Abstract
Background
ILC2s represent an important type 2 immune cell. Glucocorticoid regulation of human ILC2s is largely unknown.
Objective
To assess steroid resistance of human blood and airway ILC2s from asthmatic patients and examine its mechanism of induction.
Methods
We studied human blood and lung ILC2s from asthmatic and control subjects by flow cytometry and ELISA.
Results
Dexamethasone (Dex) inhibited (P=0.04) CRTH2 and type 2 cytokine expression by blood ILC2s stimulated with IL25 and IL33. However, it failed to do so when ILC2s were stimulated with IL7 and TSLP, two ligands of IL7Rα. Unlike blood ILC2s, BAL ILC2s from asthmatic patients were resistant to Dex. BAL from the asthmatic patients had elevated TSLP but not IL7. The BAL TSLP level correlated (r=0.74) with steroid resistance of ILC2s. TSLP was synergistically induced in epithelial cells by IL13 and human rhinovirus. Mechanistically, Dex upregulated ILC2 expression of IL7Rα, which augmented and sustained STAT5 signaling by TSLP. TSLP induced MEK, c-Fos, ID3, pSTAT3 and pSTAT5—molecules linked to steroid resistance. Dex inhibited c-Fos, ID3 and pSTAT3, but not pSTAT5 and MEK. The MEK inhibitor Trametinib, the JAK-STAT inhibitor Tofacitinib and the STAT5 inhibitor Pimozide reversed steroid resistance of BAL ILC2s.
Conclusions
Dex inhibited type 2 cytokine production by blood ILC2s. IL7 and TSLP abrogated this inhibition and induced steroid resistance of ILC2s in a MEK and STAT5-dependent manner. BAL ILC2s from asthmatic patients with elevated TSLP were steroid resistant, which was reversed by clinically available inhibitors of MEK and STAT5.
Keywords: Asthma, type 2 innate lymphoid cells, steroid resistance, TSLP, STAT5, MEK
Introduction
Innate lymphoid cells (ILC) represent a lineage of lymphoid cells that diverge from the lymphoid cells (T and B cells) of the adaptive immune system at an early developmental stage due to high expression of ID2 (inhibitor of differentiation 2) (reviewed in ref. 1). ILCs are akin to NK cells and readily respond to a tissue insult or injury. ILC2s are an important source of type 2 cytokines. They produce relatively high quantities of IL4, IL5, and IL13 among others. They are most prevalent in the tissue, especially in the mucosal tissue.
Growing number of studies implicated them in the pathogenesis of allergic diseases and in defense against parasites. The number of ILC2s was increased in the blood from patients with many allergic diseases—allergic rhinitis (2), asthma (3), eosinophilic sinusitis (4), eosinophilic esophagitis (5) and atopic dermatitis (6). We reported increased frequency of ILC2s in the bronchoalveolar lavage from allergic asthmatic patients (7). An increased frequency of ILC2s was also reported in the sputum from asthmatic patients (8). By producing high quantities of IL5 and IL13 ILC2s are likely to contribute to eosinophilia and airway hyperreactivity. Th2 cells and Th2-driven eosinophilia are usually sensitive to inhibition by steroids (glucocorticoids), although they can become steroid-resistant under certain circumstances (9–11).
In a subgroup of patients asthma does not satisfactorily respond to high dose inhaled steroids and oral steroids (12). These patients are labeled with severe refractory asthma. These patients continue to manifest eosinophilia in the blood and airways while on steroids. The mechanism of steroid resistance of this type 2 inflammation is not fully understood. Recently, Kabata et al reported that mouse ILC2s developed relative steroid resistance in a TSLP-dependent manner (13). This study suggested that ILC2s could contribute to steroid resistance in type 2 inflammatory diseases. In contrast, Walford et al reported that both mouse and human ILC2s were sensitive to steroids (14). Treatment with steroids caused apoptosis of ILC2s in vitro and in vivo. The effect of steroids on ILC2s, especially airway ILC2s from human asthma is unknown. Since the number of ILC2s is increased in asthma, we asked in this manuscript if ILC2 developed steroid resistance in refractory asthma.
Materials and Methods
Human subjects
We studied blood and BAL ILC2 from allergic asthmatic patients, disease controls and healthy donors. Allergic asthmatic patients and disease controls were recruited from the outpatient clinics at National Jewish Health. Bronchoscopy and BAL were performed as a part of their clinical work-up for poorly controlled asthma. None of the disease control patients met the ATS diagnostic criteria for asthma. The protocols for blood and BAL studies of lymphoid cells from asthmatic patients and disease controls were approved by the institutional IRB. Written informed consent was obtained from each participant. Healthy donors were recruited from the blood bank of National Jewish Health. Asthmatic patients and disease control subjects maintained their controller medications at the time of bronchoscopy. Demographic and clinical characteristics of asthma and disease control subjects are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study patients
| Parameters | Asthmatic patients | Disease controls |
|---|---|---|
| N | 50 | 35 |
| Diagnoses | 50 patients with asthma; 47 patients with allergic rhinitis, 29 with chronic sinusitis, 42 with GERD, 3 with bronchiectasis and 5 with aspiration | 21 patients with chronic cough and concurrent allergic rhinitis & GERD, 6 patients with bronchiectasis, 7 with chronic aspiration and 1 with COPD |
| Male/female | 28/22 | 19/16 |
| Age | 53.1 ± 4 (52) | 54.3 ± 4 (53) |
| FEV1 (%) | 71.4 ± 4 (72α)* [64–81β] | 89.4 ± 4 (89) [78–99] |
| Reversibility (%) | 17.0 ± 6 (14)*[10–21] | 2.8 ± 0.6 (2) [0–6] |
| PC20 (mg/ml) for methacholine | 2.7 ± 0.6 (2.3)*a [0.3–6] | 24.2 ± 0.8 (25) [20–25] |
| Allergy skin test positivity | 44 | 24 |
| Eosinophils/μL blood | 490 ± 88 (300)* [200–700] | 100 ± 21 (100) [0–200] |
| Total IgE (KIU/L) | 277 ± 34 (177) [88–525] | 132 ± 72 (59) [32–266] |
| BMI | 28.1 ± 2 (28) | 27.6 ± 1 (27) |
| Asthma medications | systemic steroids: 10 patients Omalizumab: 13 ICS/LABA: 50 LTI: 19 SABA: 50 Tiotropium: 9 |
ICS/LABA: 7 SABA: 18 |
number in the parenthesis () indicates median;
numbers in [] indicate the range in the group.
P<0.05, Mann-Whitney U test;
performed with 35 patients
Isolation of PBMC, lin- cells, culture and flow cytometry for ILC2s
PBMC were isolated from EDTA-treated blood by density centrifugation with Ficoll-Hypaque. PBMC were cultured in RPMI 1640 plus 10% FBS in the medium alone or with the cytokine combo (as indicated in the text) with Dexamethasone (10−7M) or vehicle (0.1% ethanol) as described previously (15). The following cytokines were used at 20 ng/ml concentration: IL2, IL7, IL25, IL33 and TSLP (all from Peprotech, Rocky Hills, NJ). The concentration of the allergen extract Aspergillus sp. (Greer Labs, Lenoir, NC) in the PBMC culture was 5 μg/ml. In select experiments lin- cells were isolated fom PBMCs by negative selection using antibody-coated magnetic beads (lineage cell depletion kit) from Miltenyi, Inc. San Diego, CA. PBMCs and lin- cells were cultured for 5 days unless otherwise stated in the text. Monensin (2 μM, Biolegend, Inc., San Diego, CA) was added to all cultures 4 hr before conclusion. Following culture cells were pelleted and then stained for flow cytometry as described previously (15).
Processing and culture of BAL cells for ILC2 studies
BAL cells were pelleted, washed and cultured in the medium (RPMI 1640 plus 10% FBS) alone or with cytokines (as described for PBMCs in the previous section) with vehicle (0.1% DMSO) or Dexamethasone (10−7 M, from Sigma-Aldrich, Inc. St. Louis, MO). In select experiments Trametinib (10 μM) from LC Laboratories, Tofacitinib (0.2 μM) from ApexBio, or Pimozide (5 μM) from Tocris Bioscience was added to the culture. BAL cells were cultured for 3 days until otherwise stated. Monensin (2 μM) was added to all cultures 4 hr before conclusion. Following culture cells were pelleted and then stained for flow cytometry as described previously (7, 15).
Flow cytometric staining of cells
Staining for flow cytometry was carried out as described previously (7, 15). Fluorophore-labeled antibodies were: human lineage cocktail (CD3, CD14, CD16, CD19, CD20, CD56), FcεRI, CRTH2, IL7Rα, CD25, IL5 from Biolegend; PFVD eFluor 780, pSTAT3, pSTAT5 from e-Bioscience; ST2 and IL25 receptor (IL17RB) from R&D Systems; ID3 and MEK from BD Bioscience; c-Fos from Cell signaling Technology, and glucocorticoid receptor antibody from Bioss Antibodies. The labeled FcεRI antibody was added to the lineage cocktail. We stained cell surface markers on live cells first. Then, we fixed cells using 4% paraformaldehyde, permeabilized them with 0.1% saponin and performed staining for intracellular molecules (cytokines, signaling molecules and transcription factors). Flow cytometry was performed on LSRII (BD Biosciences). The data was analyzed by the FlowJo software. Isotype antibody controls and FMO (fluorescence minus one) were used to develop the gating and data analysis strategies.
Airway Epithelial Cell Culture and Real-time PCR
Freshly isolated epithelial cells from bronchial brushing were obtained from allergic asthmatic patients and were cultured in air-liquid interface as described previously (16). Confluent cells were cultured with IL13 (10 ng/ml), IFNγ (10 ng/ml), LPS (1 μg/ml, Sigma-Aldrich, Inc., St. Louis, MO), poly-IC (50 ng/ml) or human rhinovirus-16 (8 × 104 TCID50/well) for 24 hr. Isolation of mRNA and real-time PCR was performed as described previously (17). The primers for TSLP were: forward 5′-ACCTTCAATCCCACCGCCGGC-3′; reverse 5′-GGCAGCCTTAGTTTTCATGGCGA-3′ (synthesized by Integrated DNA technologies, Inc. Coralville, Iowa).
ELISA for cytokines
IL7 and TSLP in the BAL from asthmatic patients and disease controls and TSLP from epithelial cell cultures were measured by ELISA. IL5 was measured in the culture supernatant from lin- cells. The IL5 ELISA kit was from Thermo Fisher Scientific, Inc., Walthma, MA (detection threshold 4 pg/ml) and the IL7 ELISA kit was from R&D, Inc., Minneapolis, MN (detection threshold 15.6 pg/ml) and the TSLP ELISA kit was from e-Bioscience, Inc. (detection threshold 15.6 pg/ml). ELISA was performed as per manufacturer’s instruction as described previously (7, 17).
Statistical Analyses
Comparison between the study groups was done by Mann-Whitney U test unless otherwise stated. Comparison among multiple study groups was performed by Kruskal Wallis test. Pearson correlation coefficient was used to calculate correlation coefficient.
Results
Study Subjects
We studied blood and BAL from a total of 50 asthmatic patients and 35 disease controls. We also obtained blood from the National Jewish Health Blood Bank for Research, which recruits healthy donors under an institutional IRB. The demographics and disease characteristics for the patient populations are shown in Table 1
Effect of Dexamethasone on blood ILC
We examined the effect of Dexamethasone (Dex) on survival of blood ILC. To this goal we cultured PBMC in medium and with a combination of cytokines IL2/IL7/IL33 with and without Dex for 5 days. Cell death was initially analyzed by gating for live and dead cells by flow cytometry using forward and side scatters. We observed a modest increase in dead cells in cultures with Dex (Online Repository Figure E1). There were more dead cells in cultures stimulated with cytokines, which perhaps reflects activation-induced cell death. The live cell population was gated for further analysis for dead cells using a fixable viability dye e-Fluor 780 (e-Bioscience). We observed a modest increase in dead cells in cultures with Dex, cytokines and cytokines plus Dex. The difference in the dead cell number among these cultures was not significant.
Airway but not blood-derived ILC2s are relatively steroid resistant
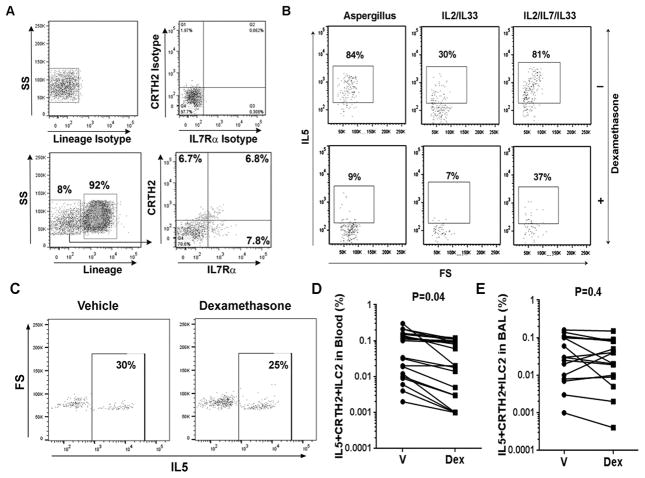
Next we cultured PBMC from an allergic asthmatic patient with the sensitizing allergen extract of Aspergillus or a combination of cytokines—IL2/IL33 and IL2/IL7/IL33 in the presence or absence of Dex for 5 days. After culture the cells were gated for lineage-negative (lin-) cells, then CRTH2+IL7Rα+ cells (referred to as CRTH2+ cells, Figure 1A), and then IL5+ cells (Figure 1B). We found that Dex potently inhibited IL5+ ILC2s from Aspergillus and IL2/IL33-stimulated cultures. However, Dex was less effective in inhibiting IL5+ ILC2s from the IL2/IL7/IL33-stimulated culture (Figure 1B). We performed a similar experiment with Dex using BAL cells from an allergic asthmatic patient. Cells were cultured without IL2/IL7/IL33. We observed negligible inhibition of BAL IL5+ ILC2 (Figure 1C). Figure 1D & E show cumulative data on Dex-inhibition of IL5+ CRTH2+ ILC2 from the blood and BAL obtained from 14 allergic asthmatic patients. Dex significantly inhibited IL2/IL33-stimulated IL5+ ILC2s from the blood but failed to inhibit IL5+ ILC2sfrom the BAL. We analyzed the effect of Dex on fluorescence intensity of IL5 staining in BAL ILC2s. Dex did not reduce the mean fluorescence intensity (MFI) of IL5 in ILC2s (online repository figure E2A). We measured the effect of Dex on BAL cell viability using two viability dyes—the fixable viability dye e-Fluor 780 and the dye Zombie aqua. Both dyes stain dead cells. Dex caused a modest increase in dead cells in BAL when stained with e-Fluor 780 but not when stained with Zombie aqua (online repository figure E2B&C). The clinical characteristics of the BAL donors are shown in Table 1. The patients had moderate to severe asthma as defined by the EPR3 guidelines. Dex sensitivity (% inhibition of IL5+ cells) of BAL ILC2s correlated negatively with BAL eosinophils (r=−0.77, P=0.00003) and blood eosinophils (r=−0.69, P=0.003), but positively with baseline FEV1 (r=0.58, P=0.003) and FEV1/FVC ratio (r=0.51, P=0.01) (Table 2).
Figure 1.
Effect of Dexamethasone (Dex) on IL5+ ILC2s. PBMCs from an allergic asthmatic patient were cultured with Aspergillus, IL2/IL33 or IL2/IL7/IL33 for 5 days with and without Dex. A: PBMCs were stained with labeled antibodies against the lineage markers, CRTH2, IL7Rα, and their isotype antibody controls (shown at the top). Live and lineage-negative cells were gated for CRTH2+ and IL7Rα+ cells. Dual CRTH2+IL7Rα+ cells were then analyzed for IL5 expression. B: Expression of IL5 in lin-CRTH2+IL7Rα+ cells in cultures with Aspergillus, IL2/IL33 or IL2/IL7/IL33 in presence or absence of Dex. C: Effect of Dex on BAL lin-CRTH2+IL7Rα+IL5+ cells. BAL cells from an allergic asthmatic patient was cultured with and without Dex for 5 days and then analyzed for live CD45+lin-CRTH2+IL7Rα+IL5+ cells. D & E: Cumulative data on the effect of Dex on blood (D) and BAL (E) lin-CRTH2+IL7Rα+IL5+ cells 14 allergic asthmatic patients. V: vehicle; Dex: Dexamethasone
Table 2.
Correlation of BAL ILC2 steroid sensitivity with clinical parameters
| Variables | Correlation (r) with Dex inhibition of ILC2s | P |
|---|---|---|
| BAL eosinophils | −0.77 | 0.00003 |
| Blood eosinophils | −0.69 | 0.0003 |
| Baseline FEV1 | 0.58 | 0.003 |
| FEV1/FVC ratio | 0.51 | 0.01 |
The IL7Rα ligands—IL7 and TSLP induce steroid resistance in vitro
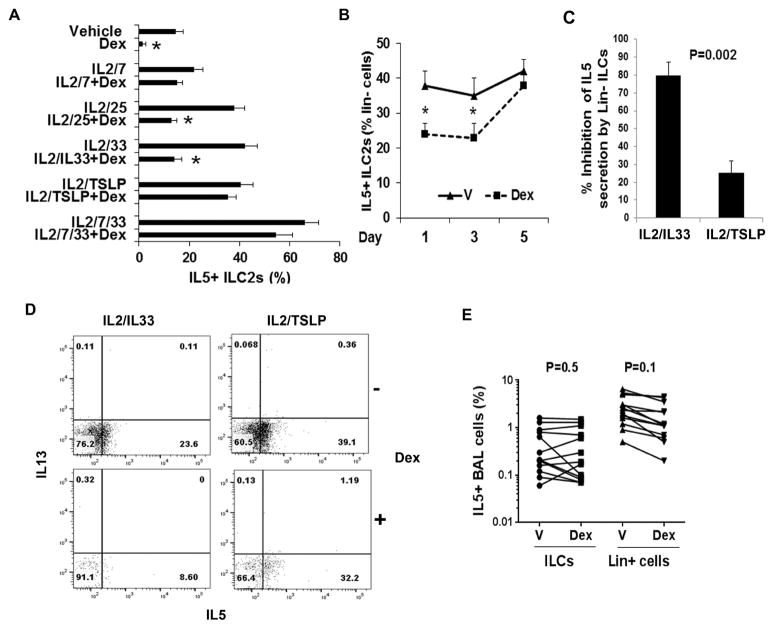
Next we examined the effect of various combinations of ILC2-stimulating cytokines on induction of steroid resistance in vitro. We found that the presence of IL7 and TSLP but not IL25 or IL33 in the cytokine combo was associated with the development of steroid resistance (Figure 2A). IL7 and TSLP attenuated Dex-inhibition of IL5+ ILC2 in the blood. Note that IL2 was added to all cultures as an ILC growth factor. It did not make any difference for steroid resistance. Kinetics analysis showed that the development of steroid resistance required a prolonged, 5 days of exposure to these cytokines (Figure 2B). Dex did not significantly change the number of lin- cells in the culture.
Figure 2.
A: Effect of cytokines on development of steroid resistance. PBMCs from allergic asthmatic patients were cultured with a combination of ILC2-stimulating cytokines in the presence or absence of Dex for 5 days. The cells were then stained for FCM and gated for IL5+ ILC2s as described under Figure 1A. *P<0.05 (n=6). B: Kinetics of development of resistance against inhibition of IL5+ ILC2s by Dex. PBMCs were cultured with IL2/TSLP plus vehicle or Dex for 1, 3 and 5 days and then analyzed for IL5+ ILC2s by FCM. *P<0.05 (n=4). C&D: Lin- cells (0.4 × 106 per culture) from peripheral blood obtained from 3 allergic rhinitis (non-asthmatic) patients were cultured with IL2/IL33 or IL2/TSLP with and without Dex (10-7 M) for 5 days. Cell-free supernatant was analyzed for IL5 by ELISA. Cells were analyzed by FCM for intracellular IL5. Dex inhibition of IL5 secretion and intracellular IL5 expression are presented in panel C and D respectively. P value for data on panel C was calculated by paired t test due to low N. E: Comparison of the effect of Dex on IL5+ ILCs (lin-CRTH2+IL7Rα+) and IL5+ lineage+ cells in BAL. BAL cells were cultured with vehicle or Dex for 5 days, stained and first gated for CD45+ cells and then for lin-, CRTH2+, IL7Rα+, IL5+ cells (IL5+ ILCs) and lin+ IL5+ cells. Each data point is an individual asthma patient (n=9).
Next we examined the direct effect of Dex on isolated lineage- negative (Lin-) cells (representing ILCs) from the peripheral blood. The effect of Dex was measured by two approaches: measurement of secreted IL5 by ELISA and measurement of the expression of intracellular IL5 by FCM. Dex inhibited IL5 secretion by IL2/IL33-stimulated ILCs by nearly 79% (Figure 2C). In contrast, it inhibited IL5 secretion by IL2/TSLP-stimulated ILCs by only 25%. The measurement of intracellular IL5 in ILCs from the same experiment showed a similar result. Dex inhibited intracellular IL5 expression in IL2/IL33- and IL2/TSLP- stimulated ILCs by 65% and 18%, respectively (Figure 2D).
We examined steroid resistance of IL5+lineage+ cells (which represent T cells, NK cells, myeloid cells and mast cells) in the BAL and compared them with ILC2s. IL5+lineage+ BAL cells from these asthmatic subjects were also steroid resistant (Figure 2E). However, their steroid resistance was milder when compared to the steroid resistance of ILC2s. Note that the frequency of IL5+lineage+ cells was much higher than that of IL5+ ILC2s in BAL. In some patients Dex actually increased the number of IL5+ ILC2s likely due to the increased number of CRTH2+IL7Rα+ cells (see below).
The foregoing experiments were performed with cells from asthmatic patients. In a next set of experiments we examined the effect of Dex on ILCs from healthy controls. PBMCs from healthy subjects had low levels of CRTH2+ ILC2s (online repository figure E3A) and type 2 cytokine expression (online repository figure E3B). Culture of these cells with IL2/IL33 and IL2/TSLP marginally increased their cytokine expression. Dex inhibited IL2/IL33- but not IL2/TSLP-induced IL5 expression in ILC2s from healthy subjects (online repository figure E3C). The results suggest that TSLP induces steroid resistance in ILC2s not only from asthmatic patients but also from healthy subjects.
TSLP is increased in BAL from asthmatic patients and correlated with steroid resistance of ILC2
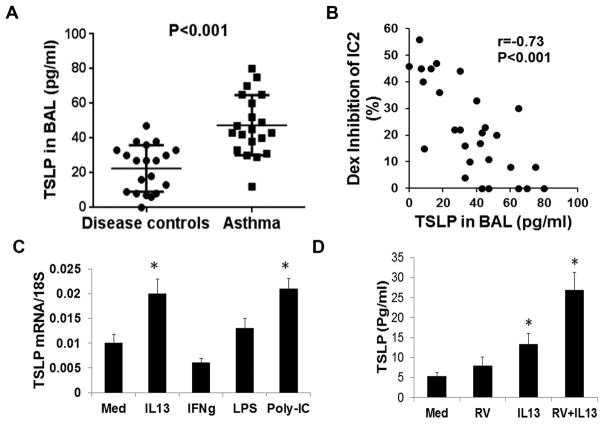
Since BAL ILC2s from asthmatic patients were relatively resistant to Dex-mediated inhibition we asked if IL7 and TSLP contributed to this resistance. We examined the level of IL7 and TSLP in BAL from asthmatic patients. IL7 was largely undetectable in BAL from asthmatic patients (data not shown). In contrast, we detected higher levels of TSLP in BAL from asthmatic patients as compared to disease controls (Figure 3A). We examined the relevance of TSLP for ILC2 steroid resistance. The level of TSLP negatively correlated with Dex-inhibition of BAL IL5+ ILC2 (Figure 3B).
Figure 3.
C: TSLP Level in BAL from asthmatic patients and disease controls. TSLP was measured by ELISA. n= 20). D: Correlation between BAL TSLP levels and Dex inhibition of BAL IL5+ ILC2s. E: Effect of select cytokines and TLR ligands on TSLP production by airway epithelial cells. Human airway epithelial cells, cultured in air-liquid interface, were stimulated with IL13, IFNγ, LPS or poly-IC for 24 hr and then expression of TSLP mRNA was analyzed by real-time PCR. *P<0.05 (paired t test, n=5) as compared to medium (Med). F: Effect of IL13 and human rhinovirus-16 infection on TSLP production by airway epithelial cells. Human airway epithelial cells cultured as above, were stimulated with IL13, human rhinovirus-16 (RV) or a combination of both for 48 hr. Supernatant was assayed for TSLP by ELISA. *P<0.05 (paired t test, n=3 replicates) as compared to medium (Med).
TSLP is synergistically induced by IL13 and human rhinovirus-16
Next we examined the expression of TSLP by human airway epithelial cells cultured under air-liquid interface. We initially studied TSLP mRNA induction by the type 2 cytokine IL13, the type 1 cytokine IFNγ, the TLR4 agonist LPS and the TLR3 agonists poly-IC. IL13 and poly-IC induced the expression of mRNA for TSLP (Figure 3C). Since poly-IC is a viral mimic, we examined the effect of human rhinovirus with and without IL13 on TSLP production at the protein level. We chose rhinovirus because it is a frequent cause of asthma exacerbation (18). Human rhinovirus-16 induced modest production of TSLP, which was synergistically augmented by IL13 (Figure 3D).
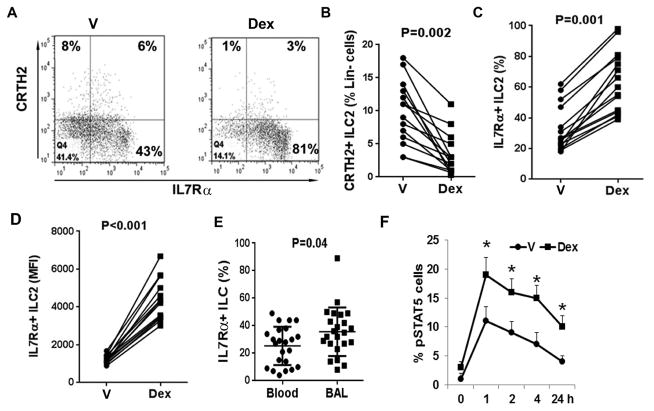
Dex strongly induces IL7Rα on ILC2s
We studied the mechanism of IL7 and TSLP induction of steroid resistance. We cultured PBMC in the presence or absence of Dex. Dex inhibited the frequency of CRTH2+ but strongly upregulated the frequency of IL7Rα+ ILCs (Figure 4A–C). More importantly, it increased the expression level (mean fluorescence intensity) of IL7Rα on cells (Figure 4D). Since most of our asthmatic patients are on inhaled steroids and some are on systemic steroids, we compared IL7Ra+ lineage- cells in the blood and BAL. We found increased number of IL7Rα+ cells in BAL (Figure 4E). We recognize that this increase could be due to a number of reasons, which include the underlying disease process and the treatment with steroids. We examined the effect of Dex with and without the cytokines (IL7 and TSLP) on expression of the IL2 receptor CD25, IL25 receptor and IL33 receptor (ST2). Dex with and without the cytokines had negligible effects on expression of these receptors on ILC2s (Online Repository Figure E4).
Figure 4.
A–D: Effect of Dex on CRTH2 and IL7Rα expression on ILC2s. PBMCs were cultured with vehicle or Dex for 5 days and then stained for flow cytometry. Live lin- cells were gated for CRTH2 and IL7Rα. A representative flow cytogram is shown in A. Cumulative data from 14 asthmatic patients for the frequency of CRTH2+ and IL7Rα+ cells are shown in B & C, respectively. D shows the mean fluorescence intensity of IL7Rα+ expression from the same study subjects. E: Comparison of the frequency of IL7Rα+ ILCs between blood and BAL. PBMCs and BAL cells from asthmatic patients were studied ex vivo for IL7Rα on gated live lin-CRTH2+ cells. Each symbol represents a donor (n=24). F: Effect of Dex on TSLP signaling. PBMCs from allergic asthmatic patients were cultured with vehicle (V) or Dex for 3 days and stimulated IL2/TSLP for increasing period of time. The frequency of pSTAT5+ cells on gated live lin-CRTH2+ cell population was measured. P*<0.05, N=4, paired t test).
IL7Rα is the common receptor for IL7 and TSLP (19). The increase in IL7Rα raised an intriguing possibility that IL7 and TSLP signaling was altered in Dex-treated cells, which secondarily contributed to steroid resistance. To test this hypothesis we cultured cells with vehicle or Dex for 3 days and then stimulated with IL2/TSLP for increasing period of time. We monitored the level of pSTAT5, which is a major signaling molecule downstream of IL7Rα. Dex pre-treated cells showed a significantly higher frequency of pSTAT5+ cells (Figure 4F). Further, this increased level of pSTAT5 was sustained over a longer period of time as compared to vehicle-treated cells.
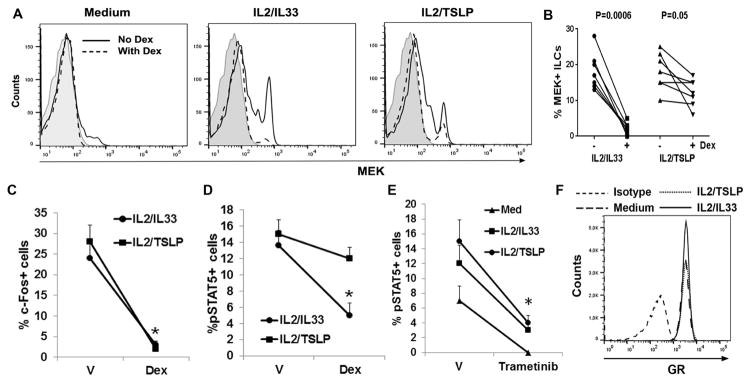
Dex is less effective in suppressing MEK1 and STAT5 signaling in the presence of TSLP
In a next set of experiments we examined the signaling mechanism that contributed to steroid resistance of ILC2s. We previously reported that MEK1 (referred to as MEK) conferred steroid resistance to T cells (14). MEK translocated to the nucleus, interacted with the glucocorticoid receptor co-repressor SMRT and induced its nuclear export (20). MEK also directly bound to the c-Fos promoter. Recently, a functional genomics study utilizing shRNA against all known genes identified MEK2 and MEK4 as top inducers of steroid resistance in leukemic cells (21). MEK is expressed at a low level in quiescent cells and is upregulated upon stimulation (11). We examined the induction of MEK by ILC2-regulating cytokines in the presence and absence of Dex. Note that Dex and the cytokines were added simultaneously to the culture for these experiments. IL2/IL33 and IL2/TSLP increased the expression of MEK in ILC2s (Figure 5A). Dex caused a near complete inhibition of MEK induction by IL2/IL33 but only modestly affected the induction by IL2/TSLP. Figure 5B shows cumulative data on MEK suppression by Dex from 7 asthmatic patients. The results show that Dex was a stronger inhibitor for IL33 than for TSLP in suppressing MEK. MEK activates ERK1/2 and induces c-Fos among many other genes. C-Fos, a component of the AP1 complex, has previously been shown to antagonize GR at transcriptional level (22). We examined c-Fos induction in ILC2s by IL2/IL33 and IL2/TSLP. Dex caused a dramatic inhibition of c-Fos induction in ILC2s stimulated with IL2/IL33 and IL2/TSLP (Figure 5C and online repository figure E5A). STAT5 is another signaling molecule that induces steroid resistance (23). STAT5 binds to the GR and sequesters it in the cytosol. We examined the effect of Dex on pSTAT5 induction in ILC2s. Dex was effective in inhibiting IL2/IL33-induced but not IL2/TSLP-induced pSTAT5 (Figure 5D). MEK and STAT5 are known to regulate each other (24). Treatment with the MEK inhibitor Trametinib significantly inhibited pSTAT5 induction by IL2/IL33 and IL2/TSLP (Figure 5E). This suggested that MEK functioned upstream of STAT5. We examined if TSLP and IL33 differentially affected GR expression on ILC2s upon culture. ILC2s expressed high levels of GR (Figure 5F). TSLP did not alter GR expression when compared with IL33.
Figure 5.
A&B: Effect of Dex on cytokine-induced MEK1 (MEK) induction. PBMCs from an asthmatic patient were cultured with medium or IL2 plus one of the two epithelial ILC-inducing cytokines—IL33 and TSLP with and without Dex for 5 days. The expression of MEK on gated live lin-CRTH2+ cells was analyzed by FCM. Representative flow cytograms are shown in panel A and cumulative data from 7 asthmatic patients is shown in panel B. C&D: PBMCs were cultured as under Figure 5A and then the frequency of c-Fos+ and pSTAT5+ cells in the live lin-CRTH2+ cell population was assessed. P*<0.05 (paired t test, N=4) as compared to vehicle (V)-treated cells. E: Effect of the MEK inhibitor Trametinib on pSTAT5 in ILCs. PBMCs from allergic asthmatic patients were cultured with medium, IL2/IL33 or IL2/TSLP with and without Trametinib (10 μM) for 5 days and then assessed for pSTAT5. (n=3, *<0.05, paired t test). F: Expression of glucocorticoid receptor (GR) in cytokine-treated cells. PBMCs were cultured with medium, IL2/TSLP or IL2/IL33 for 5 days. The expression of GR was studied in gated live lin-CRTH2+ cells. A representative flow cytogram from one of 3 similar experiments is shown.
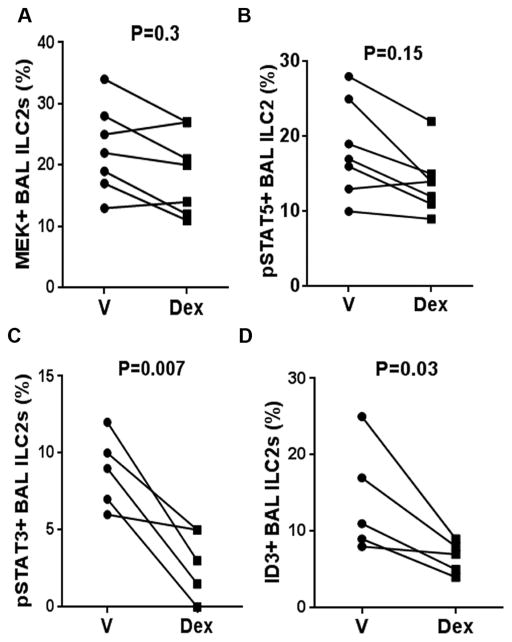
Next we studied the effect of Dex on MEK and pSTAT5 in BAL ILC2s from asthmatic patients. Dex was unable to inhibit MEK and pSTAT5 in BAL ILC2s (Figure 6A & B). TSLP is known to activate STAT3 (25). Interestingly, pSTAT3 expression in BAL ILC2s was susceptible to Dex-mediated inhibition (Figure 6C and online repository figure E5B). ILC2s express ID3 (inhibitor of DNA binding 3), a transcription factor that is involved in developmental processes of lymphocytes (26). ID3 is positively regulated by the MEK-ERK signaling pathway and induces steroid resistance (27). We examined ID3 expression by BAL ILC2s in the presence and absence of Dex. Dex significantly inhibited ID3 expression in BAL ILC2s (Figure 6D and online repository figure E5C).
Figure 6.
Effect of Dex on select signaling molecules in BAL ILC2s. BAL cells were cultured with vehicle (V) or Dex for 5 days and then the expression of MEK+ (A), pSTAT5+ (B), pSTAT3+ (C) and ID3+ (D) cells were analyzed on gate live lin-CRTH2+ cells by flow cytometry. Each symbol represents a single asthma patient (n=5–7).
Since MEK seems to play an important role in steroid resistance of ILC2s, we asked if its level was higher in BAL as compared to blood ILCs. For this purpose we studied BAL and blood ILCs from the same patients (n=4). A small fraction of BAL ILCs, studied ex vivo, expressed MEK (online repository figure E6 A& B). In contrast, blood ILCs expressed very little MEK. The difference in the MEK level between BAL and blood was statistically significant. Blood ILCs increased their MEK expression when cultured in medium alone. This increase was further augmented upon culture with IL2/TSLP (online repository figure E6C).
Inhibition of MEK and STAT signaling reverses steroid resistance
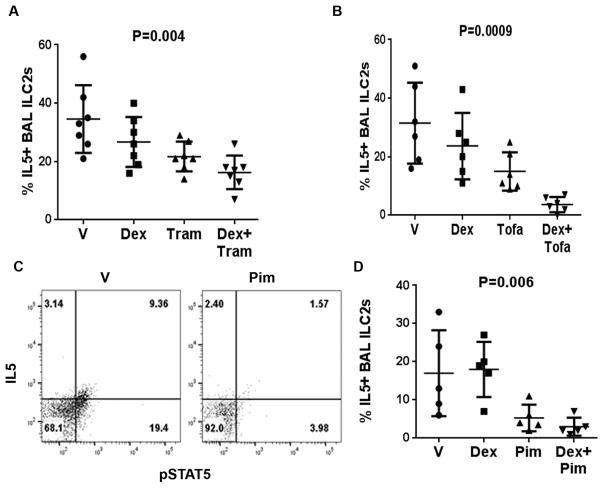
In order to establish the relevance of MEK and STAT5 signaling in steroid resistance we cultured BAL cells with Trametinib (28) and Tofacitinib (29), inhibitors of MEK and the JAK-STAT pathway, respectively. Both inhibitors reduced the frequency of IL5+ ILC2s (Figure 7A & B). More importantly they reversed the steroid resistance of BAL ILC2s. Since Tofacitinib inhibits all STATs downstream of Jak kinases, we examined the effect of the STAT5 inhibitor, Pimozide (30). Pimozide inhibited the frequency of pSTAT5+ cells as well as IL5+ ILC2s in BAL (Figure 7C & D). It also reversed the steroid resistance of BAL ILC2s. Both Tofacitinib and Pimozide were more effective than Trametinib in inhibiting IL5+ILC2s.
Figure 7.
Effect of MEK, Jak and STAT5 inhibitors on steroid resistance of BAL ILC2s. BAL cells were cultured for 3 days with vehicle, Dex, the MEK inhibitor Trametinib with and without Dex (A), the Jak3 inhibitor Tofacitinib with and without Dex (B) or the STAT5 inhibitor Pimozide with and without Dex (C&D). The effect of Dex and the inhibitors with and without Dex on BAL IL5+ ILC2s (A, B & D) is shown. The flow cytogram in C shows the effect of Pimozide on pSTAT5. Each symbol represents a single asthma patients (n=5–7). P values were calculated using the non-parametric Kruskal Wallis test.
Discussion
In this paper we identified a selective, stimulus-specific pathway in ILC2 conveying lack of response to Dex, a pathway that could be implicated in steroid-resistant asthma. We showed that IL33- and IL25- inducible IL5+ ILC2s in the blood were sensitive to inhibition by Dex. In contrast, IL7- and TSLP-inducible IL5+ ILC2s were relatively steroid resistant. We elucidated the mechanism of development of this steroid resistance. Dex increased the expression of IL7Rα, which is the common receptor for IL7 and TSLP. The increased expression of IL7Rα promoted heightened and sustained induction of pSTAT5 and MEK in ILC2s by TSLP. Sustained induction of pSTAT5 and MEK converted steroid sensitive ILC2s into steroid resistant cells. Pharmacological inhibition of MEK and STAT5 signaling abrogated steroid resistance of ILC2s.
Steroid resistance of mouse ILC2s was previously examined by Kabata et al (13). Similar to human ILC2s, mouse ILC2s became steroid resistant upon exposure to IL7 and TSLP. The authors demonstrated the development of steroid resistance in a mouse model of allergic asthma, which was reversed by an anti-TSLP antibody, and the STAT5 inhibitor Pimozide. The results established an important role for the TSLP-STAT5 axis in steroid resistance of ILC2 in a mouse model of asthma. There are differences in steroid resistance between mouse and human ILC2s. Mouse ILC2 became steroid resistant when cultured with IL2. In contrast, human ILC2s remained steroid sensitive in presence of IL2. We believe that the upregulation of IL7Rα was critical for development of steroid resistance in human ILC2s. This is supported by the finding that only IL7Rα agonists but no other ILC2-stimulating cytokines were capable of inducing steroid resistance. Although IL2 induces STAT5, this induction is of short duration and lower amplitude when compared to TSLP induction of STAT5 in Dex-treated cells. This also goes along with the finding that the induction of steroid resistance required prolonged incubation with TSLP.
The effect of dexamethasone on lymphocytes has previously studied by many labs. A microarray study showed that IL7Rα was one of the top upregulated genes by Dex in lymphocytes (31). Consequently, Dex actually increased, not reduced the survival of lymphocytes upon stimulation with IL7. In our study Dex increased the number of IL5+ ILC2s in some asthmatic patients (Figure 2B). Other labs have reported similar positive effects of Dex on lymphocytes. Dex increased the number of IL5+ lymphocytes in a mouse model of chronic asthma (32). Dex also increased the differentiation of human Th17 cells (33). These findings suggest that glucocorticoids exert both antagonistic and protagonistic effects on development and function of lymphocytes including innate lymphoid cells.
Steroids are the mainstay of anti-inflammatory therapy in asthma. Yet, a significant number of patients do not adequately respond to steroids (12). Steroid resistance in asthma is likely to occur at multiple cellular levels. Steroids induce inflammatory cell apoptosis, and inhibit their proliferation and cytokine production. A moderation of these anti-inflammatory effects has been reported for lymphocytes and monocytes obtained from severe asthmatic patients (reviewed in ref. 10). This is the first report on steroid resistance of ILC2s from a group of refractory asthmatic patients.
Many studies have delineated the mechanism of steroid resistance at the signaling and molecular levels. Steroid resistance of mononuclear cells can be induced in vitro with microbial superantigens in an ERK1/2-dependent manner (34). As mentioned previously, we reported induction of steroid resistance in T cells by MEK1, upstream activator of ERK1/2 (11). In addition to ERK1/2, the p38 MAPK promotes steroid resistance of cells by phosphorylating unliganded GR and sequestering it in the cytosol (35). Both ERK1/2 and p38 MAPK induce c-Fos. The inhibition of c-Fos by Dex in ILC2 suggests that the former is unlikely to be involved in steroid resistance of ILC2s. The ERK pathway induces ID3 in lymphocytes. ID3 confers steroid resistance through inhibition of the transcription factor SRG3 (27). We observed inhibition of ID3 by Dex in BAL ILC2s. Therefore, we do not believe that this pathway is important for steroid resistance of ILC2.
One of the mechanisms of transrepression of genes by GR is its interaction with SMRT (36). The latter forms a multimolecular complex with HDACs (37). GR utilizes SMRT to recruit HDAC to the target genes and causes deacetylation of the chromatin. As mentioned MEK translocates to the nucleus, interacts with SMRT and causes its nuclear export (18, 38). The fact that MEK inhibitor reversed steroid resistance, yet c-Fos and ID3 were not involved in steroid resistance raises two possibilities: 1). Another pathway downstream of ERK1/2 mediates steroid resistance; 2). MEK directly mediates steroid resistance through its interaction with SMRT. These possibilities need to be addressed in future studies. Direct interventions in HDAC function also induce steroid resistance. The phosphoinositide kinase δ (PI-3 kinase δ) inhibits histone deacetylase 2 (HDAC2), which leads to steroid resistance (39).
As mentioned previously, TSLP and IL7 signal through STAT3 and especially, through STAT5 (40). Both STAT3 and STAT5 have a complex relationship with GR. They can function as protagonists and antagonists at the transcriptional level depending upon the cell type and the culture milieu (41). The activation of GR inhibits expression of mRNA for STAT5, and Jak3, the upstream activator of STAT5 (42, 43). STAT5 binds GR in the cytosol and prevents its nuclear translocation (23). STAT5 knockdown reverses steroid resistance. In our study two clinically available drugs—the Jak-STAT inhibitor Tofacitinib and the STAT inhibitor Pimozide strongly inhibited BAL IL5+ ILC2s and reversed their steroid resistance. These findings suggest that STAT5 plays a critical role not only in induction of IL5+ ILC2s but also in development of steroid resistance.
Three epithelial cytokines-IL25, IL33 and TSLP are now considered to play an important role in inception of a type 2 immune response, allergic sensitivity and asthma (44). Of these three cytokines only TSLP, which is a Dex-regulated IL7Rα ligand, induced steroid resistance of ILC2s. Most asthmatic patients are steroid sensitive and are well-controlled with inhaled steroids. However, they transiently develop steroid resistance during an asthma exacerbation and require systemic administration of a much higher dose of a steroid. Viral infection, especially, rhinovirus infection is a frequent cause of asthma exacerbation (18). Our finding that the combination of IL13 and rhinovirus augment TSLP production by epithelial cells provides a mechanistic understanding of steroid resistance during asthma exacerbation. An anti-TSLP antibody has previously been shown to block allergen-induced bronchospasm in asthma (45). Our results provide a rationale for trial of an anti-TSLP antibody, Trametinib and especially, Tofacitinib and Pimozide in steroid resistance asthma.
Supplementary Material
Clinical Implications.
ILC2s become steroid resistant upon exposure to TSLP. TSLP correlates with the steroid resistance of BAL ILC2s. The ILC2-dependent steroid resistance of asthma may benefit from theinhibitors of MEK and STAT5.
Acknowledgments
Funding support: The work was supported by NIH grants RO1 AI091614, HL126895, AI102943 and HL126895.
Abbreviations
- BAL
bronchoalveolar lavage
- Dex
Dexamethasone
- ID3
inhibitor of DNA binding 3
- ILC2
innate lymphoid cell type 2
- MEK
MEK-ERK kinase
- PBMC
peripheral blood mononuclear cell
- STAT
signal transducer and activator of transcription
- TSLP
thymic stromal cell lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–74. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 2.Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69(9):1154–61. doi: 10.1111/all.12440. [DOI] [PubMed] [Google Scholar]
- 3.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134(3):671–678e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155(1):126–35. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136(3):792–794e3. doi: 10.1016/j.jaci.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–50. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Jr, Rollins DR, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015;136(1):59–68e14. doi: 10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O’Byrne PM, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137(1):75–86e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Sher ER, Leung DY, Surs W, Kam JC, Zieg G, Kamada AK, et al. Steroid-resistant asthma. Cellular mechanisms contributing to inadequate response to glucocorticoid therapy. J Clin Invest. 1994;93(1):33–9. doi: 10.1172/JCI116963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006;27(2):413–26. doi: 10.1183/09031936.06.00125404. [DOI] [PubMed] [Google Scholar]
- 11.Liang Q, Guo L, Gogate S, Karim Z, Hanifi A, Leung DY, et al. IL-2 and IL-4 stimulate MEK1 expression and contribute to T cell resistance against suppression by TGF-beta and IL-10 in asthma. J Immunol. 2010;185(10):5704–13. doi: 10.4049/jimmunol.1000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Center. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol. 2007;119(1):73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. doi: 10.1038/ncomms3675. [DOI] [PubMed] [Google Scholar]
- 14.Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, Bethel KJ, Scott DR, Khorram N, Miller M, Broide DH, Doherty TA. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155:126–35. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134(5):1175–1186e7. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu HW, Thaikoottathil J, Rino JG, Zhang G, Wu Q, Moss T, et al. Function and regulation of SPLUNC1 protein in Mycoplasma infection and allergic inflammation. J Immunol. 2007;179(6):3995–4002. doi: 10.4049/jimmunol.179.6.3995. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Tundwal K, Liang Q, Goplen Q, Rozario S, Quayum N, et al. Establishment of ERK1/2 bistability and sustained activation through sprouty 2 and its relevance for epithelial function. Mol Cell Biol. 2010;30(7):1783–99. doi: 10.1128/MCB.01003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125(5):1001–1006e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192(5):659–70. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo L, Chen C, Liang Q, Karim MZ, Gorska MM, Alam R. Nuclear translocation of MEK1 triggers a complex T cell response through the corepressor silencing mediator of retinoid and thyroid hormone receptor. J Immunol. 2013;190(1):159–67. doi: 10.4049/jimmunol.1201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones CL, Gearheart CM, Fosmire S, Delgado-Martin C, Evensen NA, Bride K, et al. MAPK signaling cascades mediate distinct glucocorticoid resistance mechanisms in pediatric leukemia. Blood. 2015;126(19):2202–12. doi: 10.1182/blood-2015-04-639138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adcock IM, Lane SJ, Brown CR, Lee TH, Barnes PJ. Abnormal glucocorticoid receptor-activator protein 1 interaction in steroid-resistant asthma. J Exp Med. 1995;182(6):1951–8. doi: 10.1084/jem.182.6.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goleva E, Kisich KO, Leung DY. A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J Immunol. 2002;169(10):5934–40. doi: 10.4049/jimmunol.169.10.5934. [DOI] [PubMed] [Google Scholar]
- 24.Tan AH, Lam KP. Pharmacologic inhibition of MEK-ERK signaling enhances Th17 differentiation. J Immunol. 2010;184(4):1849–57. doi: 10.4049/jimmunol.0901509. [DOI] [PubMed] [Google Scholar]
- 25.Shan L, Redhu NS, Saleh A, Halayko AJ, Chakir J, Gounni AS. Thymic stromal lymphopoietin receptor-mediated IL-6 and CC/CXC chemokines expression in human airway smooth muscle cells: role of MAPKs (ERK1/2, p38, and JNK) and STAT3 pathways. J Immunol. 2010;184(12):7134–43. doi: 10.4049/jimmunol.0902515. [DOI] [PubMed] [Google Scholar]
- 26.Verykokakis M, Krishnamoorthy V, Iavarone A, Lasorella A, Sigvardsson M, Kee BL. Essential functions for ID proteins at multiple checkpoints in invariant NKT cell development. J Immunol. 2013;191(12):5973–83. doi: 10.4049/jimmunol.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko M, Ahn J, Lee C, Chung H, Jeon SH, Chung HY, et al. E2A/HEB and Id3proteins control the sensitivity to glucocorticoid-induced apoptosis in thymocytes by regulating the SRG3 expression. J Biol Chem. 2004;279(21):21916–23. doi: 10.1074/jbc.M402145200. [DOI] [PubMed] [Google Scholar]
- 28.Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, DeMarini DJ, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):782–9. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302(5646):875–8. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 30.Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117(12):3421–9. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franchimont D, Galon J, Vacchio MS, Fan S, Visconti R, Frucht DM, et al. Positive effects of glucocorticoids on T cell function by up-regulation of IL-7 receptor alpha. J Immunol. 2002;168(5):2212–8. doi: 10.4049/jimmunol.168.5.2212. [DOI] [PubMed] [Google Scholar]
- 32.Wiley RE, Cwiartka M, Alvarez D, Mackenzie DC, Johnson JR, Goncharova S, et al. Transient corticosteroid treatment permanently amplifies the Th2 response in a murine model of asthma. J Immunol. 2004;172(8):4995–5005. doi: 10.4049/jimmunol.172.8.4995. [DOI] [PubMed] [Google Scholar]
- 33.Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013;132(2):297–304e3. doi: 10.1016/j.jaci.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 34.Li LB, Goleva E, Hall CF, Ou LS, Leung DY. Superantigen-induced costicosteroid resistance of human T cell occurs through activation of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK-ERK) pathway. J Allergy Clin Immunol. 2004;114(5):1059–69. doi: 10.1016/j.jaci.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109(4):649–57. doi: 10.1067/mai.2002.122465. [DOI] [PubMed] [Google Scholar]
- 36.Emont MP, Mantis S, Kahn JH, Landeche M, Han X, Sargis RM, et al. Silencing Mediator of Retinoid and Thyroid Hormone Receptors (SMRT) regulates glucocorticoid action in adipocytes. Mol Cell Endocrinol. 2015;407:52–6. doi: 10.1016/j.mce.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hua G, Ganti KP, Chambon P. Glucocorticoid-induced tethered transrepression requires SUMOylation of GR and formation of a SUMO-SMRT/NCoR1-HDAC3 repressing complex. Proc Natl Acad Sci U S A. 2016;113(5):E635–43. doi: 10.1073/pnas.1522826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuderland D, Konson A, Seger R. Identification and characterization of a general nuclear translocation signal in signaling proteins. Mol Cell. 2008;31(6):850–61. doi: 10.1016/j.molcel.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 39.To Y, Ito K, Kizawa Y, Failla M, Ito M, Kusama T, et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(7):897–904. doi: 10.1164/rccm.200906-0937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong J, Kim MS, Chaerkady R, Wu X, Huang TC, Getnet D, et al. TSLP signaling network revealed by SILAC-based phosphoproteomics. Mol Cell Proteomics. 2012;11(6):M112.017764. doi: 10.1074/mcp.M112.017764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertucci PY, Quaglino A, Pozzi AG, Kordon EC, Pecci A. Glucocorticoid-induced impairment of mammary gland involution is associated with STAT5 and STAT3 signaling modulation. Endocrinology. 2010;151(12):5730–40. doi: 10.1210/en.2010-0517. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Li WP, Meng C, Ivashkiv LB. Inhibition of IFN-gamma signaling by glucocorticoids. J Immunol. 2003;170(9):4833–9. doi: 10.4049/jimmunol.170.9.4833. [DOI] [PubMed] [Google Scholar]
- 43.Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc Natl Acad Sci U S A. 2000;97(17):9573–8. doi: 10.1073/pnas.160099797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012 May 4;18(5):684–92. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 45.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.