 13.9 (14) | 0.69 | (0.40, 27) |
| Lac/H2O | 13.0 ± 4.6 (13) | 12.3 ± 2.6 (11) | 0.65 | (−0.47, 22) |
| NAA/H2O | 56.0 ± 8.1 (14) | 55.7 ± 8.7 (14) | 0.93 | (−0.09, 26) |
| Tau/H2O | 141.4 ± 17.1 (14) | 137.8 ± 25.8 (14) | 0.67 | (−0.43, 26) |
| tCho/H2O | 17.6 ± 2.8 (15) | 16.0 ± 3.8 (14) | 0.20 | (−1.31, 27) |
| tCr/H2O | 90.8 ± 12.1 (14) | 92.5 ± 19.9 (14) | 0.78 | (0.28, 26) |
|
| ||||
| H2O | 230.8 ± 17.8 (15) | 236.2 ± 20.8 (14) | 0.46 | (0.76, 27) |
Sal = Saline-treated for 7d; Dox = Dox-treated for 7d; df = degrees of freedom; Gln = glutamine; Glu = glutamate; Gly = glycine; GSH = glutathione; H2O = unsuppressed water; Ins = myo-inositol; Lac = lactate; NAA = N-acetylaspartate; Tau = Taurine; tCho = total choline; tCr = total creatine;
Mean ± SD (N);
2-sided values,
p ≤ 0.05;
p ≤ 0.01;
Effect size (Cohen’s d)=1.15.
Only measurements with Cramér-Rao Lower Bound (CRLB) values ≤ 30% are included. Measures with Ns < 14 and dfs < 27 indicate that some CRLB values exceeded 30% and/or that some values exceeded two standard deviations from respective group means.
Figure 5.
Tat protein induction increases medial frontal cortex (MFC) temperature. GT-tg mice were treated for 7 days with saline (open circles) or Dox (100 mg/kg, i.p.; filled circles) and scanned on day 8. Shown are means ± SEMs with Ns=12 mice per group. Effects of treatment: ANOVA F(1, 22)=5.42; p=0.030. Effects of partial acquisition order (e.g., time): ANOVA F(4,88)=2.65; p=0.038. *p=0.036, post-hoc test. First partial acquisition temperature difference was 1.2±1.1ºC. Effect size (Cohen’s d)=1.11.
DISCUSSION
Conditional Tat protein expression rapidly increased depression-like behaviors with near-maximal effects achieved after one day of Tat expression. Dox treatment had no effect in G-tg mice, further implicating Tat protein in depression-like behaviors. Tat’s rapid effects in GT-tg mice are consistent with reports that intracerebroventricular Tat infusion induces depression-like behaviors within 24 hours (13) and that conditional Tat expression rapidly elevates intracranial self-stimulation reward thresholds (14). Tat’s depression-like effects in the TST persisted for up to 28 days after terminating Dox treatments. As Tat levels return to baseline within 14 days of terminating Dox exposure in GT-tg mice (57), Tat’s depression-like effects appear to outlive its detectible expression by at least 2 weeks. If Tat protein exerts similar effects in HIV-infected individuals including in those taking antiretroviral therapy, in whom new Tat protein is sporadically produced and excreted by HIV reservoirs (11), this could contribute to the high prevalence of major depression in those with HIV.
Tat’s depression-like effects were blunted by MSM. In neuronal culture, MSM substantially neutralized Tat-induced oxidative stress and mitigated GSH depletion (30). Consistent with these effects, MSM pretreatment prevented Tat-induced ROS/RNS accumulation in Dox-induced GT-tg mouse brain. This suggests that Tat-induced oxidative stress promotes depression-like behavior. Twenty-eight days after terminating treatments, TST immobility time was lower in MSM/saline-treated mice, suggesting an antidepressant-like effect of MSM. This is consistent with reports indicating that antioxidants can reduce depression-like behaviors in animals (38–42,46,47,63) and can improve symptoms of major depression in humans (33,43–45).
A single pretreatment with DEM, which rapidly depletes brain and peripheral GSH levels (65), increased immobility time in saline-treated GT-tg mice, indicating that GSH depletion by itself has rapid depression-like effects. By contrast, a single DEM pretreatment in Dox-treated GT-tg mice did not further increase TST immobility time. In vitro studies of ROS/RNS levels confirmed that Tat induction and DEM increased and MSM decreased oxidative stress, respectively. Collectively, these data support the hypothesis that both Tat and DEM induce depression-like behavior by increasing, and that MSM reduces depression-like behavior by decreasing, oxidative stress. A limitation of our MSM and DEM studies is that we only tested a single dose of these agents at single pretreatment time points, and therefore it is possible that different pretreatment conditions (doses/timings/durations) might yield different effects. While outside the scope of the present study, future testing will examine these relationships.
In MRS studies, conditional Tat protein expression for 7 days increased MFC GSH levels. No other MRS metabolite changes were apparent, suggesting that the GSH increase is a selective effect of Tat expression. Since GSH is the principal endogenous antioxidant molecule (70), it might be expected that the higher GSH levels we detected reflect lower rather than higher oxidative stress levels. However, postmortem and in vivo imaging studies in healthy humans and animals indicate that brain GSH levels are relatively stable in mature mice until late adulthood/senescence, at which point GSH may increase or decrease in different brain regions (71,94–98). Thus, our observation of increased GSH levels in young adult (8–13 weeks old) Tat-expressing mice over the relatively short interval studied (8 days in MRS studies) likely indicates a pathophysiological state induced by Tat protein. As noted above, Tat protein induces oxidative stress and pro-oxidants can increase brain GSH levels (99). Elevated GSH levels also have been reported in animal models of other disorders involving oxidative stress such as Huntington’s Disease, and were interpreted to reflect an endogenous compensatory response to oxidative stress (100,101). Accordingly, we hypothesize that the Tat-induced GSH increase we detected with MRS represents a compensatory response to Tat-induced oxidative stress. Apparently, this response is insufficient to overcome oxidative stress or depression-like behavior over the time frame we studied, the latter of which persisted long after Tat protein induction was terminated and after elevated Tat protein levels normalize (57,60).
Among its signaling properties, Tat protein increases Tumor necrosis factor alpha expression (13,49,74,76) which in the short-term can increase expression of the antioxidant response element transcription factor Nuclear factor-erythroid 2-related factor 2 (Nrf2), resulting in increased GSH production (102). Tat protein also increases Nrf2 expression and activity through the activation of glutamatergic N-methyl-D-aspartate (NMDA) receptors and the enzyme spermine oxidase (103). Thus, the GSH increase we detected could be mediated by multiple mechanisms. Future MRS studies in GT-tg bigenic mice administered Dox for longer periods and DEM or MSM at different doses and durations are needed to determine which mechanisms drive Tat-induced GSH changes and over what time courses GSH increases occur.
Conditional Tat protein expression also increased MFC temperature. Post-hoc analysis revealed that temperature was higher only transiently, during the first partial MRS spectrum. This may be a consequence of isoflurane anesthesia, which substantially increases cerebral perfusion (104–106), a primary mechanism for cooling the brain (107). Thus, sustained cerebral cooling by isoflurane-enhanced perfusion could normalize MFC temperature in Tat-expressing mice in later partial spectra. The temperature increase we detected could result from neuronal hyperactivity (107–109), inflammation (110,111), and/or oxidative stress, the latter of which increases expression of heat-generating mitochondrial uncoupling proteins (77–82). Tat induces neuronal hyperexcitability (112,113), inflammation (13,49,114–120), and as noted above, oxidative stress. Our finding of a strong correlation between initial brain temperature and GSH level (Figure S1) suggests that oxidative stress in part mediated the temperature effect.
Oxidative stress mitigation, which reduced depression-like behavior in Tat-expressing GT-tg bigenic mice, also could be adopted as an adjunct treatment for HIV. Antioxidant treatment for HIV was proposed more than two decades ago when it was discovered that plasma levels of cysteine and the cysteine dimer cystine, both precursors for intracellular GSH, as well as GSH itself, are depleted in HIV (121). Consistent with this early work, a recent postmortem study reported low frontal cortex GSH levels in HIV+ subjects (122). As oxidative stress increases and antioxidants can suppress HIV virus replication (123–126), antioxidants not only might reduce depression-like behavior but also could help maintain HIV latency. Antioxidants may even counteract undesirable effects of antiretroviral therapy including oxidative stress (127,128), which is higher in patients with better antiretroviral therapy compliance (127).
CONCLUSIONS
Our data demonstrate that HIV-Tat protein can induce depression-like behaviors by increasing oxidative stress. Moreover, our data suggest that treatments capable of mitigating oxidative stress may be effective for reducing depression severity. In light of these findings, care may be necessary when applying HIV reservoir eradication strategies involving oxidative stress-exacerbation (129), which could enhance depression severity. The noninvasive nature of MRS and the improved ability of newly-developed MRS protocols to detect GSH in humans (130,131) may facilitate research in this area, including research into novel treatments for HIV and major depression.
Supplementary Material
Table S1. Study Conditions
Figure S1: First MRS partial acquisition medial frontal cortex temperature correlates with medial frontal cortex glutathione (GSH) level (r=0.47, p=0.024). GT-tg mice were treated for 7 days with saline (open circles, N=11) or Dox (100 mg/kg, i.p.; filled circles, N=12) and scanned on day 8. Shown is the regression line (solid) and the 95% confidence interval (dashed lines).
Acknowledgments
Support was provided in part by NIH grants R01-MH085607, R01-DA039044, T32DA015036, K99-DA039791, K08DA037465, and S10-RR019356, and by the Counter-Drug Technology Assessment Center, an office within the Office of National Drug Control Policy, via contract number DBK39-03-C-0075, awarded by the Army Contracting Agency. The content of the information does not necessarily reflect the position or the policy of the Government and no official endorsement should be inferred. The funding sources played no role in study design, execution, data analysis, or decision to publish.
We thank Ms. Anna Rock for technical assistance with this project.
Footnotes
Portions of these data were presented previously in abstract form at the 78th Annual Scientific Meeting of the College on Problems of Drug Dependence, June 11–16, 2016, La Quinta, CA.
Disclosures
GDV has an ownership interest in EXQOR Technologies Inc. that owns patent rights to clathrin nanotechnologies. MJK receives funding from the National Institute on Drug Abuse and National Institute of Mental Health. He is an inventor of a technology using xenon gas to treat aversive memory disorders including Post-Traumatic Stress Disorder, which has been licensed by Nobilis Therapeutics, Inc. MJK also currently holds leadership roles in the College on Problems of Drug Dependence. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 2.Bengtson AM, Pence BW, Crane HM, Christopoulos K, Fredericksen RJ, Gaynes BN, et al. Disparities in Depressive Symptoms and Antidepressant Treatment by Gender and Race/Ethnicity among People Living with HIV in the United States. PLoS One. 2016;11:e0160738. doi: 10.1371/journal.pone.0160738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47:384–390. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58:181–187. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 2012;16:2119–2143. doi: 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11:291–307. doi: 10.1007/s11904-014-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. HIV Epidemiology Research Study Group. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 9.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 10.White JR, Chang CC, So-Armah KA, Stewart JC, Gupta SK, Butt AA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: Veterans Aging Cohort Study. Circulation. 2015;132:1630–1638. doi: 10.1161/CIRCULATIONAHA.114.014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mediouni S, Darque A, Baillat G, Ravaux I, Dhiver C, Tissot-Dupont H, et al. Antiretroviral therapy does not block the secretion of the human immunodeficiency virus tat protein. Infect Disord Drug Targets. 2012;12:81–86. doi: 10.2174/187152612798994939. [DOI] [PubMed] [Google Scholar]
- 12.Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110:13588–13593. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain Behav Immun. 2011;25:1569–1575. doi: 10.1016/j.bbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesby JP, Markou A, Semenova S. The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology. 2016;109:205–215. doi: 10.1016/j.neuropharm.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- 16.Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D. Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J Neurovirol. 1998;4:281–290. doi: 10.3109/13550289809114529. [DOI] [PubMed] [Google Scholar]
- 17.Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, et al. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- 18.Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Price TO, Uras F, Banks WA, Ercal N. A novel antioxidant N-acetylcysteine amide prevents gp120- and Tat-induced oxidative stress in brain endothelial cells. Exp Neurol. 2006;201:193–202. doi: 10.1016/j.expneurol.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal L, Louboutin JP, Strayer DS. Preventing HIV-1 Tat-induced neuronal apoptosis using antioxidant enzymes: mechanistic and therapeutic implications. Virology. 2007;363:462–472. doi: 10.1016/j.virol.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free Radic Biol Med. 2010;48:1388–1398. doi: 10.1016/j.freeradbiomed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buccigrossi V, Laudiero G, Nicastro E, Miele E, Esposito F, Guarino A. The HIV-1 transactivator factor (Tat) induces enterocyte apoptosis through a redox-mediated mechanism. PLoS One. 2011;6:e29436. doi: 10.1371/journal.pone.0029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiede LM, Cook EA, Morsey B, Fox HS. Oxygen matters: tissue culture oxygen levels affect mitochondrial function and structure as well as responses to HIV viroproteins. Cell Death Dis. 2011;2:e246. doi: 10.1038/cddis.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 Tat neurotoxicity: a model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2012;45:657–470. doi: 10.1016/j.nbd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Chen F, Lewis W, Hollander JM, Baseler W, Finkel MS. N-acetylcysteine reverses cardiac myocyte dysfunction in HIV-Tat proteinopathy. J Appl Physiol. 2012;113:105–113. doi: 10.1152/japplphysiol.00068.2012. [DOI] [PubMed] [Google Scholar]
- 26.Fitting S, Zou S, El-Hage N, Suzuki M, Paris JJ, Schier CJ, et al. Opiate addiction therapies and HIV-1 Tat: interactive effects on glial [Ca2+]i, oxyradical and neuroinflammatory chemokine production and correlative neurotoxicity. Curr HIV Res. 2014;12:424–434. doi: 10.2174/1570162X1206150311161147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozzi SJ, Borelli G, Ryan K, Steiner JP, Reglodi D, Mocchetti I, et al. PACAP27 is protective against tat-induced neurotoxicity. J Mol Neurosci. 2014;54:485–493. doi: 10.1007/s12031-014-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samikkannu T, Rao KV, Kanthikeel SP, Atluri VS, Agudelo M, Roy U, et al. Immunoneuropathogenesis of HIV-1 clades B and C: role of redox expression and thiol modification. Free Radic Biol Med. 2014;69:136–144. doi: 10.1016/j.freeradbiomed.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens PR, Gawryluk JW, Hui L, Chen X, Geiger JD. Creatine protects against mitochondrial dysfunction associated with HIV-1 Tat-induced neuronal injury. Curr HIV Res. 2014;12:378–387. doi: 10.2174/1570162x13666150121101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SH, Smith AJ, Tan J, Shytle RD, Giunta B. MSM ameliorates HIV-1 Tat induced neuronal oxidative stress via rebalance of the glutathione cycle. Am J Transl Res. 2015;7:328–338. [PMC free article] [PubMed] [Google Scholar]
- 31.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 32.Duffy SL, Lagopoulos J, Cockayne N, Hermens DF, Hickie IB, Naismith SL. Oxidative stress and depressive symptoms in older adults: A magnetic resonance spectroscopy study. J Affect Disord. 2015;180:29–35. doi: 10.1016/j.jad.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Duffy SL, Lagopoulos J, Cockayne N, Lewis SJ, Hickie IB, Hermens DF, et al. The effect of 12-wk ω-3 fatty acid supplementation on in vivo thalamus glutathione concentration in patients “at risk” for major depression. Nutrition. 2015;31:1247–1254. doi: 10.1016/j.nut.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Black CN, Penninx BW, Bot M, Odegaard AO, Gross MD, Matthews KA, et al. Oxidative stress, anti-oxidants and the cross-sectional and longitudinal association with depressive symptoms: results from the CARDIA study. Transl Psychiatry. 2016;6:e743. doi: 10.1038/tp.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camkurt MA, Fındıklı E, İzci F, Kurutaş EB, Tuman TC. Evaluation of malondialdehyde, superoxide dismutase and catalase activity and their diagnostic value in drug naïve, first episode, non-smoker major depression patients and healthy controls. Psychiatry Res. 2016;238:81–85. doi: 10.1016/j.psychres.2016.01.075. [DOI] [PubMed] [Google Scholar]
- 36.Han C, Lim YH, Hong YC. The Association Between Oxidative Stress and Depressive Symptom Scores in Elderly Population: A Repeated Panel Study. J Prev Med Public Health. 2016;49:260–274. doi: 10.3961/jpmph.16.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirose A, Terauchi M, Akiyoshi M, Owa Y, Kato K, Kubota T. Depressive symptoms are associated with oxidative stress in middle-aged women: a cross-sectional study. Biopsychosoc Med. 2016;10:12. doi: 10.1186/s13030-016-0066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zafir A, Ara A, Banu N. Invivo antioxidant status: a putative target of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:220–228. doi: 10.1016/j.pnpbp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Moretti M, Colla A, de Oliveira Balen G, dos Santos DB, Budni J, de Freitas AE, et al. Ascorbic acid treatment, similarly to fluoxetine, reverses depressive-like behavior and brain oxidative damage induced by chronic unpredictable stress. J Psychiatr Res. 2012;46:331–340. doi: 10.1016/j.jpsychires.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK, et al. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci. 2012;32:9690–9699. doi: 10.1523/JNEUROSCI.0794-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendez-David I, Tritschler L, Ali ZE, Damiens MH, Pallardy M, David DJ, et al. Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci Lett. 2015;597:121–126. doi: 10.1016/j.neulet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 42.Holzmann I, da Silva LM, Corrêa da Silva JA, Steimbach VM, de Souza MM. Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol Biochem Behav. 2015;136:55–63. doi: 10.1016/j.pbb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder—a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Berk M, Dean O, Cotton SM, Gama CS, Kapczinski F, Fernandes BS, et al. The efficacy of N-acetylcysteine as an adjunctive treatment in bipolar depression: an open label trial. J Affect Disord. 2011;135:389–394. doi: 10.1016/j.jad.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Mazereeuw G, Herrmann N, Andreazza AC, Scola G, Ma DW, Oh PI, et al. Oxidative stress predicts depressive symptom changes with omega-3 fatty acid treatment in coronary artery disease patients. Brain Behav Immun. doi: 10.1016/j.bbi.2016.10.005. (in press) [DOI] [PubMed] [Google Scholar]
- 46.Uchihara Y, Tanaka K, Asano T, Tamura F, Mizushima T. Superoxide dismutase overexpression protects against glucocorticoid-induced depressive-like behavioral phenotypes in mice. Biochem Biophys Res Commun. 2016;469:873–877. doi: 10.1016/j.bbrc.2015.12.085. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Su WJ, Chen Y, Wu TY, Gong H, Shen XL, et al. Effects of hydrogen-rich water on depressive-like behavior in mice. Sci Rep. 2016;6:23742. doi: 10.1038/srep23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mediouni S, Jablonski J, Paris JJ, Clementz MA, Thenin-Houssier S, McLaughlin JP, et al. Didehydro-cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice. Curr HIV Res. 2015;13:64–79. doi: 10.2174/1570162x13666150121111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paris JJ, Singh HD, Carey AN, McLaughlin JP. Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res. 2015;291:209–218. doi: 10.1016/j.bbr.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Y, Gao X, Chen J, Liu Y, He JJ. HIV Tat Impairs Neurogenesis through Functioning As a Notch Ligand and Activation of Notch Signaling Pathway. J Neurosci. 2016;36:11362–11373. doi: 10.1523/JNEUROSCI.1208-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct. 2015;220:605–623. doi: 10.1007/s00429-013-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paris JJ, Zou S, Hahn YK, Knapp PE, Hauser KF. 5α-reduced progestogens ameliorate mood-related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV-1 Tat. Brain Behav Immun. 2016;55:202–214. doi: 10.1016/j.bbi.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marks WD, Paris JJ, Schier CJ, Denton MD, Fitting S, McQuiston AR, et al. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neurovirol. 2016;22:747–762. doi: 10.1007/s13365-016-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carey AN, Liu X, Mintzopoulos D, Paris JJ, Muschamp JW, McLaughlin JP, et al. Conditional Tat protein expression in the GT-tg bigenic mouse brain induces gray matter density reductions. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:49–54. doi: 10.1016/j.pnpbp.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl) 2014;231:2349–2360. doi: 10.1007/s00213-013-3385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLaughlin JP, Ganno ML, Eans SO, Mizrachi E, Paris JJ. HIV-1 Tat protein exposure potentiates ethanol reward and reinstates extinguished ethanol-conditioned place preference. Curr HIV Res. 2014;12:415–423. doi: 10.2174/1570162x1206150311160133. [DOI] [PubMed] [Google Scholar]
- 59.Paris JJ, Carey AN, Shay CF, Gomes SM, He JJ, McLaughlin JP. Effects of conditional central expression of HIV-1 tat protein to potentiate cocaine-mediated psychostimulation and reward among male mice. Neuropsychopharmacology. 2014;39:380–388. doi: 10.1038/npp.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 62.Harkin A, Houlihan DD, Kelly JP. Reduction in preference for saccharin by repeated unpredictable stress in mice and its prevention by imipramine. J Psychopharmacol. 2002;16:115–123. doi: 10.1177/026988110201600201. [DOI] [PubMed] [Google Scholar]
- 63.Rosa JM, Dafre AL, Rodrigues AL. Antidepressant-like responses in the forced swimming test elicited by glutathione and redox modulation. Behav Brain Res. 2013;253:165–172. doi: 10.1016/j.bbr.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 64.Nemeth CL, Glasper ER, Harrell CS, Malviya SA, Otis JS, Neigh GN. Meloxicam blocks neuroinflammation, but not depressive-like behaviors, in HIV-1 transgenic female rats. PLoS One. 2014;9:e108399. doi: 10.1371/journal.pone.0108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber CA, Duncan CA, Lyons MJ, Jenkinson SG. Depletion of tissue glutathione with diethyl maleate enhances hyperbaric oxygen toxicity. Am J Physiol. 1990;258:L308–L312. doi: 10.1152/ajplung.1990.258.6.L308. [DOI] [PubMed] [Google Scholar]
- 66.Sasaki T, Toyama H, Oda K, Ogihara-Umeda I, Nishigori H, Senda M. Assessment of antioxidative ability in brain: technetium-99m-meso-HMPAO as an imaging agent for glutathione localization. J Nucl Med. 1996;37:1698–1701. [PubMed] [Google Scholar]
- 67.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 68.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 71.Tong J, Fitzmaurice PS, Moszczynska A, Mattina K, Ang LC, Boileau I, et al. Do glutathione levels decline in aging human brain? Free Radic Biol Med. 2016;93:110–117. doi: 10.1016/j.freeradbiomed.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 72.Pang X, Panee J. Roles of glutathione in antioxidant defense, inflammation, and neuron differentiation in the thalamus of HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2014;9:413–423. doi: 10.1007/s11481-014-9538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu M, Bashir A, Ackerman JJ, Yablonskiy DA. Improved calibration technique for in vivo proton MRS thermometry for brain temperature measurement. Magn Reson Med. 2008;60:536–541. doi: 10.1002/mrm.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, et al. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278:13512–13519. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- 75.Sawaya BE, Thatikunta P, Denisova L, Brady J, Khalili K, Amini S. Regulation of TNFalpha and TGFbeta-1 gene transcription by HIV-1 Tat in CNS cells. J Neuroimmunol. 1998;87:33–42. doi: 10.1016/s0165-5728(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 76.Steiner JP, Bachani M, Wolfson-Stofko B, Lee MH, Wang T, Li G, et al. Interaction of paroxetine with mitochondrial proteins mediates neuroprotection. Neurotherapeutics. 2015;12:200–216. doi: 10.1007/s13311-014-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 78.Ho JW, Ho PW, Zhang WY, Liu HF, Kwok KH, Yiu DC, et al. Transcriptional regulation of UCP4 by NF-kappaB and its role in mediating protection against MPP+ toxicity. Free Radic Biol Med. 2010;49:192–204. doi: 10.1016/j.freeradbiomed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Ho JW, Ho PW, Liu HF, So DH, Chan KH, Tse ZH, et al. UCP4 is a target effector of the NF-κB c-Rel prosurvival pathway against oxidative stress. Free Radic Biol Med. 2012;53:383–394. doi: 10.1016/j.freeradbiomed.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Mailloux RJ, Fu A, Robson-Doucette C, Allister EM, Wheeler MB, Screaton R, et al. Glutathionylation state of uncoupling protein-2 and the control of glucose-stimulated insulin secretion. J Biol Chem. 2012;287:39673–39685. doi: 10.1074/jbc.M112.393538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodríguez-Mora S, Mateos E, Moran M, Martín MÁ, López JA, Calvo E, et al. Intracellular expression of Tat alters mitochondrial functions in T cells: a potential mechanism to understand mitochondrial damage during HIV-1 replication. Retrovirology. 2015;12:78. doi: 10.1186/s12977-015-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramsden DB, Ho PW, Ho JW, Liu HF, So DH, Tse HM, et al. Human neuronal uncoupling proteins 4 and 5 (UCP4 and UCP5): structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction. Brain Behav. 2012;2:468–478. doi: 10.1002/brb3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.National Research Council. Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academies Press (US); Washington, DC: 2003. [Google Scholar]
- 84.Magnuson BA, Appleton J, Ames GB. Pharmacokinetics and distribution of [35S]methylsulfonylmethane following oral administration to rats. J Agric Food Chem. 2007;55:1033–1038. doi: 10.1021/jf0621469. [DOI] [PubMed] [Google Scholar]
- 85.Amirshahrokhi K, Bohlooli S. Effect of methylsulfonylmethane on paraquat-induced acute lung and liver injury in mice. Inflammation. 2013;36(5):1111–1121. doi: 10.1007/s10753-013-9645-8. [DOI] [PubMed] [Google Scholar]
- 86.Phimister AJ, Lee MG, Morin D, Buckpitt AR, Plopper CG. Glutathione depletion is a major determinant of inhaled naphthalene respiratory toxicity and naphthalene metabolism in mice. Toxicol Sci. 2004;82:268–278. doi: 10.1093/toxsci/kfh258. [DOI] [PubMed] [Google Scholar]
- 87.Frahm J, Merboldt KD, Hänicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson. 1987;72:502–508. doi: 10.1002/mrm.1910170113. [DOI] [PubMed] [Google Scholar]
- 88.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 89.Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 90.Puhl MD, Mintzopoulos D, Jensen JE, Gillis TE, Konopaske GT, Kaufman MJ, et al. In vivo magnetic resonance studies reveal neuroanatomical and neurochemical abnormalities in the serine racemase knockout mouse model of schizophrenia. Neurobiol Dis. 2015;73:269–274. doi: 10.1016/j.nbd.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990;14:26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- 92.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 93.Soher BJ, Young K, Bernstein A, Aygula Z, Maudsley AA. GAVA: spectral simulation for in vivo MRS applications. J Magn Reson. 2007;185:291–299. doi: 10.1016/j.jmr.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benzi G, Pastoris O, Marzatico F, Villa RF. Influence of aging and drug treatment on the cerebral glutathione system. Neurobiol Aging. 1988;9:371–375. doi: 10.1016/s0197-4580(88)80083-6. [DOI] [PubMed] [Google Scholar]
- 95.Chen TS, Richie JP, Jr, Lang CA. The effect of aging on glutathione and cysteine levels in different regions of the mouse brain. Proc Soc Exp Biol Med. 1989;190:399–402. doi: 10.3181/00379727-190-42879. [DOI] [PubMed] [Google Scholar]
- 96.Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 97.Emir UE, Raatz S, McPherson S, Hodges JS, Torkelson C, Tawfik P, et al. Noninvasive quantification of ascorbate and glutathione concentration in the elderly human brain. NMR Biomed. 2011;24:888–894. doi: 10.1002/nbm.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duarte JM, Do KQ, Gruetter R. Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy. Neurobiol Aging. 2014;35:1660–1668. doi: 10.1016/j.neurobiolaging.2014.01.135. [DOI] [PubMed] [Google Scholar]
- 99.Chang ML, Klaidman LK, Adams JD., Jr The effects of oxidative stress on in vivo brain GSH turnover in young and mature mice. Mol Chem Neuropathol. 1997;30:187–197. doi: 10.1007/BF02815097. [DOI] [PubMed] [Google Scholar]
- 100.Tkac I, Dubinsky JM, Keene CD, Gruetter R, Low WC. Neurochemical changes in Huntington R6/2 mouse striatum detected by in vivo 1H NMR spectroscopy. J Neurochem. 2007;100:1397–1406. doi: 10.1111/j.1471-4159.2006.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ribeiro M, Rosenstock TR, Cunha-Oliveira T, Ferreira IL, Oliveira CR, Rego AC. Glutathione redox cycle dysregulation in Huntington’s disease knock-in striatal cells. Free Radic Biol Med. 2012;53:1857–1867. doi: 10.1016/j.freeradbiomed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Correa F, Mallard C, Nilsson M, Sandberg M. Dual TNFα-induced effects on NRF2 mediated antioxidant defence in astrocyte-rich cultures: role of protein kinase activation. Neurochem Res. 2012;37:2842–2855. doi: 10.1007/s11064-012-0878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mastrantonio R, Cervelli M, Pietropaoli S, Mariottini P, Colasanti M, Persichini T. HIV-Tat Induces the Nrf2/ARE Pathway through NMDA Receptor-Elicited Spermine Oxidase Activation in Human Neuroblastoma Cells. PLoS One. 2016;11:e0149802. doi: 10.1371/journal.pone.0149802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuroda Y, Murakami M, Tsuruta J, Murakawa T, Sakabe T. Preservation of the ratio of cerebral blood flow/metabolic rate for oxygen during prolonged anesthesia with isoflurane, sevoflurane, and halothane in humans. Anesthesiology. 1996;84:555–561. doi: 10.1097/00000542-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 105.Okamoto H, Meng W, Ma J, Ayata C, Roman RJ, Bosnjak ZJ, et al. Isoflurane-induced cerebral hyperemia in neuronal nitric oxide synthase gene deficient mice. Anesthesiology. 1997;86:875–884. doi: 10.1097/00000542-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 106.Lenz C, Rebel A, van Ackern K, Kuschinsky W, Waschke KF. Local cerebral blood flow, local cerebral glucose utilization, and flow-metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology. 1998;89:1480–1488. doi: 10.1097/00000542-199812000-00026. [DOI] [PubMed] [Google Scholar]
- 107.Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci (Landmark Ed) 2010;15:73–92. doi: 10.2741/3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kiyatkin EA, Brown PL, Wise RA. Brain temperature fluctuation: a reflection of functional neural activation. Eur J Neurosci. 2002;16:164–168. doi: 10.1046/j.1460-9568.2002.02066.x. [DOI] [PubMed] [Google Scholar]
- 109.Rango M, Bonifati C, Bresolin N. Post-Activation Brain Warming: A 1-H MRS Thermometry Study. PLoS One. 2015;10:e0127314. doi: 10.1371/journal.pone.0127314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sundgren-Andersson AK, Ostlund P, Bartfai T. Simultaneous measurement of brain and core temperature in the rat during fever, hyperthermia, hypothermia and sleep. Neuroimmunomodulation. 1998;5:241–247. doi: 10.1159/000026344. [DOI] [PubMed] [Google Scholar]
- 111.Imeri L, Ceccarelli P, Mariotti M, Manfridi A, Opp MR, Mancia M. Sleep, but not febrile responses of Fisher 344 rats to immune challenge are affected by aging. Brain Behav Immun. 2004;18:399–404. doi: 10.1016/j.bbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Brailoiu GC, Brailoiu E, Chang JK, Dun NJ. Excitatory effects of human immunodeficiency virus 1 Tat on cultured rat cerebral cortical neurons. Neuroscience. 2008;151:701–710. doi: 10.1016/j.neuroscience.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zucchini S, Pittaluga A, Brocca-Cofano E, Summa M, Fabris M, De Michele R, et al. Increased excitability in tat-transgenic mice: role of tat in HIV-related neurological disorders. Neurobiol Dis. 2013;55:110–119. doi: 10.1016/j.nbd.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 114.Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 115.Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, et al. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–237. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 116.Flora G, Pu H, Hennig B, Toborek M. Cyclooxygenase-2 is involved in HIV-1 Tat-induced inflammatory responses in the brain. Neuromolecular Med. 2006;8:337–352. doi: 10.1385/NMM:8:3:337. [DOI] [PubMed] [Google Scholar]
- 117.Im JY, Kim D, Paik SG, Han PL. Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Radic Biol Med. 2006;41:960–972. doi: 10.1016/j.freeradbiomed.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 118.Pu H, Hayashi K, Andras IE, Eum SY, Hennig B, Toborek M. Limited role of COX-2 in HIV Tat-induced alterations of tight junction protein expression and disruption of the blood-brain barrier. Brain Res. 2007;1184:333–344. doi: 10.1016/j.brainres.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 119.Blanco A, Alvarez S, Fresno M, Muñoz-Fernández MA. Extracellular HIV-Tat induces cyclooxygenase-2 in glial cells through activation of nuclear factor of activated T cells. J Immunol. 2008;180:530–540. doi: 10.4049/jimmunol.180.1.530. [DOI] [PubMed] [Google Scholar]
- 120.Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS. Oxidative Stress Is Associated with Neuroinflammation in Animal Models of HIV-1 Tat Neurotoxicity. Antioxidants (Basel) 2014;3:414–438. doi: 10.3390/antiox3020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dröge W, Eck HP, Mihm S. HIV-induced cysteine deficiency and T-cell dysfunction--a rationale for treatment with N-acetylcysteine. Immunol Today. 1992;13:211–214. doi: 10.1016/0167-5699(92)90156-2. [DOI] [PubMed] [Google Scholar]
- 122.Saing T, Lagman M, Castrillon J, Gutierrez E, Guilford FT, Venketaraman V. Analysis of glutathione levels in the brain tissue samples from HIV-1-positive individuals and subject with Alzheimer’s disease and its implication in the pathophysiology of the disease process. BBA Clin. 2016;6:38–44. doi: 10.1016/j.bbacli.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Legrand-Poels S, Vaira D, Pincemail J, van de Vorst A, Piette J. Activation of human immunodeficiency virus type 1 by oxidative stress. AIDS Res Hum Retroviruses. 1990;6:1389–1397. doi: 10.1089/aid.1990.6.1389. [DOI] [PubMed] [Google Scholar]
- 124.Kalebic T, Kinter A, Poli G, Anderson ME, Meister A, Fauci AS. Suppression of human immunodeficiency virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N-acetylcysteine. Proc Natl Acad Sci U S A. 1991;88:986–990. doi: 10.1073/pnas.88.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roederer M, Raju PA, Staal FJ, Herzenberg LA, Herzenberg LA. N-acetylcysteine inhibits latent HIV expression in chronically infected cells. AIDS Res Hum Retroviruses. 1991;7:563–567. doi: 10.1089/aid.1991.7.563. [DOI] [PubMed] [Google Scholar]
- 126.Kameoka M, Kimura T, Ikuta K. Superoxide enhances the spread of HIV-1 infection by cell-to-cell transmission. FEBS Lett. 1993;331:182–186. doi: 10.1016/0014-5793(93)80322-l. [DOI] [PubMed] [Google Scholar]
- 127.Mandas A, Iorio EL, Congiu MG, Balestrieri C, Mereu A, Cau D, et al. Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. J Biomed Biotechnol. 2009;2009:749575. doi: 10.1155/2009/749575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Manda KR, Banerjee A, Banks WA, Ercal N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free Radic Biol Med. 2011;50:801–810. doi: 10.1016/j.freeradbiomed.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Benhar M, Shytaj IL, Stamler JS, Savarino A. Dual targeting of the thioredoxin and glutathione systems in cancer and HIV. J Clin Invest. 2016;126:1630–1639. doi: 10.1172/JCI85339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bednařík P, Moheet A, Deelchand DK, Emir UE, Eberly LE, Bareš M, et al. Feasibility and reproducibility of neurochemical profile quantification in the human hippocampus at 3 T. NMR Biomed. 2015;28:685–693. doi: 10.1002/nbm.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Godlewska BR, Near J, Cowen PJ. Neurochemistry of major depression: a study using magnetic resonance spectroscopy. Psychopharmacology (Berl) 2015;232:501–507. doi: 10.1007/s00213-014-3687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study Conditions
Figure S1: First MRS partial acquisition medial frontal cortex temperature correlates with medial frontal cortex glutathione (GSH) level (r=0.47, p=0.024). GT-tg mice were treated for 7 days with saline (open circles, N=11) or Dox (100 mg/kg, i.p.; filled circles, N=12) and scanned on day 8. Shown is the regression line (solid) and the 95% confidence interval (dashed lines).