Abstract
Erythropoietin (Epo) is a fundamental hormone in the regulation of hematopoiesis, and other secondary roles mediated by the binding of the hormone to its specific receptor (EpoR), which leads to an activation of key signaling pathways that induce an increase in cell differentiation, apoptosis control and neuroprotection. It has been suggested that their function depends on final conformation of glycosylations, related with affinity to the receptor and its half-life. The presence of EpoR has been reported in different tissues including central nervous system, where it has been demonstrated to exert a neuroprotective function against oxidative stress conditions, such as ischemic injury and neurodegenerative diseases. There is also evidence of an increase in EpoR expression in brain cell lysates of Alzheimer's patients with respect to healthy patients. These results are related with extensive in vitro experimental data of neuroprotection obtained from cell lines, primary cell cultures and hippocampal slices. Additionally, this data is correlated with in vivo experiments (water maze test) in mouse models of Alzheimer's disease where Epo treatment improved cognitive function. These studies support the idea that receptor activation induces a neuroprotective effect in neurodegenerative disorders including dementias, and especially Alzheimer's disease. Taken together, available evidence suggests that Epo appears to be a central element for EpoR activation and neuroprotective properties in the central nervous system. In this review, we will describe the mechanisms associated with neuroprotection and its relation with the activation of EpoR in order with identify new targets to develop pharmacological strategies.
Keywords: erythropoietin, erythropoietin receptor, neuroprotection, anti-apoptosis, Alzheimer's disease
Erythropoietin (Epo) and Its Physiological Role
Epo is a glycoprotein hormone belonging to the superfamily of type I cytokines and is mainly responsible for the proliferation, differentiation and maturation of erythroid cells, in both embryonic and adult stages (Bunn, 2013). Epo is composed of 165 amino acids arranged into a globular three-dimensional structure, consisting of four amphipathic helices connected by loops and stabilized by two internal disulfide bridges between cysteines 7–161 and 29–33, which are important to maintain its biological activity, and this basic conformation represents about 60% of its molecular mass (~ 34 kDa) (Batmunkh et al., 2006; Bunn, 2013). This hormone is a glycoprotein characterized by having a glycosylation pattern conformed by 4 glycosylated chains, 3 of them being N-glycosylations located at positions Asn-24, Asn-38 and Asn-83, and has a fourth string O-glycosylated at residue Ser-126 (Sinclair, 2013), responsible for 40% of its molecular weight (Figure 1). Glycosylation distribution gives a great heterogeneity to the mature protein, while the absence of glycosylation reduces the stability of intermediate species and causes changes in the binding kinetics of the Epo receptor (EpoR) (Jiang et al., 2014). Epo glycosylations are mainly conformed by tetra-antennas with α(2-6) N-acetylneuraminic acid (Neu5Ac α2-6) and N-acetylglucosamine (GlcNAc) terminations attached to the mannose core (Toledo et al., 2006; Montesino et al., 2008; Parra and Rodriguez, 2012). Neu5Ac terminals have a direct relation to molecular half-life, while also protecting it from free radical degradation (Ingley, 2012; Parra and Rodriguez, 2012). On the other hand, the negative charge contributed by the sialic acid is responsible for the acidic properties of the molecule, having an isoelectric point between 3.31 and 4.11 (Morimoto et al., 1996).
Figure 1.
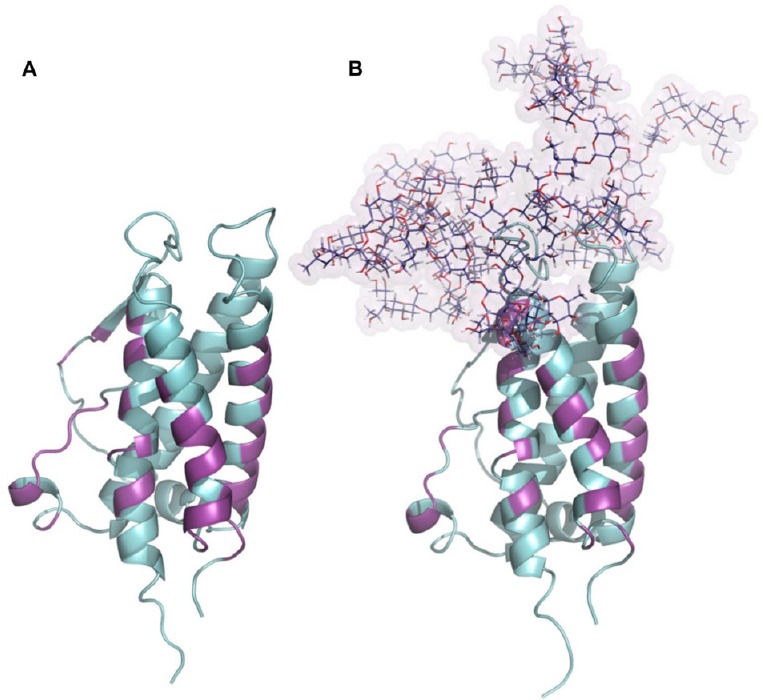
Glycosylation of erythropoietin.
(A) Structure of human erythropoietin obtained from the PDB: 1EER. (B) N-glycosylated model of erythropoietin generated combining each tetra-antennas glycan to their respective amino acids in erythropoietin sequence, N24, N38 and N83. The presence of N-glycosylations did not compromise the binding site to the erythropoietin receptor. All amino acids involved in erythropoietin-erythropoietin receptor interaction were colored magenta and define regions which remain largely conserved between both glycosylation states.
Additionally, the oligosaccharide chains attached to Epo increase its molecular size and thereby prevent glomerular filtration, while the presence of terminal neuraminic acids prevent the exposure of galactose residues, which are taken up by hepatic transporters, increasing the plasma half-life of the hormone (Jelkmann, 2008). Epo glycosylations are necessary to facilitate its transport in plasma, and for its passage from blood to bone marrow through endothelial cells in the blood-bone marrow barrier (Jelkmann and Wagner, 2004). During fetal development, Epo is produced in the liver, while during adulthood it is mainly synthesized in the peritubular cells of the kidney (80%) in order to compensate for oxygen decrease during hypoxia (Bunn, 2013). This function is developed through the hypoxia inducible factor (HIF-1) transcription factor (Toledo et al., 2006; Parra and Rodriguez, 2012), which stimulates Epo gene transcription by binding to an enhancer that flanks the 3′ region of the gene, increasing hormonal production and release into the plasma. Epo effects are produced by Epo/EpoR interaction, which activates signaling cascades that act on the control of apoptosis, decreasing the rate of cell death in the bone marrow during the final stages of the development of erythroid progenitor cells, especially during the colony forming unit-erythroid (CFU-E) stage (Fisher, 2003). This activation induces cell proliferation and maturation from normoblasts to reticulocytes, which are characterized by elimination of the nucleus and release from the bone marrow to blood circulation where final differentiation to erythrocytes occurs, having a half-life of approximately 120 days in normal adults (Sinclair, 2013). The circulating hormone promotes the proliferation, differentiation and maturation of erythroid progenitors to erythrocytes, which ultimately improves the oxygen transport as their key function (Maiese et al., 2012; Bunn, 2013). The normal plasma range of Epo in healthy individuals is 10–20 (mIU/mL) with a half-life of 7–8 hours (Toledo et al., 2006; Bunn, 2013).
Furthermore, in addition to being produced in the kidney, secondary Epo production sites have been described in retinal cells and astrocytes, suggesting that this hormone has a function not only in hematopoiesis, but also in different tissue types participating in cellular protection from stress and preventing apoptosis. Epo expression in non-erythroid tissue accounts for about 15–20% of the total production throughout the organism (Ponce et al., 2013).
Epo deficiency induces severe anemia in patients with chronic renal failure and renal failure associated with trauma, intensive drug treatments (patients with human immunodeficiency virus (HIV) or chemotherapy) or in patients with kidney transplants (Rabie and Marti, 2008). In these cases, external supply of the hormone is a key treatment. Epo was the first hematopoietic growth factor cloned and is currently one of the most sold biopharmaceutical products worldwide, according to the Food and Drug Administration (FDA). Recombinant human Epo (rhEpo) is indicated in the treatment of patients with anemia and diseases associated with low concentrations of Epo in plasma; additionally, it has been described that these patients show significant cognitive improvement (Sargin et al., 2011; Jiang et al., 2014).
EpoR
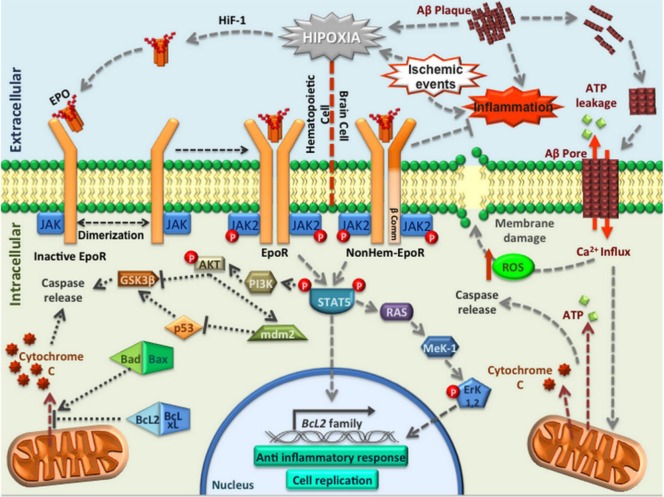
EpoR is a transmembrane receptor member of the type I cytokine superfamily, with a molecular weight of 66 kDa, which is pre-formed as homodimers on the cell surface with a density of about 1,000/cell (CFU-E) in bone marrow (Bunn, 2013). The binding of Epo to its receptor induces a conformational change that results in the autophosphorylation by Janus kinase-2 (JAK-2). The protein trans-phosphorylates, which induce phosphorylation on tyrosine residues located in the cytoplasmic region of EpoR, create a binding site for other proteins during the amplification of the signaling cascade (Losdish et al, 2013). Signaling pathways activated by EpoR include signal transducer and activator of transcription 5 (STAT-5), phosphoinositol 3′-kinase (PI3K), mitogen-activated protein kinase (MAPK), and protein kinase c (PKC) (Figure 2). This eventually leads to the regulation of cell cycle progression through phosphorylated protein kinase B (AKT), and also positively regulates anti-apoptotic proteins such as B-cell lymphoma 2 (Bcl2) and B-cell lymphoma-extra large (BclxL) (Shen et al., 2010). The activation of EpoR also acts by directly inhibiting the action of pro-apoptotic proteins such as cytochrome c, or through p53 activation (Brines and Cerami, 2005). The Epo region that binds with higher affinity to EpoR is characterized by possession of several highly hydrophobic residues, such as Phe-138, Phe-142, Tyr-145, Phe-148, Leu-153 and Tyr-156, that are attached to the nonpolar chains of Epo, forming a hydrophobic core which interacts with a specific intrasubunit receptor motif (“WSEWS motif”, residues 209–213) directly related to receptor expression, translocation to cell membrane and its activation (Cheetham et al., 1998). It was shown that there are two regions of the Epo molecule that bind to the receptor: the first binds to a region that has a high affinity (KM of 1 nM), and a second region that has a lower affinity, one thousand times smaller (KM 1 μM) (Cheetham et al., 1998).
Figure 2.
Pathways involved in EpoR activation.
Principal signal pathways activated to induce neuroprotection when Epo interacts with EpoR or EpoRβ (expressed in CNS) in response to hypoxia or different types of stress, such as ischemic brain events or inflammation induced by Alzheimer′s disease. Epo or EPO: Erythropoietin; EpoR: Epo receptor; CNS: central nervous system; HiF-1: hypoxia inducible factor; Aβ: beta amyloid peptide; ATP: adenosine triphosphate; JAK: Janus kinase; ROS: reactive oxygen species; GSK-3β: glycogen synthase kinase 3 beta; AKT: protein kinase B; PI3K: phosphoinositol 3′-kinase; STAT5: signal transducer and activator of transcription 5; MeK-1: mitogen-activated protein kinase kinase; Erk1,2: extracellular-regulated kinases 1,2; Bcl2: B-cell lymphoma 2; BclxL: B-cell lymphoma-extra large.
EpoR production is inefficient, only 10% of the receptor is transported to the cell membrane, and its transport requires the binding of the N-terminal portion of JAK-2 to the cytoplasmic region of the receptor in the endoplasmic reticulum in order to promote proper folding of the protein and sorting to the surface of the cell membrane. Hence, JAK-2 is essential for EpoR signaling when it is activated (Javadi et al., 2012).
However, expression of EpoR has also been observed in non-erythroid tissues, explaining the pleiotropic effects described for Epo (Lappin et al., 2002; Jelkmann and Wagner, 2004; Wagner et al., 2004; Brines and Cerami, 2005). The Epo non-canonical receptor expressed in non-erythroid tissues is structurally different from the classical EpoR because it is a heterodimer composed of a monomer of the canonical EpoR and another subunit of the “β-common receptor”, also known as CD131 (EpoR-β comm), which is identical to the beta region of cytokine receptors such as interleukin-3 (IL3) receptors or granulocyte macrophage colony-stimulating factor (GMSCF) receptors (Brines et al., 2004; Su et al., 2011) (Figure 3). It is important to mention that heterodimers of the non-canonical receptor are involved in the neuroprotective effects exerted by Epo on in vitro assays, similar to the ones observed with classical homodimer EpoR conformations. These results reinforce the idea that the activation of EpoRs on homodimer or heterodimer conformations is necessary to obtain neuroprotective effects (Jelkmann and Wagner, 2004; Wei et al., 2006; Yu et al., 2016).
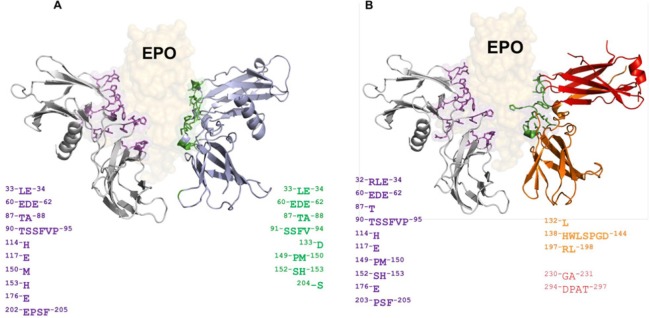
Figure 3.
In silico prediction of interaction between Epo and EpoR/EpoRβ.
(A) Complex Epo/EpoR generated by molecular docking where amino acids on the surface of EpoR involved in the interaction with Epo have been highlighted and colored according to their corresponding chain. For the classical EpoR, its functional conformation corresponds to a homodimer structure, and the two colors used (gray and light blue) for each chain differ only for schematic purposes. (B) Complex Epo/EpoRβ generated in a manner similar to the previous complex, with the exception that EpoRβ is a functional heterodimer, incorporating the beta common subunit formed by two interlaced chains colored red and orange, respectively. The number of amino acids in the interface is slightly lower than Epo/EpoR, however, both receptors are able to interact with Epo in a stable and energetically favorable manner. Epo: Erythropoietin; EpoR: Epo receptor.
EpoR is expressed in the central nervous system (CNS) as a heterodimer (EpoR-β comm), suggesting that Epo may play a specific role in this tissue (Brines and Cerami, 2005; Rabie and Marti, 2008). It has been reported that the involvement of EpoR is critical for in vitro neuroprotection. For example, a neuroprotective effect was observed in PC12 cells pretreated with Epo for 1 hour previous to treatment with beta amyloid peptide (Aβ)25–35 peptide during 6 or 12 hours, and this effect was lost when an inhibitor of STAT-5 (a major signaling pathway activated by EpoR) was used (Ma et al., 2014). Similarly, in vivo neuroprotection was observed in rats pretreated with Epo for 10 minutes before inducing ischemia by a middle cerebral artery occlusion model (MCAO), where the neuroprotective effect was evaluated by measuring the area of damaged tissue in histological sections, showing a decrease in necrotic tissue and in the percentage of edema in animals pretreated with Epo (Ratilal et al., 2014). Also, an increased expression of EpoR has been reported in ischemic brain regions in animals treated with different doses of Epo (Yu et al., 2005; Castañeda-Arellano et al., 2014). Similar neuroprotective effects with pretreatments of Epo were reported in other animal models, such as Drosophila melanogaster that were subjected to stress periods, under conditions of hypoxia, which also showed that this effect is dependent on activation of EpoR (Miljus et al., 2014; Yu et al., 2016).
Different Isoforms of Epo
One disadvantage of Epo as a neuroprotective agent is its natural capacity to increase the hematocrit, which can quickly elevate blood influx to brain tissue, increasing the risk of cerebrovascular reperfusion. Thus, it is necessary to increase the range of doses to obtain neuroprotective effects when Epo is used as a peripheral treatment. Therefore, different ways to activate EpoR with minimal hematopoietic effects have been described (Pankratova et al., 2010). Since then, different isoforms of Epo have been designed, especially with chemical modifications to increase its efficacy. Masuda et al. (1999) demonstrated that when sialic acids are removed, the molecule increases its affinity for EpoR. Furthermore, a difference has been observed between systemic-Epo and CNS-Epo due to a modification in the glycosylation tree (Masuda et al., 1993). Epo produced in the retina and brain also differs from the variant produced by the peritubular cells in kidney, with respect to its sialic acid substitutions; the former almost completely devoid of sialic acid substituents called neuro-EPO (Moon et al., 2006; Parra and Rodriguez, 2012).
Another chemical modification reported to reduce the hematopoietic effect is carbamylation of lysine residues that cause a conformational and functional change on Epo because all lysines are replaced by homocitrulline, totally depleting the hematopoietic effect (Leist et al., 2004; Wang et al., 2004). On the other hand, the synthesis of peptides that mimic the interaction zone between Epo and EpoR, promoting its activation without hematopoietic effects, has been described, but the half-life of the hormone is extremely short (around 4 minutes) (Brines and Cerami, 2008; Brines et al., 2008). These peptides were modified to improve stability and to increase its half-life, obtaining a new peptide-version with a high affinity to EpoR, converting them into potential agonists of the EpoR that can produce a decrease in inflammation, providing cytoprotection (Pankratova et al., 2010; Zellinger et al., 2011; Liu et al., 2015).
Neuroprotective Effects of Epo on Ischemic Injury
Ischemic injury is characterized by a decrease in adenosine triphosphate (ATP), disruption of the Na+/K+ pump and ATPase, which results in severe ionic dyshomeostasis. In the brain, those events are followed by an enhanced excitotoxic tone mediated by glutamate, acidosis, calcium channel dysfunction, release of nitric oxide (NO), plasma membrane disruption, and release of pro-inflammatory cytokines, finally leading to activation of “death receptors” and apoptosis. Thus, caspase activation, especially caspase 3 which is overexpressed in neuronal apoptosis processes, could be the cause of cell death by ischemic injury (Lu et al., 2014; Grupke et al., 2015; Siddiqui et al., 2015).
Epo treatment (in vitro or in vivo) induces neuroprotection against different forms of injury, including ischemic toxicity (Gui et al., 2011). It has been reported that Epo and EpoR expression in the brain is minimal, but in the case of injury, such as ischemia, an increase in EpoR occurs, especially on endothelial cells, astrocytes, and even neurons (Sargin et al., 2011; Wu et al., 2011; Zhang et al., 2011; Ott et al., 2015). In fact, it has been reported that Epo can protect neurons in vitro during hypoxia events, where EpoR is up-regulated by those same events (Parra and Rodriguez, 2012). On the other hand, it has been reported that Epo can modulate the activity of channels by regulating the cell volume and neurogenesis on areas affected by ischemic injury and reperfusion (Iwai et al., 2007, 2010). The neuroprotective effects of Epo in vivo have been reported in the MCAO model, where a 35% reduction was observed for the edema and necrotic zones, versus the healthy zone (Zhang et al., 2014). In addition, Epo treated animals showed an increase in EpoR expression in brain ischemic zones (Yu et al., 2005, 2016; Castañeda-Arellano et al., 2014; Ratilal et al., 2014).
In hypoxia models developed on cardiomyocytes, different doses of Epo induced a dose-dependent upregulation of peroxisome proliferator-activated receptor γ co-activator 1 α (PCG1α) mRNA, a key regulator of mitochondria biogenesis, and also increased the number of mitochondria. These results suggest a positive effect of the hormone in mitochondrial biogenesis, and anti-apoptotic mechanisms (Qin et al., 2014). Epo has been associated with an inhibition of mitochondrial permeability transition pore opening, reduction in brain edema and caspase-3 expression in rats with traumatic brain injury, suggesting a direct relationship between neuroprotection and mitochondrial health (Millet et al., 2016). In parallel, Epo pretreatment reverts the decrease in mitochondrial membrane potential induced by ischemic injury (Wang et al., 2015).
Neuroprotective effects were observed in patients with acute ischemic stroke and treated using rhEpo at 6, 24 and 48 hours after injury (by Barthel index). However, a negative effect was observed when Epo was used in conjunction with thrombolysis therapy, using recombinant tissue plasminogen activator (rtPA), at the same times as post treatment protocol, suggesting that Epo is unsuitable for use in combination with a thrombolytic drug such as rtPA (Ehrenreich et al., 2002, 2009).
Neuroprotection of Epo on Neurodegeneration
The main clinical use of Epo is focused on treatment of anemic conditions in patients with special conditions like renal chronic failure, however, an increase in cognitive function observed in these patients (Rabie and Marti, 2008) has been correlated with an increase in EpoR expression in hippocampus, amygdala and prefrontal cortex induced by hypoxia. This increase has been associated to the activation of HIF-1, which is also activated by other important factors with high pro-inflammatory activity like tumor necrosis factor alpha (TNFα), interleukin-1 beta (IL1β) or lipopolysaccharide (LPS) (Rabie and Marti, 2008; Ponce et al., 2013).
Many diseases related to the CNS have a very important pro-inflammatory component, for example, cerebrovascular infarction, schizophrenia, amyotrophic lateral sclerosis (ALS) and neurodegenerative diseases like Alzheimer's disease (AD) or Parkinson′s disease (PD) (Chong et al., 2013). AD and PD are the most prevalent types of dementia in people over 60 years of age (World Health Organization, 2011). In the case of AD, it has been suggested that accumulation of Aβ1–42 peptide is directly related with cellular apoptosis and DNA fragmentation. Those processes have been stopped in some experimental approaches using rat hippocampal neurons pretreated with Epo during 1 hour, versus neuronal cells that were not treated with Epo (Chong et al., 2005; Maiese et al., 2005; Shang et al., 2012). These results correlate with an increase in cognitive function, as evaluated by the Morris water maze test, and with a decrease in apoptotic signals observed in brain lysates of mice injected intraventricularly with Aβ25–35 and pretreated for 4 days with 3 daily doses of Epo. Additionally, Epo treatments have been associated with stimulation of neuronal proliferation in the dentate gyrus of hippocampus in an AD animal model (Arabpoor et al., 2012; Maurice et al., 2013). These data correlate with results that show an increase in EpoR expression in brain lysates from AD patients, when compared with brain lysates of healthy patients (Assaraf et al., 2007).
Also, similar to AD, a neuroprotective effect has been observed in various models of neurodegenerative diseases, in particular PD, where prevention of cell death, mediated by activation of EpoR activation and the 1,4,5-triphosphate (IP3) pathway, was seen in different in vitro tests (Park et al., 2009, 2011; Won et al., 2009). Similar effects were observed in other neurodegenerative alterations such as ALS, where there is selective death of superior motoneurons (motor cortex and brain stem) and inferior motoneurons (spinal cord). This causes a gradual loss of muscle innervation leading to paralysis and atrophy-like symptoms in patients between 40 and 70 years old. ALS is characterized by muscle weakness, incoordination and spasms, finally causing general paralysis which results in patient death produced by respiratory failure after 3 or 4 years. It has been reported that only 10% of these cases are associated with a familial component; while 20% are associated with mutations in the superoxide dismutase 1 (SOD1) gene (Noh et al., 2014). In animal models of ALS (transgenic mouse SOD1, G93A), treatment with Epo during 120 days prevented the death of motoneurons (Noh et al., 2014). Also, pretreatment with Epo prevented the release of pro-inflammatory cytokines and promoted the synthesis and release of anti-inflammatory cytokines in the same model (Nagańska et al., 2010; Noh et al., 2014).
These data support a neuroprotective role of Epo against several diseases related to the CNS, especially AD and PD, suggesting that EpoR could represent an interesting target to develop new therapeutic strategies (Noh et al., 2014).
Epo and AD
The Aβ in AD is responsible for the loss in ionic homeostasis, due to its ability to perforate the cell membrane, forming a pore which allows the massive entrance of cations, especially calcium, inducing apoptosis (Kumar et al., 2015). It has been described the existence of molecules that are able to prevent the activation of the apoptotic pathway in cellular models pretreated with amyloid beta peptide, Epo being one of these. Activation of EpoR initiates transcription of Bcl2 genes that block apoptosis and prevent cell death (Sargin et al., 2010; Esmaeili Tazangi et al., 2015). It has been proposed that Epo-induced neuroprotection is mediated by EpoR activation, followed by an increase in nuclear factor kappa-light-chain-enhancer activity of activated B cells (NFκB), causing an inhibition of apoptotic proteins and blocking the activation of caspases induced after the typical accumulation of TNFα observed in AD patients (Stefani et al., 2006). The blockade of caspase activity is directly related with other pathways activated by EpoR, e.g., the activation of Bcl-xL that causes a decrease in ROS (Stefani et al., 2006; Pasqualetti et al., 2015; Buendia et al., 2016). The relationship between these two effects and the activation of EpoR has been demonstrated through the silencing of EpoR in astroglia, using siRNA techniques (Lourhmati et al., 2013; Zhou and Yu, 2013), and also, in vitro experiments using PC12 cells demonstrated the neuroprotective effect of Epo against toxicity induced by Aβ. Studies have shown that the protective effects of Epo depend on its concentration, the activation of its receptor and also the activation of JAK/STAT, PI3K, and RAS pathways that increase phosphorylated AKT, which was significantly increased in cells treated with Aβ25–35 and Epo, and confirmed by the use of the PI3K inhibitor LY294002 (Ma et al., 2009; Shen et al., 2010; Shang et al., 2012). Also, it has been found that Epo increases the phosphorylation of glycogen synthase kinase 3 beta (GSK-3β) via the PI3K/AKT pathway (Zhou and Yu, 2013), causing its inhibition. This event may represent a downstream mechanism by which Epo exerts its protective effects against Aβ25–35-induced apoptosis (Ma et al., 2014). Furthermore, it was demonstrated that Epo treatments induced up-regulation of Bcl-2, contributing to the neuroprotection against Aβ toxicity (Chong et al., 2013; Jia et al., 2014; Ma et al., 2014).
The cytoprotective and anti-apoptotic effects of Epo have also been described in studies using the microglial cell line EOC2, where these effects could be associated with Wnt1 and PI3K-mediated pathways, also suggesting the involvement of the mammalian target of rapamycin (mTOR) pathway in the protection of microglial cells during Aβ toxicity (Ott et al., 2015). Thus, it has been shown that Wnt1 is a central component in the promotion of microglial integrity, preventing the loss of these cells during early and late apoptotic injury by Aβ exposure (Ott et al., 2015). Wnt1 regulates the apoptotic cascade by maintaining the mitochondrial membrane potential, phosphorylating and fostering the translocation of Bad from the mitochondria to the cytosol, reducing the formation of Bad/Bcl-xL complex, and increasing the formation of the Bcl-xL/Bax complex, and blocking the activation of caspase 1 and caspase 3 through Bcl-xL (Maiese et al., 2012). Application of Epo (10 ng/mL) in microglia significantly maintained the expression of Wnt1 at 6 hours after Aβ exposure, which may suggest another probable mechanism to prevent Aβ-induced toxicity (Shang et al., 2012).
In addition, this study also shows that Epo treatments can maintain mitochondrial membrane potential during Aβ exposure in microglial models, indicating that Epo can block apoptotic signaling like caspase activity during brain injury and neurodegeneration (Shang et al., 2012).
On the other hand, an interesting role has been suggested for EpoR regarding neurite growth during neurogenesis of hippocampal cells, and also in decreasing apoptosis in cells from the dentate gyrus in an AD model, via STAT-5 activation pathway, among others (Pankratova et al., 2010; Zellinger et al., 2011; Arabpoor et al., 2012). In the parallel, several studies have assessed the improvement in cognitive functions and spatial memory in animal AD models treated with several intraperitoneal doses of Epo (Adamcio et al., 2008; Pankratova et al., 2010). In the hippocampus, the effect of Epo was shown to be selective for memory, since no effect was seen on anxiety, spontaneous activity, exploratory behavior, or motor performance (Adamcio et al., 2008). All this evidence suggests the exciting idea that the selective effect of Epo on the enhancement of memory could be mediated by the activation of EpoR on hippocampus, representing a novel target for the treatment of neurodegenerative diseases.
Other studies have found a decrease of lipid peroxidation in brain samples from AD animals treated with Epo, which correlates with an increase of Bax levels (Chen et al., 2015). Also, it has been shown that Epo selectively induces the synthesis of the neuroglobin protein in damaged regions, promoting angiogenesis and protection of the vascular endothelium, which are key factors that contribute to neurogenesis and neuroplasticity in injured brain tissue (Maurice et al., 2013; Esmaeili Tazangi et al., 2015; Maiese, 2016a, b; Ding et al., 2017).
According to the previously commented results, the Epo treatment on AβPP/PS1 transgenic mice showed reduction of the cortical areas positive for Aβ plaques in around 20%, after treatment during 3 days/week for 4 weeks, correlating this evidence with results showing an enhancement in the performance of these transgenic mice on V-maze trials (Armand-Ugón et al., 2015; Ott et al., 2015).
In agreement with results for the requirement of EpoR activation, other studies have reinforced this idea with more data using carbamylated Epo (CEPO), and histological analysis found that the area covered by Aβ plaques in the cerebral cortex (calculated with respect to the total cerebral cortex area) of AβPP/PS1 transgenic mice was 1.3%, and that this value was reduced by 20% after treatment with Epo during 3 days/week for 4 weeks. Later, these mice were evaluated by the V-maze test to assess memory function and these data indicate that chronic treatment with EPO and CEPO improved memory of AβPP/PS1 animals to levels similar to wild type animals (Armand-Ugón et al., 2015; Ott et al., 2015).
Using field stimulation experiments, it has been demonstrated that Epo treatments on the Schaffer collateral-CA1 hippocampal areas are able to revert the extreme inhibition in long-term potentiation (LTP) induced by Aβ25–35, also showing a decrease in glutamate release in the same animal model of AD (Esmaeili Tazangi et al., 2015). Other research suggests Epo is able to recover GSK-3β activity, which is inhibited by Aβ42 (Gan et al., 2012; Ponce et al., 2013; Li et al., 2015).
Finally, current evidence suggests that Epo exerts its neuroprotective effects through activation of its receptor (EpoR), resulting in the activation of several pathways that block apoptosis and thereby reducing the damage induced by different types of stress, such as inflammatory or ischemic brain processes or neurodegenerative diseases that produce synaptic disconnection and/or silencing. Therefore, the development of new Epo isoforms lacking hematopoietic effects could be considered new potential targets, able to exert neuroprotection without the risk of vascular damage.
Footnotes
Funding: This work was supported by the Innova Proyect, No. 13IDL218688, Fondecyt Proyect, No. 1130747, 1161078, PhD CONICYT Grant, No. 21130386.
Conflicts of interest: None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Abhisek Mukherjee, University of Texas, USA.
References
- Adamcio B, Sargin D, Stradomska A, Medrihan L, Gertler C, Theis F, Zhang M, Muller M, Hassouna I, Hannke K, Sperling S, Radyushkin K, El-Kordi A, Schulze L, Ronnenberg A, Wolf F, Brose N, Rhee JS, Zhang W, Ehrenreich H. Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol. 2008;6:37. doi: 10.1186/1741-7007-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabpoor Z, Hamidi G, Rashidi B, Shabrang M, Alaei H, Sharifi MR, Salami M, Dolatabadi HR, Reisi P. Erythropoietin improves neuronal proliferation in dentate gyrus of hippocampal formation in an animal model of Alzheimer's disease. Adv Biomed Res. 2012;1:50. doi: 10.4103/2277-9175.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand-Ugón M, Aso E, Moreno J, Riera-Codina M, Sánchez A, Vegas E, Ferrer I. Memory improvement in the AbetaPP/PS1 mouse model of familial Alzheimer's disease induced by carbamylated-erythropoietin is accompanied by modulation of synaptic genes. J Alzheimers Dis. 2015;45:407–421. doi: 10.3233/JAD-150002. [DOI] [PubMed] [Google Scholar]
- Assaraf MI, Diaz Z, Liberman A, Miller WH, Jr, Arvanitakis Z, Li Y, Bennett DA, Schipper HM. Brain erythropoietin receptor expression in Alzheimer disease and mild cognitive impairment. J Neuropathol Exp Neurol. 2007;66:389–398. doi: 10.1097/nen.0b013e3180517b28. [DOI] [PubMed] [Google Scholar]
- Batmunkh C, Krajewski J, Jelkmann W, Hellwig-Bürgel T. Erythropoietin production: Molecular mechanisms of the antagonistic actions of cyclic adenosine monophosphate and interleukin-1. FEBS Lett. 2006;580:3153–3160. doi: 10.1016/j.febslet.2006.04.069. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ, Pobre E, Diaz D, Gomez D, Hand C, Coleman T, Cerami A. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M, Patel NS, Villa P, Brines C, Mennini T, De Paola M, Erbayraktar Z, Erbayraktar S, Sepodes B, Thiemermann C, Ghezzi P, Yamin M, Hand CC, Xie QW, Coleman T, Cerami A. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci U S A. 2008;105:10925–10930. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendia I, Parada E, Navarro E, León R, Negredo P, Egea J, López MG. Subthreshold concentrations of melatonin and galantamine improves pathological AD-hallmarks in hippocampal organotypic cultures. Mol Neurobiol. 2016;53:3338–3348. doi: 10.1007/s12035-015-9272-5. [DOI] [PubMed] [Google Scholar]
- Bunn HF. Erythropoietin. Cold Spring Harb Perspect Med. 2013;3:a011619. doi: 10.1101/cshperspect.a011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda-Arellano R, Feria-Velasco AI, Rivera-Cervantes MC. Activity increase in EpoR and Epo expression by intranasal recombinant human erythropoietin (rhEpo) administration in ischemic hippocampi of adult rats. Neurosci Lett. 2014;583:16–20. doi: 10.1016/j.neulet.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Cheetham JC, Smith DM, Aoki KH, Stevenson JL, Hoeffel TJ, Syed RS, Egrie J, Harvey TS. NMR structure of human erythropoietin and a comparison with its receptor bound conformation. Nat Struct Biol. 1998;5:861–866. doi: 10.1038/2302. [DOI] [PubMed] [Google Scholar]
- Chen J, Yang Z, Zhang X. Carbamylated erythropoietin: a prospective drug candidate for neuroprotection. Biochem Insights. 2015;8:25–29. doi: 10.4137/BCI.S30753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Mu Y, Cui S, Yao Q, Maiese K. Targeting erythropoietin for chronic neurodegenerative diseases. Expert Opin Ther Targets. 2013;17:707–720. doi: 10.1517/14728222.2013.780599. [DOI] [PubMed] [Google Scholar]
- Ding J, Wang J, Li QY, Yu JZ, Ma CG, Wang X, Lu CZ, Xiao BG. Neuroprotection and CD131/GDNF/AKT pathway of carbamylated erythropoietin in hypoxic neurons. Mol Neurobiol. 2017;54:5051–5060. doi: 10.1007/s12035-016-0022-0. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jahnig P, Herrmann M, Knauth M, Bähr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009:e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Esmaeili Tazangi P, Moosavi SM, Shabani M, Haghani M. Erythropoietin improves synaptic plasticity and memory deficits by decrease of the neurotransmitter release probability in the rat model of Alzheimer's disease. Pharmacol Biochem Behav. 2015;130:15–21. doi: 10.1016/j.pbb.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- Gan Y, Xing J, Jing Z, Stetler RA, Zhang F, Luo Y, Ji X, Gao Y, Cao G. Mutant erythropoietin without erythropoietic activity is neuroprotective against ischemic brain injury. Stroke. 2012;43:3071–3077. doi: 10.1161/STROKEAHA.112.663120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupke S, Hall J, Dobbs M, Bix GJ, Fraser JF. Understanding history, and not repeating it. Neuroprotection for acute ischemic stroke: from review to preview. Clin Neurol Neurosurg. 2015;129:1–9. doi: 10.1016/j.clineuro.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Gui DM, Yang Y, Li X, Gao DW. Effect of erythropoietin on the expression of HIF-1 and iNOS in retina in chronic ocular hypertension rats. Int J Ophthalmol. 2011;4:40–43. doi: 10.3980/j.issn.2222-3959.2011.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingley E. Integrating novel signaling pathways involved in erythropoiesis. IUBMB Life. 2012;64:402–410. doi: 10.1002/iub.1024. [DOI] [PubMed] [Google Scholar]
- Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, Cao G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41:1032–1037. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi M, Hofstatter E, Stickle N, Beattie BK, Jaster R, Carter-Su C, Barber DL. The SH2B1 adaptor protein associates with a proximal region of the erythropoietin receptor. J Biol Chem. 2012;287:26223–26234. doi: 10.1074/jbc.M112.382721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelkmann W. ‘O’, erythropoietin carbamoylation versus carbamylation. Nephrol Dial Transplant. 2008;23:3033. doi: 10.1093/ndt/gfn342. author reply 3033-3034. [DOI] [PubMed] [Google Scholar]
- Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83:673–686. doi: 10.1007/s00277-004-0911-6. [DOI] [PubMed] [Google Scholar]
- Jia Y, Mo SJ, Feng QQ, Zhan ML, OuYang LS, Chen JC, Ma YX, Wu JJ, Lei WL. EPO-dependent activation of PI3K/Akt/FoxO3a signalling mediates neuroprotection in in vitro and in vivo models of Parkinson's disease. J Mol Neurosci. 2014;53:117–124. doi: 10.1007/s12031-013-0208-0. [DOI] [PubMed] [Google Scholar]
- Jiang J, Tian F, Cai Y, Qian X, Costello CE, Ying W. Site-specific qualitative and quantitative analysis of the N- and O-glycoforms in recombinant human erythropoietin. Anal Bioanal Chem. 2014;406:6265–6274. doi: 10.1007/s00216-014-8037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Singh A, Ekavali A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Lappin TR, Maxwell AP, Johnston PG. EPO's alter ego: erythropoietin has multiple actions. Stem Cells. 2002;20:485–492. doi: 10.1634/stemcells.20-6-485. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Li YP, Yang GJ, Jin L, Yang HM, Chen J, Chai GS, Wang L. Erythropoietin attenuates Alzheimer-like memory impairments and pathological changes induced by amyloid beta42 in mice. Brain Res. 2015;1618:159–167. doi: 10.1016/j.brainres.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhu B, Zou H, Hu D, Gu Q, Liu K, Xu X. Carbamylated erythropoietin mediates retinal neuroprotection in streptozotocin-induced early-stage diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2015;253:1263–1272. doi: 10.1007/s00417-015-2969-3. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Kaiser CA, Krieger M, Bretscher A, Ploegh H, Amon A, Scott MP. Molecular Cell Biology. 7th ed. New York: WH Freeman and Company; 2013. [Google Scholar]
- Lourhmati A, Buniatian GH, Paul C, Verleysdonk S, Buecheler R, Buadze M, Proksch B, Schwab M, Gleiter CH, Danielyan L. Age-dependent astroglial vulnerability to hypoxia and glutamate: the role for erythropoietin. PLoS One. 2013;8:e77182. doi: 10.1371/journal.pone.0077182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Jiang L, Zhu H, Zhang L, Wang T. Hypoxia-inducible factor-1alpha and erythropoietin expression in the hippocampus of neonatal rats following hypoxia-ischemia. J Nanosci Nanotechnol. 2014;14:5614–5619. doi: 10.1166/jnn.2014.8728. [DOI] [PubMed] [Google Scholar]
- Ma R, Hu J, Huang C, Wang M, Xiang J, Li G. JAK2/STAT5/Bcl-xL signalling is essential for erythropoietin-mediated protection against apoptosis induced in PC12 cells by the amyloid beta-peptide Abeta25-35. Br J Pharmacol. 2014;171:3234–3245. doi: 10.1111/bph.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Xiong N, Huang C, Tang Q, Hu B, Xiang J, Li G. Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology. 2009;56:1027–1034. doi: 10.1016/j.neuropharm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Maiese K. Charting a course for erythropoietin in traumatic brain injury. J Transl Sci. 2016a;2:140–144. doi: 10.15761/jts.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K. Regeneration in the nervous system with erythropoietin. Front Biosci (Landmark Ed) 2016b;21:561–596. doi: 10.2741/4408. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Wang S. Erythropoietin: new directions for the nervous system. Int J Mol Sci. 2012;13:11102–11129. doi: 10.3390/ijms130911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S, Kada E, Nagao M, Sasaki R. In vitro neuroprotective action of recombinant rat erythropoietin produced by astrocyte cell lines and comparative studies with erythropoietin produced by Chinese hamster ovary cells. Cytotechnology. 1999;31:179–184. doi: 10.1023/A:1008028423510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S, Nagao M, Takahata K, Konishi Y, Gallyas F, Jr, Tabira T, Sasaki R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J Biol Chem. 1993;268:11208–11216. [PubMed] [Google Scholar]
- Maurice T, Mustafa MH, Desrumaux C, Keller E, Naert G, de la CG-BM, Rodriguez Cruz Y, Garcia Rodriguez JC. Intranasal formulation of erythropoietin (EPO) showed potent protective activity against amyloid toxicity in the Aβ25_35 non-transgenic mouse model of Alzheimer's disease. J Psychopharmacol. 2013;27:1044–1057. doi: 10.1177/0269881113494939. [DOI] [PubMed] [Google Scholar]
- Miljus N, Heibeck S, Jarrar M, Micke M, Ostrowski D, Ehrenreich H, Heinrich R. Erythropoietin-mediated protection of insect brain neurons involves JAK and STAT but not PI3K transduction pathways. Neuroscience. 2014;258:218–227. doi: 10.1016/j.neuroscience.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Millet A, Bouzat P, Trouve-Buisson T, Batandier C, Pernet-Gallay K, Gaide-Chevronnay L, Barbier EL, Debillon T, Fontaine E, Payen JF. Erythropoietin and its derivates modulate mitochondrial dysfunction after diffuse traumatic brain injury. J Neurotrauma. 2016;33:1625–1633. doi: 10.1089/neu.2015.4160. [DOI] [PubMed] [Google Scholar]
- Montesino R, Toledo JR, Sanchez O, Sanchez A, Harvey DJ, Royle L, Dwek RA, Rudd PM, Gerwig GJ, Kamerling JP, Cremata JA. Monosialylated biantennary N-glycoforms containing GalNAc-GlcNAc antennae predominate when human EPO is expressed in goat milk. Arch Biochem Biophys. 2008;470:163–175. doi: 10.1016/j.abb.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, Sollott SJ, Lakatta EG, Talan MI. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006;316:999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Tsuda E, Said AA, Uchida E, Hatakeyama S, Ueda M, Hayakawa T. Biological and physicochemical characterization of recombinant human erythropoietins fractionated by Mono Q column chromatography and their modification with sialyltransferase. Glycoconj J. 1996;13:1013–1020. doi: 10.1007/BF01053197. [DOI] [PubMed] [Google Scholar]
- Nagańska E, Taraszewska A, Matyja E, Grieb P, Rafalowska J. Neuroprotective effect of erythropoietin in amyotrophic lateral sclerosis (ALS) model in vitro. Ultrastructural study. Folia Neuropathol. 2010;48:35–44. [PubMed] [Google Scholar]
- Noh MY, Cho KA, Kim H, Kim SM, Kim SH. Erythropoietin modulates the immune-inflammatory response of a SOD1(G93A) transgenic mouse model of amyotrophic lateral sclerosis (ALS) Neurosci Lett. 2014;574:53–58. doi: 10.1016/j.neulet.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Ott C, Martens H, Hassouna I, Oliveira B, Erck C, Zafeiriou MP, Peteri UK, Hesse D, Gerhart S, Altas B, Kolbow T, Stadler H, Kawabe H, Zimmermann WH, Nave KA, Schulz-Schaeffer W, Jahn O, Ehrenreich H. Widespread expression of erythropoietin receptor in brain and its induction by injury. Mol Med. doi: 10.2119/molmed.2015.00192. doi: 10.2119/molmed.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratova S, Kiryushko D, Sonn K, Soroka V, Kohler LB, Rathje M, Gu B, Gotfryd K, Clausen O, Zharkovsky A, Bock E, Berezin V. Neuroprotective properties of a novel, non-haematopoietic agonist of the erythropoietin receptor. Brain. 2010;133:2281–2294. doi: 10.1093/brain/awq101. [DOI] [PubMed] [Google Scholar]
- Pankratova S, Kiryushko D, Sonn K, Soroka V, Kohler LB, Rathje M, Gu B, Gotfryd K, Clausen O, Zharkovsky A, Bock E, Berezin V. Neuroprotective novel, non-haematopoietic receptor. Brain. 2010;133:2281–2294. doi: 10.1093/brain/awq101. [DOI] [PubMed] [Google Scholar]
- Park KH, Choi NY, Koh SH, Park HH, Kim YS, Kim MJ, Lee SJ, Yu HJ, Lee KY, Lee YJ, Kim HT. L-DOPA neurotoxicity is prevented by neuroprotective effects of erythropoietin. Neurotoxicology. 2011;32:879–887. doi: 10.1016/j.neuro.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Park SS, Park J, Ko J, Chen L, Meriage D, Crouse-Zeineddini J, Wong W, Kerwin BA. Biochemical assessment of erythropoietin products from Asia versus US Epoetin alfa manufactured by Amgen. J Pharm Sci. 2009;98:1688–1699. doi: 10.1002/jps.21546. [DOI] [PubMed] [Google Scholar]
- Parra AL, Rodriguez JC. Nasal neuro EPO could be a reliable choice for neuroprotective stroke treatment. Cent Nerv Syst Agents Med Chem. 2012;12:60–68. doi: 10.2174/187152412800229143. [DOI] [PubMed] [Google Scholar]
- Pasqualetti G, Brooks DJ, Edison P. The role of neuroinflammation in dementias. Curr Neurol Neurosci Rep. 2015;15:17. doi: 10.1007/s11910-015-0531-7. [DOI] [PubMed] [Google Scholar]
- Ponce LL, Navarro JC, Ahmed O, Robertson CS. Erythropoietin neuroprotection with traumatic brain injury. Pathophysiology. 2013;20:31–38. doi: 10.1016/j.pathophys.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Zhou S, Xiao Y, Chen L. Erythropoietin enhances mitochondrial biogenesis in cardiomyocytes exposed to chronic hypoxia through Akt/eNOS signalling pathway. Cell Biol Int. 2014;38:335–342. doi: 10.1002/cbin.10205. [DOI] [PubMed] [Google Scholar]
- Rabie T, Marti HH. Brain protection by erythropoietin: a manifold task. Physiology (Bethesda) 2008;23:263–274. doi: 10.1152/physiol.00016.2008. [DOI] [PubMed] [Google Scholar]
- Ratilal BO, Arroja MM, Rocha JP, Fernandes AM, Barateiro AP, Brites DM, Pinto RM, Sepodes BM, Mota-Filipe HD. Neuroprotective effects of erythropoietin pretreatment in a rodent model of transient middle cerebral artery occlusion. J Neurosurg. 2014;121:55–62. doi: 10.3171/2014.2.JNS132197. [DOI] [PubMed] [Google Scholar]
- Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol. 2010;24:573–594. doi: 10.1016/j.bpa.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Sargin D, El-Kordi A, Agarwal A, Muller M, Wojcik SM, Hassouna I, Sperling S, Nave KA, Ehrenreich H. Expression of constitutively active erythropoietin receptor in pyramidal neurons of cortex and hippocampus boosts higher cognitive functions in mice. BMC Biol. 2011;9:27. doi: 10.1186/1741-7007-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012;4:187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Wu Y, Xu JY, Zhang J, Sinclair SH, Yanoff M, Xu G, Li W, Xu GT. ERK- and Akt-dependent neuroprotection by erythropoietin (EPO) against glyoxal-AGEs via modulation of Bcl-xL, Bax, and BAD. Invest Ophthalmol Vis Sci. 2010;51:35–46. doi: 10.1167/iovs.09-3544. [DOI] [PubMed] [Google Scholar]
- Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- Sinclair AM. Erythropoiesis stimulating agents: approaches to modulate activity. Biologics. 2013;7:161–174. doi: 10.2147/BTT.S45971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Martorana A, Bernardini S, Panella M, Mercati F, Orlacchio A, Pierantozzi M. CSF markers in Alzheimer disease patients are not related to the different degree of cognitive impairment. J Neurol Sci. 2006;251:124–128. doi: 10.1016/j.jns.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Su KH, Shyue SK, Kou YR, Ching LC, Chiang AN, Yu YB, Chen CY, Pan CC, Lee TS. β Common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J Cell Physiol. 2011;226:3330–3339. doi: 10.1002/jcp.22678. [DOI] [PubMed] [Google Scholar]
- Toledo JR, Sánchez O, Seguí RM, García G, Montañez M, Zamora PA, Rodríguez MP, Cremata JA. High expression level of recombinant human erythropoietin in the milk of non-transgenic goats. J Biotechnol. 2006;123:225–235. doi: 10.1016/j.jbiotec.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Wagner LM, Billups CA, Furman WL, Rao BN, Santana VM. Combined use of erythropoietin and granulocyte colony-stimulating factor does not decrease blood transfusion requirements during induction therapy for high-risk neuroblastoma: a randomized controlled trial. J Clin Oncol. 2004;22:1886–1893. doi: 10.1200/JCO.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Wang R, Wu X, Liang J, Qi Z, Liu X, Min L, Ji X, Luo Y, Zhao H. Intra-artery infusion of recombinant human erythropoietin reduces blood-brain barrier disruption in rats following cerebral ischemia and reperfusion. Int J Neurosci. 2015;125:693–702. doi: 10.3109/00207454.2014.966354. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu C, Wang X, Gerwien JG, Schrattenholz A, Sandberg M, Leist M, Blomgren K. The nonerythropoietic asialoerythropoietin protects against neonatal hypoxia-ischemia as potently as erythropoietin. J Neurochem. 2004;91:900–910. doi: 10.1111/j.1471-4159.2004.02769.x. [DOI] [PubMed] [Google Scholar]
- Wei L, Han BH, Li Y, Keogh CL, Holtzman DM, Yu SP. Cell death mechanism and protective effect of erythropoietin after focal ischemia in the whisker-barrel cortex of neonatal rats. J Pharmacol Exp Ther. 2006;317:109–116. doi: 10.1124/jpet.105.094391. [DOI] [PubMed] [Google Scholar]
- Won HH, Park I, Lee E, Kim JW, Lee D. Comparative analysis of the JAK/STAT signaling through erythropoietin receptor and thrombopoietin receptor using a systems approach. BMC Bioinformatics. 2009;10(Suppl 1):S53. doi: 10.1186/1471-2105-10-S1-S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World Health Statistics 2011. 2011. http://www.who.int/whosis/whostat/2011/en/
- Wu JH, Gao Y, Ren AJ, Zhong M, Liu L. Erythropoietin receptor antibody inhibits oxidative stress induced retinal neovascularization in mice. Int J Ophthalmol. 2011;4:243–246. doi: 10.3980/j.issn.2222-3959.2011.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Fan Y, Sun X, Yao L, Chai W. Effects of erythropoietin preconditioning on rat cerebral ischemia-reperfusion injury and the GLT-1/GLAST pathway. Exp Ther Med. 2016;11:513–518. doi: 10.3892/etm.2015.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 2005;387:5–10. doi: 10.1016/j.neulet.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Zellinger C, Seeger N, Hadamitzky M, Fischborn S, Russmann V, Wendt H, Pankratova S, Bock E, Berezin V, Potschka H. Impact of the erythropoietin-derived peptide mimetic Epotris on the histopathological consequences of status epilepticus. Epilepsy Res. 2011;96:241–249. doi: 10.1016/j.eplepsyres.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Zhang G, Lehmann HC, Bogdanova N, Gao T, Zhang J, Sheikh KA. Erythropoietin enhances nerve repair in anti-ganglioside antibody-mediated models of immune neuropathy. PLoS One. 2011;6:e27067. doi: 10.1371/journal.pone.0027067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang Q, Xiang H, Xin Y, Chang M, Lu H. Neuroprotection with erythropoietin in preterm and/or low birth weight infants. J Clin Neurosci. 2014;21:1283–1287. doi: 10.1016/j.jocn.2013.10.040. [DOI] [PubMed] [Google Scholar]
- Zhou TF, Yu JG. Recombinant human erythropoietin attenuates neuronal apoptosis and cognitive defects via JAK2/STAT3 signaling in experimental endotoxemia. J Surg Res. 2013;183:304–312. doi: 10.1016/j.jss.2012.11.035. [DOI] [PubMed] [Google Scholar]