Abstract
Background
Prior evidence for a possible link between vitamin D status and hematologic malignancy (HM) in humans comes from observational studies, leaving unresolved the question of whether a true causal relationship exists.
Methods
We performed a secondary analysis of data from the Women’s Health Initiative (WHI) Calcium/Vitamin D (CaD) Trial, a large randomized controlled trial (RCT) of CaD supplementation compared to placebo in older women. Kaplan-Meier and Cox proportional hazards survival analysis methods were used to evaluate the relationship between treatment assignment and (a) incident HM and (b) HM-specific mortality over 10 years following randomization. HMs were classified by cell type (lymphoid, myeloid or plasma cell) and analyzed as distinct endpoints in secondary analyses.
Results
34,763 WHI CaD trial participants (median age = 63 years) had complete baseline covariate data and were eligible for analysis. Women assigned to CaD had a significantly lower risk of incident HM (HR = 0.80, 95% CI: 0.65, 0.99) but not HM-specific mortality (in entire cohort: HR = 0.77, 95% CI: 0.53, 1.11; among incident HM cases following diagnosis: 1.03, 95% CI: 0.70, 1.51). In secondary analyses, protective associations were most robust for lymphoid malignancies, with HRs of 0.77 (95% CI: 0.59, 1.01) and 0.46 (95% CI: 0.24, 0.89) for cancer incidence and mortality in those assigned to CaD supplementation.
Conclusions
This post hoc analysis of data from a large and well-executed RCT demonstrates a protective association between modest CaD supplementation and HM risk in older women. Additional research on the relationship between vitamin D and HM is warranted.
MeSH Keywords: Epidemiology, Hematologic Neoplasms, Lymphoma, Risk Factors, Vitamin D, Women
INTRODUCTION
Vitamin D deficiency may be a modifiable risk factor for hematologic malignancies and may impact survival following diagnosis. Receptors for the active metabolite of vitamin D, hormone 1,25(OH)2D3, are present in a variety of tissues throughout the body, including hematopoietic cells. Animal and in vitro studies indicate that 1,25(OH)2D3 has tumor-suppressive functions, and contributes to the regulation of cellular differentiation, proliferation, and apoptosis through a variety of mechanisms. Vitamin D deficiency may contribute to carcinogenesis by impairing these normal regulatory processes.1–4 To date, the evidence for a link between vitamin D deficiency and hematologic malignancy is observational in nature, and a causal relationship has not been established definitively. Calcium intake has also been associated with a reduced risk of some forms of cancer (e.g., colorectal);5 however, there is currently little epidemiologic or laboratory evidence that would support an effect of calcium intake on the risk of hematologic malignancy.
In this study, we performed a secondary analysis of data from the Women’s Health Initiative (WHI) Calcium/Vitamin D (CaD) Trial. The WHI CaD Trial was a large randomized controlled trial (RCT) of CaD supplementation in 36,282 older women with a primary endpoint of skeletal fractures and secondary endpoints of incident colorectal cancer and breast cancer. No association was found between CaD trial assignment and the risk of either colorectal or breast cancer.6,7 In a subsequent post hoc re-analysis of the WHI CaD trial data, a possible reduction in the risk of breast cancer (HR = 0.80) and all invasive cancers (HR = 0.88) was found in women randomized to CaD supplementation who were not taking calcium or vitamin D supplements at baseline.8 Data from the WHI CaD trial provide a unique opportunity to augment the existing literature with experimental evidence on the effect of CaD supplementation on the risk of incident hematologic cancers and hematologic cancer-specific mortality following diagnosis. We hypothesized that women in the active treatment arm would have a lower risk of both outcomes.
METHODS
Study sample and follow-up
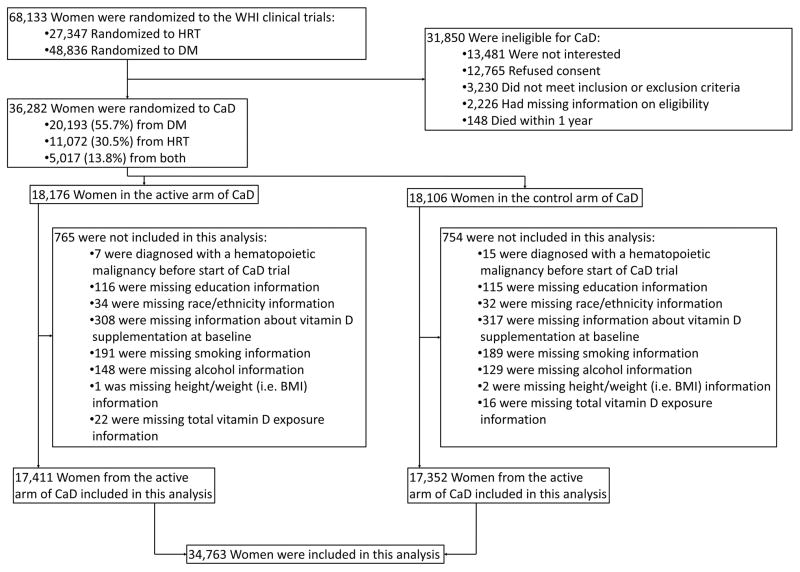
This study was a secondary analysis of data from the WHI randomized trial of CaD supplementation. Enrollment criteria have been described in detail elsewhere.9,10 Briefly, participants in the WHI hormone therapy trials and/or diet modification trial were invited to join the CaD trial at their first or second follow-up visits (Figure 1). Women were eligible to participate if they were determined not to be at high risk of adverse events associated with CaD supplementation, study drop-out, or poor adherence.
Figure 1.
Flow diagram showing eligibility for the Women’s Health Initiative (WHI) calcium / vitamin D (CaD) randomized controlled trial (RCT) and the present study of hematologic malignancy risk and mortality.
Between 1995 and 2000, 36,282 women were enrolled in the CaD trial and randomized to active treatment (1000 mg elemental calcium carbonate and 400 IU vitamin D3 daily) or placebo (allocation ratio: 1:1). Treatment allocation was determined by a permuted-block algorithm with stratification on clinic site and participant age. Both participants and research staff were blinded to treatment assignment. The study pills were identical in appearance, and were provided in identical bottles.
Participants took the study medication in two divided daily doses, and were advised to take it with a meal. Use of non-study dietary supplements of calcium (up to 1000 mg/day) and vitamin D (up to 600 IU/day initially, revised upward to 1000 IU/day in 1999, following an Institute of Medicine report on safe upper limits of calcium and vitamin D) was allowed.
Follow-up was continued until the end of the study, death of the participant, loss to follow-up, or the participant asked for no further contact. Upon completion of the CaD trial in 2005, 93% of the original study cohort were still enrolled; 4% had died, and 3% had either withdrawn from the study or were lost to follow-up. Median length of follow-up was seven years. After CaD trial close-out, 29,862 participants (82% of the original cohort) consented to participate in the WHI Extension Study, a five-year post-trial observational follow-up study. No significant differences by CaD trial assignment were observed in length of follow-up or participation in the WHI Extension Study.11
In the present study, we analyzed outcomes at 10 years following CaD trial enrollment, a median of three years following the end of study medication use in the CaD trial. At this time point, 79% of the original study cohort remained enrolled in the WHI Extension Study. In secondary analyses, we censored participants at the end of the WHI CaD trial, when the use of all study medication stopped.
Protection of Human Subjects and Research Ethics
The original WHI CaD trial was approved by the institutional review boards (IRBs) of all participating institutions, and written informed consent was obtained from all trial participants. The WHI studies, including the CaD trial, were initially registered with ClinicalTrials.gov in October 1999 (NCT00000611).
This secondary reanalysis of de-identified data from the WHI CaD trial was deemed by the University of Iowa IRB to have IRB exempt status. The study proposal and manuscript were reviewed and approved by the WHI Publications & Presentations Committee.
Exposure to Vitamin D
The primary exposure of interest was the additional vitamin D intake attributable to the trial participants’ random assignment to supplementation (400 IU daily) rather than placebo. Baseline data were available for several non-study sources of vitamin D, including diet, use of non-study nutritional supplements, and sun exposure. Vitamin D intake from dietary sources was estimated from participants’ food frequency questionnaire responses.12 Use of non-study vitamin D supplements (yes/no) was characterized based on participant self-report at baseline. Finally, as described below in the covariates section, several proxies for sun exposure and endogenous vitamin D synthesis were considered.
Covariates
Trial, demographic, clinical, and behavioral covariates (see Table 1) were identified as potential covariates based on their known or suspected relationship to vitamin D status, the risk of hematologic malignancy, and/or the probability of study drop-out. In secondary subgroup analyses, we explored whether factors related to vitamin D status (e.g., dietary intake of vitamin D, race, proxies for sun exposure such as recreational physical activity and latitude) modified the relationship between CaD supplementation and the risk of hematologic malignancy.
Table 1.
Baseline characteristics of eligible WHI calcium / vitamin D (CaD) trial participants.
| Covariate* | Placebo (N=17352) | Active treatment (N=17411) | P Value** |
|---|---|---|---|
| Age in years – median (interquartile range [IQR]) | 63 (58 – 69) | 63 (58 – 69) | 0.799 |
| Education | 0.950 | ||
| College or post-graduate degree – N (%) | 6320 (36.4) | 6339 (36.4) | |
| Some post-secondary education – N (%) | 6905 (39.8) | 6953 (39.9) | |
| High school or less – N (%) | 4127 (23.8) | 4119 (23.7) | |
| Race | 0.313 | ||
| Black – N (%) | 1538 (8.9) | 1576 (9.1) | |
| White – N (%) | 14545 (83.8) | 14496 (83.3) | |
| Other – N (%) | 1269 (7.3) | 1339 (7.7) | |
| Latitude | 0.960 | ||
| Southern (<35 degrees North) – N (%) | 5163 (29.8) | 5164 (29.7) | |
| Middle (35–40 degrees North) – N (%) | 4811 (27.7) | 4818 (27.7) | |
| Northern (>40 degrees North) – N (%) | 7378 (42.5) | 7429 (42.7) | |
| Hormone therapy trial treatment assignment | 0.421 | ||
| Not enrolled – N (%) | 9613 (55.4) | 9704 (55.7) | |
| Active treatment – N (%) | 3943 (22.7) | 3855 (22.1) | |
| Placebo – N (%) | 3796 (21.9) | 3852 (22.1) | |
| Diet modification trial treatment assignment | 0.466 | ||
| Not enrolled – N (%) | 5271 (30.4) | 5336 (30.6) | |
| Intervention – N (%) | 4650 (26.8) | 4564 (26.2) | |
| Control – N (%) | 7431 (42.8) | 7511 (43.1) | |
| Dietary vitamin D intake >50th percentile – N (%) | 8727 (50.3) | 8655 (49.7) | 0.276 |
| Taking non-study vitamin D supplements – N (%) | 4827 (27.8) | 4820 (27.7) | 0.780 |
| Body mass index (BMI; kg/m2) – median (IQR) | 28.0 (24.7 – 32.2) | 28.0 (24.7 – 32.1) | 0.176 |
| Current smoker – N (%) | 1310 (7.5) | 1352 (7.8) | 0.450 |
| Alcohol intake | 0.549 | ||
| Non-drinker – N (%) | 4917 (28.3) | 4885 (28.1) | |
| <1 drink/week – N (%) | 6063 (34.9) | 6181 (35.5) | |
| 1 or more drinks/week – N (%) | 6372 (36.7) | 6345 (36.4) | |
| Recreational physical activity >50th percentile – N (%) | 7842 (45.2) | 7927 (45.5) | 0.667 |
All variables were included as covariates in our Cox regression models with the following two exceptions. Recreational physical activity was missing for a substantial fraction of participants (8.8%), so it was included as a covariate only in the subgroup analysis where we stratified on recreational physical activity. Second, in our main analyses, we analyzed participants as they were randomized (intent-to-treat) and did not adjust for study medication adherence. Participants were stratified by adherence during the first year of follow-up in a subgroup analysis.
P values reflect the significance of the differences between treatment arms and are based on the Pearson chi-square and Wilcoxon rank sum tests for categorical and continuous covariates, respectively.
Details on how covariates were defined are provided in Supplementary Table 1. Recreational physical activity was considered as a covariate only in a secondary subgroup analysis because values were missing for ~9% of participants. With the exception of study medication adherence, covariates were assessed at WHI enrollment. In a secondary analysis where we evaluated possible effect modification by degree of adherence, we used participants’ study medication adherence at the first year of follow-up to define participants as adherent (took ≥50% of pills, or on average at least one pill daily) or non-adherent, and restricted to participant follow-up that came after this initial adherence assessment.
Outcomes
In the present study, our primary endpoints were (a) incident hematologic malignancy (all types) and (b) hematologic cancer-specific mortality. In secondary analyses of cancer incidence and cancer-specific mortality, we classified hematologic cancers by subtype and considered cases of lymphoid, myeloid, and plasma cell malignancy separately.
Total incident invasive cancers and death from any cause were secondary outcomes in the WHI CaD trial.13 During the trial period and the Extension Study, all invasive cancers were documented and coded according to primary site. Most incident cancers were ascertained through biannual self-report. After identification of possible cancers by participant or proxy report, reported cancers were physician-adjudicated. Death was ascertained by proxy report and periodic National Death Index linkages. Cause of death was physician-adjudicated based on information in the participant’s death certificate, charts from any medical encounters that directly preceded the participant’s death, and the autopsy report if available. For both endpoints, adjudication was conducted locally at each study center by a physician, and the adjudication decisions were then reviewed centrally at the WHI Clinical Coordinating Center and coded by trained cancer coders. All adjudicators received extensive training and were blinded to participants’ treatment assignment(s) in the WHI trials.13
In the present study, participants were followed until the earliest of the following: the occurrence of an endpoint event, year 10 following CaD trial enrollment, the end of the WHI Extension Study (five years after CaD trial close-out), loss to follow-up, or death unrelated to hematologic malignancy. In a secondary analysis, we censored participants at CaD trial close-out, when use of study medication ended.
Statistical methods
Non-parametric Kaplan-Meier and Cox proportional hazards regression models were used to assess the association between CaD trial assignment and the risks of (a) incident hematologic malignancy and (b) mortality attributable to hematologic malignancy. Because the primary purpose of our study was etiologic, we sought to estimate a cause-specific hazard ratios for these endpoints, and death due to unrelated causes was treated as a censoring event in these analyses.14,15 We also report absolute risks and risk differences using non-parametric cumulative incidence estimates; to avoid overestimating the absolute risks of the study endpoints, mortality unrelated to hematologic malignancy was treated as a competing risk for the cumulative incidence estimates.16,17 All statistical analyses were performed with SAS software (SAS 9.4 for Windows, SAS Institute, Cary, NC).
Since our data came from a large RCT of CaD supplementation, we did not anticipate meaningful bias due to confounding by measured or unmeasured risk factors. Due to concerns about model overfitting and sparse data bias18 (particularly for secondary subgroup analyses), we present unadjusted hazard ratios (HRs) from univariate Cox regression models. However, in sensitivity analyses, we adjusted for potential confounders to evaluate the possibility of bias attributable to covariate imbalance or covariate-dependent censoring.
RESULTS
Descriptive information
Of the 36,282 women enrolled in the WHI CaD trial, 34,763 (96%) had data for all required covariates, were not diagnosed with hematologic malignancy prior to the start of the CaD trial, and were eligible for analysis (Figure 1). At CaD trial baseline, median participant age was 63 years (interquartile range: 57, 68). Seventeen percent of participants were non-white; 28% reported taking non-study vitamin D supplements at baseline. Additional cohort characteristics are provided in Table 1. A biomarker assessment in a sample of 576 trial participants found that serum 25(OH)D3 concentrations were non-significantly different prior to randomization (group means of 20.1 and 20.8 ng/mL in the CaD supplementation and control arms, respectively), and were approximately one-third higher among women in the CaD supplementation arm compared to women in the placebo arm at year two following randomization (group means of 24.3 and 18.2 ng/mL, respectively).19
Risk of incident hematologic malignancy
Our primary analyses focused on participant outcomes over 10 years following CaD randomization, and included a median of seven on-trial and three post-trial years of participant follow-up. During this period, 349 cases of hematologic malignancy were diagnosed, including 44 myeloid malignancies (of which 28 were acute myeloid leukemia), 70 plasma cell dyscrasias (of which 60 were multiple myeloma), and 235 lymphoid malignancies (including 13 Hodgkin lymphoma, 6 T-cell lymphoma, 152 B-cell lymphoma, and 60 chronic lymphocytic leukemia / small lymphocytic lymphoma cases). A detailed breakdown of all hematologic malignancies by cell lineage is provided in Supplementary Table 2.
The risk of hematologic malignancy was significantly lower (log-rank test: p = 0.04) in the intervention arm compared to the control arm (Cox HR = 0.80, 95% CI: 0.65, 0.99, p = 0.04; 10-year cumulative incidences: 0.94% vs. 1.17%; Figure 2). The HR changed little after covariate adjustment, but was closer to the null after restricting to on-trial follow-up (Figure 3; estimates for all covariates are provided in Supplementary Figure 1). When cancers were analyzed separately by cell type, the HRs were 0.77 (95% CI: 0.59, 1.01), 0.71 (95% CI: 0.37, 1.38), and 0.90 (95% CI: 0.54, 1.49) for lymphoid, myeloid and plasma cell malignancies. Subgroup analyses revealed no significant (p < 0.05) evidence of effect modification on the ln(HR) scale (Figure 3). There was some variation in effect estimates across predefined subgroups, but none of these subgroup differences reached statistical significance. Marginally significant (p < 0.10) interactions with CaD treatment assignment were observed for vitamin D dietary intake and use of vitamin D supplements at baseline (Figure 3).
Figure 2. Risk of incident hematologic cancer by calcium / vitamin D (CaD) trial arm.
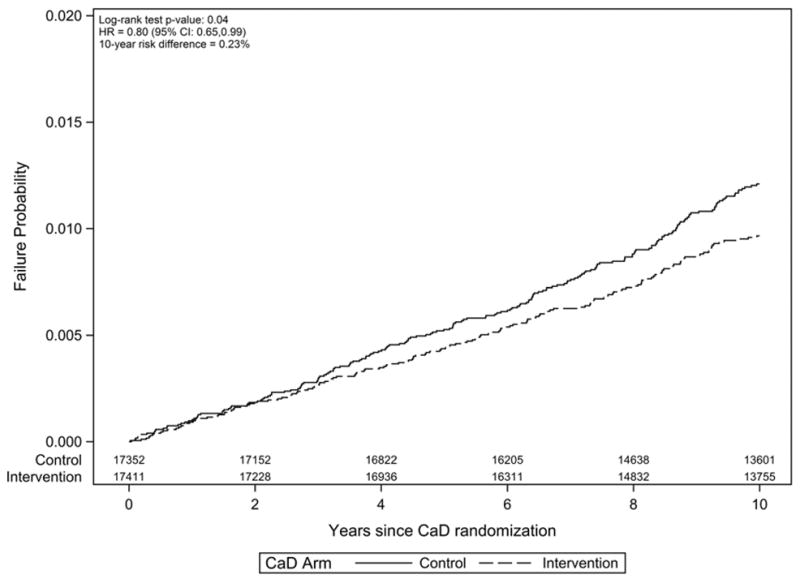
Kaplan-Meier failure plot (one minus survival) with number-at-risk shown by treatment arm. The risk difference at year 10 reflects the difference in cumulative incidence estimates, with death not attributable to hematologic cancer treated as a competing risk.
Figure 3. Incident hematologic cancer hazard ratio (HR) associated with calcium / vitamin D (CaD) supplementation vs. placebo.
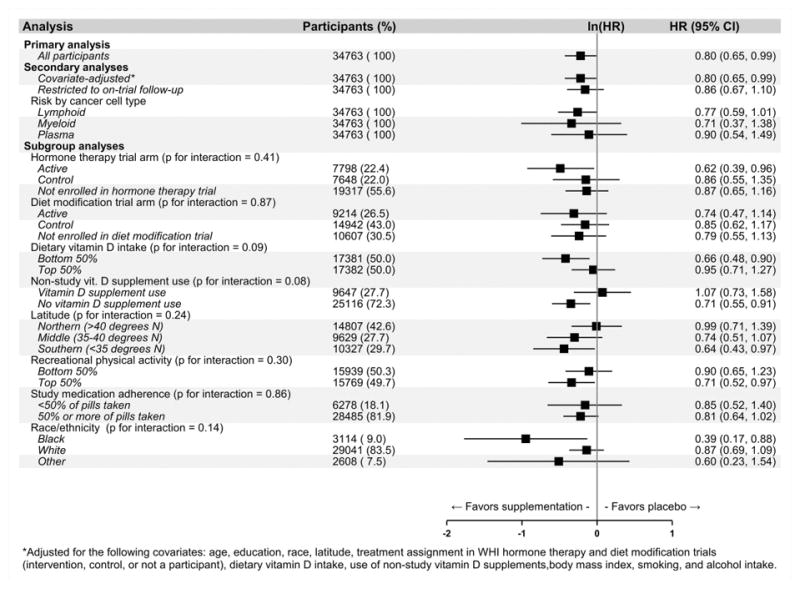
*Adjusted for the following covariates: age, education, race, latitude, treatment assignment in WHI hormone therapy and diet modification trials (intervention, control, or not a participant), dietary vitamin D intake, use of non-study vitamin D supplements, body mass index, smoking, and alcohol intake.
Risk of mortality due to hematologic malignancy in entire study cohort
During the study period, 117 participants died of hematologic malignancy. There was no significant association between CaD supplementation and hematologic cancer-specific mortality (HR = 0.77, 95% CI: 0.53, 1.11, p = 0.16; log-rank test: p = 0.15; Supplementary Figure 1). When analyzed separately by cell type, HRs for cancer-specific mortality were 0.46 (95% CI: 0.24, 0.89), 0.83 (95% CI: 0.36, 1.92), and 1.16 (95% CI: 0.54, 2.50) for lymphoid, myeloid, and plasma cell malignancies. In subgroup analyses, no tests for interaction reached statistical significance (Figure 4).
Figure 4. Hematologic cancer-specific mortality hazard ratio (HR) associated with calcium / vitamin D (CaD) supplementation vs. placebo in the entire study cohort (N = 34,763).
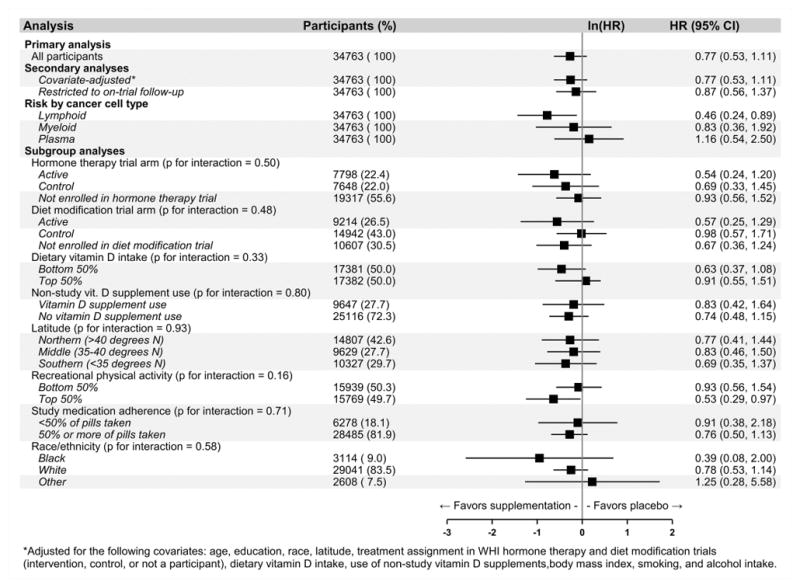
*Adjusted for the following covariates: age, education, race, latitude, treatment assignment in WHI hormone therapy and diet modification trials (intervention, control, or not a participant), dietary vitamin D intake, use of non-study vitamin D supplements, body mass index, smoking, and alcohol intake.
Risk of mortality due to hematologic malignancy following diagnosis
Among participants diagnosed with hematologic malignancy, there was no association between CaD treatment assignment and cancer-specific mortality during the five years following diagnosis (HR = 1.03, 95% CI: 0.70, 1.51, p = 0.87, log-rank test: p = 0.87; Supplementary Figure 2). When cases of hematologic malignancy were distinguished based on cell type, the cancer-specific mortality HRs following diagnosis were 0.66 (95% CI: 0.32, 1.37), 1.24 (95% CI: 0.53, 2.87), and 1.39 (95% CI: 0.62, 3.09) for lymphoid, myeloid, and plasma cell malignancies. In subgroup analyses, no tests for interaction reached statistical significance (Figure 5).
Figure 5. Hematologic cancer-specific mortality hazard ratio (HR) associated with calcium / vitamin D (CaD) supplementation vs. placebo among participants diagnosed with hematologic cancer (N = 349) during the five years following diagnosis.
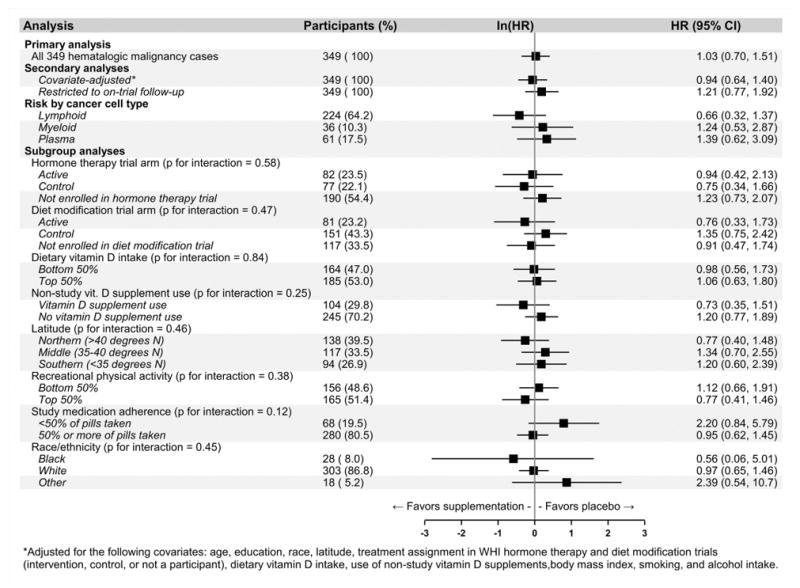
*Adjusted for the following covariates: age, education, race, latitude, treatment assignment in WHI hormone therapy and diet modification trials (intervention, control, or not a participant), dietary vitamin D intake, use of non-study vitamin D supplements, body mass index, smoking, and alcohol intake.
DISCUSSION
In this secondary analysis of the WHI CaD Trial, we found evidence for a protective effect of CaD supplementation on hematologic malignancy risk (HR = 0.80) over 10 years of follow-up. Overall, no significant association with hematologic cancer-specific mortality was found. In secondary analyses evaluating malignancy risk by cancer cell type, protective associations were most consistent for lymphoid malignancies, with trends toward a reduced risk of incident cancer (HR = 0.77) and cancer-specific mortality (HR = 0.46). While our study lacked power to detect five-year survival among cases following diagnosis, the suggestive findings for lymphoid malignancies (HR = 0.66, 95% CI: 0.32, 1.37) warrant further research.
In predefined subgroup analyses, we found evidence for some variation in effect estimates, but no test for interaction reached statistical significance. More pronounced protective associations between CaD active treatment and incident hematologic malignancy were generally observed in subgroups expected to have lower baseline levels of vitamin D (e.g., those with low dietary vitamin D intake, no use of non-study vitamin D supplements, black women), but also in some groups expected to have higher baseline levels (e.g., those with higher levels of recreational physical activity, those living at lower latitudes). While this pattern suggests that CaD supplementation was more beneficial in those with greater baseline vitamin D deficiency, sampling variability may also explain these differences.
Most prior studies examining vitamin D and hematologic malignancy have focused on lymphoid cancers. In several prior observational studies, sun exposure—an important determinant of vitamin D status—has been inversely associated with the risk of non-Hodgkin’s lymphoma (NHL) incidence. In a pooled analysis of 8,243 NHL cases and 9,697 controls,20 higher lifetime recreational sun exposure were associated with a lower risk of incident NHL (odds ratio for highest vs. lowest quartile: 0.76), with the protective association strongest for the 10-year exposure interval prior to diagnosis.
Epidemiologic evidence for a relationship between serum vitamin D levels and NHL risk is inconsistent. In a case-control study nested within the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study cohort of 29,133 male smokers,21 higher serum 25(OH)D3 levels at baseline were not associated with a reduced risk of lymphoid malignancies overall, but were associated with a reduced risk of NHL over the first seven years of follow-up. However, in a larger pooled analysis of data from 10 cohort studies by the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers, no association was found between serum 25(OH)D3 levels at baseline and subsequent risk NHL risk.22
Evidence from prior studies for an association between vitamin D levels and prognosis in patients with hematologic malignancy is more robust. Lower vitamin D levels have been associated with worse overall survival in patients with acute myeloid leukemia,23 chronic lymphocytic leukemia,24 and NHL,25,26 as well as the NHL subtypes diffuse large B-cell lymphoma,26,27 follicular lymphoma,28 and T-cell lymphoma.26,29 Due to the sampling variability of our effect estimates for cancer-specific mortality following diagnosis, our study cannot definitively confirm or disconfirm these findings. However, the suggestive protective associations we observed for mortality attributable to lymphoid malignancy provide some support for these reports.
In comparing our results to those from prior studies, it should be noted that 400 IU/day vitamin D3 in the WHI CaD trial represents a modest dose, and adherence was not ideal: 81% of participants took ≥50% of their doses (on average, at least one pill per day). For these reasons, the difference in mean serum 25(OH)D3 levels between treatment arms at year two (24.3 vs. 18.2 ng/mL) was small in comparison with the considerable degree of between-person variability in serum 25(OH)D3 levels assessed as a prognostic marker in prior epidemiologic studies. For example, prior studies of prognosis in hematologic malignancies have defined insufficient 25(OH)D3 levels as <25 ng/mL,24,26 which would include the majority of WHI participants in the CaD active treatment arm after supplementation. Our findings may not be generalizable to those with higher baseline levels of 25(OH)D3, or reflect the effects of more vigorous supplementation strategies.
Several biologic observations support a role for vitamin D as protective against hematologic malignancies. Vitamin D has effects on T cells, B cells, natural killer cells, and dendritic cells in the lymphoid compartment, where it may impact the malignant transformation of these cells or affect their ability to mitigate the emergence of other malignant hematologic clones.30 Vitamin D also decreases Janus-associated kinase – signal transducer and activator of transcription (JAK-STAT) pathway activation in vitro, providing a potential rationale for protective effects against myeloid malignancies.31,32 Further, vitamin D supports the differentiation of myeloid blasts and facilitates apoptosis in lymphoid and leukemic cell lines.33
Strengths of our study include subject randomization to CaD supplementation or placebo, adjudication of study endpoints by trained clinicians blinded to treatment assignment, the large number of participants, and extended length of follow-up—consistent with an induction period of 7–10 years as suggested by prior observational studies showing protective associations for NHL risk. In addition, participant retention was high in the WHI CaD trial through year 10 of follow-up (79%).
Our study also has limitations. First, type I error is a possible explanation for our results due to the modest effect size and the fact that our study was a secondary post hoc analysis of WHI trial data. Second, the low incidence of hematologic malignancy meant that we had limited power to assess the relationship between CaD treatment assignment and survival following diagnosis. Third, we were not able to evaluate the effect of CaD supplementation on the risk of subclinical hematopoietic disorders such as monoclonal gammopathy of undetermined significance (MGUS), since these were not outcomes adjudicated in the WHI trial. Finally, because the study intervention was a combination of 1000 mg calcium carbonate and 400 IU vitamin D3 daily, we are unable to attribute the protective associations observed in our study solely to vitamin D. While some observational data suggest possible protective associations between calcium intake and reduced cancer risk;5 both the associations and the case for biological plausibility are strongest for digestive system cancers.5,34,35 For hematopoietic malignancies, it is less biologically plausible that calcium supplementation would affect carcinogenesis. While calcium does play an essential role in apoptotic signaling pathways,36 serum and extracellular fluid calcium levels are tightly regulated,37 and would not be meaningfully changed by modest CaD supplementation in an unselected population of older women.38
In conclusion, this post hoc analysis of data from a large and well-executed RCT demonstrates a protective effect on hematologic malignancy incidence with modest vitamin D and calcium supplementation in older women. Considered in absolute terms, the size of the effect is not likely to be clinically significant, but may have public health relevance when considered at the population level. The most consistent evidence for both cancer risk and prognosis was seen for lymphoid malignancies. Given the substantial associations between vitamin D insufficiency and inferior outcome in hematologic malignancies and the current interest in evaluating the role of vitamin D treatment of these diseases, clinical trials investigating vitamin D supplementation are underway (NCT02553447 and NCT02553447). The findings of the current analysis provide support for the further allocation of resources to vitamin D clinical trials.
Supplementary Material
Acknowledgments
Funding: This work was made possible by the University of Iowa Holden Comprehensive Cancer Center Population Research Core, which is supported in part by a Cancer Center Support Grant from the National Cancer Institute (P30 CA086862). The funding for the original WHI calcium and vitamin D trial was provided by the National Heart, Lung, and Blood Institute and the General Clinical Research Center program of the National Center for Research Resources, Department of Health and Human Services. The active study drug and placebo were supplied by GlaxoSmithKline Consumer Healthcare (Pittsburgh, PA).
Footnotes
Conflict-of-interest statements:
Eric M. Ammann is now an employee in the Medical Device Epidemiology division at Johnson & Johnson. The analyses for this paper were completed and the manuscript drafted prior to his start in that role. The other authors report no conflicts.
Author contributions:
Eric M. Ammann conceptualized the study, supervised the data analysis, wrote the original manuscript draft, and reviewed and edited the manuscript.
Matthew T. Drake conceptualized the study and reviewed and edited the manuscript.
Bjarni Haraldsson performed the data analysis and reviewed and edited the manuscript.
Robert B. Wallace reviewed and edited the manuscript.
Karen C. Johnson reviewed and edited the manuscript.
Pinkal Desai reviewed and edited the manuscript.
Emily Lin reviewed and edited the manuscript.
Brian K. Link conceptualized the study and reviewed and edited the manuscript.
References
- 1.Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs--a brief overview. Mol Cell Biochem. 2003;253(1–2):247–254. doi: 10.1023/a:1026072118217. [DOI] [PubMed] [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 3.Hobaus J, Thiem U, Hummel DM, Kallay E. Role of calcium, vitamin D, and the extrarenal vitamin D hydroxylases in carcinogenesis. Anticancer Agents Med Chem. 2013;13(1):20–35. [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 5.Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med. 2009;169(4):391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 7.Chlebowski RT, Johnson KC, Kooperberg C, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100(22):1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24(2):567–580. doi: 10.1007/s00198-012-2224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 Suppl):S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 10.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 11.Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt) 2013;22(11):915–929. doi: 10.1089/jwh.2013.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 13.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 14.Allison PD. Survival analysis using SAS: A practical guide. 2. Cary, NC: SAS Institute; 2010. [Google Scholar]
- 15.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marubini E, Valsecchi MG. Analyzing survival data from clinical trials and observationl studies. West Sussex, England: John Wiley & Sons, Ltd; 2004. Competing risks; pp. 331–363. [Google Scholar]
- 17.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 18.Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. BMJ. 2016;352:i1981. doi: 10.1136/bmj.i1981. [DOI] [PubMed] [Google Scholar]
- 19.Schnatz PF, Jiang X, Vila-Wright S, et al. Calcium/vitamin D supplementation, serum 25-hydroxyvitamin D concentrations, and cholesterol profiles in the Women’s Health Initiative calcium/vitamin D randomized trial. Menopause. 2014;21(8):823–833. doi: 10.1097/GME.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kricker A, Armstrong BK, Hughes AM, et al. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122(1):144–154. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 21.Lim U, Freedman DM, Hollis BW, et al. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124(4):979–986. doi: 10.1002/ijc.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purdue MP, Freedman DM, Gapstur SM, et al. Circulating 25-hydroxyvitamin D and risk of non-hodgkin lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):58–69. doi: 10.1093/aje/kwq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HJ, Muindi JR, Tan W, et al. Low 25(OH) vitamin D3 levels are associated with adverse outcome in newly diagnosed, intensively treated adult acute myeloid leukemia. Cancer. 2014;120(4):521–529. doi: 10.1002/cncr.28368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shanafelt TD, Drake MT, Maurer MJ, et al. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood. 2011;117(5):1492–1498. doi: 10.1182/blood-2010-07-295683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aref S, Ibrahim L, Azmy E. Prognostic impact of serum 25-hydroxivitamin D [25(OH)D] concentrations in patients with lymphoid malignancies. Hematology. 2013;18(1):20–25. doi: 10.1179/1607845412Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 26.Drake MT, Maurer MJ, Link BK, et al. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(27):4191–4198. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bittenbring JT, Neumann F, Altmann B, et al. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J Clin Oncol. 2014;32(29):3242–3248. doi: 10.1200/JCO.2013.53.4537. [DOI] [PubMed] [Google Scholar]
- 28.Kelly JL, Salles G, Goldman B, et al. Low Serum Vitamin D Levels Are Associated With Inferior Survival in Follicular Lymphoma: A Prospective Evaluation in SWOG and LYSA Studies. J Clin Oncol. 2015;33(13):1482–1490. doi: 10.1200/JCO.2014.57.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tretli S, Schwartz GG, Torjesen PA, Robsahm TE. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control. 2012;23(2):363–370. doi: 10.1007/s10552-011-9885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83(7):1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 32.Furqan M, Mukhi N, Lee B, Liu D. Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res. 2013;1(1):5. doi: 10.1186/2050-7771-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006;102(1–5):156–162. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96(13):1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 35.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 36.Distelhorst CW, Dubyak G. Role of calcium in glucocorticosteroid-induced apoptosis of thymocytes and lymphoma cells: resurrection of old theories by new findings. Blood. 1998;91(3):731–734. [PubMed] [Google Scholar]
- 37.Houillier P, Froissart M, Maruani G, Blanchard A. What serum calcium can tell us and what it can’t. Nephrol Dial Transpl. 2006;21(1):29–32. doi: 10.1093/ndt/gfi268. [DOI] [PubMed] [Google Scholar]
- 38.Samozai MN, Kulkarni AK. Do calcium supplements increase serum and urine calcium levels in post-menopausal women? J Nutr Health Aging. 2015;19(5):537–541. doi: 10.1007/s12603-014-0532-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.