Abstract
Cognitive decline leading to dementia represents a global health burden. In the absence of targeted pharmacotherapy, lifestyle approaches remain the best option for slowing the onset of dementia. However, older adults spend very little time doing moderate to vigorous exercise and spend a majority of time in sedentary behavior. Sedentary behavior has been linked to poor glycemic control and increased risk of all-cause mortality. Here, we explore a potential link between sedentary behavior and brain health. We highlight the role of glycemic control in maintaining brain function and suggest that reducing and replacing sedentary behavior with intermittent light-intensity physical activity may protect against cognitive decline by reducing glycemic variability. Given that older adults find it difficult to achieve current exercise recommendations, this may be an additional practical strategy. However, more research is needed to understand the impact of poor glycemic control on brain function and whether practical interventions aimed at reducing and replacing sedentary behavior with intermittent light intensity physical activity can help slow cognitive decline.
Keywords: Dementia, Alzheimer's disease, Cognitive function, Diabetes, Glucose metabolism, Exercise, Physical activity, Light-intensity activity, Sitting, Sedentary behavior, Breaks in sedentary time
Highlights
-
•
Older adults spend a majority of time in sedentary behavior.
-
•
Sedentary behavior may be linked to cognitive decline via glycemic control.
-
•
Replacing sitting with intermittent light activity can improve glycemic control.
-
•
Future research should determine if light activity can forestall cognitive decline.
-
•
Engaging in more light activity may be an achievable public health target.
1. Introduction
Dementia, which is an umbrella term for conditions characterized by cognitive decline, is a growing global health issue. Combined projections from a meta-analysis predict that global dementia prevalence will double every 20 years [1]. Dementia prevalence also represents a huge global economic cost, estimated to be US $604 billion in 2010 [2]. Strategies that can delay or prevent dementia are urgently needed given the burden it places on individuals, families, and the wider community. It has been estimated that if interventions could delay by 1 year the onset of Alzheimer's disease (AD), the main cause of dementia worldwide, compared with no change in onset, there would be 11.8 million fewer cases of the disease by 2050 [3]. Because there is currently no targeted pharmacotherapy to reduce the risk of dementia in older adults, there is a need to investigate modifiable behavioral risk factors that can attenuate cognitive decline.
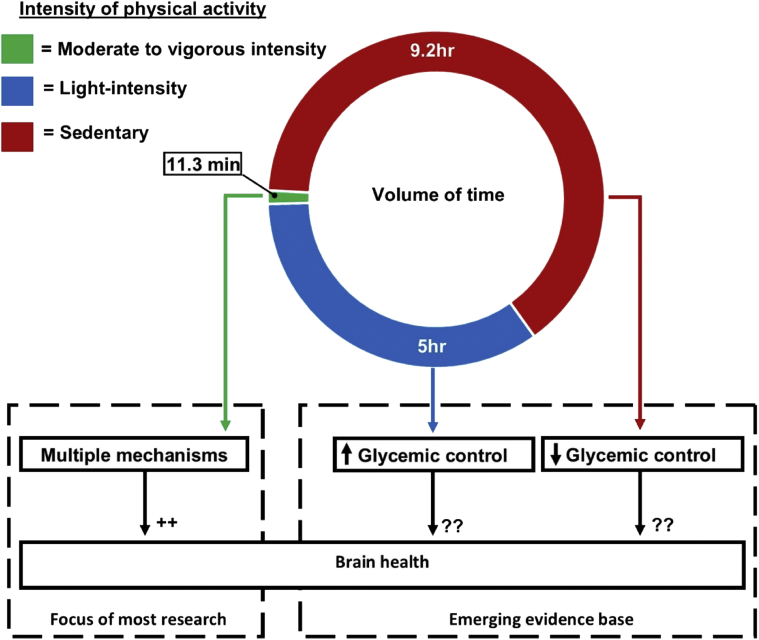
Physical activity acts on multiple mechanisms to elicit improvements in brain health [4]. Most randomized controlled trials supporting the benefits of physical activity for brain function have focused on moderate to vigorous intensity physical activity (MVPA) [4], [5]. This focus is embodied within current public health guidelines, which are based on achieving a minimal level of MVPA. For adults (18–64 years) and older adults (≥65 years), this is set at 150 minutes/week, accumulated in bouts >10 minutes [6]. However, nearly one-third of people worldwide do not achieve this minimum recommended level of MVPA [7]. Moreover, adherence is lowest in older adults, with some estimates indicating that 55% to 70% do not achieve the minimum recommended level of MVPA [8]. Fig. 1 highlights the small volume of time during waking hours, which is spent in MVPA, based on accelerometer data from a sample of older adults [9]. In contrast, a considerably larger volume of time is spent in sedentary behavior and light-intensity activity, but little is known about the implications of these behaviors for brain health.
Fig. 1.
This figure is based on accelerometer data from the U.S. National Health and Nutrition Examination Survey, which shows how 1367 older, overweight adults (mean age = 70.5 years; mean body mass index = 29.7 kg/m2) allocate their time on average throughout the day [9]. Most research on physical activity and brain health focuses on MVPA. However, only a very small proportion of the day is spent in MVPA. Emerging evidence suggests that replacing time spent in sedentary behavior with light-intensity physical activity can improve glycemic control. However, little is known about the implications of this for brain health. Abbreviation: MVPA, moderate to vigorous intensity physical activity.
Time spent in sedentary behavior and light-intensity activity may not be benign. Evidence suggests that excessive sedentary time can increase risk for all-cause mortality and chronic disease such as type 2 diabetes (T2D), even in the presence of regular MVPA to the level advocated within current public health guidelines [10], [11]. Extending these investigations to brain function is a fascinating topic of current research, with early evidence hinting that sedentary behavior may also be detrimental to cognitive function [12]. However, more studies investigating this association are needed, specifically high-quality studies attempting to tease out the independent effects of sedentary behavior from physical activity using objective measures. Also of importance is understanding how sedentary behavior might affect brain function. Controlled experiments suggest that excessive sedentary time has deleterious effects on glycemic control, but reducing and replacing sedentary behavior with intermittent light-intensity activity can ameliorate this effect [13]. In this perspectives article, we outline the potential implications of this for brain function. To do this we first establish how hyperglycemia, hypoglycemia, and cerebral blood flow (CBF) affect brain physiology. We next discuss how dynamic interactions between glycemic control and CBF may influence brain health. Finally, we propose a hypothesis that reducing and replacing sedentary behavior with light-intensity physical activity may protect against cognitive decline by reducing glycemic variability and increasing CBF. In doing so, we highlight future opportunities for both researchers and health practitioners. We argue that not only have current public health approaches failed to motivate a large proportion of the population to reach targets for MVPA, but focusing solely on MVPA ignores the potential health benefits of regular engagement in activities of a lighter intensity. Such evidence may be especially useful in the context of older adults who find it difficult to achieve targets for MVPA.
2. Glycemic control and brain health
The human brain, which accounts for about 2% of body weight, consumes about 20% of the energy required for resting metabolic rate—the minimum energy expenditure required to sustain life [14]. Most of this energy demand is met by utilizing glucose as a fuel, which places importance on glycemic control for maintaining brain health. At the cellular level, glycemic control is dependent on glucose transporters. The transport of glucose across the blood-brain barrier into the extracellular space and subsequent uptake by brain parenchymal cells is facilitated by a concentration gradient, as the transporters responsible are insulin-insensitive transporters such as glucose transporters 1 and 3 (GLUT1 and GLUT3) [15]. In this way, circulating glucose concentration regulates central glucose levels, a mechanism which may play a role in the neuropathology associated with chronic perturbations of circulating glucose [16].
Indeed, older adults with T2D are some 50% more likely to develop dementia relative to those with normal glucose metabolism [17]. Even in individuals without diabetes, higher fasting glucose and HbA1c, which is a measure of average glucose levels over 2 to 3 months, are associated with an increased risk for dementia [18]. Imaging techniques in cognitively normal adults with both prediabetes and T2D indicate that functional change related to glucose metabolism may be an early indicator of AD risk [19]. To help understand the potential link between glucose levels and dementia risk, we explore the dynamics of hyperglycemia, hypoglycemia, and CBF. We acknowledge that this is a glucose-centric view and that other mechanisms including insulin resistance, chronic low-grade inflammation, and oxidative stress contribute to neuropathology, but these aspects are beyond the scope of the present article.
3. Hyperglycemia and cerebral glucose kinetics
Exposure to hyperglycemia can result in decreased blood to brain glucose transport. This concept was first put forward in 1958 when Wyke described “relative cerebral hypoglycemia” [20]. This condition was identified in patients who displayed symptoms of hypoglycemia such as headache, confusion, and motor seizure in the presence of a normal circulating glucose concentration, where symptoms were relieved after an increase in plasma glucose. The first experimental evidence of altered blood to brain glucose transport after prolonged hyperglycemia was published in 1981 [21]. It was reported that in rats prolonged exposure to hyperglycemia followed by a return of glucose levels to normal values inhibited glucose transport into the brain by some 20%, compared with controls [21]. In cognitively normal humans with prediabetes and T2D, insulin resistance—a marker of disease severity—was associated with reduced cerebral glucose metabolism [19]. Taken together, this indicates that hyperglycemia may reduce cerebral glucose metabolism, which could serve initially as a protective mechanism. However, we hypothesize that this protective mechanism may work to exaggerate the effects of subsequent hypoglycemia and ultimately damage brain parenchymal cells by disrupting vital energy supply.
4. Hypoglycemia and brain function
Exposure to hypoglycemia can impair cognition, especially for complex tasks, which may be more sensitive to hypoglycemic impairment. For example, participants with type 1 diabetes who were tested in a driving simulator made more errors when blood glucose was 3.4 to 4.0 mmol/L, compared with driving in the range of 5.6 to 8.3 mmol/L [22]. However, repeated exposure to hypoglycemia may have more serious implications as there is a dose-dependent relationship between a higher number of severe hypoglycemic episodes and increased risk for dementia [23]. The biological mechanisms underpinning the detrimental impact of hypoglycemia on the brain are not fully understood, although insights may come from evidence which highlights a role for glucose concentration in regulating apoptosis, the process of cell death.
Glucose concentration may regulate apoptosis in the brain via a key enzyme of glucose metabolism, hexokinase II (HKII). In neurons and other cells, HKII catalyzes the formation of glucose-6-phosphate, which is the first step in most glucose metabolism pathways. However, HKII has also been described as a metabolic switch capable of turning on and off apoptosis [24]. Under conditions of glucose deprivation, HKII activates apoptosis. This places neurons, with their heavy reliance on glucose, as being particularly susceptible to apoptosis when fuel supply is disrupted [24]. Neuronal apoptosis has serious consequences, as this process is activated in neurodegenerative diseases like AD [25]. Importantly, controlled regulation of glucose protects neurons from apoptosis [24]. The exact concentration of glucose that represents the threshold for initiation of apoptosis is unknown. However, given the brain's high demand for energy, it is logical to suspect that repeated exposures below such a threshold may induce an energy crisis and allow progressive damage to accumulate over time.
5. Defense against neuroglycopenia
Because of the danger posed by a lack of glucose in the brain, known as neuroglycopenia, humans have evolved defensive mechanisms that are initiated when blood glucose falls below the range of 3.6 to 3.8 mmol/L [26]. As glucose levels fall, there is a decrease in secretion of insulin and increased secretion of glucagon and epinephrine to increase glucose levels [26]. However, this defense can be blunted by a single antecedent exposure to hypoglycemia in healthy people [27]. In those with type 1 diabetes and advanced T2D, this compromised defense can be more exaggerated as it occurs in a setting of relative pancreatic β cell failure and failure of endocrine regulation of hypoglycemia. This compromising milieu has been called “hypoglycemia-associated autonomic failure,” which has several purported mechanisms, the details of which can be found elsewhere [28].
Although antecedent hypoglycemia blunts the endocrine defense against hypoglycemia, a backup mechanism exists that acts to increase glucose transport into the brain. This is likely achieved via increases in CBF and upregulation of glucose transporters (GLUT1 and GLUT3), although human evidence for the latter is lacking. However, this backup mechanism takes time to manifest. For example, in healthy participants, 56 hours exposure to interprandial hypoglycemia (2.9 mmol/L) effectively blunted endocrine counter regulation to subsequent hypoglycemia [29]. However, this occurred in the presence of adaptive increases in CBF and brain glucose uptake which preserved cognitive function, relative to hypoglycemic exposure following normal glucose levels, in the same participants [29]. In another study of healthy participants, three exposures to a 30-minute bout of hypoglycemia (2.8 mmol/L) over a 24-hour period effectively blunted endocrine counter-regulation without any adaptive change in blood to brain glucose transport or cerebral glucose metabolism [30]. Taken together, these findings indicate that brief exposures to hypoglycemia may be more conducive to neuroglycopenia than prolonged exposure, because endocrine counter-regulation is compromised faster than the brain can increase blood to brain glucose transport.
6. Blood flow: supplying vital energy to the brain
Brain function is subserved by CBF as the mechanism of substrate delivery. In turn, energy demand in the brain can tightly regulate CBF. The mechanisms that match local neuronal energy demand to glucose and oxygen delivery are dynamic, to protect the brain from potentially hazardous declines in blood glucose. Neuronal energy demand is communicated to the vasculature by vasoactive neurotransmitters, particularly glutamate and by-products of synaptic signaling [31]. Regional CBF is regulated at the level of arterioles via smooth muscle cells and at the capillary level via the pericyte cells, which surround capillaries and can induce constriction or dilation. A comprehensive review of the mechanisms involved is available elsewhere [32]. Because of the tight coupling of CBF to brain function, impaired CBF can have serious consequences for brain health.
Hypoperfusion of the brain may be both a consequence and a cause of early neurodegeneration in both vascular dementia and AD. In healthy participants, ingestion of glucose has been shown to reduce regional CBF [33]. This hints that CBF is acutely sensitive to glucose levels. Both prolonged exposure to hyperglycemia and repeated exposure to hypoglycemia can induce microvascular damage and impair endothelial function leading to cerebral hypoperfusion [34], [35]. In a rat model of chronic hypoperfusion, oxygen and nutrient supply to the brain is reduced, damaging the blood brain barrier, neurons, astrocytes and microglial cells, as well as impairing learning and memory [36]. Reduced CBF also slows the clearance of proteins like amyloid β from the perivascular space [37]. Amyloid β accumulation, which is implicated in the pathogenesis of AD, is toxic to the pericytes surrounding capillaries [38]. Amyloid β accumulation may also eventually affect larger blood vessels causing endothelial cell damage, vasoconstriction, and a decrease in CBF [39]. In those with AD, faster deterioration in regional CBF is positively associated with a more rapid decline in cognition [40]. Thus, once established, hypoperfusion may result in a vicious cycle leading to further decreases in perfusion conducive to neurodegenerative disease. Taken together, these findings highlight the importance of protecting the brain from declines in CBF.
7. Brain exposure to glucose excursions
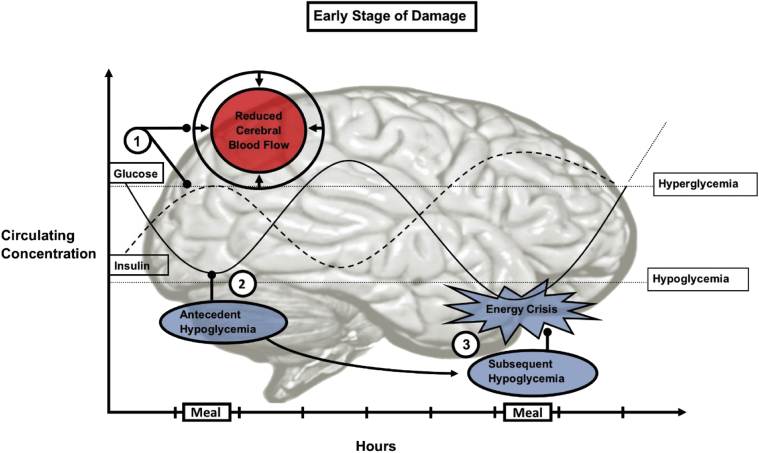
On a daily basis there are dynamic interactions among hyperglycemia, hypoglycemia, and CBF, the severity of which may have implications for brain health. Clarifying how these dynamic physiological states interact with each other may help us understand the effects of poor glycemic control on the brain. We hypothesize that under repetitive conditions of hyperglycemia and hypoglycemia, such that when one follows the other in a continued pattern, a negative feedback loop is established, which can shift brain physiology toward pathophysiology. Fig. 2 illustrates brain exposure to circulating glucose excursions at an early stage of damage in a hypothetical individual.
Fig. 2.
The effects of circulating glucose on the brain at an early stage of damage. This schematic illustrates circulating glucose excursions in response to meals in a hypothetical individual. (1) Acute hyperglycemia in this scenario causes a reduction in regional CBF and a spike in insulin levels to facilitate glucose clearance. (2) These two factors combine to result in a glucose nadir. This glucose nadir can act to impair endocrine counter-regulation to a subsequent dip in glucose, exaggerating the hypoglycemic episode. As this happens over the space of a day, there is not enough time for the brain to compensate via increased CBF or upregulation of glucose transporters. (3) The result is an exaggerated hypoglycemic episode, which can impair endothelial function. This hypoglycemic episode may also be mirrored in the central concentration, depriving neurons of glucose, resulting in an energy crisis. Such a pattern, if continued, may progressively damage the brain. Abbreviation: CBF, cerebral blood flow.
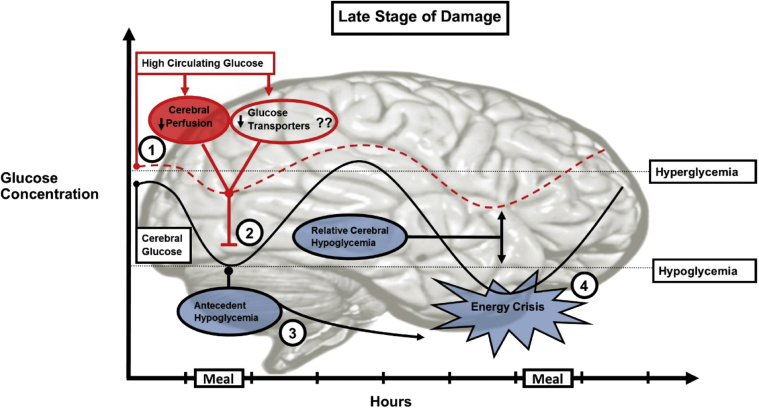
In dysglycemic individuals, the damage sustained by the brain from poor glycemic control may be exacerbated. Fig. 3 depicts both circulating and central glucose levels in a hypothetical individual with an increased fasting glucose level. The increased time spent in hyperglycemia results in adaptation to inhibit blood to brain glucose transport. This adaptation works to lower central glucose concentration relative to circulating concentration; therefore, under these circumstances, it is conceivable that at a normal peripheral concentration of glucose the brain can experience hypoglycemia. This phenomenon is termed relative cerebral hypoglycemia. Ensuing exposure to hypoglycemia can cause an energy crisis, which can induce neuroglycopenia and further impair CBF, paving the way for a recurring pattern of hypoglycemia and associated damage. This accumulating damage may ultimately result in measurable cognitive dysfunction and neuropathology.
Fig. 3.
Brain exposure to glucose excursions at a late stage of damage. This schematic illustrates both circulating and central glucose excursions in response to meals in a hypothetical individual with increased fasting glucose level. (1) The increased time spent in hyperglycemia induces damage to pericytes and endothelial dysfunction of brain arterioles, resulting in chronic hypoperfusion and decreased blood to brain glucose transport. Downregulation of glucose transporters may also contribute to decreased glucose transport, although human evidence for this is lacking. (2) This protective mechanism works to lower central glucose relative to circulating concentration. This means that the brain may experience hypoglycemia at a normal circulating glucose level, a phenomenon known as relative cerebral hypoglycemia. (3) The ensuing exposure to hypoglycemia can disable endocrine counter-regulation to subsequent hypoglycemia. (4) Exposure to subsequent hypoglycemia is exaggerated and the ensuing energy crisis may induce neuroglycopenia and the accumulating damage could move the brain toward neuropathology.
Taken together, in an acute setting, switching between hyperglycemia and hypoglycemia allows for an exaggerated glucose nadir and insufficient time for the brain to induce protective mechanisms. This effect is inflated in those with increased fasting glucose levels who may experience relative cerebral hypoglycemia. Given the damage associated with neuroglycopenia, a continued and repeated pattern of excessive glucose excursions could shift brain physiology toward pathophysiology. In the following sections, we will discuss how interventions might manipulate glycemic control with the aim of preserving cognitive function.
8. Implications of glycemic control for brain health
Evidence from a recent systematic review supports the notion that over time, poor glycemic control can impair brain structure and function [41]. However, randomized controlled trials which have investigated this show mixed results. For example, a large trial did not support the idea that intensive glycemic control (HbA1c <6.0%) was associated with improved cognitive function relative to standard therapy (HbA1c < 7.0%–7.9%) after 40 weeks for those with T2D [42]. However, glycemic control was achieved in this trial using antidiabetic drugs (physician guided, individually tailored to reach target HbA1c). Therefore, the adverse effects of this pharmacotherapy may have influenced cognitive function directly. Moreover, glycemic control was defined by HbA1c—a measure of average glucose—which does not capture daily glucose excursions or variability. In a cross-sectional study of 121 older adults with T2D, a measure of glucose variability called the mean amplitude of glycemic excursions (average amplitude of glucose excursions, upward and downward, that are >1 standard deviation) was associated with measures of cognitive function (Mini–Mental State Examination and a composite score of attention and executive functioning) [43]. Importantly, this association was independent of fasting glucose, postprandial glycemia and HbA1c—suggesting that glycemic variability itself may be worthy of consideration. Although glycemic variability is more difficult to measure than HbA1c, future trials investigating the effects of glycemic control on brain health should attempt to include it as a measure.
This evidence highlights the potential complications associated with pharmacotherapy for improving cognition by targeting glycemic control. Achieving glycemic control via pharmacotherapy may even be counterproductive, resulting in an increased number of hypoglycemic events, weight gain, and increased mortality following intensive glycemic control, relative to standard therapy [42]. Physical activity, on the other hand, has multiple benefits including better glycemic control and improved cognitive function, and may represent a superior treatment option for protecting brain health. However, there has been increasing attention given to the notion that physical activity behaviors exist across a spectrum of intensity, which could have important challenges and practical implications for older adults.
9. Intensity of physical activity: implications for older individuals
Physical activity behaviors exist across a spectrum of energy expenditure, from sedentary (e.g., quiet sitting), to light-intensity (e.g., gentle walking), through to highly intense (e.g., sprinting) activities. Each of these behaviors may have different implications for glycemic control and brain health. To date, most evidence has investigated the health implications of MVPA. However, for older adults, the vast majority of time is spent in activities of a lower intensity, particularly sedentary behavior, which is defined by its low energy expenditure and a sitting or lying posture [44]. The implications of sedentary behavior (too much sitting) is a topic that has gained much interest lately. For example, a recent meta-analysis of 16 prospective studies with more than 1 million participants indicated a dose-dependent relationship of increased risk for all-cause mortality with higher levels of sitting combined with lower levels of moderate intensity exercise [10]. However, only a high volume of moderate intensity exercise (60–75 minutes/day) appeared to eliminate the increased risk of death from a high amount of sitting (8+ hours/day) [10]. Achieving this amount of exercise may be less feasible, particularly for older adults, than reducing sitting time, in which the goal is to replace or interrupt prolonged sitting with more light-intensity activity. Indeed, objectively measured sedentary behavior and light-intensity activity are strongly and inversely correlated (r = −0.98) [45]. Therefore, reductions in sedentary time are most likely to be achieved via replacement with light-intensity activities. However, whether this can help protect from cognitive decline is unknown.
A recent systematic review of observational evidence, which included three prospective studies, examined the association between sedentary behavior and cognitive function [12]. The authors concluded that increased sedentary behavior is associated with lower cognitive function. However, there were only eight studies included and heterogeneity prevented a calculation of the magnitude of this association. To further inform this observational evidence, controlled experimental evidence is needed. In addition, further insights may come from the evidence linking sedentary behavior to glycemic control, given the importance of glycemic control in maintaining optimal cognitive function.
10. Reducing sedentary behavior: implications for glycemic control
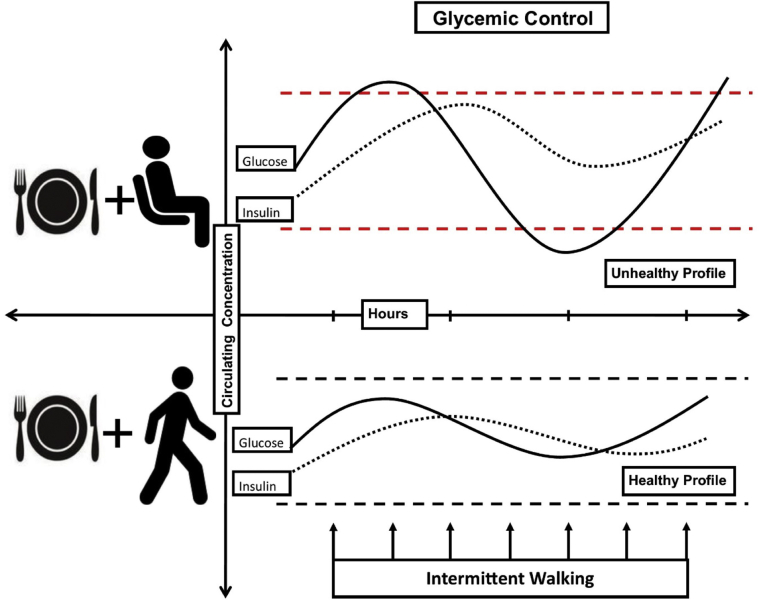
Observational and experimental evidence highlights the detrimental associations that exist between sedentary behavior and glycemic control. A meta-analysis of observational studies indicates that excessive sitting time can increase the risk of developing T2D, an association that remains after controlling for currently recommended levels of MVPA [11]. Moreover, a number of experimental studies in healthy participants and those with T2D have demonstrated that regularly interrupting prolonged sitting with brief light-intensity activity breaks can improve postprandial glycemic control [13]. Potential mechanisms for this improvement are likely to involve muscle contraction and localized increases in skeletal muscle glucose uptake, mediated by both the insulin and contraction-mediated (insulin-independent) glucose uptake pathways [46]. Fig. 4 depicts the acute effects of sedentary behavior and light-intensity activity on glycemic control in response to a meal in two hypothetical scenarios.
Fig. 4.
The effects of sedentary behavior versus light-intensity activity on postprandial glucose profile. This figure illustrates circulating glucose and insulin levels in response to a meal, in two hypothetical scenarios. Dashed lines represent the optimal glucose range between hyperglycemia and hypoglycemia. During prolonged sitting, a lack of contraction-stimulated glucose uptake leads to more extreme glucose excursions. In the presence of intermittent light-intensity activity, glucose levels are more likely to stay within the optimal range.
The greatest improvements in glycemic control in response to reducing and breaking up sitting time have been observed in participants with T2D [47]. This makes sense as T2D is characterized by disordered regulation of glucose levels. In one study of 19 overweight/obese participants with T2D, the effects of three different activity regimens were compared [48]. Each regimen lasted 4 days in duration and were performed in a randomized order, with a 10 day washout between regimens that were as follows: (1) Sitting: 4415 steps/day with sitting 14 hours/day; (2) Exercise: 4823 steps/day with 1.1 hour/day of sitting replaced with three consecutive 20-minute bouts of moderate to vigorous cycling, performed at least 2 hours after breakfast; and (3) “Sit Less”: 17,502 steps/day with 4.7 hours/day of sitting replaced with intermittent standing (2.5 hours) and light-intensity walking (2.2 hours) across the day. All meals and snacks were standardized for days 3 and 4 of each regimen and the primary outcome was 24-hour glycemic control measured on day 4 of each regimen. Results demonstrated that the “Sit Less” regimen reduced mean 24-hour glucose, 24-hour glucose excursions, and duration of hyperglycemia (glucose >10 mmol/L) compared with the “Sitting” regimen. In addition, an estimate of insulin resistance called the homeostatic model assessment for insulin resistance (HOMA-IR), was also reduced following the “Sit Less” compared with the “Sitting” regimen. The “Exercise” regimen also tended to improve these outcomes, albeit to a lesser magnitude than “Sit Less” despite a comparable energy expenditure. The results of this study have generated some important discussion around potential implications [49]. The implications pertaining to glycemic control are that: (1) exercise bouts performed in the morning may not fully compensate for the negative effects of prolonged sitting for the rest of the day; and (2) the duration and frequency of physical activity, aided by timing around meals, may be more important than the intensity of physical activity.
Thus, when it comes to glycemic control, reducing and breaking up sitting time with intermittent light-intensity activity may offer an additional option to structured bouts of MVPA. Considering that the kinetics of central glucose is determined mainly by a concentration gradient, it could be speculated that to some extent, changes in circulating glucose levels may be mirrored in the central concentration, especially in the acute setting. With this in mind, we now discuss some evidence for the effects of light-intensity activity on central glucose kinetics and brain function.
11. Light-intensity activity: implications for central glucose and brain function
Light-intensity activity may elicit beneficial effects on central glucose kinetics, which, in turn, could serve to protect the brain from excessive glucose excursions. In one study of healthy male participants, a 35-minute bout of light-intensity cycling (30% of the maximum volume of oxygen uptake, which is a measure of exercise intensity) resulted in 30% more brain glucose uptake compared with higher intensity cycling (75% of the maximum volume of oxygen uptake) [50]. This is likely because of the preferential use of lactate by the brain in place of glucose during higher intensity activity. In addition, light-intensity cycling has been shown to increase CBF relative to seated baseline in healthy male participants [51]. This suggests that light-intensity activity could increase delivery of glucose to the brain and may be protective during hypoglycemia. However, research investigating whether light-intensity activity has a meaningful protective effect on the brain during hypoglycemia and whether this has implications for cognitive function is lacking.
There is a paucity of evidence on the acute effects of light-intensity activity on cognitive function. A recent trial in 19 healthy but overweight adults (mean age = 57.9 years, mean body mass index = 31.7 kg/m2) compared two 7-hour conditions separated by a 6-day washout: (1) prolonged sitting; and (2) sitting interrupted by intermittent light-intensity walking breaks (3 minutes walking every 30 minutes, total of 30 minutes walking) [52]. Cognitive function, neuroendocrine biomarkers, and subjective fatigue were measured at multiple time points across the day. Although a trend for improvement in episodic memory was observed in the light-intensity walking condition, the difference was nonsignificant. However, there was a significant improvement in subjective fatigue. The study was likely underpowered to detect any effect on cognition attributable to the 30 minutes of total accumulated walking. In another study, healthy but overweight adults performed 2.5 hours of accumulated walking breaks across an 8-hour day, the results demonstrated improved scores on a battery of cognitive tests (z-score effect size of d = 0.71), relative to 8 hours of sitting [53].
This said, it may be that the greatest benefit of light-intensity activity for the brain does not manifest in the acute setting, but rather over a longer period of time following protection from repeated glucose excursions. However, longer term trials investigating whether light-intensity activity can protect against cognitive decline are lacking. In one study of dementia patients in a nursing home setting, a 9-month tai chi intervention successfully preserved cognitive function relative to a control group who performed simple handicrafts [54]. However, tai chi may be different to other forms of light-intensity activity, as it requires memorization of complex moves, making it difficult to generalize to other forms of light-intensity activity. A recent meta-analysis examined randomized controlled trials of walking interventions in sedentary older adults with executive function as an outcome, showing a small overall benefit but only for those without cognitive impairment [55]. However, low adherence in those with cognitive impairment may have confounded results and the intensity of all walking interventions was not reported and likely contained a mix of light and moderate intensity interventions.
Taken together, the evidence to date hints that excessive sedentary time and thus insufficient light-intensity activity could be detrimental to both glycemic control and cognition, however, more research is needed. Experimental models in the acute setting have highlighted the benefits of replacing sedentary behavior with light-intensity activity for glycemic control, especially after meals. Given the potential damage to the brain caused by poor glycemic control over time, future research should investigate whether chronic interventions aimed at increasing intermittent light-intensity activity may also protect from cognitive decline. From a public health perspective, this is likely to have many advantages, most notably due to broad opportunities to imbed more light-intensity activity throughout the day. This may be especially important for older adults who find it difficult to engage in MVPA.
12. Conclusion
There is a clear need for effective and feasible intervention strategies to help preserve brain health and cognition in older adults. Evidence-based public health messages have emphasized MVPA for its ability to improve cognitive function. Despite this, older adults spend very little time doing MVPA on a daily basis. The greatest proportion of time is spent in activities of a lower intensity, which may have implications for glycemic control and ultimately brain health. The take home message is that reducing and breaking up sitting time with intermittent light-intensity activity may play a role in maintaining glycemic control and optimal brain health. While structured MVPA retains distinct physiological adaptations important for improving cognitive function, reducing and replacing sedentary behavior with intermittent light-intensity activity may be important in forestalling cognitive decline. However, the evidence-base supporting this idea is currently lacking and should be a target for future research. Such evidence would provide an additional option, alongside structured MVPA, in the arsenal of targeted interventions, policies, and programme development aimed at preventing dementia.
Research in context.
-
1.
Systematic review: We reviewed the literature relating to sedentary behavior, glycemic control, and cognitive decline on relevant databases (Medline and Embase).
-
2.
Interpretation: Older adults spend most of time in sedentary behavior and this may contribute to cognitive decline via effects on glycemic control. We propose a hypothesis that reducing and replacing sedentary behavior with light-intensity physical activity may protect against cognitive decline by reducing glycemic variability and increasing cerebral blood flow.
-
3.
Future directions: Specifically future research should focus on understanding: (1) the mechanisms underpinning neuroglycopenia; (2) whether reducing and replacing sedentary behavior with intermittent light-intensity activity has any additive benefit to glycemic control or cognition over and above exercise at a moderate to vigorous intensity; (3) whether engaging in intermittent light-intensity activity is a feasible intervention, which can forestall cognitive decline in those who struggle to engage in moderate to vigorous intensity physical activity.
Acknowledgments
This work was supported by funding from the National Health and Medical Research Council of Australia (NHMRC) Grant 1062338 and by the Victorian Government's Operational Infrastructure Support Program. M.J.W. was supported by the University of Western Australia and the Baker Heart and Diabetes Institute, Melbourne, Australia. P.C.D. was supported by Swinburne University, Melbourne, Australia. M.S.G. was supported by the Baker Heart and Diabetes Institute Flack Fellowship. K.A.E. was supported by the University of Melbourne. P.A.G was supported by a NHMRC and Australian Research Council (ARC) joint Dementia Research Development Fellowship (NHMRC-ARC APP1103311). D.J.G. was supported by a NHMRC Principal Research Fellowship (APP1080914) and D.W.D. was supported by a NHMRC Senior Research Fellowship (NHMRC APP1078360).
Footnotes
Declarations: The authors declare that this work is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and if accepted, it will not be published elsewhere including electronically in the same form in English or any other language, without the written consent of the copyright holder.
References
- 1.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9 doi: 10.1016/j.jalz.2012.11.007. 63–75.e2. [DOI] [PubMed] [Google Scholar]
- 2.Wimo A., Jönsson L., Bond J., Prince M., Winblad B. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9 doi: 10.1016/j.jalz.2012.11.006. 1–11.e3. [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 4.Jensen C.S., Hasselbalch S.G., Waldemar G., Simonsen A.H. Biochemical markers of physical exercise on mild cognitive impairment and dementia: systematic review and perspectives. Front Neurol. 2015;6:187. doi: 10.3389/fneur.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng G., Xia R., Zhou W., Tao J., Chen L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016;50:1443. doi: 10.1136/bjsports-2015-095699. [DOI] [PubMed] [Google Scholar]
- 6.Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.-M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 7.Hallal P.C., Andersen L.B., Bull F.C., Guthold R., Haskell W., Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 8.Keadle S.K., McKinnon R., Graubard B.I., Troiano R.P. Prevalence and trends in physical activity among older adults in the United States: a comparison across three national surveys. Prev Med (Baltim) 2016;89:37–43. doi: 10.1016/j.ypmed.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempsey P.C., Owen N., Biddle S.J.H., Dunstan D.W. Managing sedentary behavior to reduce the risk of diabetes and cardiovascular disease. Curr Diab Rep. 2014;14:522. doi: 10.1007/s11892-014-0522-0. [DOI] [PubMed] [Google Scholar]
- 10.Ekelund U., Steene-Johannessen J., Brown W.J., Fagerland M.W., Owen N., Powell K.E. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet (London, England) 2016;388:1302–1310. doi: 10.1016/S0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- 11.Biswas A., Oh P.I., Faulkner G.E., Bajaj R.R., Silver M.A., Mitchell M.S. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults. Ann Intern Med. 2015;162:123. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 12.Falck R.S., Davis J.C., Liu-Ambrose T. What is the association between sedentary behaviour and cognitive function? A systematic review. Br J Sports Med. 2017;51:800–811. doi: 10.1136/bjsports-2015-095551. [DOI] [PubMed] [Google Scholar]
- 13.Benatti F.B., Ried-Larsen M. The effects of breaking up prolonged sitting time: a review of experimental studies. Med Sci Sports Exerc. 2015;47:2053–2061. doi: 10.1249/MSS.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 14.Rolfe D.F., Brown G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 15.Nijland P.G., Michailidou I., Witte M.E., Mizee M.R., Van Der Pol S.M.A., Van Het Hof B. Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia. 2014;62:1125–1141. doi: 10.1002/glia.22667. [DOI] [PubMed] [Google Scholar]
- 16.Shah K., DeSilva S., Abbruscato T. The role of glucose transporters in brain disease: diabetes and Alzheimer’s disease. Int J Mol Sci. 2012;13:12629–12655. doi: 10.3390/ijms131012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng G., Huang C., Deng H., Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 18.Crane P.K., Walker R., Hubbard R.A., Li G., Nathan D.M., Zheng H. Glucose levels and risk of dementia. N Engl J Med. 2013;369:540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker L.D., Cross D.J., Minoshima S., Belongia D., Watson G.S., Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyke B.D. Electroencephalographic studies in the syndrome of relative cerebral hypoglycaemia. Electroencephalogr Clin Neurophysiol. 1958;11:602. [Google Scholar]
- 21.Gjedde A., Crone C. Blood-brain glucose transfer: repression in chronic hyperglycemia. Science. 1981;214:456–457. doi: 10.1126/science.7027439. [DOI] [PubMed] [Google Scholar]
- 22.Cox D.J., Gonder-Frederick L.A., Kovatchev B.P., Julian D.M., Clarke W.L. Progressive hypoglycemia’s impact on driving simulation performance. Occurrence, awareness and correction. Diabetes Care. 2000;23:163–170. doi: 10.2337/diacare.23.2.163. [DOI] [PubMed] [Google Scholar]
- 23.Whitmer R.A., Karter A.J., Yaffe K., Quesenberry C.P., Jr., Selby J.V. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mergenthaler P., Kahl A., Kamitz A., van Laak V., Stohlmann K., Thomsen S. Mitochondrial hexokinase II (HKII) and phosphoprotein enriched in astrocytes (PEA15) form a molecular switch governing cellular fate depending on the metabolic state. Proc Natl Acad Sci U S A. 2012;109:1518–1523. doi: 10.1073/pnas.1108225109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sastry P.S., Rao K.S. Apoptosis and the nervous system. J Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- 26.Cryer P.E. Hierarchy of physiological responses to hypoglycemia: relevance to clinical hypoglycemia in type I (insulin dependent) diabetes mellitus. Horm Metab Res. 1997;29:92–96. doi: 10.1055/s-2007-978997. [DOI] [PubMed] [Google Scholar]
- 27.Heller S.R., Cryer P.E. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40:223–226. doi: 10.2337/diab.40.2.223. [DOI] [PubMed] [Google Scholar]
- 28.Longo D.L., Cryer P.E. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369:362–372. doi: 10.1056/NEJMra1215228. [DOI] [PubMed] [Google Scholar]
- 29.Boyle P.J., Nagy R.J., O'Connor A.M., Kempers S.F., Yeo R.A., Qualls C. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci U S A. 1994;91:9352–9356. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Criego A.B., Tkac I., Kumar A., Thomas W., Gruetter R., Seaquist E.R. Brain glucose concentrations in healthy humans subjected to recurrent hypoglycemia. J Neurosci Res. 2005;82:525–530. doi: 10.1002/jnr.20654. [DOI] [PubMed] [Google Scholar]
- 31.Busija D.W., Bari F., Domoki F., Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-D-aspartate in cerebral cortex. Brain Res Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attwell D., Buchan A., Charpak S., Lauritzen M., MacVicar B., Newman E. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–241. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page K.A., Chan O., Arora J., Belfort-Deaguiar R., Dzuira J., Roehmholdt B. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309:63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joy N.G., Tate D.B., Younk L.M., Davis S.N. Effects of acute and antecedent hypoglycemia on endothelial function and markers of atherothrombotic balance in healthy humans. Diabetes. 2015;64:2571–2580. doi: 10.2337/db14-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geraldes P., Hiraoka-Yamamoto J., Matsumoto M., Clermont A., Leitges M., Marette A. Activation of PKCδ and SHP1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farkas E., Luiten P.G.M., Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Ball K.K., Cruz N.F., Mrak R.E., Dienel G.A. Trafficking of glucose, lactate, and amyloid-β from the inferior colliculus through perivascular routes. J Cereb Blood Flow Metab. 2010;30:162–176. doi: 10.1038/jcbfm.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelmus M.M., Otte-Holler I., van Triel J.J., Veerhuis R., Maat-Schieman M.L.C., Bu G. Lipoprotein receptor-related protein-1 mediates amyloid-beta-mediated cell death of cerebrovascular cells. Am J Pathol. 2007;171:1989–1999. doi: 10.2353/ajpath.2007.070050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas T., Thomas G., McLendon C., Sutton T., Mullan M. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 40.Hanyu H., Sato T., Hirao K., Kanetaka H., Iwamoto T., Koizumi K. The progression of cognitive deterioration and regional cerebral blood flow patterns in Alzheimer’s disease: a longitudinal SPECT study. J Neurol Sci. 2010;290:96–101. doi: 10.1016/j.jns.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Geijselaers S.L., Sep S.J., Stehouwer C.D.A., Biessels G.J. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3:75–89. doi: 10.1016/S2213-8587(14)70148-2. [DOI] [PubMed] [Google Scholar]
- 42.Launer L.J., Miller M.E., Williamson J.D., Lazar R.M., Gerstein H.C., Murray A.M. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10:969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizzo M.R., Marfella R., Barbieri M., Boccardi V., Vestini F., Lettieri B. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care. 2010;33:2169–2174. doi: 10.2337/dc10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37:540–542. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 45.Healy G.N., Matthews C.E., Dunstan D.W., Winkler E.A.H., Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 200306. Eur Heart J. 2011;32:590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergouignan A., Latouche C., Heywood S., Grace M.S., Reddy-Luthmoodoo M., Natoli A.K. Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: ancillary analysis from randomized clinical trials. Sci Rep. 2016;6:32044. doi: 10.1038/srep32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dempsey P.C., Owen N., Yates T.E., Kingwell B.A., Dunstan D.W. Sitting less and moving more: improved glycemic control for type 2 diabetes prevention and management. Curr Diab Rep. 2016;16:114. doi: 10.1007/s11892-016-0797-4. [DOI] [PubMed] [Google Scholar]
- 48.Duvivier B.M.F.M., Schaper N.C., Hesselink M.K.C., van Kan L., Stienen N., Winkens B. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycemic control and insulin sensitivity in type 2 diabetes. Diabetologia. 2017;60:490–498. doi: 10.1007/s00125-016-4161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dempsey P.C., Grace M.S., Dunstan D.W. Adding exercise or subtracting sitting time for glycemic control: where do we stand? Diabetologia. 2017;60:390–394. doi: 10.1007/s00125-016-4180-4. [DOI] [PubMed] [Google Scholar]
- 50.Kemppainen J., Aalto S., Fujimoto T., Kalliokoski K.K., Langsjo J., Oikonen V. High intensity exercise decreases global brain glucose uptake in humans. J Physiol. 2005;568:323–332. doi: 10.1113/jphysiol.2005.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith K.J., Wong L.E., Eves N.D., Koelwyn G.J., Smirl J.D., Willie C.K. Regional cerebral blood flow distribution during exercise: influence of oxygen. Respir Physiol Neurobiol. 2012;184:97–105. doi: 10.1016/j.resp.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 52.Wennberg P., Boraxbekk C.-J., Wheeler M., Howard B., Dempsey P.C., Lambert G. Acute effects of breaking up prolonged sitting on fatigue and cognition: a pilot study. BMJ Open. 2016;6:e009630. doi: 10.1136/bmjopen-2015-009630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mullane S.L., Buman M.P., Zeigler Z.S., Crespo N.C., Gaesser G.A. Acute effects on cognitive performance following bouts of standing and light-intensity physical activity in a simulated workplace environment. J Sci Med Sport. 2016;20:489–493. doi: 10.1016/j.jsams.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Cheng S.-T., Chow P.K., Song Y.-Q., Yu E.C.S., Chan A.C.M., Lee T.M.C. Mental and physical activities delay cognitive decline in older persons with dementia. Am J Geriatr Psychiatry. 2014;22:63–74. doi: 10.1016/j.jagp.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 55.Scherder E., Scherder R., Verburgh L., Konigs M., Blom M., Kramer A.F. Executive functions of sedentary elderly may benefit from walking: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2014;22:782–791. doi: 10.1016/j.jagp.2012.12.026. [DOI] [PubMed] [Google Scholar]