Abstract
Both schizophrenia (SZ) and bipolar disorder (BD) are highly heritable psychiatric disorders. The significant genomic risk loci are of great importance but with no guarantee of known functional impact and they cannot totally explain the genetic inheritance. In this study we present regional enrichment analyses across the genome, aiming to strike a balance between individual risk loci and integrated regional effects. Chromosomes were partitioned into 2 million base-pair regions (indicated by an underscore sign in the cytogenetic bands) on which enrichment tests are performed. In the discovery phase, we leverage the Psychiatric Genomics Consortium SZ and BD initial association test results for European Ancestry (EA) population and dbGAP SNP data for African Ancestry (AA) population. 78 and 48 regions show significantly enriched associations with SZ and BD respectively in the EA population, and nine are in common including MHC, 3p21.1, 7p22.3_2, 2q32.3_2, 8q24.3_4, and 19q13.33_1. The most unique SZ associated region is 1p21.3_3, while the most unique BD associated region is 6q25.2_1. For the AA population fewer regions are discovered with only 10% overlapping with that of EA population. A replication test using Wellcome Trust Case Control Consortium data for EA population verified 9% of the SZ enriched regions and 40% of the BD enriched regions. In summary, we showed that regional enrichment analyses produce reliable genetic association profiles using about one tenth of samples compared to single base-pair genome wide association approach. The identified association regions will be useful for further genetic functional studies.
Keywords: schizophrenia, bipolar disorder, genetic association, regional enrichment, European ancestry, African ancestry
Introduction
Schizophrenia (SZ) and bipolar disorder (BD) have long been debated for their uniqueness given shared clinical symptoms and neurological aberrance, common treatment, and genetic heritability(Ayalew et al., 2012; Potash and Bienvenu, 2009). This viewpoint has been strengthened by recent genetic discoveries from genome-wide association studies (GWAS) (Ripke et al., 2011; Ruderfer et al., 2014; Sklar et al., 2011). For example, polygenetic risk scores generated using SNPs associated with SZ can explain about 15% of the variance for BD disorder (Lee et al., 2013). Combined analyses on five psychiatric disorders, including SZ and BD, have identified SNPs in association with either SZ or BD, as well as both disorders (Smoller et al., 2013). Notably, the genome-wide significant top risk SNPs, though of great interest, do not guarantee direct functional impact or causality (Franke et al., 2016). And the polygenetic nature of these two disorders (Purcell et al., 2009; Stefansson et al., 2009) cannot exclude the importance of SNPs with nominal risks yet possibly with functional impact.
In parallel, enrichment test of relevance to a disease on preselected genes, pathways or networks is designed to strike a balance between significant individual mutations and interactive nature of biological function(Holden et al., 2008; Liu et al., 2010). Gene ontology categories, pathways defined by KEGG, protein-protein-interaction networks have all been tested for both BD and SZ (Holmans et al., 2009; Jia et al., 2010; Pedroso et al., 2012), and brain signaling pathways and cellular basic functions have all shown enrichment (Jia et al., 2010; Pedroso et al., 2012; Torkamani et al., 2008). Yet, the Psychiatric Genomics Consortium (PGC) SZ working group found that many pathways previously reported to be associated with SZ were not significantly enriched for SZ risk in the large patients vs. control data (PGC, 2014). It likely signals the limitation of such enrichment tests that rely heavily on knowledge base of gene annotation, pathway function, and protein interaction, all of which are not yet complete and undergo rapid changes (Khatri et al., 2012). Recently, enrichment analyses have extended to sets of loci derived from genomic or methylomic studies, many of which are not in coding gene regions but with promising regulation effects, and are tested for enrichment of diseases’ association (GTEx-Consortium, 2015; Jaffe et al., 2016; Nicolae et al., 2010).
To our knowledge no enrichment test has been done on natural consecutive chromosome regions of the genome. The reason for regional enrichment tests is twofold. First, genetic loci within a close distance are potentially functionally related, not limited to within genes or linkage disequilibrium (LD) blocks, as documented most of regulatory cis-effects (Maston et al., 2006; Siepel and Arbiza, 2014). Second, increasing evidence supports the functional impact of SNPs outside protein coding regions (Maurano et al., 2012; Roussos et al., 2014). In fact SNPs associated with diseases are enriched within non-coding functional elements (ENCODE, 2012). Thus, we hypothesize that SNPs within a close distance, not limited to coding regions, might contribute to a disease in an integrative manner. Our research interest here is not to pinpoint the top risk carrying individual loci. Instead we aim to identify genetic regions with disease association, from where further functional studies such as association with gene expression or brain structural and functional variation can be launched.
Most genetic association studies for both disorders are based on European Ancestry (EA) population, less on African Ancestry (AA) population. Here we leveraged public genetic databases and PGC initial test results, and conducted regional enrichment test for SZ and BD in both EA and AA populations separately, followed by a verification using the Wellcome Trust Case Control Consortium (WTCCC) dataset. Then, we compared regional association profiles between SZ and BD, and between EA and AA populations.
Methods
Samples and Data
We studied four cohorts in the discovery phase including EA SZ cohort, EA BD cohort, AA SZ cohort, and AA BD cohort, and two cohorts in the replication phase including a replication EA SZ cohort and a replication EA BD cohort. For the EA population we started our analyses with the genomic individual SNP association results reported by PGC1 SZ and BD working groups derived from large EA samples (Ripke et al., 2011; Sklar et al., 2011). For the AA population, SNP data from three projects in the dbGaP database were used: Genome-Wide Association Study of Schizophrenia, Molecular Genetics of Schizophrenia–nonGAIN, and Whole Genome Association Study of Bipolar Disorder. For replication WTCCC data were used. Sample and SNP information for each cohort are listed in Table 1 and the preprocessing of SNP data is explained in supplementary text. Note that part of WTCCC data have been used in the PGC analyses. PGC SZ and BD analyses used 2333 and 3502 samples from WTCCC project, respectively. We used 4618 WTCCC samples for SZ association and 3301 WTCCC samples for BP association. Thus, given PGC analyses leveraged much large samples, our replication is more of a subset verification.
Table 1.
Samples and SNPs of each cohort investigated
| Discovery data: European Ancestry | Discovery data: African Ancestry | |
|---|---|---|
| SZ | 1,252,901 SNPs from 12,462 HCs & 9,394 SZ patients; 1,929 chromosomal regions with an average of 927 SNPs per region |
809,498 SNPs from 957 HCs &1,256 SZ patients (Affymetrix 6.0); 1,932 chromosomal regions with an average of 602 SNPs per region |
| BD | 2,427,220 SNPs from 9,250 HCs & 7,481 BD patients; 1,928 chromosomal regions with an average of 1806 SNPs per region |
809,498 SNPs from 957 HCs & 140 BD patients (Affymetrix 6.0); 1,932 chromosomal regions with an average of 602 SNPs per region |
| Replication WTCCC data: European Ancestry | ||
| SZ | 623,669 SNPs from 2,491 HCs & 2,127 SZ patients (Affymetrix 6.0); 1941 chromosomal regions with an average of 435 SNPs per region |
|
| BD | 348,374 SNPs from 1,456 HCs & 1,845 BD patients (Affymetrix 500K); 1930 chromosomal regions with an average of 256 SNPs per region |
|
Analysis Approaches
Enrichment tests on a continuous segment of chromosome were implemented. To partition the whole genome into chromosomal regions, we first leveraged the natural cytogenetic band structure. According to genome build hg19 reference (http://hgdownload.cse.ucsc.edu/goldenPath/hg19/database/cytoBand.txt.gz), there are 862 cytogenetic bands across the whole genome and 811 in autosomes with average size 3.5 million (M) base-pairs (bps). Considering the size of 108 SZ risk regions reported by PGC (PGC, 2014) ranging from 331 bps to 857Kbps, we tested 1Mbp and 2Mbp windows as a chromosome region to cover a possible continuous risk region within a cytoband. The results from 1Mbp- and 2Mbp-windows are very similar and results from 2Mbp windows are reported here (see supplementary text for results of 1Mbp windows). Specifically, for any cytoband larger than 3Mbps within chromosomes 1–22, we applied a moving window of 2Mbps with 1Mbps overlapping. And any cytoband of less than 3Mbps was treated as one window. Windows with more than 5 SNPs available in our data, leading to averaged 1938 regions, were tested for enrichment. See Table 1 for region information of each cohort.
Enrichment test requires: 1) univariate association tests to get individual SNPs’ p values, and 2) statistical tests on enrichment of a preselected set of SNPs. For univariate association tests, we applied standard logistic regression on disease status to each SNP with covariates for the top 3 population structure factors in MATLAB, i.e. logit (disease status) = b0 +b1 SNP+ b2–5 population structures, similar model as used in PGC1 SZ and BD studies (Ripke et al., 2011; Sklar et al., 2011). No regression for age, sex or sub-study was included. We implemented univariate tests on dbGaP AA data and WTCCC replication data, and used PGC initial univariate test results. While many enrichment statistical tests have been proposed, we compared three algorithms: a versatile gene-based association enrichment test (VEGAS) (Liu et al., 2010), a gene score from gene-set enrichment analyses (Holden et al., 2008), and a Fisher’s exact test. After comparison we chose to report VEGAS results as it properly controls for gene (region) size and LD structure based on nonparametric simulation (Mirina et al., 2012). Two levels of significance, p<0.01 uncorrected and false discovery rate (FDR) corrected p<0.05, were applied to capture the significant disease associated chromosome regions as well as the promising highly suggestive ones. Here we used FDR correction instead of Bonferroni correction due to relatedness of nearby windows.
In replication significance p<0.05 was applied to verify regions derived from the discovery EA population. In addition, we also compared our results with the 108 SZ-associated regions reported in PGC2 SZ project that leveraged data from 36,989 SZ cases and 113,075 controls (PGC, 2014).
Results
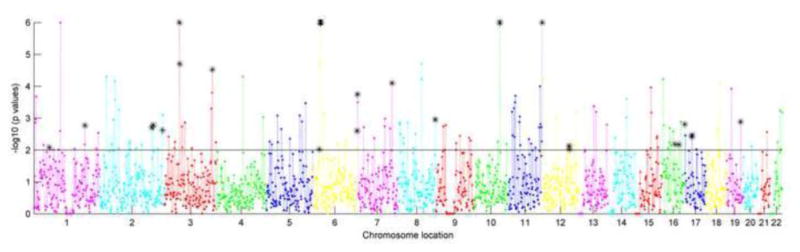
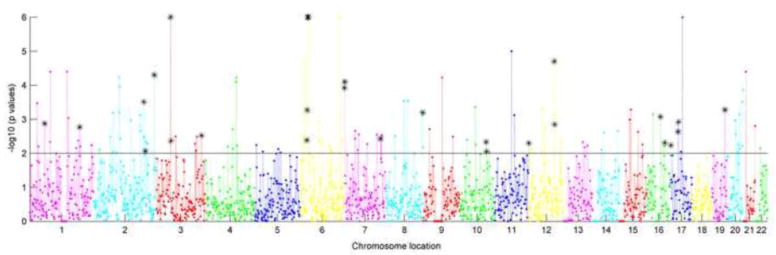
Regional genetic association profiles for SZ and BD in EA and AA populations are plotted in Figures 1–4. As showed in Figure 1, enrichment tests for SZ in EA population identified 163 regions with p<0.01 and 78 passed FDR correction. We listed the top 10 significant regions in Table 2, and the full list can be seen in supplementary table 1. The extra number after the underscore of cytoband indicates the 2-million bp moving window. Figure 2 presents enrichment results for BD in EA population. 133 regions showed enrichment with p<0.01 and 48 passed FDR correction (supplementary table 1). The top 10 significant regions are in Table 2. With the nominal significance threshold of p<0.01, there were 28 overlapping regions between SZ and BD in EA population, covering 17% of SZ enriched regions and 21% of BD enriched regions. With the strict FDR corrected significance threshold, nine regions overlapped (Table 3), covering 12% and 19% of enriched regions for SZ and BD, respectively.
Figure 1.
Regional risk profile for SZ in EA population. X axis presents the chromosome location in chromosomes 1 to 22, and Y axis plots the −log10 transformed enrichment p values of each region. The solid line separates out p-values less than 0.01, and the asterisk indicates the overlapping regions between SZ and BD based on p<0.01.
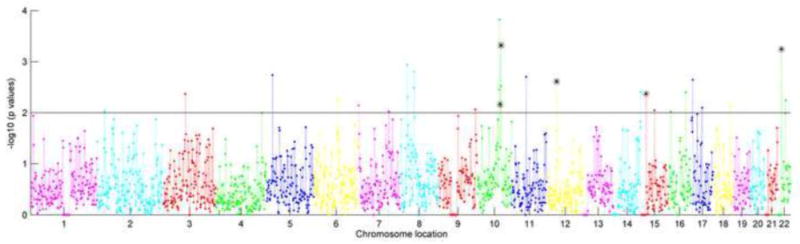
Figure 4.
Regional risk profile for BD in AA population. X axis presents the chromosome location in chromosomes 1 to 22, and Y axis plots the −log10 transformed enrichment p values of each region. The solid line separates out p-values less than 0.01, and the asterisk indicates the overlapping regions between SZ and BD based on p<0.01.
Table 2.
Top 10 regions associated with SZ and BP in EA population
| SZ in EA population | BD in EA population | ||||
|---|---|---|---|---|---|
| Chromosome Region | # of SNPs | p value | Chromosome Region | # of SNPs | p value |
| 1p21.3_3 | 1375 | <1.0E-5 | 3p21.1_1 | 1419 | <1.0E-5 |
| 3p21.1_1 | 771 | <1.0E-5 | 6p21.32 | 2800 | <1.0E-5 |
| 6p22.1_1 | 980 | <1.0E-5 | 6p21.33 | 2927 | <1.0E-5 |
| 6p22.1_2 | 1898 | <1.0E-5 | 6p22.1_2 | 4017 | <1.0E-5 |
| 6p21.33 | 2829 | <1.0E-5 | 6q25.2_1 | 2561 | <1.0E-5 |
| 6p21.32 | 2102 | <1.0E-5 | 17q21.31_2 | 1774 | <1.0E-5 |
| 10q24.32 | 645 | <1.0E-5 | 11q13.2_1 | 614 | 1.0E-05 |
| 10q24.33 | 316 | <1.0E-5 | 12q23.1_3 | 1691 | 2.0E-05 |
| 11q25_3 | 1352 | 2.0E-5 | 6p22.3_2 | 1713 | 2.0E-05 |
the extra number after the dash indicates the 2-million bp moving window index.
Figure 2.
Regional risk profile for BD in EA population. X axis presents the chromosome location in chromosomes 1 to 22, and Y axis plots the −log10 transformed enrichment p values of each region. The solid line separates out p-values less than 0.01, and the asterisk indicates the overlapping regions between SZ and BD based on p<0.01.
Table 3.
Overlapping regions enriched for both SZ and BD association in the EA population
| Chromosome Region* | P value (EA SZ) | p value (EZ BD) | example of genes involved with SNPs showing association p<0.005 |
|---|---|---|---|
| 2q32.3_2 | 1.98E-03 | 3.10E-04 | TMEFF2, PCGEM1, |
| 3p21.1_1 | <1.00E-05 | <1.00E-05 | GNL3, ITIH3-ITIH4, CACNA1D, CACNA2D3, PBRM1, NT5DC2, |
| 6p22.1_1 | <1.00E-05 | 5.40E-04 | MHC gene family, HIST1H1B, HIST1H2BL, |
| 6p22.1_2 | <1.00E-05 | <1.00E-05 | MHC gene family, PGBD1, ZNF311, ZSCAN23, GABBR1, MOG, |
| 6p21.33 | <1.00E-05 | <1.00E-05 | MHC gene family, ABCF1, DDR1, DPCR1, GTF2H4, HCP5, MICB, VARS2 |
| 6p21.32 | <1.00E-05 | <1.00E-05 | MHC gene family, NOTCH4, PSMB9, |
| 7p22.3_2 | 1.80E-04 | 8.00E-05 | MAD1L1, PRKAR1B, PDGFA, |
| 8q24.3_4 | 1.11E-03 | 6.40E-04 | JRK, TSNARE1, |
| 19q13.33_1/2** | 1.31E-03 | 5.30E-04 | IZUMO1, RASIP1, PRRG2, NOSIP |
the extra number after the dash indicates the 2-million bp moving window index.
9q13.33_1 is enriched for SZ and 19q13.33_2 is enriched for BD, these two regions overlap.
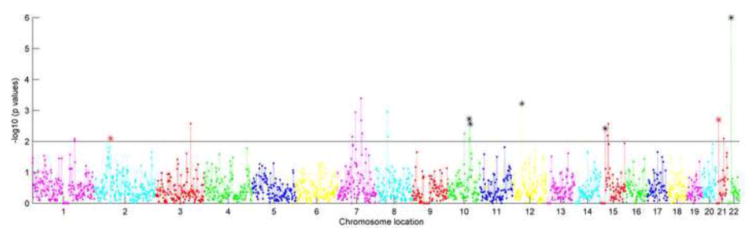
Figures 3 and 4 present enrichment results for SZ and BD in AA population. Twenty-nine regions showed enrichment with SZ and 22 regions with BD using p<0.01(supplementary table 2). Only one region, 22q11.1_2 associated with BD passed FDR correction (p<1e-5). Five risk regions overlapped between SZ and BD using p <0.01(10q23.31_1, 10q23.33_1, 12p11.1_2, 15q11.2_1, and 22q11.1_2), but none passed FDR correction.
Figure 3.
Regional risk profile for SZ in AA population. X axis presents the chromosome location in chromosomes 1 to 22, and Y axis plots the −log10 transformed enrichment p values of each region. The solid line separates out p-values less than 0.01, and the asterisk indicates the overlapping regions between SZ and BD based on p<0.01.
Between EA and AA populations, there were no overlaps in enriched regions for SZ or BD after FDR correction. Using p<0.01 threshold three regions showed enrichment of association with SZ in both EA and AA populations: 7p22.3_1 (p=0.0025, p=0.007 respectively; it is basically 7p22.3 region since 7p22.3 has 2,100,000 bps in hg18), 10q23.33_2 (p=0.0054, p=0.0075), and 22q12.2_2 (p=0.0056, p=0.0057). Similarly, two regions, 21p11.1_1 (p=0.00004, p= 0.002) and 2p14_1 (p=0.00329, p=0.008), were enriched for association with BD in both EA and AA populations.
The replication tests verified 12 out of 163 regions associated with SZ with p<0.01 in the discovery data, and seven out of 78 regions with FDR corrected significance (replication rate of 9%). In contrast, 35 out of 133 regions associated with BD in the discovery data were verified, and 19 out of 48 regions with FDR corrected significance were verified (replication rate of 40%). The details of replication results are in supplementary table 3. Compared with the 108 SZ-associated regions reported by PGC2 SZ project (PGC, 2014), 68 out of the 163 regions (p<0.01) and 48 out of the FDR corrected 78 regions (62%) overlapped with the 108 regions. Interestingly the 9 regions common to SZ and BD identified here were all within the 108 regions.
Discussions and conclusions
Our analyses on EA population are based on the initial test results provided by PGC1 projects. Not surprisingly, the uncovered regional association profiles for SZ and BD include almost all the top SNPs and genes reported by the PGC1 studies. For example, nine of the ten SNPs associated with SZ in (Ripke et al., 2011) are within our FDR corrected enriched regions. 26 out of the top 38 SNPs associated with BD in (Sklar et al., 2011) are within our BD enriched regions of p<0.01, while 22 are in FDR corrected regions. The disparity comes in part from the 2Mbp window used in the analyses. For instance the one SNP (rs10503253) associated with SZ in (Ripke et al., 2011) that we do not cover is within the significant enrichment regions when 1 Mbp window was applied. To select a proper window size is to strike the balance between regional effects and individual strong effects. In this study we focused more on regional effects. The remarkable similarity between results derived from window sizes of 1Mbps and 2Mbps suggests that we have chosen the proper window size to reach a stable result.
Regional enrichment tests discover, excitingly, much more regions than those via individual GWAS tests. To get a sense of how reliable these regions are, we compared our SZ enriched regions with the recently reported 108 SZ associated regions (PGC, 2014). These 108 regions were uncovered using first genomic significant individual SNPs and then extending to neighboring LD blocks. 62% of our identified SZ-associated regions overlap with the 108 regions, and the nine regions common to SZ and BD are all within the 108 regions. This overlapping rate suggests that with about one tenth of the sample size enrichment test is able to reliably discover the disease susceptible regions compared with traditional GWAS tests.
In the EA population, depending on the significance threshold 12–17% of SZ associated regions are also enriched for BD association, while 19–25% of BD associated regions are enriched for SZ association. This overlapping ratio is in line with reported 15–30% overlap in heritability attributable to common genetic variation between two disorders (Lee et al., 2013; Lichtenstein et al., 2009). Focusing on the common regions, we compared our results with that derived from PGC Cross-Disorder group, which conducted single SNP based GWAS on samples of five disorders including SZ and BD (Smoller et al., 2013). Four GWAS significant SNPs identified by the PGC group locate in 3p21.1 (ITIH3), 10q24.32 (AS3MT), 12p13.33_2 (CACNA1C), and 10p12.33 (CACNB2). In our analyses, 3p21.1 is a FDR corrected significantly associated common region for SZ and BD, 10q24.32 carries FDR corrected significant association for SZ and p<0.005 association for BD, 12p13.33_2 carries FDR corrected association for SZ and p=0.01 association for BD, and 10p12.33 is a p=0.02 association region for SZ and p=0.02 association region for BD. Besides, SNPs in MHC and 7p22.3 were also reported by PGC group as promising despite not exceeding the cutoff for genome-wide significance. Thus, overall 6 out of 9 common regions in our analyses are in line with PGC cross-disorder group results despite different significance level, indicating that different analytic approaches in fact converge to the similar discoveries.
MHC, 6p22.1-p21.32, is one of regions associated with both SZ and BP in our results, encoding sets of proteins essential for functions in the immune system. Beyond the GWAS findings from PGC groups, several follow-up studies have demonstrated its functional impact and possible pathogenetic mechanism on diseases through analyzing gene expression, brain imaging, and clinical presentation data (Ruderfer et al., 2014; Sanders et al., 2013; Walters et al., 2013). Other regions such as 3p21.1 and 7p22.3_2 have been previously reported for hosting genes associated with the diseases, such as GNL3 (Scott et al., 2009), NT5DC2 (Struyf et al., 2008), PBRM1 (Vassos et al., 2012) and MAD1L1 (Su et al., 2016). New common susceptible regions that we indentified include 2q32.3_2, 8q24.3_4, and 19q13.33_1, of which the PCGEM1 gene is related to white matter volume across the psychosis spectrum (Oertel-Knochel et al., 2015), JRK has been mapped to epileptic seizures (Morita et al., 1998), and NOSIP down-regulates nitric oxide expression in brain for which SZ patients have higher values (Reif et al., 2006). While these representative genes carry great interest, they should not shadow the importance of intergenic SNPs.
The most unique SZ associated region significantly different from BD is 1p21.3_3, hosting DPYD and MIR137. The MIR137 is well documented for its risk for SZ (Ripke et al., 2011; Wright et al., 2015), and PGC cross-disorder group showed it is also risk for autism but not BD (Smoller et al., 2013). The most unique BD associated region is 6q25.2_1, hosting SYNE1 whose association with BD has been implicated in a number of independent studies (Green et al., 2013; Sklar et al., 2011; Xu et al., 2014). And its risk seems restrict to BD, not the other four psychiatric disorders studied by PGC cross-disorder group (Smoller et al., 2013). These two regions together with other unique regions for either disorder, we believe, will provide a promising start point for studies on the distinct pathophysiology and etiology of each disorder.
Few disease associated regions were identified for AA population. One possibility for this includes that the smaller sample size in the range of 1–2 thousand, instead of over ten thousand, may limit our detection power and yield only few strongest disease associated regions. In fact we observed similar situations in WTCCC data where 0 and 20 disease associated regions were identified for SZ and BP respectively using 3–4 thousand of samples. Thus we speculate that about ten thousand samples are needed to discover a relatively complete genomic disease association profile. A second possibility is the true racial difference. It is known that AA population carries larger genetic variability (Tishkoff et al., 2009), shorter LD blocks than other racial groups (Bryc et al., 2010), and may show different genetic risk profiles for diseases. In our data, when focusing only on the regions identified in AA population from the smaller sample size, with the hope of being replicated in the EA larger samples, no FDR corrected common regions were observed between AA and EA. Only three regions (out of 29) showed nominal association for SZ in both populations, and two regions (out of 22) showed nominal association for BD in both. About 10% overlapping in disease association profiles may reflect the different genetic background of races, and suggests that genetic studies for SZ and BD should be conducted separately for EA and AA populations.
WTCCC replication rates are 9% for SZ enriched regions and 40% for BD enriched regions. Besides the aforementioned sample size differences which may yield low replication rates, the significantly higher rate of replication for BD than for SZ, as well as fewer number of risk regions, suggests that the genetic predisposition for SZ may be more complex and heterogeneous. This echoes the clinical symptom diversity in schizophrenia (McGlashan and Fenton, 1991), and recent extension of the envelope of SZ subtype diversity (Arnedo et al., 2015).
The findings of this study need to be considered with several limitations. First, we aim at identifying coarse yet broad disease genetic susceptibility profiles, a start point to investigate genetic functional impact leveraging endophenotypes such as gene expressional and neurological anomalies. Second, sample size differences between two population data and the replication data are a major limiting factor. Future independent relatively balanced data may further validate the conclusion. Nonetheless, comparison between SZ and BD and overlapping rates based on findings of small sample size data are valid and provide guidance for future population based or disease based studies. Another related limitation is that the WTCCC replication data are not totally independent from the discovery data, and replication results more reflect the stability of findings on a small subsample data. Finally, window size selected may influence the identified regions. We did not test whether different window size would be employed for difference genetic background, such as EA and AA populations.
Supplementary Material
Acknowledgments
Funding body agreements and policies
This work is supported by National Institutes of Health, grant number: P20GM103472.
Footnotes
Conflict of Interest
All authors claimed no any actual or potential conflicts of interest.
Contributors
Author JL designed the study and analyzed the data. Author JC analyzed the data. Authors NP, JT, and VC all participated in study design. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnedo J, Svrakic DM, Del Val C, Romero-Zaliz R, Hernandez-Cuervo H, Fanous AH, Pato MT, Pato CN, de Erausquin GA, Cloninger CR, Zwir I. Uncovering the hidden risk architecture of the schizophrenias: confirmation in three independent genome-wide association studies. Am J Psychiatry. 2015;172(2):139–153. doi: 10.1176/appi.ajp.2014.14040435. [DOI] [PubMed] [Google Scholar]
- Ayalew M, Le-Niculescu H, Levey DF, Jain N, Changala B, Patel SD, Winiger E, Breier A, Shekhar A, Amdur R, Koller D, Nurnberger JI, Corvin A, Geyer M, Tsuang MT, Salomon D, Schork NJ, Fanous AH, O’Donovan MC, Niculescu AB. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryc K, Auton A, Nelson MR, Oksenberg JR, Hauser SL, Williams S, Froment A, Bodo JM, Wambebe C, Tishkoff SA, Bustamante CD. Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc Natl Acad Sci U S A. 2010;107(2):786–791. doi: 10.1073/pnas.0909559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE PC. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B, Stein JL, Ripke S, Anttila V, Hibar DP, van Hulzen KJ, Arias-Vasquez A, Smoller JW, Nichols TE, Neale MC, McIntosh AM, Lee P, McMahon FJ, Meyer-Lindenberg A, Mattheisen M, Andreassen OA, Gruber O, Sachdev PS, Roiz-Santianez R, Saykin AJ, Ehrlich S, Mather KA, Turner JA, Schwarz E, Thalamuthu A, Yao Y, Ho YY, Martin NG, Wright MJ, O’Donovan MC, Thompson PM, Neale BM, Medland SE, Sullivan PF. Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci. 2016;19(3):420–431. doi: 10.1038/nn.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Forty L, Gordon-Smith K, Russell E, Farmer A, Hamshere M, Jones IR, Jones L, McGuffin P, Moran JL, Purcell S, Sklar P, Owen MJ, O’Donovan MC, Craddock N. Association at SYNE1 in both bipolar disorder and recurrent major depression. Mol Psychiatry. 2013;18(5):614–617. doi: 10.1038/mp.2012.48. [DOI] [PubMed] [Google Scholar]
- GTEx-Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden M, Deng S, Wojnowski L, Kulle B. GSEA-SNP: applying gene set enrichment analysis to SNP data from genome-wide association studies. Bioinformatics. 2008;24(23):2784–2785. doi: 10.1093/bioinformatics/btn516. [DOI] [PubMed] [Google Scholar]
- Holmans P, Green EK, Pahwa JS, Ferreira MA, Purcell SM, Sklar P, Owen MJ, O’Donovan MC, Craddock N. Gene ontology analysis of GWA study data sets provides insights into the biology of bipolar disorder. Am J Hum Genet. 2009;85(1):13–24. doi: 10.1016/j.ajhg.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AE, Gao Y, Deep-Soboslay A, Tao R, Hyde TM, Weinberger DR, Kleinman JE. Mapping DNA methylation across development, genotype and schizophrenia in the human frontal cortex. Nat Neurosci. 2016;19(1):40–47. doi: 10.1038/nn.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P, Wang L, Meltzer HY, Zhao Z. Common variants conferring risk of schizophrenia: a pathway analysis of GWAS data. Schizophr Res. 2010;122(1–3):38–42. doi: 10.1016/j.schres.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol. 2012;8(2):e1002375. doi: 10.1371/journal.pcbi.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Mowry BJ, Thapar A, Goddard ME, Witte JS, Absher D, Agartz I, Akil H, Amin F, Andreassen OA, Anjorin A, Anney R, Anttila V, Arking DE, Asherson P, Azevedo MH, Backlund L, Badner JA, Bailey AJ, Banaschewski T, Barchas JD, Barnes MR, Barrett TB, Bass N, Battaglia A, Bauer M, Bayes M, Bellivier F, Bergen SE, Berrettini W, Betancur C, Bettecken T, Biederman J, Binder EB, Black DW, Blackwood DH, Bloss CS, Boehnke M, Boomsma DI, Breen G, Breuer R, Bruggeman R, Cormican P, Buccola NG, Buitelaar JK, Bunney WE, Buxbaum JD, Byerley WF, Byrne EM, Caesar S, Cahn W, Cantor RM, Casas M, Chakravarti A, Chambert K, Choudhury K, Cichon S, Cloninger CR, Collier DA, Cook EH, Coon H, Cormand B, Corvin A, Coryell WH, Craig DW, Craig IW, Crosbie J, Cuccaro ML, Curtis D, Czamara D, Datta S, Dawson G, Day R, De Geus EJ, Degenhardt F, Djurovic S, Donohoe GJ, Doyle AE, Duan J, Dudbridge F, Duketis E, Ebstein RP, Edenberg HJ, Elia J, Ennis S, Etain B, Fanous A, Farmer AE, Ferrier IN, Flickinger M, Fombonne E, Foroud T, Frank J, Franke B, Fraser C, Freedman R, Freimer NB, Freitag CM, Friedl M, Frisen L, Gallagher L, Gejman PV, Georgieva L, Gershon ES, Geschwind DH, Giegling I, Gill M, Gordon SD, Gordon-Smith K, Green EK, Greenwood TA, Grice DE, Gross M, Grozeva D, Guan W, Gurling H, De Haan L, Haines JL, Hakonarson H, Hallmayer J, Hamilton SP, Hamshere ML, Hansen TF, Hartmann AM, Hautzinger M, Heath AC, Henders AK, Herms S, Hickie IB, Hipolito M, Hoefels S, Holmans PA, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hultman CM, Hus V, Ingason A, Ising M, Jamain S, Jones EG, Jones I, Jones L, Tzeng JY, Kahler AK, Kahn RS, Kandaswamy R, Keller MC, Kennedy JL, Kenny E, Kent L, Kim Y, Kirov GK, Klauck SM, Klei L, Knowles JA, Kohli MA, Koller DL, Konte B, Korszun A, Krabbendam L, Krasucki R, Kuntsi J, Kwan P, Landen M, Langstrom N, Lathrop M, Lawrence J, Lawson WB, Leboyer M, Ledbetter DH, Lee PH, Lencz T, Lesch KP, Levinson DF, Lewis CM, Li J, Lichtenstein P, Lieberman JA, Lin DY, Linszen DH, Liu C, Lohoff FW, Loo SK, Lord C, Lowe JK, Lucae S, MacIntyre DJ, Madden PA, Maestrini E, Magnusson PK, Mahon PB, Maier W, Malhotra AK, Mane SM, Martin CL, Martin NG, Mattheisen M, Matthews K, Mattingsdal M, McCarroll SA, McGhee KA, McGough JJ, McGrath PJ, McGuffin P, McInnis MG, McIntosh A, McKinney R, McLean AW, McMahon FJ, McMahon WM, McQuillin A, Medeiros H, Medland SE, Meier S, Melle I, Meng F, Meyer J, Middeldorp CM, Middleton L, Milanova V, Miranda A, Monaco AP, Montgomery GW, Moran JL, Moreno-De-Luca D, Morken G, Morris DW, Morrow EM, Moskvina V, Muglia P, Muhleisen TW, Muir WJ, Muller-Myhsok B, Murtha M, Myers RM, Myin-Germeys I, Neale MC, Nelson SF, Nievergelt CM, Nikolov I, Nimgaonkar V, Nolen WA, Nothen MM, Nurnberger JI, Nwulia EA, Nyholt DR, O’Dushlaine C, Oades RD, Olincy A, Oliveira G, Olsen L, Ophoff RA, Osby U, Owen MJ, Palotie A, Parr JR, Paterson AD, Pato CN, Pato MT, Penninx BW, Pergadia ML, Pericak-Vance MA, Pickard BS, Pimm J, Piven J, Posthuma D, Potash JB, Poustka F, Propping P, Puri V, Quested DJ, Quinn EM, Ramos-Quiroga JA, Rasmussen HB, Raychaudhuri S, Rehnstrom K, Reif A, Ribases M, Rice JP, Rietschel M, Roeder K, Roeyers H, Rossin L, Rothenberger A, Rouleau G, Ruderfer D, Rujescu D, Sanders AR, Sanders SJ, Santangelo SL, Sergeant JA, Schachar R, Schalling M, Schatzberg AF, Scheftner WA, Schellenberg GD, Scherer SW, Schork NJ, Schulze TG, Schumacher J, Schwarz M, Scolnick E, Scott LJ, Shi J, Shilling PD, Shyn SI, Silverman JM, Slager SL, Smalley SL, Smit JH, Smith EN, Sonuga-Barke EJ, St Clair D, State M, Steffens M, Steinhausen HC, Strauss JS, Strohmaier J, Stroup TS, Sutcliffe JS, Szatmari P, Szelinger S, Thirumalai S, Thompson RC, Todorov AA, Tozzi F, Treutlein J, Uhr M, van den Oord EJ, Van Grootheest G, Van Os J, Vicente AM, Vieland VJ, Vincent JB, Visscher PM, Walsh CA, Wassink TH, Watson SJ, Weissman MM, Werge T, Wienker TF, Wijsman EM, Willemsen G, Williams N, Willsey AJ, Witt SH, Xu W, Young AH, Yu TW, Zammit S, Zandi PP, Zhang P, Zitman FG, Zollner S, Devlin B, Kelsoe JR, Sklar P, Daly MJ, O’Donovan MC, Craddock N, Sullivan PF, Smoller JW, Kendler KS, Wray NR. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM, Hayward NK, Montgomery GW, Visscher PM, Martin NG, Macgregor S. A versatile gene-based test for genome-wide association studies. Am J Hum Genet. 2010;87(1):139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maston GA, Evans SK, Green MR. Transcriptional regulatory elements in the human genome. Annu Rev Genomics Hum Genet. 2006;7:29–59. doi: 10.1146/annurev.genom.7.080505.115623. [DOI] [PubMed] [Google Scholar]
- Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, Reynolds AP, Sandstrom R, Qu H, Brody J, Shafer A, Neri F, Lee K, Kutyavin T, Stehling-Sun S, Johnson AK, Canfield TK, Giste E, Diegel M, Bates D, Hansen RS, Neph S, Sabo PJ, Heimfeld S, Raubitschek A, Ziegler S, Cotsapas C, Sotoodehnia N, Glass I, Sunyaev SR, Kaul R, Stamatoyannopoulos JA. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337(6099):1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Fenton WS. Classical subtypes for schizophrenia: literature review for DSM-IV. Schizophr Bull. 1991;17(4):609–632. doi: 10.1093/schbul/17.4.609. [DOI] [PubMed] [Google Scholar]
- Mirina A, Atzmon G, Ye K, Bergman A. Gene size matters. PLoS ONE. 2012;7(11):e49093. doi: 10.1371/journal.pone.0049093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Miyazaki E, Fong CY, Chen XN, Korenberg JR, Delgado-Escueta AV, Yamakawa K. JH8, a gene highly homologous to the mouse jerky gene, maps to the region for childhood absence epilepsy on 8q24. Biochem Biophys Res Commun. 1998;248(2):307–314. doi: 10.1006/bbrc.1998.8947. [DOI] [PubMed] [Google Scholar]
- Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, Cox NJ. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010;6(4):e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knochel V, Lancaster TM, Knochel C, Stablein M, Storchak H, Reinke B, Jurcoane A, Kniep J, Prvulovic D, Mantripragada K, Tansey KE, O’Donovan MC, Owen MJ, Linden DE. Schizophrenia risk variants modulate white matter volume across the psychosis spectrum: evidence from two independent cohorts. Neuroimage Clin. 2015;7:764–770. doi: 10.1016/j.nicl.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso I, Lourdusamy A, Rietschel M, Nothen MM, Cichon S, McGuffin P, Al-Chalabi A, Barnes MR, Breen G. Common genetic variants and gene-expression changes associated with bipolar disorder are over-represented in brain signaling pathway genes. Biol Psychiatry. 2012;72(4):311–317. doi: 10.1016/j.biopsych.2011.12.031. [DOI] [PubMed] [Google Scholar]
- PGC, S.W.G.o.t.P.G.C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potash JB, Bienvenu OJ. Neuropsychiatric disorders: Shared genetics of bipolar disorder and schizophrenia. Nature reviews Neurology. 2009;5(6):299–300. doi: 10.1038/nrneurol.2009.71. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Herterich S, Strobel A, Ehlis AC, Saur D, Jacob CP, Wienker T, Topner T, Fritzen S, Walter U, Schmitt A, Fallgatter AJ, Lesch KP. A neuronal nitric oxide synthase (NOS-I) haplotype associated with schizophrenia modifies prefrontal cortex function. Mol Psychiatry. 2006;11(3):286–300. doi: 10.1038/sj.mp.4001779. [DOI] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin DY, Duan J, Ophoff RA, Andreassen OA, Scolnick E, Cichon S, St Clair D, Corvin A, Gurling H, Werge T, Rujescu D, Blackwood DH, Pato CN, Malhotra AK, Purcell S, Dudbridge F, Neale BM, Rossin L, Visscher PM, Posthuma D, Ruderfer DM, Fanous A, Stefansson H, Steinberg S, Mowry BJ, Golimbet V, De Hert M, Jonsson EG, Bitter I, Pietilainen OP, Collier DA, Tosato S, Agartz I, Albus M, Alexander M, Amdur RL, Amin F, Bass N, Bergen SE, Black DW, Borglum AD, Brown MA, Bruggeman R, Buccola NG, Byerley WF, Cahn W, Cantor RM, Carr VJ, Catts SV, Choudhury K, Cloninger CR, Cormican P, Craddock N, Danoy PA, Datta S, de Haan L, Demontis D, Dikeos D, Djurovic S, Donnelly P, Donohoe G, Duong L, Dwyer S, Fink-Jensen A, Freedman R, Freimer NB, Friedl M, Georgieva L, Giegling I, Gill M, Glenthoj B, Godard S, Hamshere M, Hansen M, Hansen T, Hartmann AM, Henskens FA, Hougaard DM, Hultman CM, Ingason A, Jablensky AV, Jakobsen KD, Jay M, Jurgens G, Kahn RS, Keller MC, Kenis G, Kenny E, Kim Y, Kirov GK, Konnerth H, Konte B, Krabbendam L, Krasucki R, Lasseter VK, Laurent C, Lawrence J, Lencz T, Lerer FB, Liang KY, Lichtenstein P, Lieberman JA, Linszen DH, Lonnqvist J, Loughland CM, Maclean AW, Maher BS, Maier W, Mallet J, Malloy P, Mattheisen M, Mattingsdal M, McGhee KA, McGrath JJ, McIntosh A, McLean DE, McQuillin A, Melle I, Michie PT, Milanova V, Morris DW, Mors O, Mortensen PB, Moskvina V, Muglia P, Myin-Germeys I, Nertney DA, Nestadt G, Nielsen J, Nikolov I, Nordentoft M, Norton N, Nothen MM, O’Dushlaine CT, Olincy A, Olsen L, O’Neill FA, Orntoft TF, Owen MJ, Pantelis C, Papadimitriou G, Pato MT, Peltonen L, Petursson H, Pickard B, Pimm J, Pulver AE, Puri V, Quested D, Quinn EM, Rasmussen HB, Rethelyi JM, Ribble R, Rietschel M, Riley BP, Ruggeri M, Schall U, Schulze TG, Schwab SG, Scott RJ, Shi J, Sigurdsson E, Silverman JM, Spencer CC, Stefansson K, Strange A, Strengman E, Stroup TS, Suvisaari J, Terenius L, Thirumalai S, Thygesen JH, Timm S, Toncheva D, van den Oord E, van Os J, van Winkel R, Veldink J, Walsh D, Wang AG, Wiersma D, Wildenauer DB, Williams HJ, Williams NM, Wormley B, Zammit S, Sullivan PF, O’Donovan MC, Daly MJ, Gejman PV. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43(10):969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Mitchell AC, Voloudakis G, Fullard JF, Pothula VM, Tsang J, Stahl EA, Georgakopoulos A, Ruderfer DM, Charney A, Okada Y, Siminovitch KA, Worthington J, Padyukov L, Klareskog L, Gregersen PK, Plenge RM, Raychaudhuri S, Fromer M, Purcell SM, Brennand KJ, Robakis NK, Schadt EE, Akbarian S, Sklar P. A role for noncoding variation in schizophrenia. Cell Rep. 2014;9(4):1417–1429. doi: 10.1016/j.celrep.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderfer DM, Fanous AH, Ripke S, McQuillin A, Amdur RL, Gejman PV, O’Donovan MC, Andreassen OA, Djurovic S, Hultman CM, Kelsoe JR, Jamain S, Landen M, Leboyer M, Nimgaonkar V, Nurnberger J, Smoller JW, Craddock N, Corvin A, Sullivan PF, Holmans P, Sklar P, Kendler KS. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2014;19(9):1017–1024. doi: 10.1038/mp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AR, Goring HH, Duan J, Drigalenko EI, Moy W, Freda J, He D, Shi J, Gejman PV. Transcriptome study of differential expression in schizophrenia. Hum Mol Genet. 2013;22(24):5001–5014. doi: 10.1093/hmg/ddt350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Muglia P, Kong XQ, Guan W, Flickinger M, Upmanyu R, Tozzi F, Li JZ, Burmeister M, Absher D, Thompson RC, Francks C, Meng F, Antoniades A, Southwick AM, Schatzberg AF, Bunney WE, Barchas JD, Jones EG, Day R, Matthews K, McGuffin P, Strauss JS, Kennedy JL, Middleton L, Roses AD, Watson SJ, Vincent JB, Myers RM, Farmer AE, Akil H, Burns DK, Boehnke M. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc Natl Acad Sci U S A. 2009;106(18):7501–7506. doi: 10.1073/pnas.0813386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepel A, Arbiza L. Cis-regulatory elements and human evolution. Curr Opin Genet Dev. 2014;29:81–89. doi: 10.1016/j.gde.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Ripke S, Scott LJ, Andreassen OA, Cichon S, Craddock N, Edenberg HJ, Nurnberger JI, Jr, Rietschel M, Blackwood D, Corvin A, Flickinger M, Guan W, Mattingsdal M, McQuillin A, Kwan P, Wienker TF, Daly M, Dudbridge F, Holmans PA, Lin D, Burmeister M, Greenwood TA, Hamshere ML, Muglia P, Smith EN, Zandi PP, Nievergelt CM, McKinney R, Shilling PD, Schork NJ, Bloss CS, Foroud T, Koller DL, Gershon ES, Liu C, Badner JA, Scheftner WA, Lawson WB, Nwulia EA, Hipolito M, Coryell W, Rice J, Byerley W, McMahon FJ, Schulze TG, Berrettini W, Lohoff FW, Potash JB, Mahon PB, McInnis MG, Zollner S, Zhang P, Craig DW, Szelinger S, Barrett TB, Breuer R, Meier S, Strohmaier J, Witt SH, Tozzi F, Farmer A, McGuffin P, Strauss J, Xu W, Kennedy JL, Vincent JB, Matthews K, Day R, Ferreira MA, O’Dushlaine C, Perlis R, Raychaudhuri S, Ruderfer D, Hyoun PL, Smoller JW, Li J, Absher D, Thompson RC, Meng FG, Schatzberg AF, Bunney WE, Barchas JD, Jones EG, Watson SJ, Myers RM, Akil H, Boehnke M, Chambert K, Moran J, Scolnick E, Djurovic S, Melle I, Morken G, Gill M, Morris D, Quinn E, Muhleisen TW, Degenhardt FA, Mattheisen M, Schumacher J, Maier W, Steffens M, Propping P, Nothen MM, Anjorin A, Bass N, Gurling H, Kandaswamy R, Lawrence J, McGhee K, McIntosh A, McLean AW, Muir WJ, Pickard BS, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Jones IR, Kirov G, Moskvina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Williamson R, Young AH, Ferrier IN, Stefansson K, Stefansson H, Thornorgeirsson T, Steinberg S, Gustafsson O, Bergen SE, Nimgaonkar V, Hultman C, Landen M, Lichtenstein P, Sullivan P, Schalling M, Osby U, Backlund L, Frisen L, Langstrom N, Jamain S, Leboyer M, Etain B, Bellivier F, Petursson H, Sigur Sson E, Muller-Mysok B, Lucae S, Schwarz M, Schofield PR, Martin N, Montgomery GW, Lathrop M, Oskarsson H, Bauer M, Wright A, Mitchell PB, Hautzinger M, Reif A, Kelsoe JR, Purcell SM. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, Ripke S, Santangelo S, Sullivan PF. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf J, Dobrin S, Page D. Combining gene expression, demographic and clinical data in modeling disease: a case study of bipolar disorder and schizophrenia. BMC Genomics. 2008;9:531. doi: 10.1186/1471-2164-9-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Shen T, Huang G, Long J, Fan J, Ling W, Jiang J. Genetic association of GWAS-supported MAD1L1 gene polymorphism rs12666575 with schizophrenia susceptibility in a Chinese population. Neurosci Lett. 2016;610:98–103. doi: 10.1016/j.neulet.2015.10.061. [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, Hirbo JB, Awomoyi AA, Bodo JM, Doumbo O, Ibrahim M, Juma AT, Kotze MJ, Lema G, Moore JH, Mortensen H, Nyambo TB, Omar SA, Powell K, Pretorius GS, Smith MW, Thera MA, Wambebe C, Weber JL, Williams SM. The genetic structure and history of Africans and African Americans. Science. 2009;324(5930):1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkamani A, Topol EJ, Schork NJ. Pathway analysis of seven common diseases assessed by genome-wide association. Genomics. 2008;92(5):265–272. doi: 10.1016/j.ygeno.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassos E, Steinberg S, Cichon S, Breen G, Sigurdsson E, Andreassen OA, Djurovic S, Morken G, Grigoroiu-Serbanescu M, Diaconu CC, Czerski PM, Hauser J, Babadjanova G, Abramova LI, Muhleisen TW, Nothen MM, Rietschel M, McGuffin P, St Clair D, Gustafsson O, Melle I, Pietilainen OP, Ruggeri M, Tosato S, Werge T, Ophoff RA, Rujescu D, Borglum AD, Mors O, Mortensen PB, Demontis D, Hollegaard MV, van Winkel R, Kenis G, De Hert M, Rethelyi JM, Bitter I, Rubino IA, Golimbet V, Kiemeney LA, van den Berg LH, Franke B, Jonsson EG, Farmer A, Stefansson H, Stefansson K, Collier DA. Replication study and meta-analysis in European samples supports association of the 3p21.1 locus with bipolar disorder. Biol Psychiatry. 2012;72(8):645–650. doi: 10.1016/j.biopsych.2012.02.040. [DOI] [PubMed] [Google Scholar]
- Walters JT, Rujescu D, Franke B, Giegling I, Vasquez AA, Hargreaves A, Russo G, Morris DW, Hoogman M, Da Costa A, Moskvina V, Fernandez G, Gill M, Corvin A, O’Donovan MC, Donohoe G, Owen MJ. The role of the major histocompatibility complex region in cognition and brain structure: a schizophrenia GWAS follow-up. Am J Psychiatry. 2013;170(8):877–885. doi: 10.1176/appi.ajp.2013.12020226. [DOI] [PubMed] [Google Scholar]
- Wright C, Calhoun VD, Ehrlich S, Wang L, Turner JA, Bizzozero NI. Meta gene set enrichment analyses link miR-137-regulated pathways with schizophrenia risk. Front Genet. 2015;6:147. doi: 10.3389/fgene.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Cohen-Woods S, Chen Q, Noor A, Knight J, Hosang G, Parikh SV, De Luca V, Tozzi F, Muglia P, Forte J, McQuillin A, Hu P, Gurling HM, Kennedy JL, McGuffin P, Farmer A, Strauss J, Vincent JB. Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1. BMC Med Genet. 2014;15:2. doi: 10.1186/1471-2350-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.