Abstract
Generation of a potent antibody response that can be sustained over time is highly challenging in young infants. Our previous studies using a nursery-reared nonhuman primate model identified R848 conjugated to inactivated influenza virus as a highly immunogenic vaccine for neonates. Here we determined the effectiveness of this vaccine in mother-reared infants as well as its ability to promote improved responses at 6 months compared to vaccination in the absence of R848. In agreement with our nursery study, R848 conjugated to influenza virus induced a higher antibody response in neonates compared to the non-adjuvanted vaccine. Further, the increase in the response relative to that induced by the non-adjuvanted vaccine was maintained at 6 months suggesting the increased antibody secreting cells that resulted from inclusion of conjugated R848 production were capable of surviving long term. There was no significant difference in quality of antibody (i.e. neutralization or affinity), suggesting the beneficial effect of conjugated R848 during vaccination of neonates with inactivated influenza virus is likely manifest during the early generation of antibody secreting cells.
Keywords: influenza vaccine, neonate, adjuvant, TLR7/8, R848, memory
INTRODUCTION
Vaccines are undoubtedly the most effective mechanism to limit the morbidity and mortality that results from pathogen infection. However utilization of vaccines to protect the very young has met with significant challenges which limits their use [1]. For example, the widely administered trivalent or quadrivalent inactivated influenza vaccine (TIV or QIV) is not approved for use in infants less than 6 months of age as a result of its poor immunogenicity in this group [2, 3]. The efficacy of TIV/QIV in older individuals rests in its ability to produce high level neutralizing antibody, with an HI titer of ≥1:32 considered protective [2]. Achieving this goal is challenging in infants as the production of high level, high affinity IgG is depressed through the first year of life [4, 5]. In addition, neonates are impaired in the production and survival of long-lived plasma cells; as such antibody levels wane rapidly over time [6–10].
The mechanism responsible for this is likely multi-factorial. For example, recruitment of T cell help to B cells is hampered as a result of low MHC II and reduced antigen processing and presentation [11]. In addition, decreased dendritic cell maturation contributes to poor CD4+ T cell generation necessary to support antibody responses [4, 12, 13]. Finally, neonates demonstrate impairment in the CD40-CD40L pathway which plays a critical role in regulating B cells differentiation and function [14, 15]. While these attributes present obstacles, there is evidence that the immune system of neonates can respond under appropriate stimulatory conditions, e.g. hepatitis B vaccination [16], opening the door to development of vaccine approaches that can be utilized to protect this at risk population.
We have established a nonhuman primate (NHP) model for use in development of an influenza vaccine that will be safe and effective in neonates [17–19]. The experimental inactivated influenza vaccine assessed is comprised of the TLR7/8 agonist R848 conjugated to inactivated influenza virus A/Puerto Rico/8/34(H1N1) (IPR8-R848). Our earlier studies performed in nursery-reared neonates showed this experimental vaccine could induce robust acute antibody and T cell responses that were capable of increased virus clearance and reduced pathology following challenge [17]. Here we tested this vaccine for its ability to induce a long-lived antibody response (e.g. 6 months). As this was administered in infants that were mother-reared, we were also able to assess the ability of this vaccine to elicit responses in infants that were exposed to broader environmental immune stimulatory signals (e.g. other animals, outdoors, etc) compared to those housed in the nursery. We find that conjugated R848 is a potent adjuvant in mother-reared neonates. Further, the increase in antibody response observed at early times post-vaccination is maintained over time, i.e. 6 months. Thus, R848 conjugation results in a vaccine with sustained long lived immunity.
MATERIALS AND METHODS
Animals
African green monkey (AGM) infants (Caribbean-origin Chlorocebus aethiops sabaeus) used in this study were housed at the Vervet Research Colony at Wake Forest School of Medicine. Infants were mother-reared. Animals were housed in social groups of 15–20 individuals with access to inside and outside spaces. All animal protocols were approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine. The WFSM animal care and use protocol adhered to the U.S. Animal Welfare Act and Regulations.
Influenza A/PR/8/34 (H1N1)
A/Puerto Rico/8/34(H1N1) (PR8) virus was purchased from Charles River Laboratories International, Inc. Virus was propagated in SPF eggs in the allantoic cavity. Clarified allantoic fluid was concentrated and resuspended in Hepes-Saline and layered on a sucrose gradient. The interface band was diluted, pelleted and resuspended in a minimal volume of Hepes-Saline. Antigen was assessed for protein concentration using a Bio-Rad colorimetric protein assay.
Vaccination
At 4–6 days of age, infants were vaccinated with 45μg of 0.74% formaldehyde inactivated R848 conjugated virus (IPR8-R848) in the presence or absence of 10μg of flagellin (flg) or with inactivated IPR8 (IPR8) mixed with the same amount of an inactive flagellin (m229) [20]. The R848 conjugated virus was prepared as previously described [17]. Briefly an amine derivative of R848 was linked to SM(PEG)4 (Thermo Scientific) and subsequently to influenza virus. Flagellin from Salmonella enteritidis was prepared as previously described [20]. All injections were delivered intramuscularly in the deltoid muscle (500μl volume). Animals were boosted 21 days later.
ELISA for the detection of influenza virus-specific antibody
ELISAs were performed as previously described [17]. Threshold titer was defined as the value that reached 3 times the assay background, i.e. wells that received only sample diluent.
Neutralization assay
Heat-inactivated samples were serially diluted and mixed with 7.5×106 EID50 of PR8-GFP (kindly provided by Dr. Adolfo Garcia-Sastre [21]). Following a 2 hour incubation, 2×105 U937 cells were and incubated overnight at 37°C. Samples were acquired on a BD FACSCalibur and analyzed with CellQuest Pro software (Becton Dickinson) to determine the percentage of U937 cells that were positive for GFP. Nonlinear regression (Graphpad Prism) used to determine the dilution at which the 50% maximum PR8-GFP infected cells was achieved.
Statistical analysis
Descriptive statistics were examined for each measure. If variables were found to be non-normally distributed a logarithmic transformation was used and the normality of the variable after transformation was considered. Next, comparisons were made among the groups using a two-step approach. First, for comparisons made over time, a 2-way repeated measures analysis of variance model was fit with time and group included as fixed effects and the primate was included as a random effect in this model. Of primary interest was the time by group interaction which was examined to see whether there were differential changes in outcome (i.e., IgG) over time. If the interaction was found to be significant, then comparisons were made at each time point among groups using one-way analysis of variance ANOVA models, followed by 2-sample t-tests if the ANOVA model was significant. If the time by group interaction in the repeated measures model was not significant this would suggest that the groups could be compared across all time points pooled together and the interaction term was removed from the model and group comparisons were made using the overall model. Data were analyzed using Prism 5 software (GraphPad) or SAS Version 9.3.
RESULTS
Vaccination of mother-reared infants with IPR8-R848 results in an increased influenza-specific IgG response that is maintained through 6 months
Newborn AGM (3–6 days old) were vaccinated in the right arm with inactivated PR8 (IPR8) conjugated to R848 (IPR8-R848), IPR8-R848 admixed with the TLR5 agonist flagellin (IPR8+flg), or IPR8 admixed with an inactive flagellin protein m229 (IPR8+m229), the last of which served as a non-adjuvanted control. Neonates received a boost dose 21 days following initial vaccination. Virus-specific antibody was assessed at days 10 and 21 post primary vaccination and boost, as well as at approximately d100 and 6 months following initial vaccination. Non-vaccinated infants were also assessed at the 6 month timepoint as a measure of baseline antibody. Infants in the study remained with their mothers and were housed in social groups with access to the outdoors.
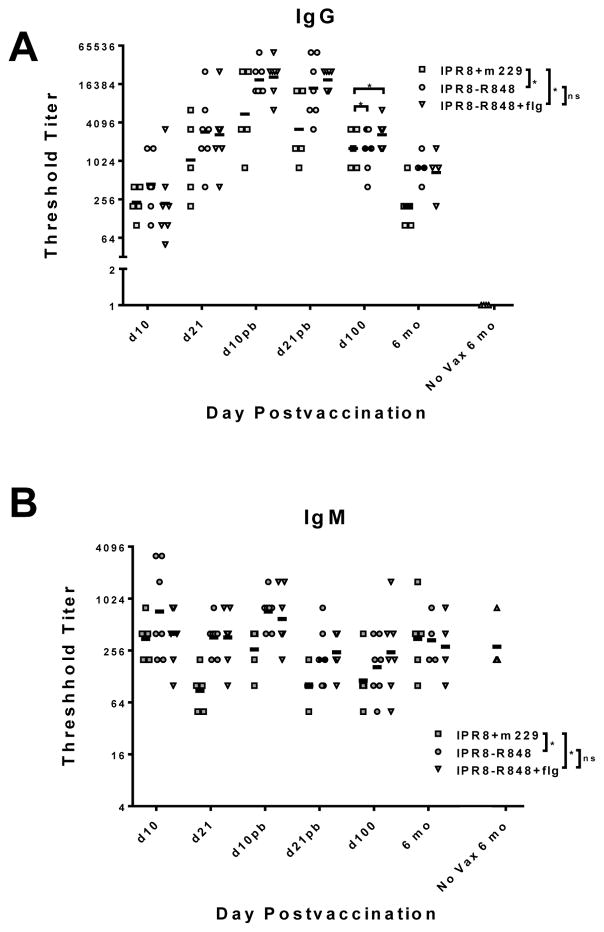
As was observed in our previous analysis performed in nursery reared infants [17], R848 conjugation to IPR8 resulted in a significantly increased influenza-specific IgG and IgM response at early times post vaccination (through d21 post boost (pb)) (Fig. 1). Addition of flagellin did not further enhance the response (Fig. 1), in agreement with our previous findings [17]. Regardless of vaccine utilized, antibody levels increased and decreased with similar kinetics, the latter resulting in a substantial reduction in the level of antibody at the d100 and 6 month timepoint in all groups compared to the peak response (Fig. 1A). Nonetheless, at 6 months, the level of virus-specific IgG antibody in infants vaccinated with IPR8-R848 or IPR8-R848+flg groups remained significantly higher than the IPR8+m229 vaccinated infants (Fig. 1A). The increase afforded at this time by conjugation with R848 was similar to that apparent during the early (d10–d21pb) response. As expected, no influenza-specific IgG antibody was detected at this timepoint in infants that were not vaccinated (Fig. 1A). The IgM response over the course of the experiment was also significantly higher for R848 adjuvanted versus non-adjuvanted vaccine recipients, although analysis of the 6 month showed this was not maintained over time (Fig. 1B). Interestingly influenza-specific IgM antibody levels were similar at 6 months in vaccinated and non-vaccinated infants, showing the lack of a sustained vaccine induced IgM response (Fig. 1B). Together, these data show that inclusion of conjugated R848 in the IPR8 vaccine resulted in significantly increased virus-specific IgG antibody responses in mother reared infants both at early times and at 6 months post vaccination.
Figure 1. Influenza-specific IgG in infants vaccinated with IPR8-R848, IPR8-R848+flg or IPR8+m229.
PR8-specific IgG (A) or IgM (B) was measured in the plasma of vaccinated (IPR8-R848 and IPR8-R848+flg, IPR8+m229) and non-vaccinated infants. The threshold titer for individual animals and the geometric mean for each group are shown. Infants with no detectable titer are assigned a value of 1 for visualization purposes. For the timepoints through d100 there were 5 animals for the IPR8+m229, and seven animals for the IPR8-R848 and IPR8-R848+flg groups. Five animals were maintained through 6 months for the IPR8+m229 group and 4 animals for the IPR8-R848 and IPR8-R848+flg groups. Significance over the time course was assessed by one-way ANOVA (indicated on the legend). The 6 month timepoint was also individually assessed given our focus on maintenance of the immune response. Influenza-specific IgG but not IgM was significantly increased at this time (indicated over 6 month timepoint data). *p<0.05.
The combined presence of flagellin and R848 results in significantly increased neutralizing antibody generation in the acute response that is lost at 6 months
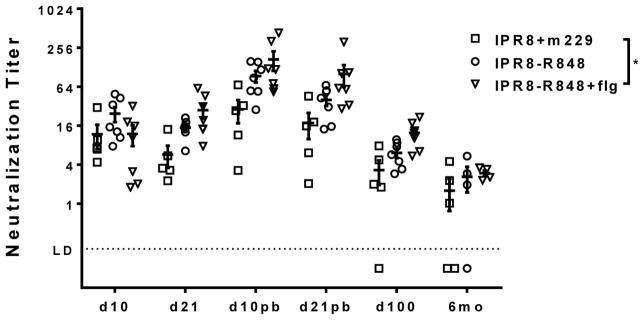
We assessed neutralizing antibody using an assay that directly measures inhibition of infection by a GFP-expressing PR8 virus [21]. The presence of conjugated R848 together with flagellin resulted in a significant increase in neutralizing antibody compared to infants vaccinated with IPR8+m229 when evaluated across the time course (Fig. 2). As was observed for total virus specific antibody, the level of neutralizing antibody dropped significantly by d100 and further declined by 6 months (Fig. 2). While IPR8-R848+flg vaccinated infants continued to have significantly higher neutralizing antibody levels at d100, this was lost at 6 months. Nonetheless, all IPR8-R848+flg infants had detectable neutralizing antibody at 6 months. Three of four IPR8-R848 vaccinated infants had detectable neutralizing antibody at this time. Two of five infants vaccinated with IPR8+m229 had undetectable neutralizing antibody at this timepoint. In all groups, the infants with detectable neutralizing antibody had levels that were very similar (Fig. 2). These data show the combination of flagellin and conjugated R848 is the most effective inducer of early neutralizing antibody; however the increased in neutralizing antibody compared to the non-adjuvanted vaccine was not maintained over time.
Figure 2. Neutralizing antibody in infants vaccinated with IPR8-R848, IPR8-R848+flg or IPR8+m229.
Neutralizing antibody titers in plasma were measured over the course of vaccination by assessing inhibition of the ability of a GFP expressing PR8 virus to infect U937 cells. Neutralization titer was defined as the half maximal inhibitory concentration (IC50), i.e. the dilution factor at which 50% infectivity was blocked. Individual animals and geometric means are shown. Significance over the time course was assessed by one-way ANOVA (significance indicated on the legend). *p<0.05
Affinity of antibody at 6 months in vaccinated infants
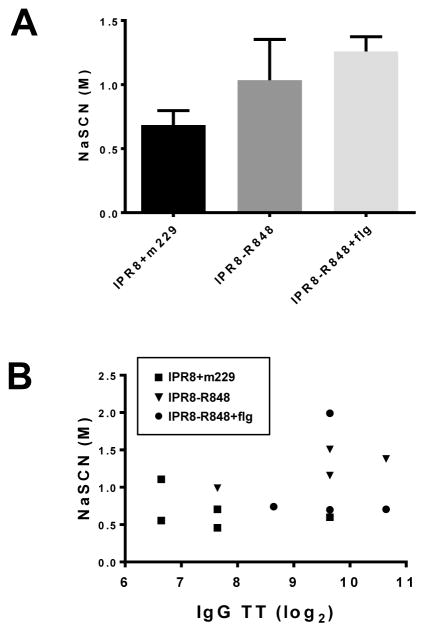
Finally, we assessed the affinity of virus-specific IgG antibody present at 6 months in the vaccinated infants. While there was a trend towards higher affinity in the animals that received IPR8-R848 or IPR8-R848+flg, this did not reach statistical significance (Fig. 3A). Assessment of significance was hampered by notable variability in the IPR8-R848 group. We asked whether this may relate to the magnitude of the IgG response. To address this, we plotted the threshold titer versus the concentration of NaSCN necessary to disrupt binding. No relationship between the calculated affinity and the amount of antibody present in the infants was observed at 6 months post vaccination (Fig. 3B), suggesting other factors are contributing to the variability in affinity of the influenza-specific antibody.
Figure 3. Affinity of influenza-specific IgG antibody present at 6 months following vaccination with IPR8-R848, IPR8-R848+flg or IPR8+m229.
(A) Titrated concentrations of sodium isothyocyanate were added following binding of PR8-specific antibody in our standard ELISA protocol. Affinity was calculated based on the concentration of NaSCN necessary to reduce the OD by 50%. Control animals that did not receive vaccine had no detectable virus-specific IgG and as such were not assessed. (B) The affinity and threshold titer measured at 6 months post vaccination for individual animals is shown.
DISCUSSION
In this study we assessed the ability of an inactivated influenza virus vaccine adjuvanted by conjugation to R848 or dual adjuvanted with conjugated R848 and soluble flagellin to induce a higher quality and/or longer lived antibody response compared to a non-adjuvanted inactivated virus in a nonhuman primate neonate model. Our results reveal the capacity for conjugated R848 to support increased IgG antibody responses that are long lived (i.e. 6 months following vaccination). Together with the increased antibody at early times post vaccination, the sustained increase strengthens the case for the use of conjugated R848 as an effective adjuvant in the context of the neonate. Additional support of the utility of TLR7/8 agonists in neonates comes from a recent study reporting the ability of a lipidated form of 3M-052 to increase antibody responses elicited in rhesus macaque neonates in the context of pneumococcal PCV13 vaccination [22]. In agreement with our study, the authors showed increases in antibody that were maintained at 150 days post vaccination [22].
Our data are most consistent with R848 affecting the quantity as opposed to the quality of antibody generated following vaccination. This is based on a number of observations garnered from comparison of the acute (d10–42) and late (6 months) immune response. First, the magnitude of the increase in influenza-specific antibody resulting from inclusion of conjugated R848 is similar at 6 months versus d42 post vaccination. This suggests R848 does not improve the survival of antibody secreting cells. If it had, we would have expected the difference in antibody levels in infants that received the adjuvanted versus non-adjuvanted vaccine to widen at the 6 month timepoint. Secondly, the affinity or neutralizing capacity of the antibody was not higher at 6 months in infants that received the R848 adjuvanted vaccines. Thus, by these measures, the quality of the antibody induced was not improved.
The increase in the quantity of antibody could result from improved differentiation of activated B cells into antibody secreting cells, increased antibody output per cell, or an increase in the number of B cells activated as a result of vaccination. TLR7 is expressed on human B cells [14, 15] and given the similarity in the expression and function of TLR in NHP and humans we would predict that NHP B cells also express TLR7. In support of this, we know from studies in our laboratory that AGM B cells have the capacity to directly respond to R848 (manuscript submitted). Therefore, one possibility is that R848 increases antibody by facilitating B cell activation or proliferation. Further, the enhancing effect of R848 may promote differentiation into ASC. With regard to the latter, in studies of mouse B cells, TLR7 was found to synergize with the BCR and CD40 to promote differentiation into ASC [23]. TLR7 stimulation was associated with increased expression of Blimp-1, the master regulator of ASC differentiation as well as AID, the enzyme required for class switching and somatic hypermutation [23]. Studies using human B cells similarly reported a role for TLR7 in promoting differentiation into ASC [24, 25]. If R848 is promoting differentiation into ASC, it does not appear to be at the expense of memory B cell generation. We propose this based on preliminary studies of infants who received a third dose of vaccine at the 6 month timepoint. Admittedly, this was a small number of infants (2/group); however, the antibody in the infants that received R848-containig vaccines was higher than the control group, a profile similar to what has been detected throughout the analysis (data not shown). It is tempting to speculate that the augmenting effect of R848 could be further improved with increased CD40 signaling in the context of the neonate where the CD40-CD40L pathway is impaired [14, 15].
It was disappointing that the presence of R848 did not promote a higher affinity response or more neutralizing antibody at 6 months following vaccination. This remains a goal and an area of future development for the vaccine. Previous studies have reported the positive correlation between the number of GC B cells and Tfh cells [26, 27]. Further, in humans Tfh helper cells have been reported to contribute to the production of high affinity antibodies following influenza vaccination [28]. Thus, one approach to achieve higher quality antibody is provision of additional modulatory signals that promote Tfh, e.g. ICOS or OX40 agonists [29–32]. ICOS binding is well established to promote differentiation of Tfh [31] and OX40 engagement has been reported to promote generation, proliferation and survival of Tfh [33–36]. Given the impaired generation of Tfh cell responses reported in infants [37–39], targeting these cells holds promise for the development of more potent vaccines.
Our study supports the ability of conjugated R848 to serve as an adjuvant outside of the unique environment of a nursery where infants were highly restricted with regard to the environmental conditions to which they were exposed. In the current study infants remained with their mothers (and were thus breast fed) and were housed in social groups in pens that allowed access to the outdoor environment. It could be argued that this is more reflective of the exposures of human infants. We have an increasing appreciation for the effects of the microbiome on the ability of the immune system to respond to challenge [40–42]. The microbiome established in newborns is the result of exposure in the birth canal as well as from environmental encounters in the first days of life. As there is evidence to suggest the microbiome is a contributor to vaccine responsiveness [43], it is likely that this as well as other immune challenges impact the developing immune system in the early days following birth. Given the potential for immune modulation, it is important that the beneficial effects of the conjugated R848 adjuvant are not restricted to neonates housed in the unique environment of the nursery.
The current clinical recommendation is that pregnant individuals receive the influenza vaccine. This is based on studies suggesting a protective benefit in young infants, predominantly during the first 8 weeks of life [44, 45]. Thus, in addition to overcoming impaired immunity in neonates, the potential for interference from maternal antibody will be another consideration for optimizing vaccines in this age group. With that said, identification of vaccine formulations that can induce robust immune responses in neonates is a necessary first step in achieving the goal of protecting this at-risk population. Subsequent studies to understand the role of maternal antibody, its impact on vaccination, and how to circumvent any negative effects will be an important next step.
In summary, our studies in the NHP model strongly support conjugated R848 as a potent adjuvant for neonates. This approach results in an enhanced antibody level during the acute response that is maintained at 6 months. While these results are highly promising, the failure to see significant increases in antibody affinity or neutralizing capacity at 6 months with the R848-conjugated vaccine is impetus for exploration of additional immune modulators that could promote an optimally efficacious long term antibody response.
Acknowledgments
This work was supported by National Institutes of Health grants 5R01AI098339 (to M.A.A.-M.). The Vervet Research Colony is supported in part by P40 OD010965 (to M.J.J.). We acknowledge services provided by the Cell and Viral Vector Core, Synthetic Chemistry Core and Flow Cytometry Core Laboratories of the Wake Forest Comprehensive Cancer Center, supported in part by NCI P30 CA121291-37. We thank Dr. Karen Haas for helpful comments regarding this manuscript.
Footnotes
Conflict of interest
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prelog M. Differential approaches for vaccination from childhood to old age. Gerontology. 2013;59:230–9. doi: 10.1159/000343475. [DOI] [PubMed] [Google Scholar]
- 2.Groothuis JR, Levin MJ, Rabalais GP, Meiklejohn G, Lauer BA. Immunization of high-risk infants younger than 18 months of age with split-product influenza vaccine. Pediatrics. 1991;87:823–8. [PubMed] [Google Scholar]
- 3.Halasa NB, Gerber MA, Chen Q, Wright PF, Edwards KM. Safety and immunogenicity of trivalent inactivated influenza vaccine in infants. J Infect Dis. 2008;197:1448–54. doi: 10.1086/587643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J Comp Pathol. 2007;137(Suppl 1):S4–9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 6.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–64. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 7.Paunio M, Hedman K, Davidkin I, Valle M, Heinonen OP, Leinikki P, et al. Secondary measles vaccine failures identified by measurement of IgG avidity: high occurrence among teenagers vaccinated at a young age. Epidemiol Infect. 2000;124:263–71. doi: 10.1017/s0950268899003222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliano M, Mastrantonio P, Giammanco A, Piscitelli A, Salmaso S, Wassilak SG. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. J Pediatr. 1998;132:983–8. doi: 10.1016/s0022-3476(98)70395-6. [DOI] [PubMed] [Google Scholar]
- 9.Tiru M, Hallander HO, Gustafsson L, Storsaeter J, Olin P. Diphtheria antitoxin response to DTP vaccines used in Swedish pertussis vaccine trials, persistence and projection for timing of booster. Vaccine. 2000;18:2295–306. doi: 10.1016/s0264-410x(99)00539-3. [DOI] [PubMed] [Google Scholar]
- 10.Whittle HC, Aaby P, Samb B, Jensen H, Bennett J, Simondon F. Effect of subclinical infection on maintaining immunity against measles in vaccinated children in West Africa. Lancet. 1999;353:98–102. doi: 10.1016/S0140-6736(98)02364-2. [DOI] [PubMed] [Google Scholar]
- 11.Pichichero ME. Challenges in vaccination of neonates, infants and young children. Vaccine. 2014;32:3886–94. doi: 10.1016/j.vaccine.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander-Miller MA. Vaccines against respiratory viral pathogens for use in neonates: opportunities and challenges. J Immunol. 2014;193:5363–9. doi: 10.4049/jimmunol.1401410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol. 2014;10:1171–84. doi: 10.1586/1744666X.2014.942288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Min B, Legge KL, Bell JJ, Gregg RK, Li L, Caprio JC, et al. Neonatal exposure to antigen induces a defective CD40 ligand expression that undermines both IL-12 production by APC and IL-2 receptor up-regulation on splenic T cells and perpetuates IFN-gamma-dependent T cell anergy. J Immunol. 2001;166:5594–603. doi: 10.4049/jimmunol.166.9.5594. [DOI] [PubMed] [Google Scholar]
- 15.Donckier V, Flamand V, Abramowicz D, Goldman M. Increased IL-4 production and decreased CD40L expression by newborn T cells contribute to transplantation tolerance. Transplant Proc. 1999;31:782–3. doi: 10.1016/s0041-1345(98)01764-3. [DOI] [PubMed] [Google Scholar]
- 16.Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Whittle H, Lambert PH, et al. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine. 2004;22:511–9. doi: 10.1016/j.vaccine.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Holbrook BC, Kim JR, Blevins LK, Jorgensen MJ, Kock ND, D’Agostino RB, Jr, et al. A novel R848-conjugated inactivated influenza virus vaccine is efficacious and safe in a neonate nonhuman primate model. J Immunol. 2016;197:555–64. doi: 10.4049/jimmunol.1600497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JR, Holbrook BC, Hayward SL, Blevins LK, Jorgensen MJ, Kock ND, et al. Inclusion of flagellin during vaccination against influenza enhances recall responses in nonhuman primate neonates. J Virol. 2015;89:7291–303. doi: 10.1128/JVI.00549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holbrook BC, D’Agostino RB, Jr, Parks GD, Alexander-Miller MA. Adjuvanting an inactivated influenza vaccine with flagellin improves the function and quantity of the long-term antibody response in a nonhuman primate neonate model. Vaccine. 2016;34:4712–7. doi: 10.1016/j.vaccine.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDermott PF, Ciacci-Woolwine F, Snipes JA, Mizel SB. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect Immun. 2000;68:5525–9. doi: 10.1128/iai.68.10.5525-5529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci U S A. 2010;107:11531–6. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowling DJ, van Haren SD, Scheid A, Bergelson I, Kim D, Mancuso CJ, et al. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight. 2017;2:e91020. doi: 10.1172/jci.insight.91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boeglin E, Smulski CR, Brun S, Milosevic S, Schneider P, Fournel S. Toll-like receptor agonists synergize with CD40L to induce either proliferation or plasma cell differentiation of mouse B cells. PLoS One. 2011;6:e25542. doi: 10.1371/journal.pone.0025542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douagi I, Gujer C, Sundling C, Adams WC, Smed-Sorensen A, Seder RA, et al. Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. J Immunol. 2009;182:1991–2001. doi: 10.4049/jimmunol.0802257. [DOI] [PubMed] [Google Scholar]
- 25.Simchoni N, Cunningham-Rundles C. TLR7-and TLR9-responsive human B cells share phenotypic and genetic characteristics. J Immunol. 2015;194:3035–44. doi: 10.4049/jimmunol.1402690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LM, et al. Phosphoinositide 3-kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol. 2010;185:4042–52. doi: 10.4049/jimmunol.1001730. [DOI] [PubMed] [Google Scholar]
- 27.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Bentebibel SE, Khurana S, Schmitt N, Kurup P, Mueller C, Obermoser G, et al. ICOS+PD- 1+CXCR3+ T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci Rep. 2016;6:26494. doi: 10.1038/srep26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–7. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 30.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–46. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2009;106:20371–6. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–32. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 33.Brocker T, Gulbranson-Judge A, Flynn S, Riedinger M, Raykundalia C, Lane P. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur J Immunol. 1999;29:1610–6. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–22. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacquemin C, Schmitt N, Contin-Bordes C, Liu Y, Narayanan P, Seneschal J, et al. OX40 Ligand contributes to human Lupus pathogenesis by promoting T follicular helper response. Immunity. 2015;42:1159–70. doi: 10.1016/j.immuni.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tahiliani V, Hutchinson TE, Abboud G, Croft M, Salek-Ardakani S. OX40 cooperates with ICOS to amplify follicular Th cell development and germinal center reactions during infection. J Immunol. 2017;198:218–28. doi: 10.4049/jimmunol.1601356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Debock I, Jaworski K, Chadlaoui H, Delbauve S, Passon N, Twyffels L, et al. Neonatal follicular Th cell responses are impaired and modulated by IL-4. J Immunol. 2013;191:1231–9. doi: 10.4049/jimmunol.1203288. [DOI] [PubMed] [Google Scholar]
- 38.Mastelic Gavillet B, Eberhardt CS, Auderset F, Castellino F, Seubert A, Tregoning JS, et al. MF59 mediates its B cell adjuvanticity by promoting T follicular helper cells and thus germinal center responses in adult and early life. J Immunol. 2015;194:4836–45. doi: 10.4049/jimmunol.1402071. [DOI] [PubMed] [Google Scholar]
- 39.Mastelic B, Kamath AT, Fontannaz P, Tougne C, Rochat AF, Belnoue E, et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol. 2012;189:5764–72. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 40.Kramer CD, Genco CA. Microbiota, immune subversion, and chronic inflammation. Front Immunol. 2017;8:255. doi: 10.3389/fimmu.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghazal P, Dickinson P, Smith CL. Early life response to infection. Curr Opin Infect Dis. 2013;26:213–8. doi: 10.1097/QCO.0b013e32835fb8bf. [DOI] [PubMed] [Google Scholar]
- 42.Gritz EC, Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr. 2015;3:17. doi: 10.3389/fped.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YG. Microbiota influences vaccine and mucosal adjuvant efficacy. Immune Netw. 2017;17:20–4. doi: 10.4110/in.2017.17.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abu Raya B, Edwards KM, Scheifele DW, Halperin SA. Pertussis and influenza immunisation during pregnancy: a landscape review. Lancet Infect Dis. 2017;17:e209–e22. doi: 10.1016/S1473-3099(17)30190-1. [DOI] [PubMed] [Google Scholar]
- 45.Nunes MC, Cutland CL, Jones S, Hugo A, Madimabe R, Simoes EA, et al. Duration of infant protection against influenza illness conferred by maternal immunization: Secondary analysis of a randomized clinical trial. JAMA Pediatr. 2016;170:840–7. doi: 10.1001/jamapediatrics.2016.0921. [DOI] [PubMed] [Google Scholar]