Abstract
The incidence and severity of acute kidney injury (AKI) is rising globally, and the associated morbidity and mortality remain high despite promising advances in experimental therapeutics. The reasons include (a) an incomplete understanding of the complex pathophysiology, (b) an inability to reliably identify risk factors for AKI and (c) a lack of biomarkers for the early prediction of AKI and its outcomes. Functional genomics, bioinformatics and proteomics have begun to uncover candidates that are emerging as biomarkers and therapeutic targets. This review will update the reader on current technologies in genomics (including targeted sequencing, genome wide association studies and transcriptome profiling) and proteomics (including gel electrophoresis and mass spectrometry methods) and their application on human AKI.
Keywords: Acute kidney injury, Genomics, Proteomics, Biomarkers, Pathophysiology
Introduction
Acute kidney injury (AKI) is an increasingly frequent cause of morbidity and mortality, developing in about 5% of all hospitalized patients and in more than 25% of critically ill patients. It is now evident that AKI also results in subsequent development of chronic kidney disease. Experimental studies have uncovered biologic pathways and mechanisms that have evolved into promising novel therapeutic approaches for the prevention and treatment of AKI in animal models. However, translational efforts in human AKI have yielded disappointing results. Progress has been limited by (a) a still incomplete understanding of the multifaceted and interdependent mechanisms, (b) an inability to reliably identify clinical and biologic risk factors for human AKI and (c) a lack of reliable biomarkers for the early prediction of AKI and its outcomes. Fortunately, innovative technologies such as functional genomics, bioinformatics and proteomics have begun to uncover candidates that are emerging as new players in the mechanistic pathways that can be targeted for therapies. Genes and proteins that confer an increased risk for AKI are being discovered and evaluated. Newly identified proteins that participate in the early stress response of the injured kidney have also proven to represent predictive biomarkers that can be non-invasively measured. This review will briefly update the reader on current technologies in genomics and proteomics and their potential application on clinical AKI.
Genomics
The tools of modern functional genomics allow for the identification of genetic variations and changes in gene expression with unprecedented speed and accuracy. Unbiased genome-wide methodologies have already contributed to our understanding of AKI and identified novel biomarkers for the early prediction of AKI. The most commonly employed genomic techniques are listed in table 1, and briefly detailed below.
Table 1.
Genomic and proteomic methods to characterize AKI
| Method | Advantages | Disadvantages | Role in AKI |
|---|---|---|---|
| Targeted sequencing | Candidate gene approach Hypothesis-based Standard DNA sequencing |
Biased approach Low throughput |
Not yet clinically applicable to AKI |
| GWAS | Unbiased approach Especially applicable to complex diseases |
False-positives High cost Complex analysis |
Not yet clinically applicable to AKI |
| Transcriptome profiling | Unbiased approach Affordable Data in public repositories |
Identifies changes in gene expression and not genetic risk | AKI biomarkers (e.g., NGAL) identified |
| Gel electrophoresis | Established, inexpensive Suited for low molecular weight proteins |
Labor intensive Protein identification often not successful |
AKI biomarkers (e.g., fetuin-A, α-1-microglobulin, NGAL) identified |
| MALDI-TOF | Established, reproducible Suited for low molecular weight proteins |
Labor intensive Protein identification often not successful |
Not yet clinically applicable to AKI |
| SELDI-TOF | Rapid, high throughput Suited for middle molecular weight proteins |
Not reproducible Protein identification often not successful |
AKI biomarkers (e.g., NGAL, α-1-microglobulin, hepcidin-25) identified |
| CE-MS | High sensitivity and efficiency with minimal sample volumes | Expensive Peptide-based validation difficult |
AKI biomarkers (albumin, β-2-microglobulin, α-1- antitrypsin) identified |
| Differential labeling | Sensitive Choice of several labeling methods |
Expensive Dependent on labeling efficiency |
Chitinase 3-like and NGAL identified as biomarkers |
Targeted Sequencing
Several decades of experimental AKI studies have identified critical epithelial, vascular and inflammatory components, and the molecular mechanisms that underlie these responses. Variations in the genes responsible for these molecular cascades may play a role in determining the risk of AKI. Hypothesis-driven targeted sequencing employs a candidate gene approach, in which the investigator seeks polymorphisms in those genes whose protein products are known to be associated with AKI. Samples (most commonly blood) from AKI populations are compared to control non-AKI populations, using standard DNA Sanger sequencing.
A recent systematic review examined the published literature for results of targeted sequencing in subjects with AKI [1]. The 16 included studies investigated 35 single nucleotide polymorphisms (SNPs) for associations with AKI in 21 genes. Only one polymorphism, Apolipoprotein E (APO E) e2/e3/e4, was found to associate with AKI incidence in more than one study. Apo E is known to mediate inflammatory responses and regulate nitric oxide release from macrophages, suggesting a role for APO E polymorphisms to render an increased risk for AKI. However, the positive association found in 2 studies was contradicted by larger studies, and the evidence to date is inadequate to implicate APO E polymorphisms in AKI. Eight other polymorphisms in biologically plausible genes were associated with AKI in a single study. These include genes involved in oxidative stress (NADPH oxidase and catalase), vasomotor regulation (ACE), inflammation (TNF-α, IL-10, IL-6) and cytoprotection (HSP72, haptoglobin). However, polymorphisms in most of these genes have also been linked to many other disease states. In conclusion, current targeted sequencing studies have not provided definitive evidence linking a specific genetic variation with increased AKI risk.
Genome-Wide Association Studies
Genome wide association studies (GWAS) employ an unbiased approach to identify candidate SNPs in all the genes that may be associated with a given disease state. DNA obtained from blood or buccal smears from patients and control subjects is scanned using commercially available array chips that now routinely contain well over 1 million SNPs. The major advantages of GWAS are the abilities to identify candidate SNPs in a hypothesis-independent manner and to detect gene–gene interactions. These characteristics are especially pertinent to complex disease states such as AKI, where interactions between multiple SNPs may be required to confer increased risk. Pertinent negatives of GWAS include the high cost, the high potential for false-positive results and the need for advanced statistical and bioinformatics analyses.
GWAS have been successfully harnessed to explore genetic polymorphisms associated with high blood pressure [2]. This study examined over 2.5 million SNPs in 200,000 individuals of European descent. Twenty-nine independent SNPs at 28 loci were significantly associated with high blood pressure, and 16 of these were novel gene loci. Six of these loci contain genes that were previously known to regulate blood pressure, lending plausibility to this approach. A genetic risk score based on the 29 genome-wide significant variants was associated with hypertension, left ventricular wall thickness, stroke and coronary artery disease. These findings provide new insights into the genes and proteins that regulate blood pressure, and they suggest potential novel therapeutic pathways to target for cardiovascular disease prevention. In another recent study, a GWAS meta-analysis among 63,558 participants of European descent with serial kidney function measurements, followed by independent replication, identified SNPs in genes encoding uromodulin, GALNT11 and cadherin-related 23 to be strongly associated with the decline of chronic kidney function [3]. A small GWAS study of AKI has been completed [4]. In this study of 158 patients with blunt trauma and no previous evidence for kidney disease, 33 developed AKI. No differences in genomic sequences were detected between the AKI and non-AKI groups. Much larger studies are required to reliably identify genes that confer AKI risk, which are currently in progress.
Transcriptome Profiling
Complementary DNA microarray methods have been extensively and successfully employed in animal and human models of AKI to uncover pathophysiologic pathways, novel biomarkers and potential therapies. In this unbiased approach, mRNA from normal and diseased tissues or cells is extracted, and the complementary DNA strands are synthesized and conjugated to fluorescent probes. Following hybridization to commercially available microarray chips, the slides are scanned and analyzed for differential gene expression. The results are typically deposited in public repositories, including in the Gene Expression Omnibus, which provides easily search-able information for data mining (http://www.ncbi.nih.gov/geo). A recent data mining study obtained gene expression profiles from 150 distinct microarray experiments from 21 different models of AKI (including mouse, rat, pig and human models) [5]. A meta-analysis using expression-based GWAS identified 46 upregulated and 1 downregulated genes, of which 26 (55%) were previously well known to be associated with AKI, including LCN2, CCL2, HMOX1, clusterin and ANXA1, which validated the approach (table 2). Genes with as yet unclear roles in AKI that were identified include ANXA2, CLDN4 and TYROBP, all of which are important candidates for future mechanistic and therapeutic AKI studies based on their known biological functions.
Table 2.
Most upregulated genes in AKI determined by meta-analysis of transcriptome profiling data
| Symbol | Gene name | Biological role | Role in AKI |
|---|---|---|---|
| AXNA1 | Annexin A1 | Neutrophil anti-migratory | Anti-inflammatory, protective in AKI |
| LCN2 | Lipocalin 2 (NGAL) | Iron chelation, anti-apoptotic, anti-bacterial | Anti-apoptotic, protective in AKI, biomarker of AKI |
| CCL2/MCP-1 | Chemokine (C-C motif) ligand 2 | Regulates macrophage migration and infiltration | Pro-inflammatory, mediates/worsens AKI |
| HMOX1 | Heme oxygenase (decycling) 1 | Anti-oxidant, regulates immune cell trafficking | Anti-inflammatory, protective in AKI |
| CLU | Clusterin | Cytoprotection, clearance of cellular debris | Epithelial proliferation, protective in AKI |
| ARPC1B | Actin-related protein complex subunit 1B | Controls actin polymerization, regulates cell mitosis | Possible role in tubule cell repair after AKI |
| EGR2 | Early growth response 2 | Transcription factor, immunomodulation | Possible role in tubule cell repair after AKI |
| CDKN1A/p21 | Cyclin-dependent kinase inhibitor 1A | Cell cycle regulation, growth arrest | Limits tubule cell death, protective in AKI |
| ATF3 | Activating transcription factor 3 | Regulates cell proliferation and cell stress response | Inhibits inflammatory gene transcription, protective |
| CXCL3 | Chemokine (C-X-C) ligand 3 | Controls migration of macrophages and neutrophils | Pro-inflammatory, mediates/worsens AKI |
| CLDN4 | Claudin 4 | Maintains epithelial barrier, regulates tight junctions | Unknown role in AKI |
| RASD1 | RAS, dexamethasone-induced 1 | Steroid-induced changes in cell growth and morphology | Unknown role in AKI |
| ADAMTS1 | ADAM metallopeptidase with TS1 | Cleaves capillary basement membrane, anti-angiogenic | Peritubular capillary loss, impaired recovery from AKI |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | Inhibits ECM degradation, promotes cell proliferation | Unknown role in AKI |
| ANXA2 | Annexin A2 | Diverse calcium-dependent cellular processes | Unknown role in AKI |
| TYROBP | TYRO tyrosine kinase binding protein | Activation of neutrophils and macrophages | Unknown role in AKI |
Proteomics
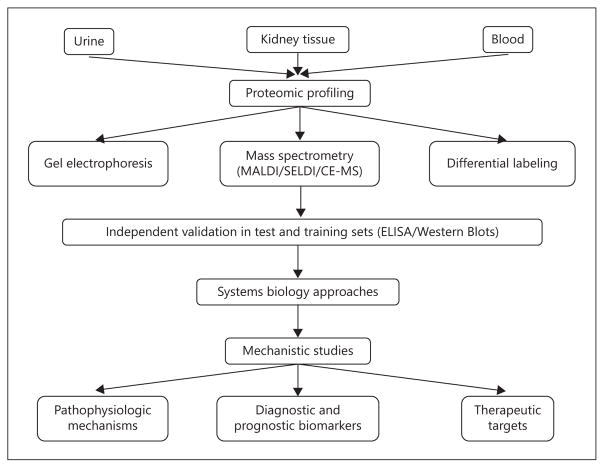
We are in the post-genomic era, and recognize that the 23,000 identified human genes encode for more than 125,000 human proteins that change rapidly and specifically with pathophysiologic changes in the individual. The science of proteomics can identify and quantify these changes, and is therefore indispensable to predictive and personalized medicine. A typical AKI proteomics work-flow is illustrated in figure 1. Urine is most commonly the starting material utilized to study human kidney diseases by proteomic approaches. Urine is a direct window to the kidney, which is non-invasive and easily available. The normal urine proteins are in low abundance and well described, with approximately 75% of urinary proteins originating from the kidney. Most urinary biomarkers are stable, protease resistant and do not degrade even after prolonged storage at −80°C. However, urinary proteomics do present some challenges. First, urine is a dilute biofluid that requires concentration. Different methods of concentrating the urine (e.g., precipitation, size fractionation, ultracentrifugation and lyophilization) will yield distinct proteomes that make comparisons difficult. Second, the composition of urine can be influenced by non-renal factors (including age, gender, time of collection and dilution). Correcting for urinary dilution (for example, using urine creatinine) is often recommended, but is fraught with inaccuracies in the setting of AKI. Third, depletion of high abundance proteins, such as albumin, is often required to highlight relevant low abundance urinary proteins. However, this process also removes albumin-bound proteins that are of potential importance to AKI. Standardized protocols for urine collection, processing, concentration and storage are still works in progress (Human Kidney and Urine Proteome Project, www.hkupp.org). Table 1 lists the most common proteomic methods utilized to study AKI, which are briefly detailed below.
Fig. 1.
AKI proteomics workflow.
Gel Electrophoresis
The separation of proteins based on mass and charge using gel electrophoresis has been the mainstay of proteomics for several decades. The version that is most commonly in current use (2-dimensional fluorescent differential in gel electrophoresis [2-D DIGE]) involves extraction of proteins from samples obtained from individuals with the disease of interest, as well as from normal controls and disease controls. The protein extracts are differentially labeled with fluorescent dyes, separated by 2-D gel electrophoresis and images obtained for each fluoroprobe. Following image analysis, spots containing differentially expressed proteins are picked and the proteins identified using mass spectrometry. This technique is especially well suited for low molecular weight proteins, but remains time- and labor-intensive, and subsequent identification and validation is only moderately successful.
Zhou et al. [6] employed 2-D DIGE to examine urinary exosomes in an animal model of nephrotoxic AKI due to cisplatin. While they initially uncovered 74 peptide spots that showed differential expression, only 28 were identified, and only 2 of these were confirmed by Western blotting (fetuin-A and annexin V). While fetuin-A was shown to represent a potential biomarker of AKI in a limited human study, the relatively low rate with which differentially expressed proteins could be identified and confirmed exemplifies the limitations of the 2-D DIGE methodology. Similarly, Holly et al. [7] used 2-D DIGE in a rat model of septic AKI to identify 97 differentially expressed spots but could identify only 30. The few peptides that were identified to be upregulated included previously known players in AKI, such as albumin, aminopeptidase and neutrophil gelatinase-associated lipocalin (NGAL). A recent human study by Aregger et al. [8] compared 12 patients who had early recovery from AKI and 12 matching patients with late/non-recovery. The urinary proteomes were analyzed by 2-D DIGE, and 8 prognostic candidates were identified including α-1-microglobulin, α-1-antitrypsin, apolipoprotein D, calreticulin, cathepsin D, CD59, insulin-like growth factor-binding protein 7 (IGFBP-7) and NGAL, all of which were previously known to be associated with AKI. Subsequent quantification by ELISA in an independent verification group validated IGFBP-7 and NGAL as discriminators between early and late/non-recovery patients and patients with and without AKI. Significant upregulation of the urinary markers predicted mortality (IGFBP-7: AUC 0.68; NGAL: AUC 0.81), recovery (IGFBP-7: AUC 0.74; NGAL: AUC 0.70) and severity of AKI (IGFBP-7: AUC 0.77; NGAL: AUC 0.69).
Mass Spectrometry
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and the related surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) are based on the principle of proteins immobilized on a matrix and subsequently excited by a laser and differentially detected based on protein mass and charge. While the SELDI-TOF methods are high throughput and relatively inexpensive, they are best suited for middle molecular weight proteins and have suffered from difficulties with reproducibility and downstream protein identification. In comparison, MALDI-TOF is well suited for low molecular weight proteins and has better reproducibility, but tends to be more laborious.
Nguyen et al. [9] employed SELDI-TOF-MS to identify protein peaks in the urine of children with cardiac surgery-associated AKI. All peaks were subsequently identified as albumin fragments, α-1-acid glycoprotein and 2 lipocalins (α-1-microglobulin and NGAL), and all have now been validated as early biomarkers of AKI [10, 11]. Ho et al. [12] demonstrated a number of upregulated peaks in patients with AKI using SELDI-TOF-MS, including the presence of known AKI proteins such as β-2-microglobulin, α-1-microglobulin and NGAL. In addition, a unique protein peak was detected in non-AKI patients, which was subsequently identified as hepcidin-25, an iron-chelating renoprotective molecule that has a strong negative predictive value for ruling out AKI.
Capillary electrophoresis mass spectrometry (CE-MS) combines high efficiency separation of proteins (usually by electrospray ionization or by MALDI) with MS to achieve differential peptide identification with high sensitivity in minimal sample volumes. Limitations include the need for sophisticated equipment and technical expertise and difficulties with obtaining antibodies directed at peptide epitopes for downstream validation. Metzger et al. [13] utilized CE-MS to identify 20 peptides significantly associated with human AKI, representing the degradation products of 6 proteins. Peptides of albumin, α-1-antitrypsin and β-2-microglobulin were upregulated (as previously known) while, additionally, fragments of fibrinogen α and collagens 1 α(I) and 1 α(III) were downregulated in AKI. In a validation set of 20 patients with AKI, the identified peptide marker pattern was found to be of superior prognostic value when compared to previously established markers.
Differential Labeling
Techniques for differentially labeling proteins, including isotope coded affinity tags and isobaric tags for relative and absolute quantitation, followed by MS, are sensitive methods for identifying differentially expressed proteins or peptides. Limitations include the need for sophisticated equipment and the extent to which the label used is incorporated. Maddens et al. [14] have used this method to identify 8 candidate biomarkers of AKI in a rat-septic AKI model, including NGAL and chitinase 3-like protein, which were also found to be elevated in a small study of humans with septic AKI.
Conclusions and Future Directions
Genomic and proteomic technologies have already identified a number of candidates that play important roles in AKI, which are presented in tables 2 and 3, respectively. Large-scale validation and commercial launching of some of the identified biomarkers is well under way [15], as is the experimental testing of some of the identified therapeutic targets [16]. First, in the near future, it is likely that ongoing GWAS will provide us with a genetic profile that will accurately identify individuals at risk for developing AKI. Powerful techniques such as next-generation sequencing can then rapidly and accurately screen a subject’s entire genome for AKI risk at very affordable (and constantly diminishing) costs. Second, concomitant advances in proteomics will likely continue to identify biomarker panels to accurately predict the development of AKI and its adverse outcomes. Finally, the tools of bioinformatics and systems biology will incorporate the identified genes and proteins into pathways and algorithms to target in therapeutic trials. It is hoped that such advances will dramatically alter our current dismal approach of retrospective diagnosis and merely supportive care of AKI to one that is personalized, predictive and effective.
Table 3.
Most commonly reported upregulated proteins in AKI determined by proteomic profiling studies
| Protein | Source and biological role | Role in AKI |
|---|---|---|
| NGAL | Distal nephron, iron chelator, normally present in low concentrations | Rapidly induced by tubular injury, limits kidney injury, early biomarker of AKI |
| Albumin | Liver, circulating protein, normally not filtered by the glomerulus | Reflects altered glomerular permeability or impaired tubular reabsorption |
| α-1-microglobulin | Liver, unknown function, normally freely filtered and fully reabsorbed by the kidney | Reflects impaired tubular reabsorption due to acute injury, early biomarker of AKI |
| IGFBP-7 | Proximal tubule, cell cycle arrest protein, normally present in low concentrations | Reflects impaired tubular reabsorption due to acute injury, early biomarker of AKI |
| β-2-microglobulin | MCH class 1 molecules, especially on WBCs, normally filtered and reabsorbed | Reflects impaired tubular reabsorption due to acute injury, possible early biomarker of AKI |
| α-1-antitrypsin | Liver, protease inhibitor, acute phase reactant, normally filtered and reabsorbed | Reflects impaired tubular reabsorption due to acute injury, possible early biomarker of AKI |
Footnotes
Contribution from the AKI & CRRT 2015 Symposium at the 20th International Conference on Advances in Critical Care Nephrology, Manchester Grand Hyatt, San Diego, Calif., USA, February 17–20, 2015.
Disclosure Statement
The author has no conflicts of interest to declare.
References
- 1.Lu JC, Coca SG, Patel UD, Cantley L, Parikh CR. Translational Research Investigating Bio-markers and Endpoints for Acute Kidney Injury (TRIBE-AKI) Consortium: Searching for genes that matter in acute kidney injury: a systematic review. Clin J Am Soc Nephrol. 2009;4:1020–1031. doi: 10.2215/CJN.05411008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Consortium for Blood Pressure Genome-Wide Association Studies. Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorski M, Tin A, Garnaas M, McMahon GM, et al. Genome-wide association study of kidney function decline in individuals of European descent. Kidney Int. 2015;87:1017–1029. doi: 10.1038/ki.2014.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bihorac A, Delano MJ, Schold JD, Lopez MC, Nathens AB, Maier RV, Layon AJ, Baker HV, Moldawer LL. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg. 2010;252:158–165. doi: 10.1097/SLA.0b013e3181deb6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigoryev DN, Cheranova DI, Heruth DP, Huang P, Zhang LQ, Rabb H, Ye SQ. Meta-analysis of molecular response of kidney to ischemia reperfusion injury for the identification of new candidate genes. BMC Nephrol. 2013;14:231. doi: 10.1186/1471-2369-14-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou H, Pisitkun T, Aponte A, Yuen PS, Hoffert JD, Yasuda H, Hu X, Chawla L, Shen RF, Knepper MA, Star RA. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006;70:1847–1857. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holly MK, Dear JW, Hu X, Schechter AN, Gladwin MT, Hewitt SM, Yuen PS, Star RA. Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int. 2006;70:496–506. doi: 10.1038/sj.ki.5001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aregger F, Uehlinger DE, Witowski J, Brunisholz RA, Hunziker P, Frey FJ, Jörres A. Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney Int. 2014;85:909–919. doi: 10.1038/ki.2013.363. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen MT, Ross GF, Dent CL, Devarajan P. Early prediction of acute renal injury using urinary proteomics. Am J Nephrol. 2005;25:318–326. doi: 10.1159/000086476. [DOI] [PubMed] [Google Scholar]
- 10.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 11.Devarajan P, Krawczeski CD, Nguyen MT, Kathman T, Wang Z, Parikh CR. Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. Am J Kidney Dis. 2010;56:632–642. doi: 10.1053/j.ajkd.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho J, Lucy M, Krokhin O, Hayglass K, Pascoe E, Darroch G, Rush D, Nickerson P, Rigatto C, Reslerova M. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am J Kidney Dis. 2009;53:584–595. doi: 10.1053/j.ajkd.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Metzger J, Kirsch T, Schiffer E, Ulger P, Mentes E, Brand K, Weissinger EM, Haubitz M, Mischak H, Herget-Rosenthal S. Urinary excretion of twenty peptides forms an early and accurate diagnostic pattern of acute kidney injury. Kidney Int. 2010;78:1252–1262. doi: 10.1038/ki.2010.322. [DOI] [PubMed] [Google Scholar]
- 14.Maddens B, Ghesquière B, Vanholder R, Demon D, Vanmassenhove J, Gevaert K, Meyer E. Chitinase-like proteins are candidate biomarkers for sepsis-induced acute kidney injury. Mol Cell Proteomics. 2012;11:M111.013094. doi: 10.1074/mcp.M111.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014;51(pt 3):335–351. doi: 10.1177/0004563214521795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124:2355–2363. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]