Summary
Light affects sleep/wake behaviors by providing an indirect cue that entrains circadian rhythms and also by inducing a direct and rapid regulation of behavior. While circadian entrainment by light is well characterized at the molecular level, mechanisms that underlie the direct effect of light on behavior are largely unknown. In zebrafish, a diurnal vertebrate, we found that both overexpression and mutation of the neuropeptide prokineticin 2 (Prok2) affect sleep/wake behaviors in a light-dependent but circadian-independent manner. In light, Prok2 overexpression increases sleep and induces expression of galanin (galn), a hypothalamic sleep-inducing peptide. We also found that light-dependent, Prok2-induced sedation requires prokineticin receptor 2 (prokr2) and is strongly suppressed in galn mutants. These results suggest that Prok2 antagonizes the direct wake-promoting effect of light in zebrafish, in part through the induction of galn expression in the hypothalamus.
Keywords: prokineticin 2, galanin, sleep, circadian rhythm, masking, zebrafish
eTOC Blurb
Chen et al. show that Prok2 regulates zebrafish sleep/wake behaviors in a light-dependent but circadian-independent manner, and that Prok2-induced sleep requires the neuropeptide galn. These results suggest that Prok2 antagonizes the direct effect of light on sleep via a known sleep-promoting center.
Introduction
A classical model postulates that sleep is regulated by homeostatic and circadian processes (Borbely, 1982). First, homeostatic pressure increases during wakefulness and dissipates during sleep in response to internal cues. Second, circadian entrainment by environmental cues ensures that sleep occurs at the appropriate time during the 24-hour day/night cycle. Sleep/wake behaviors can also be directly and rapidly affected by light, a phenomenon known as “masking” or as “direct effects” of light and dark (Hubbard et al., 2013). Thus, light exposure at night promotes arousal in diurnal animals, including humans (Lockley et al., 2006), and sleep in nocturnal animals (Altimus et al., 2008; Lupi et al., 2008; Tsai et al., 2009). Conversely, exposure to dark during the day inhibits arousal in diurnal animals (Gander and Moore-Ede, 1983) and sleep in nocturnal rodents (Altimus et al., 2008; Tsai et al., 2009). Several factors have been implicated in the homeostatic process (Brown et al., 2012), and the circadian clock mechanism has been well-described (Hastings and Herzog, 2004), with melatonin required for circadian gating of sleep in a diurnal animal (Gandhi et al., 2015). While the photoreceptors mediating the direct effect of light on sleep in mammals have been identified (Altimus et al., 2008; Hattar et al., 2003; Lupi et al., 2008; Tsai et al., 2009), little is known about downstream mechanisms.
While factors that mediate the direct effect of light on behavior are unknown, several factors have been proposed to transmit circadian information from master circadian pacemakers such as the mammalian suprachiasmatic nucleus (SCN) to other tissues (Li et al., 2012). However, these factors have mostly been studied in nocturnal rodents and it is unknown if they function similarly in diurnal animals. One of these factors, prokineticin 2 (Prok2), has been implicated in diverse functions, including neurogenesis, angiogenesis and nociception (Gardiner et al., 2010; LeCouter et al., 2001; Li et al., 2001; Ng et al., 2005). Loss of prok2 in humans and rodents results in Kallmann syndrome, which is characterized by hypogonadism and defects in olfactory bulb development (Cole et al., 2008; Dode et al., 2006; Matsumoto et al., 2006; Pitteloud et al., 2007). Several lines of evidence suggest that Prok2 also plays a role in regulating sleep in nocturnal rodents. First, prok2 mRNA levels oscillate in a circadian manner in the rodent SCN (Cheng et al., 2005; Cheng et al., 2002). Second, intracerebroventricular (ICV) injection of Prok2 at night, the active phase of nocturnal rodents, suppresses locomotor activity (Cheng et al., 2002), while mice that lack prok2 or prokineticin receptor 2 (prokr2) exhibit attenuated circadian rhythms of locomotor activity, thermoregulation, and circulating corticosteroid and glucose levels (Li et al., 2006; Prosser et al., 2007). Interestingly, loss of prok2 in mice reduces daytime sleep and sleep recovery after deprivation (Hu et al., 2007), suggesting a direct role for prok2 in regulating sleep. In contrast to mice, a study of humans with a prok2 loss of function mutation found no abnormalities in several processes regulated by the circadian clock (Balasubramanian et al., 2014).
The zebrafish, a diurnal vertebrate, exhibits behavioral, genetic, anatomical and pharmacological conservation of mammalian sleep (Oikonomou and Prober, 2017). To explore the function of Prok2 in regulating behavior in a diurnal vertebrate, here we analyze the effects of Prok2 gain- and loss-of-function perturbations on sleep/wake behaviors in larval and adult zebrafish. Surprisingly, we found that Prok2 affects behavior in a light-dependent but circadian-independent manner. We also found that Prok2 overexpression induces expression of galanin (galn), a sleep-promoting neuropeptide, in a light-specific manner in the hypothalamus, and that Prok2-induced sedation in light is suppressed in galn mutant animals. These results suggest that Prok2 is not an output of the circadian clock in zebrafish but instead antagonizes the direct effect of light on behavior, doing so in part by inducing galn expression in the hypothalamus.
Results
prok2 is expressed in the larval zebrafish ventral hypothalamus but does not co-localize with orthologs of genes expressed in the mammalian SCN
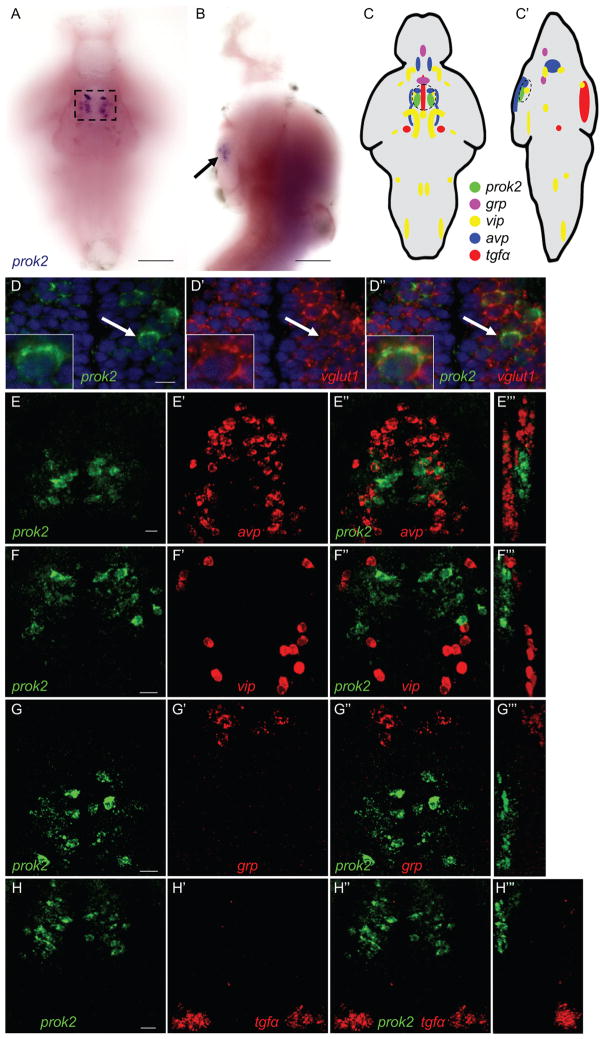
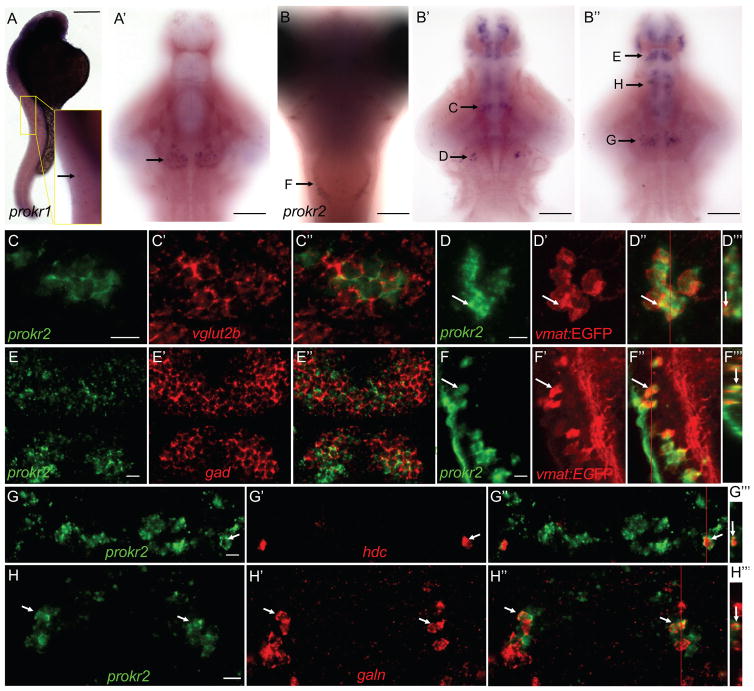
A previous study identified a single zebrafish prok2 ortholog (Ayari et al., 2010) but its expression in larvae was not described. Using in situ hybridization (ISH), we found that prok2 is exclusively expressed in a bilateral cluster of neurons in the ventral hypothalamus at 5-days post-fertilization (dpf) (Figures 1A–1C′). Each cluster consists of ~10 neurons and is located adjacent to the optic chiasm, a location similar to the mammalian SCN. Double fluorescent ISH (FISH) revealed that all prok2-expressing neurons express vesicular glutamate transporter 1 (vglut1), and thus are glutamatergic (Figures 1D––1D″).
Figure 1. prok2 expression in the larval zebrafish brain.
(A, B) prok2 mRNA (black box and arrow) is expressed in a bilateral cluster of ~10 neurons in the ventral hypothalamus at 5 dpf. Dashed box in (A) indicates the region shown in (D). In (B), only the purple stain indicated by the arrow near the ventral brain surface is specific staining. (C, C′) Schematic drawings summarize gene expression patterns from (E–H), as well as brain regions not shown in those panels. Dashed black circles indicate anatomical region potentially analogous to the mammalian SCN. (D) prok2-expressing neurons co-express vglut1. DAPI nuclear stain is shown in blue. A representative cell, indicated by a white arrow, is enlarged in the inset. A 0.46 μm confocal section is shown. (E–H) In the ventral hypothalamus, prok2 is expressed caudal and dorsal to populations that express avp (E), and caudal to cells that express vip (F). grp (G) and tgfα (H) are not expressed in the ventral hypothalamus. 34 μm (E–G) and 60 μm (H) thick confocal projections are shown. (E‴, F‴, G‴ and H‴) show orthogonal projections of each confocal stack. All samples are at 5 dpf. Ventral views with rostral at top (A, C–H, D′-H′, D″-H″) and lateral views with rostral at top and dorsal at right (B, C′, E‴-H‴) are shown. Scale bar: (A, B) 100 μm, (D–H) 10 μm. See also Figure S1.
Because prok2 is highly expressed in the rodent SCN and has been proposed to act as a circadian output factor, we compared the expression of prok2 in larval zebrafish with that of zebrafish orthologs of mammalian SCN markers using double FISH. In mammals, vasoactive intestinal peptide (vip)- and gastrin-releasing peptide (grp)-expressing neurons are found in the core of the SCN, while arginine vasopressin (avp)-expressing neurons are found in the shell of the SCN (Hastings and Herzog, 2004). Transforming growth factor alpha (tgfα) is expressed throughout the SCN but is more abundant in the core (Li et al., 2012). In the rat, prok2 is expressed in both the shell and core of the SCN, and over 50% of prok2-expressing neurons also express vip, grp or avp (Masumoto et al., 2006). In zebrafish, avp is expressed in the ventral hypothalamus rostral to prok2, and in cells ventral to prok2 (Figures 1E––1E‴). avp is also expressed in the neurosecretory preoptic area (NPO) (Figures 1C and 1C′), which corresponds to the mammalian paraventricular nucleus of the hypothalamus (Herget and Ryu, 2015). vip is expressed in cells just rostral to prok2, as well as in nuclei in the diencephalon, midbrain and hindbrain (Figures 1F––1F‴, 1C and 1C′). grp is expressed near the NPO avp-expressing neurons, but rostral and dorsal to prok2 (Figures 1G––1G‴, 1C and 1C′). tgfα is expressed in the midbrain (Figures 1H––1H‴) and along the diencephalic ventricle (Figures 1C and 1C′), but not in the ventral hypothalamus. Thus, three of four mammalian SCN markers are expressed in the ventral hypothalamus close to the optic chiasm in larval zebrafish. However, zebrafish prok2-expressing neurons are glutamatergic, whereas most mammalian SCN neurons are GABAergic (Moore and Speh, 1993), and prok2 is not co-expressed with mammalian SCN marker genes in larval zebrafish. These observations suggest that this brain region may have different functions in larval zebrafish and mammals.
prok2 expression does not oscillate in a circadian manner in larval zebrafish
prok2 mRNA oscillates in a circadian manner in rodents (Cheng et al., 2005; Cheng et al., 2002). To test whether prok2 expression similarly oscillates in zebrafish larvae, we entrained larvae to a 14:10 hour light:dark (LD) cycle until 6 dpf and quantified prok2 mRNA using quantitative reverse-transcription PCR (qRT-PCR). In contrast to rodents, we did not observe a significant change in prok2 mRNA level, whereas expression of the circadian clock gene period3 oscillated in a circadian manner (Figure S1A), as expected. Consistent with this result, we did not observe circadian changes in prok2 expression by FISH (Figures S1B and S1C). Circadian gene expression often results from the binding of CLOCK/BMAL1 heterodimers to E-box motifs (Hastings and Herzog, 2004). A 2.4 kb genomic region directly upstream of the mouse prok2 gene contains 4 copies of the canonical E-box motif CACGTG (Cheng et al., 2002), but this motif is absent within 5 kb upstream of the zebrafish prok2 gene, which may explain the lack of circadian oscillations in prok2 expression in zebrafish. Thus, in contrast to rodents, prok2 expression does not oscillate in a circadian manner in zebrafish larvae. Because prok2 expression is influenced by light in rodents (Cheng et al., 2005), we quantified prok2 mRNA in larvae raised in constant light (LL) compared to those raised in constant dark (DD) (Figures S1D and S1E) and in larvae exposed to changes in lighting conditions (Figure S1G). We did not observe significant changes in prok2 mRNA level under any of these conditions. Finally, changes in lighting conditions did not induce expression of c-fos, a marker of neuronal activity, in prok2-expressing neurons (Figures S1I-S1L). This result suggests that changes in lighting conditions do not activate prok2-expressing neurons, although c-fos expression is not always induced in activated neurons (Cruz et al., 2013).
Prok2-overexpressing zebrafish larvae are less active and sleep more during the day
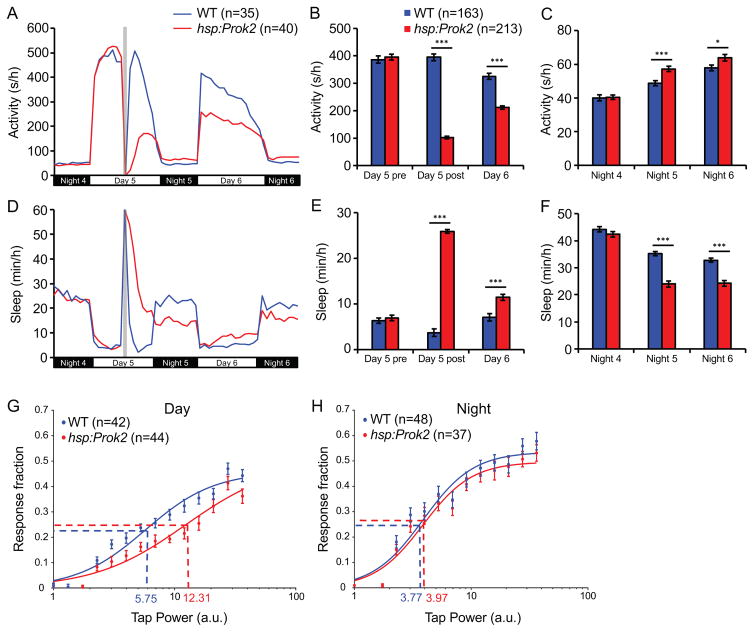
A previous study showed that ICV infusion of in vitro synthesized Prok2 protein in nocturnal rats at night, when they are active, suppresses locomotor activity (Cheng et al., 2002). Since prok2 expression levels in the SCN are high during the day, the inactive phase of nocturnal rodents, this result could mean that Prok2 normally acts as a signal to promote day-specific behaviors. If this “day-specific” hypothesis is correct, Prok2 overexpression should inhibit sleep in diurnal animals. Alternatively, Prok2 may act as a signal for behaviors specific for the inactive phase of the circadian period. If this “inactive phase” hypothesis is correct, Prok2 overexpression should promote sleep in diurnal animals. To distinguish between these possibilities, we generated transgenic zebrafish in which a heat shock inducible promoter regulates expression of the zebrafish Prok2 ortholog (Tg(hsp:Prok2)) and assayed effects of Prok2 overexpression on locomotor behaviors (Prober et al., 2006). Heat shock resulted in high levels of prok2 mRNA throughout the brain at 1 hour post-heat shock, with reduced levels at 4 hours post-heat shock and a return to baseline levels by 9 hours post-heat shock (Figure S2A). We generated an antibody specific for zebrafish Prok2, which revealed high levels of Prok2 protein throughout the brain at 4-hours post heat shock that remained elevated for up for to 48 hours (Figure S2B). We did not observe any differences in locomotor activity or sleep between Tg(hsp:Prok2) larvae and their wild type (WT) siblings before heat shock (Figures 2A–2F). However, after heat shock, Tg(hsp:Prok2) larvae were less active and slept more during the day (Figures 2A, 2B, 2D and 2E). This phenotype was caused by an increase in both the number and length of sleep bouts, with a corresponding decrease in the length of wake bouts (Figures S2D–S2F). This result suggests that Prok2 in zebrafish larvae acts as a signal to promote inactivity and/or sleep, consistent with the “inactive phase” hypothesis, rather than as a signal for day-specific behaviors.
Figure 2. Prok2 overexpression increases sleep during the day and decreases sleep at night.
Heat shock (gray bar in (A, D)) during the day decreases locomotor activity during the day (A, B) and increases locomotor activity at night (A, C) for Tg(hsp:Prok2) larvae compared to their WT siblings. Prok2 overexpression also increases sleep during the day (D, E) and decreases sleep at night (D, F). These phenotypes were observed for over 36 hours after heat shock. Data from one representative experiment (A, D) and five experiments combined (B, C, E, F) are shown. Bar graphs show mean ± SEM. n indicates number of larvae analyzed. *p<0.05, ***p<0.001 by two-tailed Student’s t test. (G, H) Stimulus-response curve for Tg(hsp:Prok2) larvae compared to their WT siblings during the day (G) and at night (H) following heat shock during the day. Each data point represents mean ± SEM. Dashed lines mark the ETP50 value for each genotype. During the day (G), Tg(hsp:Prok2) larvae have an ETP50 value of 12.31 compared to 5.75 for WT (114.1% increase, p<0.01 by extra sum-of-squares F test). At night (H), Tg(hsp:Prok2) larvae have an ETP50 value of 3.77 compared to 3.97 for WT (5.3% increase, p=0.66 by extra sum-of-squares F test). n = number of larvae. See also Figure S2.
Surprisingly, Prok2 overexpression also increased locomotor activity and decreased sleep at night (Figures 2A, 2C, 2D and 2F), the opposite of the day phenotype. The night phenotype was caused by a decrease in the length of sleep bouts, with a trend towards longer wake bouts (Figures S2H and S2I), indicating that Prok2 function cannot be explained by the “inactive phase” model alone. Consistent with this observation, ICV infusion of Prok2 at night in nocturnal rodents decreased locomotor activity at night but also increased activity the following day (Cheng et al., 2002). The opposite phenotype during the day was hypothesized to be an indirect consequence of Prok2 signaling at night, perhaps due to desensitization of Prok2 receptors or a “rebound” in activity following a night of decreased activity. However, it is possible that Prok2 signaling directly promotes opposite sleep/wake phenotypes during the day and night, thus acting as a “balancing factor” (Berridge, 2004) to maintain appropriate sleep amounts during the day and night. To distinguish between these hypotheses, we repeated the behavioral experiment but performed the heat shock at night. Following a short recovery period after the heat shock, Prok2-overexpressing larvae were more active and slept less at night (Figures S2J, S2L, S2M and S2O). Similar to the daytime heat shock results, Prok2-overexpressing larvae showed the opposite phenotype the following day (Figures S2J, S2K, S2M and S2N). These results suggest that Prok2 overexpression directly induces opposite sleep/wake phenotypes during the day and night.
Prok2 overexpression decreases arousal threshold during the day
Because Prok2 overexpression affects sleep, we asked whether it also alters arousal threshold. To do so, we monitored behavioral responses to a mechano-acoustic stimulus applied over a range of intensities (Singh et al., 2015) during the day and night after heat shock. When the assay was performed during the day following heat shock, the effective tap intensity at which we observed the half maximal response (effective tap power 50, ETP50) was significantly higher for Tg(hsp:Prok2) larvae than their WT siblings (Figure 2G). This result indicates that Prok2 overexpression increases arousal threshold during the day. In contrast, we did not observe a significant change in ETP50 between the two genotypes during the night following heat shock (Figure 2H), indicating that Prok2 overexpression does not affect arousal threshold at night. However, because the Prok2 overexpression phenotype is weaker at night than during the day, it is possible that an effect on arousal at night was too subtle to detect using this assay.
The Prok2 overexpression phenotype depends on light and not on circadian rhythms
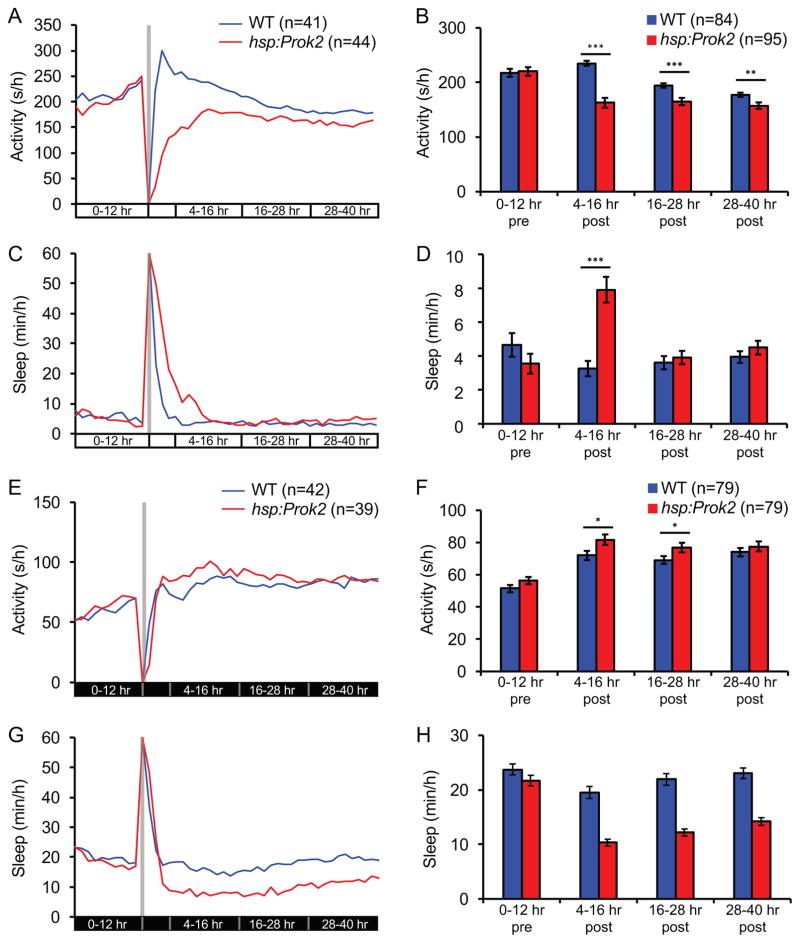
The opposite sleep/wake phenotypes induced by Prok2 overexpression during the day and night might result from effects of the circadian clock or lighting conditions on Prok2 function. We used three strategies to distinguish between these possibilities. First, we raised and tested larvae in either LL or DD from birth, both of which result in larvae that lack overt entrained circadian rhythms (Hurd and Cahill, 2002). In LL, Prok2 overexpression decreased locomotor activity for at least 36 hours and increased sleep for 12 hours (Figures 3A–3D). The lack of change in sleep after 12 hours could be due to a floor effect because both Tg(hsp:Prok2) and WT larvae slept very little in LL. We observed the opposite phenotype in DD; Prok2 overexpression increased locomotor activity for 24 hours and decreased sleep for at least 36 hours (Figures 3E–3H). Heat shock did not induce circadian oscillations in per3 expression in animals raised in either LL or DD (Figures S3A–S3C), suggesting that the heat shock did not induce or synchronize circadian rhythms in these animals. These results suggest that lighting condition, and not circadian rhythm, determines the behavioral effect of Prok2 overexpression.
Figure 3. The Prok2 overexpression phenotype depends on lighting condition.
(A–D) Following heat shock at 5 dpf (gray bar in (A, C), larvae raised and tested in LL are less active for at least 36 hours (A, B) and sleep more for up to 16 hours (C, D) than WT siblings. (E–H) Following heat shock at 5 dpf (gray bar in (E, G)), larvae raised and tested in DD are more active for up to 28 hours (E, F) and sleep less for at least 36 hours (G, H) than WT siblings. Data from one representative experiment (A, C, E, G) and two experiments combined (B, D, F, H) are shown. Bar graphs show mean ± SEM. n = number of larvae. *p<0.05, **p<0.01, ***p<0.001 by two-tailed Student’s t test. Note that (B, D, F, H) exclude the first four hours after heat shock to allow larvae to recover from the heat shock. See also Figure S3.
Second, we entrained larvae in 14:10 hour LD, performed a heat shock to induce Prok2 overexpression during the day, and then monitored behavior in either LL or DD. In these conditions, circadian rhythms persist following the shift from LD to either LL or DD. If the effects of Prok2 overexpression depend on the circadian clock, opposite sleep/wake phenotypes should be observed during subjective day and subjective night. However, if the phenotype depends on lighting condition, larvae should exhibit light-specific behaviors in LL and dark-specific behaviors in DD. We observed the latter result (Figures S3D–S3O), consistent with the hypothesis that lighting condition determines the behavioral effect of Prok2 overexpression.
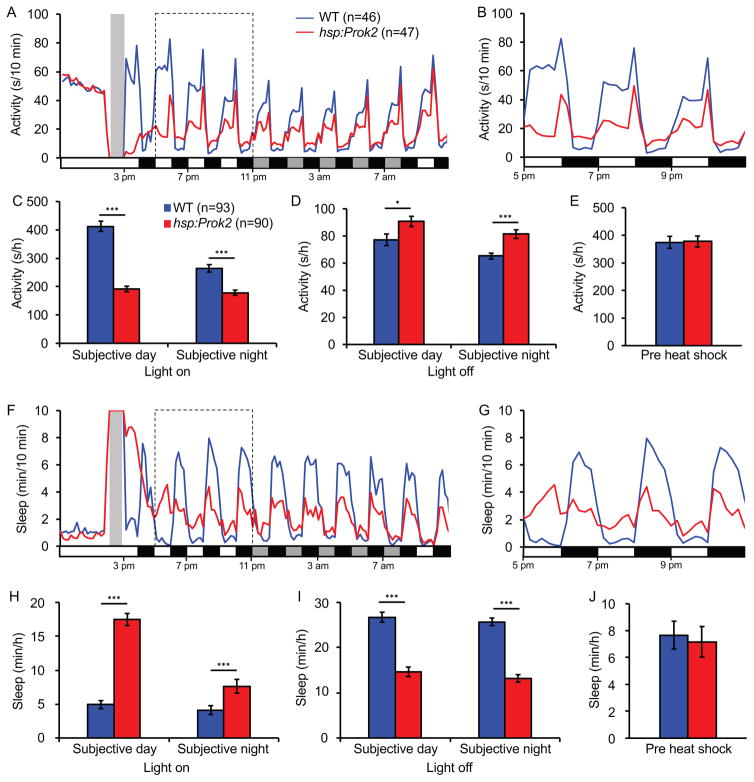
Third, we entrained larvae in 14:10 hour LD and then subjected them to alternating 1 hour periods of light and dark during the day and night following heat shock (Figure 4). WT larvae were more active and slept less during light periods than during dark periods, as expected due to the direct effects of light and dark on behavior. Strikingly, Prok2-overexpressing larvae were less active and slept more than WT during light periods, and were more active and slept less than WT during dark periods. These differences were apparent during both subjective day and subjective night. These results indicate that Prok2 overexpression antagonizes the direct effects of both light and dark on behavior, consistent with the hypothesis that the behavioral effects of Prok2 overexpression are determined by lighting condition.
Figure 4. Prok2 overexpression results in behavior that opposes the direct effect of light.
Larvae were entrained in 14:10 hour LD for 5 days and then subjected to alternating 1 hour light and dark periods following heat shock at 5 dpf (gray bar in (A, F)). During light periods, Tg(hsp:Prok2) larvae are less active (A–C) and sleep more (F–H) than WT siblings. During dark periods, Prok2 overexpressing larvae are more active (A, B, D) and sleep less (F, G, I) than WT siblings. During the day prior to heat shock, transgenic and WT larvae show similar levels of activity (E) and sleep (J). Data from one representative experiment (A, B, F, G) and two experiments combined (C–E, H–J) are shown. In (A, F), white and black boxes along x-axes indicate 1-hour periods of light and dark, and gray boxes indicate periods of light during subjective night. Bar graphs show mean ± SEM. n = number of larvae. *p<0.05, ***p<0.001 by two-tailed Student’s t test. See also Figure S4.
To test whether these phenotypes are specific to the larval stage of zebrafish development, we assayed the effect of Prok2 overexpression in adult zebrafish. We exposed adult Tg(hsp:Prok2) fish and their WT siblings to temperatures up to 37°C overnight (increased slowly over 9 hours to avoid lethality) and monitored their behavior during alternating 30 minute periods of light and dark during the following day (Figure S4A). As in larvae, adult WT zebrafish were more active in light than in dark, and Prok2 overexpression suppressed activity in light and enhanced activity in dark (Figures S4B–S4E). In particular, Prok2-overexpressing fish spent more time at lower swim speeds in light and higher swim speeds in dark compared to their WT siblings (Figures S4D–S4E). Because the light effect was strongest in the initial minutes following each dark to light transition (Figure S4B), we performed a second overnight heat shock experiment followed by continued light exposure (Figures S4F–S4H). Similar to larvae, Prok2 overexpression in adults suppressed locomotor activity during 10 hours of light exposure (Figure S4G), in particular by shifting swim speeds to lower values (Figure S4H). Taken together, these results suggest that the opposite effects of Prok2 overexpression on behavior during the day and night are due to lighting condition rather than interaction with the circadian system or developmental stage.
prok2 mutant zebrafish lack overt developmental defects
To examine the function of endogenous prok2 in a diurnal animal, we generated two independent prok2 mutant zebrafish lines (Figure 5A). Both predicted mutant proteins lack several conserved domains, including 5 of 10 conserved cysteine residues and the peptide proteolytic cleavage site, and thus are likely non-functional (Bullock et al., 2004). prok2 mutant mice exhibit a high rate of embryonic and neonatal lethality, and surviving animals are hypoactive and have defective olfactory, reproductive and gonadotropin releasing hormone (GnRH) neuron development (Dode et al., 2006; Ng et al., 2005; Pitteloud et al., 2007). In contrast, prok2−/− zebrafish are viable, fertile and comparable in appearance to their prok2+/− and WT siblings (Figure S5B). Furthermore, a prok2+/− incross generated progeny of the expected Mendelian ratio (12/45 prok2+/+, 22/45 prok2+/− and 11/45 prok2−/−). To test whether the zebrafish mutant has developmental defects similar to the mouse mutant, we performed ISH using markers for the olfactory system (tyrosine hydroxylase 1 (th1) and empty spiracles homeobox 1 (emx1)), and the two zebrafish gnrh paralogs, gnrh2 and gnrh3. We failed to detect abnormal expression of these markers, suggesting that the olfactory system and gnrh-expressing neurons develop normally in zebrafish prok2−/− larvae (Figure S5C). This result accords with our observation that prok2 is only expressed in the larval zebrafish ventral hypothalamus (Figures 1A and 1B), whereas in rodents prok2 is expressed in many other brain regions (Cheng et al., 2002). We also did not observe differences in the expression level or pattern of three hypothalamic genes (hypocretin (hcrt) (Prober et al., 2006), qrfp (Chen et al., 2016), and prok2), or in the number of cells expressing these genes, in prok2−/− larvae compared to sibling controls (Figure S5D), suggesting that hypothalamus development is normal in prok2−/− larvae.
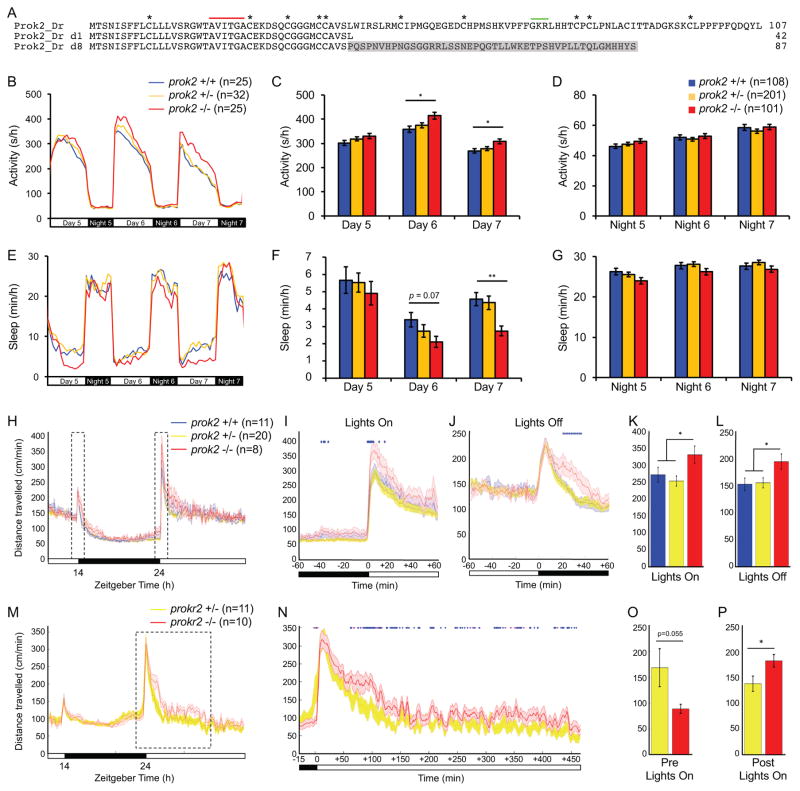
Figure 5. prok2 mutant larvae and prokr2 mutant adults are more active during the day.
(A) Amino acid sequences of two zebrafish Prok2 mutant proteins (d1 and d8) compared to WT. Asterisks mark conserved cysteine residues. Red line indicates a region conserved in human, rodent, frog and zebrafish. Green line indicates putative peptide cleavage site. Gray shading indicates altered sequence in mutant d8. Both mutants exhibited similar phenotypes and d8 was used for all reported experiments. (B–G) During the day at 6 and 7 dpf, prok2−/− larvae are more active (B, C) and sleep less (E, F) than their prok2+/− and prok2+/+ siblings. Data from one representative experiment (B, E) and five experiments combined (C, D, F, G) are shown. Bar graphs show mean ± SEM. (H–L) prok2−/− adults are more active at dawn and twilight. (H) 24-hour time course of mean ± SEM distance traveled, averaged over 3 days. Boxed regions are expanded to show dawn (I) and twilight (J). Mean ± SEM distance traveled during the first hour of light at dawn (K) and the first hour of dark at twilight (L). (M–P) prokr2−/− adults are more active during the day and show a trend of less activity before dawn. (M) 24-hour time course of mean ± SEM distance traveled, averaged over 2 days. The boxed region is expanded in (N). The data are smoothed over a 5-minute sliding window. Mean ± SEM distance traveled during 15 minutes of dark before dawn (O) and 450 minutes of light after dawn (P). Females and prokr2+/+ animals were not analyzed because too few such individuals were present in the dataset to allow statistically robust analysis. In (I, J, N), blue and magenta diamonds indicate one-minute periods for which p<0.05 or 0.05<p<0.07, respectively (repeated measures ANOVA). n = number of animals. *p<0.05 and **p<0.01 by one-way ANOVA with post-hoc Tukey’s test (K, L) or Dunnett’s test for comparisons to WT (C, F). See also Figure S5.
prok2 mutants sleep less and are more active in a light-dependent manner
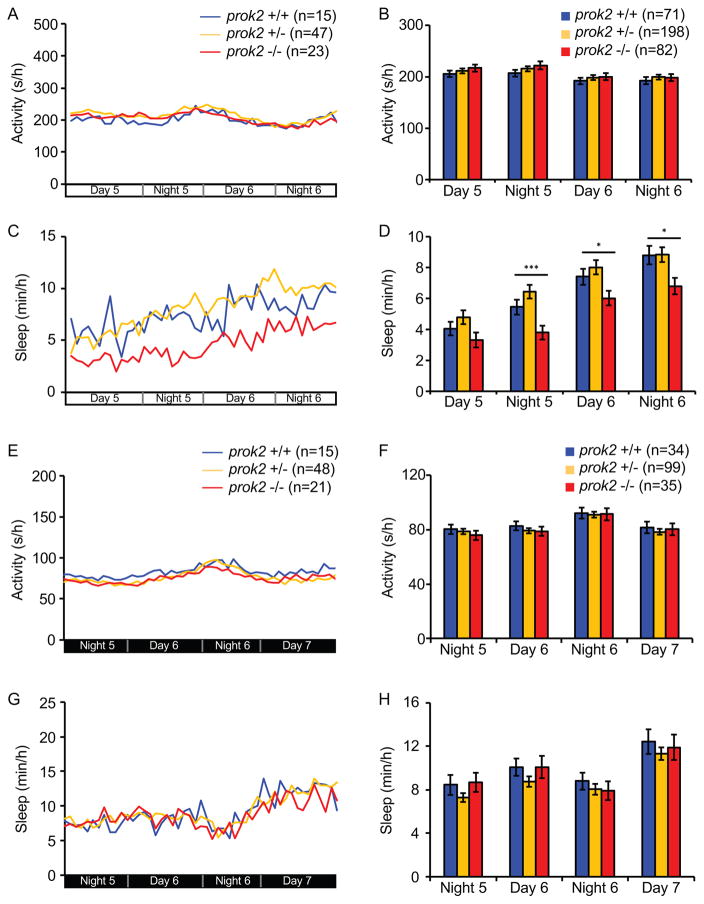
To test whether prok2 is required for normal sleep/wake behaviors in larval zebrafish, we compared the behavior of prok2−/− larvae to their prok2+/− and prok2+/+ siblings. Larvae of all three genotypes showed similar amounts of locomotor activity and sleep at night (Figures 5B, 5D, 5E and 5G). However, prok2−/− larvae were more active and slept less compared to sibling controls during the day at 6 and 7 dpf (Figures 5B, 5C, 5E and 5F). The effect on sleep was particularly striking, with a ~40% decrease at 6 and 7 dpf. The phenotype was due to fewer sleep bouts, with little or no effect on the length of sleep or wake bouts (Figures S5E, S5G and S5I). These results indicate that Prok2 is required for normal daytime sleep/wake levels in zebrafish larvae.
To determine whether the prok2−/− phenotype is specific to the circadian day or light, we raised and tested prok2−/− larvae and their sibling controls in LL or DD from birth. prok2−/− larvae slept significantly less than sibling controls in LL (Figures 6C and 6D), although there was no effect on locomotor activity (Figures 6A and 6B). In contrast, locomotor activity and sleep were indistinguishable among the three genotypes in DD (Figures 6E–6H). These results suggest that prok2 is required for normal sleep levels in a light-dependent manner in larval zebrafish.
Figure 6. prok2 mutant larvae sleep less when raised in constant light.
(A–D) When raised and tested in LL, prok2−/− larvae show similar locomotor activity (A, B) but significantly less sleep (C, D) than their prok2+/− and prok2+/+ siblings. (E–H) When raised and tested in DD, there is no significant difference in activity (E, F) or sleep (G, H) among the three genotypes. Data from one representative experiment (A, C, E, G) and two experiments combined (B, D, F, H) are shown. Bar graphs show mean ± SEM. n = number of larvae. *p<0.05 and ***p<0.001 by one-way ANOVA with post-hoc Dunnett’s test for comparisons to WT. See also Figure S5.
To test whether prok2 is differentially required for sleep/wake behaviors in larval and adult zebrafish, we assayed locomotor activity in prok2−/− adults. These animals were significantly more active than their prok2+/− and prok2+/+ siblings immediately after lights on in the morning (Figures 5H, 5I and 5K). prok2−/− adults were also more active than sibling controls during the first hour after lights off at night (Figures 5H, 5J and 5L). Thus, similar to prok2 mutant larvae, prok2 mutant adults show a light-dependent locomotor activity defect following transitions between light and dark.
prok2 is not required for circadian regulation of sleep/wake behaviors
To test whether prok2 is required for circadian regulation of locomotor activity and sleep in zebrafish, we entrained prok2−/− larvae and their sibling controls in 14:10 hour LD conditions for 5 days and then monitored their behavior in DD. The amount and timing of locomotor activity and sleep were indistinguishable for all three genotypes (Figures S5K–S5P), indicating that prok2 is not required for circadian regulation of locomotor activity or sleep in zebrafish larvae.
Zebrafish Prokr2 is expressed in brain regions that regulate sleep
Mammals have two putative Prok2 receptors, Prokr1 and Prokr2 (Lin et al., 2002). The zebrafish genome contains two Prokr paralogs, designated prokr1a and prokr1l (Figures S6A and S6B). Zebrafish Prokr1a protein is 62% and 59% identical to mouse Prokr1 and Prokr2, respectively. Zebrafish Prokr1l protein is 66% and 69% identical to mouse Prokr1 and Prokr2, respectively. Based on this and expression pattern similarities with mammals (see below), we refer to zebrafish prokr1a as prokr1 and prokr1l as prokr2.
prokr2 mRNA is widely expressed in the rodent brain, including regions that regulate sleep, such as the hypothalamus, periaqueductal gray, dorsal raphe and mammillary nuclei (Masumoto et al., 2006). prokr1 expression is more restricted, including the olfactory region, dentate gyrus, zona incerta and dorsal motor vagal nucleus (Masumoto et al., 2006). To determine the expression patterns of zebrafish Prok2 receptors, we performed ISH using animals fixed at 24-hours post-fertilization (hpf) and at 5 dpf. We found that prokr1 is expressed in a row of cells along the spinal cord at 24 hpf and in a bilateral cluster of cells in the ventral hypothalamus at 5 dpf (Figures 7A and 7A′). In contrast, prokr2 is expressed in several clusters of cells in the telencephalon, thalamus, hypothalamus, tuberomammillary nucleus, locus coeruleus and medulla oblongata (Figures 7B––7B″).
Figure 7. Expression patterns of prokr1 and prokr2 in larval zebrafish.
prokr1 is expressed in a row of cells in the spinal cord at 24 hpf (A, enlarged in inset), and in a bilateral cluster of cells in the ventral hypothalamus at 5 dpf (arrow in A′). Most of the embryo in (A) is overstained in order to see the weak prokr1-expressing cells in the spinal cord. prokr2 is expressed in discrete clusters of cells in the hindbrain (B), midbrain (B′, B″) and forebrain (B′, B″) at 5 dpf. Arrows indicate domains of prokr2-expressing cells that are shown at higher magnification in the indicated panels. Side (A), ventral (A′, B′, B″) and dorsal (B) views are shown, with rostral at top. (B′) and (B″) show dorsal and ventral optical sections, respectively. prokr2-expressing neurons express vesicular glutamate transporter 2b (vglut2b) in the thalamic region (C) and glutamate decarboxylase (gad65 and gad67 probes combined) in the forebrain (E). prokr2-expressing neurons in the locus coeruleus (D) and medulla oblongata (F) express vmat:EGFP, which labels noradrenergic neurons. prokr2 is expressed in a subset of hypothalamic neurons that express hdc (G) and galn (H). Images show 0.46 (C, E), 10 (D, F), 2.3 (G) and 2.9 (H) μm thick confocal projections. Ventral views are shown in (C–H, C′-H′, C″-H″). (D‴, F‴, G‴, H‴) show orthogonal views of (D″, F″, G″, H″) at positions indicated by red lines. Arrows in (D, F, G, H) indicate representative neurons. In (C–H), samples were fixed at 5 dpf and are oriented with rostral at top. Scale bar: (A, B) 100 μm, (C–H) 10 μm. See also Figures S6 and S7.
To characterize prokr2-expressing cells, we performed FISH using cell type-specific markers. We observed that prokr2-expressing cells in the telencephalon are GABAergic (Figures 7E––7E″), while a bilateral cluster in the thalamic region is glutamatergic (Figures 7C––7C″). All prokr2-expressing cells in the locus coeruleus and medulla oblongata express EGFP in ET(vmat2-EGFP) animals (Figures 7D––7D″, 7F–7F″), and thus produce norepinephrine (NE) (Wen et al., 2008). Approximately 30% (14/46 cells, n = 4 larvae) of histidine decarboxylase (hdc)-expressing cells in the tuberomammillary nucleus express prokr2, and thus produce histamine (HA) (Figures 7G––7G″). We also observed co-expression of prokr2 and galanin (galn) in the anterior hypothalamus. At least 38% (29/76 cells, n = 5 larvae) of galn-expressing cells in the anterior hypothalamus express prokr2 (Figures 7H––7H‴), although this is likely an underestimate because we only counted cells that strongly expressed both genes, and not cells that weakly expressed either gene. Thus, prokr2 is expressed in brain regions that are thought to regulate sleep and arousal.
The Prok2 overexpression phenotype requires Prokr2 but not Prokr1
Mammalian Prok2 has similar binding affinities for Prokr1 and Prokr2 in vitro (Lin et al., 2002) but roles for these receptors in Prok2 signaling in vivo have not been tested. To test whether these receptors are required for Prok2 signaling in vivo, we generated zebrafish with predicted null mutations in prokr1 and prokr2 (Figures S6A and S6B). Like the zebrafish prok2 mutant, prokr1 and prokr2 single and double mutant zebrafish are homozygous viable and fertile and lack overt developmental defects (Figures S5B and S5C). We found that the Prok2 overexpression phenotype was abolished in prokr2−/− larvae (Figures S7A–S7F), but was unaffected in prokr1−/− larvae (Figures S7G–S7L), indicating that Prok2 overexpression-induced behavioral effects require prokr2 but not prokr1. In contrast to zebrafish prok2 mutant larvae, prokr1 and prokr2 single and double mutant larvae exhibited normal sleep/wake behaviors (Figures S6C–S6T). However, similar to prok2−/− larvae and adults, prokr2−/− adults were more active during the day than their prokr2+/− siblings (p<0.05, repeated measures ANOVA), especially during the first seven hours of the morning (Figures 5M, 5N and 5P). prokr2−/− adults were also less active than sibling controls in dark at the end of the night, although the effect did not reach statistical significance (p=0.055, repeated measures ANOVA) (Figures 5M, 5N and 5O). Unlike prok2−/− adults, prokr2−/− adults did not exhibit a behavioral phenotype after lights off at night (Figure 5M). The prokr2−/− adult phenotype of less activity in dark and more activity in light is consistent with the more activity in dark and less activity in light observed in Prok2-overexpressing larvae and adults.
The Prok2 overexpression phenotype does not require several sleep regulatory pathways
Because prokr2 is expressed in NE- and HA-producing neurons, we hypothesized that Prok2 overexpression-induced phenotypes require NE or HA. To test this hypothesis, we overexpressed Prok2 in larvae containing a null mutation in dopamine beta hydroxylase (dbh), which lack NE and exhibit less activity and more sleep (Singh et al., 2015), and in hdc mutant larvae, which lack HA yet exhibit largely normal behavior (Chen et al., 2017). The Prok2 overexpression phenotype was additive with that of the dbh mutant (Figures S7M–S7R) and was unaffected in the hdc mutant (data not shown), suggesting that the Prok2 overexpression phenotype does not require NE or HA. We further tested whether the Prok2 overexpression phenotype requires melatonin synthesis, or hypocretin or glucocorticoid signaling, using aanat2 (Gandhi et al., 2015), hcrt receptor (Yokogawa et al., 2007) and glucocorticoid receptor (Ziv et al., 2013) mutants. We also tested a dopamine receptor 1 antagonist (Monti et al., 1990) and exogenous melatonin (Gandhi et al., 2015). None of these perturbations of sleep regulatory systems blocked the Prok2 overexpression phenotype (data not shown), suggesting that these pathways are not required for the effects of Prok2 overexpression. While it is possible that Prok2-induced phenotypes act via more than one of these pathways, these results suggest that Prok2 affects sleep via other mechanisms.
Prok2 overexpression increases hypothalamic galanin expression in the presence of light
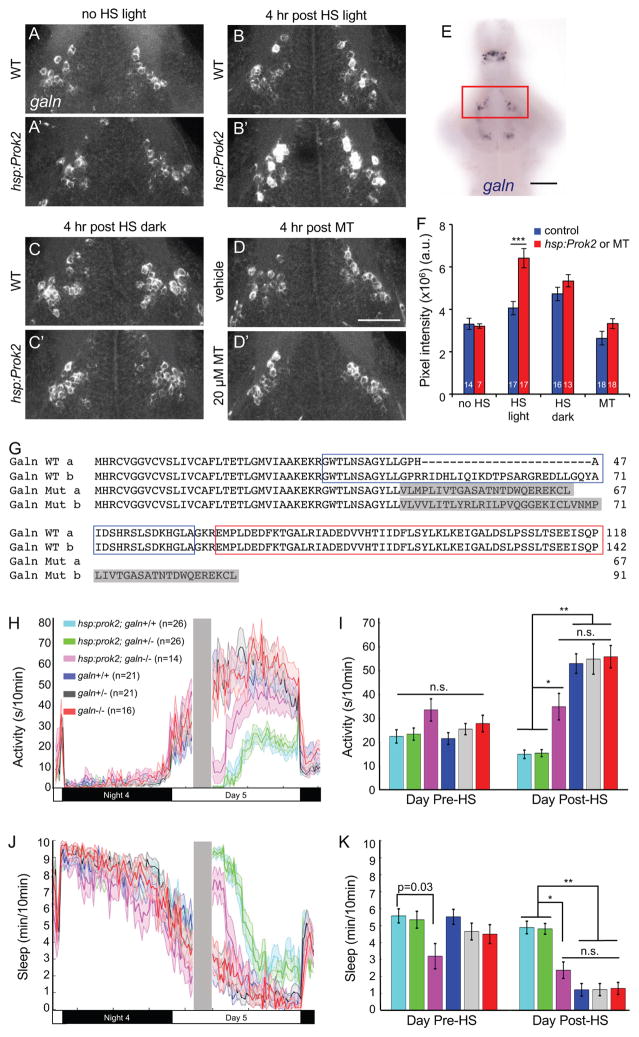
Because prokr2 and galn are co-expressed in the anterior hypothalamus (Figure 7H″), we hypothesized that Prok2 signaling interacts with Galn to affect behavior. Consistent with this hypothesis, we observed dense projections from prok2-expressing neurons in the ventral hypothalamus, near the location of the anterior hypothalamic galn-expressing neurons (Figure S8I). Galn is an inhibitory neuropeptide that is expressed in rodent ventrolateral preoptic area (VLPO) sleep-active neurons (Sherin et al., 1998) and is sufficient to induce sleep in zebrafish (Woods et al., 2014). Similar to rodents (Cheung et al., 2001), galn is expressed in several nuclei in the larval zebrafish brain, including an anterior hypothalamic population that may be a homolog of the mammalian VLPO (Figure 8E). To test whether Prok2 overexpression might induce daytime sleep via Galn, we compared galn expression in Tg(hsp:Prok2) larvae to their WT siblings by ISH during the day following heat shock. We observed a significant increase in galn expression in galn-expressing anterior hypothalamic neurons (Figures 8B, 8B′ and 8F), with no effect on forebrain galn-expressing neurons (data not shown) or on hypothalamic galn-expressing neurons in non-heat shocked animals (Figures 8A, 8A′ and 8F). Strikingly, we did not observe this effect if larvae were maintained in dark after heat shock (Figures 8C, 8C′ and 8F). Consistent with the requirement of prokr2 for Prok2 overexpression-induced sleep, the effect of Prok2 overexpression on galn mRNA level was abolished in prokr2−/− animals (Figures S8A and S8B). Consistent with the Prok2 overexpression behavioral phenotype in animals exposed to alternating 1 hour periods of light and dark, increased galn mRNA was observed following 1 hour of light, but not following 1 hour of dark (Figures S8C and S8D). Thus, increased hypothalamic galn expression is correlated with Prok2 overexpression-induced sleep in light, but not with Prok2 overexpression-induced arousal in dark.
Figure 8. Light-dependent sleep induction by Prok2 is correlated with increased galn expression and requires galn.
(A–D) 29 μm thick projections from representative brains showing galn expression in the anterior hypothalamus. Larvae were raised in LD until 5 dpf and then were not heat shocked (A, A′), heat shocked from 3–4 p.m. and kept in light (B, B′), or heat shocked from 3–4 p.m. and kept in dark (C, C′) and then fixed at 8 p.m. (D, D′) WT larvae were treated with either DMSO vehicle or 20 μM melatonin at 4 p.m. and fixed at 8 p.m. Ventral views with rostral at top are shown. (E) galn ISH in a 5 dpf brain. Red box indicates the area shown in (A–D) and quantified in (F). (F) Mean ± SEM fluorescence intensity of galn ISH. ***p<0.001 by two-way ANOVA for pair-wise comparisons of the mean of each control sample (blue) with its corresponding hsp:Prok2 or melatonin-treated sample (red), with Bonferroni correction for multiple comparisons. White numbers indicate number of animals. Scale bar: (AD) 50 μm, (E) 100 μm. (G) Amino acid sequences of WT and mutant zebrafish Galn (isoforms a and b). Blue and red boxes indicate Galn peptide and Galn message-associated peptide, respectively. Gray shading indicates altered amino acids in the mutant. (H–K) Larval progeny from a Tg(hsp:Prok2)/+; galn+/− to galn+/− mating were heat shocked (gray bar in (H, J)) during the day. This resulted in a significant decrease in locomotor activity (H, I) and increase in sleep (J, K) in Tg(hsp:Prok2)/+; galn+/+ and Tg(hsp:Prok2)/+; galn+/− animals compared to their non-transgenic galn−/−, galn+/− and galn+/+ siblings. This phenotype was significantly suppressed in Tg(hsp:Prok2)/+; galn−/− animals, particularly following 1 hour after heat shock, reaching non-transgenic control levels after 3 hours. Line and bar graphs show mean ± SEM from a representative of 4 experiments, with different heat shock times that preclude combining datasets. n.s. = not significant, *p<0.001, **p<1×10−10 by one-way ANOVA with post-hoc Tukey’s test. See also Figure S8.
To test whether increased galn expression is a consequence of increased sleep, we induced daytime sleep by treating WT larvae with melatonin. We found that melatonin had no significant effect on galn expression (Figures 8D, 8D′ and 8F), consistent with the hypothesis that increased galn expression is due to Prok2 overexpression and not simply to increased sleep. Taken together with the co-expression of prokr2 and galn, these results suggest that Prok2 signaling in the presence of light may directly induce hypothalamic galn expression, although indirect effects are also possible. Intriguingly, we observed fibers from prok2-expressing neurons in the optic tectum (Figures S8I and S8J), which receives direct visual input from the retina, providing a potential link between light, the Prok2 system and hypothalamic galn-expressing neurons.
In contrast to Prok2 overexpression, we observed no significant difference in galn mRNA level during either the day or night in prok2−/− larvae compared to their prok2+/− or prok2+/+ siblings (Figures S8E and S8F). This result suggests that galn expression is subject to compensation in response to chronic lack of Prok2 signaling in prok2 mutants, that the ISH assay is not sensitive enough to detect changes in galn mRNA level in prok2−/− larvae, which is already low during the day in WT siblings, or that the effect of Prok2 overexpression on galn mRNA is an overexpression artifact.
Prok2 overexpression-induced sleep is suppressed in galn mutants
Based on the correlation of Prok2 overexpression-induced sleep and increased galn expression in light, we hypothesized that increased galn expression underlies the sedating effect of Prok2 overexpression. If this hypothesis is correct, Prok2-induced sleep should be suppressed in galn mutant animals. To test this hypothesis, we generated zebrafish with a predicted null mutation in galn (Figure 8G). We mated Tg(hsp:Prok2); galn+/− animals to galn+/− and compared the behavior of their larval progeny before and after heat shock during the day (Figures 8H–8K). galn−/−, galn+/− and galn+/+ siblings that lacked the Tg(hsp:Prok2) transgene had statistically indistinguishable levels of locomotor activity both before and after heat shock. As expected, Prok2 overexpression decreased locomotor activity and increased sleep in Tg(hsp:Prok2); galn+/− and Tg(hsp:Prok2); galn+/+ larvae. During the first hour after heat shock, Tg(hsp:Prok2); galn−/− larvae also were less active and slept more, although the effect was smaller than that of Prok2-overexpressing larvae that had one or two functional copies of galn. However, by three hours after heat shock, the locomotor activity and sleep levels of Tg(hsp:Prok2); galn−/− larvae had returned to levels similar to those of non-transgenic animals, and remained statistically indistinguishable from non-transgenic animals for the rest of the day. These observations suggest that galn is required for the maintenance of Prok2 overexpression-induced sleep and also acts with other mechanisms to initiate this effect. Alternatively, it is possible that the very high Prok2 protein levels present for the first hour after heat shock affect behavior via a mechanism that is not physiologically relevant, and is thus not fully suppressed in galn−/− animals. In either case, our results suggest that Prok2 overexpression-induced sleep in light is at least partially mediated by upregulated galn expression in the hypothalamus.
Discussion
Prok2 regulates sleep/wake states in a light-dependent manner in zebrafish
Here we describe the first diurnal vertebrate genetic gain- and loss-of-function models for Prok2 and use them to investigate the function of Prok2 in regulating zebrafish sleep. Surprisingly, we found that Prok2 overexpression has opposite behavioral effects during the day and night. Prok2-overexpressing larvae are less active and sleep more during the day, whereas they are more active and sleep less at night. The day- and night-specific phenotypes are observed regardless of whether overexpression is induced during the day or night, suggesting they are not “rebound” effects resulting from the effect during the previous day or night. The lighting-specific phenotype is observed during both subjective day and subjective night after entrained larvae are shifted to LL or DD, and is also observed in animals that are raised and tested in either LL or DD, and thus lack overt entrained circadian rhythms. The phenotype is also observed during each light and dark period when Prok2-overexpressing larvae and adults are subjected to alternating short periods of light and dark. These results indicate that the Prok2 overexpression phenotype depends on lighting conditions and not on circadian rhythms, suggesting that Prok2 acts as a signal for light/dark cues, rather than day/night circadian cues. Consistent with this hypothesis, prok2 mutant larvae and adults are more active in a light-dependent manner, and prokr2 mutant adults are more active in light and less active in dark. These results suggest that Prok2 signaling regulates the direct effects of light and dark on behavior. While these effects are widely observed in the animal kingdom, including in humans (Lockley et al., 2006), little is known about how they are mediated downstream of light detection in the retina (Hubbard et al., 2013). To our knowledge, our results provide the first evidence for a non-photopigment pathway that affects this phenomenon, and provide an entry-point to explore its genetic and neuronal underpinnings.
Based on our observations, we propose two alternative models for the role of Prok2 in regulating zebrafish sleep/wake states. First, we propose that Prok2 is necessary and sufficient to promote sleep in a manner that depends on lighting conditions and not on circadian rhythms. This model is supported by our observations that Prok2 overexpression decreases locomotor activity and increases sleep in 1) entrained larvae during the day; 2) entrained larvae during exposure to light pulses during the day and night; 3) entrained larvae transferred to constant light; 4) non-entrained larvae raised and tested in constant light; and 5) entrained adults exposed to constant light or light pulses. This model is also supported by our findings that entrained prok2 mutant larvae are more active and sleep less during the day, and non-entrained prok2 mutant larvae sleep less in constant light. Similarly, we found that prok2, and especially prokr2, adult mutant zebrafish are more active following the dark to light transition in the morning.
Second, we propose the more speculative model that Prok2 acts as a “balancing factor” (Berridge, 2004) that antagonizes the direct effects of light and dark on behavior. While most sleep occurs at night in zebrafish, they also sleep a significant amount during the day. Similarly, nocturnal rodents sleep more during the day than at night, but still sleep a significant amount at night. It would thus seem useful to not only have mechanisms that promote wake during the day and sleep at night (e.g. for diurnal animals), but to also have antagonizing mechanisms that maintain the correct balance of sleep and wake. This model is consistent with our Prok2 overexpression studies in diurnal zebrafish larvae and adults, and with Prok2 infusion studies in nocturnal rodents (Berridge, 2004). These studies have shown that Prok2 overexpression in light, when diurnal animals are active and nocturnal animals are inactive, promotes inactivity in diurnal zebrafish and activity in nocturnal rodents. Conversely, Prok2 overexpression in dark promotes activity in diurnal zebrafish and inactivity in nocturnal rodents. If this hypothesis is correct, diurnal prok2 mutants should be more active during the day and less active at night. Indeed, we found that prok2 mutant zebrafish larvae are more active and sleep less during the day, and prok2 mutant larvae raised and tested in constant light sleep less than sibling controls. Furthermore, we found that adult prok2 and prokr2 mutants are more active following lights-on in the morning, and adult prokr2 mutants are also less active before lights-on in the morning. However, this model is not supported by our observations that prok2 mutant larvae and adults exhibit normal sleep/wake states at night, and that prok2 mutant larvae raised and tested in constant dark lack sleep/wake phenotypes. The lack of mutant phenotypes at night or in the dark could result from redundant sleep-regulating mechanisms in dark but not in light, which would not be surprising since most zebrafish sleep occurs during the dark phase. Alternatively, since the zebrafish Prok2 overexpression phenotype is weaker in dark than in light, our behavioral assays may not be sensitive enough to detect subtle prok2 mutant phenotypes in dark. It is also possible that chronic absence of prok2 in mutant animals may induce compensatory mechanisms during development. Indeed, strong effects of acute pharmacological manipulation compared to subtle phenotypes for constitutive genetic loss-of-function have been observed for several systems that regulate sleep, including HA and adenosine (Brown et al., 2012), suggesting that this compensation may be a general feature of sleep control. More sensitive arousal assays or methods to acutely block Prok2 signaling may reveal a requirement for endogenous Prok2 in dark and at night.
Prok2 may act as a circadian output factor in nocturnal rodents but not in diurnal animals
prok2 and prokr2 mutant mice exhibit attenuated circadian rhythms of body temperature and circulating glucose and cortisol levels (Li et al., 2006; Prosser et al., 2007), suggesting that Prok2 acts as a circadian output factor. However, these mutants suffer from embryonic and neonatal lethality, and exhibit defects in the olfactory and reproductive systems (Li et al., 2006; Matsumoto et al., 2006; Ng et al., 2005; Pitteloud et al., 2007; Prosser et al., 2007), which confounds interpretation of circadian phenotypes. In contrast, humans that lack prok2 have normal circadian rhythms of body temperature, and circulating cortisol and melatonin levels (Balasubramanian et al., 2014). Our results in zebrafish agree with the human study, as circadian sleep/wake patterns are normal in larval zebrafish prok2 mutants, which also lack apparent developmental defects.
A potentially important difference between larval zebrafish and both nocturnal and diurnal rodents is that prok2 expression oscillates in a circadian manner in the rodent SCN (Cheng et al., 2005; Cheng et al., 2002; Lambert et al., 2005) but not in larval zebrafish. Consistent with these observations, E box motifs, which mediate circadian gene expression, are present in the prok2 promoter in mice but not in zebrafish. A previous study using qRT-PCR suggested that prok2 levels may be elevated at the transition from the dark to light phase in adult zebrafish, although it was not shown where this change occurs (Noonin et al., 2013). While we did not detect changes in prok2 expression or in the activity of prok2-expressing neurons in response to light transitions in zebrafish larvae, the Noonin et al. observation is consistent with our finding that prok2 and prokr2 mutant adult zebrafish display increased activity following the dark to light phase transition.
It is unclear whether regulation of prok2 expression differs between nocturnal and diurnal animals, or between mammals and teleosts. The latter possibility is consistent idea that zebrafish do not need a master circadian pacemaker analogous to the mammalian SCN because zebrafish tissues can be directly entrained by light (Whitmore et al., 2000). We found that several markers of the mammalian SCN are expressed in a similar anatomical region of the larval zebrafish brain. However, unlike mammals, none of these markers are co-expressed in the same neurons in zebrafish larvae, and many of these neurons are glutamatergic in zebrafish, whereas most mammalian SCN neurons are GABAergic (Moore and Speh, 1993). Tools that allow ablation of these neurons are needed to test whether this region of the zebrafish brain has an SCN-like function.
Effects of Prok2 on sleep are mediated by Prokr2
Previous studies identified two GPCRs, Prokr1 and Prokr2, that bind Prok2 with similar affinity in vitro (Lin et al., 2002). Prokr2 was suggested to be the endogenous receptor for Prok2 based on its expression in SCN target regions (Cheng et al., 2002), but this hypothesis had not been tested. We found that Prok2 overexpression phenotypes are abolished in prokr2, but not prokr1, mutant zebrafish, suggesting that Prokr2 mediates the effects of Prok2 on sleep/wake behaviors. Despite the sleep phenotype observed in prok2 mutant larvae, we failed to detect sleep defects in either single or double prokr1 and prokr2 mutant larvae. This discrepancy suggests there may be additional receptors for Prok2 at larval stages that are not required for Prok2 overexpression phenotypes but are sufficient to maintain normal sleep/wake behaviors in the absence of prokr1 and prokr2. Alternatively, there may be other ligands for these receptors that have effects on sleep opposite to those of Prok2. However, in contrast to larval zebrafish, we found that prokr2 mutant adult zebrafish were less active before, and more active after, lights-on in the morning, consistent with the opposing effects of Prok2 overexpression on behavior in light and dark.
The direct effect of light on sleep in nocturnal and diurnal animals may converge on hypothalamic Galn neurons
We found that Prok2 overexpression significantly increased expression of the sleep-promoting neuropeptide galn (Woods et al., 2014) in a hypothalamic population of galn-expressing neurons in light, but not dark, conditions. These neurons are located in a similar anatomical region as the mammalian VLPO, whose ablation results in reduced sleep (Lu et al., 2000), and which contains sleep-active galn-expressing neurons (Sherin et al., 1998). We found that at least a third, and possibly most or all, of these galn neurons co-express prokr2, suggesting that Prok2 signaling directly affects these neurons. Finally, we found that Prok2 overexpression-induced sleep is largely suppressed in galn mutant animals. Taken together, these results suggest that Prok2 overexpression induces sleep by increasing hypothalamic galn levels. Similar to our findings, a recent study using nocturnal rodents found that sleep induced by green light at night is associated with increased galn expression in the VLPO (Pilorz et al., 2016). These results suggest that the direct effect of light on sleep may act via galn-expressing hypothalamic neurons in both diurnal zebrafish and nocturnal rodents.
In summary, we have shown that a gene thought to be a circadian clock output factor in nocturnal rodents instead regulates the direct effect of light on sleep/wake behaviors in the zebrafish, a diurnal vertebrate. Our results are consistent with observations in humans with prok2 mutations, suggesting that zebrafish may be more appropriate than nocturnal rodents as a model to understand how Prok2 regulates sleep in humans. It will be important to determine whether our findings are broadly applicable to other proteins thought to act as circadian clock output factors, and thus reveal general principles for how sleep regulatory mechanisms described in nocturnal animals may be translated to diurnal animals. Our results also provide an avenue to explore genetic and neuronal mechanisms that underlie the direct effects of light and dark on sleep/wake behaviors.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for reagents may be directed to, and will be fulfilled by, the Lead Contact David A. Prober (dprober@caltech.edu).
Experimental Model and Subject Details
Zebrafish experiments and husbandry followed standard protocols in accordance with Caltech 890 Institutional Animal Care and Use Committee guidelines. Experimental procedures at UCL were conducted under project license (70/7612) awarded to JR from the UK Home Office, according to the UK Animals (Scientific Procedures) Act 1986. Larval zebrafish were studied before the onset of sexual differentiation. Adult zebrafish experiments were performed using both males and females, unless stated otherwise. The age of animals used in each experiment are described in the manuscript and in each figure legend.
Transgenic and mutant zebrafish
Tg(hsp:Prok2) ct841
Full-length zebrafish prok2 cDNA was isolated using 5′ and 3′ RACE (FirstChoice RLM-RACE, AM1700, Thermo Fisher Scientific) and the open reading frame was cloned downstream of the zebrafish hsp70c promoter (Prober et al., 2006) in a vector containing flanking I-SceI endonuclease recognition sites. Stable transgenic lines were generated by injecting plasmids with I-SceI (R0694, New England Biolabs Inc.) into zebrafish embryos at the one-cell stage. Transgenic founders were identified by outcrossing potential founders, heat shocking progeny at 5 dpf, fixing larvae 30 minutes after heat shock and performing ISH using a prok2-specific probe. Tg(hsp:Prok2) fish were genotyped using the primers 5′-CGGGCCACCATGACAT-3′ and 5′-GGTTTGTCCAAACTCATCAATGT-3′, which generate a 404 bp band.
prok2 ct842 mutant
prok2 and prok receptor mutants were generated using the TALEN method (Reyon et al., 2012). For the prok2 mutant, TALEN target sites were 5′-TGGCATGTGTTGTGCAGT-3′ and 5′-TGCACATTCGGAGACT-3′. Two mutants were isolated and tested. Mutant d1 contains a 1 bp deletion (nucleotide 127 of the open reading frame: 5′-G-3′), which results in a change in reading frame after amino acid 41 and a premature stop codon after amino acid 42 compared to 107 amino acids for the WT protein. Mutant d8 contains an 8 bp deletion (nucleotides 124-131 of the open reading frame: 5′-TGTGGATC -3′), which results in a change in reading frame after amino acid 41 and a premature stop codon after amino acid 87. The predicted mutant protein lacks key conserved domains including 5 of 10 cysteine residues and the dibasic proteolytic cleavage site, and is thus likely non-functional (Bullock et al., 2004). This assertion is supported by the observation that a mutant form of prok2 identified in humans with Kallmann syndrome, that was shown to be inactive in vitro, was truncated at amino acid 54 (Pitteloud et al., 2007). Both mutants exhibited similar phenotypes and mutant d8 was used for all reported experiments. prok2 mutants were genotyped using the primers 5′-CAAGTGGACACACCGAACAC-3′ and 5′-ATCCTGGAATGGAAATGGTG-3′. PCR products were digested with BamHI (R0136, New England Biolabs Inc.). Mutant (404 bp) and WT (155 bp + 257 bp) bands were distinguished by running the digested PCR product on a 2% agarose gel. prokr1 (ENSDARG00000074182, also known as prokr1a) ct843 mutant: TALEN target sites were 5′-TACTTGAGGACTGTGTC-3′ and 5′-TGGCCAGCAGAGCATT-3′. Two mutants were isolated and tested. Mutant d7 contains a 7 bp deletion (nucleotides 411-422 of the open reading frame: 5′-TACGTGTCCAC-3′ were replaced by 5′-TTGGT3′), which results in a change in reading frame after amino acid 136 and a premature stop codon after amino acid 156 compared to 385 amino acids for the WT protein. Mutant d22 contains a 22 bp deletion (nucleotides 405-426 of the open reading frame: 5′-TCTCTCTACGTGTCCACCAATG-3′), which results in a change in reading frame after amino acid 134 and a premature stop codon after amino acid 151. Both predicted mutant proteins lack more than 4 transmembrane domains and are thus likely non-functional (Lin et al., 2002). Both mutants exhibited similar phenotypes and mutant d7 was used for all reported experiments. prokr1 mutants were genotyped using the primers 5′-GGCGTTGGTAATTGCGTATT-3′ and 5′-TATCAGCCACCAGCACTCTG-3′. PCR products were digested with AflIII (R0541, New England Biolabs Inc.). Mutant (527 bp) and WT (218 bp + 316 bp) bands were distinguished by running the digested PCR reaction on a 2% agarose gel.
prokr1 (ENSDARG00000074182, also known as prokr1a) ct843 mutant
TALEN target sites 929 were 5′-TACTTGAGGACTGTGTC-3′ and 5′-TGGCCAGCAGAGCATT-3′. Two mutants were isolated and tested. Mutant d7 contains a 7 bp deletion (nucleotides 411-422 of the open reading frame: 5′-TACGTGTCCAC-3′ were replaced by 5′-TTGGT3′), which results in a change in reading frame after amino acid 136 and a premature stop codon after amino acid 156 compared to 385 amino acids for the WT protein. Mutant d22 contains a 22 bp deletion (nucleotides 405-426 of the open reading frame: 5′-TCTCTCTACGTGTCCACCAATG-3′), which results in a change in reading frame after amino acid 134 and a premature stop codon after amino acid 151. Both predicted mutant proteins lack more than 4 transmembrane domains and are thus likely non-functional (Lin et al., 2002). Both mutants exhibited similar phenotypes and mutant d7 was used for all reported experiments. prokr1 mutants were genotyped using the primers 5′-GGCGTTGGTAATTGCGTATT-3′ and 5′-TATCAGCCACCAGCACTCTG-3′. PCR products were digested with AflIII (R0541, New England Biolabs Inc.). Mutant (527 bp) and WT (218 bp + 316 bp) bands were distinguished by running the digested PCR reaction on a 2% agarose gel.
prokr2 (ENSDARG00000090315, also known as prokr1l) ct844 mutant
TALEN target sites were 5′-TCTCACAGAAACAGCCAT-3′ and 5′-TACAGCTGCCACGTGGCT-3′. Two mutants were isolated and tested. Mutant d1 contains a 1 bp deletion (nucleotide 12 of the open reading frame: 5′-C-3′), which results in a change in reading frame after amino acid 4 and a premature stop codon after amino acid 13 compared to 396 amino acids for the WT protein. Mutant d14 contains a 14 bp deletion (nucleotides 7-20 of the open reading frame: 5′-GACGCCAATATCAG-3′), which results in a change in reading frame after amino acid 2 and a premature stop codon after amino acid 21. Both predicted mutant proteins lack all 7 transmembrane domains and are thus likely non-functional (Lin et al., 2002). Both mutants exhibited similar phenotypes and mutant d1 was used for all reported experiments. prokr2 mutants were genotyped using the primers 5′-TGAGCGTAATGCTAATGGTCT-3′ and 5′-CCAGAGTGGCGATAAACACA-3′. PCR products were digested with BsaHI (R0556, New England Biolabs Inc.). Mutant (428 bp) and WT (190 bp + 239 bp) bands were distinguished by running the digested PCR reaction on a 2% agarose gel.
galn u4017 mutant
The zebrafish galn gene encodes two isoforms resulting from alternative splicing, the longer of which (isoform b) contains an additional 72-base pair exon that inserts 42 amino acids into the Galn mature peptide domain. We generated a mutant using CRISPR/Cas9 (Hwang et al., 2013). The sgRNA target sequence 5′-CGGACTCACGAGGACCGAGGA-3′ is located in exon 3, which is present in both isoform a and isoform b. The mutant contains a 1 bp deletion (G) after nucleotide 130 of the coding sequence of both isoforms. The mutation results in a frame shift affecting the protein sequence after amino acid 43, and a stop codon after amino acid 67 (isoform a) or amino acid 91 (isoform b), compared to the 118 and 142 amino acid WT proteins. The predicted mutant proteins contain the signal peptide sequence of the Galn precursor pro-peptide but lack the mature Galn and Galn message-associated peptide (GMAP) sequences, and are thus predicted to be non-functional. Mutants were genotyped using the primers 5′ATGTACTGTCCTCATGGCAAAG-3′ and 5′-AAATGTAGACCTGAGAGCAGC-3′, which produce a 512 bp band. Digestion with BsaJI (R0536, New England Biolabs Inc.) for 3 hours at 60°C produces 394 and 118 bp bands for the WT allele, whereas the restriction enzyme site is missing in the galn mutant allele. galn mutants are morphologically indistinguishable from WT siblings and lack overt phenotypes during the stages analyzed (until 10 dpf). A detailed description of this mutant is in preparation for a separate publication (Reichert et al., in preparation).
The ET(vmat2:EGFP) pku2ET line (Wen et al., 2008), and aanat2 ct801 (Gandhi et al., 2015), hdc ct836 (Chen et al., 2017), hypocretin receptor hu2098 (Yokogawa et al., 2007) and glucocorticoid receptor s357 (Ziv et al., 2013) mutants have been described.
Tg(prok2:GFP-Aequorin)
A GFP-Aequorin (Naumann et al., 2010) transgene was inserted at the prok2 start codon of a BAC (CH211-36N11, BACPAC resource center) containing the prok2 gene, and 110 kb upstream and 40 kb downstream genomic sequence, as previously described (Gong et al., 2002). The iTol2 sequence was inserted into the backbone vector (pTARBAC2.1). Recombined BAC was purified using the Nucleobond BAC kit (740579, Macherey-Nagel), and diluted to a final concentration of 50 ng/μL in water. 1 nL of this solution was injected into embryos at the 1-cell stage, along with tol2 transposase mRNA at a concentration of 50 ng/μL.
Method Details
Locomotor activity assays
Larval zebrafish videotracker experiments were performed as previously described (Prober et al., 2006). Larval zebrafish were raised on a 14:10 hour light:dark cycle at 28.5°C with lights on at 9 a.m. and off at 11 p.m., except for experiments in which larvae were raised in constant light or constant dark. On the fourth day of development, individual larvae were placed in each well of a 96-well plate (7701-1651, GE Healthcare Life Sciences) containing 650 μL of E3 embryo medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, pH 7.4). Plates were sealed with an optical adhesive film (4311971, Applied Biosystems) to prevent evaporation. Locomotor activity was monitored using an automated videotracking system (Viewpoint Life Sciences) with a Dinion one-third inch Monochrome camera (Dragonfly 2, Point Grey) fitted with a variable-focus megapixel lens (M5018-MP, Computar) and infrared filter. The movement of each larva was recorded using the quantization mode. The 96-well plate and camera were housed inside a custom-modified Zebrabox (Viewpoint Life Sciences) that was continuously illuminated with infrared lights and with customizable white light. The 96-well plate was housed in a chamber filled with recirculating water to maintain a constant temperature of 28.5°C. The parameters used for detection were: detection threshold, 15; burst, 29; freeze, 3; bin size, 60 seconds, which were determined empirically. Heat shocks were performed by removing the 96-well plate from the videotracker and placing it in a 37°C water bath for 1 hour. At the end of each experiment, each well was examined and those containing bubbles (introduced during the sealing process) or more or less than one larva were excluded from analysis.
Videotracker sleep/wake data was processed using custom PERL and Matlab (The Mathworks, Inc) scripts. Any one minute period with less than 0.1 second of total movement was defined as one minute of sleep (Prober et al., 2006). A sleep bout was defined as a continuous string of sleep minutes. Average activity was defined as the average amount of activity in seconds/hour, including all rest bouts. Statistical tests were performed using Prism (GraphPad).
For adult behavioral assays, individual four to six-month old zebrafish were randomly placed into a 7 cm × 12 cm by × 8.5 cm (WxLxH) open topped, transparent plastic chamber, with three small holes (5 mm) to allow water exchange. The chambers (8 per tracking session) were placed into a semi-transparent, plastic 46 cm × 54 cm chamber, which was supplied with aquarium fish water (water height in chamber: 4.5 cm), pumped from a 45 L reservoir with an aquarium pump (Maxi-Jet, MJ500) at a flow rate of 1.3 L/min and heated to 28°C with an aquarium heater (HT100; Tetra). The chamber was continuously illuminated from below with two panels of infrared lights (60 Degree, 54 LED Video Camera Red Infrared Illuminator Lamp, Sourcing Map) with the detector covered, and illuminated from above with white light (180 lux at water surface) from 9 a.m. to 11 p.m. The chambers were monitored with a ceiling mounted (143 cm from chamber to lens) Dinion one-third inch Monochrome camera (LTC0385; Bosch) fitted with a 13–36 mm, 1:2:8, 2/3″ lens (Computar). The entire setup was housed in an isolated darkroom. Fish were continuously tracked for one to four days at 15 Hz using an automated videotracking system (Viewpoint Life Sciences) in tracking mode, with a background threshold of 40, inactive/small movement cutoff of 1.3 cm/sec, and small/large movement cutoff of 8 cm/sec, which were determined empirically. Each track was analyzed using custom Matlab scripts. Mixed gender siblings were raised in groups and genotyped by fin clip after the behavioral assay.
Arousal threshold assay
The arousal threshold assay was performed as described (Singh et al., 2015). Taps of 14 different intensities were applied in a random order from 12:30 p.m. to 7:30 p.m. (day experiments) and from 12:30 a.m. to 7:30 a.m. (night experiments) during the fifth day of development. Thirty trials were performed at each stimulus intensity, with a 1-minute inter-trial interval. The background probability of movement was calculated by identifying for each genotype the fraction of larvae that moved 5 seconds prior to all stimuli delivered. This value was subtracted from the average response fraction value for each tap event. A response is defined as any movement that occurred within 1 second after a tap is delivered. Curves were constructed using the non-linear variable slope module in Prism and fitted using ordinary least squares. Three independent day experiments and two independent night experiments were performed. One representative experiment was shown for each condition.
in situ hybridization (ISH) and immunohistochemistry (IHC)
For ISH, samples were fixed in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) for 16 hours at room temperature. ISH was performed using digoxigenin (DIG)-labeled antisense riboprobes (DIG RNA Labeling Kit, 11175025910, Sigma-Aldrich), a sheep anti-DIG antibody conjugated to alkaline phosphatase (1:2000; 11093274910, Sigma-Aldrich), and developed using NBT/BCIP (11681451001, Sigma-Aldrich). Antisense prok2 riboprobe for was generated from a zebrafish EST (Genbank EB931495; GE Healthcare Life Sciences). Antisense prokr1 riboprobe was generated using a RT-PCR fragment as template. The fragment was amplified using primers 5′-TGACTCGCAGTCACACAGTTC-3′ and T7 promoter-coupled 5′-GAATTGTAATACGACTCACTATAGGGTCTTCCAAAGTATGGGTCGAA-3′. Antisense prokr2 riboprobe was generated using a RT-PCR fragment as template. The fragment was amplified using primers 5′-GCACAGAGAATGAGCGTCTG-3′ and T7 promoter-coupled 5′-GAATTGTAATACGACTCACTATAGGGTCACTGAGGCTGAGGGTATAAA-3′. Antisense grp riboprobe was generated using a RT-PCR fragment as template. The fragment was amplified using primers 5′-CTATGTGCCTGGTGTGGAGA-3′ and T7 promoter-coupled 5′-GAATTGTAATACGACTCACTATAGGGGCAGAAAGCCCAACAAGTTC-3′. Antisense vip riboprobe was generated from a zebrafish EST (Genbank EH437720, a kind gift from C. Wei, Genome Institute of Singapore). Antisense tgfα riboprobe was generated using a RT-PCR fragment as a template. The fragment was amplified using primers 5′-CGCGTGCCTTCATCTTTATT-3′ and T7 promoter-coupled 5′-GAATTGTAATACGACTCACTATAGGGTCCCACTGCCCATATTGAAC-3′. Antisense avp (Herget and Ryu, 2015), c-fos (Singh et al., 2015), egfp (Singh et al., 2015); hcrt (Prober et al., 2006), hdc (Sundvik et al., 2011), qrfp (Chen et al., 2016), vglut1, vglut2b, gad65, and gad67 (Higashijima et al., 2004) riboprobes have been previously described. Antisense galn riboprobe was generated from a zebrafish EST (Genbank AL918581, a kind gift from J. Peng, Institute of Molecular and Cell Biology, Singapore). Images were acquired using a Zeiss AxioImagerM1 microscope. Fluorescent ISH used DIG-, 2, 4-dinitrophenol (DNP)- and fluorescein-labeled antisense riboprobes with the TSA Plus DNP (HRP) System (NEL747A001KT, PerkinElmer) and the TSA Plus Cyanine 3 and Fluorescein System (NEL753001KT, PerkinElmer).
For IHC, samples were fixed in 4% PFA/PBS overnight at 4°C and then washed with 0.25% Triton X-100/PBS (PBTx). Brains were manually dissected and blocked for 2 hours in 2% goat serum/2% DMSO/PBTx. Antibody incubations were performed in blocking solution overnight at 4°C using rabbit polyclonal anti-zebrafish Prok2 (1:500; SDIX LLC) and rabbit polyclonal anti-GFP (1:1000; MBL International, #598) primary antibodies, and Alexa Fluor 568 goat anti-rabbit (1:500; Invitrogen, #A-11011) secondary antibody. The Prok2-specific antibody was generated against the full-length zebrafish Prok2 protein. 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) (268298, EMD Millipore) staining was performed at 1:5000 in 2% DMSO/PBTx for at least 4 hours at room temperature. Samples were mounted in 50% glycerol/PBS, and imaged with a Zeiss LSM 780 confocal microscope with 405 nm, 488 nm, and 561 nm lasers and 40x objective. Images were processed using Fiji (Schindelin et al., 2012).
Analysis of galn expression using ISH
For quantification of galn expression, galn ISH was performed by incubating fixed 5 dpf larval brains with a DIG-labeled galn antisense riboprobe, followed by a sheep anti-DIG antibody conjugated to alkaline phosphatase (1:2000; 11093274910, Sigma-Aldrich), and developed using NBT/BCIP (11681451001, Sigma-Aldrich) for approximately 40 minutes to obtain a dark purple stain. Development was stopped before the hypothalamic staining was saturated. Brains were imaged with a Zeiss LSM 510 laser-scanning confocal microscope using a 633 nm laser and 40x objective. For experiments examining galn expression in Tg(hsp:prok2) in prokr2 mutants, Tg(hsp:prok2) animals in alternating 1 hour light/dark conditions, and prok2 mutants, samples were imaged using a Zeiss LSM 410 laser-scanning confocal microscope using a 633 nm laser and 40x objective because the LSM 510 microscope was no longer available. Raw integrated pixel density was quantified in a 200 μm × 100 μm region of interest in the hypothalamus. Only sections containing galn cells were quantified and background was subtracted from the raw integrated pixel density. . Quantification was performed using Fiji. Transgenic and non-transgenic sibling larvae were processed for ISH in the same tube, imaged, quantified and then genotyped by PCR.
Analysis of prok2 expression using fluorescent ISH (FISH)
Larvae were raised in on a 14:10 hour light:dark cycle at 28.5°C with lights on from 9 a.m. to 11 p.m. until 6 dpf, or in constant light or constant dark. Samples were fixed in 4% PFA/PBS overnight at room temperature and FISH was performed on dissected brains using a DIG-labeled antisense riboprobe specific for prok2 and the TSA Plus System (NEL753001KT, PerkinElmer). Samples were developed using Cy3 amplification reagent at 1:200 for 10 minutes. Imaging was performed using a Zeiss 780 confocal microscope using a 40x objective and the same settings for all samples. A box encompassing all prok2-expressing cells was drawn for each brain and the raw integrated pixel density was measured for a 50 μm stack consisting of approximately 30 sections. Background, defined as the mean pixel intensity of an 80 μm × 10 μm region above the region of interest multiplied by the total area of the box encompassing all prok2-expressing cells, was then subtracted from the raw integrated pixel density. The final value was then normalized to the lowest ZT value. Quantification was performed using Fiji.
Analysis of prok2, prokr2 and per3 expression using qRT-PCR
Larval zebrafish were raised on a 14:10 hour light:dark cycle at 28.5°C with lights on from 9 a.m. to 11 p.m., or in constant light or constant dark. Total RNA was isolated using the RNeasy Mini Kit (74106, Qiagen) from 25 pooled larvae every 4 hours starting at 12 p.m. at 6 dpf for prok2, and every 6 hours starting at 6 p.m. at 6 dpf for prokr2. cDNA was synthesized from 5 μg total RNA using Superscript III Reverse Transcriptase (18080051, Thermo Fisher Scientific) and quantitative PCR was carried out using SYBR green master mix (4364346, Thermo Fisher Scientific) in triplicate using an ABI PRISM 7900HF (Life Technologies) instrument. Δ Ct was calculated using actin as a reference gene. Relative expression levels were calculated using the 2−ΔΔCt method by normalizing to the sample with the highest ΔCt value for each gene. Primers for amplification: prok2, 5′-GGGGCATGTGAGAAGGACTCT-3′ and 5′-TCTTCTCCCTCCTGACCCATT-3′; prokr2, 5′-GTGTCCACCAACGCCTTACT -3′ and 5′-CTCCTGTGATCAAACAGTACGC-3′; per3, 5′-CTCCAGCTTTCACAGCACTCA-3′ and 5′-ACGCTTCTTCATCTCCTGCAC-3′; actin, 5′-TCCTCCCTGGAGAAGAGCTATG-3′ and 5′-TCCATACCCAGGAAGGAAGG-3′.
Quantification and Statistical Analysis
For behavioral experiments with two genotypes, statistical significance was assessed using a two-tailed Student’s t test. For prok2 ligand and receptor mutant experiments, one-way ANOVA with post-hoc Dunnett’s test for comparisons to WT was used. For experiments in which Prok2 was overexpressed in various mutant backgrounds, statistical significance was assessed using two-way ANOVA with post-hoc Tukey’s HSD test. For quantification of galn expression, statistical significance was assessed using two-way ANOVA for pair-wise comparisons of the mean of each control sample with its corresponding hsp:Prok2 or melatonin-treated sample, with post-hoc Bonferroni correction for multiple comparisons. For prok2 qPCR and FISH, one-way ANOVA was used. Adult experiments were analyzed by repeated measures ANOVA and by one-way ANOVA with post-hoc Tukey’s HSD test. galn mutant behavior was analyzed by one-way ANOVA, with post-hoc Tukey’s HSD test. Excel (Microsoft), Prism and Matlab were used for statistical analyses. Statistical tests and number of animals used are stated in each figure or figure legend.
Data and Software Availability
Custom MATLAB code used for zebrafish behavioral analysis is available upon request.
Supplementary Material
Highlights.
Prok2 overexpression induces sleep in light and suppresses sleep in dark
Prok2-induced sleep is independent of circadian rhythms and requires prokr2
prok2 mutant exhibits reduced sleep in light independently of circadian rhythms
Prok2-induced sleep in light induces hypothalamic galn expression and requires galn
Acknowledgments
We thank Daisy Chilin, Alex Mack Cruz and Kenna Molinder for animal husbandry, Viveca Sapin, Brett Niles and Jae Chu for genotyping, and Catherine Oikonomou and members of the Prober lab for comments on the manuscript. This work was supported by grants from the NIH (SC: NS077842; DAP: NS070911, NS094390, NS095824), the Mallinckrodt, Rita Allen and Brain and Behavior Research Foundations (DAP), a Sir Henry Wellcome Trust Fellowship (SR), and a UCL Excellence Fellowship and European Research Council Starting Grant (JR). We declare no conflicts of interest.
Footnotes
Author contributions
SC, CS, GO and DAP generated all reagents, and designed and performed all experiments, except that SR and JR generated the galn mutant, and designed and performed the adult and galn mutant experiments. SC and DAP wrote the paper with assistance from SR and JR. DAP supervised the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayari B, El Hachimi KH, Yanicostas C, Landoulsi A, Soussi-Yanicostas N. Prokineticin 2 expression is associated with neural repair of injured adult zebrafish telencephalon. J Neurotrauma. 2010;27:959–972. doi: 10.1089/neu.2009.0972. [DOI] [PubMed] [Google Scholar]
- Balasubramanian R, Cohen DA, Klerman EB, Pignatelli D, Hall JE, Dwyer AA, Czeisler CA, Pitteloud N, Crowley WF. Absence of central circadian pacemaker abnormalities in humans with loss of function mutation in prokineticin 2. The Journal of clinical endocrinology and metabolism. 2014;99:E561–566. doi: 10.1210/jc.2013-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiological reviews. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock CM, Li JD, Zhou QY. Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Molecular pharmacology. 2004;65:582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- Chen A, Chiu CN, Mosser EA, Kahn S, Spence R, Prober DA. QRFP and Its Receptors Regulate Locomotor Activity and Sleep in Zebrafish. J Neurosci. 2016;36:1823–1840. doi: 10.1523/JNEUROSCI.2579-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Singh C, Oikonomou G, Prober DA. Genetic Analysis of Histamine Signaling in Larval Zebrafish Sleep. eNeuro. 2017;4 doi: 10.1523/ENEURO.0286-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bittman EL, Hattar S, Zhou QY. Regulation of prokineticin 2 expression by light and the circadian clock. BMC neuroscience. 2005;6:17. doi: 10.1186/1471-2202-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Hohmann JG, Clifton DK, Steiner RA. Distribution of galanin messenger RNA-expressing cells in murine brain and their regulation by leptin in regions of the hypothalamus. Neuroscience. 2001;103:423–432. doi: 10.1016/s0306-4522(01)00012-4. [DOI] [PubMed] [Google Scholar]
- Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, Pignatelli D, Hughes VA, Dwyer AA, Raivio T, Hayes FJ, et al. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. The Journal of clinical endocrinology and metabolism. 2008;93:3551–3559. doi: 10.1210/jc.2007-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–754. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, et al. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006;2:e175. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gander PH, Moore-Ede MC. Light-dark masking of circadian temperature and activity rhythms in squirrel monkeys. The American journal of physiology. 1983;245:R927–934. doi: 10.1152/ajpregu.1983.245.6.R927. [DOI] [PubMed] [Google Scholar]
- Gandhi AV, Mosser EA, Oikonomou G, Prober DA. Melatonin is required for the circadian regulation of sleep. Neuron. 2015;85:1193–1199. doi: 10.1016/j.neuron.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner JV, Bataveljic A, Patel NA, Bewick GA, Roy D, Campbell D, Greenwood HC, Murphy KG, Hameed S, Jethwa PH, et al. Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake. Diabetes. 2010;59:397–406. doi: 10.2337/db09-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Yang XW, Li C, Heintz N. Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kgamma origin of replication. Genome Res. 2002;12:1992–1998. doi: 10.1101/gr.476202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Herzog ED. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. Journal of biological rhythms. 2004;19:400–413. doi: 10.1177/0748730404268786. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget U, Ryu S. Coexpression analysis of nine neuropeptides in the neurosecretory preoptic area of larval zebrafish. Front Neuroanat. 2015;9:2. doi: 10.3389/fnana.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Mandel G, Fetcho JR. Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J Comp Neurol. 2004;480:1–18. doi: 10.1002/cne.20278. [DOI] [PubMed] [Google Scholar]
- Hu WP, Li JD, Zhang C, Boehmer L, Siegel JM, Zhou QY. Altered circadian and homeostatic sleep regulation in prokineticin 2-deficient mice. Sleep. 2007;30:247–256. [PMC free article] [PubMed] [Google Scholar]
- Hubbard J, Ruppert E, Gropp CM, Bourgin P. Non-circadian direct effects of light on sleep and alertness: lessons from transgenic mouse models. Sleep Med Rev. 2013;17:445–452. doi: 10.1016/j.smrv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Hurd MW, Cahill GM. Entraining signals initiate behavioral circadian rhythmicity in larval zebrafish. Journal of biological rhythms. 2002;17:307–314. doi: 10.1177/074873002129002618. [DOI] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. Analysis of the prokineticin 2 system in a diurnal rodent, the unstriped Nile grass rat (Arvicanthis niloticus) Journal of biological rhythms. 2005;20:206–218. doi: 10.1177/0748730405275135. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JD, Hu WP, Zhou QY. The circadian output signals from the suprachiasmatic nuclei. Prog Brain Res. 2012;199:119–127. doi: 10.1016/B978-0-444-59427-3.00028-9. [DOI] [PubMed] [Google Scholar]
- Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Molecular pharmacology. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- Masumoto KH, Nagano M, Takashima N, Hayasaka N, Hiyama H, Matsumoto S, Inouye ST, Shigeyoshi Y. Distinct localization of prokineticin 2 and prokineticin receptor 2 mRNAs in the rat suprachiasmatic nucleus. Eur J Neurosci. 2006;23:2959–2970. doi: 10.1111/j.1460-9568.2006.04834.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Fernandez M, Jantos H. Sleep during acute dopamine D1 agonist SKF 38393 or D1 antagonist SCH 23390 administration in rats. Neuropsychopharmacology. 1990;3:153–162. [PubMed] [Google Scholar]
- Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- Naumann EA, Kampff AR, Prober DA, Schier AF, Engert F. Monitoring neural activity with bioluminescence during natural behavior. Nat Neurosci. 2010;13:513–520. doi: 10.1038/nn.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- Noonin C, Watthanasurorot A, Winberg S, Soderhall I. Circadian regulation of melanization and prokineticin homologues is conserved in the brain of freshwater crayfish and zebrafish. Developmental and comparative immunology. 2013;40:218–226. doi: 10.1016/j.dci.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Oikonomou G, Prober DA. Attacking sleep from a new angle: contributions from zebrafish. Curr Opin Neurobiol. 2017;44:80–88. doi: 10.1016/j.conb.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorz V, Tam SK, Hughes S, Pothecary CA, Jagannath A, Hankins MW, Bannerman DM, Lightman SL, Vyazovskiy VV, Nolan PM, et al. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 2016;14:e1002482. doi: 10.1371/journal.pbio.1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2007;104:17447–17452. doi: 10.1073/pnas.0707173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Rihel J, Onah AA, Sung RJ, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Bradley A, Chesham JE, Ebling FJ, Hastings MH, Maywood ES. Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc Natl Acad Sci U S A. 2007;104:648–653. doi: 10.1073/pnas.0606884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyon D, Khayter C, Regan MR, Joung JK, Sander JD. Engineering designer transcription activator-like effector nucleases (TALENs) by REAL or REAL-Fast assembly. Curr Protoc Mol Biol. 2012;Chapter 12(Unit 12):15. doi: 10.1002/0471142727.mb1215s100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh C, Oikonomou G, Prober DA. Norepinephrine is required to promote wakefulness and for hypocretin-induced arousal in zebrafish. eLife. 2015;4:e07000. doi: 10.7554/eLife.07000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundvik M, Kudo H, Toivonen P, Rozov S, Chen YC, Panula P. The histaminergic system regulates wakefulness and orexin/hypocretin neuron development via histamine receptor H1 in zebrafish. FASEB J. 2011;25:4338–4347. doi: 10.1096/fj.11-188268. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(−/−) mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Wei W, Gu W, Huang P, Ren X, Zhang Z, Zhu Z, Lin S, Zhang B. Visualization of monoaminergic neurons and neurotoxicity of MPTP in live transgenic zebrafish. Dev Biol. 2008;314:84–92. doi: 10.1016/j.ydbio.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS, Sassone-Corsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature. 2000;404:87–91. doi: 10.1038/35003589. [DOI] [PubMed] [Google Scholar]
- Woods IG, Schoppik D, Shi VJ, Zimmerman S, Coleman HA, Greenwood J, Soucy ER, Schier AF. Neuropeptidergic signaling partitions arousal behaviors in zebrafish. J Neurosci. 2014;34:3142–3160. doi: 10.1523/JNEUROSCI.3529-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa T, Marin W, Faraco J, Pezeron G, Appelbaum L, Zhang J, Rosa F, Mourrain P, Mignot E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007;5:e277. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv L, Muto A, Schoonheim PJ, Meijsing SH, Strasser D, Ingraham HA, Schaaf MJ, Yamamoto KR, Baier H. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Molecular psychiatry. 2013;18:681–691. doi: 10.1038/mp.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.