Abstract
Overexposure to manganese (Mn) leads to toxic effects, such as promoting the development of Parkinson’s-like neurological disorders. The gut microbiome is deeply involved in immune development, host metabolism, and xenobiotics biotransformation, and significantly influences central nervous system (CNS) via the gut-brain axis, i.e. the biochemical signaling between the gastrointestinal tract and the CNS. However, it remains unclear whether Mn can affect the gut microbiome and its metabolic functions, particularly those linked to neurotoxicity. In addition, sex-specific effects of Mn have been reported, with no mechanism being identified yet. Recently, we have shown that the gut microbiome is largely different between males and females, raising the possibility that differential gut microbiome responses may contribute to sex-selective toxicity of Mn. Here, we applied high-throughput sequencing and gas chromatography–mass spectrometry (GC-MS) metabolomics to explore how Mn2+ exposure affects the gut microbiome and its metabolism in C57BL/6 mice. Mn2+ exposure perturbed the gut bacterial compositions, functional genes and fecal metabolomes in a highly sex-specific manner. In particular, bacterial genes and/or key metabolites of neurotransmitter synthesis and pro-inflammatory mediators are significantly altered by Mn2+ exposure, which can potentially affect chemical signaling of gut-brain interactions. Likewise, functional genes involved in iron homeostasis, flagellar motility, quorum sensing, and Mn transportation/oxidation are also widely changed by Mn2+ exposure. Taken together, this study has demonstrated that Mn2+ exposure perturbs the gut microbiome and its metabolic functions, which highlights the potential role of the gut microbiome in Mn2+ toxicity, particularly its sex-specific toxic effects.
Keywords: Manganese exposure, gut microbiome, sex-specific toxicity, gut-brain axis, metabolome, metagenomics
Introduction
Trillions of bacteria reside in human gastrointestinal tract and they are deeply involved in human metabolism and health (Ley et al., 2006). Besides food digestion and energy harvest, the gut microbiome plays a crucial role in neurodevelopment, immune response, inflammation and xenobiotic biotransformation (Guarner and Malagelada, 2003; Bäckhed et al., 2005). The gut microbiome is a highly dynamic system and can be influenced by environmental factors, such as heavy metals and antibiotics (Jakobsson et al., 2010; Lu et al., 2014). Multiple xenobiotics can alter bacteria community compositions and disturb the production of key metabolites, which can largely influence the interactions between the gut microbiome and host (Maurice et al., 2013; Lu et al., 2014). On the other hand, gut bacteria can modulate the effects of xenobiotics on the host. For example, gut bacteria transform Hg2+ and Cr (VI) to Hg0 and Cr(III) to reduce their toxicity, while other xenobiotics, such as nitrazepam, are converted to more toxic species by gut bacteria (Takeno and Sakai, 1991; Upreti et al., 2004; Monachese et al., 2012; Younan et al., 2016). Therefore, bi-directional interactions between the gut microbiome and exposure actually exist.
Manganese (Mn) is an essential trace element for mammals and many microorganisms (Jakubovics and Jenkinson, 2001; Aschner and Aschner, 2005). Mn is necessary for normal brain function and amino acid, lipid, and carbohydrate metabolism (Greger, 1998; Aschner and Aschner, 2005). Mn also functions as the cofactor of numerous key enzymes, such as arginase, glutamine synthetase, manganese catalase, and manganese superoxide dismutase (Greger, 1998; Jakubovics and Jenkinson, 2001). However, Mn overload is toxic and associated with a series of diseases, including chronic liver failure, cardiovascular diseases, bone loss and neurodegeneration (Roth and Garrick, 2003; Crossgrove and Zheng, 2004; Milatovic et al., 2009). Mn can cross the blood-brain barrier, accumulate in the brain and cause neurodegenerative disorders, such as Parkinson’s disease (PD) (Crossgrove and Zheng, 2004; Reaney et al., 2006). Mn-induced tissues and neuron damages involve multiple mechanisms, including mitochondrial dysfunction, oxidative stress (Milatovic et al., 2009), activation of pro-inflammatory mediators and neuroinflammation (Chen et al., 2006; Milatovic et al., 2009), and alterations of ion homeostasis (Klaassen and Amdur, 1996; Zheng et al., 1999; Roth and Garrick, 2003; Zhang et al., 2003; Crossgrove and Zheng, 2004). Manganese toxicity is also species-dependent. Mn3+ is more reactive and toxic than Mn2+ (Crossgrove and Zheng, 2004; Reaney et al., 2006). A previous study revealed that Mn3+ exposure caused significantly higher blood manganese levels than Mn2+, and Mn3+ accumulated in brain more efficiently than Mn2+ (Reaney et al., 2006). Since a considerable amount of Mn comes from food and water, gut bacteria are being exposed to Mn before it is absorbed to the body. However, it is largely unknown whether Mn exposure can perturb the gut microbiome and its functions. It is also unclear whether gut bacteria can influence the toxicity and physiological effects of Mn.
In particular, a compelling body of evidence demonstrates that the gut microbiome significantly affects central nervous system (CNS) via the gut-brain axis, i.e. the bidirectional biochemical signaling between the gastrointestinal tract and the CNS. The gut microbiome can largely influence behaviors and diseases in the host, such as depression and schizophrenia (Collins et al., 2012; Cryan and Dinan, 2012; Foster and Neufeld, 2013; Dinan et al., 2014). Animals with depression and anxiety were generally associated with alterations of gut bacteria (O’Mahony et al., 2009; Park et al., 2013). Oral administration of Lactobacilli rhamnosus to mice could alter the GABA receptor expression in key CNS stress-related brain regions and influence anxiety-like behaviors (Foster and Neufeld, 2013). Gut microbiome perturbation has been proposed to play a role in neurodegenerative disorders such as PD (Ghaisas et al., 2016). Previous studies clearly showed that the gut produced a large amount of neurotransmitters and related compounds (O’Mahony et al., 2015; Yano et al., 2015). For example, intestinal cells, but not brain cells, generate more than 90% of serotonin in the body (Gershon and Tack, 2007; Yano et al., 2015). Gut compounds play key roles in the cross-talk of microbiome-gut-brain. Inflammatory signaling is another important type of interaction in the gut-brain axis (Bercik et al., 2010; Hanamsagar and Bilbo, 2016; Rea et al., 2016). It has been shown that chronic gastrointestinal inflammation induces anxiety-like behaviors and alters central nervous system biochemistry (Bercik et al., 2010). However, it remains unknown whether Mn exposure perturbs the gut microbiome, which leads to altered chemical signaling involved in the gut-brain axis.
It has been reported that Mn has sex-selective toxicity (Zhang et al., 2003; Madison et al., 2011; Mergler, 2012). For example, MnCl2 exposure had reverse effects on the body weight of male and female SD rats (Zhang et al., 2003). Another study found that Mn2+-exposed female mice had long-lasting effects in neuronal morphology, which was absent in male mice (Madison et al., 2011). However, the mechanism underlying sex-specific effects of Mn is poorly understood. Recently, we and others have shown that the gut microbiome is largely different between male and female animals (Chi et al., 2016; Cong et al., 2016), raising the possibility that differential gut microbiome responses may contribute to sex-selective toxicity. In fact, toxicants, such as arsenic and organophosphate pesticides, cause sex-specific perturbations of the gut bacteria (Chi et al., 2016; Gao et al., 2017), which may further affect toxicity and disease susceptibility in males and females when exposed to these toxicants.
This study was designed to address three questions: Will Mn exposure alter the gut microbiome and its metabolic functions? Are there any changes of chemical signaling involved in the gut-brain interactions? Are these changes sex-specific? Therefore, both male and female C57BL/6 mice were exposed to MnCl2 in drinking water for 13 weeks, followed by the assessment with multi-omics, including 16S rRNA gene sequencings, metagenomics and GC-MS metabolomics. 16S rRNA sequencing and metagenomics sequencing were used to define the alterations of bacterial compositions and functional pathways of gut bacteria. GC-MS metabolomics was employed to analyze the metabolic changes related to the gut microbiome. To the best of our knowledge, this is the first study to examine the sex-specific effects of Mn exposure on the gut microbiome and associated metabolic functions.
Materials and Methods
Animals and manganese exposure
C57BL/6 mice (7 weeks old, Jackson Laboratory, Bar Harbor, ME) were housed in the University of Georgia animal facility for a week before exposure, as well as throughout the duration of the experiment in static microisolator cages with Bed-O-Cob combination bedding under environmental conditions of 22°C, 40–70% humidity, and a 12:12 hr light:dark cycle. Before experimentation, all mice were allowed to consume tap water ad libitum, and were provided with standard pelleted rodent diet before and during experimentation. At the experimental period, mice were randomly assigned into either the control group, or 100 ppm MnCl2 treatment group (consumption of Mn is ~20 mg/kg body weight/day) (n=20, with 5 male mice and 5 female mice per group). The Mn dose used in this study was modeled according to several previous studies that demonstrated neurotoxicity of Mn at similar concentrations (Moreno et al., 2009; Avila et al., 2010; Krishna et al., 2014). The animals were treated humanely and every effort was made to alleviate suffering. The animal protocol was approved by the University of Georgia Institutional Animal Care and Use Committee. At the start of experiment, MnCl2 (Pfaltz & Bauer, Inc., Waterbury, CT) was dissolved in tap water and was administered to individual animal (~ 8 weeks of age) in drinking water for 13 weeks. The mice were allowed to consume ad libitum. Drinking water with MnCl2 was made fresh every week. Control mice (~8 weeks of age) continued to receive tap water in their drinking water bottles which they were allowed to consume ad libitum.
16S rRNA sequencing
Mice fecal pellets from individual mouse were collected for 16S rRNA analysis at 0 and 13 weeks, and stored under liquid nitrogen before being transferred to -80°C until further analysis. DNA was isolated from fecal pellets using a PowerSoil® DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA) according to the manufacturer’s instructions. The resultant DNA was quantified by Nanodrop® and stored at −80°C until further analysis. Purified DNA (1 ng) was used to amplify the V4 region of 16S rRNA of bacteria using universal primers of 515 (5′-GTGCCAGCMGCCGCGGTAA) and 806 (5′-GGACTACHVGGGTWTCTAAT). The resultant DNA products were barcoded and quantified by Qubit 2.0 Fluorometer using Qubit dsDNA HS Assay kit (Life Technologies, Grand Island, NY) according to manufacturer’s instructions and pooled to be sequenced. Sequencing was performed on an Illumina Miseq at the Georgia Genomics Facility to generate pair-end 250 × 250 (PE250, v2 kit) reads. The raw mate-paired fastq files were merged and quality-filtered using Geneious 8.0.5 (Biomatters, Auckland, New Zealand) with error probability limit set as 0.01. The data then were analyzed using quantitative insights into microbial ecology (QIIME, version 1.9.1). UCLUST was used to get the operational taxonomic units (OTUs) with 97% sequence similarity. The data was assigned at five different levels: phylum, class, order, family and genus.
Metagenomics sequencing
For metagenomics sequencing, DNA (10 ng/μL) was fragmented using the Bioruptor UCD-300 sonication device, followed by sequencing library construction using the Kapa Hyper Prep Kit according to the manufacturer’s instructions. The resulting DNA was pooled, quantified, and sequenced at the Georgia Genomics Facility using an Illumina NextSeq High Output Flow Cell. The raw fastq files were imported into the MG-RAST metagenomics analysis server (version 4.0) with MG-RAST ID: Control (male): 4689984.3, 4689979.3, 4689977.3, 4689967.3, and 4689960.3; MnCl2 treatment (male): 4707697.3, 4707696.3, 4707700.3, 4707701.3, and 4707704.3; Control (female): 4689988.3, 4689986.3, 4689982.3, 4689978.3, and 4689973.3; MnCl2 treatment (female): 4707703.3, 4707702.3, 4707699.3, 4707698.3, and 4707695.3 (Meyer et al., 2008). Sequences were assigned to M5NR Subsystems database for functional analysis with maximum e-Value cutoff 10–5, 75% minimum identity cutoff, and minimum alignment length cutoff of 35. Metagenomics analyzed and compared the normalized sequencing counts of bacterial genes between the controls and treatment samples.
Gas chromatography mass spectrometry metabolomics profiling
Metabolites were extracted from fecal samples collected from individual mouse using methanol and chloroform as described previously (Lu et al., 2014). Briefly, 20mg feces was vortexed with 1ml of methanol/chloroform/water solution (2:2:1) for 1 hour, followed by centrifugation at 3,200 × g for 15 minutes. The resultant upper phase and lower phase were transferred to a HPLC vial and dried for about 4 hours in SpeedVac, and derivatized using N,O Bis(trimethylsilyl)trifluoroacetamide (BSTFA). The derivatized samples were analyzed using an Agilent Technologies 6890N Network GC System/5973 Mass-Selective Detector (Agilent Technologies, Santa Clara, CA) with an Agilent DB5-MS column (5% Phenyl and 95% dimethylarylene siloxane as the stationary phase, 30 m length; 0.250 mm diameter (narrowbore); film thickness 0.25 μm) (Agilent Technologies, Santa Clara, CA) under the following conditions: initial oven temperature was set at 60° C for 2 minutes, ramped to 320°C by 8°C/minute, and then held at 320°C for 10.5 minutes. 2 μL of sample solution was injected with helium as the carrier gas at a flow rate of 0.8 mL/minute. The temperature of the injector, ion source, and MS Quadrupole were set at 275°C, 230°C, and 150°C respectively. The mass spectrometer was operated in full scan mode from 50 to 600 m/z. The XCMS Online tool was used to pick up and align peaks and calculate the accumulated peak intensity. To identify the metabolite represented by a particular feature, retention time and m/z data from the XCMS Online output was used to filter the total ion chromatogram. The compounds were tentatively identified after matching with the NIST MS database and the identification of a few selected metabolites, such as amino acids, was further validated using authentic standards.
Statistical analysis
Principal coordinate analysis (PCoA) was used to compare the gut microbiome profiles between the control and treatments, while a nonparametric t-test was conducted by Metastats, integrated with Mothur software (Schloss et al., 2009), to determine statistically significant changes (p<0.05) to the gut-microbial community structure between treatments and control as previously described (White et al., 2009). DESeq2 (version 3.4) has been applied to calculate the statistically significant changes of functional genes (Love et al., 2014). To generate difference of metabolic profile between the control and treatment group, a two-tail Welch’s t-test (p<0.05) was used.
Results
1. Mn2+ exposure perturbed the gut microbiome in a sex-specific manner
We first used 16s rRNA gene sequencing to examine the changes of gut bacterial compositions over time using beta diversity and alpha diversity metrics. Beta diversity evaluates the diversity in microbial community between samples, while alpha diversity reflects species richness in given samples. As shown in Fig. 1A and 1B, PCoA analysis shows that gut bacterial communities of mice initially clustered together at the baseline before exposure. After a 13-week Mn2+ treatment, the controls and exposed animals clearly separated into their own groups, which indicated Mn2+ exposure perturbed the trajectories of gut microbiome development. The alpha diversities (PD whole tree) in Mn2+-treated groups were lower than control groups (Fig. 1C and 1D), indicating that Mn2+ treatment reduced phylogenetic diversity of gut bacteria. Notably, a strong sex-specific alteration of gut microbiome was found. For example, the relative abundance of the phylum Firmicutes significantly increased in Mn2+-exposed male mice, while it decreased in Mn2+-treated female mice (Fig. 2A). Moreover, Mn2+ exposure significantly reduced the phylum Bacterodetes in male mice only. Likewise, sex-dependent perturbations of gut bacteria were also evident at the genus level, as illustrated in Fig. 2B and 2C. These results clearly show that the gut bacterial community structures have been differentially altered in male and female mice by Mn2+ exposure.
Fig. 1.
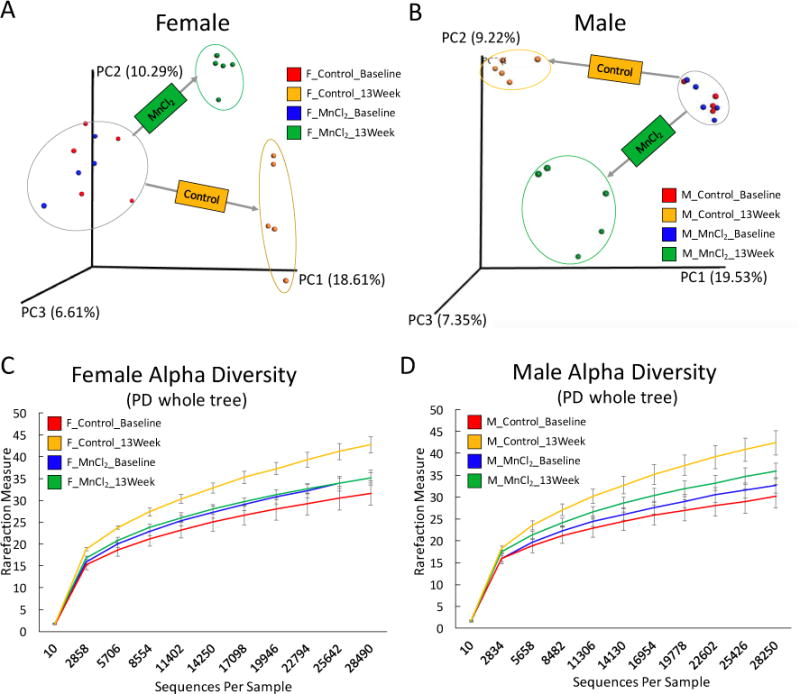
The effects of Mn2+ exposure on the gut microbiome in male and female C57BL/6 mice, as revealed by 16S rRNA gene sequencing. Based on the PCoA analysis of beta diversity, the gut microbiome community structures of female (A) and male (B) mice were significantly altered by Mn2+ exposure. The phylogenetic diversity, as evaluated by PD whole tree (alpha diversity), of the gut microbiome was significantly decreased in Mn2+-treated female (C) mice and male (D) mice.
Fig. 2.
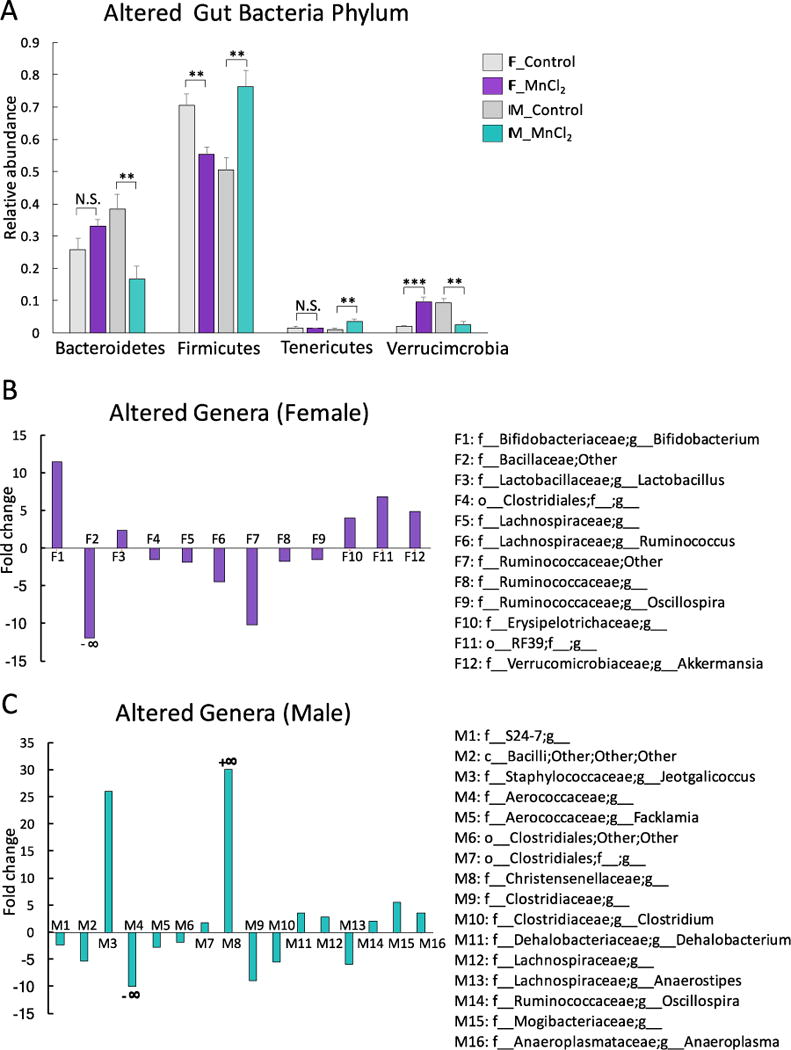
Mn2+ altered the relative abundance of gut bacteria at the phylum level in a sex-specific manner (A) (***p<0.001, **p<0.01, *p<0.05, N.S., no statistically significant change); Sex-dependent perturbations of gut bacteria at the genus level, as illustrated by the fold changes of significantly altered gut bacterial genera (B: Females; C: Male) (Only genera with p<0.05 are listed here; Fold changes were calculated using the relative abundance of each genus in Mn2+-treated mice divided by the relative abundance of the same genus in control mice. c: class; f: family; O: order; G: genus. +∞: only appeared after Mn exposure; -∞: abolished by Mn exposure)
2. Mn2+ exposure altered the abundance of bacterial genes of tryptophan and GABA metabolism pathways
Perturbed gut microbiome profiles are frequently associated with the alterations of functional bacterial genes. Neurotransmitters serve as key signaling molecules for the gut microbiota to influence brain activities (Sampson and Mazmanian, 2015), so we examined whether Mn2+ exposure altered relevant genes in the gut microbiome. As shown in Fig. 3A, genes in tryptophan biosynthesis pathways, including anthranilate phosphoribosyltransferase, indole-3-glycerol phosphate synthase, and tryptophan synthase (beta and alpha chain), were significantly altered by Mn2+ in a sex-specific manner. For example, the gene encoding tryptophan synthase (beta chain) was decreased and increased in Mn2+-treated female and male mice, respectively (Fig. 3A). Mn2+ also induced a different effect on the genes of phenylalanine synthesis, with biosynthetic aromatic amino acid aminotransferase and prephenate dehydratase being increased and decreased in females and males (Fig. 3B). Likewise, a sex-specific effect of Mn2+ on GABA/putrescine metabolism is evident, as shown in Fig. 3C. For instance, the gene encoding glutamate decarboxylase, which synthesizes GABA from glutamate, was significantly decreased in Mn2+-exposed male mice only (Fig. 3C). As an important source of GABA, the metabolism of putrescine plays a role in GABA homeostasis (Sequerra et al., 2007). The putrescine transport gene, potA, was increased in Mn2+-exposed male mice, while potA and potG were decreased in female mice (Fig. 3C). Putrescine biosynthesis genes were also significantly changed by Mn2+ exposure in a sex-selective fashion, as revealed by altered agmatine deiminase in females and arginine decarboxylase and N-carbamoylputrescine amidase in males (Fig. 3C).
Fig. 3.
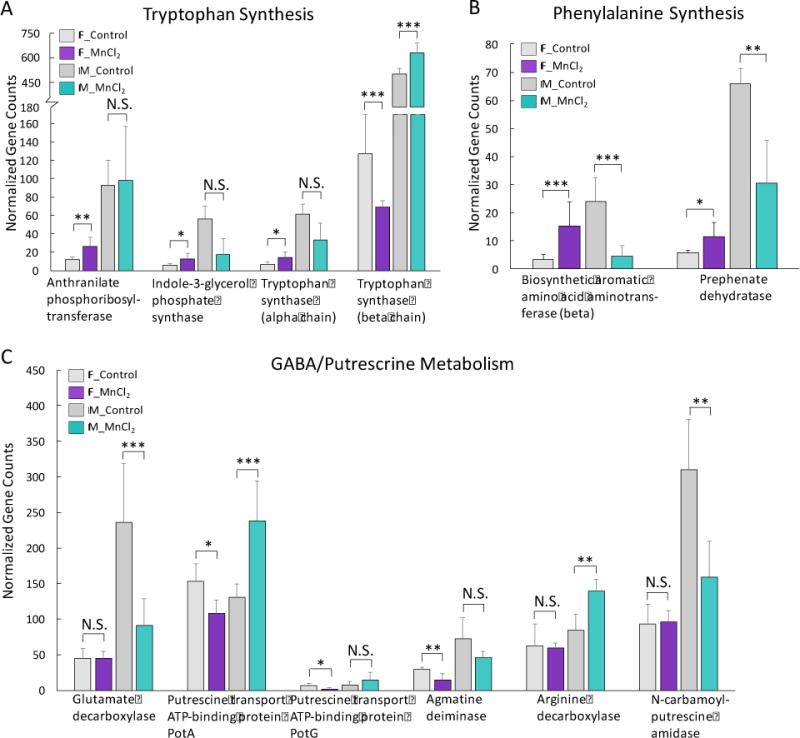
Sex-selective effects of Mn2+ on bacterial genes involved in neurotransmitter pathways. (A: tryptophan synthesis genes; B: phenylalanine synthesis genes; C: GABA/putrecrine metabolism genes) (***p<0.001, **p<0.01, *p<0.05, N.S., no statistically significant change)
3. LPS synthesis and DNA repair genes were specifically enriched in female mice by Mn2+ exposure
LPS plays an important role in the host inflammation and contributes to the gut-brain interactions (Cryan and Dinan, 2012; Sampson and Mazmanian, 2015). We found that the abundances of LPS biosynthesis genes were widely increased in Mn2+-treated female mice, but reduced in male animals, as shown in Fig. 4A and 4B. For example, bacterial genes involved in Kdo2 and lipid A synthesis, the important components of LPS, were increased in females only by Mn2+ treatment (Fig. 4A). Moreover, LPS assembly related genes were largely increased in female mice by Mn2+ exposure, but were either reduced or not significantly changed in male mice (Fig. 4B). The oxidative stress response gene, cytochrome c551 peroxidase, as well as multiple DNA repair genes, was significantly increased in female mice but decreased in male mice under Mn2+ exposure (Fig. 4C).
Fig. 4.
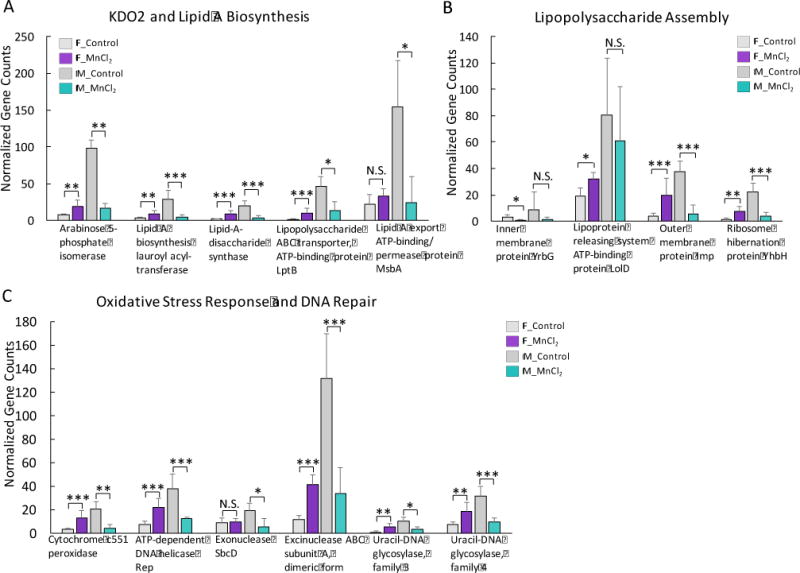
Gut bacterial genes involved in Kdo2 and lipid A synthesis (A), LPS assembly (B) and oxidative stress response and DNA repair (C) were significantly increased in Mn2+-exposed female mice and decreased in male mice. (***p<0.001, **p<0.01, *p<0.05, N.S., no statistically significant change)
4. Genes related to iron homeostasis were altered by Mn2+ exposure
Mn can interact with iron and perturb normal physiological processes (Zheng et al., 1999; Crossgrove and Zheng, 2004). So, we next explored whether the iron homeostasis in the gut microbiota was affected by Mn2+. In fact, many iron transport related genes were significantly altered, such as ferrichrome-iron receptor, outer membrane receptor protein (Fe transport), ferric iron ABC transporter, iron compound ABC uptake transporter, and ferric uptake regulation protein (Fig. 5). Again, a sex-selective impact of Mn2+ exposure on iron acquisition and metabolism was found. These results suggest that Mn exposure could differentially affect the iron acquisition and metabolism in the gut microbiome of female and male mice.
Fig 5.
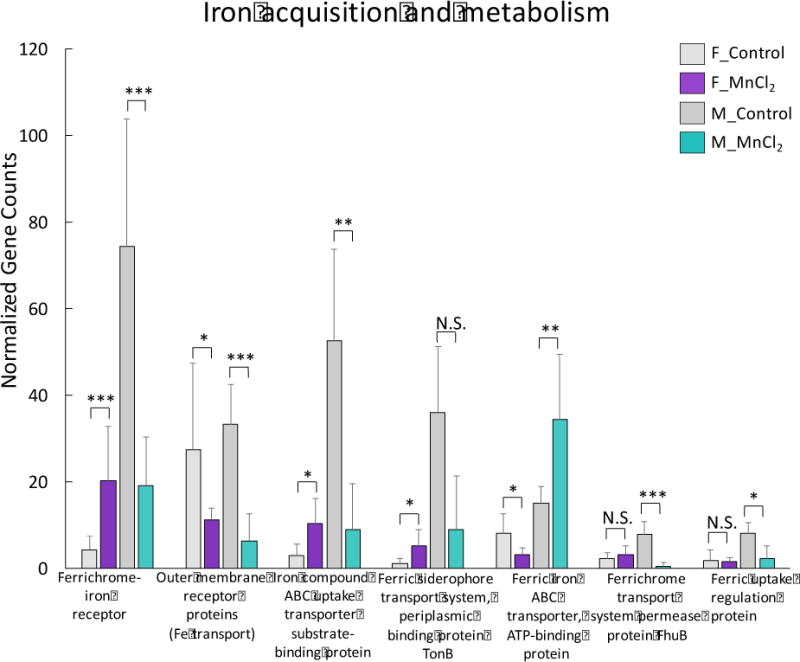
Iron acquisition and metabolism related genes were widely altered in Mn2+-exposed female and male mice. (***p<0.001, **p<0.01, *p<0.05, N.S., no statistically significant change)
5. Mn2+ exposure altered genes in quorum sensing, motility and chemotaxis and metal/drug resistance
Bacteria control behaviors including motility and virulence according to the population density fluctuation by the cell-cell signaling process called quorum sensing. The detection of an autoinducer at the threshold concentration leads to the alteration of gene expression. We found that quorum sensing genes were specifically increased in Mn2+-exposed male mice only, including autoinducer 2 (AI-2) kinase LsrK, S-ribosylhomocysteine lyase and autoinducer-2 production protein LuxS (Fig. 6A). Consequently, bacterial genes involved in flagellar motility and chemotaxis were largely increased in male mice (Fig. 6B and 6C). Interestingly, multiple metal or drug resistance related genes were significantly increased in Mn2+-treated female mice but consistently decreased in male mice (Fig. 6D). Our results again highlight a sex-selective effect of Mn2+ on these critical bacterial functional genes.
Fig. 6.
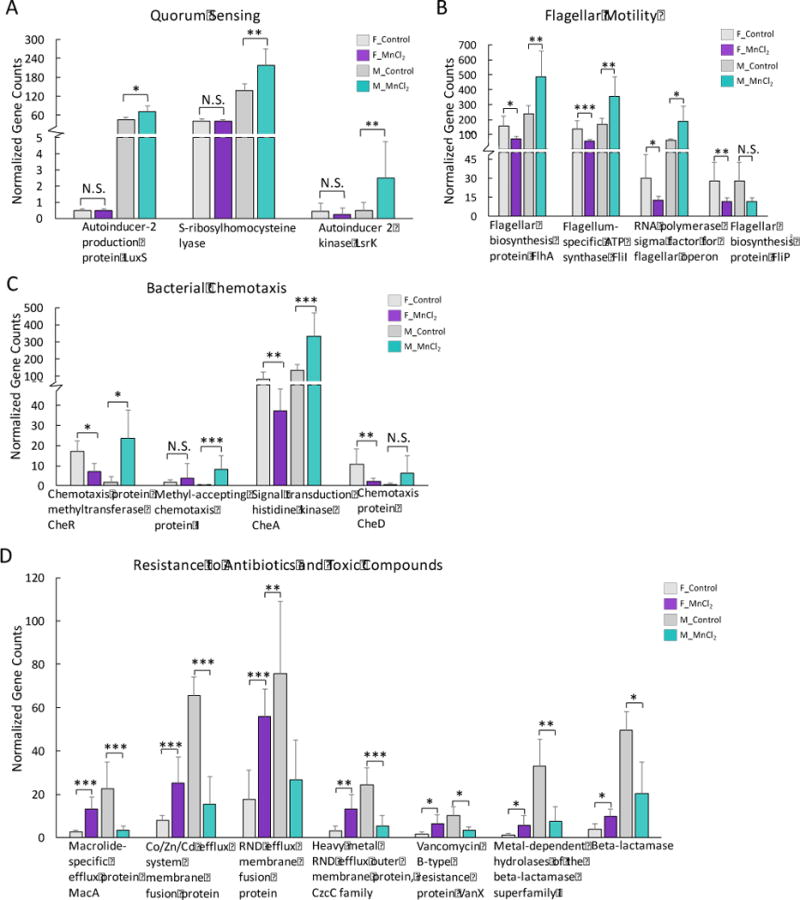
Quorum sensing genes specifically increased in Mn2+-exposed male mice (A). Bacterial genes of flagellar motility (B) and chemotaxis (C) increased in Mn2+-exposed male mice but significantly decreased in female mice. Heavy metal or antibiotic resistance genes decreased in the gut bacteria of Mn2+-exposed male mice, but increased in Mn2+-exposed female mice (D). (***p<0.001, **p<0.01, *p<0.05, N.S., no statistically significant change)
6. Sex-selective regulation of genes of Mn transportation and oxidation
As an essential trace metal, Mn can be absorbed and utilized by many bacteria species (Jakubovics and Jenkinson, 2001). We next investigated whether Mn metabolism-related bacterial genes were regulated by exogenous Mn2+ exposure. Interestingly, two manganese transporter genes, manganese ABC transporter SitB and SitD, were specifically enriched in the gut microbiome of Mn2+-treated female mice, while they were largely reduced in male mice (Fig. 7A and 7B). The gene encoding multicopper oxidase that oxidizes Mn2+ to Mn3+ significantly increased in females but decreased in males (Fig. 7C) (Webb et al., 2005). These results suggest the gut bacteria of male and female animals may have different capacities or responses to mediate Mn utilization and oxidation.
Fig. 7.
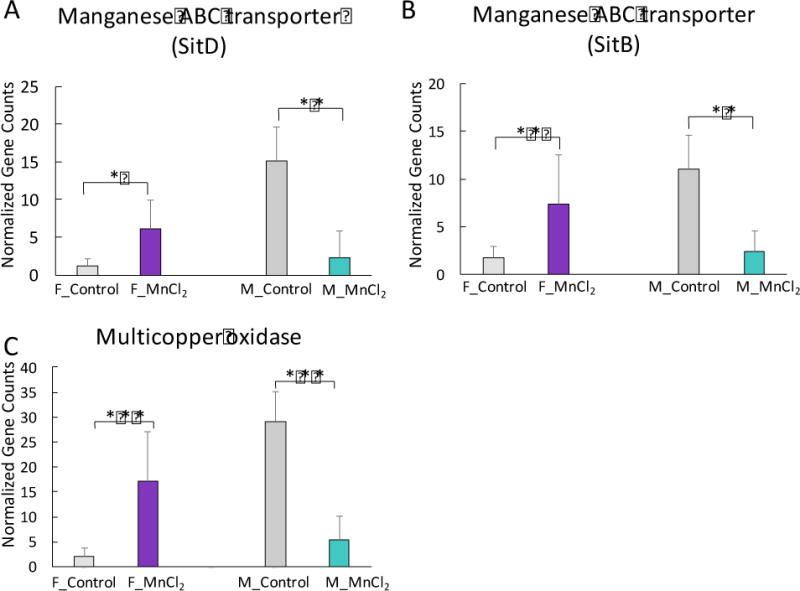
Sex-selective alterations of bacterial genes of Mn transportation and oxidation in mice exposed to Mn2+. Mn ABC transporter genes (A and B) and Mn oxidation gene (C) were significantly increased in female mice but decreased in male mice after Mn2+ treatment. (***p<0.001, **p<0.01, *p<0.05)
7. Mn2+ exposure disturbed the fecal metabolome of mice
The communication between gut bacteria and host is largely dependent on metabolites with various functions. We further examined whether the fecal metabolomes were altered by Mn2+ exposure. As shown in Fig. 8, the fecal metabolomic profiles of male and female mice were perturbed by a 13-week Mn2+ treatment in a sex-selective manner. In particular, the majority of fecal metabolites of male mice were down-regulated, while numerous up- and down-regulated metabolites were captured for female mice (Fig. 8A and 8B). For example, we observed the aphla-tocopherol and gama-tocopherol were decreased in Mn2+-treated male and female mice (Fig. 8C and 8D), with a stronger response being observed for female animals. In addition, several neurotransmitters or the precursors of neurotransmitter synthesis, such as glycine, glutamic acid and phenylalanine, were altered in a sex-selective manner, with up-regulation and down-regulation in the fecal metabolomes of female and male mice, respectively (Fig. 8E–8G). More identified significantly changed metabolites (p<0.05) are listed in Table S1 and S2.
Fig. 8.
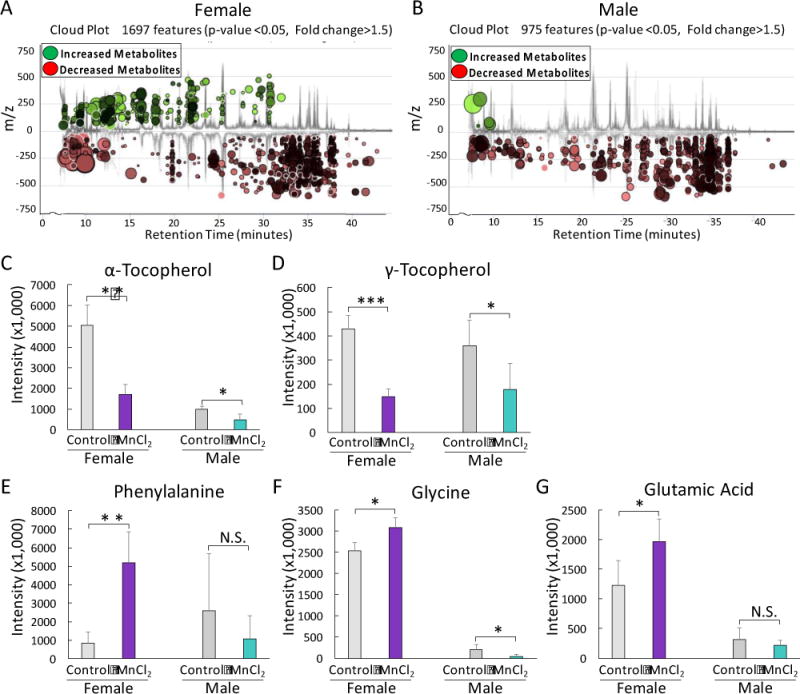
Mn exposure perturbed the metabolic profiles of fecal samples of female (A) and male mice (B). Antioxidants, such as α-tocopherol (C) and γ-tocopherol (D), were significantly decreased in the fecal samples of Mn2+-exposed female and male mice, with a stronger effect being observed in female animals. Relative intensity of phenylalanine (E), glycine (F), and glutamic acid (G) was significantly increased and decreased in Mn2+-exposed female mice and male mice, respectively. (***p<0.001, **p<0.01, *p<0.05, N.S., no statistically significant change)
Discussion
In this study, we explored the sex-specific effects of Mn2+ exposure on the gut microbiome compositions, functional genes and fecal metabolomes by high-throughput sequencing and GC-MS metabolomics. Our results reveal that the gut microbiome has been significantly perturbed in C57BL/6 mice by Mn2+ treatment. Our data also show that Mn2+ may affect the gut-brain axis by influencing the synthesis of several neurotransmitters or their precursors, including GABA and tryptophan. Mn2+ also modulates the bacteria-related pro-inflammatory mediators, such as LPS, which may also impair gut-brain interactions. Moreover, our data highlight several potential mechanisms of how Mn2+ perturbs the gut microbiome, including altering quorum sensing, inducing oxidative stress, and disturbing iron homeostasis. Notably, we found strong sex-specific effects of Mn2+ exposure on the gut bacteria. Our data also suggest that the bi-directional interactions between Mn2+ exposure and the gut microbiome was impaired: Mn2+ exposure influences the gut bacteria and their functions, and Mn2+-induced perturbations of gut bacteria may mediate the toxicity of Mn in the host.
Recently, much attention has been paid to the interactions between the gut microbiome and CNS (Foster and Neufeld, 2013; Ghaisas et al., 2016). Compelling evidence indicates that the gut microflora has profound effects on CNS activities and host behaviors (Collins et al., 2012; Cryan and Dinan, 2012; Foster and Neufeld, 2013). For example, previous studies suggested that bacterial colonization in the gut played a critical role in neural system development (Barbara et al., 2005; Stilling et al., 2014; Sampson and Mazmanian, 2015). Genes of some key neurotransmitter receptors like serotonin receptor 1A in germ-free mice are different compared with conventionally raised mice (Neufeld et al., 2011). Likewise, oral administration of some species such as Lactobacillus rhamnosus and Bifidobacterium longum can alter the gene expression in CNS and influence anxiety-like behaviors (Bercik et al., 2011b; Bravo et al., 2011). Other experiments also demonstrated that the perturbation of gut microbiota by non-absorbable antimicrobials was associated with the changes of CNS activities and behaviors (Bercik et al., 2011a). One of the main potential mechanisms for gut bacteria to interact with host nervous systems is through neurotransmitters or their metabolic precursors (Sharon et al., 2014; Sampson and Mazmanian, 2015), such as GABA and tryptophan. Tryptophan is a central precursor of serotonin or 5-hydroxytryptamine (5-HT) (Leathwood, 1990). 5-HT is an important neurotransmitter involved in multiple physiological processes, such as modulating colonic motility (Fukumoto et al., 2003). Previous studies show that gut microbiota participated in the 5-HT turnover modulation (Reigstad et al., 2015; Yano et al., 2015). Tryptophan is generated by the gut microbiota and then can cross the blood brain barrier to be synthesized to 5-HT (O’Mahony et al., 2015). Here, we found multiple genes involved in synthesizing tryptophan from chorismate were increased in Mn2+-treated female mice (Fig. 3A). GABA, as an inhibitory neurotransmitter, also functions in multiple physiological processes and is directly associated with anxiety and depression (Kalueff and Nutt, 2007; Hall et al., 2014). Similar to 5-HT, the concentration of GABA in the host is also affected by gut microbiota (Barrett et al., 2012; Sampson and Mazmanian, 2015). In this study, the gene encoding glutamate decarboxylase was altered in Mn2+-treated male mice (Fig. 3C). Bacteria can synthesize GABA from glutamate by glutamate decarboxylase (Barrett et al., 2012). Previous research has also found that putrescine is an important source of GABA in the brain of rats (Sequerra et al., 2007). Herein, genes involved in putrescine synthesis and transportation were changed by Mn2+ treatment (Fig. 3C). In addition, two phenylalanine synthesis genes also have significantly higher abundances in Mn2+-treated mice than controls (Fig. 3B). Consistently, at the metabolite level, several neurotransmitters or the precursors of neurotransmitter synthesis, such as glycine, glutamic acid and phenylalanine, were perturbed by Mn2+ exposure (Fig. 8). Glycine and glutamic acid are well-known neurotransmitters, while phenylalanine is the precursor of the neurotransmitter dopamine (Daubner et al., 2011). Collectively, these results suggest that Mn-induced perturbation of gut microbiome might disturb the normal metabolism of neurotransmitters or related precursors in gut, which could further interfere with normal gut-brain interactions.
Modulation of immune response is another mechanism that the gut flora can influence the neuron systems (Collins et al., 2012; Cryan and Dinan, 2012; Sampson and Mazmanian, 2015). The gut microbiome plays a critical role in host inflammation, and multiple bacteria-derived molecules can activate immune systems, such as LPS and bacterial lipoprotein (Hirschfeld et al., 1999; Cani et al., 2008; Hooper et al., 2012). LPS can stimulate immune cells to produce various pro-inflammatory cytokines, such as IL-1b, TNFa, and IL-6 (Bruunsgaard et al., 1999). Cytokines can transport to neural systems and function as signaling molecules to influence neuron activities and behaviors (Cryan and Dinan, 2012). Here, we observed that genes involved in LPS synthesis and assembly are widely increased in Mn2+-treated female mice (Fig. 4A and 4B). Interestingly, a previous study found that Mn2+ enhanced LPS-induced NOS2 expression and cytokines release (Barhoumi et al., 2004). Therefore, Mn2+ exposure might increase inflammatory response not only by increasing LPS secretion in gut bacteria, but also by potentiating the physiological response of LPS, which in turn leads to a more pronounced effect on the gut-brain axis.
In this study, Mn exposure significantly altered the gut microbiome. Likewise, we also demonstrated that other environmental toxicants, like arsenic, could also largely alter the bacterial compositions, abundance and community structures of gut bacteria (Lu et al., 2014; Chi et al., 2016). What mechanism is responsible for regulating the bacterial community? This is an important question to be addressed in microbiome-exposure research. In this context, our data may provide new insights into how Mn causes gut microbiome perturbations. Mn exposure may result in shifted gut microbiome structures by altering the quorum sensing, Mn availability, oxidative stress and iron homeostasis, as briefly discussed as below.
Mn2+ exposure alters the quorum sensing system. Bacteria control behaviors such as sporulation, motility and virulence according to the population density fluctuation by quorum sensing (Nasser and Reverchon, 2007). Autoinducers play a key role in quorum sensing. Key quorum sensing genes, including AI-2 kinase LsrK, S-ribosylhomocysteine lyase and AI-2 production protein LuxS, were increased in Mn2+-exposed male mice (Fig. 6A), indicating that Mn2+ exposure could perturb the bacterial community compositions via altering quorum sensing. Besides regulating the density of the bacterial population, alterations of quorum sensing could also lead to the regulation of many critical downstream genes and pathways. As the consequence, we observed genes involved in bacteria motility and chemotaxis were enriched in gut bacteria of Mn2+-treated male mice (Fig. 6B and 6C). Flagellar motility and bacterial chemotaxis play an important role in bacteria survival, which allows bacteria to respond to favorable or unfavorable environmental stimuli (Haefele and Lindow, 1987). Moreover, quorum sensing and flagella genes are also necessary for the biofilm formation in many bacteria, which could promote bacteria aggregation and adhesion (Pratt and Kolter, 1998; Singh et al., 2006). Chemotaxis ability is critical in the spreading of biomass in mature biofilm, which help bacteria find optimum conditions for growth and survival (Pandey and Jain, 2002; Singh et al., 2006).
Mn2+ exposure may also perturb the gut bacteria by mediating Mn2+ availability. As an essential metal, Mn is critical in multiple physiological processes in mammals and bacteria (Jakubovics and Jenkinson, 2001; Aschner and Aschner, 2005). Mn in gastrointestinal tract is normally maintained at low levels and bacteria that require Mn for growth are inhibited due to limited availability of Mn. In fact, one of effective strategies for the host to fight against bacterial infections is Mn sequestration (Diaz-Ochoa et al., 2014). The host can express proteins, such as calprotectin in mucosa, which can directly bind Mn2+ to reduce Mn available in gastrointestinal tract and then inhibit microbial growth. This process is called nutritional immunity (Diaz-Ochoa et al., 2014). However, Mn2+ exposure significantly increases Mn in gastrointestinal tract, which might weaken or even destroy nutritional immunity. Under such a scenario, bacteria limited by Mn availability can greatly benefit from Mn2+ exposure. For example, previous studies found that the growth of Lactobacillus species required extremely high concentrations of Mn2+ (Archibald and Fridovich, 1981; Archibald and Duong, 1984). In this study, Lactobacillus was enriched in Mn2+-treated female animals (Fig. 2B). Consistently, two genes encoding Mn ABC transporter were significantly increased in Mn2+-treated female mice (Fig. 7A and 7B). Mn ABC transporter genes are actually encoded in the genome of Lactobacillus species (Groot et al., 2005). Taken together, our data suggest that Mn2+ may perturb gut bacterial profiles by altering the availability of Mn2+.
However, overexposure to Mn2+ potentially affects gut bacteria by inducing oxidative stress (Milatovic et al., 2009). Previous studies demonstrated that Mn2+ accumulated in mitochondria and could disrupt oxidative phosphorylation, leading to ROS generation (Gunter et al., 2006; Milatovic et al., 2009). Mitochondria dysfunction and DNA fragmentation is one of main toxic effects of Mn2+ on rat neurons (Malecki, 2001; Milatovic et al., 2009). In this study, the oxidative stress response gene, cytochrome c551 peroxidase, and multiple DNA repair genes were significantly activated (Fig. 4C). Likewise, we found that antioxidants, such as alpha-tocopherol and gama-tocopherol, were decreased in the fecal samples of Mn2+-exposed mice. These evidence suggests that Mn2+ may induce oxidative stress in gut bacteria, which could influence the growth and survival of selected components of gut bacteria.
In addition to oxidative stress, high concentrations of Mn2+ can disturb the balance of ions, especially iron homeostasis (Zheng et al., 1999). Numerous evidence shows that the interaction with iron is one of the mechanisms of Mn2+ toxicity (Zheng et al., 1999; Roth and Garrick, 2003; Crossgrove and Zheng, 2004). For example, previous studies demonstrate that Mn transportation through blood-brain barrier to CNS relies on the binding with transferrin, an iron transporting protein (Aschner and Aschner, 1990). Mn can change the catalytic function of aconitase potentially through competing with iron in the active center of this enzyme (Zheng et al., 1998). Likewise, Mn exposure causes excessive accumulation of iron in neurons, leading to oxidative stress and neuron damage (Zheng et al., 1999; Crossgrove and Zheng, 2004). Iron and Mn homeostasis are important for the survival of microorganisms (Jakubovics and Jenkinson, 2001). Thus, disturbance of iron acquisition and metabolism may contribute to shaping the gut microbiome compositions. Indeed, we found multiple bacterial genes involved in the uptake and transport of iron were significantly perturbed by Mn exposure (Fig. 5). Besides the effects of Mn on the gut bacteria, Mn may affect iron metabolism in gut bacteria by perturbing host genes or responses. Mn absorption is mainly occurred in gastrointestinal tract by transferrin-dependent and transferrin-independent pathways, which are also used for iron absorption (Roth and Garrick, 2003). Transferrin and transferrin receptors can also be expressed in mucosa for iron sequestration (Diaz-Ochoa et al., 2014). Mn can activate the expression of host ferritin and transferrin receptor to increase the iron absorption in tissues (Zheng et al., 1999). Therefore, enhanced nutritional immunity by increasing iron absorption in GI tract may potentially affect bacterial growth and microbiome community structures.
As already mentioned above, strong sex-specific effects of Mn exposure on the gut microbiome were observed. For example, Firmicutes significantly increased in Mn2+-exposed males, but decreased in female animals (Fig. 2A). Bacterial genes in multiple pathways, such as LPS synthesis and DNA repair, were increased in females but decreased in males (Fig. 4). Fecal metabolomes were also differentially altered in male and female mice (Fig. 8). Likewise, multiple genes encoding heavy metal resistance protein and efflux system protein, as well as several antibiotic resistance genes, were significantly increased in treated female mice (Fig. 6D). These genes can improve bacterial survival by exporting heavy metals out of cells. As discussed previously, the genus Lactobacillus and Mn ABC transporter genes were specifically enriched in Mn2+-exposed female mice (Fig. 2B and Fig. 7). Collectively, differential enrichment of specific genes may reflect sex-specific gut microbiome responses to Mn2+ exposure. The mechanism underlying sex-specific gut microbiome response remain elusive. It could arise from the initial gut microbiome difference, driven by sex hormones, between male and female mice. Additionally, sex-selective host response may also participate in mediating gut-microbiome response to exposure. Nevertheless, sex-specific gut microbiome response may play a role in differential toxicity of Mn in males and females.
The bi-directional interactions between the gut microbiome and exposure exist. Besides the widespread effects of Mn2+ on the gut microbiome, our data suggest that the gut microbiome may also influence the toxic effects of Mn. For example, the increase of Mn transportation genes in female mice may enrich Mn2+ in gut bacteria, thus reducing its toxic effects in the host by limiting Mn adsorption in host cells. On the other hand, the gut microbiota may increase the toxicity by oxidizing Mn2+ to Mn3+. Mn3+ is much more reactive and toxic than Mn2+ (Chen et al., 2001). Previous studies indicated that Mn3+ had higher affinity with transferrin and could accumulate at higher concentrations in brain than Mn2+ (Reaney et al., 2002; Kearns, 2010). Here, we found the gene encoding multicopper oxidase was highly enriched in Mn2+-exposed female mice only (Fig. 7C). Bacteria can oxidize Mn2+ to Mn3+ by multicopper oxidase (Webb et al., 2005). These data suggest that the gut microbiota in females may have a higher capability of oxidizing Mn2+ to more toxic Mn3+, which can enhance the toxic effects of Mn in the host. Interestingly, previous studies show that the toxic effects of manganese are different in males and females (Madison et al., 2011; Mergler, 2012). Thus, differential biotransformation of Mn2+ by gut bacteria in males and females may play a role in the sex-specific effects of Mn exposure. Future studies are warranted to elucidate the role of the gut microbiome in mediating Mn toxicity in the host, particularly via a sex-specific manner.
Supplementary Material
Acknowledgments
The authors thank the University of Georgia, University of North Carolina and NIH/NIEHS for partial financial support (R01ES024950).
References
- Archibald FS, Duong MN. Manganese acquisition by Lactobacillus plantarum. J Bacteriol. 1984;158:1–8. doi: 10.1128/jb.158.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald FS, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Aschner JL. Manganese transport across the blood-brain barrier: relationship to iron homeostasis. Brain Res Bull. 1990;24:857–860. doi: 10.1016/0361-9230(90)90152-p. [DOI] [PubMed] [Google Scholar]
- Avila DS, Colle D, Gubert P, Palma AS, Puntel G, Manarin F, Noremberg S, Nascimento PC, Aschner M, Rocha JB. A possible neuroprotective action of a vinylic telluride against Mn-induced neurotoxicity. Toxicol Sci. 2010;115:194–201. doi: 10.1093/toxsci/kfq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560–2568. doi: 10.1111/j.1572-0241.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Barhoumi R, Faske J, Liu X, Tjalkens RB. Manganese potentiates lipopolysaccharide-induced expression of NOS2 in C6 glioma cells through mitochondrial-dependent activation of nuclear factor kappaB. Mol Brain Res. 2004;122:167–179. doi: 10.1016/j.molbrainres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Barrett E, Ross R, O’toole P, Fitzgerald G, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011a;141:599–609.e593. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park A, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett P, Fahnestock M, Moine D. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol Motil. 2011b;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112 e2101. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen B. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin Exp Immunol. 1999;118:235–241. doi: 10.1046/j.1365-2249.1999.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Ou YC, Lin SY, Liao SL, Chen SY, Chen JH. Manganese modulates pro-inflammatory gene expression in activated glia. Neurochem Int. 2006;49:62–71. doi: 10.1016/j.neuint.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Chen JY, Tsao GC, Zhao Q, Zheng W. Differential cytotoxicity of Mn (II) and Mn (III): special reference to mitochondrial [Fe-S] containing enzymes. Toxicol Appl Pharmacol. 2001;175:160–168. doi: 10.1006/taap.2001.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Bian X, Gao B, Ru H, Tu P, Lu K. Sex-Specific Effects of Arsenic Exposure on the Trajectory and Function of the Gut Microbiome. Chem Res Toxicol. 2016;29:949–951. doi: 10.1021/acs.chemrestox.6b00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, Maas K, Graf J. Gut Microbiome Developmental Patterns in Early Life of Preterm Infants: Impacts of Feeding and Gender. PLoS One. 2016;11:e0152751. doi: 10.1371/journal.pone.0152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004;17:544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ochoa VE, Jellbauer S, Klaus S, Raffatellu M. Transition metal ions at the crossroads of mucosal immunity and microbial pathogenesis. 2014 doi: 10.3389/fcimb.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T, Borre Y, Cryan J. Genomics of schizophrenia: time to consider the gut microbiome? Mol Psychiatry. 2014;19:1252–1257. doi: 10.1038/mp.2014.93. [DOI] [PubMed] [Google Scholar]
- Foster JA, Neufeld KAM. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269–R1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- Gao B, Bian X, Mahbub R, Lu K. Sex-specific effects of organophosphate diazinon on the gut microbiome and its metabolic functions. Environ Health Perspect. 2017;125:198. doi: 10.1289/EHP202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Ghaisas S, Maher J, Kanthasamy A. Gut microbiome in health and disease: Linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther. 2016;158:52–62. doi: 10.1016/j.pharmthera.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger J. Nutrition versus toxicology of manganese in humans: evaluation of potential biomarkers. Neurotoxicology. 1998;20:205–212. [PubMed] [Google Scholar]
- Groot MNN, Klaassens E, de Vos WM, Delcour J, Hols P, Kleerebezem M. Genome-based in silico detection of putative manganese transport systems in Lactobacillus plantarum and their genetic analysis. Microbiology. 2005;151:1229–1238. doi: 10.1099/mic.0.27375-0. [DOI] [PubMed] [Google Scholar]
- Guarner F, Malagelada JR. Gut flora in health and disease. The Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gavin CE, Aschner M, Gunter KK. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology. 2006;27:765–776. doi: 10.1016/j.neuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Haefele DM, Lindow SE. Flagellar motility confers epiphytic fitness advantages upon Pseudomonas syringae. Appl Environ Microbiol. 1987;53:2528–2533. doi: 10.1128/aem.53.10.2528-2533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Prokic E, McAllister C, Ronnqvist K, Williams A, Yamawaki N, Witton C, Woodhall G, Stanford I. GABA-mediated changes in inter-hemispheric beta frequency activity in early-stage Parkinson’s disease. Neuroscience. 2014;281:68–76. doi: 10.1016/j.neuroscience.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. 2016;160:127–133. doi: 10.1016/j.jsbmb.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubovics NS, Jenkinson HF. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology. 2001;147:1709–1718. doi: 10.1099/00221287-147-7-1709. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Kearns DB. A field guide to bacterial swarming motility. Nature Rev Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Amdur MO. Casarett and Doull’s toxicology: the basic science of poisons. McGraw-Hill; New York: 1996. [Google Scholar]
- Krishna S, Dodd CA, Hekmatyar SK, Filipov NM. Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water. Arch Toxicol. 2014;88:47–64. doi: 10.1007/s00204-013-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathwood PD. Tryptophan availability and serotonin synthesis, Serotonin. Springer. 1990:221–225. doi: 10.1079/pns19870018. [DOI] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Ryan PA, Schlieper KA, Graffam ME, Levine S, Wishnok JS, Swenberg JA, Tannenbaum SR, Fox JG. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect. 2014;122:284. doi: 10.1289/ehp.1307429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology. 2011;32:896–906. doi: 10.1016/j.neuro.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki EA. Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res Bull. 2001;55:225–228. doi: 10.1016/s0361-9230(01)00456-7. [DOI] [PubMed] [Google Scholar]
- Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler D. Neurotoxic exposures and effects: gender and sex matter! Hänninen Lecture 2011. Neurotoxicology. 2012;33:644–651. doi: 10.1016/j.neuro.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Meyer F, Paarmann D, D’Souza M, Olson R, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A. The metagenomics RAST server–a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol. 2009;240:219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monachese M, Burton JP, Reid G. Bioremediation and tolerance of humans to heavy metals through microbial processes: a potential role for probiotics? Appl Environ Microbiol. 2012;78:6397–6404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Streifel KM, Sullivan KA, Legare ME, Tjalkens RB. Developmental exposure to manganese (Mn) increases adult susceptibility to inflammatory activation of glia and neuronal protein nitration. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp221. kfp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld K, Kang N, Bienenstock J, Foster J. Reduced anxiety‐like behavior and central neurochemical change in germ‐free mice. Neurogastroenterol Motil. 2011;23:255–e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- O’Mahony S, Clarke G, Borre Y, Dinan T, Cryan J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Pandey G, Jain RK. Bacterial chemotaxis toward environmental pollutants: role in bioremediation. Appl Environ Microbiol. 2002;68:5789–5795. doi: 10.1128/AEM.68.12.5789-5795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Collins J, Blennerhassett P, Ghia J, Verdu E, Bercik P, Collins S. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733–e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaney SH, Bench G, Smith DR. Brain accumulation and toxicity of Mn (II) and Mn (III) exposures. Toxicol Sci. 2006;93:114–124. doi: 10.1093/toxsci/kfl028. [DOI] [PubMed] [Google Scholar]
- Reaney SH, Kwik-Uribe CL, Smith DR. Manganese oxidation state and its implications for toxicity. Chem Res Toxicol. 2002;15:1119–1126. doi: 10.1021/tx025525e. [DOI] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. The FASEB Journal. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol. 2003;66:1–13. doi: 10.1016/s0006-2952(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequerra E, Gardino P, Hedin-Pereira C, De Mello F. Putrescine as an important source of GABA in the postnatal rat subventricular zone. Neuroscience. 2007;146:489–493. doi: 10.1016/j.neuroscience.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014;20:719–730. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Paul D, Jain RK. Biofilms: implications in bioremediation. Trends Microbiol. 2006;14:389–397. doi: 10.1016/j.tim.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour–epigenetic regulation of the gut–brain axis. Genes, Brain and Behav. 2014;13:69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- Takeno S, Sakai T. Involvement of the intestinal microflora in nitrazepam‐induced teratogenicity in rats and its relationship to nitroreduction. Teratology. 1991;44:209–214. doi: 10.1002/tera.1420440209. [DOI] [PubMed] [Google Scholar]
- Upreti R, Shrivastava R, Chaturvedi U. Gut microflora & toxic metals: chromium as a model. Indian J Med Res. 2004;119:49–59. [PubMed] [Google Scholar]
- Webb SM, Dick GJ, Bargar JR, Tebo BM. Evidence for the presence of Mn (III) intermediates in the bacterial oxidation of Mn (II) Proc Natl Acad Sci U S A. 2005;102:5558–5563. doi: 10.1073/pnas.0409119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5:e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younan S, Sakita GZ, Albuquerque TR, Keller R, Bremer-Neto H. Chromium (VI) bioremediation by probiotics. J Sci Food Agric. 2016;96:3977–3982. doi: 10.1002/jsfa.7725. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhou Z, Fu J. Effect of manganese chloride exposure on liver and brain mitochondria function in rats. Environ Res. 2003;93:149–157. doi: 10.1016/s0013-9351(03)00109-9. [DOI] [PubMed] [Google Scholar]
- Zheng W, Ren S, Graziano JH. Manganese inhibits mitochondrial aconitase: a mechanism of manganese neurotoxicity. Brain Res. 1998;799:334–342. doi: 10.1016/s0006-8993(98)00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–132. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


