Abstract
Response selection is foundational to adaptive behavior, and considerable attention has been devoted to investigating this behavior under conditions in which the mapping between stimuli and responses is fixed. Results from prior studies implicate the left supramarginal gyrus (SMg), premotor and prefrontal cortices, as well as the cerebellum in this essential function. Yet, many goal-directed motor behaviors have multiple solutions with flexible mappings between stimuli and responses whose solutions are believed to involve prospective planning. Studies of selection under conditions of flexible mappings also reveal involvement of the left SMg, as well as bilateral premotor, superior parietal cortex (SPL) and pre-supplementary motor (pre-SMA) cortices, along with the cerebellum. This evidence is, however, limited by exclusive reliance on tasks that involve selection in the absence of overt action execution and without complete control of possible confounding effects related to differences in stimulus and response processing demands. Here, we address this limitation through use of a novel fMRI repetition suppression (FMRI-RS) paradigm. In our prime-probe design, participants select and overtly pantomime manual object rotation actions when the relationship between stimuli and responses is either flexible (experimental condition) or fixed (control condition). When trials were repeated in prime-probe pairs of the experimental condition, we detected improvements in performance accompanied by a significant suppression of blood oxygen-level dependent (BOLD) responses in: left SMg extending into and along the length of the intraparietal sulcus (IPS), right IPS, bilateral caudal superior parietal lobule (cSPL), dorsal premotor cortex (dPMC), pre-SMA, and in the lateral cerebellum. Further, region-of-interest analyses revealed interaction effects of fMRI-RS in the experimental versus control condition within left SMg and cerebellum, as well as in bilateral caudal SPL. These efficiency effects cannot be attributed to the repetition of stimulus or response processing, but instead are planning-specific and generally consistent with earlier findings from conventional fMRI investigations. We conclude that repetition-related increases in the efficiency of planning-based selection appears to be associated with parieto-cerebellar networks.
Introduction
Adaptive behavior demands the selection of contextually appropriate responses. A longstanding approach to investigating response selection in psychological and neuroscience research is to employ tasks with fixed mappings that unambiguously specify the relationships between presented stimuli and required responses. The classic example is the choice reaction time task pioneered by Donders in the 2th century in which the identity of the stimulus indicates which of two response keys to press (Donders, 1969). Multiple lines of evidence converge to indicate asymmetrical involvement of the left cerebral hemisphere in the selection and preparation of responses under these circumstances, independent of the hand involved (Rushworth, Johansen-Berg, Gobel & Devlin, 2003; Rushworth, Nixon, Wade, Renowden & Passingham, 1998; Schluter, Krams, Rushworth & Passingham, 2001).
Outside the laboratory, many goal-directed actions are instead typified by flexible mappings between stimuli and responses, in which more than one effective option is available to solve a given task. For instance, either an under- or over-hand grip may suffice for turning a door handle, although one grip-type may enable a more forceful, more precise and less awkward movement than the other. The fact that actors do consistently prefer such solutions has been attributed to the ability to anticipate accurately the costs (energetic, biomechanical) associated with the available response alternatives through prospective motor planning (Janssen, Meulenbroek & Steenbergen, 2011; Johnson, 2000; Rosenbaum & Jorgensen, 1992). Similar to fixed mappings, fMRI studies of response selection under these flexible mapping circumstances also indicate asymmetrical involvement of left hemisphere; specifically SMg and adjacent cortex within the intraparietal sulcus (IPS), as well as the left vPMC. In addition, these investigations report bilateral increases in activity of the caudal superior parietal lobule (cSPL), dorsal premotor cortex (dPMC), pre-SMA, and the cerebellum (Jacobs, Danielmeier & Frey, 2010; Johnson, 2002; Marangon, Jacobs & Frey, 2011; Martin, Jacobs & Frey, 2011). There is, however, a potentially significant limitation to this body of work: in an effort to isolate prospective action planning and selection mechanisms from those involved in execution, prior investigations avoided having participants actually perform their chosen responses (e.g., an under- or over-hand grasp of a stimulus handle). Instead, actors expressed their action preferences via button presses, a task subcomponent that itself involves utilizing a fixed response mapping. Whether similar results would be found under more ecological conditions—in which goal-directed actions are both planned and produced, therefore remains unclear.
Here, we address these limitations through use of a repetition suppression (RS) fMRI design in which each trial consists of two events, a prime action and a subsequent probe action. The overall logic of this approach is that successive actions involving repeated task demands (repeated trial) will result in more efficient processing than successive actions with different task demands (changed trial). This increased processing efficiency would be evident in faster response times (RTs) and reduced blood oxygenation level-dependent (BOLD) signal specifically within involved brain mechanisms (i.e., RS) (Grillspector, Henson & Martin, 2006; Henson & Rugg, 2003; Horner & Henson, 2008; Valyear & Frey, 2015). This improved processing of a repeated event, measured via a behavioral variable such as lower RT, is known as behavioral priming.
Critically, this design uses identical stimuli and motor responses in both repeated and changed trials. The only difference between repeated trials and changed trials is whether the planning demands are held constant allowing a single plan to be repeated; or changed, thereby increasing planning demands. However, repeated events may lead to more generalized decreases in processing demands associated with repeated task elements, such as stimulus and response processing. To address this possibility, we included a control condition (“Rule” condition) that utilized nearly identical stimuli and the same motor responses as the Plan experimental condition. However, actions in this Rule condition were prescribed by a rule that unambiguously specified a single fixed mapping between stimuli and responses, thereby eliminating all demand for prospective planning. Therefore, any planning-specific effects should only appear in the Plan and not the Rule task.
Indeed, a prior behavioral investigation involving this paradigm revealed significantly reduced RTs to initiate the same actions within a flexible stimulus-response mapping (Plan) condition compared to a fixed stimulus-response mapping (Rule) condition (Randerath, et al., 2015). In the current study, we expected lower probe RTs and reduced neural activity (i.e., a RS effect) for repeated vs. changed trials in the Plan condition only. We predicted that RS in the Plan condition would occur specifically within brain regions previously implicated in prospective action planning and selection (i.e., left SMg and IPS, vPMC, as well as bilateral pre-SMA, cSPL, dPMC and lateral cerebellum). If the predicted effects are specific to planning-based action selection, then they should be minimal (or absent) in the control condition (Rule).”
Materials & Methods
Participants
Twenty individuals (twelve female; mean age = 23 ± 4.6 years) from the University of Oregon participated in the study. All participants were right-handed according to the Edinburgh Handedness Inventory (Oldfield 1971), had normal or corrected-to-normal vision, and were naïve to the specific goals of the study. Participants provided informed consent in accordance with the local IRB. The study took approximately 2.5 hours to complete (1 hour for training, 1.5 hours for scanning), and participants received financial compensation. Due to technical difficulties, behavioral data from one individual was not recorded.
Material and procedure
The used paradigm was adapted from Randerath, Valyear, Hood and Frey (2015). Both the Plan and Rule conditions required the same behavioral responses. Specifically, participants demonstrated a hand posture (pronated/overhand or supinated/underhand) as if they were to insert the flattened right hand into a slotted disk and rotate it to a cued orientation for each the prime and the probe event in a trial. Participants lay supine in the scanner (Figure 1). Both arms were extended along the participants’ sides and the palms facing downward toward the MRI table. Participants wore a paddle on their hand to ensure that they maintained a flat hand posture throughout the study, and that responses across trials only varied with respect to wrist and forearm rotation. Each trial began with the right hand in the start position, with palm down on a response button device. Unless noted otherwise, the procedures were the same for both conditions.
Figure 1.
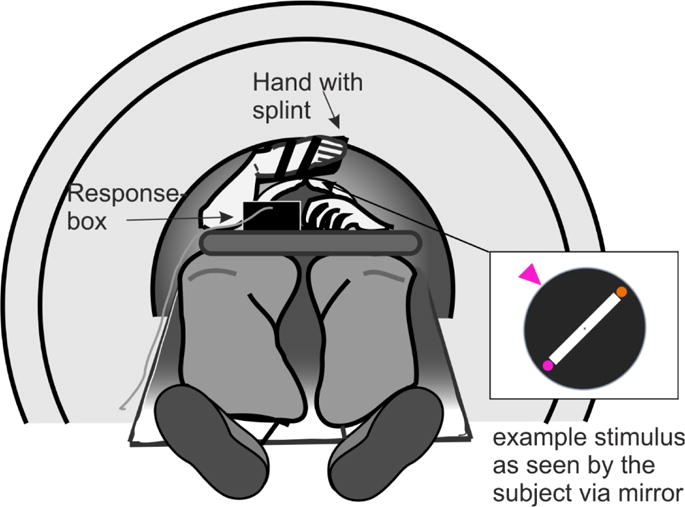
Experimental setup
Stimuli were single images projected onto a back-projection screen positioned at the rear of the scanner bore and viewed in a mirror attached to the head coil. Stimuli and response recording were controlled using Superlab 4.5 (Cedrus Corp, San Pedro CA).
A disk with a central diagonal hand slot was visible on each trial (Figure 2). The orientation of the slot was constant throughout the experiment at 315 degree. On each trial, an orange and a pink circle appeared at opposite ends of the slot, and their respective positions (upper or lower end of slot) varied across trials. A rotation cue (pink or orange “arrow”) was displayed in either the upper left or lower right corner of the disk. A central cue was present throughout the study. Participants were asked to fixate the cue during the entire experiment. Within a trial, the shape of the fixation cue specified which task to perform, and the identity of the mapping between the shape of the fixation cue (diamond/cross) and condition (Rule/Plan) was counterbalanced across participants. Trials from each condition were presented in an optimally counterbalanced order.
Figure 2. Experimental task.
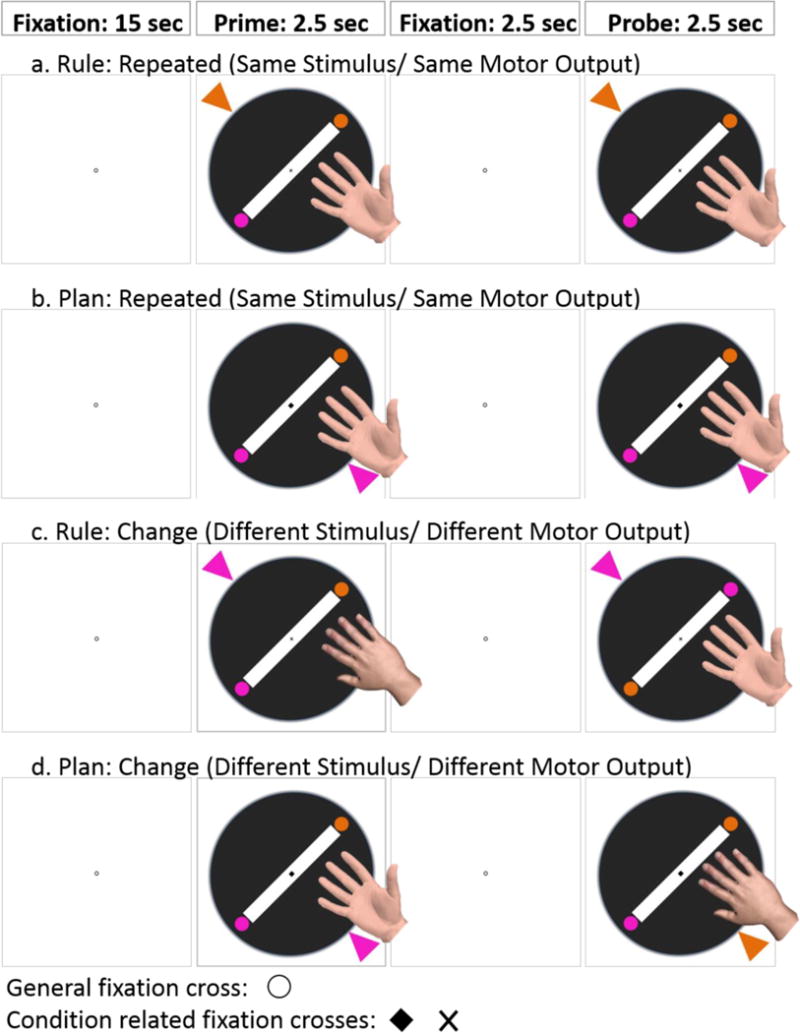
Example trials for Rule and Plan condition. One trial includes two events of the same condition (Rule or Plan). The shape of the fixation-cross indicates what task to execute; here: x=Rule, diamond=Plan. One trial consists of 15 sec fixation, followed by 2.5 sec prime event requiring the first response. Then 2.5 sec fixation cue are presented before the probe event starts, which requires the second response. Responses are over- or underhand postures, which are predetermined by the stimulus configuration (Rule condition) or selected based on the end-state comfort (Plan condition). In the Plan condition participants had to choose the most comfortable way to rotate the disk and align the same colored circle cue with the arrow. In the Rule condition subjects had to insert their hand with the thumb towards the same colored circle as the arrow, then rotate the disk to align the same colored circle cue with the arrow. The displayed example trials with correct responses show repeated events (a./b.) or changed events (c./d). By using similar stimuli and actions for both conditions, we controlled for effects of stimulus and response, but manipulate the approach for action selection.
Based on prior awkwardness rating results (Johnson, 2000), the slot angle was chosen that was comfortable for both underhand and overhand insertion postures produced with the right hand, reflecting a diagonal plane at 315 degree. The distance from the start-position to either the underhand or overhand insertions was identical. Response times (RTs) were measured from stimulus onset until the participant released the button at movement onset. Participants were asked to respond as quickly but also as accurately as they could. Errors were determined based on video-evaluation determining the type of selected posture.
Critically, the actual motor responses issued in the two conditions were identical, but differed in the processing strategies required to select responses. In the experimental Plan condition, subjects were asked to select the hand posture (under- or over-hand) that would allow them to complete the cued rotation of the plate most comfortably, given the required rotation and the prevailing biomechanical constraints on right forearm rotation. In the Rule condition, responses were prescribed by the stimulus configuration; specifically, participants were instructed to insert their hand into the slot in such a way that the thumb shows towards the circle that had the same color as the arrow cue. Participants were then required to rotate their hand as if to turn the disk in order to align the same colored circle with the arrow cue. For example, if the arrow cue was pink, then participants were to position the thumb toward the pink circle, and then rotate the hand in a manner that would align the pink circle with the arrow cue. After demonstrating the hand posture and rotation, participants returned their hands to the start position.
The use of different locations of the arrow cue for the Plan condition (lower right corner) and the Rule condition (upper left corner) was necessary to avoid any overlap of task-instructions; otherwise, participants may have chosen their responses based on one approach (Plan or Rule) for all trials. However, the participant was not informed about this technical configuration. To keep participants eyes fixated on the center of the screen, they instead were instructed that the task was indicated by the form of the fixation cue, as described above.
A single trial comprised the following events: 1) 2.5s prime stimulus, resultant motor response and return to the start position, 2) 2.5s inter-stimulus interval, 3) 2.5s probe stimulus, resultant motor response and return to the start position, 4) 15s inter-trial interval. Coincident with the onset of the prime stimulus, the fixation cue changed from a circle to either a diamond or cross to indicate the condition (Rule or Plan) to be performed on that trial. Note that prime and probe events occurring within a single trial were always of the same condition. Within trials of each condition, we manipulated whether the probe involved stimuli and motor responses identical to (i.e., repeated) or different from (i.e., changed) the immediately preceding prime event. The experiment comprised six unique run orders. Each run comprised 16 trials: 2 conditions (Rule, Plan) × 2 levels of prime-probe congruency (Repeated, Changed) × 4 trials). Trial history (N-1) was balanced within runs according to condition × congruency. Run-order was counterbalanced across participants.
Two to five days prior to scanning, participants took part in a behavioral training session to become familiar with the task, and to practice responding without moving the head. Initially, participants performed one run (16 trials) of the task using a rotatable apparatus that closely resembled the graphic stimuli used in the fMRI task. This allowed them to practice both conditions while actually inserting their hands into and rotating a disk to cued orientations. Next, they performed 2 runs of the actual experiment while lying supine in a mock scanner that closely simulated the spatial constraints of the MRI system. Participants were instructed to perform movements smoothly, and in a manner consistent with those issued during the initial training session involving the physical device. They were given feedback about incorrect and/or spatially imprecise movements.
During debriefing after the experiment, five participants reported developing alternative strategies during the experiment to perform the experimental task, but only 3 were able to describe their approach. All three cases used the opposite prescription of the rule task (i.e. select the posture that would place the pinky towards the circle with the same color as the arrow) in order to solve the plan condition. Twelve subjects found the Plan condition to be more difficult, 4 found the Rule condition to be more difficult, and one person found both equivalent.
Behavioral data analysis
The dependent variable was response times. Trials were removed from analysis if their response time exceeded 2 standard deviations above/below each condition mean, or if they included an error. An error was recorded if the participant: moved awkwardly (e.g. ended up in a different position), changed response mid-movement, failed to respond, or used the wrong hand posture. Errors were defined via post-hoc video analysis. Statistical analysis were done with IBM SPSS 21. RTs for probe events were submitted to a 2 (Condition: Plan, Rule) × 2 (Congruency: Repeated, Changed) repeated measures analysis of variance (RM-ANOVA). Because only few subjects used strategies we ran the analyses with and without the five participants who reported using an alternative strategy. Because this led to no difference in the pattern of findings, the data are reported with all participants included.
MRI data acquisition
Functional images were acquired on a Siemens (Erlangen, Germany) Allegra 3T MRI system, equipped with echo planar imaging (EPI) capabilities, using a standard birdcage coil for radiofrequency transmission and signal reception. BOLD-sensitive functional images were acquired using a single-shot gradient EPI sequence (TE/TR = 30/2500ms, flip angle = 80°, 41 axial slices, voxel size = 3.13 × 3.13 mm; field of view = 200 mm, slice thickness = 4.0 mm). Siemens’ PACE was used for prospective online motion correction (Thesen, Heid, Mueller & Schad, 2000). High-resolution anatomic images were acquired using a three-dimensional MP-RAGE pulse sequence (TE/TR = 4.38/2500ms; flip angle = 8.0°, 176 contiguous axial slices, slice thickness = 1.0 mm, voxel size = 1.0 × 1.0 mm; field of view = 256 mm). Siemen’s AutoAlign scout and TrueFISP sequences were executed for each participant at the beginning of each functional data collection run to ensure that the head was in a good position. DICOM image files were converted to NIfTI format using MRIConvert software (http://lcni.uoregon.edu/~jolinda/MRIConvert/).
MRI processing and analysis
FMRI data were preprocessed using the FSL toolbox version 4.1 (http://www.fmrib.ox.ac.uk/fsl) (Smith, Jenkinson, Woolrich, Beckmann, Behrens, Johansen-Berg, Bannister, De Luca, Drobnjak, Flitney, Niazy, Saunders, Vickers, Zhang, De Stefano, Brady & Matthews, 2004). Skull and surrounding non-brain tissue were removed using a brain extraction tool (Smith, 2002). PACE (Thesen, et al., 2000) was used for MELODIC independent components analysis to identify artifactual components for removal (Beckmann & Smith, 2004). Volumes were spatially smoothed with a Gaussian kernel of 5 mm (FWHM), intensity was normalized and filtered with a nonlinear high-pass temporal filter (70.0s) and delays and undershoots in the hemodynamic response were accounted for by convolving the model with a double-gamma HRF function. Registration to high-resolution structural images was carried out using FLIRT (Jenkinson, Bannister, Brady & Smith, 2002; Jenkinson & Smith, 2001), and registration to standard space was performed using FNIRT nonlinear registration (Andersson, Jenkinson & Smith, 2007).
For each fMRI run, we created a model with four explanatory variables (EVs) and their temporal derivatives to encode the conditions in our 2 (Condition: Plan, Rule) × 2 (Congruency: Repeated, Changed) factorial design. These EVs were time-locked to the onset of the stimulus and included the subsequent 7500ms (2500ms prime + 2500ms fixation + 2500ms probe). The remaining time (15s fixation) was treated as resting baseline. Orthogonal contrasts were used to test for differences between all factors and resting baseline, as well as all combinations of the factorial design. The resulting first-level contrasts of parameter estimates (COPEs) served as inputs to second-level analyses (within subjects, across runs). COPEs from the second-level analyses then served as inputs to third-level analyses (across participants). Z-statistic (Gaussianized T/F) images were thresholded using Z>2.3 (corresponding to a p <.01 for a one-tailed hypothesis) and a corrected cluster significance threshold of p=.05 (Worsley, 2001). Estimated smoothness and critical cluster size are reported in supplementary table 1.
The third-level analyses controlled for between-condition differences in RT by entering participant mean RT (demeaned across participants) as a covariate in the model. These third-level, group analyses were performed using FLAME Stage 1 to model and estimate random-effects components of mixed-effects variance (Beckmann, Jenkinson & Smith, 2003; Woolrich, Behrens, Beckmann, Jenkinson & Smith, 2004). Third level analyses were constrained to the set of areas with significant activity for Plan > Rest or Rule > Rest, to focus analysis on areas exhibiting task-relevant increases in activity. We examined the following contrasts to elucidate plan specific effects: Plan > Rule, Plan Change > Plan Repeated, Rule Change > Rule Repeated, and the interaction analysis of (Plan Change > Plan Repeated) > (Rule Change > Rule Repeated). In addition, the below-described hypothesis driven anatomical ROI analysis was performed. The focus on a priory defined regions reduces the amount of analyzed voxels and therefor has the advantage to be less vulnerable to the multiple testing problem going along with conservative thresholds for whole brain corrections, i.e. for ROI analyses statistical inferences are restricted to fewer simultaneous tests, leading to a more sensitive analysis (see also Poldrack, 2007).
Region-of-Interest Analyses
Based on our hypotheses and prior results, we also used FSL’s Featquery to calculate mean percent signal change within the following regions of interest defined anatomically based on the FSL Harvard-Oxford Cortical Structural Atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html): left SMg and vPMC, bilateral cSPL, dPMC and cerebellum. Similar to the voxel-wise analysis, voxels were included in the ROI analysis if they satisfied two conditions: i) were located within the boundaries defined using the atlas, and ii) demonstrated significant increases in activity within our inclusive mask defined by the conditions (Rule vs. Rest + Plan vs. Rest). Separate repeated-measures ANOVAS were conducted with IBM SPSS 21 on results. First we ran a 2 (Condition: Plan, Rule) × 2 (Congruency: Repeated, Changed) × 8 (ROI) factorial design. Subsequently, within each ROI, differences in the 2 (Condition: Plan, Rule) × 2 (Congruency: Repeated, Changed) factorial design were tested. Post hoc pairwise comparisons were reported for significant interactions with p<.1.
Results
In both the Plan and Rule conditions, we expected increased processing efficiency (lower probe RTs and BOLD signal amplitudes) when probe events were preceded by primes that involved identically repeated stimuli and motor responses, compared to trials where stimuli or motor responses changed between prime and probe.
Behavioral Data
Participants made few errors on average (1.6% and 0.3% for Plan and Rule conditions, respectively). An additional 3.8% and 3.9% of Plan and Rule trials, respectively, were identified as RT outliers, using the procedure detailed in the method section. As shown in Figure 2, results of the RM-ANOVA revealed a significant main effect of Condition [F(1,17)=30.78, p<.001], wherein RTs were shorter for the Rule vs. Plan condition. Likewise, as expected there was a main effect of Congruency [F(1,17),=56.29, p<.001], with shorter RTs when probe events were an identical repetition of the preceding prime versus changed. We hypothesized that if the repetition effects are specific to planning-based action selection, then they should be minimal (or absent) in the control condition (Rule). Although the Condition × Congruency interaction term did not reach statistical significance [F(1,17)=3.80,p=.068], we found a trend toward greater repetition effects in the Plan as opposed to Rule condition, consistent with prior findings (Randerath, et al., 2015).
fMRI Data
Plan vs. Rule conditions contrast
The contrast of the Plan versus Rule condition revealed activations bilaterally along the intraparietal sulcus and adjacent angular (Ang) and supramariginal (SMg) gyri (Figure 4). These parietal effects exhibited substantial left cerebral asymmetry. Activations had larger circumference in the left hemisphere including the bilateral superior parietal lobule (SPL). We also found activations in left cerebellar regions and peaks in the right temporal pole and adjacent Insula, frontal orbital cortex and inferior frontal gyrus. Furthermore, the contrast showed activations in the left lateral occipital cortex, as well as the occipital pole in the right hemisphere.
Figure. 4.
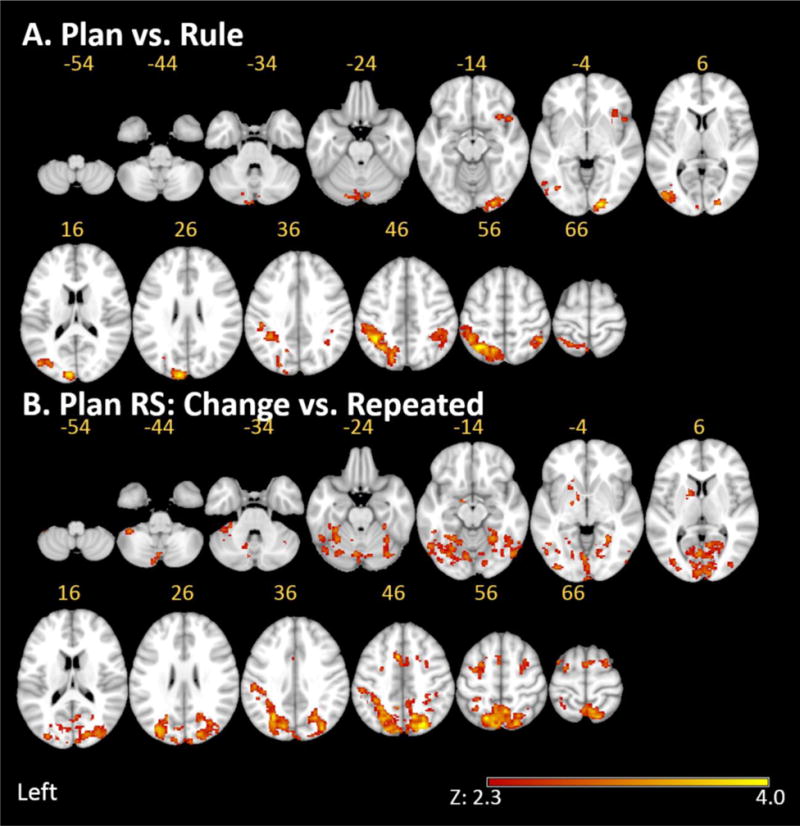
A. Contrast between the experimental Plan and the control Rule condition. Activations bilaterally along the intraparietal sulcus and adjacent angular (Ang) and supramariginal (SMg) gyri are prominent. B. Repetition suppression of neural activity. The Plan repetition suppression effects are appear bilaterally in the posterior parietal cortex, dPMC, pre-SMA and cerebellum. The left IPS and SMg are accentuated. As described in the text, we failed to detect any regions exhibiting greater RS for the Rule condition.
BOLD Repetition Suppression
In the Plan condition, the significant priming effect in RT noted above indicates that participants used the information in the prime. This increase in processing efficiency was associated with a lower amplitude BOLD response within the left posterior parietal cortex (left SMg extending into and along the length of the IPS and bilateral caudal SPL), dPMC, pre-SMA and cerebellum (hemispheres and vermis) (Figure 4). We also found unexpected bilateral RS in the lateral and medial occipital cortex, as well as left-lateralized basal ganglia. In short, we found Plan-specific RS in, but not exclusively in, cortical and cerebellar areas previously implicated in prospective action planning and selection. Because the Rule condition exhibited no significant main effects of Changed vs. Repeated trials, we have avoided to test the interaction at the whole brain level.
Similar to the Plan condition, the significant priming effect in the behavioral data from the Rule Condition indicates that participants did indeed benefit from rule repetition. Nevertheless, we found no evidence of statistically significant RS anywhere in the brain. Importantly, this suggests that the changes detected in the Plan condition, are indeed specific to repeated planning and not to a generalized decrease in task difficulty on repeated vs. changed trials. Using a more liberal test that did not correct for multiple comparisons, we did detect RS in this control condition within bilateral medial and lateral occipital cortex, as well as caudal SPL. These weaker RS effects overlapped with those evident at reliable statistical thresholds for the Plan condition (Figure 5).
Figure 5. Condition overlay of repetition suppression maps (Plan Change > Plan Repeated and Rule Change > Rule repeated).
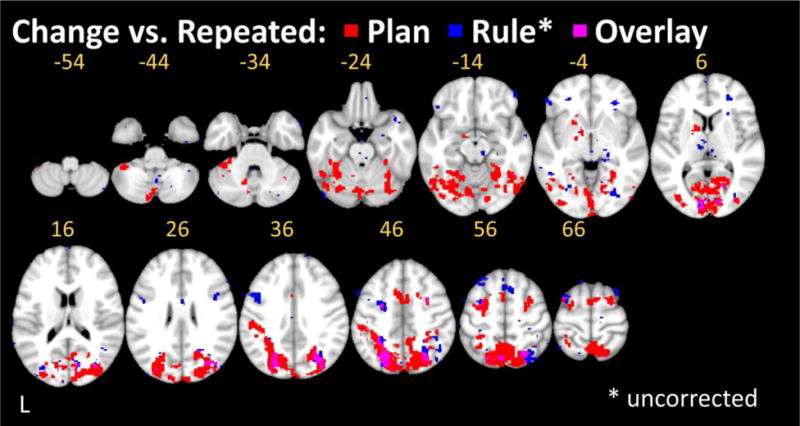
Overlap is prominent in the left IPS, whereby results for the Rule condition are uncorrected for multiple comparisons.
In addition we computed the Plan RS > Rule RS interaction to further analyze selective effects of Plan RS. The interaction of (Plan Change > Repeat) > (Rule Change > Repeat) activated only the right SPL and left Cerebellum (see Supplementary Figure 1). The overlap of the uncorrected Rule RS map and the Plan RS map in Figure 5 illustrates why the interaction does not reach significance in the left parietal lobe. It reveals that the effects are in the same directions - working against a significant interaction, mirroring our behavioral data.
Region-of-interest analysis
To elucidate the effects of repetition suppression in each condition further, we undertook an ROI analysis within regions chosen a priori on the basis of past research findings as detailed above. Planning-specific effects would be demonstrated by a Plan RS > Rule RS interaction. A repeated measures ANOVA with a 2 (Condition: Plan, Rule) × 2 (Congruency: Repeated, Changed) × 8 (ROI) factorial design resulted in a significant three-way interaction of condition*congruency*ROI (Greenhouse Geisser: F(2.48, 47.16)=3.86, p=.021). The subsequent 2 (Condition: Plan, Rule) × 2 (Congruency: Repeated, Changed) analysis for each ROI revealed a significant interaction in the left SMG [F (1, 19)=5.03. p=.037] and marginal effects for the left Cerebellum [F (1, 19)=3.82. p=.065] and bilateral caudal SPL [left: F (1, 19)=4.16. p=.056; right: F (1, 19)=3.87. p=.064] (Figure 6). Post-hoc t-tests for those ROI regions, confirmed reduced activity for repeated vs. changed trials exclusively in the Plan condition within the left SMG (p=.009), bilateral caudal SPL (p<.001) and left cerebellum (p=.009), whereby according to Bonferroni correction for multiple comparisons, significance should only be considered at p<.006. For detailed ANOVA results and post hoc statistics please see supplementary tables 2 to 4.
Figure 6. The figure displays percent signal change in selected ROIs separately per condition (Plan, Rule) and trialtype (Congruency: Repeat, Change).
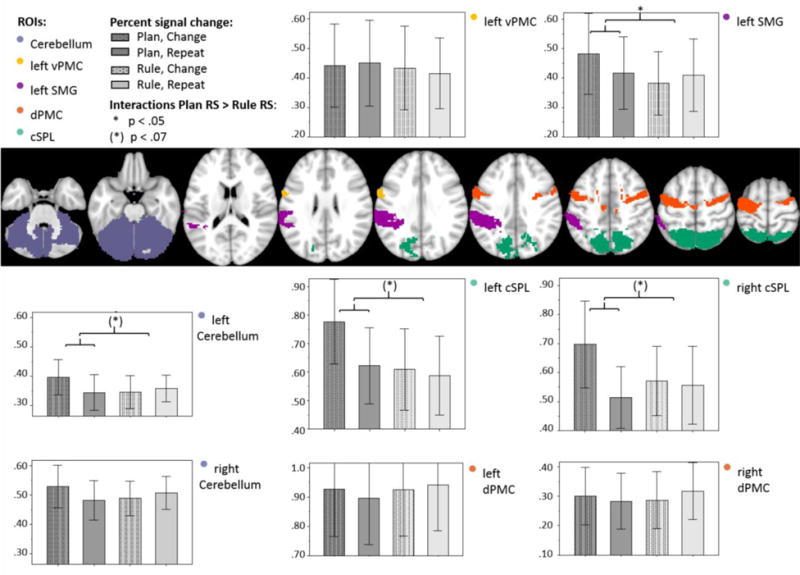
Percent signal change from the resting baseline within anatomical ROIs which were further delimited by the Rule + Plan vs baseline contrast. The interaction effect of condition × congruency was found to be significant in the left SMG (p<.05). Further effects were found in the cerebellum and bilateral cSPL (p<.07). The interaction results demonstrate planning specific efficiency effects. Errorbars represent 95% CI.
Discussion
While a number of prior studies have used RS-fMRI to investigate mechanisms involved in action production, to our knowledge, this is the first study to employ fMRI-RS to isolate mechanisms involved in action selection under conditions of flexible stimulus-response mappings that are believed to involve the use of prospective planning. Importantly, in contrast with prior studies that examined prospective planning with conventional fMRI designs, this RS approach allowed us to isolate planning mechanisms while controlling fully for stimulus and response processing demands. Consistent with prior behavioral work (Randerath, et al., 2015), we found evidence for greater behavioral priming of action selection in both the experimental (Plan) and the control condition: in each circumstance, participants benefitted significantly (decreased RTs) on repeated vs. changed trials, and the magnitude of this priming effect showed a non-significant tendency to be larger for the Plan condition. Behavioral priming in the Plan condition was furthermore associated with significant RS (i.e., a reduced BOLD response for repeated vs. changed trials) in left SMg extending into and along the length of the IPS, in the bilateral caudal SPL, and the cerebellar hemispheres and vermis. In frontal cortex, RS in the Plan condition was present bilaterally within dPMC and the pre-SMA. However, interaction analyses of Plan RS > Rule RS did not demonstrate any plan specific efficiency effects in frontal regions. Instead, the ROI analysis showed a significant interaction of condition and congruency for the left SMG. A strong trend (p<.07) for Plan-specific RS were also found for the left Cerebellum and bilateral SPL. Thus, the RS contrast within the Plan condition, the Plan versus Rule contrast and the ROI analysis exploring RS specific effects for the Plan condition all demonstrated strong involvement of parietal and cerebellar regions and that these effects are substantially left lateralized. These results are consistent with prior findings from conventional fMRI designs with tasks involving prospective planning in the absence of overt execution of selected actions (Jacobs, et al., 2010; Johnson, 2002; Marangon, et al., 2011; Martin, et al., 2011). Demonstration of these effects under conditions where stimulus and response demands are fully controlled and in which selected actions are chosen strengthens the assertion that parieto-cerebellar networks are engaged in the prospective action planning.
In the conventional whole brain analysis, we also found unexpected bilateral RS in the lateral and medial occipital cortex, as well as left-lateralized basal ganglia. In our previous work, we have frequently observed increased activity in lateral occipital and/or adjacent caudal temporal cortex in association with motor planning (Jacobs, et al., 2010; Johnson, 2002; Marangon, et al., 2011; Martin, et al., 2011). There are several possible interpretations of these effects including imagined motion of the limb driving activity in motion processing centers, or possible involvement of the extrastriate body area in action planning.
One concern for the interpretation of selective RS effects in the Plan condition is that the Plan RS > Rule RS interaction results were not entirely concordant across analysis approaches. However, the occurring differences appear to be due to focused ROI tests being more sensitive compared to whole-brain analyses approaches. The hypothesis-driven ROI analyses found a significant Condition * Congruency interaction in left SMG (Figure 6) and a trend (p<.07) for bilateral SPL and left cerebellum whereas the whole-brain analysis detected a significant interaction only in right SPL and left cerebellum (Supplementary Figure 1). The difference likely can be explained by the Rule RS effects appearing below threshold in left parietal regions (see also Figure 5). Here the conventional interaction contrast may not be sensitive enough to detect the different magnitude in RS effects between conditions. Thus the more focused and sensitive ROI results demonstrate effects that are otherwise missed by the conventional analysis. Further, the interaction results in the ROI analysis are in line with both, the main effect found for Plan RS and the results of the Plan > Rule condition-contrast. Overall, the accumulated evidence supports the interpretation of a substantial left cerebral asymmetry of activations in parietal regions associated with action selection under conditions of flexible stimulus-response mappings.
Despite evidence for behavioral priming, significant accompanying RS effects were absent in the control condition where action selection was based on a simple rule. This strengthens the claim that participants were compliant in using planning, rather than some heuristic, when selecting actions in the Plan condition. It also helps to rule out the possibility that RS effects in the Plan condition are attributable to some generalized decrease in processing demands associated with repeated task elements including stimulus and/or response processing. We now discuss these main results in greater detail, along with their implications and limitations.
Behavioral Priming
Consistent with earlier results (Randerath, et al., 2015), RTs to probe events were significantly longer in the Plan vs. Rule condition. In both conditions, RTs were reduced when preceded by a prime that was identical in both stimulus and response, and there was a non-significant tendency (p = .068) for this priming effect to be greater in the Plan condition. These findings suggest that our Plan condition was more demanding than the Rule condition, and indicate that that processing efficiency in both conditions is facilitated by repetition.
Parietofrontal Repetition Suppression for Plan-based Action Selection
Consistent with our a priori expectations based on earlier fMRI findings from activation paradigms involving similar prospective planning tasks (Jacobs, et al., 2010; Johnson, 2002; Marangon, et al., 2011; Martin, et al., 2011), behavioral priming effects in the Plan condition were accompanied with RS in the left SMg/IPS, bilateral caudal SPL, dPMC, pre-SMA and cerebellum.
A justifiable criticism of earlier fMRI activation studies on prospective planning concerns imperfect control for processes related to stimulus and/or response processing. Because these potentially confounding factors are matched in comparisons between repeated and changed trials, replication of key aspects of these earlier results regarding mechanisms of planning with an RS paradigm is an important advance.
Planning-specific Repetition Suppression Effects
Our results for the Plan condition have implications for the interpretation of prior RS findings involving repeated hand actions. Previous studies reveal RS in some of the same parietal and frontal regions during repeated grasping (Króliczak, Mcadam, Quinlan & Culham, 2008; Monaco, Cavina-Pratesi, Sedda, Fattori, Galletti & Culham, 2011; Monaco, Chen, Medendorp, Crawford, Fiehler & Henriques, 2014) or hand gestures (Chouinard & Goodale, 2009; Dinstein, Hasson, Rubin & Heeger, 2007; Hamilton & Grafton, 2009). While several of these studies distinguished motor- from visually-driven RS (Chouinard & Goodale, 2009; Króliczak, et al., 2008; Monaco, et al., 2011; Monaco, et al., 2014), it has remained unclear whether motor-driven effects reflected the repetition of planning/selection, execution-related processes, or both. Our Condition * Congruency interaction with Plan RS > Rule RS revealed effects in left SMg, bilateral cSPL, as well as the left Cerebellum. These Plan-condition specific effects cannot be explained by the repetition of either the stimulus or the basic motoric elements of responses. We therefore propose that this specific set of areas exhibits relative increases in neural processing efficiency when tasked with action selection based on repeated prospective motor planning, and that these changes contribute to the reductions in RTs (improved behavioral efficiency) noted earlier. In other words, RS effects in the Plan condition appear due to the unique cognitive demands of action selection based on prospective motor planning.
Possible Neural Bases of Planning-based Repetition Suppression
Neuronal-level mechanisms underpinning RS of the BOLD response remain unsettled (Ewbank & Henson, 2012; Grill-Spector, Henson & Martin, 2006; Larsson & Smith, 2012). One way of conceptualizing the plan-based effects reported here derives from the Affordance Competition Hypothesis (ACH) (Cisek, 2007; Cisek & Kalaska, 2010), which posits that action selection is mediated by resolving competition between simultaneously-activated neural populations within sensorimotor control areas that specify the spatiotemporal parameters of possible actions. Indeed, the current RS effects of prospective planning-based selection involve parietal areas that have previously been implicated in the control of hand actions. Activity localized to anterior aspects of the IPS and adjacent SMg corresponds well with the expected location of areas important for grasp control according to prior neuroimaging (Binkofski, Dohle, Posse, Stephan, Hefter, Seitz & Freund, 1998; Cavina-Pratesi, Monaco, Fattori, Galletti, Mcadam, Quinlan, Goodale & Culham, 2010; Culham, Danckert, Desouza, Gati, Menon & Goodale, 2003; Frey, Vinton, Norlund & Grafton, 2005), transcranial magnetic stimulation (TMS) (Rice, Tunik & Grafton, 2006; Tunik, 2005), and neuropsychological (Binkofski, et al., 1998) results. Neuroimaging studies involving reaching/pointing actions similarly reveal regions of increased activity in parietofrontal cortex (Astafiev, Shulman, Stanley, Snyder, Van Essen & Corbetta, 2003; Beurze, De Lange, Toni & Medendorp, 2007; Connolly, Andersen & Goodale, 2003; Medendorp, Goltz, Crawford & Vilis, 2005; Prado, Clavagnier, Otzenberger, Scheiber, Kennedy & Perenin, 2005; Tosoni, Galati, Romani & Corbetta, 2008), TMS to the more superior parietal areas impairs reaching performance (Vesia, Prime, Yan, Sergio & Crawford, 2010), and damage here can result in optic ataxia—a disorder characterized by difficulties using visual information to guide and control the arm/hand for actions (Bálint, 1909; Jakobson, Archibald, Carey & Goodale, 1991; Karnath & Perenin, 2005; Perenin & Vighetto, 1988).
According to the ACH, populations encoding similar metrics of possible actions excite one another, while populations encoding distinct metrics inhibit one another (Cisek, 2007). When the activity of one population reaches a particular threshold, its activity levels further increase and competing populations are inhibited. Baseline activity that remained elevated from recently selected (or suppressed for recently non-selected) populations would result in more (versus less) efficient selection for repeated versus non-repeated trials (for similar interpretation see also Valyear & Frey, 2015). Similarly, if the range of responsive populations competing for selection were to be narrowed for repeated vs. changed successive actions, reduced fMRI activity levels would arise. This latter account coincides with ‘sharpening’ models of priming and fMRI-RS (Wiggs & Martin, 1998).
Absence of Significant Repetition Suppression for Rule-based Action Selection
In stark contrast to the Plan condition, and despite significant behavioral priming (see Figure 2), we failed to detect any significant RS effects for the Rule condition. Though caution is needed when interpreting the failure to detect significant RS for the Rule condition, several possibilities that may stimulate further research are worth considering. One potential explanation is that the RS effects are related to overall difficulty. Indeed, RTs for the Plan condition exceeded those of the Rule condition. Because RTs were statistically controlled for in the fMRI analyses, however, this seems unlikely to provide a complete account of these differences.
Another possibility is that the reduction in processing demands between repeated versus changed trials in the Rule condition are negligible, and thus fail to yield significant RS effects. Our behavioral results indicate that the efficiency of processes underlying response selection for the Rule condition is already high relative to the Plan condition, i.e., overall RTs are lower than in the Plan condition and although significant, decreases for repeated vs. changed trials tended to be more modest. The two prescriptions used in the Rule condition were relatively simple and easily learned. Both repeated and changed trials in the Rule condition are furthermore heavily dependent on holding information online. Prime stimuli serve to unambiguously cue (prescribe) the required response and once this representation is activated it can either be repeated in the probe event, or they can receive a new cue instructing them to switch to the other rule and associated action. In either case, because only two simple rules are used, the processing load may be quite similar in repeated and changed trials, and these modest demands on rule-based selection may not engage expected prefrontal and premotor mechanisms. If so, then RS effects might be apparent in a similar condition with more complex rules (cf.(Chouinard & Goodale, 2009; Dinstein, et al., 2007). A related possibility is that in the Rule condition, participants may treat the probe as unique from the preceding prime; i.e., encoding the stimuli and determining the associated response anew rather than holding on to information from the prime event. In other words, the rule representation activated by the prime may be more fleeting, decaying prior to the onset of the probe. An argument against this explanation is the modest yet significant reduction in RTs for repeated vs. changed trials. However, these behavioral effects could reflect repetition of stimulus and/or response processes. Clearly, the discrepancy between our results and the larger existing literature on the involvement of frontal and prefrontal regions in rule-based selection mandates additional research.
Supplementary Material
Figure 3. Behavioral response times.
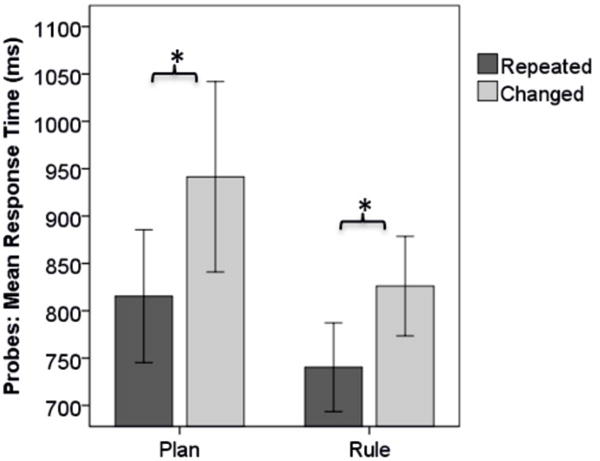
Planning is apparent through significantly prolonged RT in the Plan- compared to the Rule condition. Priming occurs in both tasks, showing faster responses to the probe for repeated events. (Error bars: 95% Confidence Interval)
Highlights.
Stronger effects of repetition in flexible (Planning) vs. fixed (Rules) responses
These effects were apparent in behavioral priming and fMRI repetition suppression
Planning-specific effects were found predominantly in the parietal lobule
Acknowledgments
This work was funded by grants from the James S McDonnell Foundation (#220020190) and NIH/NINDS (#NS053962) to S.H.F. Thanks to Nathan Baune and Ronny Wolfram for their assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests.
References
- Andersson JLR, Jenkinson M, Smith SM. Non-linear optimisation 2007 [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bálint D. Seelenlähmung des “Schauens”, optische Ataxie, räumliche Störung der Aufmerksamkeit. European Neurology. 1909;25:51–66. 51–66. [Google Scholar]
- Beckmann C, Smith S. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beurze SM, De Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. Journal of Neurophysiology. 2007;97:188–199. doi: 10.1152/jn.00456.2006. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–1259. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Monaco S, Fattori P, Galletti C, Mcadam TD, Quinlan DJ, Goodale MA, Culham JC. Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. The Journal of neuroscience. 2010;30:10306–10323. doi: 10.1523/JNEUROSCI.2023-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Goodale MA. FMRI adaptation during performance of learned arbitrary visuomotor conditional associations. Neuroimage. 2009;48:696–706. doi: 10.1016/j.neuroimage.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2007;362:1585–1599. doi: 10.1098/rstb.2007.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annual review of neuroscience. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a ‘parietal reach region’ in the human brain. Exp Brain Res. 2003;153:140–145. doi: 10.1007/s00221-003-1587-1. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, Desouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res. 2003;153:180–189. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger D. Brain Areas Selective for Both Observed and Executed Movements. Journal of Neurophysiology. 2007;98:1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders FC. On the speed of mental processes. Acta Psychologica. 1969;30:412–431. doi: 10.1016/0001-6918(69)90065-1. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Henson RN. Explaining away repetition effects via predictive coding. Cogn Neurosci. 2012;3:239–240. doi: 10.1080/17588928.2012.689960. [DOI] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Res Cogn Brain Res. 2005;23:397–405. doi: 10.1016/j.cogbrainres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grillspector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Repetition suppression for performed hand gestures revealed by fMRI. Human brain mapping. 2009;30:2898–2906. doi: 10.1002/hbm.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R, Rugg M. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Horner A, Henson R. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46:1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Danielmeier C, Frey SH. Human anterior intraparietal and ventral premotor cortices support representations of grasping with the hand or a novel tool. Journal of Cognitive Neuroscience. 2010;22:2594–2608. doi: 10.1162/jocn.2009.21372. [DOI] [PubMed] [Google Scholar]
- Jakobson L, Archibald Y, Carey D, Goodale MA. A kinematic analysis of reaching and grasping movements in a patient recovering from optic ataxia. Neuropsychologia. 1991;29:803–809. doi: 10.1016/0028-3932(91)90073-h. [DOI] [PubMed] [Google Scholar]
- Janssen L, Meulenbroek RG, Steenbergen B. Behavioral evidence for left-hemisphere specialization of motor planning. Experimental Brain Research. 2011;209:65–72. doi: 10.1007/s00221-010-2519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnson S. Selective Activation of a Parietofrontal Circuit during Implicitly Imagined Prehension. Neuroimage. 2002;17:1693–1704. doi: 10.1006/nimg.2002.1265. [DOI] [PubMed] [Google Scholar]
- Johnson SH. Thinking ahead: the case for motor imagery in prospective judgements of prehension. Cognition. 2000;74:33–70. doi: 10.1016/s0010-0277(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Perenin MT. Cortical control of visually guided reaching: evidence from patients with optic ataxia. Cereb Cortex. 2005;15:1561–1569. doi: 10.1093/cercor/bhi034. [DOI] [PubMed] [Google Scholar]
- Króliczak G, Mcadam T, Quinlan D, Culham J. The human dorsal stream adapts to real actions and 3D shape processing: a functional magnetic resonance imaging study. J Neurophysiol. 2008;100:2627–2639. doi: 10.1152/jn.01376.2007. [DOI] [PubMed] [Google Scholar]
- Larsson J, Smith AT. fMRI repetition suppression: neuronal adaptation or stimulus expectation? Cerebral Cortex. 2012;22:567–576. doi: 10.1093/cercor/bhr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangon M, Jacobs S, Frey SH. Evidence for context sensitivity of grasp representations in human parietal and premotor cortices. J Neurophysiol. 2011;105:2536–2546. doi: 10.1152/jn.00796.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K, Jacobs S, Frey SH. Handedness-dependent and -independent cerebral asymmetries in the anterior intraparietal sulcus and ventral premotor cortex during grasp planning. Neuroimage. 2011;57:502–512. doi: 10.1016/j.neuroimage.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Crawford JD, Vilis T. Integration of target and effector information in human posterior parietal cortex for the planning of action. Journal of Neurophysiology. 2005;93:954–962. doi: 10.1152/jn.00725.2004. [DOI] [PubMed] [Google Scholar]
- Monaco S, Cavina-Pratesi C, Sedda A, Fattori P, Galletti C, Culham JC. Functional magnetic resonance adaptation reveals the involvement of the dorsomedial stream in hand orientation for grasping. Journal of Neurophysiology. 2011;106:2248–2263. doi: 10.1152/jn.01069.2010. [DOI] [PubMed] [Google Scholar]
- Monaco S, Chen Y, Medendorp WP, Crawford JD, Fiehler K, Henriques DY. Functional magnetic resonance imaging adaptation reveals the cortical networks for processing grasp-relevant object properties. Cerebral Cortex. 2014;24:1540–1554. doi: 10.1093/cercor/bht006. [DOI] [PubMed] [Google Scholar]
- Perenin MT, Vighetto A. Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain. 1988;111(Pt 3):643–674. doi: 10.1093/brain/111.3.643. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2:67. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J, Clavagnier S, Otzenberger H, Scheiber C, Kennedy H, Perenin MT. Two cortical systems for reaching in central and peripheral vision. Neuron. 2005;48:849–858. doi: 10.1016/j.neuron.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Randerath J, Valyear KF, Hood A, Frey SH. Two routes to the same action: an action repetition priming study. J Mot Behav. 2015;47:142–152. doi: 10.1080/00222895.2014.961891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice NJ, Tunik E, Grafton ST. The anterior intraparietal sulcus mediates grasp execution, independent of requirement to update: new insights from transcranial magnetic stimulation. J Neurosci. 2006;26:8176–8182. doi: 10.1523/JNEUROSCI.1641-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DA, Jorgensen MJ. Planning macroscopic aspects of manual control. Human Movement Science. 1992;11:61–69. [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20(Suppl 1):S89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Nixon PD, Wade DT, Renowden S, Passingham RE. The left hemisphere and the selection of learned actions. Neuropsychologia. 1998;36:11–24. doi: 10.1016/s0028-3932(97)00101-2. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MFS, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Tosoni A, Galati G, Romani GL, Corbetta M. Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nature Neuroscience. 2008;11:1446–1453. doi: 10.1038/nn.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST. Virtual lesions of the human anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nature Neuroscience. 2005;8:505–511. doi: 10.1038/nn1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyear KF, Frey SH. Human posterior parietal cortex mediates hand-specific planning. Neuroimage. 2015;114:226–238. doi: 10.1016/j.neuroimage.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesia M, Prime SL, Yan X, Sergio LE, Crawford JD. Specificity of human parietal saccade and reach regions during transcranial magnetic stimulation. The Journal of neuroscience. 2010;30:13053–13065. doi: 10.1523/JNEUROSCI.1644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. USA: Oxford University Press; 2001. pp. 251–270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


