Abstract
Objective
Autophagy is a cellular homeostasis mechanism that facilitates normal cell function and survival. Objectives of this study were to determine associations between autophagic responses with meniscus injury, joint aging, and osteoarthritis (OA), and to establish the temporal relationship with structural changes in menisci and cartilage.
Methods
Constitutive activation of autophagy during aging was measured in GFP-LC3 transgenic reporter mice between 6 and 30 months. Menisus injury was created by surgically destabilizing the medial meniscus (DMM) to induce posttraumatic OA in C57BL/6J mice. Levels of autophagy proteins and activation were analyzed by confocal microscopy and immunohistochemistry. Associated histopathological changes, such as cellularity, matrix staining, and structural damage, were graded in the meniscus and compared to changes in articular cartilage.
Results
In C57BL/6J mice, basal autophagy was lower in the meniscus than in articular cartilage. With increasing age, expression of the autophagy proteins ATG5 and LC3 was signficantly reduced by 24 months. Age-related changes included abnormal Safranin-O staining and reduced cellularity, which preceded structural damage in the meniscus and articular cartilage. In mice with DMM, autophagy was induced in the meniscus while it was suppressed in cartilage. Articular cartilage exhibited the most profound changes in autophagy and structure that preceded meniscus degeneration. Systemic administration of rapamycin to mice with DMM induced autophagy activation in cartilage and reduced degenerative changes in both meniscus and cartilage.
Conclusion
Autophagy is significantly affected in the meniscus during aging and injury and precedes structural damage. Maintenance of autophagic activity appears critical for meniscus and cartilage integrity.
Keywords: Autophagy, Meniscus, Cartilage, Aging, Osteoarthritis
Introduction
Knee meniscus injuries and degeneration are closely linked to the initiation and progression of osteoarthritis (OA), the most prevalent joint disease1. OA is characterized by degradation and loss of articular cartilage; menisci are often implicated in OA pathogenesis. More than 75% of patients with OA concomitantly suffer from meniscal lesions; 50% of patients with meniscal injury develop posttraumatic OA within 10 to 20 years2,3. While the exact pathogenic processes of meniscus degeneration are unclear, injury or damage to the meniscus disrupts the biomechanical properties and is associated with cell death, abnormal cell activation, and differentiation4. Injury also disrupts tissue homeostasis, which leads to progressive extracellular matrix degeneration (ECM) that ultimately manifests as OA of the whole joint 5.
Autophagy is a critical cellular homeostasis mechanism that supports normal cellular function and survival under stress-induced conditions such as nutrient deprivation and hypoxia6 and injury7. This catabolic process regulates energy and nutrients through the removal of damaged or dysfunctional proteins and organelles. Aggregate-prone proteins and organelles accumulate with deficient autophagy, resulting in cell death5,8. Aging increases susceptibility to defects in autophagy, which are implicated in many pathogenic processes, including cancer9 and neurodegeneration10 In OA, autophagy defects in articular cartilage have been linked to cartilage degeneration11,12.
In articular cartilage tissue, resident chondrocyte population cannot be replenished via vasculature. Autophagy is thus essential for maintaining cell and tissue homeostasis. We previously reported low levels of basal autophagy activation in the articular cartilage of normal young, skeletally mature mice under physiological conditions12. Articular cartilage underwent age-dependent reduction in expression of autophagy proteins, ATG-5 and LC3, in both human and mice11–13. These changes were accompanied by reduced cellularity and increased apoptotic cell death along with ECM degradation and OA development. When mice subjected to DMM were treated with rapamycin, a pharmacological activator of autophagy, chondrocyte cellularity was preserved and severe damage and degeneration was prevented in the articular cartilage14. Together, these findings suggest that articular chondrocyte autophagy plays an important role in joint aging and OA development. Autophagy may also play a role in the relationship between meniscal injury and degeneration, cartilage damage, and posttraumatic OA. However, very little is known about the role of autophagy in meniscus in health, injury, and degeneration.
This study was conducted as an extension of previous studies that examined autophagy in articular cartilage during aging and OA12 to specifically analyze autophagy in meniscus. The objectives were to determine the changes in autophagy in mouse meniscus (1) during normal aging, (2) following meniscus injury, and (3) as a consequence of treatment with rapamycin, a pharmacological activator of autophagy, after meniscus injury.
Materials and methods
Mice and tissue collection
All animal experiments were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute (TSRI). Reporter mice that ubiquitously express green fluorescence protein (GFP) fused to LC3 with a C57BL/6J genetic background (GFP-LC3 transgenic mice) were obtained from the RIKEN BioResource Center 15. Pathogen-free C57BL/6J mice were purchased from the breeding facility at TSRI. Mice were housed in a temperature-controlled environment with 12-hour light/dark cycles and received food and water ad libitum. Mice were euthanized at the various ages and knee joints collected for analysis. Both male and female mice were included in this study. At least 5 mice were analyzed per each age group (6, 18, 24, 30 months).
Surgically induced meniscus injury in mice
C57BL/6J mice at 2 months old were subjected to surgical transection of the medial meniscotibial ligament and medial collateral ligament in the right knee, as previously described16. The left knee, not subjected to surgery, was used as a control. The mice were euthanized at 10 weeks post-surgery and knee joints were harvested for analysis.
Rapamycin treatment
Rapamycin was obtained from LC Laboratories (Woburn, Massachusetts, USA) and was dissolved in dimethyl sulfoxide (DMSO) at 25 mg/ml and stored at −20 °C. For injection, the stock solution was diluted in PBS at 1 mg/kg bodyweight/dose in a total injection volume of 0.3 ml. Mice with DMM surgery received daily intraperitoneal injections, starting 1 day post-surgery for 10 weeks; control mice received the DMSO vehicle at 0.4% in a total injection volume of 0.3 ml14.
Histological analysis of knee joints
A detailed protocol for tissue collection has been previously described12. Briefly, knee joints were fixed with zinc buffered formalin (Z-Fix; Anatech, Battle Creek, MI) and decalcified in a Shandon TBD-2 decalcifier for 12 hours. Knee joints were washed and stored in PBS at 4°C, and cut into ~70 μm sections using a Leica VT 1000 S Vibratome. Knee joints were also embedded in paraffin for histology and immunochemistry.
Knee joint serial sections (4 μm thick) were stained with Hematoxylin to evaluate cellularity. Images were taken under 40X magnification and the total number of cells per 100 μm2 in each section was counted. Sections were stained with Safranin O-fast green for histological analysis using a semiquantitative grading system for meniscus degeneration17. Briefly, 3 parameters, tissue structure, cellularity, and Safranin-O staining were scored to determine the degenerative states of the meniscus as follows: 0 = absent, 1 = slight (grade 1), 2 = moderate (grade 2), or 3 = severe (grade 3).
Immunohistochemistry
Knee joint serial sections (4-μm thick) were first deparaffinized in Pro-Par Clearant (Anatech) and rehydrated in a series of graded ethanol and water. Sections were blocked with 5% serum for 30 minutes at room temperature (RT) and incubated with ATG-5 antibody (1:500 dilution, Novus Biologicals. Littleton CO. NB110-53818), LC3-antibody (1:600 dilution); MBL International (Woburn, MA) PM036), and poly(ADP-ribose) polymerase (PARP) p85 (1:60 dilution, G7341, Promega, Madison, WI) overnight at 4°C. After washing with PBS, sections were incubated with biotinylated goat anti-rabbit secondary antibody for 30 minutes at RT and then with Vectastain ABC-AP alkaline phosphatase (Vector Laboratories) for 30 minutes at RT. Slides were washed and developed in alkaline phosphatase substrate for 10 to 15 minutes. Sections were dehydrated in graded ethanol and cleared in Pro-Par Clearant (Anatech), and mounted with a coverslip.
Immunostaining and fluorescent imaging of autophagosome formation
A detailed protocol for immunostaining of knee joint sections has been previously described12. Briefly, sections were incubated with Hoechst 33342 (1:1,000 dilution, Life Technologies, Carlsbad, CA) for 1 hour at RT to label nuclei. For localization of LC3, sections were incubated with anti-LC3 antibody (1:5,000 dilution, MBL International) in PBS with 1% normal goat serum and 0.3% Triton X-100 for 1 hour at RT and 2 days at 4°C on a vertical shaker. Sections were also incubated with Alexa Fluor® 568 conjugated goat anti-rabbit antibody (1:400 dilution) in PBS/0.3% Triton X-100/1% normal goat serum for 1 hour at RT. After incubation, sections were mounted with ProLong® Gold antifade reagent (Life Technologies) for confocal microscopy.
A detailed protocol for quantitative imaging of autophagosome formation in GFP-LC3 transgenic mice has been previously described12,18. Individual autophagosomes can be detected as discrete signals of high fluorescence, which allows quantification of vesicles per cell as well as the area, perimeter, and circularity. Briefly, optical Z-series images were obtained with a Zeiss 780 laser scanning confocal microscope equipped with gallium arsenide phosphide detectors using the Zen 2011 software (Zeiss, Jena, Germany). The images were reconstructed using Imaris software (Bitplane) to generate a 3-dimensional tissue representation. The images were imported into Image-Pro Plus Software (Media Cybernetics, Rockville, MD) for quantitative image analysis. The results were reported as the average number of vesicles per cell.
Quantification of Atg5 and LC3 expressing cells
For each mouse, images of anterior and posterior menisci, and articular cartilage (regions covered by menisci) were taken at the tissue surface (~100 μm in depth) under 40X magnification. The total number of ATG-5 and LC3 expressing cells per 100 X 100 μm2 area were counted. The results are reported as density of ATG-5 and LC3 expressing cells per 100 μm2.
Statistical analysis
Statistically significant differences between 2 groups were determined using the Mann-Whitney-Wilcoxon test. Statistically significant differences between multiple groups were determined using the Kruskal-Wallis test. Pair-wise differences between young and aged mice, and between normal (grade 0) and higher grades of degeneration, were assessed using Dunn’s method. In cases involving 2 dependent groups, such as comparison between knees in the same mouse, we used the Wilcoxon signed rank test. P values less than 0.05 were considered significant. The results are displayed as box and whisker plots.
Results
Autophagosome formation in menisci and age-related changes
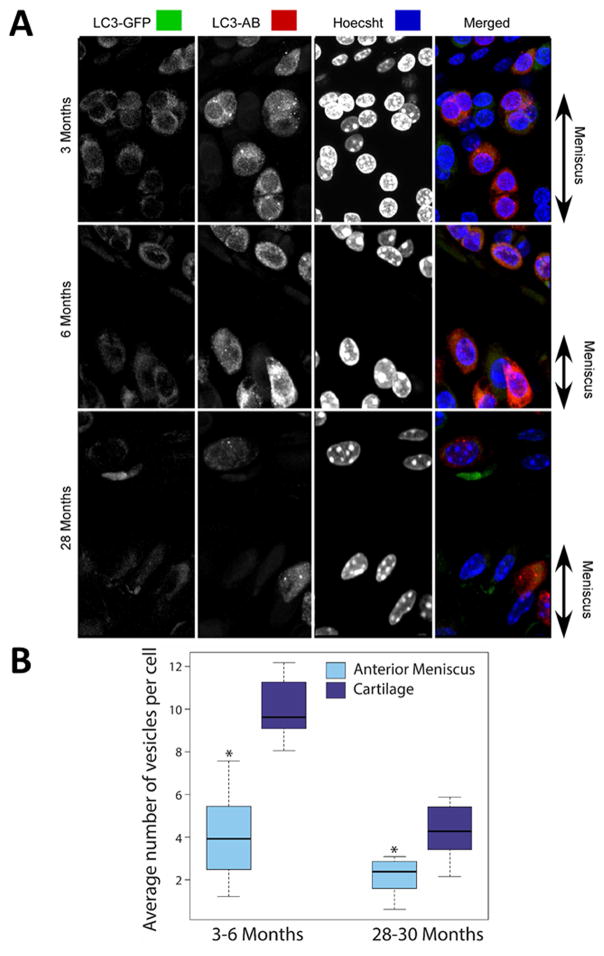
GFP-LC3 transgenic mice15 ubiquitously express GFP-LC3, which serves as a specific biomarker for autophagic vesicles. The accumulation of GFP puncta indicates the formation of autophagosomes, which can be individually detected as discrete signals of high fluorescence. These signals were analyzed as a measure of autophagy activation based on the average number of vesicles per cell (Fig. 1A).
Fig. 1.
Autophagosome formation in meniscus from GFP-LC3 transgenic mice. Vibratome cut sagittal sections (70 μm) of mouse knee joints were stained with anti-LC3 antibody (AB) and Hoechst 33342 to label nuclei and were analyzed by confocal microscopy. (A) Representative images of GFP-LC3 signal in 3-, 6-, and 28-month-old mice showing the surface superficial layer of meniscus for each condition. Original magnification = 63X. (B) Quantitative analysis of autophagosomes presented as average number of autophagic vesicles per cell. Box and whisker plots (5 mice per group); * = P < 0.05 meniscal cells versus cartilage cells.
Autophagosome formation was analyzed in menisci from young, skeletally mature mice (3–6 months old; n = 5) and older mice (28–30 months old; n = 5). GFP-LC3 puncta detected in young mice established the basal levels of normal autophagy activity under physiological conditions. The average number of autophagic vesicles per cell was significantly lower in menisci compared to cartilage (P = 0.003 at 3–6 months and P = 0.005 at 28–30 months, Fig. 1B). This result indicated lower basal level of autophagy activity in menisal cells compared to articular chondrocytes. In older mice (28–30 months), the average number of vesicles per cell was reduced in both meniscus and articular cartilage.
Age-related changes in autophagy protein expression
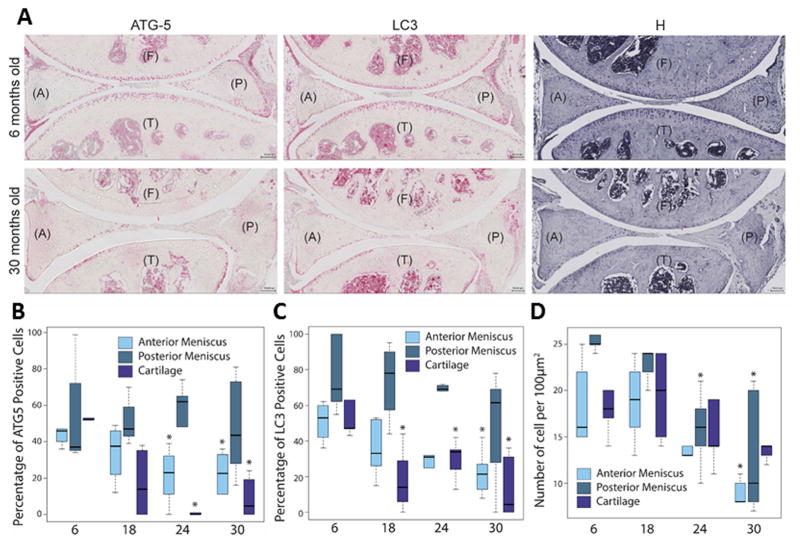
For further confirmation of autophagy activity, we performed immunohistochemistry for key autophagy proteins ATG-5 and LC312,19, in knee joints from mice ages 6, 18, 24, and 30 months. Because ATG-5 is an important regulator of autophagy and LC3 an effector of autophagy, their expression indicated the level of autophagy activity12. ATG-5 and LC3 were expressed mostly in round cells (chondrocyte-like) in the superficial zone of meniscus and much weaker in fusiform cells (fibroblast-like) in the deep zone of young menisci (Fig. 2A). ATG-5 and LC3 expression were greater in the posterior region of the meniscus compared to the anterior region (Fig. 2B and 2C). The number of ATG-5 and LC3 positive cells in the superifical zone of the meniscus decreased with increasing age. ATG-5-positive cells were significantly reduced at 24 (P = 0.03) and 30 months (P = 0.03) in the anterior region (Fig. 2B). LC3-positive cells were significantly reduced (Fig. 2C, P = 0.008) at 30 months in the anterior region. No significant changes in ATG-5 and LC3 expression were found in the posterior region. In addition, age-dependent reduction of ATG-5 and LC3 positive cells was accompanied by a decrease in total cell density (Fig. 2D), which was statistically significant at 30 months, in both the anterior (P = 0.005) and posterior (P = 0.005) menisci; at 24 months in the posterior meniscus (P = 0.02).
Fig. 2.
Age-related changes in ATG-5 and LC3 expression and cellularity. Immunohistochemistry for ATG-5 and LC3 were performed on mouse knee joint sections. (A) Representative images of knee joints from 6- and 30-month-old mice stained with ATG-5, LC3, and Hematoxylin (H), showing the anterior (A) and posterior meniscus, femur (F) and tibia (T). Original magnification = 10X, Scale bar = 100 μm. Quantitative analysis of ATG-5 (B) and LC3 (C) positive cell density per 100 μm2 area. Hematoxylin (H) stained sections were analyzed by total cell density per 100-μm2 area (D). Results show a significant decrease in ATG-5 and LC3 cell number and reduced cellularity. Box and whisker plots (5 mice per group); * = P < 0.05 versus 6-month-old mice.
These findings were similar to those found in articular caritlage12. ATG-5 and LC3 expressing cells were primarily located in the superficial zones in young cartilage and were fewer in number in aged mice (Fig. 2A). In aged mice, the number of ATG-5 and LC3 positive cells were already signficantly reduced (P < 0.05) at 18 months. There was less autophagy activity, reflected by fewer cells and lower GFP signals, in articular cartilage compared to meniscus (Fig. 1B, 30-months old), suggesting a spatial and temporal relationship in autophagy deficiencies across the knee joint.
Autophagy in relationship to meniscus degeneration
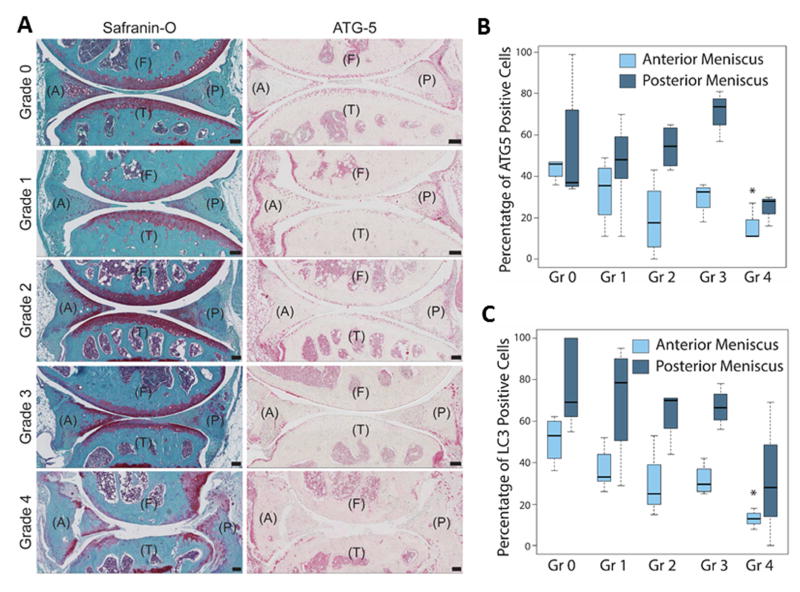
To determine the relationship between autophagy deficiencies and meniscus degeneration, we performed histopathological analysis using a semiquantitative grading system that we recently developed17. The 3 criteria scored were: tissue structure (smooth, fibrillation, undulating), cellularity (normal, hypercellularity, hypocellularity), and matrix staining of Safranin O/Fast Green (normal and disrupted staining). Safranin O stain intensity for the meniscus was often irregular, varied with degeneration, and associated with cellular changes as previously described17. The total score was summed and categorized into the following grades: Grade 0, normal menisci; Grade 1, early degeneration; Grade 2, mild degeneration; Grade 3, moderate degeneration; and Grade 4, severe degeneration). We graded both the anterior and posterior sections of each murine meniscus, (n = 12; 4 per age group). There are cells resembling chondrocytes in the avascular region of the mouse meniscus. Unlike the human meniscus, mice (and several animal species) have larger areas that are positive for Safranin-O stain. This is likely due to the fact that in animals the meniscus function more as load-bearing tissue as evidenced by its relatively increased thickness and larger coverage of the tibial plateau. The results showed that with increasing grade, indicating advancing degeneration, the autophagy protein expression was reduced (Fig. 3A). The reduction of ATG-5 expression was statistically signifcant (P = 0.02) at Grade 4 in the anterior menisci (Fig. 3B). LC3 expression was also signficiantly (P = 0.004) reduced at Grade 4 in the anterior menisci (Fig. 3C). Changes in ATG-5 and LC3 expression were not statistically significant in the posterior menisci.
Fig. 3.
Changes in ATG-5 expression associated with meniscus degeneration. Mouse knee joints were analyzed by a semiquantitative histological grading system for meniscal degeneration in mouse models17. Grades (Gr) are defined from 0 to 4 ranging from healthy normal tissue to severe degeneration. (A) Representative images of mouse knee joints stained with Safranin-O and ATG-5, showing the anterior (A) and posterior meniscus, femur (F) and tibia (T). Original magnification = 10X, Scale bar = 100 μm. (B) Correlation of grading with ATG-5 positive cell density per 100 μm2 area. Results show a significant decrease in ATG-5 positive cells with advancing degeneration. Box and whisker plots (5 mice per group); * = P < 0.05 versus Grade 0 (healthy, normal menisci).
Autophagy changes in response to meniscal injury in experimental OA
To identify differences in age-related autophagy function from those in response to acute meniscal injury, we examined mouse knee joints injured by surgical destabilization of the medial menisci (DMM) in the right knee. The knee joints were harvested 10 weeks after injury and analyzed by histology and immunohistochemistry for ATG-5 and LC3.
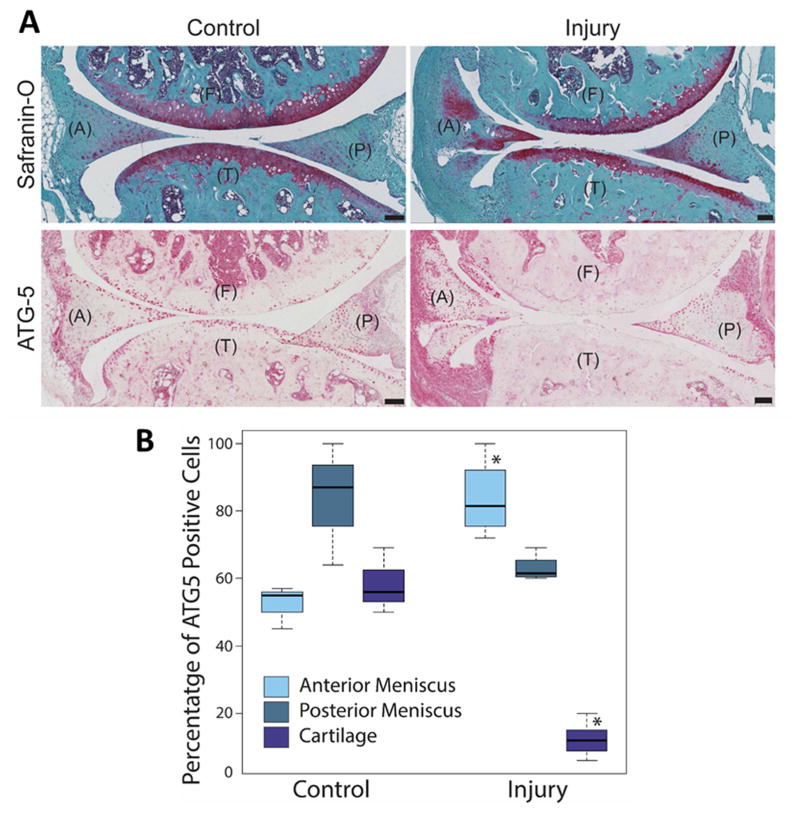
DMM resulted in a rapid development of OA, as reflected by structural damage to menisci and articular cartilage compared to the control knee joints (without injury/surgery) (Fig. 4A). Articular cartilage appeared to be more severely damaged than menisci. Injury led to a significant increase (P = 0.05) in the ATG-5 expression around the injury site in menisci in the anterior horn, while ATG-5 expression was reduced in the posterior region. Autophagy protein expression was almost completely depleted in articular cartilage (Fig. 4B, P = 0.04). Compared to normal aging, the density of ATG-5 expressing cells was much greater in menisci from the injured mice; which demonstrated that autophagy in meniscus cells is rapidly activated in response to acute local injury and suggests lower levels of autophagy proteins in articular cartilage may render it more susceptible to structural damage compared to menisci.
Fig. 4.
Autophagic response following meniscus injury. Two-month-old C57BL/6J transgenic mice were subjected to surgical transection of the medial meniscotibial ligament and medial collateral ligament in the right knee. The left knee was not subjected to surgery but was used as a control. The knee joints were analyzed by Safranin-O staining and immunohistochemistry for ATG-5. (A) Representative images of the control and injured mice knee joints stained with Safranin-O and ATG-5, showing the anterior (A) and posterior meniscus, femur (F) and tibia (T). Original magnification = 10X, Scale bar = 100 μm. (B) Quantitative analysis of ATG-5 positive cell density per 100 μm2 area. Results show significant increase in ATG-5 expressing cells in the anterior menisci. Box and whisker plots (5 mice per group); * = P < 0.05 versus control.
Effect of rapamycin treatment on autophagy in protecting against post-traumatic OA
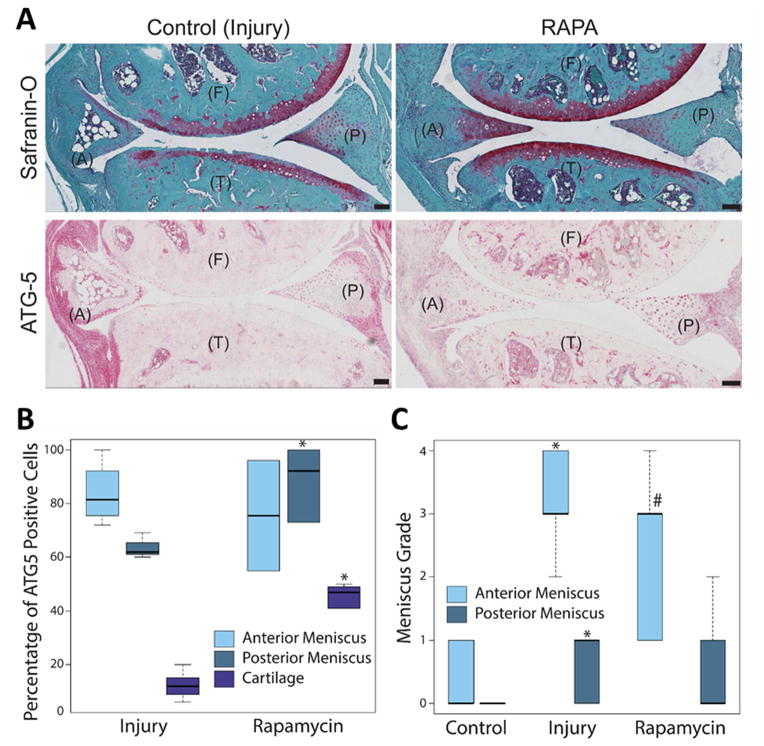
To determine whether the induction of autophagy can reduce meniscus degeneration and OA, we treated injured mice with rapamycin, which directly inhibits the mTOR signaling pathway and activates autophagy20,21. Previously, we investigated the potential of rapamycin in preserving articular cartilage damage and found that it significantly reduced the cartilage degradation and synovial inflammation in mice subjected to DMM14. In this study, we examined menisci from the same mice. Rapamycin treatment protected the articular cartilage from damage and OA development (Fig. 5A). Rapamycin treatment significantly increased the number of ATG-5 expressing cells in the posterior menisci (P = 0.02) and articular cartilage (P = 0.009), but not in the anterior menisci at the injury site (Fig. 5B). Reduction in grade of meniscal degeneration by rapamycin approached statistical significance in the anterior meniscus (Fig. 5C, P = 0.07) but not in the posterior meniscus.
Fig. 5.
Effect of rapamycin treatment on meniscus injury and degeneration. C57BL/6J transgenic mice with surgically induced meniscus injuries and received daily intraperitoneal injections of rapamycin for 10 weeks. The knee joints were analyzed by Safranin-O staining and immunohistochemistry for ATG-5. (A) Representative images of the control (injury) and rapamycin (RAPA) treated mice knee joints stained with Safranin-O and ATG-5, showing the anterior (A) and posterior meniscus, femur (F) and tibia (T). Original magnification = 10X, Scale bar = 100 μm. (B) Quantitative analysis of ATG-5 positive cell density per 100-μm2 area. Results show significant increase in ATG-5 expressing cell number in the posterior menisci and articular cartilage. (C) Meniscus degeneration increases with injury. Rapamycin treatment does not improve degeneration in posterior meniscus. Box and whisker plots (5 mice per group); * = P < 0.05 versus control; # = P = 0.07 versus injury.
Discussion
Menisci play an important role in knee joint load bearing and transmission, shock absorption, and mechanical stability22. These biomechanical functions protect the articular cartilage from degeneration, damage, and injury. While articular cartilage degeneration is central to the pathogenesis of primary OA, meniscus degeneration commonly accompanies moderate or severe OA, and meniscus injury is a significant risk factor for posttraumatic OA2,4,23,24. We previously established a role for dysfunctional autophagy in cartilage mechanical injury and OA11,12,25. In this study, we investigated autophagy using 3 animal models, including meniscal aging and injury and OA secondary to meniscal injury treated with an autophagy activator.
The primary mechanism of the tissue degradation is abnormal cell function and cell death because of homeostatic imbalance that involves autophagy8,26. Autophagy, an essential cellular homeostasis mechanism, protects against cell dysfunction by regulating nutrients and energy and by removing damaged or dysfunctional proteins and organelles27. Thus, deficiencies in autophagy can lead to uncontrolled cellular dysfunction that ultimately manifests as widespread tissue and organ disease and failure28–30.
In our previous study, we evaluated defects in autophagy in articular cartilage degradation and its implications for joint aging and OA in mice12. Normal cartilage in mice exhibited a steady level of constitutive autophagy, progressing to an aging-dependent reduction in autophagy activation, which preceded cell death and structural damage12. The defects in autophagy were most profound in the superficial and upper middle zones, where OA-like structural damages first appeared, suggesting that normal autophagic activity plays a major role in preventing matrix degradation, cell death, and OA development in cartilage.
The purpose of the present study was to analyze the role of autophagy in meniscus degeneration during aging and injury in mice. We used the same GFP-LC3–transgenic reporter mice12 to establish the baseline constitutive level of autophagy activity in normal menisci. Meniscus cells exhibited lower constitutive autophagy activity compared to chondrocytes, reflected in the number of autophagosomes per cell. This difference between meniscal cells and chondrocytes may be due to differences in cell phenotype, tissue vascularity, and mechanical loading between the two tissues.
Cells expressing ATG-5 and LC3, in menisci appeared to be predominantly fibrochondrocytic or chondrocytic in shape. These cells were primarily located in the superficial zones; very few positive cells were found in the deep zone. A similar pattern of autophagy activation existed in the superficial and middle zones of articular cartilage, suggesting that chondrocyte-like cells have a higher level of constitutive autophagy.
Similar to articular cartilage12, expression of autophagy proteins, ATG-5 and LC3 in menisci were also reduced with age. The deficit in autophagy was associated with meniscus degeneration characterized by our previously reported histological grading system17. More severe degeneration was observed in mouse menisci exhibiting lower levels of autophagy proteins compared to menisci with greater autophagy expression. Reduced autophagy corresponded with structural degeneration that included surface fibrillation and undulation, while histological analysis revealed loss of proteoglycan content and reduction in cellularity. These observations support a major role for deficient autophagy in meniscus degeneration during aging.
We also evaluated the temporal relationship between autophagy activation in articular cartilage and menisci during aging. In most mouse knee joints, reduced expression of LC3 in articular cartilage preceded the reduction in meniscus, and cartilage damage was relatively greater than meniscus degeneration. It is possible that autophagy in articular chondrocytes is more susceptible to suppression or dysregulation than in meniscus cells. In fact, this result was also observed in the presence of surgically induced meniscus injury, where autophagy suppression was more profound in articular cartilage than in menisci.
In addition to aging, we previously reported that mechanical injury suppresses autophagy in articular cartilage25. By contrast, meniscus showed increased autophagy after injury despite visible damage to both the menisci and cartilage. This increase was more notable in the anterior horn. These regional differences in autophagy response to injury are possibly due to local variations in the extent of abnormal mechanical loading induced by DMM or due to regional differences in meniscal cells response to injury. In the DMM model and in contrast to aging, ATG-5 expressing cells were located throughout the injured meniscus and were especially numerous in the region near the injury site. The increase in cells exhibiting high levels of autophagy could also be a result of hypercellularity within the menisci, or cell infiltration from the synovium; the latter being a consequence of the inflammatory and repair response observed at the meniscus injury site.
These observations motivated further study to examine whether systemic administration of pharmacological autophagy activator, rapamycin, can reduce the severity of meniscus degeneration in injured mice as previously demonstrated for articular cartilage14. While rapamycin treatment resulted in more protection against structural damage in articular cartilage, it still significantly reduced the severity of meniscus degeneration. These findings suggest that pharmacological activation of autophagy may be effective not only in primary OA but also in preventing or delaying the progression of secondary cartilage degeneration resulting from meniscus injury31 through beneficial effects on both menisci and cartilage.
Analysis of the injury model revealed region-specific changes in the meniscus. The basal expression of ATG-5 and LC3 in normal meniscus was greater in the posterior region compared to the anterior region. Injury led to a significant increase in the ATG-5 expression around the injury site in menisci in the anterior horn, while ATG-5 expression was reduced in the posterior region. Rapamycin treatment significantly increased the number of ATG-5 expressing cells in the posterior menisci, but not in the anterior menisci at the injury site. Structural damage was reduced by rapamycin in the anterior but not in the posterior region. Thus, rapamycin increases autophagy in the posterior region where it is suppressed by injury but was not associated with reduced lesion severity in the same region. These findings suggest a complex regional relationship between rapamycin effects on ATG5 and structural damage and do not support a direct correlation. Further studies are needed in the injury model to more completely examine the effects of rapamycin on structural changes and autophagy, such as effects on autophagosome formation. Nonetheless, rapamycin does have some protective effect against meniscus damage, which could also be in part secondary to its protective effect on cartilage.
Aging is associated with a reduction in autophagy while injury is associated with an increase in autophagy proteins in the anterior horn. These divergent changes indicate a capacity of cells in menisci from young animals to respond to injury with increased autophagy, which we also observed in tissue explants from young animals25. This potentially protective response is compromised in aging. Potential mechanisms by which autophagy declines with aging and injury could be related to cellular stresses, namely increased production of free radicals, including reactive oxygen and nitrogen species5. In particular, the effects of nitric oxide (NO), an inorganic, gaseous free radical, on autophagy pathways seem to play a critical role in regulating several catabolic processes such as the synthesis of collagen and proteoglycan32–35. Elevated levels of NO have been reported to inhibit autophagy during aging36,37 and disease, including OA in articular cartilage35 and meniscus38. Furthermore, NO production has been reported to be higher in articular cartilage compared to menisci and synovium, respectively39,40. Since articular cartilage seems to be the main source of NO in the knee joint, it is possible that higher NO production corresponds to higher basal autophagy in cartilage compared to menisci, as shown in the present study. This result might also increase the susceptibility for autophagy suppression in articular cartilage with aging, injury, and OA. Only limited information is available about autophagy regulation in meniscus. In cultured meniscus cells, dexamethasone-induced autophagy as well as apoptosis and autophagy protected the meniscal cells from dexamethasone-induced apoptosis41. Further investigation is warranted to elucidate mechanisms of autophagy in meniscal cells.
One limitation of this study is that the knee joint anatomy, physiology, and biomechanics of mice are different from that of humans42,43. The spatial and temporal associations between autophagy, cell density, and degeneration in cartilage with similar changes in meniscus require further experiments to establish cause and effect. Sample sizes for individual groups were small but within the range commonly reported for mouse studies of knee degeneration and osteoarthritis44–50.
In summary, this study is the first to characterize changes in meniscal autophagy in response to aging and injury. Baseline autophagic activity was lower in meniscal tissue relative to articular cartilage and underwent an age-related reduction, which was associated with meniscus degeneration. Autophagy significantly increased locally in response to meniscus injury. Treatment with rapamycin did not affect autophagy protein expression in menisci but reduced the severity of meniscus degeneration and development of posttraumatic OA. These results link compromised autophagy to meniscus degeneration and to overall joint degeneration.
Acknowledgments
Author contributions: DD and ML had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study design: DD, ML, JK, MC. Data acquisition: JK, BC, WK, MO. Data analysis and interpretation: DD, ML, JK, BC, WK, MO, SG. Manuscript preparation and approval: JK, SG, DD, ML.
We thank James Koziol, PhD for advice on statistical analysis, Lilo Creighton for technical assistance, and Judy Blake for manuscript formatting and copyediting.
Funding source: National Institute of Health grant AG-007996
Competing interests: None
Ethics approval: This study was conducted with the approval of the Institutional Animal Care and Use of Committee at The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeanie Kwok Meckes, Materials Science and Engineering Program, Department of Mechanical and Aerospace Engineering, University of California, San Diego, CA USA.
Beatriz Caramés, Instituto de Investigación Biomédica de A Coruña, Complexo Hospitalario Universitario de A Coruña, SERGAS, and Universidade da Coruña, A Coruña, Spain.
Merissa Olmer, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA, USA.
William B. Kiosses, Core Microscopy, The Scripps Research Institute, La Jolla, CA USA.
Shawn P. Grogan, Shiley Center for Orthopaedic Research and Education at Scripps Clinic, La Jolla, CA USA.
Martin K. Lotz, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA USA.
Darryl D. D’Lima, Shiley Center for Orthopaedic Research and Education at Scripps Clinic, 11025 North Torrey Pines Road, Suite 200, La Jolla, CA 92037.
References
- 1.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39:1–19. doi: 10.1016/j.rdc.2012.10.004. http://.10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonte F, Beaudoin G, Bloch DA, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–563. doi: 10.1136/ard.2004.023796. http://.10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthr Cartilage. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 4.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. http://.10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 5.Lotz MK, Carames B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7:579–587. doi: 10.1038/nrrheum.2011.109. http://.doi:10.1038/nrrheum.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizushima N. Autophagy in Infection and Immunity. Springer; 2009. Physiological functions of autophagy; pp. 71–84. [DOI] [PubMed] [Google Scholar]
- 7.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. http://.doi:10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, et al. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. http://.doi:10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. http://.doi:10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. http://.doi:10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 11.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. http://.doi:10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carames B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheum. 2015;67:1568–1576. doi: 10.1002/art.39073. http://.doi:10.1002/art.39073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez de Figueroa P, KLM, Blanco FJ, Carames B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheum. 2015;67:966–976. doi: 10.1002/art.39025. http://.doi:10.1002/art.39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carames B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz MK. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71:575–581. doi: 10.1136/annrheumdis-2011-200557. http://.doi:10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In Vivo Analysis of Autophagy in Response to Nutrient Starvation Using Transgenic Mice Expressing a Fluorescent Autophagosome Marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. http://.doi:10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. http://.doi:10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Kwok J, Onuma H, Olmer M, Lotz MK, Grogan SP, D’Lima DD. Histopathological analyses of murine menisci: implications for joint aging and osteoarthritis. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2015.11.006. http://.doi:10.1016/j.joca.2015.11.006. [DOI] [PMC free article] [PubMed]
- 18.Mizushima N, Kuma A. Autophagosomes in GFP-LC3 Transgenic Mice. Method Mol Cell Biol. 2008;445:119–124. doi: 10.1007/978-1-59745-157-4_7. http://.doi:10.1007/978-1-59745-157-4_7. [DOI] [PubMed] [Google Scholar]
- 19.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. http://.doi:10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shigemitsu K, Tsujishita Y, Hara K, Nanahoshi M, Avruch J, Yonezawa K. Regulation of translational effectors by amino acid and mammalian target of rapamycin signaling pathways. Possible involvement of autophagy in cultured hepatoma cells. J Biol Chem. 1999;274:1058–1065. doi: 10.1074/jbc.274.2.1058. [DOI] [PubMed] [Google Scholar]
- 21.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 22.Seedhom B. Transmission of the Load in the Knee Joint with Special Reference to the Role of the Menisci Part I: Anatomy, Analysis and Apparatus. Eng Med. 1979;8:207–219. [Google Scholar]
- 23.Roos H, Laurén M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. http://.doi:10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Englund M, Guermazi A, Lohmander SL. The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin North Am. 2009;47:703–712. doi: 10.1016/j.rcl.2009.03.003. http://.10.1016/j.rcl.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Carames B, Taniguchi N, Seino D, Blanco FJ, D’Lima D, Lotz MK. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012;64:1182–1192. doi: 10.1002/art.33444. http://.doi:10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. http://.doi:10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 27.Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A. Autophagy and Aging: The Importance of Maintaining “Clean” Cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. http://.doi:10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 28.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. http://.doi:10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 29.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. http://.doi:10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shintani T, Klionsky DJ. Autophagy in Health and Disease: A Double-Edged Sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. http://.doi:10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotz M, Carames B. Autophagy: a new therapeutic target in cartilage injury and osteoarthritis. J Am Acad Orthop Surg. 2012;20:261–262. doi: 10.5435/JAAOS-20-04-261. http://.doi:10.5435/JAAOS-20-04-261. [DOI] [PubMed] [Google Scholar]
- 32.Hauselmann HJ, Oppliger L, Michel BA, Stefanovic-Racic M, Evans CH. Nitric oxide and proteoglycan biosynthesis by human articular chondrocytes in alginate culture. FEBS Lett. 1994;352:361–364. doi: 10.1016/0014-5793(94)00994-5. [DOI] [PubMed] [Google Scholar]
- 33.Murrell GA, Jang D, Williams RJ. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun. 1995;206:15–21. doi: 10.1006/bbrc.1995.1003. http://.doi:10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- 34.Cao M, Westerhausen-Larson A, Niyibizi C, Kavalkovich K, Georgescu HI, Rizzo CF, et al. Nitric oxide inhibits the synthesis of type-II collagen without altering Col2A1 mRNA abundance: prolyl hydroxylase as a possible target. Biochem J. 1997;324(Pt 1):305–310. doi: 10.1042/bj3240305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amin AR, Abramson SB. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr Opin Rheumatol. 1998;10:263–268. doi: 10.1097/00002281-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 36.McCann SM, Licinio J, Wong ML, Yu WH, Karanth S, Rettorri V. The nitric oxide hypothesis of aging. Exp Gerontol. 1998;33:813–826. doi: 10.1016/s0531-5565(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 37.McCann SM, Mastronardi C, de Laurentiis A, Rettori V. The nitric oxide theory of aging revisited. Ann N Y Acad Sci. 2005;1057:64–84. doi: 10.1196/annals.1356.064. http://.doi:10.1196/annals.1356.064. [DOI] [PubMed] [Google Scholar]
- 38.Shen C, Yan J, Erkocak OF, Zheng XF, Chen XD. Nitric oxide inhibits autophagy via suppression of JNK in meniscal cells. Rheumatology. 2014;53:1022–1033. doi: 10.1093/rheumatology/ket471. http://.doi:10.1093/rheumatology/ket471. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto S, Takahashi K, Ochs RL, Coutts RD, Amiel D, Lotz M. Nitric oxide production and apoptosis in cells of the meniscus during experimental osteoarthritis. Arthritis Rheum. 1999;42:2123–2131. doi: 10.1002/1529-0131(199910)42:10<2123::AID-ANR12>3.0.CO;2-G. http://.doi:10.1002/1529-0131(199910)42:10<2123::AID-ANR12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi K, Mishima H, Hashimoto S, Goomer RS, Harwood FL, Lotz M, et al. Chondrocyte apoptosis and regional differential expression of nitric oxide in the medial meniscus following partial meniscectomy. J Orthop Res. 2001;19:802–808. doi: 10.1016/S0736-0266(01)00023-7. http://.doi:10.1016/s0736-0266(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 41.Shen C, Gu W, Cai GQ, Peng JP, Chen XD. Autophagy protects meniscal cells from glucocorticoids-induced apoptosis via inositol trisphosphate receptor signaling. Apoptosis. 2015;20:1176–1186. doi: 10.1007/s10495-015-1146-9. http://.10.1007/s10495-015-1146-9. [DOI] [PubMed] [Google Scholar]
- 42.Joshi MD, Suh J-K, Marui T, Woo SLY. Interspecies variation of compressive biomechanical properties of the meniscus. J Biomed Mater Res. 1995;29:823–828. doi: 10.1002/jbm.820290706. http://.doi:10.1002/jbm.820290706. [DOI] [PubMed] [Google Scholar]
- 43.Sweigart MA, Zhu CF, Burt DM, deHoll PD, Agrawal CM, Clanton TO, et al. Intraspecies and Interspecies Comparison of the Compressive Properties of the Medial Meniscus. Ann Biomed Eng. 2004;32:1569–1579. doi: 10.1114/b:abme.0000049040.70767.5c. http://.doi:10.1114/B:ABME.0000049040.70767.5c. [DOI] [PubMed] [Google Scholar]
- 44.McCann MR, Yeung C, Pest MA, Ratneswaran A, Pollmann SI, Holdsworth DW, et al. Whole-body vibration of mice induces articular cartilage degeneration with minimal changes in subchondral bone. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.11.001. http://.10.1016/j.joca.2016.11.001. [DOI] [PubMed]
- 45.Longobardi L, Temple JD, Tagliafierro L, Willcockson H, Esposito A, D’Onofrio N, et al. Role of the C-C chemokine receptor-2 in a murine model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.11.004. http://.10.1016/j.joca.2016.11.004. [DOI] [PMC free article] [PubMed]
- 46.Veronesi F, Giavaresi G, Maglio M, Scotto d’Abusco A, Politi L, Scandurra R, et al. Chondroprotective activity of N-acetyl phenylalanine glucosamine derivative on knee joint structure and inflammation in a murine model of osteoarthritis. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.10.021. http://.10.1016/j.joca.2016.10.021. [DOI] [PubMed]
- 47.Kerr GJ, McCann MR, Branch JK, Ratneswaran A, Pest MA, Holdsworth DW, et al. C57BL/6 mice are resistant to joint degeneration induced by whole-body vibration. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.09.020. http://.10.1016/j.joca.2016.09.020. [DOI] [PubMed]
- 48.van Dalen SC, Blom AB, Sloetjes AW, Helsen MM, Roth J, Vogl T, et al. Interleukin-1 is not involved in synovial inflammation and cartilage destruction in collagenase-induced osteoarthritis. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.09.009. http://.10.1016/j.joca.2016.09.009. [DOI] [PubMed]
- 49.Kung LH, Zaki S, Ravi V, Rowley L, Smith MM, Bell KM, et al. Utility of circulating serum miRNAs as biomarkers of early cartilage degeneration in animal models of post-traumatic osteoarthritis and inflammatory arthritis. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.09.002. http://.10.1016/j.joca.2016.09.002. [DOI] [PubMed]
- 50.Aulin C, Lundback P, Palmblad K, Klareskog L, Erlandsson Harris H. An in vivo cross-linkable hyaluronan gel with inherent anti-inflammatory properties reduces OA cartilage destruction in female mice subjected to cruciate ligament transection. Osteoarthritis Cartilage. 2017;25:157–165. doi: 10.1016/j.joca.2016.08.011. http://.10.1016/j.joca.2016.08.011. [DOI] [PubMed] [Google Scholar]