Abstract
Epidemiological evidence implicates maternal infection as a risk factor for autism spectrum disorder and schizophrenia. Animal models corroborate this link and demonstrate that maternal immune activation (MIA) alone is sufficient to impart lifelong neuropathology and altered behaviors in offspring. This review describes common principles revealed by these models, highlighting recent findings that strengthen their relevance for schizophrenia and autism and are starting to reveal the molecular mechanisms underlying the effects of MIA on offspring. The role of MIA as a primer for a much wider range of psychiatric and neurologic disorders is also discussed. Finally, the need for more research in this nascent field and the implications for identifying, and developing new treatments for, individuals at heightened risk for neuro-immune disorders are considered.
The Zika virus and its accompanying risk of microcephaly(1) has finally turned public attention to the detrimental effects of maternal infection. While images of microcephalic newborns evoke outcry and require government action, the direct effects of Zika are only one part of a much larger global health hazard. An acute maternal immune response initiated by many common viruses is sufficient to cause lifelong changes in brain function and behavior of offspring in animal models(2). While Zika and other pathogens may confer higher risk of specific disorders, growing evidence suggests that maternal immune activation (MIA) in the absence of a pathogen may increase the risk of a broad spectrum of central nervous system (CNS) disorders in humans(3)(Figure 1).
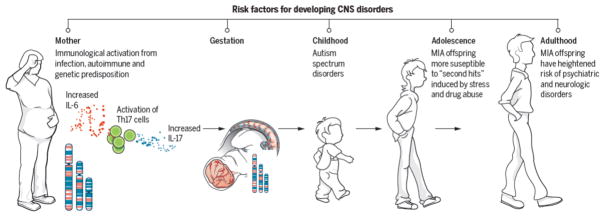
Figure 1. MIA as a disease primer.
This schematic depicts the current model for how MIA leads to psychiatric disorders in offspring. Infection leads to release of pro-inflammatory cytokines and activation of TH17 cells in the mother’s bloodstream(6, 19). A combination of genetic background, autoimmune status, and second hits during childhood and adolescence (including stress and drug abuse) combine with the consequences of maternal infection to increase the likelihood of offspring developing psychiatric disorders as adults(3, 6, 14, 37).
Maternal infection and psychiatric disorders
The association between maternal infection and neurodevelopmental disorders is longstanding, but not without controversy. Following the 1964 rubella pandemic, the incidence of two neurodevelopmental disorders, autism (ASD) and schizophrenia (SZ), rose from less than 1% in the unexposed population to about 13% and 20% respectively(2). Subsequent studies charting historic outbreaks of flu, measles, mumps, chickenpox, and polio, revealed an association with ASD, SZ, and several mood disorders(4). However, not all ecological studies have replicated these associations(5). The differing conclusions may stem from differences in estimating the exposed population(5). Nevertheless, several prospective studies following birth cohorts(3, 6) are consistent with an association between viral infection and psychiatric disorders in offspring and add other classes of pathogens to the list: namely, bacterial infections including pneumonia, sinusitis, and tonsillitis, and the parasite Toxoplasma gondii(2, 3).
How can such a diverse group of pathogens confer similar risk of neurodevelopmental disorders? Common to the implicated pathogens is the maternal immune response. In support of this, enduring fevers above a certain threshold pose the greatest risk(6). It follows that immune system activation above that threshold due to any environmental insult or genetic predisposition would also increase risk. Indeed, maternal autoimmune disorders, allergies, asthma, acute stress, and exposure to environmental pollutants—all of which lead to elevated immune responses—have been linked to an enhanced risk of ASD and SZ(3, 6). These findings may help to contextualize two recent prospective studies that failed to find a significant association between prenatal infection and SZ after adjusting for parental infection in general, parental psychiatric disorder, and socioeconomic status(7, 8). For example, in one study, the modest association between prenatal infection and SZ was not significantly different from an association with a generalized familial liability to develop severe infection(8). This finding may again point to the importance of the maternal immune background. A paternal association implicates the immunogenetic background of the fetus. Thus, the immune status of both mother and child determines the vulnerability to MIA. A second study found a synergism between maternal infection and maternal psychiatric disorders(7). Since many individuals with SZ have immune abnormalities, this association could point to maternal immune status as well as synergism with genetic risk factors. If MIA is a primer for a wide-array of disorders then further work is necessary to identify additive(9) and synergizing risk factors(7), which may be hidden in the adjusted models typically used in these studies.
Explosive growth in the human population, urbanization, and climate change combine to drive emerging infectious diseases like Zika(10). Simultaneously, pervasive poverty that limits access to childhood vaccinations, together with baseless fear of vaccinations within affluent groups, has led to a resurgence in infectious diseases of the past, like measles, mumps, rubella, whooping cough, and even polio(11). Increased exposure to new and old viruses heightens the risk of pregnant women contracting one of these diseases and thereby may increase the likelihood that her children will develop CNS disorders. Together, the increased presence of communicable diseases combined with an uptick in autoimmune disorders(6) could account for a significant proportion of the concerning recent increase in incidence of neurodevelopmental disorders(12).
Animal models of maternal infection
Evidence for these associations has been growing steadily more compelling, but epidemiology alone cannot establish a causal relationship between maternal immune activation (MIA) and risk of neurodevelopmental disorders. Thus, this association in humans will likely remain controversial at least into the near future. Humans are genetically, ecologically, and behaviorally heterogeneous, all of which can influence susceptibility to disease and therefore complicate and undermine detection of causal relationships. Clinical research is also limited in its ability to identify the molecular pathways downstream of maternal infection since humans cannot be subject to invasive experimentation. Moreover, there is currently not an effective way to identify the at-risk pregnancies. The majority of pregnancies even at high risk will lead to healthy offspring and the resulting CNS disorders in offspring often do not appear for many years after birth and appear to be influenced by postnatal risk factors that synergize with genetic and prenatal risk to act as “second hits”(3, 13–15). Clearly, there is a compelling need for long-term and large prospective studies to identify the specific aspects of infection during pregnancy (the type of pathogen, extent of fever, timing of infection, etc.), as well as synergies with postnatal exposures, that lead to heightened risk of CNS disorders in offspring.
Because of these challenges of studying MIA in humans, animal research is therefore essential for identifying causal mechanisms and developing new diagnostic tools and therapeutics. Indeed, a causal relationship between maternal infection and ASD and SZ-related behavioral abnormalities has been clearly demonstrated using rodent and, more recently, non-human primate (NHP), animal models. In these models, pregnant animals are exposed to an immunological manipulation at a specific gestational stage. The behavior and brain structure and function of MIA offspring are then compared to those of control offspring. The most common immunogens used in these studies include influenza infection and exposure to viral (polyinosinic:polycytidylic (poly(I:C)) and bacterial (lipopolysaccharide (LPS)) mimics that cause MIA(14). These MIA animal models meet all of the criteria required for validity for a disease model: they mimic a known disease-related risk factor (construct validity), they exhibit a remarkably wide range of disease-related symptoms (face validity), and they can be used to predict the efficacy of treatments (predictive validity). Each specific MIA model has important advantages and disadvantages. Differences in gestational age, immunogen, dose, and timing lead to overlapping and distinct phenotypic signatures that are critical factors in evaluating their use as preclinical models. The common principles revealed by these models are included in this overview of the field. Please see other recent reviews for details on each model(4, 14) as well as additional maternal immune(14), maternal antibody(16), autoimmune(17), and stress models(18).
Rodent MIA models
The rodent MIA models manifest a remarkably comprehensive range of SZ and ASD-related behavioral abnormalities. Offspring from the poly(I:C) rodent model, in particular, exhibit most of the core behavioral symptoms of ASD—abnormal communication, abnormal social behaviors, and increased repetitive behaviors(2–4, 6, 14). Offspring from these MIA models also show many additional SZ- and ASD-related behaviors, including decreased sensorimotor gating (which measures the ability of the brain to filter out extraneous information), deficits in working memory and cognitive flexibility, increased anxiety, and enhanced sensitivity to amphetamines(2, 3, 14). Importantly, many of these behaviors can be alleviated by antipsychotic drugs, supporting the disease-relevance of these models(3, 4, 14).
In addition to these aberrant behaviors, adult MIA offspring also exhibit neuropathologies emerging at specific developmental ages, especially SZ-associated reduced cortical thickness and hippocampal (HC) volume and increased ventricular size as well as ASD-associated aberrations in Purkinje cells(2–4, 14). Several studies have also recently reported deficits in dendritic spine density, levels of synaptic proteins, synaptic transmission, long-term plasticity, and cortical malformations(4, 19–24). However, most of these measures have been studied in single brain regions from single models at a single age. So, while it is likely that MIA causes changes in synaptic connectivity, function, and plasticity, elucidating the details and common principles remains an important goal for the future.
Recent work has also uncovered neurochemical changes in adult MIA offspring that are characteristic of SZ and ASD(3, 4, 14, 25) (Figure 2). Serotonin and dopaminergic signaling is altered in MIA offspring across models(3, 4, 14). Additionally, specific changes in inhibitory neurotransmission have been linked to both SZ and ASD(26) and similar reductions in several components of the GABA system are present in the brains of MIA offspring(3, 4, 14, 25, 27–29). One of the most exciting recent advances in the MIA field is the discovery of deficits in the function of parvalbumin (PV) cells, known to be selectively altered in SZ, in the brains of adult MIA offspring(3, 14, 22, 30). MIA causes a specific reduction in inhibition from PV cells onto pyramidal neurons that is sufficient to cause deficits in attentional set shifting and enhance anxiety-related behavior in offspring(30), similar to behavioral changes in SZ patients with confirmed evidence of gestational infection(31). Consistent with predictions from a loss of perisomatic inhibition of pyramidal cells in the prefrontal cortex (PFC)(4), MIA offspring exhibit increased power in the theta band(32) and reduced EEG coherence specifically between the HC and mPFC(4). These findings mirror reductions in long-range signaling in SZ(33).
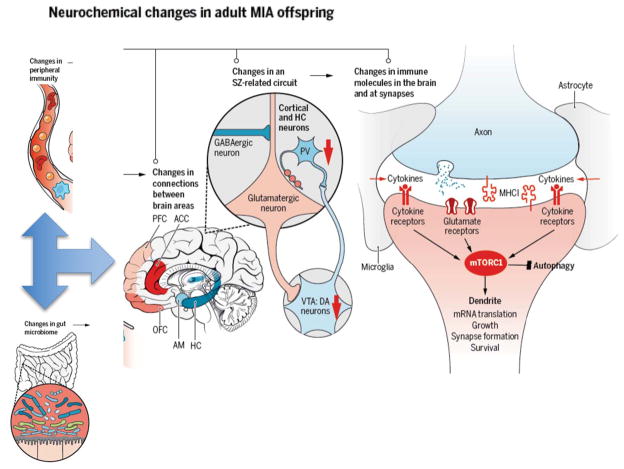
Figure 2. Mechanisms underlying the effects of MIA on brain function.
Aberrations in the microbiome following MIA can lead to altered development of peripheral immunity, which in turn leads to alterations in brain development. Deficits in long-range connections between brain regions implicated in SZ and ASD, including the hippocampus (HC), prefrontal cortex (PFC), the anterior cingulate cortex (ACC), the orbitofrontal cortex (OFC) and the amygdala (AM) have been reported in MIA(3, 4, 14). Specific alterations in activity of glutamatergic neurons in the cortex caused by decreased function of dopaminergic neurons in the ventral tegmental area (VTA) and decreased GABAergic input also occurs in SZ and ASD as well as the MIA models(3, 4, 14). Changes in expression of immune molecules in the brain and even at synapses, including cytokines and MHCI molecules, alters synaptic plasticity and function, and contributes to the changes in circuitry and connectivity between brain regions that characterize these disorders(6). Finally, alterations in immune and neuronal signaling due to MIA may converge upon the mammalian target of rapamycin (mTOR) pathway, which regulates synapse formation, growth, translation, survival, and autophagy(68).
NHP MIA models
Even though these rodent models have remarkably strong face, construct, and predictive validity for SZ and ASD, the potential of using rodents to tell us about psychiatric disorders that are so inherently human has remained controversial. To bridge the gap between rodents and humans, several groups have established rhesus macaque MIA models. Some of these NHP models display behavioral symptoms of ASD and SZ—increased repetitive behaviors, abnormal communication and impaired social interactions—that start at weaning and increase in intensity with age(4, 14, 34). MIA also alters gray and white matter volume in an immunogen-specific manner(4) and causes subtle changes in dendritic arborization(35) in neonatal NHP offspring. An outstanding question that can be addressed uniquely by NHP models is whether molecular signatures of MIA identified in rodent models underlie phenotypes similar to ASD and SZ in humans. Answering this question in the future will be a major advantage for generating new therapies.
Considerations in interpreting MIA models
MIA models utilize a surprisingly wide range of protocols that vary in the type, as well as the timing, mode of delivery, and dose of immunogen used. The type of immunogen dictates the nature of the immune response and downstream phenotypes. The timing of exposure is also key in determining the nature and severity of the outcomes (Figure 3)(14). MIA in early versus late gestation causes distinct fetal brain cytokine responses and changes in neuropathology and behavior in adult offspring(14, 36), but whether the timing of exposure leads to distinct CNS disorders remains unknown. MIA outcomes can also be dependent on the mouse strain(37, 38), individual differences in maternal immune responsiveness within a strain(39), and gender differences in offspring(4, 14). Finally, the mode of delivery and dose of the immunogen also dictate the outcome in offspring following MIA, implying that there must be a threshold of MIA required to produce ASD and SZ-like phenotypes in offspring(14, 30). A key advance would be quantifying the threshold for MIA to induce disease-relevant phenotypes. This would allow better comparison between studies and more effective use of the model in the preclinical arena(29).
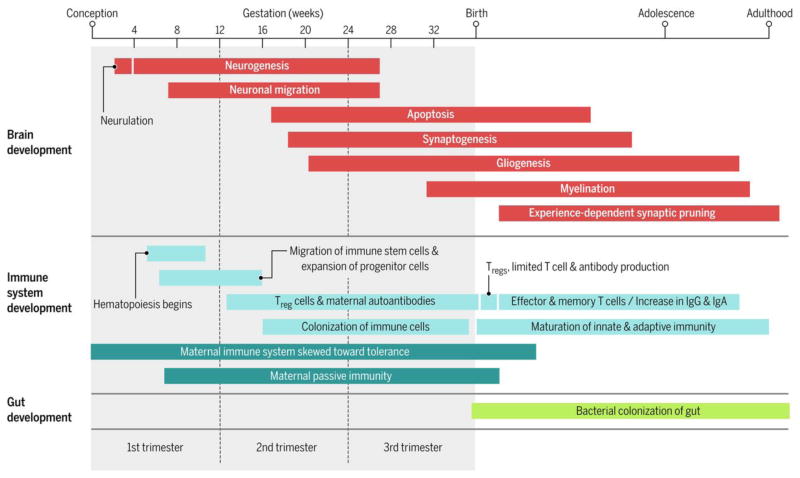
Figure 3.
Timeline of major events occurring in brain, immune system, and gut development from conception to adulthood(83–85).
MIA as a disease primer
In humans, most maternal infections do not lead to SZ or ASD in offspring(40); thus, it is currently thought that multiple “hits” (exposure to more than one risk factor) may be required for disease induction (Figure 1). MIA appears to act as a “disease primer”(14) to make an individual more susceptible to the effects of genetic mutations and environmental exposures in triggering disease-related symptoms later in life(41). Consistent with this idea, the incidence of both ASD and SZ is much higher in families with autoimmune disorders(2, 6) and the effect of maternal infection in increasing SZ risk is greater in families with a history of SZ(3). Indeed, low dose poly(I:C) MIA synergizes with mutations in SZ and ASD-associated genes, including DISC1, NRG1 (encodes Neuregulin-1), NR4A2 (encodes Nurr1), and TSC2 to cause greater effects than either insult alone(3, 4, 14).
While studies of interactions between MIA and environmental risk factors have only recently begun, the results thus far using animal models suggest that even subclinical maternal infection can make offspring much more vulnerable to second “hits” (Figure 1). Sub-threshold MIA increases the likelihood of environmental risk factors, such as stress and drug use, to cause SZ and ASD-related phenotypes in offspring(3, 14). Peripubertal stress causes synergistic effects in subclinical poly(I:C) MIA offspring on a wide range of SZ and ASD-related behaviors and molecular phenotypes(3, 4, 14). Similarly, adolescent cannabis exposure causes synergistic effects in subclinical MIA mice(4, 42). Combined insults can even change the nature of the phenotype. LPS MIA alone results in NMDAR hypofunction and a loss of HC long-term plasticity in adolescent rats but, when combined with restraint stress, the outcome switches to the opposite phenotypes(4). While the molecular mechanism of MIA as a disease primer is unknown, brain region-specific alterations in epigenetic marks at several loci including DISC1 could be a molecular signature of its priming effect(43, 44).
There is also growing evidence that MIA is associated with a much wider array of psychiatric and neurologic disorders than just ASD and SZ. Recently, MIA has been linked to anxiety, major depressive disorder (MDD), and bipolar disorder (BPD) in people (3, 45–47). These seemingly distinct disorders share a surprising number of genetic and environmental risk factors, behavioral abnormalities, and alterations in brain structure and function(3, 48, 49). MIA animal models also exhibit behavioral and neurochemical alterations consistent with depression and anxiety(3, 47, 50). MIA has even been shown recently to prime mice for degenerative diseases during aging, including Alzheimer’s disease(51).
These links of MIA to an increasing array of diseases as diverse as neurodevelopmental disorders (ASD) and neurodegenerative disease (Alzheimer’s disease) that afflict individuals at non-overlapping stages of life and result in distinct symptoms has been surprising. This conundrum is not an issue for MIA alone but rather has surfaced in the field of genetics as well with increasing evidence for associations between specific genes and a similarly wide range of CNS disorders(52–54). How MIA or genetic mutations can cause such a wide range of disorders is unknown, but both may act to prime an individual for second hits and the nature and timing of those second hits may determine when and which disease will result, if any (Figure 1).
The fact that MIA is associated with such a broad range of disorders indicates that MIA animal models can be used to identify common molecular pathways that are shared among them. This is good news from a treatment perspective since it predicts that new therapies could have efficacy in a wide range of human diseases. When, where, and how the common pathways are disrupted may be critical to understanding disease-specific vulnerabilities and can be manipulated and identified using the MIA animal models. Perhaps most important, the larger the family of MIA-associated CNS disorders, the greater the public health implications for initiatives aimed at preventing maternal infection in high risk populations.
How does MIA alter brain function and behavior in offspring?
Immune activation within the maternal compartment likely influences the developing fetal CNS through inflammatory mediators found in the blood and amniotic fluid of ASD and SZ mothers(2, 3, 6, 14)(Figure 1). Whether or not these proteins cross the placenta and act directly upon the fetal brain is unknown. MIA somehow induces alterations in multiple cytokines within the fetal brain in a matter of hours(6, 14). While MIA triggers unequivocal inflammation within the mother, the limited cytokine profiles that have been measured in the fetal brain do not by themselves signify a classic inflammatory response(29, 55). If, and how, these changes in fetal brain cytokines are related to MIA neuropathologies are critical questions for the field. More research is needed to address these issues and to quantify the dynamic changes in a large number of immune molecules across brain regions and age, with the ultimate goal of identifying immune signatures associated with specific disease-related neuropathologies and behaviors.
Nevertheless, several maternal cytokines have already been identified as critical mediators of MIA on disease-related phenotypes in offspring (Figure 1). Injection of a single inflammatory cytokine (interleukin (IL)-6, -17, or -2) is sufficient to induce several ASD and SZ-like behaviors in offspring(14, 19, 56). Moreover, co-administration of Poly I:C with an IL-6 or an IL-17 function blocking antibody, or with overexpression of the anti-inflammatory cytokine IL-10, partially prevents the effects of MIA in offspring(3, 19). However, remarkably little is known about how these maternal cytokines alter brain development. One possibility is that MIA leads to long-lasting changes in expression of immune molecules known to regulate neural connectivity and function in offspring(6). Indeed, the levels of numerous cytokines are altered throughout development and into adulthood in the brain of MIA offspring in a region and age-specific manner(6, 57). These cytokines are likely to regulate the expression of other classes of immune molecules on neurons, including major histocompatibility complex I (MHCI) molecules (Figure 2). In the immune system, MHCI levels are controlled by cytokines as an important early step in the immune response. In the healthy brain, MHCI is found on neurons where it negatively regulates synapse formation and synaptic plasticity required for activity-dependent synaptic pruning(6, 58, 59). Alterations in synaptogenesis and pruning are associated with a range of neurodevelopmental disorders and thought to play a central role in the etiology of ASD and SZ(60, 61). MIA causes a dramatic change in MHCI levels on neurons in the brains of offspring (6, 21) and the resulting increase in MHCI at birth is required for the dramatic MIA-induced deficit in the ability of newborn neurons to form synapses(62). Outstanding questions for the field include determining if these changes in cortical connectivity are long-lasting and causally related to the disease –related behaviors emerging at later stages of development.
MHCI is one of several immune proteins present at the synapse capable of mediating responses to infection while concurrently regulating activity-dependent plasticity and circuit formation. These versatile proteins—part immune defense, part brain architecture—are the most likely suspects to bring about changes in brain development in response to maternal infection(63). Although studies on this topic are only just beginning, MIA presumably alters the expression and function of these immune proteins in the brain as it does for cytokines and MHCI. The alterations may be acute, reflecting the nature, intensity, and duration of infection, or chronic through epigenetic changes that may synergize with other risk factors at different stages of life. With such heterogeneity from onset to phenotype could there be a unifying converging pathway underlying MIA’s priming effect?
If immune molecules act through similar pathways in the brain (and the same intracellular machinery is present, which remains an open question), then it is possible that immune signaling in neurons may converge upon mammalian target of rapamycin (mTOR) signaling. In the immune system, mTOR acts as a regulatory hub integrating inputs from numerous upstream intracellular signaling pathways including cytokines, trophic factors, and synaptic scaffolding proteins, many of which are altered in the brains of MIA offspring as well as individuals with ASD, SZ, MDD, and AD(64–68). Dysregulation of protein synthesis due to alterations in the mTOR pathway has been implicated in several monogenetic forms of ASD as well as the SZ-associated DISC-1 mutation in animal models(69)(68). Hyper or hypo-activation of mTOR signaling imparts mutation-specific alterations in neuronal morphology and synaptic plasticity as well as the synthesis of many synaptic proteins and glutamate receptors(70), which are common features of several MIA models. mTOR signaling also regulates macroautophagy, a homeostatic process leading to the breakdown of unused proteins and structures within cells that has been implicated in several neurodegenerative diseases and, recently, idiopathic forms of ASD(61). Although direct evidence for a role for mTOR dysfunction in MIA is lacking, this is a plausible exemplar of several potential signaling hubs that have been implicated in a similar wide range of disorders as MIA. These hubs could be altered by underlying genetic mutations or postnatal environmental risk factors to cause distinct responses in individuals following MIA, leading to the growing list of distinct disorders associated with maternal infection
The gut-immune-brain axis in MIA
Several intriguing studies indicate that some MIA-induced neuropathologies may emanate from outside the nervous system (Figure 2). MIA mice exhibit peripheral immune abnormalities including T- and myeloid cell skewing to inflammatory phenotypes, mirroring some patient studies(3, 6). These peripheral changes are likely to alter immune-brain communication, which has been demonstrated to modulate cognition(71). As such, reconstitution of MIA offspring with normal bone marrow ameliorates repetitive and anxiety-like behaviors, suggesting that some MIA phenotypes are causally related to ongoing immune imbalances(3).
As an essential nexus for nervous-immune-endocrine interactions, the gut may also play an important role in MIA and thus, may be an effective target for therapeutic interventions. During birth, the fetal gut is colonized by maternal microbiota (Figure 3), and this formative colonization primes the developing immune system and directs immune homeostasis (Figure 2). Like individuals with ASD and other disorders, MIA offspring display altered microbiota signatures. Restoring a more typical microbiome by treating MIA offspring with B. fragilis not only restores peripheral immune homeostasis, but ameliorates several aberrant behaviors(72). Exploiting the gut as a novel therapeutic route requires animal models of healthy and disease-relevant microbiota. Typical research rodents raised under sanitized conditions lack exposure to a wide variety of microbes, which can stunt immune system development(73). Exposure to specific microbiota due to housing conditions has recently been shown to drive TH17 T-cell differentiation, a cell type that may be necessary for MIA, but is absent in other mouse lines(19, 37, 74). These microbiota-induced differences in immunity have only recently become appreciated and their contribution to variability in the MIA model and the presence, type, and severity of disease in humans remains to be determined.
Implications for treatment of psychiatric disorders
The great promise of the MIA model lies in its potential to lead to novel therapeutics and to determine when particular symptoms emerge and are sensitive to those new treatments. Currently, a handful of therapeutic manipulations rescue some MIA-induced phenotypes. Further work promises to identify new classes of therapies for psychiatric disorders. Importantly, some of these therapies can be used during the presumptive prodromal phase to prevent the emergence of SZ-related phenotypes. For example, treatment of adolescent MIA rats with a COX-2 inhibitor protects them from developing several SZ-related behavioral aberrations(3, 4). Similarly, minocycline, a microglia (a type of macrophage-like cell in the brain) modulator, prevents the emergence of MIA-induced behaviors and changes in cytokines in the adult brain when given during exposure to peripubertal stress (3, 4, 75). Several SZ and ASD-related phenotypes in MIA offspring can also be prevented with probiotic treatment(72), anti-cytokine antibody treatment(19, 76), and environmental enrichment (77) or maternal dietary supplementation with zinc(78, 79), n-3 polyunsaturated fatty acids (80, 81) or N-acetyl-cysteine (NAC)(82). Finally, one of the most exciting potential treatments is anti-purinergic therapy, which completely reverses several ASD-related phenotypes in MIA offspring(4).
The Future
If MIA is a disease primer for numerous CNS disorders, as mounting evidence suggests, then MIA animal models are critical for determining the molecular pathways that mediate the resulting neuropathology and abnormal behaviors. Although the field is in its infancy, newly identified convergent pathways are already starting to serve as targets for development of new therapeutics. The altered signaling in these molecular pathways is age-, region-, and gender- specific, a critical finding that helps explain the considerable individual differences in vulnerability to MIA-related disorders. Tailoring therapies to different stages of these disorders requires MIA animal models where dynamic changes can be quantified and related to specific symptoms. Effective use of these new treatments will depend in large part on new, as yet undiscovered, approaches to identify at risk individuals. MIA models are already uncovering synergizing genetic and environmental risk factors that will help define these populations. The ultimate goal, which is now within sight, is to utilize new therapeutic interventions before deleterious symptoms appear, decreasing the incidence of, or even preventing, the emergence of immune-mediated psychiatric illness in adulthood.
Acknowledgments
The authors thank members of the McAllister laboratory for ongoing discussions about the topics covered in this Review. Due to journal guidelines, we were not permitted to cite much of the original reports, and we apologize to those whose articles are not referenced. Please see referenced reviews for primary source articles. M.L.E. has been supported by a Stanley & Jacqueline Schilling Neuroscience Postdoctoral Research Fellowship, a Dennis Weatherstone Predoctoral Fellowship from Autism Speaks (#7825), the Letty and James Callinan and Cathy and Andrew Moley Fellowship from the ARCS (Achievement Rewards for College Scientists) Foundation, and a Dissertation Year Fellowship from the University of California Office of the President. A.K.M. is supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS060125-05), the National Institute of Mental Health (NIMH; P50-MH106438-01), the Simons Foundation (SFARI #321998), and the University of California Davis Research Investments in Science and Engineering Program.
References
- 1.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. The New England journal of medicine. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 2.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Knuesel I, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nature reviews Neurology. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 4.Reisinger S, et al. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther. 2015;149:213–226. doi: 10.1016/j.pharmthera.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Selten JP, Frissen A, Lensvelt-Mulders G, Morgan VA. Schizophrenia and 1957 pandemic of influenza: meta-analysis. Schizophr Bull. 2010;36:219–228. doi: 10.1093/schbul/sbp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nature reviews Neuroscience. 2015;16:469–486. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomstrom A, et al. Associations Between Maternal Infection During Pregnancy, Childhood Infections, and the Risk of Subsequent Psychotic Disorder--A Swedish Cohort Study of Nearly 2 Million Individuals. Schizophr Bull. 2016;42:125–133. doi: 10.1093/schbul/sbv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen PR, Laursen TM, Mortensen PB. Association between parental hospital-treated infection and the risk of schizophrenia in adolescence and early adulthood. Schizophr Bull. 2013;39:230–237. doi: 10.1093/schbul/sbr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen PR, Meyer U, Mortensen PB. Individual and combined effects of maternal anemia and prenatal infection on risk for schizophrenia in offspring. Schizophr Res. 2016;172:35–40. doi: 10.1016/j.schres.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Smith KF, et al. Global rise in human infectious disease outbreaks. J R Soc Interface. 2014;11:20140950. doi: 10.1098/rsif.2014.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dube E, Vivion M, MacDonald NE. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Rev Vaccines. 2015;14:99–117. doi: 10.1586/14760584.2015.964212. [DOI] [PubMed] [Google Scholar]
- 12.Boyle CA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen PR, Benros ME, Mortensen PB. Hospital contacts with infection and risk of schizophrenia: a population-based cohort study with linkage of Danish national registers. Schizophr Bull. 2014;40:1526–1532. doi: 10.1093/schbul/sbt200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biological psychiatry. 2014;75:307–315. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Debost JP, et al. Joint Effects of Exposure to Prenatal Infection and Peripubertal Psychological Trauma in Schizophrenia. Schizophr Bull. 2016 doi: 10.1093/schbul/sbw083. [DOI] [PMC free article] [PubMed]
- 16.Camacho J, et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav Brain Res. 2014;266:46–51. doi: 10.1016/j.bbr.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brimberg L, Sadiq A, Gregersen PK, Diamond B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry. 2013;18:1171–1177. doi: 10.1038/mp.2013.101. [DOI] [PubMed] [Google Scholar]
- 18.Howerton CL, Bale TL. Prenatal programing: at the intersection of maternal stress and immune activation. Hormones and behavior. 2012;62:237–242. doi: 10.1016/j.yhbeh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi GB, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Coiro P, et al. Impaired synaptic development in a maternal immune activation mouse model of neurodevelopmental disorders. Brain Behav Immun. 2015;50:249–258. doi: 10.1016/j.bbi.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, van Praag H. Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain Behav Immun. 2015;45:60–70. doi: 10.1016/j.bbi.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Giovanoli S, et al. Late prenatal immune activation causes hippocampal deficits in the absence of persistent inflammation across aging. Journal of neuroinflammation. 2015;12:221. doi: 10.1186/s12974-015-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patrich E, Piontkewitz Y, Peretz A, Weiner I, Attali B. Maternal immune activation produces neonatal excitability defects in offspring hippocampal neurons from pregnant rats treated with poly I:C. Sci Rep. 2016;6:19106. doi: 10.1038/srep19106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr Bull. 2014;40:351–361. doi: 10.1093/schbul/sbs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15:146–167. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoftman GD, et al. Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr Bull. 2015;41:180–191. doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40:190–206. doi: 10.1038/npp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey L, Boksa P. A stereological comparison of GAD67 and reelin expression in the hippocampal stratum oriens of offspring from two mouse models of maternal inflammation during pregnancy. Neuropharmacology. 2012;62:1767–1776. doi: 10.1016/j.neuropharm.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Canetta S, et al. Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol Psychiatry. 2016 doi: 10.1038/mp.2015.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown AS, et al. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff AR, Bilkey DK. Prenatal immune activation alters hippocampal place cell firing characteristics in adult animals. Brain Behav Immun. 2015;48:232–243. doi: 10.1016/j.bbi.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature reviews Neuroscience. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 34.Machado CJ, Whitaker AM, Smith SE, Patterson PH, Bauman MD. Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biological psychiatry. 2015;77:823–832. doi: 10.1016/j.biopsych.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weir RK, et al. Preliminary evidence of neuropathology in nonhuman primates prenatally exposed to maternal immune activation. Brain Behav Immun. 2015;48:139–146. doi: 10.1016/j.bbi.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arsenault D, St-Amour I, Cisbani G, Rousseau LS, Cicchetti F. The different effects of LPS and poly I:C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain Behav Immun. 2014;38:77–90. doi: 10.1016/j.bbi.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Estes ML, McAllister AK. IMMUNOLOGY. Maternal TH17 cells take a toll on baby’s brain. Science. 2016;351:919–920. doi: 10.1126/science.aaf2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartzer JJ, et al. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Translational psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: contribution of environmental factors. Behav Brain Res. 2011;220:55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selten JP, Morgan VA. Prenatal exposure to influenza and major affective disorder. Bipolar Disord. 2010;12:753–754. doi: 10.1111/j.1399-5618.2010.00849.x. [DOI] [PubMed] [Google Scholar]
- 41.Ayhan Y, McFarland R, Pletnikov MV. Animal models of gene-environment interaction in schizophrenia: A dimensional perspective. Prog Neurobiol. 2016;136:1–27. doi: 10.1016/j.pneurobio.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollins SL, Zavitsanou K, Walker FR, Cairns MJ. Alteration of transcriptional networks in the entorhinal cortex after maternal immune activation and adolescent cannabinoid exposure. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Tang B, Jia H, Kast RJ, Thomas EA. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav Immun. 2013;30:168–175. doi: 10.1016/j.bbi.2013.01.086. [DOI] [PubMed] [Google Scholar]
- 44.Connor CM, et al. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res. 2012;140:175–184. doi: 10.1016/j.schres.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simanek AM, Meier HC. Association Between Prenatal Exposure to Maternal Infection and Offspring Mood Disorders: A Review of the Literature. Curr Probl Pediatr Adolesc Health Care. 2015;45:325–364. doi: 10.1016/j.cppeds.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA psychiatry. 2013;70:677–685. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- 47.Ronovsky M, Berger S, Molz B, Berger A, Pollak DD. Animal Models of Maternal Immune Activation in Depression Research. Curr Neuropharmacol. 2015 doi: 10.2174/1570159X14666151215095359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatric research. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.C Cross-Disorder Group of the Psychiatric Genomics et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature genetics. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Depino AM. Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience. 2015;299:56–65. doi: 10.1016/j.neuroscience.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 51.Krstic D, et al. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. Journal of neuroinflammation. 2012;9:151. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malishkevich A, et al. Activity-dependent neuroprotective protein (ADNP) exhibits striking sexual dichotomy impacting on autistic and Alzheimer’s pathologies. Translational psychiatry. 2015;5:e501. doi: 10.1038/tp.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lepeta K, et al. Synaptopathies: synaptic dysfunction in neurological disorders. J Neurochem. 2016 doi: 10.1111/jnc.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H. Fragile X mental retardation protein: from autism to neurodegenerative disease. Front Cell Neurosci. 2015;9:43. doi: 10.3389/fncel.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Estes ML, McAllister AK. Alterations in immune cells and mediators in the brain: it’s not always neuroinflammation! Brain Pathol. 2014;24:623–630. doi: 10.1111/bpa.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ponzio NM, Servatius R, Beck K, Marzouk A, Kreider T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Annals of the New York Academy of Sciences. 2007;1107:118–128. doi: 10.1196/annals.1381.013. [DOI] [PubMed] [Google Scholar]
- 57.Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glynn MW, et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nature neuroscience. 2011;14:442–451. doi: 10.1038/nn.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sekar A, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang G, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elmer BM, Estes ML, Barrow SL, McAllister AK. MHCI requires MEF2 transcription factors to negatively regulate synapse density during development and in disease. J Neurosci. 2013;33:13791–13804. doi: 10.1523/JNEUROSCI.2366-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voineagu I, Eapen V. Converging Pathways in Autism Spectrum Disorders: Interplay between Synaptic Dysfunction and Immune Responses. Front Hum Neurosci. 2013;7:738. doi: 10.3389/fnhum.2013.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caccamo A, De Pinto V, Messina A, Branca C, Oddo S. Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci. 2014;34:7988–7998. doi: 10.1523/JNEUROSCI.0777-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ignacio ZM, et al. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol. 2015 doi: 10.1111/bcp.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollins SL, Zavitsanou K, Walker FR, Cairns MJ. Alteration of imprinted Dlk1-Dio3 miRNA cluster expression in the entorhinal cortex induced by maternal immune activation and adolescent cannabinoid exposure. Translational psychiatry. 2014;4:e452. doi: 10.1038/tp.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hemmerle AM, et al. Modulation of schizophrenia-related genes in the forebrain of adolescent and adult rats exposed to maternal immune activation. Schizophr Res. 2015;168:411–420. doi: 10.1016/j.schres.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahin M. Sur, Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science. 2015:350. doi: 10.1126/science.aab3897. [DOI] [PMC free article] [PubMed]
- 69.Kim JY, et al. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–773. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santini E, Klann E. Reciprocal signaling between translational control pathways and synaptic proteins in autism spectrum disorders. Science signaling. 2014;7:re10. doi: 10.1126/scisignal.2005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kipnis J. Science. 2016 [Google Scholar]
- 72.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beura LK, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giovanoli S, et al. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Translational psychiatry. 2016;6:e772. doi: 10.1038/tp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Connors EJ, Shaik AN, Migliore MM, Kentner AC. Environmental enrichment mitigates the sex-specific effects of gestational inflammation on social engagement and the hypothalamic pituitary adrenal axis-feedback system. Brain Behav Immun. 2014;42:178–190. doi: 10.1016/j.bbi.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 78.Chua JS, Cowley CJ, Manavis J, Rofe AM, Coyle P. Prenatal exposure to lipopolysaccharide results in neurodevelopmental damage that is ameliorated by zinc in mice. Brain Behav Immun. 2012;26:326–336. doi: 10.1016/j.bbi.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Coyle P, Tran N, Fung JN, Summers BL, Rofe AM. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res. 2009;197:210–218. doi: 10.1016/j.bbr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 80.Weiser MJ, et al. Dietary docosahexaenoic acid alleviates autistic-like behaviors resulting from maternal immune activation in mice. Prostaglandins Leukot Essent Fatty Acids. 2016;106:27–37. doi: 10.1016/j.plefa.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Li Q, et al. Dietary supplementation with n-3 fatty acids from weaning limits brain biochemistry and behavioural changes elicited by prenatal exposure to maternal inflammation in the mouse model. Translational psychiatry. 2015;5:e641. doi: 10.1038/tp.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lante F, et al. Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus. 2008;18:602–609. doi: 10.1002/hipo.20421. [DOI] [PubMed] [Google Scholar]
- 83.Clancy B, et al. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 84.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35:299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]