Significance
Green fluorescent protein (GFP) is widely used as a tag to watch otherwise invisible proteins and as a sensor of its local chemical environment. Since GFP can form many partially folded states, it is critical to know how these structural changes affect its signature fluorescence. We use optical tweezers to force single molecules of GFP into folding and unfolding intermediate states and simultaneously probe single-molecule fluorescence from each state. It was found that GFP fluorescence requires complete structural integrity; none of the unfolding or refolding intermediates were observed to fluoresce, but fluorescence could be recovered by complete refolding. This feature was exploited to reversibly, mechanically switch GFP between on and off fluorescence states.
Keywords: optical tweezers, protein folding, fluorescent protein, mechanoswitch
Abstract
Green fluorescent protein (GFP) variants are widely used as genetically encoded fluorescent fusion tags, and there is an increasing interest in engineering their structure to develop in vivo optical sensors, such as for optogenetics and force transduction. Ensemble experiments have shown that the fluorescence of GFP is quenched upon denaturation. Here we study the dependence of fluorescence on protein structure by driving single molecules of GFP into different conformational states with optical tweezers and simultaneously probing the chromophore with fluorescence. Our results show that fluorescence is lost during the earliest events in unfolding, 3.5 ms before secondary structure is disrupted. No fluorescence is observed from the unfolding intermediates or the ensemble of compact and extended states populated during refolding. We further demonstrate that GFP can be mechanically switched between emissive and dark states. These data definitively establish that complete structural integrity is necessary to observe single-molecule fluorescence of GFP.
Green fluorescent protein (GFP) is a 27-kDa -barrel protein with an intrinsic chromophore. (1, 2) It is widely used in imaging applications that rely on its structural stability [to thermal and high-pressure unfolding (3), in fusion constructs (4), and to circular permutation (5)] or its optical response to environment, such as in biosensors for force transduction (6–9), calcium concentration (10), protease activity (11), and pH (12, 13). Understanding the relationship between protein structure and fluorescence is essential for these applications. Photophysical properties of the chromophore are sensitive to its hydrogen bonding, solvation, isomerization state, and binding-pocket structure. (1, 2, 14). It is known that fluorescence is quenched upon denaturation and that several intermediates have been observed in folding experiments and simulations (3, 15–21), although it has not been possible to definitively probe the fluorescence properties of partially structured intermediates in isolation from the native state. Speculation exists regarding whether single-molecule photobleaching can be reversed by unfolding and refolding the protein (22). It is not known whether tension on the native state alters fluorescence in similarity to the compressive, pressure-induced elastic effect (3) and whether its emission can be reversibly mechanically switched. In this study, we address these questions by making an explicit connection between structure and fluorescence, using single-molecule methods.
Single-molecule experiments are widely used to reveal distributions in biophysical structure and kinetics that underlie ensemble-averaged properties. Single-molecule manipulation using force experiments such as optical tweezers can be used to observe states nominally at negligible concentration in a bulk distribution (23–25). Single-molecule fluorescence experiments have been used to probe the chemical structure and environment of chromophores (14, 26, 27). The combination of these two tools can provide an entirely optical method to drive conformational changes and probe several orthogonal coordinates; however, their joint implementation is challenged by factors including rapid sample photodegradation unless the trapping and fluorescence excitation spots are temporally or spatially displaced (28–31). In this work, we reveal the structural requirements for fluorescence from the GFP chromophore by using optical trapping force experiments to prepare a range of protein structures. The fluorescence measured here directly reports on the solvation of the chromophore and rigidity of its binding pocket, rather than its more common use as a tag or a proxy distance measurement. With the ability to monitor fluorescence with millisecond time resolution while independently observing protein structure, we find that unfolding GFP disrupts the environment of the chromophore and affects a loss of fluorescence 3.5 ms before any secondary structure changes are observed with force. No fluorescence is observed from the unfolding intermediates or the ensemble of states GFP populates during refolding. We show that fluorescence can be recovered by refolding the protein and demonstrate that GFP can be switched between emissive and dark states by mechanical unfolding and refolding.
Results
Single-Molecule Fluorescence and Force-Jump Unfolding.
We designed a mutant of GFP, including enhanced fluorescence mutations (32, 33) and cysteine residues at the N- and C termini that allowed attachment of DNA handles for dual-bead optical trapping experiments (Fig. 1). The length of the DNA handles gave an 860-nm separation of GFP from the trapping laser beam foci to circumvent undesirable photophysical processes known to accompany combined optical trapping/single-molecule force experiments (28). Native single-molecule fluorescence was observed from GFP in the optical trap as characterized by unperturbed lifetime, blinking, and photobleaching (14, 34) (Figs. S1 and S2). Traces that did not show both the expected fluorescence and contour length changes upon unfolding were excluded. Single-molecule fluorescence of GFP was probed throughout unfolding and refolding events with a force-jump assay (Fig. 1 and refs. 35 and 36). To investigate unfolding, a force jump (from 0 pN to 30–60 pN) is applied by abruptly increasing the distance between the optical traps. With each such force jump, the protein had a chance to unfold that increased with the magnitude and duration of high-force application. Unfolding was evidenced by a loss of fluorescence and an increase in the end-to-end length of the protein as folded regions lose structure under force. As unfolded proteins were not observed to refold at high-force conditions, a subsequent jump back to 0 pN allowed for the folded state and fluorescence to be recovered. Periodic repetition of force jumps up and down shows that single-molecule fluorescence cycling is possible by unfolding and refolding the protein and allowed for optical characterization of the intermediates.
Fig. 1.
Schematic of the force-jump and single-molecule fluorescence assay. Cycling the trap separation (blue trace) over an 800-nm range provides periodic high (47 pN) and low (0 pN) force intervals to facilitate unfolding and refolding. The time resolutions were 4.8 ms for the fluorescence (green) and 33 s for the force (gray) measurements. The red trace shows the force smoothed to 0.66 ms. The excitation is switched on and fluorescence is detected only during the high-force intervals. Unfolding occurs at 0 s and refolding occurs during the low-force interval at 0.8 s, which is observed at 0.853 s. Laser off and laser on fluorescence backgrounds are visible during low-force intervals and when GFP is unfolded, respectively.
Fig. S1.
Fluorescence trajectories showing blinking and photobleaching. (A) The power of the excitation light can be increased to increase the emission rate and yield better time resolution at the cost of faster photobleaching. (B) Characteristic blinking of stepwise photobleaching of a GFP dimer compared with a dimer construct of an organic dye, Alexa 488. The time resolution varies between 4.8 ms and 50 ms.
Fig. S2.
Fluorescence lifetime of GFP is unperturbed by the optical trap. (A) Time-correlated single-photon counting (TCSPC) was used to measure the fluorescence lifetime of a GFP-DNA construct tethered in the optical trapping assay (12 acquisitions, 3.5–10 s duration each). The time resolution was 150 ps and limited by the pulse duration of the excitation laser. The time axis includes an arbitrary offset (11 ns) due to the delay between the laser trigger pulse and photon measurement. The early time data (0–1.5 ns from the emission peak) are contaminated by fluorescence impurities from the glass sample chamber, which bleach rapidly but peak when the laser is first switched on. These points were excluded from all fits. (B) TCSPC measurement of the same construct free in solution (60 s acquisition time) is compared with averaged data from the optical trap. Measurement of the GFP-DNA construct in solution represents an average of many single molecules that diffuse within the confocal excitation spot. These data show less contamination from the glass sample chamber, because the measurement is sufficiently long compared with the time it takes for the fluorescence impurities in the glass to bleach. All data shown fit to a fluorescence lifetime of 2.46 0.03 ns.
Characterization of Unfolding Intermediates.
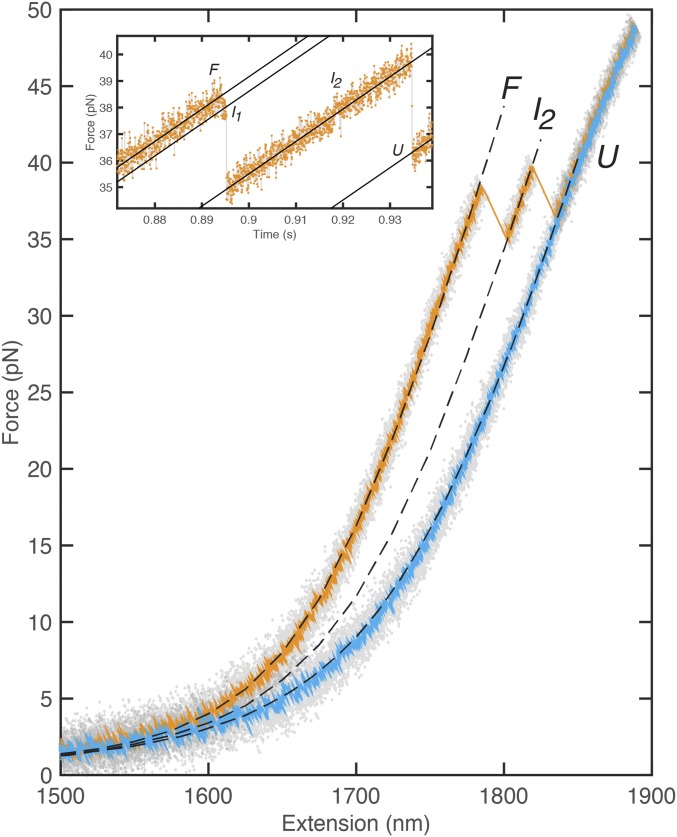
As GFP unfolds, the force measurement probes the end-to-end length of the protein with sufficient time resolution (33 s) to characterize unfolding intermediates (Fig. 2 A and B). Time resolution of the fluorescence experiment was dictated by the emission rate, which is proportional to the excitation laser intensity; for the best time resolution following the force jump (4.8 ms), laser power causing bleaching in ∼2 s was needed (Fig. S1). The length change upon complete unfolding ( = 79.1 3 nm) was consistent with that measured from force-extension experiments (Fig. S3) and the difference arising from unfolding of the structured amino acids between the attachment points ( = 225 aa × 0.365 nm/aa = 82.1 nm) and the distance between attachment points in the folded protein ( = 2 nm; = × .) Signature force changes were observed for two intermediates, and ( = 10.0 2.2 nm, = 39.4 3.1 nm), which are consistent with the 11 and 11–7 previously observed from GFP unfolding with the ClpXP mechanical protease (21). Repeated measurements with different force jumps yield the force-dependent unfolding rate [fitted to a Bell model (37, 38), (F) = exp(−F*dx/kT); with = 0.33 s−1 and ; Fig. 2B and Fig. S4]. The value of is greatly affected by the unavailability of low-force data points and is likely overestimated (16). The fluorescence was observed to cease before any evidence of unfolding was seen in the force signal. Due to the fluorescence intermittency of GFP, statistically significant sampling of the fluorescence intensity during unfolding was facilitated by averaging 30 trajectories. By synchronizing all trajectories to the F force transition, a delay of 3.5 ms was observed between loss of fluorescence and length change (Fig. 2 C and D, alternative analysis in Fig. S5). This offset—which is shorter than the detector integration time—was provided by the synchronization and averaging procedure with no deconvolution. Model calculations that demonstrate this resolution enhancement as well as independence of the offset with respect to GFP blinking are presented in SI Materials and Methods (and Fig. S6). The averaged force signal represents the statistical lifetime of the two intermediate states, and the averaged fluorescence signal represents both the statistical blinking and loss of fluorescence due to unfolding. These results show that the earliest events in unfolding GFP quench its fluorescence before any disruption of the -barrel structure is observable via change in the end-to-end distance with force.
Fig. 2.
Characterization of unfolding intermediates. (A) Sample unfolding trace showing sequential transition from folded state (F) to , , and U (33-s resolution in gray, 0.66-ms smoothed data in red). (B) Logarithm of rate of first unfolding is linearly dependent on force. (C) Fluorescence intermittency (blinking) is observed as well as quenching upon unfolding in single-molecule traces (green). The concomitant force data are shown in red (33-s resolution). Synchronization defines t = 0 ms at the first unfolding, F . (D) Average of 30 synchronized single-molecule traces shows that fluorescence is quenched before the force drop due to unfolding. Fit values: = 0 ms, = 4.5 ms (force) and = −3.5 ms, = 1.0 ms (fluorescence). The synchronization procedure yields better resolution than the time between acquisition points (4.8 ms).
Fig. S3.
Force-extension unfolding trace of GFP. The elasticity of the linker was fitted to the eWLC model (pD = 45 nm, LD = 1,720 nm, K = 630 pN). Parameters for the unfolded peptide chain were modeled by adding a WLC segment in series with the linker ( = 10.0 ± 2.2 nm, = 39.4 ± 3.1 nm, = 79.1 ± 2.74 nm, = 0.7 nm). Pulling speed was 1,000 nm/s.
Fig. S4.
Lifetime histogram of (→I2 or →U). Data were combined from all unfolding forces. Lifetime was fitted to ; = 7.2 ms.
Fig. S5.
Force/fluorescence synchronization scheme and rupture control. (A and B) Illustration of synchronization schemes (A) to the midpoint of the or (B) to the force drop. (C) Averaged unfolding force and fluorescence with synchronization scheme 1. (E) Averaged unfolding force and fluorescence with synchronization scheme 2 (as in Fig. 2D). (G) Control experiment: force and fluorescence signal due to handle rupture showing instrument response of both signals to a correlated stepwise change. (D, F, and H) Zoom-in at synchronization point.
Fig. S6.
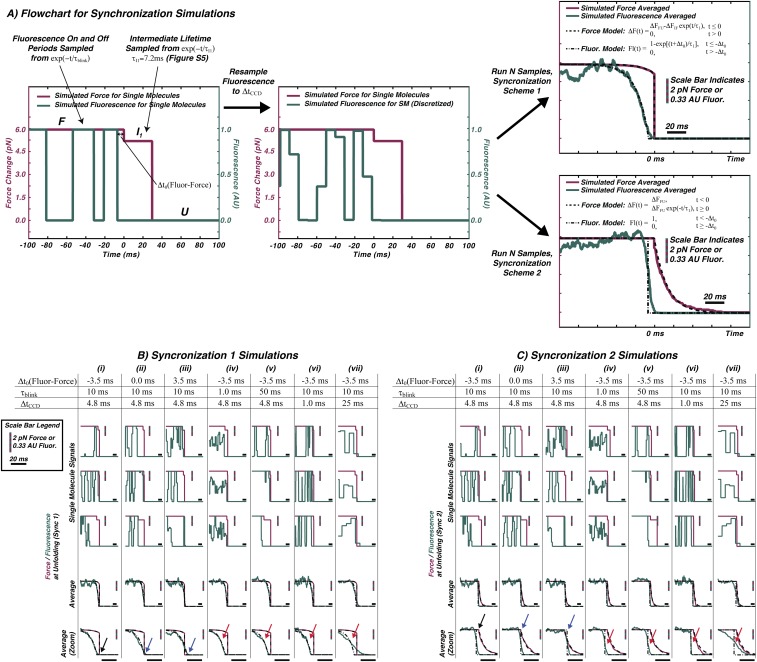
Synchronization data analysis simulations. Simulations are presented to illustrate how the averaged unfolding data and analysis reflect the effects of GFP blinking, the time resolution of the CCD, and the temporal offset between the formation of the first unfolding intermediate and the loss of fluorescence. The fluorescence signal is shown in green and the force signal is shown in red. (A) A visual description of the model. (B and C) The single-molecule trajectories produced by the simulation are synchronized to either the I1 → U (synchronization 1, B) or the F → I1 transition (synchronization 2, C). Seven different parameter sets (B and C, i–vii) are chosen to highlight the effects of the GFP blinking timescale, , and the finite-time resolution of the CCD detector, , on determination of the force/fluorescence offset, . The simulation results are compared with an analytical model shown in A for the force changes (dashed lines) and fluorescence changes (dotted-dashed lines).
Refolding Intermediates.
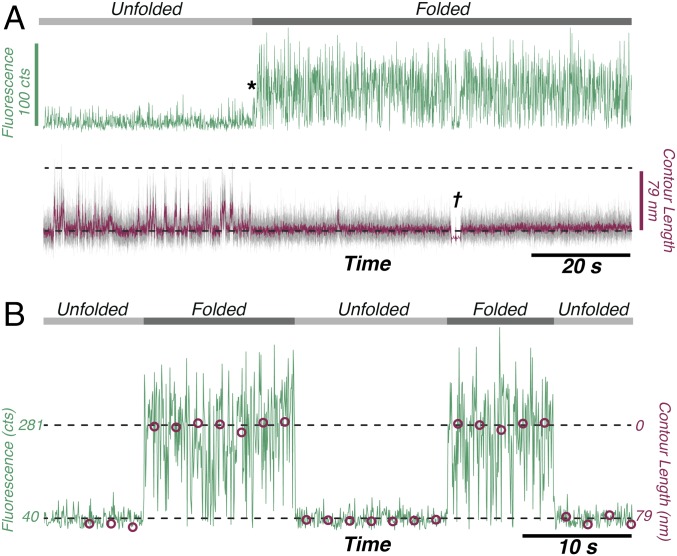
In a different set of measurements, real-time recovery of fluorescence with refolding was observed. Unfolding occurred at high force as described above and the fluorescence was probed continuously at a low force of 3 pN (Fig. 3A). The observed contour length fluctuations indicate that GFP samples an ensemble of compact and extended states (35, 39) during refolding, although these low forces did not provide sufficient length resolution for structural assignment. (This refolding ensemble was also observed in force-extension measurements; Fig. S7). Fluorescence was observed abruptly upon the cessation of the contour length fluctuations when refolding was complete; as such, we conclude that the refolding ensemble did not contain any sufficiently fluorescent species with an approximately millisecond or longer lifetime(zoomed-in view in Fig. S8). Native-state stability was verified by a single force-extension cycle (at Fig. 3A, †), during which GFP was shifted away from the excitation spot. The force-jump assay was used to probe the success rate of refolding as a function of time spent at 0 pN and analyzed with a maximum-likelihood algorithm to yield a folding rate of = 0.62 0.06 s−1 (Table S1). A significant fraction of GFP was not observed to refold on the 5-min experimental timescale, which we attribute to the formation of slowly reequilibrating proline isomers (16) or nonnative disulfide linkages (40). This strict dependence of fluorescence on the native state was further verified by concatenating the fluorescence from the high-force intervals across 26 unfolding/refolding force-jump cycles (Fig. 3B). The folded/unfolded state of the protein is indicated by the average contour length during that period. These data show that force-jump cycles can be tuned to flip GFP between folded/fluorescent and unfolded/nonfluorescent states and complement the information obtained by synchronizing many traces. The reversibility of this process is limited by photobleaching and the propensity for GFP to enter a slowly refolding state. These results demonstrate that the fluorescence can be reversibly mechanically switched in single molecules of GFP.
Fig. 3.
Fluorescence recovers upon refolding and can be switched. (A) No fluorescence is observed as GFP samples different contour length states during refolding; fluorescence recovers immediately upon formation of the native state at *. † indicates where force extension was used to verify native-state formation. The time resolutions were 50 ms for the fluorescence (green) and 33 s for the force (gray) measurements. The red trace shows the force smoothed to 0.66 ms. (B) Driving GFP fluorescence off/on by repeated unfolding and refolding cycles. Data such as those shown in Fig. 1 were compressed to show the fluorescence counts (green) during the high-force intervals from 26 sequential force-jump cycles. For each interval, the average contour length is shown (red circles). The fluorescence time resolution was 4.8 ms, and the laser on background fluorescence was 40 counts.
Fig. S7.
Force extension after unfolding GFP showing the ensemble of extended and compact states sampled during refolding. An extension (lower traces, orange) and contraction (upper traces, blue, offset by +10 pN) cycle is shown for the unfolded state of GFP with a pulling speed of 10 nm/s. The raw data in gray (30.3 kHz) are overlaid with filtered traces (blue and orange = 1.51 kHz, black = 10 Hz) to highlight force changes during refolding. In these data, GFP is predominantly in a state that is well described by the unfolded state WLC fit shown in Fig. S3. However, the slower pulling speed in this measurement makes brief excursions in the 0- to 5-pN region, apparent where the force increases and contour length decreases. These contour-length fluctuations are interpreted as refolding intermediates composed of an ensemble of extended and compact states that does not ultimately yield the native folded state. These data support the claim that the contour-length fluctuations shown in Fig. 3A represent folding intermediates.
Fig. S8.
Fluorescence recovery upon refolding in detail. To better illustrate the relationship between contour-length fluctuations and fluorescence, an expanded view of Fig. 3A is provided around the refolding time. The time indicated at zero is the last fluctuation to the native contour length before native fluorescence was observed.
Table S1.
Refolding Kinetics Measurements
| Interval length at 0 pN, s | No. of times GFP failed to refold during interval before refolding |
| 0.5 | 8 |
| 0.7 | 1 |
| 0.3 | 2 |
| 0.3 | 1 |
Measurements of the refolding kinetics of GFP are shown. After GFP was unfolded by a high-force interval (30–60 pN), the number of low-force intervals that did not result in successful refolding is indicated. For example, row 1 indicates that GFP refolded during the ninth low-force interval. As contour-length resolution is impossible at 0 pN, the folded/unfolded state of GFP was determined during the subsequent high-force interval. These data were analyzed using Eq. S5.
SI Materials and Methods
Protein Expression and Purification.
The gene for cycle3 GFP was obtained from previous studies in our group (15). Cysteine residues and enhanced fluorescence mutations were inserted by QuikChange mutagenesis (Agilent Technologies Deutschland GmbH). The proteins were expressed in DE3 Escherichia coli and purified by Ni-NTA affinity column chromatography (Ni-NTA Superflow Cartridges; Qiagen) and subsequently by gel filtration chromatography (Superdex S200 column 10/300GL; GE Healthcare).
Protein Sequence.
In the following, the cysteines that are used for handle attachment are shown in boldface type, the sequence corresponding to GFP is shown in italics, and spacers are underlined:
MCGGTGGKGEELFTGVVPILVELDGDVNGHKFSVSGEGEGDATYGKLTLKFICTTGKLPVPWPTLVTTFSYGVQCFSRYPDHMKRHDFFKSAMPEGYVQERTISFKDDGNYKTRAEVKFEGDTLVNRIELKGIDFKEDGNILGHKLEYNYNSHNVYITADKQKNGIKANFKIRHNIEDGSVQLADHYQQNTPIGDGPVLLPDNHYLSTQSALSKDPNEKRDHMVLLEFVTAAGITHGMDELYCGHHHHHH.
The sequence above contains four cysteine residues; the solvent-exposed N- and C-terminal cysteines are used for DNA attachment. Potential side reactivity of C49 and C71 in forming the native state is excluded by selecting only molecules that displayed native fluorescence and the expected contour length changes upon unfolding.
DNA Tether Synthesis.
A 1:1 ratio of biotin- and dixogenin-functionalized 2,532-bp (860 nm) DNA handles was synthesized by PCR amplification of -DNA, using the following primers: (forward) 5′-GGCGAT*CT*GGT*CGTTGATTTG-3′ and 5′-GGCGAT**CT**GGT**CGTTGATTTG-3′ and (reverse) 5′-CGACTCGCTGGTCTGGTTGAACGTCAGCCCTGCC-X-GGAGAACAGGCCACCAT CACG-3′, where T* and T** indicate dixoxigenin- or biotin-functionalized thymine and X indicates an abasic site. Subsequent to PCR amplification, the product was separated using CHROMA SPIN+TE-400 columns. All oligonucleotides were purchased from IBA Lifesciences GmbH.
Protein–DNA Linkage.
Linkage between the protein and DNA handles was performed in two steps, as previously described (52). In the first step, GFP proteins were reduced with 1 mM tris(2-carboxyethyl)phosphine, exchanged into PBS (pH 7.2) with a desalting column (PD-10; GE Healthcare), and functionalized at both cysteine sites with the oligonucleotide primer (complementary to the overhang sequence in the 2,532-bp DNA tether), 5′-GGCAGGGCTGACGTTCAACCAGA CCAGCGAGTCG-3′-Mal (IBA Lifesciences GmbH), where Mal indicates a maleimide functional group. The reaction occurred with a 2:1 molar ratio of oligonucleotides to protein at room temperature for 2 h, and double-functionalized proteins were separated from unreacted oligonucleotides and single- and unfunctionalized proteins by gel filtration chromatography (Superdex S200 column 10/300GL; GE Healthcare). In the second step, oligonucleotide-functionalized proteins were annealed to the 2,532-bp DNA handles described above in the presence of 20% PEG-6000 (Sigma Aldrich) for 16 h at room temperature. A 1% wt/vol agarose gel was used to assay the extent of reaction by appearance of a band near 5 kbp. DNA tether-functionalized proteins were stored in 30% glycerol stock solutions at −20 °C for up to 4 wk.
Measurement Procedures.
To prepare the measurement sample, 0.8 pmol of DNA-functionalized GFP was mixed with 1 m streptavidin-coated silica microspheres (Bangs Laboratories). The mixture was diluted to 65 of PBS, pH 7.4, that had been filtered with 0.2- filter tips and degassed immediately before with a vacufuge. After incubation at 4 °C overnight on a rotator, 7 of this mixture was added to 1- silica microspheres (Bangs Laboratories) that had been functionalized on site with anti-digoxygenin (Roche). This mixture was diluted to 200 in PBS, pH 7.4, containing an oxygen scavenger system [0.33% glucose (Sigma), 13 units/mL glucose oxidase (Sigma), 8,500 units/mL catalase (Calbiochem)]. Approximately 30 of the final mixture was loaded into a sample chamber constructed using a 76- × 25-mm microscope slide, an 18- × 18-mm No. 1.5H coverslip, and 3 mm Parafilm strips as spacers.
Data Analysis and Fitting.
To analyze force-extension measurements and determine the force-dependent unfolding rate, bead displacements derived from the position-sensitive detector measurements were converted to force with calibrations as previously described (51, 52). Force-extension data in the region before GFP unfolding were fitted to the extensible worm-like chain (eWLC) model to derive persistence and contour lengths for the DNA tethers,
| [S1] |
with force, F; extension, x; Boltzmann constant, ; absolute temperature, T; DNA persistence length, ; DNA contour length, ; and elastic stretch modulus, K. The force extension of a segment of unfolded amino acids was described with a worm-like chain (WLC) model,
| [S2] |
with protein contour length, , and protein persistence length, . Sequential unfolding responses were fitted with for the DNA tethers in series with for the unfolded protein segment. The DNA tether fits yielded nm, nm, and K = 630 pN. The protein persistence length was fixed to 0.7 nm.
The inverse of Eqs. S1 and S2 was used to convert force into contour length to produce Fig. 3A. As the WLC does not fit equally well at high and low forces (Fig. S3), this error is highlighted when comparing contour lengths at the extremes of the measurement range (Fig. 3A, ).
To determine the unfolding rate as a function of force, , a table was constructed with the data from all jump experiments (151 measurements) including the force applied, F; the time spent at F in each jump, ; the number of unsuccessful unfolding attempts, , and the time spent at F in the observed unfolding jump, . The probability for observing such an experiment with rate constant was modeled as
| [S3] |
and the rate constant maximizing the log-likelihood was fitted. Probabilities were combined for unfolding experiments in 1.5-pN bins. The series of rate constants was fitted with a Bell-like model,
| [S4] |
to determine the zero-force unfolding rate constant, and transition state distance, . Refolding rates with 0 pN applied force, , were fitted with a similar maximum-likelihood model including the time spent at 0 pN, , and the number of unsuccessful refolding attempts, ,
| [S5] |
Error bars were estimated using the bootstrap method. To determine the lifetime of , a histogram of intermediate lifetimes from unfolding at all observed forces was combined.
For the experiments observing fluorescence changes upon unfolding, temporal synchronization between the two signals was required. This was achieved by observing the two-photon fluorescence generated by the optical trapping lasers in a 1- rhodamine B sample (Fig. S10). From these data, a corrected time axis for the fluorescence signals derived from the FPGA reference clock was fitted to two parameters: time delay and time step. The corrected time step (5.0886 ms) was within the error bounds expected from the EMCCD software exposure time (5.095 ± 0.01 ms). The traces from independent experiments were aligned to the point of protein unfolding (Fig. S5).
Fig. S10.
Cross-correlation of fluorescence and force trajectories was facilitated by observing two-photon fluorescence from the optical trapping laser oscillated through the fluorescence collection region. A trajectory that oscillates for many cycles with a distinct change in oscillation pattern near the end allows for the time delay and time step to be fitted. (A) Two-photon fluorescence collected using the EMCCD and laser position derived from a piezoelectric tip/tilt actuator encoder. (B and C) Overlay of both signals at (B) early and (C) late times (gray regions in A), using the EMCCD software exposure time (5.095 ms). (D and E) Overlay of both signals at (D) early and (E) late times, using the fitted exposure time (5.0886 ms).
As the force-jump experiment led to many handle rupture events (14/44 handle rupture events and 30/44 successful unfolding events), these data were analyzed using the same algorithm as the unfolding data as a control experiment (see Fig. S5 C–F for unfolding data analysis and Fig. S5 G and H for rupture data analysis). By comparing these data, one can discriminate the instrument response to a simultaneous force change and loss of fluorescence (handle rupture) from a noncoincident force change and loss of fluorescence (upon unfolding).
Modeling the Effect of Experimental Parameters and Synchronization Procedure.
A simple model was constructed to address whether GFP blinking affects determination of the force change/fluorescence quenching offset, how the time resolution of the fluorescence measurement affects the analysis, and the features of the two synchronization methods. The flowchart for this model construction is shown in Fig. S6A. In this simple model, folded GFP switches between emissive and dark states, both of which are characterized by an exponential distribution with the same timescale, . At an arbitrary time, unfolding occurs, which we model as a process with three different contour length states (neglecting the force-dependent probability to initiate unfolding and the second intermediate, I2). Fluorescence is set to zero for regardless of the on/off state of GFP; thus, is a temporal offset for fluorescence quenching relative to the transition. While the temporal offset between the force change and fluorescence quenching almost certainly arises from a statistical distribution, it is set to a single variable parameter, in each simulation. The lifetime of is set to the fitted value displayed in Fig. S4 (7.2 ms). The time resolution of the force experiment is = 33 , the time resolution of the fluorescence experiment is a variable parameter, ; and effects of this discrete timescale give the fluorescence intermediate values besides 0 and 1 shown throughout Fig. S6. The force change for the intermediate state and the force change for the complete unfolding are set to an average of the experimentally observed values ( = 0.73 pN, = 6.1 pN).
The simulations are run for 100 iterations to produce a signal-to-noise ratio approximating that of the experiment, and the resulting force and fluorescence trajectories are synchronized in an identical manner to that of the measured data (synchronization schemes 1 and 2 in Fig. S5) and averaged together. The averaged data are overlaid with two analytical models parameterized by the simulation variables , , , , and , which do not include the effects of finite-time resolution ( or ). These functional forms are shown in Fig. S6A.
Fig. S6 B and C displays the results of seven parameter sets that were chosen to illustrate the effects of , , and . Comparison of columns i, ii, and iii in Fig. S6 B and C shows the effect of . Comparison of columns i, iv, and v shows the effect of . Comparison of columns i, vi, and vii shows the effect of .
Model Results.
The simulation results analyzed using the two synchronization schemes display the salient features of the analyzed experimental data. Namely, the exponential distribution of lifetimes for the intermediate cause an exponential variation of the force and fluorescence signals before t = 0 when the data are synchronized to the transition and exponential decays after t = 0 when the data are synchronized to the F → I transition.
Fig. S6 B and C, columns i, ii, and iii shows that synchronizing the force and fluorescence experiments provides better time resolution than would be expected based solely upon ; positive or negative offsets in of 3.5 ms are clearly visible (black and blue arrows in Fig. S6 B and C). This enhancement is loosely akin to the manner in which some superresolution localization algorithms work; in this case, the lower time-resolution fluorescence experiment gains time resolution by synchronization with the better time-resolved force experiment, using the independent calibration shown in Fig. S10. In contrast, the statistical blinking timescale, , does not affect the value of the offset . This parameter dictates only how many trajectories are necessary for convergence (Fig. S6 B and C, columns i, iv, and v). The time resolution of the fluorescence experiment, , may further broaden the exponential variation of the fluorescence signal (Fig. S6 B and C, columns i, vi, and vii). In this model, would affect measurement of the lifetime of the nonemissive, native contour-length state but not a temporal offset in its formation, . Both the simulations and the analytical models show that the variation in the fluorescence signal crosses 50% at the offset time regardless of .
Discussion
We observe that the fluorescence of GFP requires the structural rigidity afforded by the native apoprotein; fluorescence is quenched in the first unfolding event, is not recovered while sampling compact and extended states in the refolding ensemble, and completely recovers upon full refolding. GFP unfolding begins with disruption of the chromophore fluorescence, followed by unfolding of 11 and then (7–11) before reaching the fully unfolded state. The nonfluorescent state with native end-to-end length may correspond to internal rearrangements, such as the protonated dark state (14, 22). Alternatively, fluorescence may be quenched by external solvent penetration to the chromophore with contacts remaining among all strands (3). As the unfolding is force dependent, the 3.5-ms offset in fluorescence quenching is also likely to display force dependence; if the quenched state is a protonated dark state of GFP (41), then its lifetime would be dictated by the sum of the rates for deprotonation and force-dependent unfolding. The structural changes we observe are consistent with previous single-molecule unfolding studies (21), H/D exchange pointing to 7–10 as a region of increased solvent accessibility (17), and SAXS data indicating an intermediate with native-like secondary structure (16). However, as the quintessential feature of GFP is its fluorescence, the ability of combined single-molecule force and fluorescence to make clear statements about the fluorescent properties of intermediates is key. In a mixture containing multiple potentially fluorescent species, the total fluorescence is proportional to both the concentration and the brightness (= extinction coefficient × quantum yield) of each species, which has previously limited the ability to quantify the fluorescence of GFP intermediates. Our data indicate that the weak fluorescence observed in bulk denaturation arises from residual native GFP or states not appreciably populated on the approximately millisecond timescale (16, 17).
GFP folding proceeds by transitions within an ensemble of disordered states, evidenced by rapid contour length fluctuations with no fluorescence (35, 39). From this ensemble, either the native state is reformed swiftly (0.62 s−1) and fluorescence is recovered or GFP enters a slowly reequilibrating state. For the construct studied here, both proline isomerization [which reequilibrates on a 3-min timescale (16)] and formation of a nonnative Cys49-Cys71 disulfide [potentially as rapid as 1 s−1 (40)] may contribute to the slow timescale. Previous measurements provide background that the lack of fluorescence from all partially folded GFP intermediate states observed here can be generalized to GFP variants despite structural differences (e.g., circularly permuted GFP) and may arise due to both a reduction in fluorescence quantum yield and molar extinction; studies on the isolated GFP chromophore have established that the fluorescence quantum yield may drop by a factor of 103 in a nonrigid matrix (42, 43), and the absorption of GFP is reduced by a factor of >200 upon deleting as few as five N-terminal amino acids (20).
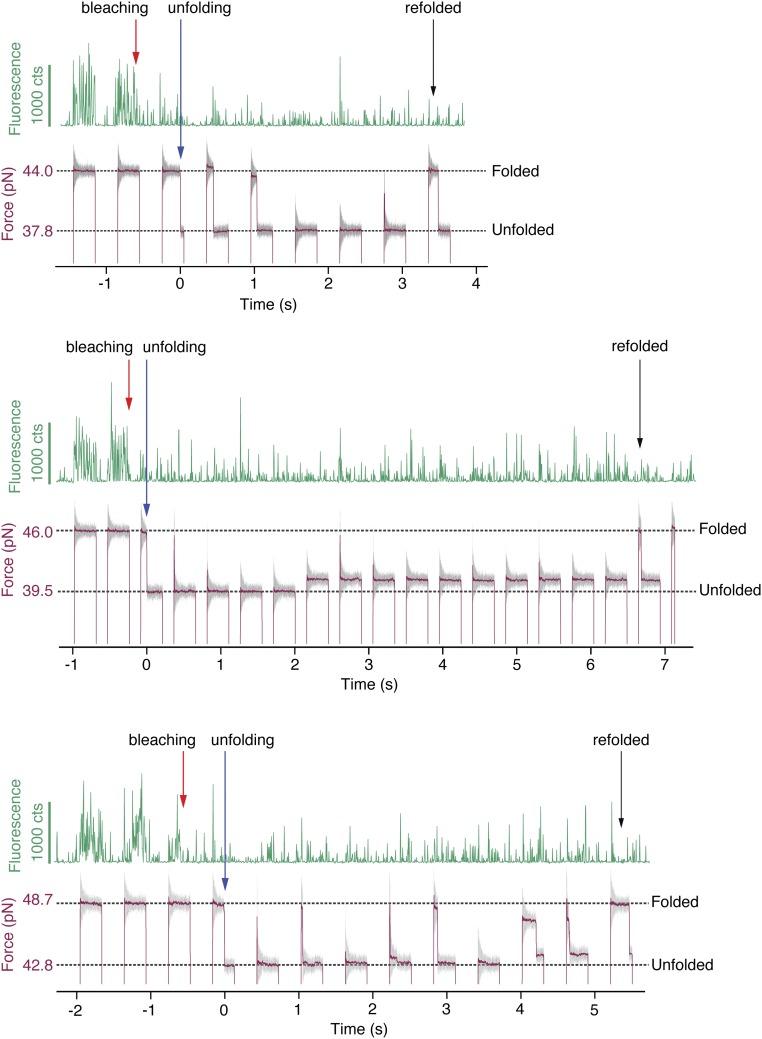
Once photobleached, fluorescence could not be recovered by unfolding and refolding, which provides evidence against the suggestion that secondary structure rearrangements can restore fluorescence (Fig. S9) (22). Finally, we have shown that cycling the fluorescence by mechanical force application is possible, which allows GFP to be calibrated as a force sensor in the 30- to 60-pN range. The data presented here can be used to expand upon results showing that integrin-mediated forces are sufficient to denature and quench the structurally comparable superfolder GFP (6). For sensing in the lower, ∼4- to 20-pN force regime, it may be possible to design mutants that are more mechanically susceptible to unfolding and to calibrate the force sensor using the methods presented here. These findings may provide additional tools to the growing community of optical force sensing (7–9).
Fig. S9.
GFP fluorescence was not recovered by unfolding and refolding. Three traces are shown (Top, Middle, Bottom), in which the force-jump cycles begin with native GFP. After bleaching and unfolding, refolding does not cause the native fluorescence to return. As these data represent failed attempts to observe the changes in fluorescence during unfolding, they were not used in any analysis. The time resolutions were 4.8 ms for the fluorescence (green) and 33 for the force (gray) measurements. The red trace shows the force smoothed to 0.66 ms.
The combination of single-molecule manipulation and complementary optical probing provides a powerful tool to systematically induce chemical changes and observe the effects on function, which may be obscured in ensemble-averaged experiments. The methods presented here may be expanded by using fluorescence to probe other degrees of freedom, such as redox state or ATP binding (26, 44). Moreover, the development of new single-molecule microscopies with chemical structural resolution—using Raman scattering (45–48), direct absorption (49), photothermal contrast (50), and modulation of conductivity (51)—provides exciting opportunities for combined manipulation and characterization of chemical structure.
Materials and Methods
The instrument for optical trapping and single-molecule fluorescence was adapted from that previously described (52, 53). Single-molecule fluorescence capability was built using a 488-nm, <100-ps excitation laser (BDL-488-SMC; Becker & Hickl) operated at 20 MHz and modulated electronically using the same field-programmable gate array (FPGA) used to steer the optical trap and acquire data (NI PCI-7833R 3M; National Instruments). A polarizer and /2 waveplate were used to control the excitation intensity, followed by a /4 waveplate to provide circularly polarized light [WPH10M-488 and WPQ10M-488 (ThorLabs) and G335719000 (Qioptiq)]. The excitation light was combined with the 1,064-nm trapping lasers, using a 1,064/visible dichroic mirror (R 1,064 nm > 90%, T 400–800 nm > 90%; Precision Photonics), and provided confocal excitation. Alignment was facilitated by a 1-mM rhodamine B sample, which allowed for two-photon fluorescence to visualize the two 1,064-nm trapping laser foci and one-photon fluorescence to visualize the excitation laser focus (Fig. S10); the excitation laser was iteratively aligned to the midpoint between the two traps by centroid fitting of the three point-spread functions. Excitation laser power at the sample was 10–110 nW, depending on the desired time resolution and observation length; data shown were acquired at 110 nW (Figs. 1 and 2), 10 nW (Fig. 3A), and 50 nW (Fig. 3B). Fluorescence passed through the 1,064/visible dichroic mirror and a dichroic mirror to block the excitation light (F52-477; AHF analysentechnik) and passed through two filters to remove scattered light from the trapping laser, 850 nm brightfield, and excitation laser (F74-750 and F33-473Z; AHF analysentechnik) before detection. An EMCCD (iXon; Andor Technology) was used to acquire all fluorescence trajectories with a time resolution of 4.8 ms (Figs. 1 and 2) or 50 ms (Fig. 3A). To enhance sensitivity, the pixels were binned to 4 × 4 superpixels. Intensity was monitored on either the superpixel centered on the sample (Figs. 1 and 3) or a sum over the five most central superpixels (Fig. 2). Tuning these integration conditions causes the fluorescence counts to vary across figures. For fluorescence lifetime measurements (Fig. S2), an avalanche photodiode (id100-50-STD; ID Quantique SA) and time-correlated single-photon counting card (TimeHarp 200 PCI; PicoQuant GmbH) were used to provide 150-ps time resolution.
In the force-jump experiments, the mobile trap was steered with a square wave to produce a trap separation as shown in Fig. 1. The rate at which the trap distance changed was limited by the speed of the two-axis piezoelectric tip/tilt actuator (Mad City Labs) with typical times of 5 ms required to jump 1,000 nm (0.2 mm/s). The 488-nm excitation spot is fixed throughout the experiment, but the position of GFP (precisely in the middle of the fixed and mobile optical traps) changes with applied force. Therefore, the excitation was aligned separately to probe fluorescence at 2 pN (1,050 nm separation between optical trap and excitation, Fig. 3A) during refolding experiments and between 30 pN and 60 pN (1,800–2,200 nm separation; Figs. 1, 2, and 3B) during unfolding experiments. (This is evident in the fluorescence dip at Fig. 3A, †.) The fluorescence laser was turned on simultaneously with the jump up (or jump down) by a signal from the FPGA when fluorescence during unfolding (or refolding) was measured. The EMCCD recorded continuously throughout the experiment with 4 × 4 pixel binning and images were saved as a stacked tagged image file (TIF). Each binned superpixel imaged light from a 560- × 560-nm region in the sample, and single-molecule fluorescence trajectories were assembled by integrating four superpixels, which resulted in maximal collection efficiency with minimal background from the optically trapped microspheres.
Acknowledgments
The authors thank Matthias Reisser and Diana Beyerlein for their assistance in the early stages of these experiments. This research was supported by an SFB 863 grant from Deutsche Forschungsgemeinschaft (to M.R.) (A02) and a postdoctoral fellowship from the Humboldt Foundation (to Z.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704937114/-/DCSupplemental.
References
- 1.Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 2.Zimmer M. Green fluorescent protein (gfp): Applications, structure, and related photophysical behavior. Chem Rev. 2002;102:759–781. doi: 10.1021/cr010142r. [DOI] [PubMed] [Google Scholar]
- 3.Scheyhing CH, Meersman F, Ehrmann MA, Heremans K, Vogel RF. Temperature-pressure stability of green fluorescent protein: A Fourier transform infrared spectroscopy study. Biopolymers. 2002;65:244–253. doi: 10.1002/bip.10237. [DOI] [PubMed] [Google Scholar]
- 4.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev. 2010;90:1103–1163. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- 5.Baird GS, Zacharias DA, Tsien RY. Circular permutation and receptor insertion within green fluorescent proteins. Proc Natl Acad Sci USA. 1999;96:11241–11246. doi: 10.1073/pnas.96.20.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galior K, Liu Y, Yehl K, Vivek S, Salaita K. Titin-based nanoparticle tension sensors map high-magnitude integrin forces within focal adhesions. Nano Lett. 2016;16:341–348. doi: 10.1021/acs.nanolett.5b03888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austen K, et al. Extracellular rigidity sensing by talin isoform-specific mechanical linkages. Nat Cell Biol. 2015;17:1597–1606. doi: 10.1038/ncb3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blakely BL, et al. A DNA-based molecular probe for optically reporting cellular traction forces. Nat Methods. 2014;11:1229–1232. doi: 10.1038/nmeth.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morimatsu M, Mekhdjian AH, Adhikari AS, Dunn AR. Molecular tension sensors report forces generated by single integrin molecules in living cells. Nano Lett. 2013;13:3985–3989. doi: 10.1021/nl4005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Do K, Boxer SG. Gfp variants with alternative beta-strands and their application as light-driven protease sensors: A tale of two tails. J Am Chem Soc. 2013;135:10226–10229. doi: 10.1021/ja4037274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crone DE, et al. GFP-based biosensors. In: Rinken T, editor. State of the Art in Biosensors: General Aspects. InTech; Rijeka, Croatia: 2013. [Google Scholar]
- 13.Mahon MJ. pHluorin2: An enhanced, ratiometric, pH-sensitive green fluorescent protein. Adv Biosci Biotechnol. 2011;2:132–137. doi: 10.4236/abb.2011.23021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haupts U, Maiti S, Schwille P, Webb WW. Dynamics of fluorescence fluctuations in green fluorescent protein observed by fluorescence correlation spectroscopy. Proc Natl Acad Sci USA. 1998;95:13573–13578. doi: 10.1073/pnas.95.23.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz H, Rief M. Exploring the energy landscape of GFP by single-molecule mechanical experiments. Proc Natl Acad Sci USA. 2004;101:16192–16197. doi: 10.1073/pnas.0404549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enoki S, et al. The equilibrium unfolding intermediate observed at pH 4 and its relationship with the kinetic folding intermediates in green fluorescent protein. J Mol Biol. 2006;361:969–982. doi: 10.1016/j.jmb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Huang JR, Craggs TD, Christodoulou J, Jackson SE. Stable intermediate states and high energy barriers in the unfolding of GFP. J Mol Biol. 2007;370:356–371. doi: 10.1016/j.jmb.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Mickler M, et al. Revealing the bifurcation in the unfolding pathways of GFP by using single-molecule experiments and simulations. Proc Natl Acad Sci USA. 2007;104:20268–20273. doi: 10.1073/pnas.0705458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy G, Liu Z, Thirumalai D. Denaturant-dependent folding of GFP. Proc Natl Acad Sci USA. 2012;109:17832–17838. doi: 10.1073/pnas.1201808109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saeger J, Hytonen VP, Klotzsch E, Vogel V. GFP’s mechanical intermediate states. PLoS One. 2012;7:e46962. doi: 10.1371/journal.pone.0046962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen M, et al. The ClpXP protease unfolds substrates using a constant rate of pulling but different gears. Cell. 2013;155:636–646. doi: 10.1016/j.cell.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung G, et al. Confocal microscopy of single molecules of the green fluorescent protein. Bioimaging. 1998;6:54–61. [Google Scholar]
- 23.Dangkulwanich M, Ishibashi T, Bintu L, Bustamante C. Molecular mechanisms of transcription through single-molecule experiments. Chem Rev. 2014;114:3203–3223. doi: 10.1021/cr400730x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stigler J, Ziegler F, Gieseke A, Gebhardt JC, Rief M. The complex folding network of single calmodulin molecules. Science. 2011;334:512–516. doi: 10.1126/science.1207598. [DOI] [PubMed] [Google Scholar]
- 25.Woodside MT, Block SM. Reconstructing folding energy landscapes by single-molecule force spectroscopy. Annu Rev Biophys. 2014;43:19–39. doi: 10.1146/annurev-biophys-051013-022754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein IH, et al. Linking single-molecule blinking to chromophore structure and redox potentials. Chemphyschem. 2012;13:931–937. doi: 10.1002/cphc.201100820. [DOI] [PubMed] [Google Scholar]
- 27.Xu W, Kong JS, Yeh YT, Chen P. Single-molecule nanocatalysis reveals heterogeneous reaction pathways and catalytic dynamics. Nat Mater. 2008;7:992–996. doi: 10.1038/nmat2319. [DOI] [PubMed] [Google Scholar]
- 28.Brau RR, Tarsa PB, Ferrer JM, Lee P, Lang MJ. Interlaced optical force-fluorescence measurements for single molecule biophysics. Biophys J. 2006;91:1069–1077. doi: 10.1529/biophysj.106.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comstock MJ, Ha T, Chemla YR. Ultrahigh-resolution optical trap with single-fluorophore sensitivity. Nat Methods. 2011;8:335–340. doi: 10.1038/nmeth.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heller I, et al. Sted nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA. Nat Methods. 2013;10:910–916. doi: 10.1038/nmeth.2599. [DOI] [PubMed] [Google Scholar]
- 31.Hohng S, et al. Fluorescence-force spectroscopy maps two-dimensional reaction landscape of the Holliday junction. Science. 2007;318:279–283. doi: 10.1126/science.1146113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crameri A, Whitehorn EA, Tate E, Stemmer WPC. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat Biotechnol. 1996;14:315–319. doi: 10.1038/nbt0396-315. [DOI] [PubMed] [Google Scholar]
- 33.Ormö M, et al. Crystal structure of the aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. doi: 10.1126/science.273.5280.1392. [DOI] [PubMed] [Google Scholar]
- 34.Peterman EJG, Brasselet S, Moerner WE. The fluorescence dynamics of single molecules of green fluorescent protein. J Phys Chem A. 1999;103:10553–10560. [Google Scholar]
- 35.Garcia-Manyes S, Dougan L, Badilla CL, Brujic J, Fernandez JM. Direct observation of an ensemble of stable collapsed states in the mechanical folding of ubiquitin. Proc Natl Acad Sci USA. 2009;106:10534–10539. doi: 10.1073/pnas.0901213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rognoni L, Most T, Zoldak G, Rief M. Force-dependent isomerization kinetics of a highly conserved proline switch modulates the mechanosensing region of filamin. Proc Natl Acad Sci USA. 2014;111:5568–5573. doi: 10.1073/pnas.1319448111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell GI, et al. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 38.Evans E, Ritchie K. Dynamic strength of molecular adhesion bonds. Biophys J. 1997;72:1541–1555. doi: 10.1016/S0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elms PJ, Chodera JD, Bustamante C, Marqusee S. The molten globule state is unusually deformable under mechanical force. Proc Natl Acad Sci USA. 2012;109:3796–3801. doi: 10.1073/pnas.1115519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosuri P, et al. Protein folding drives disulfide formation. Cell. 2012;151:794–806. doi: 10.1016/j.cell.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oltrogge LM, Wang Q, Boxer SG. Ground-state proton transfer kinetics in green fluorescent protein. Biochemistry. 2014;53:5947–5957. doi: 10.1021/bi500147n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gepshtein R, Huppert D, Agmon N. Deactivation mechanism of the green fluorescent chromophore. J Phys Chem B. 2006;110:4434–4442. doi: 10.1021/jp0540095. [DOI] [PubMed] [Google Scholar]
- 43.Wu L, Burgess K. Syntheses of highly fluorescent GFP-chromophore analogues. J Am Chem Soc. 2008;130:4089–4096. doi: 10.1021/ja710388h. [DOI] [PubMed] [Google Scholar]
- 44.Funatsu T, Harada Y, Tokunaga M, Saito K, Yanagida T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature. 1995;374:555–559. doi: 10.1038/374555a0. [DOI] [PubMed] [Google Scholar]
- 45.Dieringer JA, Lettan RB, 2nd, Scheidt KA, Van Duyne RP. A frequency domain existence proof of single-molecule surface-enhanced Raman spectroscopy. J Am Chem Soc. 2007;129:16249–16256. doi: 10.1021/ja077243c. [DOI] [PubMed] [Google Scholar]
- 46.Rao S, et al. Direct observation of single DNA structural alterations at low forces with surface-enhanced Raman scattering. Biophys J. 2013;104:156–162. doi: 10.1016/j.bpj.2012.11.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheaton S, Gelfand RM, Gordon R. Probing the Raman-active acoustic vibrations of nanoparticles with extraordinary spectral resolution. Nat Photon. 2014;9:68–72. [Google Scholar]
- 48.Yampolsky S, et al. Seeing a single molecule vibrate through time-resolved coherent anti-stokes Raman scattering. Nat Photon. 2014;8:650–656. [Google Scholar]
- 49.Celebrano M, Kukura P, Renn A, Sandoghdar V. Single-molecule imaging by optical absorption. Nat Photon. 2011;5:95–98. [Google Scholar]
- 50.Gaiduk A, Yorulmaz M, Ruijgrok PV, Orrit M. Room-temperature detection of a single molecule’s absorption by photothermal contrast. Science. 2010;330:353–356. doi: 10.1126/science.1195475. [DOI] [PubMed] [Google Scholar]
- 51.Akhterov MV, et al. Observing lysozyme’s closing and opening motions by high-resolution single-molecule enzymology. ACS Chem Biol. 2015;10:1495–1501. doi: 10.1021/cb500750v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pelz B, Zoldak G, Zeller F, Zacharias M, Rief M. Subnanometre enzyme mechanics probed by single-molecule force spectroscopy. Nat Commun. 2016;7:10848. doi: 10.1038/ncomms10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jahn M, et al. The charged linker of the molecular chaperone hsp90 modulates domain contacts and biological function. Proc Natl Acad Sci USA. 2014;111:17881–17886. doi: 10.1073/pnas.1414073111. [DOI] [PMC free article] [PubMed] [Google Scholar]