Abstract
Zika virus (ZIKV) has become a global public health emergency due to its rapidly expanding range and its ability to cause severe congenital defects such as microcephaly. However, there are no FDA-approved therapies or vaccines against ZIKV infection. Through our screening of viral entry inhibitors, we found that chloroquine (CQ), a commonly used antimalarial and a FDA-approved drug that has also been repurposed against other pathogens, could significantly inhibit ZIKV infection in vitro, by blocking virus internalization. We also demonstrated that CQ attenuates ZIKV-associated morbidity and mortality in mice. Finally, we proved that CQ protects fetal mice from microcephaly caused by ZIKV infection. Our methodology of focusing on previously identified antivirals in screens for effectiveness against ZIKV proved to be a rapid and efficient means of discovering new ZIKV therapeutics. Selecting drugs that were previously FDA-approved, such as CQ, also improves the likelihood that they may more quickly reach stages of clinical testing and use by the public.
Keywords: FDA-approved drug, Chloroquine, ZIKV entry, Microcephaly, Antiviral effects
Highlights
-
•
5 out 16 tested Ebola virus entry inhibitors can inhibit ZIKV entry efficiently
-
•
Chloroquine can inhibit ZIKV internalization in vitro and reduce ZIKV-associated morbidity and mortality in mice
-
•
Chloroquine prevents ZIKV-associated congenital microcephaly in mice
Zika virus (ZIKV) is an emerging virus which can cause birth defects, however there are currently no effective treatments or vaccines. We tested the effects of 16 verified Ebola virus cell entry inhibitors on ZIKV infection, and found that chloroquine (CQ) could prevent ZIKV infection in cell cultures, consistent with results from a previous study. We then demonstrated that CQ can reduce ZIKV-associated morbidity and mortality in mice. Most importantly, it protects fetal mice from microcephaly caused by ZIKV infection. Therefore, CQ is a potential drug which would be used to treat ZIKV infection after clinical test.
1. Introduction
Zika virus (ZIKV) is a single-stranded RNA virus primarily transmitted by mosquitos and closely related to dengue virus (DENV), another member of the Flaviviridae family (Musso and Gubler, 2016). It was first identified in 1947 from a rhesus monkey in the Zika forest of Uganda (Dick et al., 1952). Human cases were later reported in both Africa and Asia, and ZIKV strains became classified as belonging to either the African or Asian lineages (Haddow et al., 2012). Since 2013, human infection with the Asian lineage of ZIKV in the South Pacific and the Americas (Gulland, 2016) has been reported to be associated with Guillain-Barré syndrome (GBS), meningoencephalitis and congenital microcephaly (Araujo et al., 2016; Brasil et al., 2016; Driggers et al., 2016; Rasmussen et al., 2016). ZIKV was also found to be sexually transmissible (D'Ortenzio et al., 2016), and infection was found to cause male infertility in susceptible mice (Govero et al., 2016; Ma et al., 2016). We and others were able to develop mouse models in which ZIKV infection in utero could lead to microcephaly (Cugola et al., 2016; Li et al., 2016; Miner et al., 2016). Given that ZIKV can be acquired by sexual activity even with asymptomatic ZIKV carriers (Hills et al., 2016), and the extensive range of the mosquito vectors and of human travel, this virus has become a global threat.
There are currently no FDA-approved drugs available for the prevention or treatment of ZIKV infection or its associated risk of congenital defects. Although vaccines are in early trials for effective ZIKV prevention, their development and manufacture is a rigorous and time-consuming process. Antibody-based therapies have shown promise in the mouse model, however these drugs are expensive and in limited supply, and their safety has yet to be validated in humans. Drug repurposing screens have been shown in the past to be an efficient approach to speeding up drug development, and should be given priority when strategies are devised against new emerging viruses (Nabel and Zerhouni, 2016). These screens led to the discovery of potential new therapies for Entamoeba histolytica infection (Debnath et al., 2012), hepatitis C virus (HCV) infection (He et al., 2015) and also ZIKV (Barrows et al., 2016; Xu et al., 2016). During the 2014 Ebola virus (EBOV) outbreak in West Africa, researchers were desperate to find a cure that could rapidly reach those in need. Multiple FDA-approved drugs were screened for possible repurposing as EBOV treatments (Johansen et al., 2013, 2015; Kouznetsova et al., 2014). We decided to perform a similar screen of 16 EBOV inhibitors for their potential use against ZIKV. As these drugs have already passed strict FDA guidelines for human safety, any candidates with promising efficacy against ZIKV might be approved more rapidly than novel drugs as anti-ZIKV therapies. Of note, we have found that one of the drugs, chloroquine can inhibit ZIKV infection efficiently in vitro, in agreement with previous finding that CQ could inhibit ZIKV (MR766 strain and Brazilian strain) infection in Vero, human brain microvascular endothelial cells (hBMEC) and hNPC cells (Delvecchio et al., 2016). Our results further demonstrated that CQ suppresses ZIKV infection in mouse models in vivo and protects embryonic brains from ZIKV infection and its associated microcephaly.
2. Materials and Methods
2.1. Mice and Ethics Statements
A129 mice (IFN-α/β receptor deficient) were a gift from Professor Qi-Bin Leng (Institut Pasteur of Shanghai, Chinese Academy of Sciences, China). ICR mice were bought from Vital River Laboratory (Beijing, China) and BALB/c and Kunming mice were bought from Jackson Laboratory (Bar Harbor, ME) and then bred in our core animal facility. All experiments related to animals were conducted according to protocols approved by the Animal Experiment Committee of the Laboratory Animal Center, Academy of Military Medical Sciences, China (IACUC-13-2016-001).
2.2. Virus, Cells and Reagents
ZIKV strains (GZ01, GenBank: KU820898; FSS13025, GenBank: JN860885) used in this study were described in our previous work (Deng et al., 2016; Zhang et al., 2016). BHK-21, Huh7 and Vero cells were bought from ATCC and cultured in DMEM media (37 °C, 5% CO2). All media were supplemented with 10% FBS (ExCell Bio, Jiangsu), 100 units/ml penicillin, and 50 μg/ml streptomycin. NITD008 was provided by Dr. Pei-Yong Shi (Novartis Institute for Infectious Diseases). Chloroquine (Sigma, C6628) and other molecules were purchased from Sigma or Sangon (Shanghai, China).
2.3. RNA Isolation and Real Time Quantitative PCR
Total RNA from cell supernatants was extracted with the EasyPure Viral DNA/RNA Kit (TransGen Biotech, Beijing). Viral RNA copies were detected by real time quantitative PCR (qRT-PCR) (Johnson et al., 2005). ZIKV primers were described previously (Deng et al., 2016).
2.4. ZIKV Internalization Assay and Plaque Assay
ZIKV internalization assay and plaque assay were performed as described previously (Talarico et al., 2005). Briefly, BHK-21 cells seeded in 12 well plates were infected with ZIKV (200 PFU) at 4 °C for 1 h. Cells were then washed with PBS and incubated at 37 °C for 1 h to allow virus internalization. Cells were washed with PBS and treated with citric acid buffer (pH 3.0) to inactivate non-internalized viruses. Media containing 2% FBS and 1% agar was immediately added for plaque assay as described previously (Li et al., 2017). Viral plaques were counted at 4 dpi.
2.5. ZIKV Replicon Assay
The ZIKV replicon assay was conducted as described previously (Liu et al., 2013; Xie et al., 2016). Briefly, in vitro transcribed RNA was transfected with Lipofectamine 2000 reagent (Thermo Fisher) into BHK-21 cells seeded in 24 well plates. 6 hour post transfection (hpt), CQ was added to the media. 48 hpt, the renilla luciferase activity in cell lysates was measured with a microplate reader (Promega).
2.6. ZIKV Infection in Mice
3–4 week-old BALB/c mice (female) or A129 mice were treated with CQ or a control intragastrically (i.g.) 6 h before ZIKV infection and once daily for the following 5 days (A129 mice). Mice were infected intraperitoneally (i.p.) with ZIKV strain GZ01/2016. ZIKV RNA copies in serum at indicated time points were measured by qRT-PCR as described previously (Li et al., 2017).
For neonatal mouse infection, 20 μl DMEM containing 100 PFU of ZIKV was injected intracerebrally to 1-day-old Kunming mice (Deng et al., 2016). 20 mg/kg CQ was administrated by s.c. route to the mother mice as previously described (Keyaerts et al., 2009).
For the microcephaly model, 1 μl of ZIKV GZ01 virus stock (3.5 × 105 PFU/ml) or culture medium was injected transplacentally into one side of the cerebroventricular space/lateral ventricle (LV) of E13.5 ICR mouse brains and inspected 5 days later as described previously (Li et al., 2016; Wang et al., 2017). For each pregnant dam, around one half of littermates were injected with ZIKV.
2.7. Immunohistochemistry and Antibodies
For cryosections, tissues were fixed in 4% PFA, dehydrated in 30% sucrose, and frozen in TFM (tissue freezing medium). Sections (thickness: E18.5, 40 μm) were used for immunofluorescence staining as described previously (Li et al., 2016). The antibodies used for immunostaining were anti-ZIKV serum from a patient (1:1000), Activated-caspase3 (Abcam, ab2302, 1:1000), Phospho-Histone 3 (P-H3) (Abcam, ab10543, 1:1000), Sox2 (Abcam, ab97959, 1:1000), Tbr2 (Miilipore, ab15894, 1:1000), NeuN (Abcam, ab104224, 1:1000), and Tbr1 (Abcam, ab31940, 1:500). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI, Invitrogen).
2.8. Nissl Staining
Brain slices were stained with 0.1% toluidine blue for 5 min and dehydrated in turn by 70%, 96%, and 99% ethanol (45 s, twice for each). Finally, slices were hyalinized by Xylene for 5 min before being sealed with neutral balsam.
2.9. Confocal Imaging and Qualification
Slices were imaged on an LSM 700 (Carl Zeiss) confocal microscope, and the images were analyzed and qualified with Zen (Blue edition) or ImageJ as described previously (Li et al., 2016).
2.10. Statistical Analysis
Prism software (GraphPad) was used to analyze the data. All data are shown as mean ± SEM from three repeat assays. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, unpaired student t-test.
3. Results
Of the 16 EBOV entry inhibitors reported previously (Johansen et al., 2013, 2015; Kouznetsova et al., 2014), we found that the FDA-approved drugs chloroquine (CQ), clomiphene, amodiaquine, alverine and sertraline could inhibit the early stages of ZIKV infection (Fig. 1A). Consistent with the fact that ribavirin is a polymerase inhibitor, it did not disrupt ZIKV entry in our experiment, therefore it served as a negative control (Fig. 1A). Since clomiphene cannot be given safely to pregnant women (Legro et al., 2007), and amodiaquine, alverine and sertraline were less effective inhibitors, we focused on CQ, which had an efficacy similar to the known 25-hydroxycholesterol (25HC) ZIKV entry inhibitor (Li et al., 2017). The IC50 of CQ against ZIKV (GZ01, a recent Asian lineage strain (Zhang et al., 2016)) is about 4.15 μM in Vero cells and 1.72 μM in Huh7 cells (Fig. 1B-C), while there was no cytotoxicity induced by CQ even at 10 μM (Fig. S1) (Kouznetsova et al., 2014). CQ also inhibits the ZIKV FSS13025 strain (old Asian lineage), with an IC50 of 2.72 μM in Huh7 cells (Fig. 1D). In order to detect which step of the ZIKV life cycle is inhibited by CQ, an internalization assay was performed as previously described (Talarico et al., 2005) using the RNA synthesis inhibitor, NITD008, as a negative control. The results showed that CQ suppressed ZIKV internalization effectively, while NITD008 did not (Fig. 1F). However, CQ did not interfere with ZIKV binding to cells or RNA synthesis (Fig. 1E, G).
Fig. 1.
CQ inhibits ZIKV entry in vitro and in vivo.
(A) BHK-21 cells were treated for 12 h with 10 μM of 16 individual compounds known to inhibit entry by EBOV (25HC was used as positive control; Ribavirin was used as a negative control). The cells were then infected by ZIKV (GZ01/2016 strain, 200 PFU/well) for 1 h and plaque assay was performed to compare the levels of infection. 5 molecules (chloroquine, clomiphene, amodiaquine, alverine and sertraline) were identified to inhibit ZIKV infection. (B–D) Cells were pretreated with CQ for 12 h and then infected with ZIKV for 1 h. ZIKV in cell supernatants were detected with qRT-PCR at 48 hpi. The IC50 of CQ on ZIKV (GZ01) in Vero (B) or Huh7 cells (C), or on ZIKV (FSS13025) in Huh7 cells (D) are indicated. (E–G) Inhibitory effects of CQ on binding to Vero cells (E), ZIKV internalization (F) and RNA synthesis (G) are shown. (E-F) Cells were pretreated with CQ for 12 h, then were infected with ZIKV (E, 200 PFU/well; F, MOI = 1) at 4 °C for 1 h. (F) Then cells were washed once by PBS and incubated at 37 °C for 1 h. The bound ZIKV (E) and internalized ZIKV (F) were detected using plaque assay and qRT-PCR, respectively. (G) ZIKV replicon RNA was transfected into BHK-21 cells. 6 h later, CQ or NITD008 were used to treat cells for 48 h. Renilla luciferase activity was measured with a microplate reader. All data are shown as mean ± SEM from three independent experiments, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, unpaired student t-test.
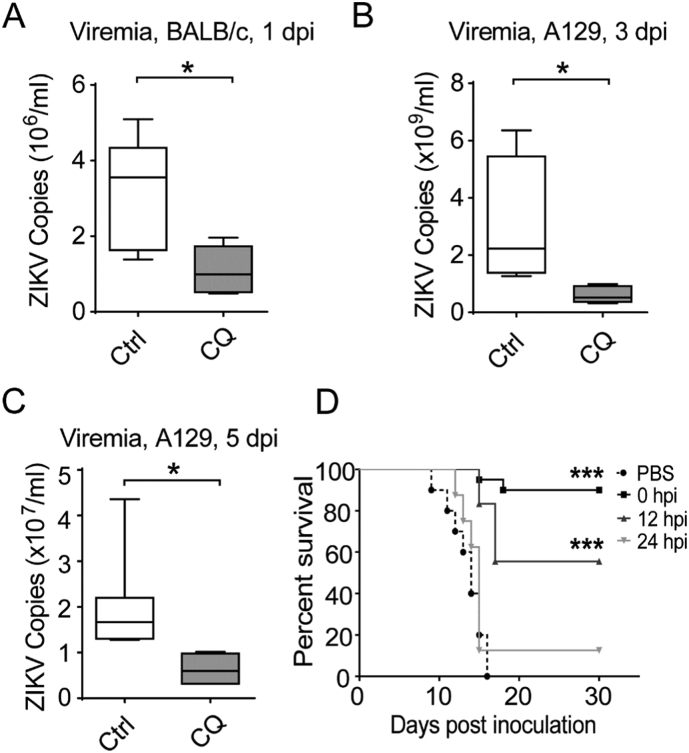
Next, we tested the ability of CQ to protect against ZIKV infection in vivo. 3–4 week-old immunocompetent BALB/c mice were treated with CQ (100 mg/kg) by intragastric administration (i.g.) 6 h before ZIKV infection by the intraperitoneal (i.p.) route (GZ01 strain, 105 PFU/mouse). CQ treatment significantly suppressed ZIKV infection in mice compared to the PBS control at 1 day post-infection (dpi) (Fig. 2A). We then used the IFN-α/β receptor (IFNAR)-deficient A129 mouse model (Dai et al., 2016; Lazear et al., 2016) to further evaluate the protective effects of CQ on ZIKV infection. A129 mice were treated with PBS or CQ (i.g., 100 mg/kg) 6 h before ZIKV infection (GZ01 strain, 103 PFU/mouse), and daily for the following 5 days. Viremia was measured at 3 and 5 dpi, and it was found to be significantly reduced in CQ-treated mice (Fig. 2B–C). Since CQ transmitted through breastmilk was found to inhibit human coronavirus OC43 infection in newborn mice through maternal milk (Keyaerts et al., 2009), we then tested the effects of CQ on ZIKV infection in a neonatal mouse model (Deng et al., 2016) (Fig. 2D). We found that CQ treatment of mother mice could improve survival of ZIKV-infected suckling neonatal mice by about 90% (Fig. 2D). If CQ was administrated at 12 hour post infection (hpi), this protective effect was reduced, and became completely absent when postponing CQ treatment to 24 hpi. These results showed that CQ can suppress ZIKV mouse infection.
Fig. 2.
CQ protects against ZIKV infection in mouse models.
(A) Antiviral effect of CQ on ZIKV infection in BALB/c mice (A) and A129 mice (B–C). 100 mg/kg of CQ was administrated to mice i.g. 6 h before ZIKV infection i.p. (105 PFU/mouse for BALB/c, 103 PFU/mouse for A129 experiment). Viremia was determined at 1 (A), 3 or 5 (B–C) dpi as indicated. (D) Improved survival of ZIKV-infected suckling neonatal mice after CQ was given to breastfeeding mothers. 1-day-old neonatal mice were infected intracerebrally with ZIKV (100 PFU/mouse, GZ01 strain). Kunming mother mice were administrated CQ subcutaneously (s.c.) at 0, 12 or 24 hpi. CQ continued to be given daily to the mothers for another 10 days. Survival of ZIKV-infected neonatal mice was followed until 30 dpi. Median values for 4–7 mice for experiments in A–C are shown. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, unpaired student t-test. Log-Rank (Mantel-Cox) test were used in D (PBS, n = 10; 0 hpi, n = 20; 12 hpi, n = 18; 24 hpi, n = 8).
Previously, we have shown that 25HC and convalescent serum from a patient who recovered from ZIKV infection both protected embryonic mice from ZIKV infection and its associated microcephaly (Li et al., 2017; Wang et al., 2017). Here we adopted the same model to investigate the effect of CQ on littermate embryos. ZIKV (~ 350 pfu) or culture medium was injected into the cerebroventricular space in the brains of embryonic day 13.5 (E13.5) ICR mice. Pregnant mice were treated with PBS (Veh) or CQ (i.g., 100 mg/kg) 6 h before infection of the embryonic brains, and then once a day from E13.5 to E18.5. The mice were sacrificed and inspected at E18.5. First, we investigated whether CQ could protect mice from microcephaly caused by ZIKV infection. Compared to the mock-infected or ZIKV-infected CQ-treated groups, the average brain sizes of ZIKV-infected but untreated mice were significantly smaller (Fig. 3A). In addition, the brains of those mice had a significantly thinner cortex compared to the brains of uninfected or CQ-treated mice (Fig. 3B). The reduced thickness of different layers of cortex was confirmed by immunostaining with NeuN and Tbr1 antibodies (Fig. 3B and Fig. S2).
Fig. 3.
CQ protects embryonic brains from ZIKV infection and microcephaly. Littermate embryonic brains were injected with ZIKV or medium at E13.5, then the pregnant mice were treated daily with CQ or vehicle until E18.5. (A) Images of brains and Nissl staining of coronal sections. (B) Measurements of cortical layer thickness. Mock + Veh: n = 9/4, Mock + CQ: n = 6/3, ZIKV + Veh: n = 6/3, ZIKV + CQ: n = 8/4. CP: cortical plate, SP: subplate, IZ: intermediate zone, SVZ: subventricular zone, VZ: ventricular zone. (C) Images of coronal sections stained with ZIKV antiserum (green) and DAPI (blue). Right panel: Quantification of relative intensity. n = 7/3 for each. (D) Images of cortices stained for the activated form of Caspase3 (white) and DAPI (blue). Right panel: quantification of relative intensity. N = 7/3 for each. (E) Images of cortices stained with phosphorylated Histone H3 (P–H3, red). Right panel: quantification of P-H3 + cells. Mock + Veh: n = 7/4, Mock + CQ: n = 8/3, ZIKV + Veh: n = 5/3, ZIKV + CQ: n = 5/3. (F) Coronal sections stained for ZIKV (green), Sox2 (red) and Tbr2 (white). Right panel: quantification of the density of Sox2 + cells and Tbr2 + cells in the cortices. Mock + Veh: n = 8/4 (Sox2 +), 9/4 (Tbr2 +); Mock + CQ: n = 6/3 (Sox2 +), 6/3 (Tbr2 +); ZIKV + Veh: n = 10/5 (Sox2 +), 10/5 (Tbr2 +); ZIKV + CQ: n = 10/5 (Sox2 +), 10/5 (Tbr2 +). All data are means ± SEM. Student's t-test. *P < 0.05, **P < 0.01, ***P < 0.001. #P < 0.05, ##P < 0.01, ###P < 0.001. ns: not significant. n: number of slices/different brains. Scale bar for 1 mm (A), 100 μm (B–F).
We next explored whether CQ treatment could suppress ZIKV infection in fetal brains. Immunostaining with an anti-ZIKV serum showed that the embryonic brains infected with ZIKV and treated with CQ had a dramatically reduced burden of ZIKV compared to untreated infected brains (Fig. 3C). Similarly, the intensity of cells positive for the activated form of Caspase 3, an indicator of apoptosis, was also reduced substantially after CQ treatment (Fig. 3D). These findings indicate that CQ can significantly reduce ZIKV infection and its associated apoptotic effects in the fetal brain, which has been shown previously to be a contributing factor in the development of microcephaly (Li et al., 2016; Wang et al., 2017).
The infection of neuronal progenitor cells (NPCs) and the dysregulation of their proliferation and differentiation may also contribute to thinner VZ/SVZ cortical layers and microcephaly (Li et al., 2016; Wang et al., 2017). We investigated whether CQ could prevent this effect on NPC proliferation after ZIKV infection. As shown in Fig. 3E, cells positive for phosphorylated histone H3 (P-H3, a marker for cells in M-phase) were significantly reduced in ZIKV-infected brains. Surprisingly, these cells were rescued by CQ therapy. Similarly, the reduction in Sox2+ (NPC marker) and Tbr2+ (intermediate/basal progenitor cell marker) cells were also reversed in those treated with CQ (Fig. 3F). These findings correlate with changes in the cortical layer thickness and overall size of ZIKV-infected brains. Therefore, CQ treatment protects embryonic mice from ZIKV infection and its associated microcephaly.
4. Discussion
Recently, Xu et al. performed a screen of ~ 6000 compounds approved or under investigation by the FDA, and found that ~ 3% (173/6096) could suppress ZIKV infection in neural cells (Xu et al., 2016). Similarly, Barrows et al. tested 700 FDA-approved drugs and found that 20 (~ 3%) had inhibitory effects on ZIKV replication in vitro (Barrows et al., 2016; Xu et al., 2016). However, in vivo testing of these drugs has not yet to be performed. We chose to begin our screening with EBOV inhibitors, which yielded a much higher percentage of promising ZIKV therapeutics (~ 31%, 5/16).
Recently, Bullard-Feibelman et al. reported that the FDA-approved anti-hepatitis C drug sofobuvir can also inhibit ZIKV infection in vitro and in vivo (Bullard-Feibelman et al., 2017). However, its ability to prevent ZIKV-associated microcephaly remains unclear, and the drug is also very expensive. CQ is a widely used anti-malarial drug, and was found to have no toxic effects in pregnant women or their infants. Previously, there were two clinical trials to investigate the antiviral effects of CQ on DENV infection (Borges et al., 2013; Tricou et al., 2010). Patients were given CQ for 3 days beginning 72 h after infection by DENV in both trials. The results showed that CQ did not reduce the duration of viral infection, but could reduce the chance of dengue hemorrhagic fever (Tricou et al., 2010), as well as decrease patients' perceived intensity of pain and improve their daily activity performance (Borges et al., 2013). Our results demonstrated that CQ can prevent ZIKV-induced mortality in suckling mice when administrated soon after infection (0 or 12 hpi), but it has no antiviral effect when administrated at 24 hpi (Fig. 2D). Together, these findings provide valuable information for setting up future clinical trials using CQ against ZIKV infection. In addition, the widespread availability of CQ, as well as its safety profile in pregnancy, are important benefits for its global distribution. Through RNASeq, we detected the induction of multiple cytokines in the embryonic brain upon ZIKV infection, and suggest that this strong immune response is likely to play an important role in the neuronal cell death that leads to microcephaly (Li et al., 2016). CQ may be protecting mice from these ZIKV-induced inflammatory changes at a critical stage in their brain development, although this would need to be further investigated. We also have noticed that it should be investigated more extensively before using CQ as for possible ZIKV therapy in humans.
Our methodology of restricting screens for ZIKV therapeutics to FDA-approved EBOV-inhibitors was both efficient and more likely to allow promising candidates, like CQ, to quickly reach clinical trials. Such a strategy is important to rapidly bring to market therapeutics which could help prevent or relieve symptoms of ZIKV infection, and should also be considered for other emerging infections in the future.
Conflicts of Interests
The funding providers have no roles in performing experiments, writing of the manuscript or the decision to submit it for publication. The authors confirm that there are no known conflicts of interest associated with this publication.
Author Contributions
G.C., Z.X. C.-F.Q and F.X.Q jointly directed the research. C.L. and X.Z. designed and performed the experiments, analyzed the data and wrote the first draft of the manuscript. X.J. helped perform mice related experiments. N.Q., Y.-Q.D., M.T., R.A., X.Z., L.Y., S.K.A., X.-F.L, J.U.J and K.N.S helped to perform experiments, revise the manuscript or contributed reagents.
Acknowledgments
Acknowledgments
This work was supported by MOST (China, 2016YFD0500304) and the State Key Laboratory of Pathogen and Biosecurity (SKLPBS1601), the NSFC Excellent Young Scientist (81522025), the Innovative Research Group (81621005), and the Newton Advanced Fellowship from the UK Academy of Medical Sciences (81661130162) to C-F.Q., the CAMS Initiative for Innovative Medicine (No. 2016-I2M-1-005), NSFC (China, 91542201, 81590765), NIH R01 AI069120, AI056154 and AI078389 502 grants, the NSFC of China (31670883) to G.C., and PUMC Youth Fund (No. 3332016125) and NSFC (No. 31500145) to C.L., NSFC (31430037), Shanghai brain-intelligence project from STCSM (16JC1420500), Beijing Brain Project (Z161100002616004) and MOST (2014CB942801 and 2012YQ03026006) to Z.X. National Science and Technology Major Project for “Significant New Drugs Innovation and Development” (2015ZX09102023) to G.C. and F.X-F.Q. We thank all members in our team for help discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.09.034.
Contributor Information
Frank Xiao-Feng Qin, Email: qinxiaof@mail.sysu.edu.cn.
Cheng-Feng Qin, Email: qincf@bmi.ac.cn.
Zhiheng Xu, Email: zhxu@genetics.ac.cn.
Genhong Cheng, Email: gcheng@mednet.ucla.edu.
Appendix A. Supplementary data
Supplementary figures
References
- Araujo L.M., Ferreira M.L., Nascimento O.J. Guillain-Barre syndrome associated with the Zika virus outbreak in Brazil. Arq. Neuropsiquiatr. 2016;74:253–255. doi: 10.1590/0004-282X20160035. [DOI] [PubMed] [Google Scholar]
- Barrows N.J., Campos R.K., Powell S.T., Prasanth K.R., Schott-Lerner G., Soto-Acosta R., Galarza-Munoz G., McGrath E.L., Urrabaz-Garza R., Gao J. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe. 2016;20:259–270. doi: 10.1016/j.chom.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges M.C., Castro L.A., Fonseca B.A. Chloroquine use improves dengue-related symptoms. Mem. Inst. Oswaldo Cruz. 2013;108:596–599. doi: 10.1590/0074-0276108052013010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P., Pereira J.P., Jr., Raja Gabaglia C., Damasceno L., Wakimoto M., Ribeiro Nogueira R.M., Carvalho de Sequeira P., Machado Siqueira A., Abreu de Carvalho L.M., Cotrim da Cunha D. Zika virus infection in pregnant women in Rio de Janeiro - preliminary report. N. Engl. J. Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard-Feibelman K.M., Govero J., Zhu Z., Salazar V., Veselinovic M., Diamond M.S., Geiss B.J. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antivir. Res. 2017;137:134–140. doi: 10.1016/j.antiviral.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola F.R., Fernandes I.R., Russo F.B., Freitas B.C., Dias J.L., Guimaraes K.P., Benazzato C., Almeida N., Pignatari G.C., Romero S. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., Song J., Lu X., Deng Y.Q., Musyoki A.M., Cheng H., Zhang Y., Yuan Y., Song H., Haywood J. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe. 2016;19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Debnath A., Parsonage D., Andrade R.M., He C., Cobo E.R., Hirata K., Chen S., Garcia-Rivera G., Orozco E., Martinez M.B. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 2012;18:956–960. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvecchio R., Higa L.M., Pezzuto P., Valadao A.L., Garcez P.P., Monteiro F.L., Loiola E.C., Dias A.A., Silva F.J., Aliota M.T. Chloroquine, an endocytosis blocking agent, inhibits Zika virus infection in different cell models. Viruses. 2016;8 doi: 10.3390/v8120322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y.Q., Zhao H., Li X.F., Zhang N.N., Liu Z.Y., Jiang T., Gu D.Y., Shi L., He J.A., Wang H.J. Isolation, identification and genomic characterization of the Asian lineage Zika virus imported to China. Sci. China Life Sci. 2016;59:428–430. doi: 10.1007/s11427-016-5043-4. [DOI] [PubMed] [Google Scholar]
- Dick G.W., Kitchen S.F., Haddow A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- D'Ortenzio E., Matheron S., Yazdanpanah Y., de Lamballerie X., Hubert B., Piorkowski G., Maquart M., Descamps D., Damond F., Leparc-Goffart I. Evidence of sexual transmission of Zika virus. N. Engl. J. Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- Driggers R.W., Ho C.Y., Korhonen E.M., Kuivanen S., Jaaskelainen A.J., Smura T., Rosenberg A., Hill D.A., DeBiasi R.L., Vezina G. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 2016;374:2142–2151. doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- Govero J., Esakky P., Scheaffer S.M., Fernandez E., Drury A., Platt D.J., Gorman M.J., Richner J.M., Caine E.A., Salazar V. Zika virus infection damages the testes in mice. Nature. 2016;540:438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulland A. Zika virus is a global public health emergency, declares WHO. BMJ. 2016;352:i657. doi: 10.1136/bmj.i657. [DOI] [PubMed] [Google Scholar]
- Haddow A.D., Schuh A.J., Yasuda C.Y., Kasper M.R., Heang V., Huy R., Guzman H., Tesh R.B., Weaver S.C. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Lin B., Chu V., Hu Z., Hu X., Xiao J., Wang A.Q., Schweitzer C.J., Li Q., Imamura M. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.3010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills S.L., Russell K., Hennessey M., Williams C., Oster A.M., Fischer M., Mead P. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission - Continental United States, 2016. MMWR Morb. Mortal. Wkly Rep. 2016;65:215–216. doi: 10.15585/mmwr.mm6508e2. [DOI] [PubMed] [Google Scholar]
- Johansen L.M., Brannan J.M., Delos S.E., Shoemaker C.J., Stossel A., Lear C., Hoffstrom B.G., Dewald L.E., Schornberg K.L., Scully C. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen L.M., DeWald L.E., Shoemaker C.J., Hoffstrom B.G., Lear-Rooney C.M., Stossel A., Nelson E., Delos S.E., Simmons J.A., Grenier J.M. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa5597. [DOI] [PubMed] [Google Scholar]
- Johnson B.W., Russell B.J., Lanciotti R.S. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2005;43:4977–4983. doi: 10.1128/JCM.43.10.4977-4983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M., Maes P. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouznetsova J., Sun W., Martinez-Romero C., Tawa G., Shinn P., Chen C.Z., Schimmer A., Sanderson P., McKew J.C., Zheng W. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg. Microb. Infect. 2014;3 doi: 10.1038/emi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Govero J., Smith A.M., Platt D.J., Fernandez E., Miner J.J., Diamond M.S. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro R.S., Barnhart H.X., Schlaff W.D., Carr B.R., Diamond M.P., Carson S.A., Steinkampf M.P., Coutifaris C., McGovern P.G., Cataldo N.A. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- Li C., Xu D., Ye Q., Hong S., Jiang Y., Liu X., Zhang N., Shi L., Qin C.F., Xu Z. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016;19:120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Li C., Deng Y.Q., Wang S., Ma F., Aliyari R., Huang X.Y., Zhang N.N., Watanabe M., Dong H.L., Liu P. 25-Hydroxycholesterol protects host against Zika virus infection and its associated microcephaly in a mouse model. Immunity. 2017;46:446–456. doi: 10.1016/j.immuni.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.Y., Li X.F., Jiang T., Deng Y.Q., Zhao H., Wang H.J., Ye Q., Zhu S.Y., Qiu Y., Zhou X. Novel cis-acting element within the capsid-coding region enhances flavivirus viral-RNA replication by regulating genome cyclization. J. Virol. 2013;87:6804–6818. doi: 10.1128/JVI.00243-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Li S., Ma S., Jia L., Zhang F., Zhang Y., Zhang J., Wong G., Zhang S., Lu X. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2016;167:1511–1524. doi: 10.1016/j.cell.2016.11.016. e1510. [DOI] [PubMed] [Google Scholar]
- Miner J.J., Cao B., Govero J., Smith A.M., Fernandez E., Cabrera O.H., Garber C., Noll M., Klein R.S., Noguchi K.K. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso D., Gubler D.J. Zika virus. Clin. Microbiol. Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G.J., Zerhouni E.A. Once and future epidemics: Zika virus emerging. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf4548. [DOI] [PubMed] [Google Scholar]
- Rasmussen S.A., Jamieson D.J., Honein M.A., Petersen L.R. Zika virus and birth defects—reviewing the evidence for causality. N. Engl. J. Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- Talarico L.B., Pujol C.A., Zibetti R.G., Faria P.C., Noseda M.D., Duarte M.E., Damonte E.B. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antivir. Res. 2005;66:103–110. doi: 10.1016/j.antiviral.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Tricou V., Minh N.N., Van T.P., Lee S.J., Farrar J., Wills B., Tran H.T., Simmons C.P. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl. Trop. Dis. 2010;4:e785. doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Hong S., Deng Y.Q., Ye Q., Zhao L.Z., Zhang F.C., Qin C.F., Xu Z. Transfer of convalescent serum to pregnant mice prevents Zika virus infection and microcephaly in offspring. Cell Res. 2017;27:158–160. doi: 10.1038/cr.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Zou J., Shan C., Yang Y., Kum D.B., Dallmeier K., Neyts J., Shi P.Y. Zika virus replicons for drug discovery. EBioMedicine. 2016;12:156–160. doi: 10.1016/j.ebiom.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Lee E.M., Wen Z., Cheng Y., Huang W.K., Qian X., Tcw J., Kouznetsova J., Ogden S.C., Hammack C. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016;22:1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.C., Li X.F., Deng Y.Q., Tong Y.G., Qin C.F. Excretion of infectious Zika virus in urine. Lancet Infect. Dis. 2016;16:641–642. doi: 10.1016/S1473-3099(16)30070-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures