Pdx1 has long been suspected to be a master transcriptional regulator with essential roles in pancreatic development and β-cells (1–5), but the upstream mechanisms that determine Pdx1 gene expression in mature β-cells are poorly understood. In embryonic pancreatic development, Pdx1 is required to initiate branching morphogenesis and early islet formation (6,7). Pdx1 also has pivotal postnatal roles in mature β-cell function and expansion (8,9). Several conserved 5′ cis-regulatory regions (areas I–III) influence Pdx1 gene expression in embryonic development (Fig. 1) (10,11). In contrast, another conserved region (area IV) has been implicated in postnatal β-cell maturation (12). However, the master themes that govern Pdx1 gene expression remain a mystery, as cell lines and global knockouts cannot deconvolute how Pdx1 is dynamically regulated in embryonic and postnatal life.
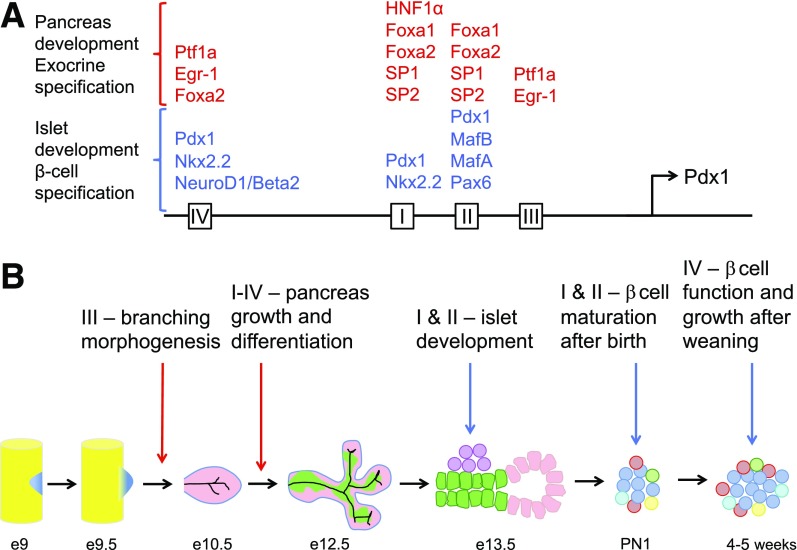
Figure 1.
Enhancer region areas I–IV of the Pdx1 promoter and the regulation of Pdx1 in pancreas development and postnatal β-cells. A: A depiction of the conserved 5′ cis-regulatory regions (areas I–IV) upstream of the Pdx1 transcription start site. Transcription factors with known binding sites within each area are described for those with roles in pancreas development and exocrine specification (red) and others in islet development and β-cell specification (blue). B: Each enhancer element (areas I–IV) is mapped to its embryonic or postnatal function. Briefly, pancreas specification occurs around embryonic day (e) 9, which leads to budding of the dorsal and ventral pancreas, initiating the “primary transition” (5). Area III control of Pdx1 is required for subsequent branching morphogenesis on e10.5, with some possibly overlapping roles for area I and II in epithelial growth of the pancreas through to e12.5 (6,11,16). Area I and II play important roles in Pdx1 specification of Ngn3+ islet endocrine progenitors beginning at ∼e13.5, initiating the secondary transition (5,15). Area I and II also regulate Pdx1 control of β-cell maturation and repression of α-cell lineage after birth (postnatal day 1 [PN1]) (15). Lastly, Pdx1 regulation by area IV has a novel role after the onset of weaning (4–5 weeks of age) to mediate β-cell function and growth (18).
Enter Stein, Wright, and colleagues, who have generated several mouse models to precisely interrogate the Pdx1 promoter (5, 10–16). In embryonic life, Pdx1 gene disruption results in pancreatic agenesis, exocrine insufficiency, and neonatal diabetes, thus revealing the essential role of Pdx1 in early pancreas development (6). This embryonic phenotype is mostly recapitulated by combined deletion of areas I, II, and III (11). Subsequent studies specified areas I and II as regulators of Pdx1 expression in β-cell development, predominated by area II and potentiated by area I (10,13–15). area III may also influence Pdx1 in embryogenesis (16). Notably, area III contains a binding site for Ptf1a, key for pancreas and acinar development (5,16).
Regulation of Pdx1 gene expression in postnatal life is complex and less well understood. Several major elements of the Pdx1 promoter may influence postnatal β-cells. While area I may have a role in mature β-cells to potentiate area II activity (14), area II may also be required for β-cell lineage commitment and maturation (15). Deletion of area II with loss of one Pdx1 allele (Pdx1∆AII/−) results in mice that are severely hyperglycemic with neonatal lethality. Area II mutant mice contain cells coexpressing insulin and glucagon after birth (15), consistent with reports that Pdx1 maintains β-cell identity in part by repressing α-cell genes (17). Similarly, area II compound deletions restricted to endocrine progenitors (Ngn3Cre) or β-cells (RIPCre) lead to elevated α-cell markers and reduced β-cell factors. Meanwhile, the postnatal role of area III is unclear, as Pdx1 becomes restricted to β- and δ-cells in late development (5).
In this issue of Diabetes, Spaeth et al. (18) shift focus to area IV, uncovering the unique and powerful impact of area IV upon postnatal β-cells. In contrast to previously described area I–III mutations, area IV–specific knockouts (Pdx1∆AIV/∆AIV) did not induce developmental or early postnatal β-cell defects. To further interrogate area IV, mice with deletion of area IV were combined with loss of one Pdx1 allele (Pdx1∆AIV/−). These area IV mutants exhibited reduced Ngn3+ progenitors and differentiated insulin+ and somatostatin+ cells (18), a milder phenotype than area II mutants (15). The primary effects of area IV were observed in postnatal life after weaning. Male area IV mutant (Pdx1∆AIV/−) mice had reduced Pdx1 at 5 weeks of age and defective β-cell function, with reduced expression of β-cell–defining genes such as insulin, MafA, Nkx6.1, and Glut2. Notably, reduced Pdx1 expression in area IV mutants was sufficient to suppress α-cell lineage genes (18), contrary to previous reports (15). As expected, β-cell proliferation and area were substantially reduced in male area IV mutants. These studies reveal that area IV regulates Pdx1 control of postnatal β-cell function and growth.
The early postweaning period appears to be a novel window for Pdx1 regulation of β-cells. Using wild-type islets, Spaeth et al. (18) demonstrated increased Pdx1 binding to area IV by chromatin immunoprecipitation analysis, perfectly concordant with the reduction in β-cell proliferation and functional genes. The authors strategically annotated published data sets describing Pdx1 DNA binding sites from mouse βTC-6 cells, with differentially expressed genes during weaning. Their alignment revealed Pdx1 binding sites within one-third of differentially expressed genes from weaned islets. Subsequent assessment of area IV mutant islets demonstrated several Pdx1-bound genes involved in replication and oxidative phosphorylation were decreased. Together, this suggests a potentiation effect of Pdx1 during a critical window for β-cell function and proliferation.
Collectively, these studies provide novel insights into Pdx1 regulation through distinct regional and temporal mechanisms. Absent or reduced Pdx1 induces neonatal diabetes and maturity-onset diabetes of the young and contributes to type 2 diabetes (5). Therapeutic strategies to increase Pdx1 through targeting of area II might benefit developmental and maturation defects associated with maturity-onset diabetes of the young and neonatal diabetes, while targeting area IV might improve mature β-cell function and growth in type 2 diabetes. Indeed, small molecules have been identified that increase Pdx1 expression (19); however, the specific regulatory region activated might dictate Pdx1 function based on growing evidence from Stein, Wright, and colleagues. Notably, these observations also have important consequences for directed differentiation of stem/pluripotent cells into endocrine progenitors and subsequently for β-cell maturation, function, and expansion.
Area IV mice exhibit sexual dimorphic phenotypes, indicating that some intricacies of Pdx1 gene regulation remain unresolved. Female area IV mutant mice were phenotypically identical to controls, in contrast to male mutants. Further investigation into the sex-specific effects of area IV will be important, as it could reveal important biology regarding the impact of sex steroids upon Pdx1 gene regulation and diabetes phenotypes (20). However, the effects of sex steroids upon Pdx1 regulation might occur through nonclassic mechanisms, as Spaeth et al. (18) did not find typical androgen or estrogen response elements within areas I–IV.
In conclusion, the interrogation of area IV regulation of Pdx1 by Spaeth et al. (18) provides a full molecular elucidation of the roles of Pdx1 played out in vivo. These studies expand the existing literature to reveal unique transcriptional regulatory and subsequent functional roles of Pdx1 in embryonic and postnatal life (Fig. 1). Area IV has distinct roles in Pdx1 expression upon postnatal β-cell function and growth during a novel period after weaning. Future studies on the upstream regulation of Pdx1 will have great value to unravel the mysteries of β-cell development, function, and adaptation to diabetes.
Article Information
Funding. The Robert and Janice McNair Foundation, the National Institutes of Health National Institute on Aging (1R01AG040110), and the Diabetes Research Center at Baylor College of Medicine (P30DK079638) provided funding.
Duality of Interest. J.A.K. has served on the scientific advisory board of Johnson & Johnson and currently serves on an advisory board for Lexicon. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 2830.
References
- 1.Wright CV, Schnegelsberg P, De Robertis EM. XlHbox 8: a novel Xenopus homeo protein restricted to a narrow band of endoderm. Development 1989;105:787–794 [DOI] [PubMed] [Google Scholar]
- 2.Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy MR. Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol Endocrinol 1993;7:1275–1283 [DOI] [PubMed] [Google Scholar]
- 3.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 1993;12:4251–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller CP, McGehee RE Jr, Habener JF. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J 1994;13:1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babu DA, Deering TG, Mirmira RG. A feat of metabolic proportions: Pdx1 orchestrates islet development and function in the maintenance of glucose homeostasis. Mol Genet Metab 2007;92:43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994;371:606–609 [DOI] [PubMed] [Google Scholar]
- 7.Oliver-Krasinski JM, Kasner MT, Yang J, et al. The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice. J Clin Invest 2009;119:1888–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 1998;12:1763–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushner JA, Ye J, Schubert M, et al. Pdx1 restores beta cell function in Irs2 knockout mice. J Clin Invest 2002;109:1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerrish K, Gannon M, Shih D, et al. Pancreatic beta cell-specific transcription of the pdx-1 gene. The role of conserved upstream control regions and their hepatic nuclear factor 3beta sites. J Biol Chem 2000;275:3485–3492 [DOI] [PubMed] [Google Scholar]
- 11.Fujitani Y, Fujitani S, Boyer DF, et al. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev 2006;20:253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerrish K, Van Velkinburgh JC, Stein R. Conserved transcriptional regulatory domains of the pdx-1 gene. Mol Endocrinol 2004;18:533–548 [DOI] [PubMed] [Google Scholar]
- 13.Samaras SE, Cissell MA, Gerrish K, Wright CV, Gannon M, Stein R. Conserved sequences in a tissue-specific regulatory region of the pdx-1 gene mediate transcription in Pancreatic beta cells: role for hepatocyte nuclear factor 3 beta and Pax6. Mol Cell Biol 2002;22:4702–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Velkinburgh JC, Samaras SE, Gerrish K, Artner I, Stein R. Interactions between areas I and II direct pdx-1 expression specifically to islet cell types of the mature and developing pancreas. J Biol Chem 2005;280:38438–38444 [DOI] [PubMed] [Google Scholar]
- 15.Yang YP, Magnuson MA, Stein R, Wright CV. The mammal-specific Pdx1 Area II enhancer has multiple essential functions in early endocrine cell specification and postnatal β-cell maturation. Development 2017;144:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiebe PO, Kormish JD, Roper VT, et al. Ptf1a binds to and activates area III, a highly conserved region of the Pdx1 promoter that mediates early pancreas-wide Pdx1 expression. Mol Cell Biol 2007;27:4093–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao T, McKenna B, Li C, et al. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab 2014;19:259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spaeth JM, Gupte M, Perelis M, et al. Defining a novel role for the Pdx1 transcription factor in islet β-cell maturation and proliferation during weaning. Diabetes 2017;66:2830–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan Y, Hartland K, Boskovic Z, et al. A small-molecule inducer of PDX1 expression identified by high-throughput screening. Chem Biol 2013;20:1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauvais-Jarvis F. Role of sex steroids in β cell function, growth, and survival. Trends Endocrinol Metab 2016;27:844–855 [DOI] [PMC free article] [PubMed] [Google Scholar]