Abstract
More sensitive biomarker is urgently needed to reduce the mortality caused by the worldwide prevalent liver cancer. This study aims to assess whether quantitative measurement of heat shock protein 90alpha (Hsp90α) in plasma can improve the diagnosis accuracy and monitor treatment response of liver cancer patients. We analyzed the data from an official (registered at ClinicalTrial.gov: NCT02324127), large-scale (1647 enrollments), and multicenter (three independent hospitals) clinical trial, which quantitatively measured plasma Hsp90α by ELISA for patients with liver cancer, patients with at-risk liver diseases (including hepatitis, liver cirrhosis, focal nodular hyperplasia), and healthy individuals. Diagnostic performance of plasma Hsp90α was evaluated by the calculated sensitivity, specificity, and area under the receiver operating characteristic curve (AUC). ROC curve showed plasma Hsp90α can discriminating liver cancer with a sensitivity of 92.7% and specificity of 91.3% from non-liver cancer control. Similar results were noted in detecting early-stage liver cancer (sensitivity 91.4%, specificity 91.3%). In a parallel study compared with AFP20, plasma Hsp90α exhibited a significantly higher diagnostic performance (sensitivity 93.3% vs 61.1%) in discriminating hepatocellular carcinoma (HCC) from the control. Furthermore, plasma Hsp90α measurement maintained distinctly excellent diagnostic accuracy in distinguishing AFP-negative HCC patients (sensitivity 93.9%, specificity 91.3%) and AFP-limited liver cancer (sensitivity 96.6%, specificity 90.3%). In the efficacy monitoring study, levels of plasma Hsp90α were dramatically decreased after surgery (P = 0.005), and correlated significantly with tumor size during interventional therapy (P ≤ 0.05). These findings highlight that plasma Hsp90α as a biomarker for the diagnosis of liver cancer, and can be used to evaluate the therapeutic efficacy of liver cancer patients underwent surgery, or interventional therapy.
Keywords: Plasma Hsp90α, Liver cancer, Biomarker
Highlights
-
•
Plasma Hsp90α is an emerging diagnosis biomarker for liver cancer patients.
-
•
Plasma Hsp90α can detect early-stage liver cancer.
-
•
Plasma Hsp90α is a better biomarker than AFP for the diagnosis in liver cancer patients.
-
•
Plasma Hsp90α can detect the AFP-negative and AFP-limited liver cancer, which both can not be detected by AFP in liver cancer patients.
-
•
The dynamic changes of Plasma Hsp90α level in liver cancer patients can monitor the efficacy of treatments, including liver surgery and interventional treatment.
Early detection is the most efficient solution to reduce the mortality caused by cancer metastasis, and more sensitive biomarker is urgently needed to facilitate early diagnosis. In this study, we developed an official, large-scale and multicenter clinical study, and assessed that quantitative measurement of heat shock protein 90alpha (Hsp90α) in plasma can improve the diagnosis accuracy and monitor treatment response of liver cancer patients, which proved Hsp90α as a liver cancer biomarker. Furthermore, Hsp90α also shows excellent accuracy in detecting early-stage liver cancer and liver cancers cannot be detected by currently widely used biomarker α-Fetoprotein (AFP).
1. Introduction
Liver cancer, mainly including hepatocellular carcinoma (HCC), cholangiocarcinoma (CC), combined hepatocellular carcinoma and cholangiocarcinoma (HCC-CC), and other hepatic malignancies (Other), has become the sixth most common malignant disease and the second leading cause of cancer-associated death (Torre et al., 2015). In 2012, about 782,500 new cases and 745,500 deaths of liver cancer patients occurred worldwide, with China alone accounting for about half of the new cases and deaths (Chen et al., 2016). Globally, 5-year overall survival rate is lower than 5% (Shariff et al., 2009). α-Fetoprotein (AFP) is a well-known blood tumor biomarker for the HCC diagnosis, but its sensitivity is only 25–65% at the commonly used cutoff value of 20 ng/mL (Tateishi et al., 2008). Due to its low sensitivity, the use of AFP as a screening indicator for HCC has been cancelled by the 2010 American Association for the Study of Liver Diseases (AASLD) guidelines (Bruix and Sherman, 2011). AFP is majorly synthesized and secreted by the HCC cells in carcinoma. Therefore, AFP diagnosis is not applicable in AFP-limited liver cancer, including CC, HCC-CC, and Other (Khan et al., 2002). Moreover, many at-risk patients with chronic liver diseases also have an elevated level of AFP (Birrer et al., 2003). In addition, other serum protein markers such as des-gamma carboxy prothrombin (DCP), Glypican-3, and Dickkopf-1 (DKK1) have also been reported to detect only HCC, either alone or in combination with AFP, but the combined accuracy is still limited (Tameda et al., 2013, Capurro et al., 2003, Shen et al., 2012). The sensitive, accurate, and timely diagnosis is the key to tackle such a daunting dilemma for patients with liver cancer.
Heat shock protein 90alpha (Hsp90α), a conserved and essential molecular chaperone (Frydman, 2001), can be translocated to the cell surface and secreted into the extracellular space by cancer cells (Eustace et al., 2004a). In addition, levels of secreted Hsp90α increase significantly in cancer patients, and correlate positively with tumor malignancy and metastatic ability (Wang et al., 2009). Accordingly, we propose that extracellular Hsp90α may serve as a potential diagnostic biomarker of various cancer. As we expected, the clinical trial with the enrollment of 2347 cases in 2012 showed the good auxiliary diagnosis effect and monitoring ability in treatment efficacy, which has proved plasma Hsp90α as a lung cancer biomarker (Luo et al., 2014). Besides, our small-scale clinical investigation has also revealed that the levels of plasma Hsp90α were elevated in many other cancer types, including liver cancer (Wang et al., 2009). Here, we designed a large-scale, multicenter clinical trial to assess the diagnostic accuracy of plasma Hsp90α for liver cancer, including discriminating liver cancer patients from non-liver cancer control, which contained patients with at-risk liver diseases and healthy individuals, or at-risk control alone, and detecting early-stage liver cancer patients, AFP-negative HCC, or AFP-limited liver cancer patients from both controls. Moreover, we explored the efficacy monitoring capability of plasma Hsp90α in liver cancer patients underwent surgery, or interventional therapy.
2. Materials and Methods
2.1. Participants Enrolled
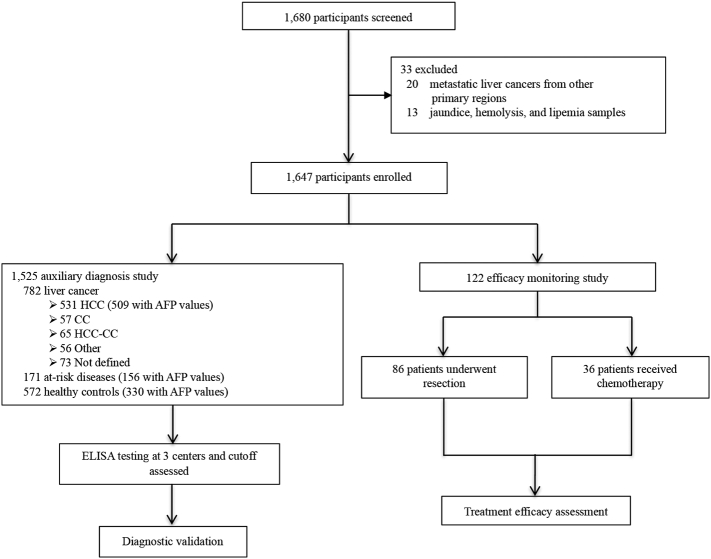
In this clinical trial, we recruited 1647 enrollments, including patients with liver cancer, patients with at-risk liver diseases (referring to hepatitis, liver cirrhosis, focal nodular hyperplasia), and healthy individuals, from The First Affiliated Hospital of Zhejiang University, Zhejiang Province People's Hospital, and The Tumor Hospital of Shandong Province, from October 2012 to May 2015. These enrollments were divided into two cohorts: the first (auxiliary diagnosis study) comprised 782 liver cancer patients, 171 with at-risk liver diseases, and 572 healthy controls; the second (efficacy monitoring group) enrolled 122 liver cancer patients, among which 86 patients received surgery, 36 patients underwent interventional therapy. We matched the participants in auxiliary diagnosis study for age, sex, classification, and differentiation as far as possible. Liver cancer was diagnosed on the basis of at least two imaging methods (ultrasound, liver CT, and MRI) and biochemistry, and further confirmed by histopathology according to the AASLD guide lines (Bruix and Sherman, 2005). Patients who received radiotherapy or had a history of other solid tumors were excluded from the study. Liver cancer patients were classified according to the TNM staging system (National Comprehensive Cancer Network, 2009), of which stage I and II liver cancer were recognized as early-stage liver cancer. Patients with at-risk liver diseases and healthy controls were enrolled according to eligibility criteria listed in the appendix. Briefly, cirrhosis confirmation was based on histopathology of liver biopsy samples, and supported by imaging evidences such as CT and liver ultrasound. Chronic HBV infection was confirmed by HBsAg presence for the last 6 months with an HBV DNA concentration to > 103 copies per mL as well as abnormal concentration of serum alanine amino transferase. Focal nodular hyperplasia (FNH) was diagnosed using contrast-enhanced magnetic resonance imaging (MRI). Healthy controls were identified as without liver or other systematic diseases, or HBV markers (HBsAg, HBeAg, anti-HBe, and anti-HBc), as well as normal concentrations of liver function enzymes. Samples of jaundice, hemolysis, and lipemia were incorporated into the exclusion criteria (Fig. 1). The study was approved by the institutional ethics review committee at all three centers. Informed consents were obtained from all participants based on each committee's regulations.
Fig. 1.
Study design. HCC indicates hepatocellular carcinoma. CC represents cholangiocarcinoma. HCC-CC means combined type of HCC and CC. Other includes rare types of primary liver cancer such as angiosarcoma, hemangiosarcoma, or squamous cell carcinoma of the liver. At-risk liver diseases include hepatitis, liver cirrhosis, focal nodular hyperplasia. AFP means α-fetoprotein.
2.2. Testing of Blood Samples
Peripheral blood samples (EDTA-K2 anticoagulant) from all participants were collected, and centrifuged at 3000 RPM for 10 min, then stored the plasma at − 20 °C, until use. In the auxiliary diagnosis study, we also compared the levels of plasma Hsp90α and serum AFP in parallel in patients with hepatocellular carcinoma (HCC), at-risk liver diseases, and healthy controls. For the efficacy monitoring group, CT scans of liver were done before and after patients receiving interventional therapy or undergoing surgery resection for assessment of diseases progression, meanwhile plasma Hsp90α were also detected. Due to the lack of bed for clinical use in China, and patients would usually be released from the hospital within about one week, it was extremely difficult to get the blood samples from patients when it's longer than one week. For patients undergoing surgery resection, blood samples were still collected before surgery and one week after surgery, though wound healing caused by surgery would take a much longer time than one week.
We used the commercially available ELISA kit (Protgen, Ltd) to quantitatively measure plasma Hsp90α concentrations according to the manufacturer's recommendations. Briefly, diluted plasma samples and standard samples were added to the 96-well microplate pre-coated with monoclonal antibody to Hsp90α. HRP-conjugated anti-Hsp90α antibody (50 μL) was added to the plate and then the plate was incubated at 37 °C for 1 h. The reaction was visualized by adding 50 μL chromogen TMB solution A and 50 μL chromogen TMB solution B sequentially to each well and incubated for 20 min at 37 °C. Finally, the reaction was stopped by adding with 50 μL Stop Solution to each well. The optical density was measured at 450 nm and referenced to 570 nm on a spectrophotometer (Thermo Fisher Scientific). The standard curve was generated by plotting the logarithm of average O.D. obtained for each of the six standard samples on the vertical (Y) axis versus the logarithm of corresponding concentrations on the horizontal (X) axis. To determine the amount of Hsp90α in plasma sample, the absorbance of samples was then calculated with the method of substitution in the standard curve. Double logarithmic curve fitting was recommended, and the coefficient of correlation (R2) was required to be > 0.980. The AFP concentrations were tested with commercially available ELISA kit (Roche Life Science) following the manufacturer's recommendations. Researchers assessing the outcomes were blinded to clinical information.
2.3. Statistical Analysis
Statistical analysis was performed using SPSS 19.0 software. The data were summarized as mean ± SE. Differences between two independent groups were tested with the Wilcoxon Mann-Whitney test. Receiver operating characteristics (ROC) curves were constructed to assess sensitivity, specificity, and respective areas under the curves (AUCs) with 95% CI. We determined the optimum cutoff value for the diagnosis by maximizing the sum of sensitivity and specificity, minimizing the square root of the sum[(1-sensitivity)2 + (1-specificity)2], and minimizing the distance between the point to the top-left corner of the ROC curve (where sensitivity = 1 and specificity = 1) (Liu, 2012). The correlation between plasma Hsp90α concentrations and clinicopathological characteristics of patients were analyzed with Fisher's test.
To assess the diagnostic performance of plasma Hsp90α either along, or combination with AFP, between patients with HCC and non-liver cancer control, the models were assessed by logistic regression (Pepe and Thompson, 2000), and the equation was:
After fitting, the new variable predicted probability (P) was subjected to ROC analysis.
Differences in efficacy evaluation for liver cancer patients were evaluated using the independent samples t-test and the paired t-test. P value < 0.05 (two sided) was considered to be statistically significant.
3. Results
3.1. Plasma Hsp90α is a Much Precise Diagnosis Biomarker for all Types of Liver Cancer
1647 participants, including 1525 in auxiliary diagnosis trial and 122 in the efficacy monitoring study, were recruited from three independent hospitals (Fig. 1, Tables S1, S2, S3 in the Supplementary Appendix). The eligibility criteria for the recruited participants and demographic information were summarized in the appendix (Table S4 in the Supplementary Appendix). Serum AFP levels were also measured in 509 of 557 patients with hepatocellular carcinoma (HCC), 156 of 171 with at-risk liver diseases, and 330 of 572 healthy controls in parallel with plasma Hsp90α, respectively.
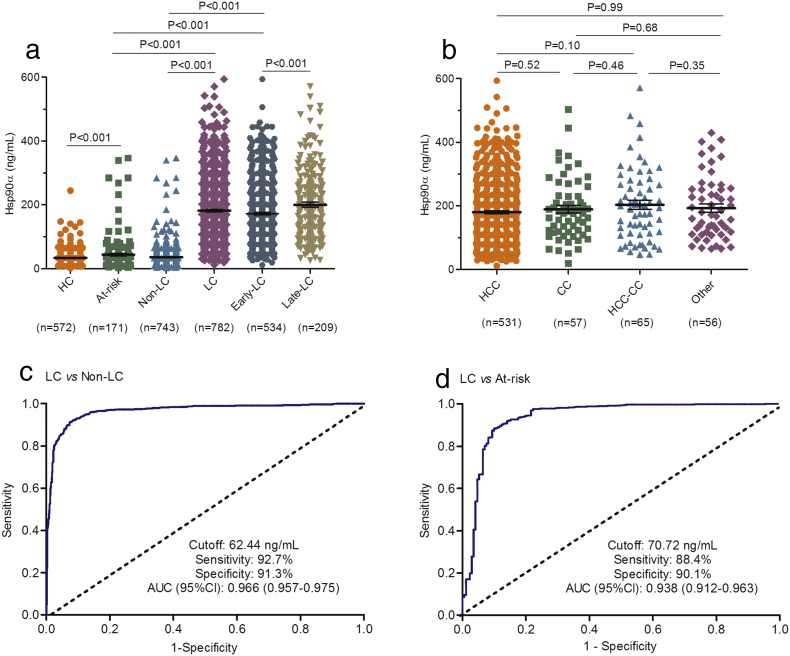
In auxiliary diagnosis trial, plasma Hsp90α concentrations detected by ELISA were significantly elevated in liver cancer patients (median 159.9 ng/mL, IQR 96.7–246.8; mean 181.5 ng/mL, SD 105.4) than those in non-liver cancer control (P < 0.001; Fig. 2A; Table S5 in the Supplementary Appendix) and those in at-risk control (P < 0.001; Fig. 2A; Table S5 in the Supplementary Appendix). For different types of liver cancer, including HCC, cholangiocarcinoma (CC), combined (HCC-CC), and other types of liver cancer (Other), levels of plasma Hsp90α were not dramatically different (Fig. 2B). Also no significant differences were observed between liver cancer of corresponding differentiation grades (Fig. S1 in the Supplementary Appendix). ROC curve was used to evaluate the sensitivity, specificity, and area under the curve (AUC) of plasma Hsp90α, and determine the best cutoff value for liver cancer diagnosis. Compared with non-liver cancer control, 62.44 ng/mL (AUC 0.966, 95% CI 0.957–0.975; sensitivity 92.7%, specificity 91.3%; Fig. 2C) was chosen as the optimum diagnostic cutoff. Positive ratio of plasma Hsp90α in CC, HCC-CC, or Other were > 94.7% (Fig. S2 in the Supplementary Appendix). Similar results were obtained from the comparison between patients with liver cancer and at-risk control (Fig. 2D). All these results demonstrate that plasma Hsp90α can be used as a much precise tumor biomarker for the diagnosis of all types of liver cancer in clinical.
Fig. 2.
Plasma Hsp90α is a much precise diagnosis biomarker for all types of liver cancer. (A) shows the plasma Hsp90α levels for liver cancer patients and different controls. (B) shows the plasma Hsp90α levels for different histological types of liver cancer. (C) shows ROC curve of plasma Hsp90α for liver cancer patients versus non-liver cancer group. (D) ROC curve of plasma Hsp90α for liver cancer patients versus at-risk control. Hsp90α denotes heat shock protein 90alpha. HC indicates healthy control. LC represents liver cancer. Non-LC means non-liver cancer control group, including patients with non-cancerous diseases and healthy individuals. Early-LC indicates patients with early-stage liver cancer. Late-LC means patients with late-stage liver cancer. HCC indicates hepatocellular carcinoma. CC represents cholangiocarcinoma. HCC-CC means combined type of HCC and CC. Other includes rare types of primary liver cancer such as angiosarcoma, hemangiosarcoma, or squamous cell carcinoma of the liver. At-risk indicates non-cancerous liver diseases, including hepatitis, liver cirrhosis, focal nodular hyperplasia. Black horizontal lines indicate means, and error bars represent standard errors (SE).
3.2. Plasma Hsp90α can be Used as an Early-diagnosis Biomarker for Patients With Liver Cancer
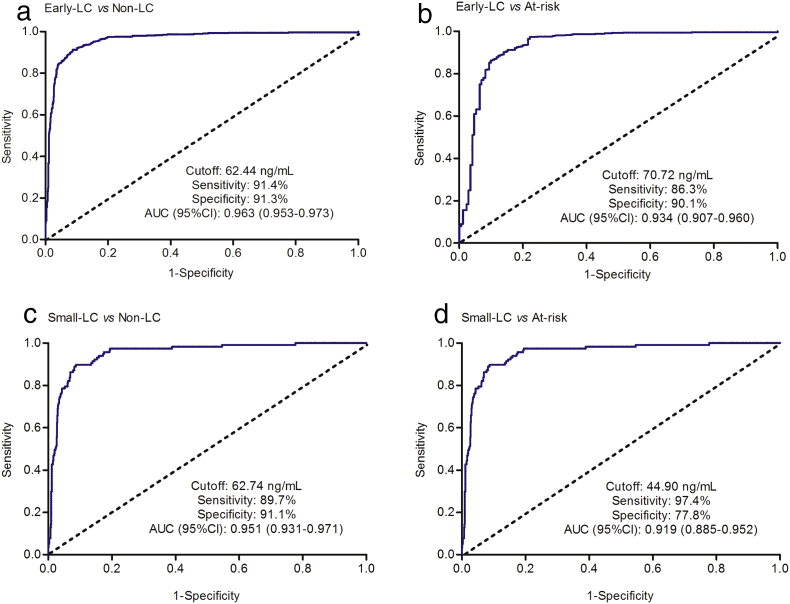
Plasma Hsp90α concentrations in late-stage liver cancer patients (TNM stage III + IV; median183.3, IQR 111.7-262.6, mean 200.5, SD 110.4) were significantly elevated than those in patients with early-stage liver cancer (TNM stage I + II; P < 0.001; Fig. 2A; Table S5 in the Supplementary Appendix), which indicated that plasma Hsp90α positively correlated with liver cancer malignancy (Table S6 in the Supplementary Appendix). We therefore attempted to assess the diagnosis performance of plasma Hsp90α in detecting early-stage liver cancer patients. Plasma Hsp90α concentrations in patients with early-stage liver cancer increased significantly than those in both non-liver cancer control and at-risk control (P < 0.001; Fig. 2A; Table S5 in the Supplementary Appendix). ROC curve showed plasma Hsp90α (AUC 0.963, sensitivity 91.4%, specificity 91.3%) a remarkable performance in the diagnosis of early-stage liver cancer patients from non-liver cancer control (Fig. 3A). Similar results were also obtained from the comparison between early-stage liver cancer patients and at-risk control (Fig. 3B). In patients with small-size liver cancer (size ≤ 3 cm), we also observed the distinguished diagnosis capability for plasma Hsp90α in discriminating patients from both controls (Fig. S3; Fig. 3C–D; Table S5 in the Supplementary Appendix). These results collectively prove that plasma Hsp90α can be used as an excellent biomarker to detect early-stage liver cancer.
Fig. 3.
Plasma Hsp90α can be used as an early-diagnosis biomarker for liver cancer patients. (A) shows ROC curve of plasma Hsp90α for early-stage liver cancer patients versus non-liver cancer group. (B) Diagnosis for plasma Hsp90α in early-stage liver cancer versus at-risk control. (C) Diagnosis for small-size liver cancer versus non-liver cancer control. (D) Diagnosis for small-size liver cancer versus at-risk control. Early-LC indicates patients with early-stage liver cancer. Small-LC means patients with small-size liver cancer. Non-LC indicates non-liver cancer control. At-risk indicates non-cancerous liver diseases, including hepatitis, liver cirrhosis, focal nodular hyperplasia.
3.3. Plasma Hsp90α is a Much Better Biomarker Than AFP for the Diagnosis of Liver Cancer
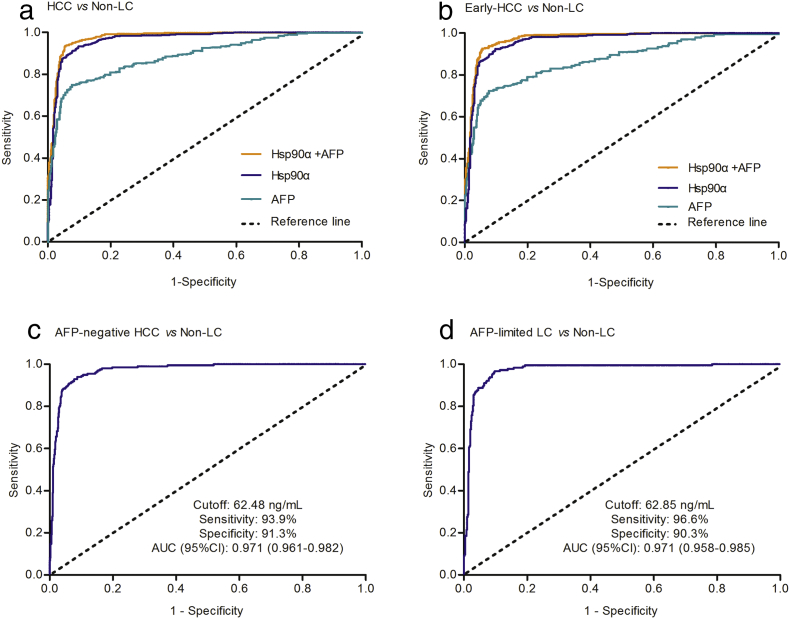
To evaluate the differential diagnosis performance of plasma Hsp90α and AFP, we analyzed the parallel clinical data from HCC (Fig. S4 in the Supplementary Appendix). It was shown that, by contrast with AFP20 (AUC 0.887, sensitivity 61.1%, specificity 96.3%), plasma Hsp90α (AUC 0.965, sensitivity 93.3%, specificity 90.3%) or the combination (AUC 0.977, sensitivity 93.7%, specificity 94.4%) improved significantly the diagnostic ability of HCC from non-liver cancer control (Fig. 4A; Table 1; Table S7 in the Supplementary Appendix). However, the combination of Hsp90α and AFP had increased only the diagnosis accuracy to a lesser extent when compared with Hsp90α alone (Fig. 4A; Table 1). The diagnosis between early-HCC and non-liver cancer control also showed that plasma Hsp90α is more sensitive than AFP (Fig. 4B; Table 1; Table S7 in the Supplementary Appendix). Similar results were also obtained from the comparison between patients with HCC, or early-stage HCC, and at-risk control (Table 1; Table S7; Figs. S5 and S6 in the Supplementary Appendix). When considering AFP status, we found that the positive ratio of Hsp90α diagnosis in AFP-negative and AFP-positive HCC patients were comparable (93.9% vs 92.9%; Fig. S7 in the Supplementary Appendix), which indicated the diagnosis of Hsp90α in HCC irrespective of AFP status. Furthermore, various comparisons of specificities (or sensitivities) at different adjusted sensitivities 90% or 85% of AFP (or specificities 90% or 85% of AFP) showed that plasma Hsp90α exhibited prominent advantages in discriminating HCC patients or early-stage HCC patients from non-liver cancer control (Table S8, S9 in the Supplementary Appendix). These results conclusively demonstrate that plasma Hsp90α is a much better diagnosis biomarker than AFP in HCC.
Fig. 4.
Plasma Hsp90α is a much better biomarker than AFP for the diagnosis of liver cancer. (A) shows ROC curve of plasma Hsp90α for HCC patients versus non-liver cancer group. (B) shows ROC curve of plasma Hsp90α for early-HCC patients versus non-liver cancer group. (C) shows ROC curve of plasma Hsp90α for AFP-negative HCC patients versus non-liver cancer group. (D) shows ROC curve of plasma Hsp90α for AFP-limited liver cancer patients versus non-liver cancer group. HCC indicates hepatocellular carcinoma. Non-LC indicates non-liver cancer control. Early-LC indicates patients with early-stage liver cancer. AFP-negative HCC indicates the HCC patients with serum AFP concentrations lower than 20 ng/mL. AFP-limited LC represents other types of liver cancer with exception of HCC, and in which AFP was not used to detect these patients. Early-HCC represents patients with early-stage hepatocellular carcinoma.
Table 1.
Results for measurement of plasma Hsp90α, AFP, or both in the diagnosis of hepatocellular carcinoma (HCC), or early-stage HCC.
| AUC (95%CI) | Cutoff (ng/mL) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR | Negative LR | |
|---|---|---|---|---|---|---|---|---|
| HCC vs Non-LC | ||||||||
| Hsp90α | 0.965(0.953–0.976) | 62.44 | 93.32 | 90.27 | 87.32 | 95.04 | 9.64 | 0.07 |
| AFP20a | 0.887(0.866–0.907) | 6.98 | 75.05 | 92.34 | 91.17 | 77.95 | 9.86 | 0.27 |
| Hsp90α + AFP | 0.977(0.968–0.985) | − | 93.70 | 94.40 | 91.91 | 95.63 | 14.69 | 0.07 |
| HCC vs At-risk | ||||||||
| Hsp90α | 0.938(0.910–0.967) | 73.23 | 89.00 | 90.20 | 96.53 | 72.64 | 8.96 | 0.12 |
| AFP20a | 0.851(0.820–0.883) | 6.171 | 76.82 | 83.01 | 93.54 | 52.23 | 4.44 | 0.28 |
| Hsp90α + AFP | 0.952(0.930–0.975) | − | 94.10 | 88.90 | 96.57 | 82.25 | 8.64 | 0.07 |
| Early-stage HCC vs Non-LC | ||||||||
| Hsp90α | 0.962(0.949–0.974) | 62.44 | 92.41 | 90.27 | 83.52 | 95.72 | 9.54 | 0.08 |
| AFP20a | 0.875(0.851–0.898) | 6.98 | 72.66 | 92.34 | 88.58 | 80.61 | 9.54 | 0.30 |
| Hsp90α + AFP | 0.974(0.964–0.983) | − | 92.70 | 94.4 | 91.13 | 94.06 | 16.68 | 0.08 |
| Early-stage HCC vs At-risk | ||||||||
| Hsp90α | 0.935(0.905–0.964) | 73.23 | 87.34 | 90.20 | 95.29 | 75.12 | 8.76 | 0.14 |
| AFP20a | 0.833(0.798–0.868) | 6.17 | 74.43 | 83.01 | 91.59 | 56.09 | 4.30 | 0.31 |
| Hsp90α + AFP | 0.949(0.925–0.972) | − | 92.70 | 88.90 | 95.56 | 82.74 | 8.50 | 0.08 |
HCC indicates hepatocellular carcinoma. Non-LC indicates non-liver cancer control. At-risk indicates non-cancerous liver diseases, including hepatitis, liver cirrhosis, focal nodular hyperplasia. Early-LC indicates patients with early-stage liver cancer. Hsp90α + AFP indicates the combined diagnosis with these two proteins. AUC represents area under curve. CI indicates confidence interval. Early-stage HCC represents patients with early-stage hepatocellular carcinoma. Hsp90α indicates heat shock protein 90alpha. AFP means α-fetoprotein. AUC represents area under curve. HC for healthy controls. PPV indicates positive predictive value. NPV means negative predictive value. LR represents likelihood ratio.
The commonly used cutoff value for AFP in HCC diagnosis.
We further assessed the diagnostic capability of plasma Hsp90α in the diagnosis of AFP-negative HCC and AFP-limited liver cancer, of which composed other types of liver cancer patients with exception of HCC (Fig. S8 in the Supplementary Appendix). ROC curves showed that plasma Hsp90α had an AUC 0.971 (95% CI 0.961–0.982) with a sensitivity of 93.9% and specificity of 91.3% at the optimum cutoff 62.48 ng/mL in discriminating AFP-negative HCC from non-liver cancer control (Fig. 4C; Table S7 in the Supplementary Appendix), and an AUC 0.971 (95% CI 0.958–0.985) with a sensitivity of 96.6% and specificity of 90.3% at the optimum cutoff 62.85 ng/mL in discriminating AFP-limited liver cancer patients from non-liver cancer control (Fig. 4D; Table S7 in the Supplementary Appendix), respectively. Similar results were obtained in evaluating the sensitivities, specificities, and AUCs of plasma Hsp90α in AFP-negative HCC or AFP-limited liver cancer patients when compared with at-risk control (Fig. S9 in the Supplementary Appendix). Above results show that plasma Hsp90α can complement the diagnosis of AFP-negative HCC and AFP-limited liver cancer patients with remarkable discriminating performance.
3.4. Dynamic Changes of Plasma Hsp90α in Liver Cancer Patients can Monitor the Efficacy of Treatment, Including Liver Surgery and Interventional Treatment
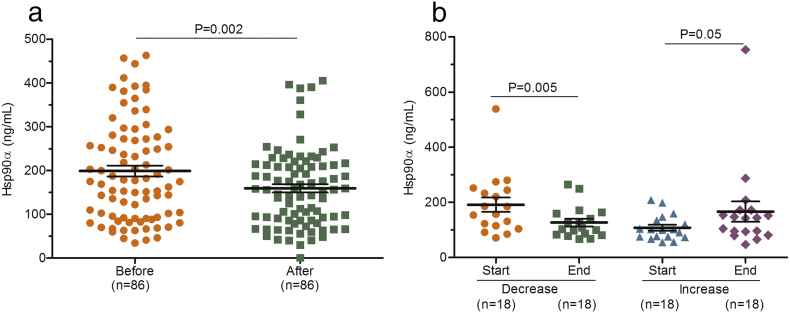
Dynamic changes reflecting the patients' condition could provide the clinical guidance for doctors. We therefore tentatively explored the efficacy monitoring capability of plasma Hsp90α in patients undergoing hepatic operation or receiving interventional therapy, respectively. The mean concentration of plasma Hsp90α before surgery was 205.9 (SD 122.5) ng/mL, and values dropped to 150.3 (SD 100.2) ng/mL after 7 days (P = 0.002; Fig. 5A; Table S10). Moreover, the levels of plasma Hsp90α in patients whose tumor volume shrunk after treatment decreased dramatically (P = 0.005; Fig. 5B; Table S10), while increased marginally in patients whose tumor volume became larger after treatment (P = 0.05; Fig. 5B; Table S10). Taken together, dynamic changes of plasma Hsp90α in liver cancer patients can monitor the treatment efficacy, including liver surgery and interventional treatment.
Fig. 5.
Concentrations of plasma Hsp90α in efficacy monitoring group. (A) shows the concentrations of plasma Hsp90α before and after surgery. (B) shows dynamic changes of plasma Hsp90α before and after interventional therapy. Before and After represent before surgery and after surgery, Respectively. Start and End means the measuring point before interventional therapy and the efficacy evaluation after interventional therapy, respectively. Decrease and Increase indicate tumors size decrease or increase after interventional therapy, respectively.
4. Discussion
In the diagnosis of liver cancer, plasma Hsp90α shows remarkable sensitivities and specificities in detecting liver cancer patients, even at the early stage or patients with small-size tumor (size ≤ 3 cm), from non-liver cancer or at-risk control. In hepatocellular carcinoma (HCC), plasma Hsp90α exhibits significantly superior sensitivities than AFP to distinguish HCC patients from both controls. Plasma Hsp90α also shows excellent distinguishing ability in the diagnosis of AFP-negative HCC and AFP-limited liver cancer patients. Moreover, dynamic changes of plasma Hsp90α in liver cancer patients can monitor the efficacy of treatment, including surgery and interventional therapy. These findings highlight plasma Hsp90α as a non-invasive diagnosis biomarker for liver cancer patients.
Hsp90α, which has evolved for almost 3.5 billion years at least (Gupta, 1995), is widely recognized as an essential molecular chaperone and a master regulator for the key cell signaling networks in human cells (El-Serag et al., 2008). Three points have made it reality that plasma Hsp90α can be used as a clinical diagnosis biomarker: It is specifically overexpressed in cancer cells, which accounts for 2–7% of total proteins (Whitesell and Lindquist, 2005); It can be translocated to the cell surface, or secreted by different types of cancer cells (Wong et al., 2016); Altered expression of Hsp90α are associated with tumor development, progression, and metastasis (Shen et al., 2012). In addition, our group (Huang et al., 2009) and Lyden's group (Kaplan et al., 2005) have reported that metastasis at molecular level occurred in the early stage of cancer progression, which provides the evidence supporting the detection for the early-stage cancer. 90% of many cancer indications, estimated by the World Health Organization, could be hopefully cured if it can be diagnosed at early stage (World Health Organization, 2016). The detection of plasma Hsp90α represents an effective and timely “liquid biopsy” means for the diagnosis of liver cancer, particularly for the patients at early stage.
Among primary liver cancer occurring worldwide, HCC accounts for the most common type (Torre et al., 2015). However, only for HCC, almost one third of them will be missed if used merely AFP as the diagnostic means (Farinati et al., 2006). The high rates of missed diagnosis of AFP is in part due to the fact that significant increase in serum (20–200 ng/mL) can be also observed in 11–58% of patients with at-risk liver diseases (Johnson, 2001). However, when used plasma Hsp90α to detect patients with liver cancer, it shows the concentrations with better data convergence in liver cancer patients or in non-liver cancer control, which facilitates improving sensitivity and decreasing the rates of missed diagnosis. Recently, two large-scale and multicenter studies reported that DKK1 (Shen et al., 2012) and serum microRNA (Lin et al., 2015) could be used as the potential biomarkers to detect HCC. However, compared with plasma Hsp90α, the lower sensitivities (69.1–71.3% in DKK1 and 70.4–85.7% in microRNA) were far inadequate for the purpose of liver cancer screening, even when combined with AFP. In addition, plasma Hsp90α can also be extended to the diagnosis of AFP-negative HCC and AFP-limited liver cancer with excellent sensitivities and specificities. The etiology between East and West is different (Monsour et al., 2013), therefore, the diagnostic value in West still needs further investigation. Our clinical trial has demonstrated that plasma Hsp90α is a much better diagnosis biomarker than AFP, or other potential biomarkers, which provides a more precise means for the diagnosis of liver cancer patients.
What are the underpinnings for the prominent diagnostic performance of plasma Hsp90α in early-stage, small-size, or AFP-limited liver cancer, or AFP-negative HCC, which underscored the much higher diagnosis accuracy than the clinical (AFP) or other potential biomarkers (DKK1 or microRNA) mentioned above? The main function of AFP is to prevent the virilization of female fetuses (Carter, 1992, Seregni et al., 1994), but not associated with tumor malignancy, or metastasis. Likewise, other potential biomarkers, such as DKK1 (Niida et al., 2004) or microRNAs (Slaby et al., 2008, Yang et al., 2014), are only related to a few aspects in cancer development, even so, their use as a tumor biomarker need to be confirmed by official clinical trials. However, hundreds of identified clients of intracellular Hsp90α, including many transcription factors (p53, PPARs, or Stat2/3), kinases (AKT, Src, MAPK, CDKs, or MET), or mutant oncoproteins (p53, EGFR, or BRAF), are broadly involved in various biological activities of cancer cells (Taipale et al., 2010, Perl and Prodromou, 2000), referring to ten hallmarks of cancer (Hanahan and Weinberg, 2011). Extracellular Hsp90α has also been reported to promote tumor progression by stabilizing MMP2 (Eustace et al., 2004b, Song et al., 2010), initiating epithelial to mesenchymal transition (EMT) (Hance et al., 2012), promoting cell motility induced by ERK through LRP1 (Bohonowych et al., 2014), activating NF-κB signaling pathway (Bohonowych et al., 2014), contributing to cancer cell growth and chemotherapy resistance (Whitesell et al., 2014), and inhibiting the normal mechanism of programmed cell death (Gallerne et al., 2013). Further studies are needed to determine the detailed mechanisms of Hsp90α in orchestrating tumor development, especially the role of extracellular Hsp90α in facilitating tumor pre-metastatic niche formation and metastasis. In addition, the tremendous progress has been made in developing Hsp90 inhibitors as anticancer agents (Whitesell and Lindquist, 2005). Therefore, it is conceivable that, instead of just a cancer indicator, plasma Hsp90α is more a functional biomarker. These findings that the central position occupied by functional Hsp90α in cancer cellular networks has emphasized the importance of Hsp90α in cancer pathophysiology, and these inherent characteristics contribute to the decisive influences on the distinguished diagnosis performance of Hsp90α for liver cancer.
Funding
This work was supported by Protgen Ltd., which is a part of the National Engineering Laboratory for Anti-Tumor Protein Therapeutics (NEL) (20130507, 201312001, 201407001). The NEL has played a role in discussing the study design and paper writing, but had no role in data collection, data analysis, and interpretation. Protgen Ltd. also provided all the ELISA kit for plasma Hsp90α.
Competing Interests
The authors declared no conflict of interests.
Author Contributions
Y.F. wrote the manuscript; Y.F., D.W.C., J.J.L., S.S.Z., and Y.Z.L. participated in discussion. X.X., D.S.H., L.S.L., J.W.L., Z.L.H., and S.S.Z. collected the data and analyzed the preclinical data; Y.Z.L. and S.S.Z. designed the experiments.
Acknowledgments
Acknowledgements
This work was supported by Protgen Ltd., which is a part of the National Engineering Laboratory for Anti-Tumor Protein Therapeutics (NEL) (20130507, 201312001, 201407001). The NEL has played a role in discussing the study design and paper writing, but had no role in data collection, data analysis, and interpretation. Protgen Ltd. also provided all the ELISA kit for plasma Hsp90α.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.09.007.
Contributor Information
Shusen Zheng, Email: shusenzheng@zju.edu.cn.
Yongzhang Luo, Email: yluo@tsinghua.edu.cn.
Appendix A. Supplementary data
References
- Birrer R.B., Birrer D., Klavins J.V. Hepatocellular carcinoma and hepatitis virus. Ann. Clin. Lab. Sci. 2003;33:39–54. [PubMed] [Google Scholar]
- Bohonowych J.E., Hance M.W., Nolan D.K., Defee M., Parsons C.H., Isaacs J.S. Extracellular Hsp90 mediates an NF-κB dependent inflammatory stromal program: implications for the prostate tumor microenvironment. Prostate. 2014;74:395–407. doi: 10.1002/pros.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruix J., Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro M., Wanless I.R., Sherman M., Deboer G., Shi W., Miyoshi E., Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- Carter C. Neuroendocrinology of sexual behavior in the female. In: Becker J., Breedlove S., Crews D., editors. Behavioral Endocrinology. MIT Press; Cambridge: 1992. pp. 71–96. [Google Scholar]
- Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., Jemal A., Yu X., He J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- El-Serag H.B., Marrero J.A., Rudolph L., Reddy K.R. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- Eustace B.K., Sakurai T., Stewart J.K., Yimlamai D., Unger C., Zehetmeier C., Lain B., Torella C., Henning S.W., Beste G., Scroggins B.T., Neckers L., Ilag L.L., Jay D.G. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat. Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- Eustace B.K., Sakurai T., Stewart J.K., Yimlamai D., Unger C., Zehetmeier C., Lain B., Torella C., Henning S.W., Beste G., Scroggins B.T., Neckers L., Ilag L.L., Jay D.G. Functional proteomic screens reveal an essential extracellular role for Hsp90α in cancer cell invasiveness. Nat. Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- Farinati F., Marino D., De Giorgio M., Baldan A., Cantarini M., Cursaro C., Rapaccini G., Del Poggio P., Di Nolfo M.A., Benvegnù L., Zoli M., Borzio F., Bernardi M., Trevisani F. Diagnostic and prognostic role of α-fetoprotein in hepatocellular carcinoma: both or neither&quest. Am. J. Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 2001;70(1):603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- Gallerne C., Prola A., Lemaire C. Hsp90 inhibition by PU-H71 induces apoptosis through endoplasmic reticulum stress and mitochondrial pathway in cancer cells and overcomes the resistance conferred by Bcl-2. BBA Mol. Cell. Res. 2013;1833:1356–1366. doi: 10.1016/j.bbamcr.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Gupta R.S. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol. Biol. Evol. 1995;12:1063–1073. doi: 10.1093/oxfordjournals.molbev.a040281. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hance M.W., Dole K., Gopal U., Bohonowych J.E., Jezierska-Drutel A., Neumann C.A., Liu H., Garraway I.P., Isaacs J.S. Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer. J. Biol. Chem. 2012;287:37732–37744. doi: 10.1074/jbc.M112.389015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Song N., Ding Y., Yuan S., Li X., Cai H., Shi H., Luo Y. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009;69:7529–7537. doi: 10.1158/0008-5472.CAN-08-4382. [DOI] [PubMed] [Google Scholar]
- Johnson P.J. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin. Liver Dis. 2001;5:145–159. doi: 10.1016/s1089-3261(05)70158-6. [DOI] [PubMed] [Google Scholar]
- Kaplan R.N., Riba R.D., Zacharoulis S., Bramley A.H., Vincent L., Costa C., MacDonald D.D., Jin D.K., Shido K., Kerns S.A., Zhu Z., Hicklin D., Wu Y., Port J.L., Altorki N., Port E.R., Ruggero D., Shmelkov S.V., Jensen K.K., Rafii S., Lyden D. VEGFR1-positive Haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.A., Davidson B.R., Goldin R., Pereira S.P., Rosenberg W.M., Taylor-Robinson S.D., Thillainayagam A.V., Thomas H.C., Thursz M.R., Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51:vi1–vi9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Chong Y., Guo Z., Xie C., Yang X., Zhang Q., Li S., Xiong Y., Yuan Y., Min J., Jia W., Jie Y., Chen M., Chen M., Fang J., Zeng C., Zhang Y., Guo R., Wu Y., Lin G., Zheng L., Zhuang S. A serum MicroRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015;16:804–815. doi: 10.1016/S1470-2045(15)00048-0. [DOI] [PubMed] [Google Scholar]
- Liu X. Classification accuracy and cut point selection. Stat. Med. 2012;31:2676–2686. doi: 10.1002/sim.4509. [DOI] [PubMed] [Google Scholar]
- Luo Y., Cui D., Fu Y. Google Patents. 2014. Tumor biomarker. [Google Scholar]
- Monsour H., Jr., Asham E., McFdden Robert, Victor D., Muthuswamy B., Zaheer I. Hepatocellular carcinoma: the rising tide from east to west – a review of epidemiology screening and tumor markers. Transl. Cancer Res. 2013;2:492–506. [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers v. 2.2009. National Comprehensive Cancer Network Available at http://www.nccn.org (accessed 25 September 2016). [DOI] [PMC free article] [PubMed]
- Niida A., Hiroko T., Kasai M., Furukawa Y., Nakamura Y., Suzuki Y., Sugano S., Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- Pepe M.S., Thompson M.L. Combining diagnostic test results to increase accuracy. Biostatistics. 2000;1:123–140. doi: 10.1093/biostatistics/1.2.123. [DOI] [PubMed] [Google Scholar]
- Perl L.H., Prodromou C. Structure and in vivo function of Hsp90. Curr. Opin. Struct. Biol. 2000;10:46–51. doi: 10.1016/s0959-440x(99)00047-0. [DOI] [PubMed] [Google Scholar]
- Seregni E., Botti C., Bombardieri E. Biochemical characteristics and clinical applications of alpha-fetoprotein isoforms. Anticancer Res. 1994;15:1491–1499. [PubMed] [Google Scholar]
- Shariff M.I., Cox I.J., Gomaa A.I., Khan S.A., Gedroyc W., Taylor-Robinson S.D. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev. Gastroenterol. Hepatol. 2009;3:353–367. doi: 10.1586/egh.09.35. [DOI] [PubMed] [Google Scholar]
- Shen Q., Fan J., Yang X., Tan Y., Zhao W., Xu Y., Wang N., Niu Y., Wu Z., Zhou J., Qiu S., Shi Y., Yu B., Tang N., Chu W., Wang M., Wu J., Zhang Z., Yang S., Gu J., Wang H., Qin W. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–826. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- Slaby O., Svoboda M., Fabian P., Smerdova T., Knoflickova D., Bednarikova M., Nenutil R., Vyzula R. Altered expression of MiR–21, MiR–31, MiR–143 and MiR–145 is related to Clinicopathologic features of colorectal cancer. Oncology. 2008;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- Song X., Wang X., Zhuo W., Shi H., Feng D., Sun Y., Liang Y., Fu Y., Zhou D., Luo Y. The regulatory mechanism of extracellular Hsp90α on matrix Metalloproteinase-2 processing and tumor angiogenesis. J. Biol. Chem. 2010;285:40039–40049. doi: 10.1074/jbc.M110.181941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M., Jarosz D.F., Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- Tameda M., Shiraki K., Sugimoto K., Ogura S., Inagaki Y., Yamamoto N., Ikejiri M., Takei Y., Ito M., Nobori T. Des-γ-carboxy prothrombin ratio measured by P-11 and P-16 antibodies is a novel biomarker for hepatocellular carcinoma. Cancer Sci. 2013;104:725–731. doi: 10.1111/cas.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi R., Yoshida H., Matsuyama Y., Mine N., Kondo Y., Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol. Int. 2008;2:17–30. doi: 10.1007/s12072-007-9038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Wang X., Song X., Zhuo W., Fu Y., Shi H., Liang Y., Tong M., Chang G., Luo Y. The regulatory mechanism of Hsp90α secretion and its function in tumor malignancy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21288–21293. doi: 10.1073/pnas.0908151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L., Lindquist S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Santagata S., Mendillo M.L., Lin N.U., Proia D.A., Lindquist S. HSP90 empowers evolution of resistance to hormonal therapy in human breast cancer models. Proc. Natl. Acad. Sci. U. S. A. 2014;111:18297–18302. doi: 10.1073/pnas.1421323111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D.S., Jay D.G. Chapter six-Emerging roles of extracellular Hsp90 in cancer. Adv. Cancer Res. 2016;129:141–163. doi: 10.1016/bs.acr.2016.01.001. [DOI] [PubMed] [Google Scholar]
- World Health Organization 2016. http://www.who.int/cancer/en Geneva. (accessed July 30, 2016)
- Yang Q., Jia C., Wang P., Xiong M., Cui J., Li L., Wang W., Wu Q., Chen Y., Zhang T. MicroRNA-505 identified from patients with essential hypertension impairs endothelial cell migration and tube formation. Int. J. Cardiol. 2014;177:925–934. doi: 10.1016/j.ijcard.2014.09.204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.