Abstract
Background
Long-term survival in patients with aggressive peripheral T-cell lymphoma (PTCL) is generally poor and there is no clear consensus on initial therapy used for these diseases. We analyzed treatment patterns and outcomes in a prospectively collected cohort of patients with a new diagnosis of nodal PTCL in the United States.
Methods
Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment (COMPLETE) is a prospective multicenter cohort study designed to identify the most common prevailing treatment patterns used for newly diagnosed PTCL patients in the United States. Patients with nodal PTCL and completed records on baseline characteristics and initial therapy were included in this analysis. All statistical tests were two-sided.
Results
Of a total of 499 patients enrolled, 256 (51.3%) had nodal PTCL and completed treatment records. Patients received as initial therapy: doxorubicin-containing regimens (41.8%), doxorubicin + etoposide-containing regimens (20.9%), other etoposide regimens (15.8%), other single agent or combination regimens (19.2%), and gemcitabine-containing regimens (2.1%). Survival was statistically significantly longer for patients who received doxorubicin (log-rank P = .03). After controlling for disease histology and International Prognostic Index, results showed a trend toward significance in mortality reduction in patients who received doxorubicin compared to those who did not (hazard ratio = 0.71, 95% confidence interval = 0.48 to 1.05, P = .09).
Conclusion
There is no clear standard of care in the treatment of PTCL in the United States. While efforts to improve front-line treatments are necessary, anthracyclines remain an important component of initial therapy of curative intent.
Keywords: prospective studies, cohort studies, lymphoma, T-cell, peripheral, drug therapy, anthracyclines, treatment outcome
Introduction
The peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of aggressive malignancies derived from mature (post-thymic) T-lymphocytes that comprise between 5% and 10% of all non-Hodgkin lymphomas (NHLs) in Western countries.1,2 Nodal PTCLs are the most common of the PTCL subtypes and include: peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), anaplastic large cell lymphoma (ALCL), and angioimmunoblastic T-cell lymphoma (AITL).3,4 While PTCL-NOS is the most common of the nodal PTCL subtypes, representing roughly 25% of all PTCLs, it is largely a diagnosis of exclusion that is made when typical features of better defined PTCL subtypes are not present.5 Less common PTCLs include extranodal and leukemic PTCLs such as extranodal NK/T-cell lymphoma, T-cell prolymphocytic leukemia, and hepatosplenic T-cell lymphoma, among others.6
Most clinical practice guidelines recommend initial treatment of the nodal PTCLs either on a therapeutic clinical trial or with regimens that were initially developed for patients with B-cell lymphoma, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone).6–8 However, the use of CHOP in PTCL has been associated with suboptimal 5-year overall survival rates ranging from 32% to 43% for PTCL-NOS, and 32% to 36% for AITL.4,9–11 A notable exception to the poor long-term prognosis associated with CHOP treatment in PTCL is ALK+ ALCL, which is associated with 5-year overall survival up to 70% after CHOP.4,12 In an effort to improve upon the poor results seen in PTCL-NOS and AITL, hematopoietic stem cell transplantation (HSCT) has been proposed for patients achieving complete remission after induction therapy; however, there are no randomized trials demonstrating a survival benefit for HSCT in this setting.13–15,16
To better understand the clinical characteristics, treatment patterns, and outcomes in patients with PTCL, the Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment (COMPLETE) study was initiated in 2010 (NCT01110733). Herein, we present the first publication of the treatments administered to COMPLETE study enrollees, focusing on those with aggressive nodal PTCL subtypes.
Methods
Study Design
COMPLETE is a prospective multicenter cohort study of patients with newly diagnosed PTCL in the United States. The primary objective of the study was to describe, in detail, patterns of care for PTCL patients. Secondary objectives were to document outcomes, identify factors influencing treatment decisions, determine incidence and severity of selected treatment toxicities and identify supportive care received (protocol available online).
Ethics
The protocol was approved by the institutional review board (IRB) of each participating institution. All study participants or participants’ guardians gave written informed consent before study entry.
Study Eligibility
Patients with a new diagnosis of histologically confirmed PTCL were eligible for enrollment in the COMPLETE study until 30 days after initiation of the first lymphoma directed therapy. The following T- and NK-cell malignancies were excluded from COMPLETE: Precursor T/NK neoplasms, T-cell large granular lymphocytic leukemia, mycosis fungoides (other than transformed mycosis fungoides), Sézary syndrome, and primary cutaneous CD30+ disorders. Patients participating in clinical trials were not excluded from participation in COMPLETE. No specific target for enrollment was pre-specified given the lack of prior studies to inform such projections.
Data Collection and Review for Accuracy
De-identified data were regularly uploaded onto the study server from each enrolling site, as new data became available. Data entered on the web-based server were subjected to automated error checking that looked for logical inconsistencies, out of range values and missing data. Verification of data accuracy was performed by either the treating physician or site principal investigator, after which the data was locked. Central review of the locked data was performed by a steering committee of experts in PTCL and queries were generated to verify any data inconsistencies. A separate analysis was performed to verify the accuracy of the histologic data entered at each site through comparison of the uploaded histologic data to the diagnostic pathology reports.17
Cohort Selection
Due to the variable natural history and treatments used for the various PTCL subtypes, the study population presented herein was confined to the nodal PTCL population with a poor prognosis: AITL, PTCL-NOS, and ALCL (ALK−, and ALK+ with international prognostic index [IPI] 2–5). For patients with ALCL, we excluded patients with ALK+ disease and a low IPI score from our analyses given their superior prognosis compared to the other nodal PTCLs in the cohort.18 The remaining patients were stratified into initial treatment groups based on the type of therapy received. The primary outcomes of interest were response rates and overall survival.
Statistical Analysis
Descriptive statistics were used to summarize the baseline characteristics of the study cohort. For categorical and ordinal variables, frequencies and percentages were calculated. For continuous variables, descriptive statistics (number of patients, mean, median, standard deviation and range) were provided. Null hypothesis testing utilized chi-square, t-test and other non-parametric tests as required, with a two tailed p value of ≤0.05 to reject the null hypothesis. Survival-based analyses were performed using Kaplan-Meier methodology with censoring as appropriate and evaluated using a log rank test with a two tailed p value of ≤0.05 to reject the null hypothesis. Multivariable Cox regression was used to assess the relationship between pre-specified variables and overall survival. Hazard ratios and their 95% confidence intervals were also calculated. Non-significant variables (e.g. p >0.05) were not removed from the final models. The assumption of proportional hazards for the multivariable Cox regression models was assessed using a test based upon weighted Schoenfeld residuals versus log(time). All analyses were performed using R version 3.1.0 or greater (The R Foundation for Statistical Computing - http://www.r-project.org/).
Results
A total of 499 patients were enrolled in the COMPLETE study between February 2010 and February 2014 from 55 academic and community sites located throughout the United States. Locked baseline records were available for 440 patients (88.2%). Patients with a diagnosis of ALCL, AITL, or PTCL-NOS comprised roughly two-thirds of the overall cohort of patients with locked baseline records (n = 287, 65.2%). After excluding patients with ALCL, AKL+ disease with an IPI of 0–1 and patients who discontinued the study for reasons other than death, locked baseline records and treatment records were available for 275 and 256 patients, respectively. The principal reason patients were removed from the baseline and treatment analyses was due to other PTCL histology (n = 150 patients). A total of 198 patients had year one follow-up records, and 119 patients had year two follow-up records (Figure 1).19
Fig. 1.
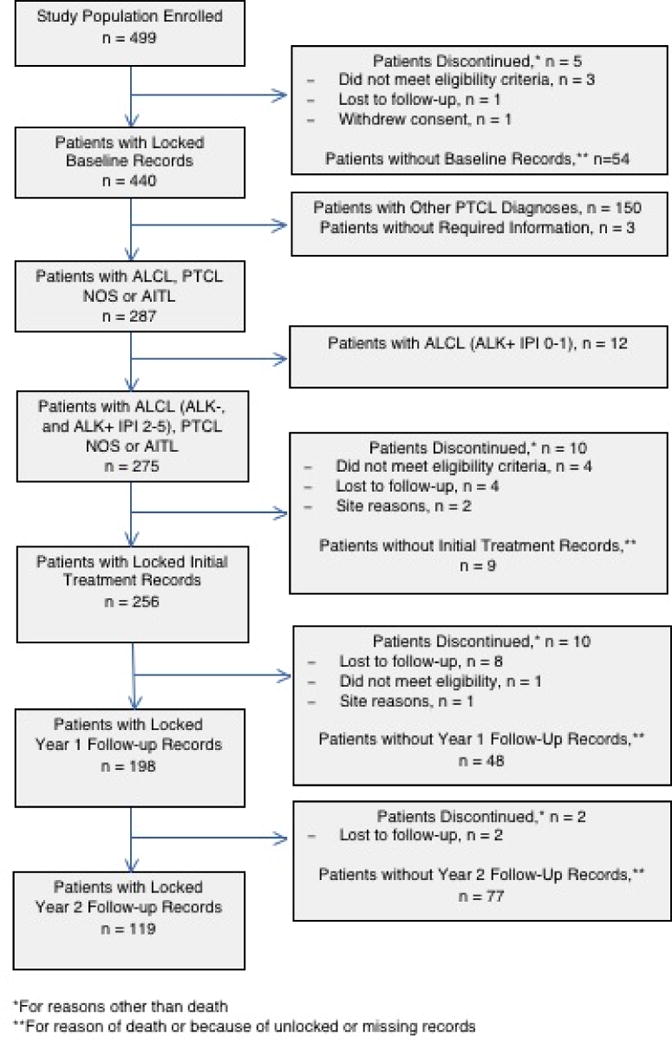
STROBE diagram
Patient Characteristics
Baseline characteristics of the patients with nodal PTCL are presented in Table 1. Consistent with data from the Surveillance, Epidemiology, and End Results (SEER) Program,20 a sizable proportion of patients were 65 years of age or older (45.1% and 44.7% for COMPLETE and SEER patients, respectively); the majority were male (66.3% and 58.9%, respectively), white (77.3% and 76.2%, respectively), and had advanced stage (Ann Arbor stage III/IV) disease (76.5% and 61.4%, respectively). The diagnosis of PTCL-NOS was roughly twice as frequent as ALCL or AITL (51.3% and 49.9%, for COMPLETE and SEER patients, respectively). Characteristics of COMPLETE patients were also comparable to patients enrolled in the International T-Cell Lymphoma Project4 (ITCP): median age (63.1 vs. 62), Stage III/IV (76.5% vs. 74.0%) and male sex (66.3% vs. 61.9%).
Table 1.
Baseline Demographics and Clinical Characteristics of Patients with PTCL-NOS, AITL, and ALCL (N = 273)
| Characteristic | No. (%) |
|---|---|
| Age (years) | |
| Median | 63.1 |
| 65+ | 123 (45.1%) |
|
| |
| Sex | |
| Male | 181 (66.3) |
| Female | 92 (33.7) |
|
| |
| Race | |
| Black | 42 (15.4) |
| White | 211 (77.3) |
| Other | 13 (4.8) |
| Unknown | 7 (2.6) |
|
| |
| Ann Arbor Stage | |
| I | 29 (10.6) |
| II | 35 (12.8) |
| III | 82 (30.0) |
| IV | 127 (46.5) |
|
| |
| Any B-Symptoms present | 126 (46.2) |
|
| |
| Serum LDH elevated* | 118 (44.4) |
|
| |
| Histopathology | |
| PTCL-NOS | 140 (51.3) |
| AITL | 71 (26.0) |
| ALCL | 62 (22.7) |
| ALK− | 49 (79.0) |
| ALK+ (IPI 2–5) | 13 (21.0) |
|
| |
| Median IPI | 2 |
|
| |
| ECOG Performance Status | |
| 0–1 | 248 (90.8) |
| 2 | 23 (8.4) |
| ≥3 | 2 (0.7) |
|
| |
| Enrolling institution | |
| Academic | 236 (86.0) |
| Community | 37 (14.0) |
|
| |
| Clinical trial participant | 57 (20.9) |
Baseline information is missing for 2 patients.
Abbreviations: LDH, lactate dehydrogenase; IPI, International Prognostic Index; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified; ECOG, Eastern Cooperative Oncology Group
Information on LDH is missing for 7 patients.
Treatment Patterns
The intent of therapy, as defined by the treating investigator, was cure in 86.9% of the patients, while palliation was the intent in the remaining 13.1%. A slightly higher proportion of patients given doxorubicin were treated with curative intent (94.4%), compared to those who did not receive doxorubicin (83.3%), but a substantial majority in both sub-groups were treated with curative intent. The top reasons for selection of a specific first-line regimen by the treating physicians were: PTCL subtype (62.5%), clinical data from the literature (55.3%), and patient age (37.9%). Cost or insurance coverage was a factor in treatment decisions for only two patients (<1.0%).
Initial treatment strategies included doxorubicin in 41.8% and doxorubicin + etoposide in 20.9% (Figure 2). The remaining patients received a variety of etoposide-based combination or single agent regimens (15.8%), other single agent or combination regimens (19.2%), or gemcitabine-based regimens (2.1%). Eight patients received rituximab as part of their PTCL treatment, half of whom were diagnosed with AITL. HSCT was performed in 52 patients (47 autologous and 5 allogeneic) as part of initial therapy, representing 20.6% of the overall study cohort. The proportion of patients receiving doxorubicin was comparable between those who received a transplant vs. those who did not (64.0% vs. 62.7%, P = .87). Radiotherapy was administered to 16 patients. Eleven patients were followed initially with observation/supportive care. First-line therapy was completed as planned in 64.3% of patients.
Fig. 2.
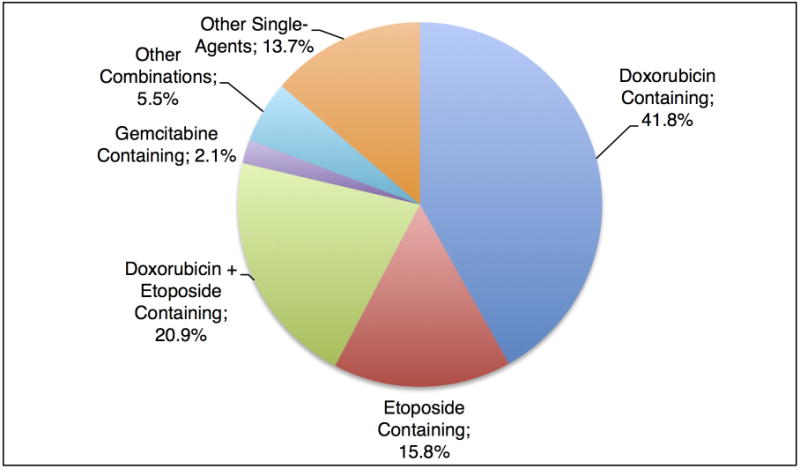
Induction Regimen for Patients with PTCL-NOS, AITL, and ALCL (N = 234).
Information on treatment regimen is missing for 4 patients.
Outcomes
Response assessment was undertaken by the treating investigator according to the Revised Response Criteria for Malignant Lymphoma.21 Complete response (CR) was reported in 57.1%, partial response in 13.3%, and stable or progressive disease in 19.1%. An additional 10.4% of patients were not evaluable for response. Best response was observed a median of 132 days after PTCL diagnosis, which approximated when 6 cycles of chemotherapy administered every 3 weeks would be completed. When responses were stratified by doxorubicin vs. non-doxorubicin treatment, a higher CR rate was seen in patients receiving doxorubicin treatment (Table 2). A higher CR rate was also reported in patients treated with curative vs. palliative intent: 60.6% vs. 24%.
Table 2.
Overall Best Response to Induction Therapy for Patients with PTCL-NOS, AITL, and ALCL, Stratified by Anthracycline Use (N = 227)
| Best Response | Did not Receive Anthracycline (%) |
Received Anthracycline (%) |
P* |
|---|---|---|---|
| Complete Response | 46.4 | 62.9 | .05 |
| Partial Response | 14.3 | 11.9 | |
| Stable Disease | 6.0 | 7.0 | |
| Progressive Disease | 16.7 | 11.9 | |
| Not evaluable | 16.7 | 6.3 |
Chi-square test
Information on response is missing for 29 patients.
Abbreviations: ALCL, anaplastic large cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified
Median follow-up in the cohort was 26.0 months (interquartile range 14.4 to 37.7 months). Estimated median survival was 43.0 months (95% confidence interval [CI]: 34.5 months to not reached; Figure 3A), with 12- and 24-month survival rates of 71.7% and 58.7%, respectively. PTCL subtype and IPI score had a significant impact on overall survival. Patients with ALCL had statistically significant better overall survival compared to those with AITL and PTCL-NOS (log-rank P = .01) (Figure 3B), and patients with lower IPI scores had statistically significant longer survival compared to those with higher scores (log-rank P = .0002) (IPI 0/1 vs. 2/3 vs. 4/5; Figure 3C). Survival by doxorubicin use is shown in Figure 4. Survival was statistically significantly longer for patients who received doxorubicin (log-rank P = .03). Cox proportional hazards analysis was performed to evaluate doxorubicin use, etoposide use, and overall survival, while controlling for IPI score and PTCL histology (Table 3); results showed a trend toward significance in reduction in mortality among those treated with doxorubicin (hazard ratio [HR] = 0.71, 95% confidence interval [CI] 0.48 – 1.05, P = .09), but no association was observed for those treated with etoposide (HR = 1.00, 95% CI 0.66 – 1.53, P = .99). Analyses surrounding HSCT and survival were not performed based on limited follow-up.
Fig. 3.
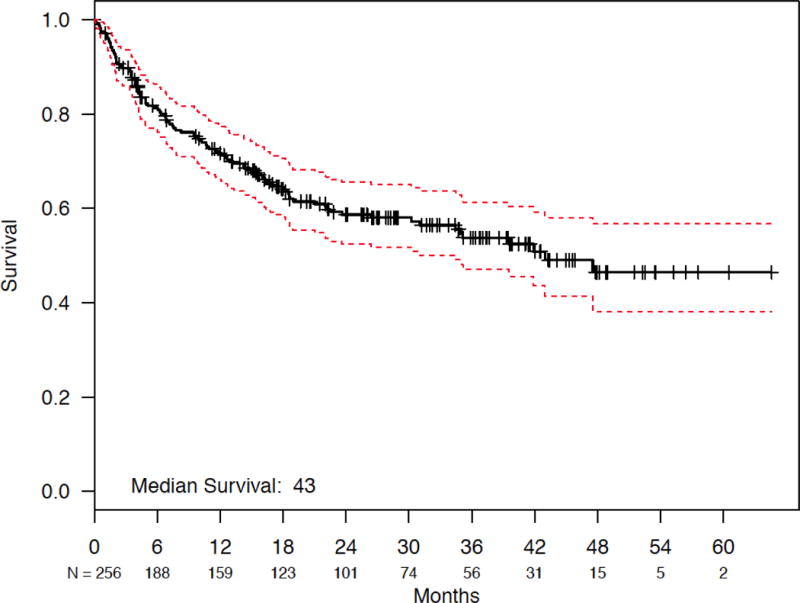
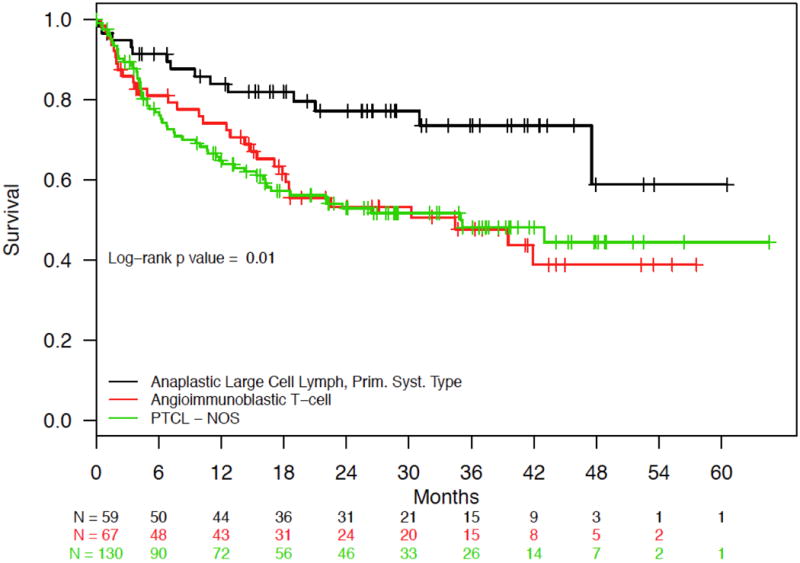
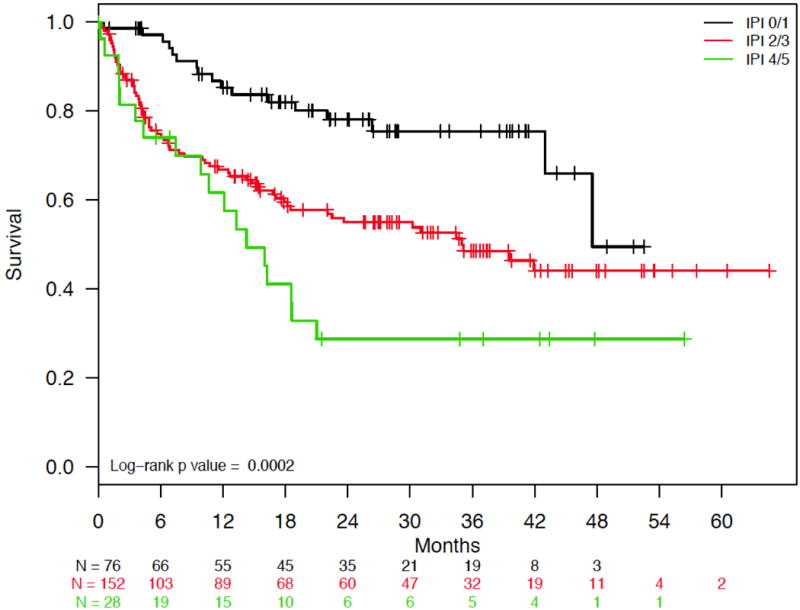
(a) Overall Survival for Patients with PTCL-NOS, AITL, and ALCL. (b) Overall Survival for Patients with PTCL-NOS, AITL, or ALCL, Stratified by PTCL Subtype. (c) Overall Survival for Patients with PTCL-NOS, AITL, and ALCL, Stratified by IPI Score.
Abbreviations: ALCL, anaplastic large cell lymphoma; prim syst type, primary systemic type; AITL, angioimmunoblastic T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified; IPI, International Prognostic Index
Fig. 4.
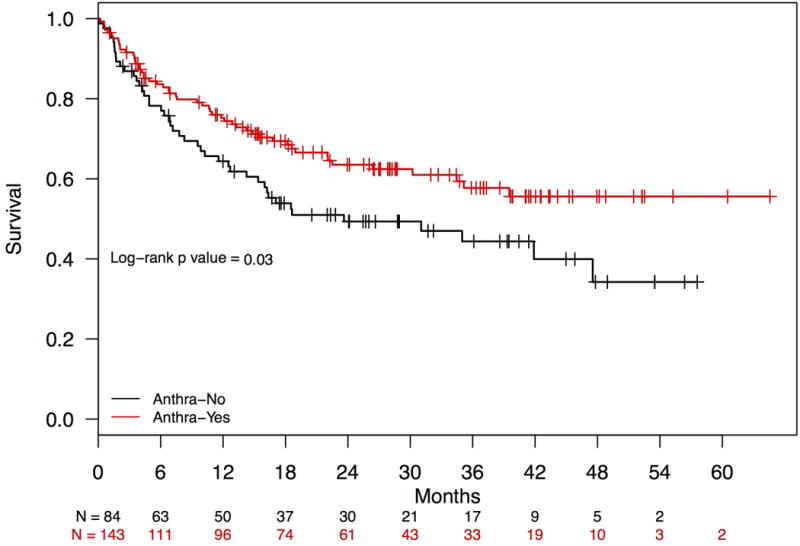
Overall Survival for Patients with PTCL-NOS, AITL, and ALCL, Stratified by Anthracycline Use.
Abbreviations: Anthra, anthracyclines; PTCL-NOS, peripheral T-cell lymphoma-not otherwise specified; AITL, angioimmunoblastic T-cell lymphoma IPI; ALCL, anaplastic large cell lymphoma
Table 3.
Cox Regression Model for Multivariable Analysis of Overall Survival (N = 250)
| Factor | Hazard Ratio | 95% CI | P value* |
|---|---|---|---|
| IPI score | |||
| 2–3 | 2.16 | 1.25–3.72 | .006 |
| 4–5 | 3.28 | 1.67–6.43 | <.001 |
|
| |||
| Histopathology | |||
| AITL | 0.93 | 0.59–1.45 | .73 |
| ALCL | 0.46 | 0.25–0.84 | .01 |
|
| |||
| Anthracycline used† | 0.71 | 0.48–1.05 | .09 |
|
| |||
| Etoposide used‡ | 1.00 | 0.66–1.53 | .99 |
Wald chi-square test
Alone or in combination with another agent including etoposide.
Alone or in combination with another agent including an anthracycline.
Abbreviations: IPI, International Prognostic Index; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; CI, confidence interval
Reference levels: IPI 0–1, peripheral T-cell lymphoma-not otherwise specified
Number of events = 100
Grade 3 and 4 or clinically significant adverse events were common among nodal PTCL patients. Roughly one third of patients (32.3%, n = 82) were hospitalized at some point during initial treatment. The most common reported adverse events were neutropenia (33.9%), thrombocytopenia (24.0%), anemia (18.9%), and febrile neutropenia (18.5%). The most common supportive care measures administered were myeloid growth factors (57.1%) and blood/platelet transfusions (21.3%).
Discussion
COMPLETE is the largest-ever prospective cohort study of patients with PTCL in the United States. As with other aggressive NHLs,21 the intent of therapy in a substantial majority of patients was cure. Treatment regimens were selected primarily based upon clinical considerations, with cost or insurance coverage concerns exerting little influence. Treatment-related toxicities occurred at a rate expected for aggressive NHL patients receiving multi-agent chemotherapy.
Over the past decade, the role of doxorubicin in the initial treatment of nodal PTCLs other than ALCL has come into question, as evidenced by the fact that more than one third of patients in COMPLETE did not receive anthracyclines in the front line.4,23,24 However, we observed that patients who received non-anthracycline regimens had inferior response rates and worse overall survival compared to patients treated with anthracyclines. Confirmation of reduced mortality associated with anthracycline use on multivariate analysis (controlling for IPI score and disease histology) argues against confounding by indication as the primary source of this observation. Of note, two recent clinical trials using combination chemotherapy regimens without doxorubicin also did not improve response rates.23,24 Our findings support the use of anthracyclines as an important part of the induction regimen for patients with nodal PTCL who are being treated with curative intent.25 Among those expected to tolerate anthracycline treatment, removal of anthracyclines should be attempted only under the auspices of a clinical trial.
The inclusion of etoposide as a component of initial therapy was not significantly associated with survival in our study after controlling for disease histology and IPI (HR = 1.00). While the German high-grade non-Hodgkin lymphoma study group has presented evidence supporting the addition of etoposide to the CHOP regimen (CHOEP) in PTCL patients, this was a post-hoc analysis of clinical trial data and the benefit was confined to an improvement in event-free survival among patients younger than age 60 with normal LDH.26 Furthermore, the majority of the benefit was observed in patients with ALCL. (We did not compare CHOP to CHOEP due to small numbers of patients.) While it is conceivable that residual confounding or small patient numbers limited our ability to detect a survival difference, consideration of our data in conjunction with the German data (which also did not demonstrate an improvement in overall survival) suggests that any overall survival benefit associated with etoposide use in PTCL is at best modest.
Despite the fact that a majority of the patients in this study were enrolled at academic institutions, only 20.6% of the nodal PTCL patients received HSCT. A recent retrospective analysis of PTCL patients in the United States demonstrated a transplant frequency of only 10%.27 Our findings highlight that regardless of the survival benefit that may be conferred by HSCT, in practice this treatment modality is utilized in only a small minority of the PTCL patient population. As a result, efforts to improve overall survival for the majority of PTCL patients must focus on improvement in outcomes with induction and salvage therapies.
The use of rituximab in patients with nodal PTCL deserves special attention. Half of the patients in the cohort who received rituximab had AITL, a disease in which abnormal T-cells are histologically associated with reactive B-cells that express CD20.28 While it was initially thought that rituximab induced B-cell reduction in patients with AITL would improve outcomes, a trial by GELA showed no difference in outcomes compared to CHOP alone.29
Front-line studies in PTCL remain challenging to perform, largely due to the rarity of PTCL. Nearly all of the recently completed large therapeutic studies in PTCL were in relapsed and refractory patients who were fit to enter clinical trials and were designed to support drug approvals. As a result, there may be differences between patients studied in clinical trials and the overall population of patients with PTCL.30 Moving forward, consideration should be given to pragmatic clinical trials to compare the effectiveness of front-line treatments in PTCL patients with both favorable and unfavorable clinical manifestations. Such an approach would enhance enrollment through broader enrollment criteria.31 In addition, more widespread adoption of the National Cancer Institute Central IRB process may streamline the opening of multicenter PTCL trials at a sufficient number of sites to allow completion of trials of comparative effectiveness.32
The limitations of this study should be highlighted. Although we cannot rule out selection bias, the similarity of characteristics of the patients enrolled in COMPLETE compared to what has been reported in SEER20 and the ITCP4 suggest this patient cohort is representative of the overall PTCL patient population. Furthermore, the prospective design of the study should reduce the risk of bias and confounding compared to retrospective studies of patients with PTCL. Data quality is also a concern in observational studies. While we did not perform onsite monitoring, the steering committee reviewed the collected data on an ongoing basis to uncover clinical inconsistencies, prompting clarification. Additionally, the level of missing data was low, with few patients discontinuing the study for reasons other than death (n = 27, 5.4%). The high proportion of patients enrolled in academic sites (86.0%) is likely a reflection of the referral patterns for rare diseases. Finally, like any analysis of data that is not randomized, we cannot rule out residual confounding from unmeasured variables as a contributor to our findings.
According to the NCCN guidelines, there is no standard of care for nodal PTCL and many regimens have been used for induction therapy. The data from COMPLETE support the concept that anthracycline-containing regimens should be utilized over non-anthracycline regimens for patients with common nodal subtypes. The lack of significant improvement in overall survival in COMPLETE compared to historical data underscores the need for further studies of novel agents and approaches for PTCL.
Condensed abstract.
There is no clear standard of care in the treatment of PTCL in the United States. While efforts to improve front-line treatments are necessary, anthracyclines remain an important component of initial therapy of curative intent.
Acknowledgments
P30 CA008748.
Funding Source: Spectrum Pharmaceuticals, Inc.
Footnotes
Conflict of Interest Disclosures: Dr. Carson: consultant, Celgene, Millennium, Genentech; research funding, Millennium, Kyowa Hakko Kirin; expert testimony, Abbvie. Dr. Horwitz: consultant, Celgene, BMS, Millennium, Amgen, Seattle Genetics, Spectrum; research funding, Celgene, Millennium, Kyowa Hakko Kirin, Seattle Genetics, Spectrum, Infinity Pharmaceuticals. Dr. Pinter-Brown: consultant, Celgene, Spectrum, Seattle Genetics. Dr. Rosen: consultant, Allos, Celgene, DAVAOncology, Defined Health, Elorac Inc., Genentech, IMC Healthcare Communications Premier, Healthcare Resources, Prostrakan Inc., Seattle Genetics, Teva, TRM Oncology; research funding, Celgene, ILEX Pharmaceuticals, Eli Lilly, Millennium; expert testimony, Fulbrite & Jawarski LLP, Hinshaw & Culbertson LLP, Hunt Suedhoff & Kalamaros LLP; stock or other ownership, AuraSense LLC, Nanosphere; honoraria, Celgene, Genentech, Genzyme, Seattle Genetics, Teva, The CM Group, The Medal Group, Therakos. Dr. Pro: consultant, Celgene; research funding, Celgene, Seattle Genetics; honoraria, Celgene, Seattle Genetics, Takeda. Dr. Hsi: research funding, Abbvie, Cellerant, Eli Lilly; honoraria, Abbvie, Cellerant, Onyx, Seattle Genetics. Dr. Federico: consultant, MedNet. Dr. Gisselbrecht: research funding, Roche/Genentech; speakers’ bureau, Roche/Genentech. Mr. Schwartz: employee, MedNet. Ms. Bellm: consultant, MedNet; stock or other ownership, BMS, Johnson & Johnson, Merck. Dr. Acosta: employee, Spectrum; stock or other ownership, Spectrum. Dr. Advani: consultant, Genentech, FortySeven, Kyowa Hakko Kirin; research funding, Millennium, Seattle Genetics, Genentech, Allos, Pharmacyclics, Janssen, Celgene, Idera, Agensys, Merck, Kura Oncology, Regeneron, Infinity Pharmaceuticals. Dr. Feldman: consultant, Celgene, Novartis, Seattle Genetics; honoraria, Celgene, Pharmacyclics, Seattle Genetics; speakers’ bureau, Celgene, Seattle Genetics, Pharmacyclics. Dr. Lechowicz: consultant, Seattle Genetics, Spectrum, Soligenix. Dr. Smith: consultant, Genentech, Onyx, Seattle Genetics, TG Therapeutics, Gilead, Immunogenix, Pharmacyclics; honoraria, Celgene, Janssen. Dr. Lansigan: consultant, Celgene; research funding, Spectrum, Teva. Dr. Kahl: consultant, Seattle Genetics, Celgene. Dr. Leach: research funding, BMS, AstraZeneca, Merck, Halozyme. Dr. Park: research funding, Teva, Seattle Genetics. Dr. Foss: consultant, Celgene, Seattle Genetics, Spectrum, Eisai; research funding, Celgene; speakers’ bureau, Celgene, Seattle Genetics. Drs. Shustov, Tulpule, Craig, Greer, Morganstein and Casulo: none.
Author Contributions: Conceptualization: KC, SH, LP-B, SR, BP, EH, MF, CG, LB, FF; Methodology: KC, SH, LP-B, SR, BP, EH, MF, CG, MS, LB, FF; Software: N/A; Validation; N/A; Formal analysis: MS; Investigation: KC, SH, LP-B, SR, BP, AS, RA, TF, MJL, SS, FL, AT, MC, JG, BK, JL, NM, CC, SP, FF; Resources: KC, SH, LP-B, SR, BP, AS, RA, TF, MJL, SS, FS, AT, MC, JG, BK, JL, NM, CC, SP, FF; Data curation: MS, LB; Writing: KC; Writing - review and editing: KC, SH, LP-B, SR, BP, EH, MF, CG, MS, LB, MA, AS, RA, TF, MJL, SS, FL, AT, MC, JG, BK, JL, NM, CC, SP, FF; Visualization: KC, MS, LB; Supervision: KC, SH, LP-B, SR, BP, EH, MF, CG, LB, MA, FF; Project administration: LB, MA; Funding: MA
References
- 1.The Non-Hodgkin’s Lymphoma Classification Project. A Clinical Evaluation of the International Lymphoma Study Group Classification of Non-Hodgkin’s Lymphoma. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 2.Ascani S, Zinzani PL, Gherlinzoni F, et al. Peripheral T-cell lymphomas. Clinico-pathologic study of 168 cases diagnosed according to the R.E.A.L Classification. Ann Oncol. 1997;8:583–592. doi: 10.1023/a:1008200307625. [DOI] [PubMed] [Google Scholar]
- 3.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization Classification of Neoplastic Diseases of the Hematopoietic and Lymphoid Tissues: Report of the Clinical Advisory Committee Meeting—Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 4.Vose J, Armitage J, Weisenburger D. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study: Pathology Findings and Clinical Outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 5.Agostinelli C, Piccaluga PP, Went P, et al. Peripheral T cell lymphoma, not otherwise specified: the stuff of genes, dreams and therapies. J Clin Path. 2008;61:1160–1167. doi: 10.1136/jcp.2008.055335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-cell lymphoma. Blood. 2011;117:6756–6767. doi: 10.1182/blood-2010-05-231548. [DOI] [PubMed] [Google Scholar]
- 7.d’Amore F, Gaulard P, Trümper L, et al. Peripheral T-cell lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v108–v115. doi: 10.1093/annonc/mdv201. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Non-Hodgkin’s Lymphomas (Version 1.2016) http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.
- 9.Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 10.Federico M, Rudiger T, Bellei M, et al. Clinicopathologic Characteristics of Angioimmunoblastic T-Cell Lymphoma: Analysis of the International Peripheral T-Cell Lymphoma Project. J Clin Oncol. 2013;31:240–246. doi: 10.1200/JCO.2011.37.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pautier P, Devidas A, Delmer A, et al. Angioimmunoblastic-Like T-Cell Non Hodgkin’s Lymphoma: Outcome After Chemotherapy in 33 Patients and Review of the Literature. Leuk Lymphoma. 1999;32:545–552. doi: 10.3109/10428199909058412. [DOI] [PubMed] [Google Scholar]
- 12.Savage KJ, Harris NL, Vose JM, et al. ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen ED, Kim HT, Ho VT, et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann Oncol. 2011;22:1608–1613. doi: 10.1093/annonc/mdq698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reimer P, Rüdiger T, Geissinger E, et al. Autologous Stem-Cell Transplantation As First-Line Therapy in Peripheral T-Cell Lymphomas: Results of a Prospective Multicenter Study. J Clin Oncol. 2009;27:106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 15.d’Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 16.Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. a study from the Swedish Lymphoma Registry. Blood. 2014;124:1570–1577. doi: 10.1182/blood-2014-04-573089. [DOI] [PubMed] [Google Scholar]
- 17.Hsi ED, Gruver AM, Foss F, et al. Biomarker Quality Assurance (QA) Findings from the Comprehensive Oncology Measures for Peripheral T-Cell Lymphoma Treatment (COMPLETE) Registry. Blood. 2012;120(21(Suppl)) abstract 4263. [Google Scholar]
- 18.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 20.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2013 Sub (1992–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
- 21.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 22.van der Poel MW, Mulder WJ, Ossenkoppele GJ, et al. Factors that influence treatment decision-making in elderly DLBCL patients: a case vignette study. Ann Hematol. 2015;94:1373–1379. doi: 10.1007/s00277-015-2358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahadevan D, Unger JM, Spier CM, et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer. 2013;119:371–379. doi: 10.1002/cncr.27733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Advani RH, Ansell SM, Lechowicz MJ, et al. A phase II study of cyclophosphamide, etoposide, vincristine and prednisone (CEOP) Alternating with Pralatrexate (P) as front line therapy for patients with peripheral T-cell lymphoma (PTCL): final results from the T-cell consortium trial. Br J Haematol. 2016;172:535–544. doi: 10.1111/bjh.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briski R, Feldman AL, Bailey NG, et al. The role of front-line anthracycline-containing chemotherapy regimens in peripheral T-cell lymphomas. Blood Cancer J. 2014;4:e214. doi: 10.1038/bcj.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 27.Abramson JS, Feldman T, Kroll-Desrosiers AR, et al. Peripheral T-cell lymphomas in a large US multicenter cohort: prognostication in the modern era including impact of frontline therapy. Ann Oncol. 2014;25:2211–2217. doi: 10.1093/annonc/mdu443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourad N, Mounier N, Brière J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463–4470. doi: 10.1182/blood-2007-08-105759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delfau-Larue M-H, de Leval L, Joly B, et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological study of the GELA. Haematologica. 2012;97:1594–1602. doi: 10.3324/haematol.2011.061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalata P, Martus P, Zettl H, et al. Differences between clinical trial participants and patients in a population-based registry: the German Rectal Cancer Study vs. the Rostock Cancer Registry Dis Colon Rectum. 2009;52:425–437. doi: 10.1007/DCR.0b013e318197d13c. [DOI] [PubMed] [Google Scholar]
- 31.Luce BR, Kramer JM, Goodman SN, et al. Rethinking Randomized Clinical Trials for Comparative Effectiveness Research: The Need for Transformational Change. Ann Intern Med. 2009;151:206–209. doi: 10.7326/0003-4819-151-3-200908040-00126. [DOI] [PubMed] [Google Scholar]
- 32.Wagner TH, Murray C, Goldberg J, Adler JM, Abrams J. Costs and benefits of the national cancer institute central institutional review board. J Clin Oncol. 2010;28:662–666. doi: 10.1200/JCO.2009.23.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]


