Abstract
Diabetic retinopathy is the leading cause of blindness in the working age population. Unfortunately, there is no cure for this devastating ocular complication. The early stage of diabetic retinopathy is characterized by the loss of various cell types in the retina, namely endothelial cells and pericytes. As the disease progresses, vascular leakage, a clinical hallmark of diabetic retinopathy, becomes evident and may eventually lead to diabetic macular edema, the most common cause of vision loss in diabetic retinopathy. Substantial evidence indicates that the disruption of connexin-mediated cellular communication plays a critical role in the pathogenesis of diabetic retinopathy. Yet, it is unclear how altered communication via connexin channel mediated cell-to-cell and cell-to-extracellular microenvironment is linked to the development of diabetic retinopathy. Recent observations suggest the possibility that connexin hemichannels may play a role in the pathogenesis of diabetic retinopathy by allowing communication between cells and the microenvironment. Interestingly, recent studies suggest that connexin channels may be involved in regulating retinal vascular permeability. These cellular events are coordinated at least in part via connexin-mediated intercellular communication and the maintenance of retinal vascular homeostasis. This review highlights the effect of high glucose and diabetic condition on connexin channels and their impact on the development of diabetic retinopathy.
Keywords: Diabetic retinopathy, connexin, connexin 43, gap junction, hemichannel
1. Introduction
1.1. Diabetes and diabetic retinopathy
Diabetes is a complex metabolic disorder characterized by chronic hyperglycemia. Type 1 diabetes accounts for 5–10% of diabetic patients whereas type 2 diabetes accounts for approximately 90% of the diabetic population. Type 1 diabetes is an autoimmune disease characterized by the destruction of β-islet cells of the pancreas resulting in insulin deficiency. Type 2 diabetes is characterized by a combination of insulin resistance and/or insufficient compensatory insulin secretion (American Diabetes, 2010). In addition to hyperglycemia, disturbances of carbohydrate, protein, and fat metabolism are also known to be present in diabetic patients. Although there are differences in the etiology and the age of onset of diabetes, long-term complications of chronic hyperglycemia are essentially the same, i.e. nephropathy, retinopathy, neuropathy, cardiovascular disease, cerebrovascular accidents, and susceptibility to infections.
Based on fundus images of retinal hemorrhages, microaneurysms, venous beading, neovascularization and vitreous hemorrhage, diabetic retinopathy (DR) is classified into 5 disease severity levels: no apparent retinopathy, mild, moderate, severe non-proliferative DR and proliferative DR (Wilkinson et al., 2003). In addition to DR classification, there are also severity levels for diabetic macular edema as described (Ding and Wong, 2012).
DR progresses from mild to severe stages if not treated. As capillary acellularity increases in the diabetic retina, ischemic areas start producing cytokines including vascular endothelial growth factor (VEGF), the most potent growth factor, which triggers neovascularization and vascular leakage ultimately leading to macular edema. Diabetic macular edema compromises vision significantly, and neovascularization promotes the development of vitreous hemorrhage and tractional retinal detachment that could lead to blindness. Moreover, DR is not exclusively confined to vascular changes. Diabetes is known to impact retinal neurons, glial cells, microglia, and retinal pigment epithelial (RPE) cells even before the vascular changes are detectable.
Hyperglycemia impacts several biochemical pathways including activation of protein kinase C (PKC), polyol pathway, accumulation of advanced glycation end product, increase of oxidative stress and pro-inflammatory cytokines, and inhibition of gap junction intercellular communication (for review, see (Ahsan, 2015; Roy et al., 2017; Roy et al., 2010)). The altered retinal microenvironment is associated with proliferation of extracellular matrix (basement membrane thickening), increased capillary permeability (retinal edema), endothelial apoptosis (acellular capillaries), and angiogenesis (neovascularization).
1.2. Effects of diabetes on cellular components of gap junction intercellular communication
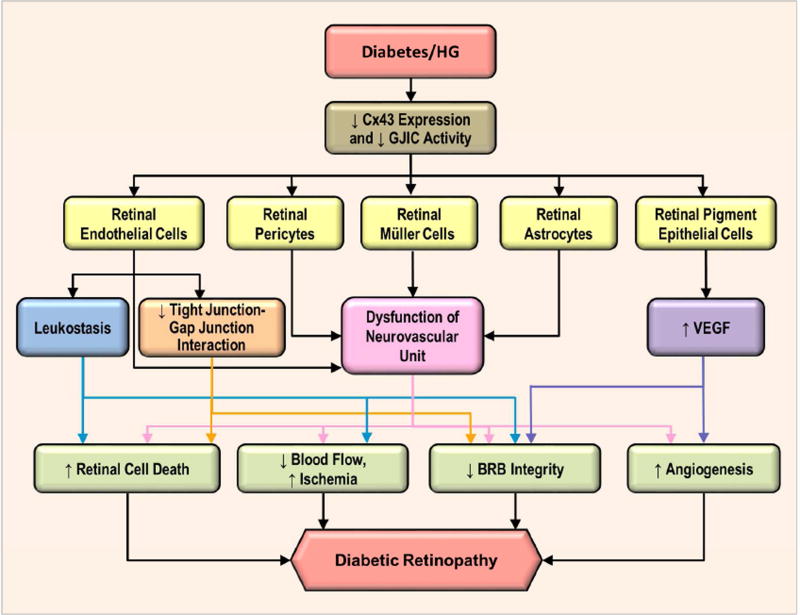
When vascular homeostasis is disrupted in the diabetic retina, it can promote the development and progression of DR by triggering retinal cell death and vascular permeability, two prominent characteristic hallmarks of DR. It is well established that gap junction intercellular communication (GJIC) allows propagation of cell survival and cell death signals between cells. The exchange of small molecules between cells and their microenvironment via connexin hemichannels, and subsequent propagation of signaling events impacting cell survival or death are of profound biological importance since they may influence pathological outcomes in disease processes.
The possibility that retinal vascular cells under high glucose (HG) stress reduced Cx43 expression and compromised cell-cell communication is of pathophysiological significance. In diabetes, cells in the retinal vasculature are known to die by apoptosis; however, these cells undergoing apoptosis die at random locations in the retinal capillaries and rarely, if at all, die next to each other. This phenomenon refutes the possibility of a bystander effect and instead supports a global HG effect on retinal vascular homeostasis where cell death may occur at different locations of the retinal vasculature. Recent work pointed towards the involvement of non-conventional role of connexins in regulating tight junctions of retinal capillaries. Recent studies suggest possible involvement of gap junctions in blood retinal barrier breakdown and vascular permeability associated with DR (Tien et al., 2013; Tien et al., 2014).
Connexin hemichannels have attracted significant attention towards understanding communication between cells and the extracellular microenvironment in disease pathogenesis. In fact, inside the retinal capillaries, endothelial cells are arranged very tightly between adjacent cells but the apical surface of these cells is in constant contact with the hyperglycemic milieu. This could allow interactions between the endothelial cells and the hyperglycemic condition leading to adverse hemichannel-mediated actions. The opening and closure of these channels may impact both autocrine and paracrine signaling and thereby influence cell functionality and survival. The effect of HG can thus be directly mediated through the hemichannels.
Studies in various cell types have shown mitochondrial connexin 43 (mtCx43) as an important regulator of apoptosis and mitochondrial function (Goubaeva et al., 2007). However, only limited information is available about the role of mtCx43 in retinal vascular cells in diabetes. We have investigated the role of mtCx43 in maintenance of mitochondrial morphology and cytochrome c release in retinal endothelial cells in the context of apoptosis in DR. Our recent studies indicate that dysfunction of mitochondrial Cx43 may contribute to the progression of DR (Trudeau et al., 2012).
While current studies and clinical trials have shown encouraging results for targeting abnormal Cx43 expression and activity in non-ocular related pathologies such as in wound healing, the promise for an ocular-based treatment in normalizing Cx43-related cellular activities has not yet materialized. Correcting Cx43-mediated abnormalities in DR remain a novel approach that holds therapeutic potential.
2. Connexin gap junction channels
2.1. Structure
Gap junction channels are comprised of two connexons or hemichannels, each of which are composed of six connexin monomers that form a hexagonal configuration as seen under electron crystallography (Unger et al., 1999; Yeager and Harris, 2007). The docking of the two hemichannels forms a gap junction channel, creating a conduit for cell-cell communication between adjacent cells. The “gap” refers to the narrow clearance of 2–4 nm between the two adjoining cells (Oshima, 2014). Members of the connexin family share similar structural characteristics. Each connexin protein is comprised of four transmembrane α-helix domains, which are connected by two extracellular loops essential for cell-cell recognition and docking (Mese et al., 2007). Conserved in connexin species, there are three cysteine residues in each of the two extracellular loops that are critical for intramolecular stabilization (Krutovskikh and Yamasaki, 2000). In addition, there are cytoplasmic N-terminal and C-terminal domains as well as a cytoplasmic loop linking the second and third transmembrane domains in connexin proteins (Mese et al., 2007).
2.2. Nomenclature
To date, twenty-one members of the connexin family with the same structural topology have been identified in the human genome and all cells in the human body express at least one type of the connexin isoforms (Mese et al., 2007; Oshima, 2014; Sohl and Willecke, 2004). Currently, two systems of nomenclatures for connexin proteins and their genes exist. First system of nomenclature designates connexins according to their molecular weight in kilodaltons. For instance, Cx43 represents connexin with a molecular weight of approximately 43 kilodaltons. Furthermore, the differences in molecular weight between connexins reflect differences primarily in the length of the cytoplasmic part of the chain and the C-terminal domain (Wilkins, 1998). While the N-terminus is conserved in all connexin species, the cytoplasmic loop and C-terminus exhibit a large variation related to sequence and length (Mese et al., 2007). Additionally, this system of nomenclature may include the initial letter of the species placed in front of the connexin protein to identify where the connexin was derived from. Alternatively, the second nomenclature system places connexins that can be separated into subgroups (α, β, or γ) with respect to their extent of sequence identity and length of the cytoplasmic domains (Sohl and Willecke, 2003, 2004). Using this nomenclature, connexin isoforms are labeled “Gj” for gap junction and serially numbered based on the order of their discovery (Sohl and Willecke, 2003).
2.3. Diversity
Homotypic gap junctions are formed when identical connexin subunits come together and dock, whereas heterotypic gap junctions are formed when two different connexons (hemichannels) dock. When different subunits come together, this could give rise to various combinations of heteromeric gap junctions. For a full review of various types of connexin gap junction channels, refer to (Evans and Martin, 2002). There are several heterotypic gap junction channels in many tissues including the retina (Evans and Martin, 2002; Yeager et al., 1998).
Studies have shown that heterotypic channels have different unitary conductance and gating properties from homotypic channels (Delmar, 2002; Rackauskas et al., 2007; Valiunas et al., 2002). In the retina, there are homologous (between same cell types) and heterologous (between different cell types) coupling within various neurons (Baldridge et al., 1998; Vaney and Weiler, 2000). Interestingly, heterologous communication between AII amacrine cells and cone bipolar cells can occur in one direction (Xin and Bloomfield, 1997). In this asymmetrical coupling, both heterotypic channels of Cx36 and Cx45 and homotypic Cx36 channels between AII amacrine and distinct types of cone bipolar cells exhibited different conductance properties (Dedek et al., 2006; Han and Massey, 2005). There is also evidence that formation of heterotypic channels can be a selective process determined by the second extracellular domain of the connexin protein (White et al., 1994; White et al., 1995). Furthermore, the functionality of gap junction channels depends on the connexin isotype. For example, Cx43 channels have 100–300 fold higher selectivity for ATP than Cx32 channels (Goldberg et al., 2002); in Xenopus oocytes expressing both Cx40 and Cx43, different pH gating properties of GJIC have been observed (Gu et al., 2000). These examples demonstrate that diversity of gap junction channels provides a high level of complexity during regulation of GJIC under different physiological conditions.
2.4. Function – Connexin-mediated classical and non-classical function
2.4.1. Classical Cx43-mediated GJIC function
Gap junction channels allow transfer of small molecules less than 1 kilodalton between adjacent cells. These molecules include amino acids, small peptides, glucose, various ions, ATP, ADP, cAMP, IP3, glutathione and glutamate. Studies have shown that there is transjunctional selectivity during GJIC activity (Harris, 2001) that facilitates physiological activities in specific tissues.
GJIC facilitates biological activities, including development, differentiation, proliferation and immune response. Studies have shown that knockout or mutation of certain connexins can lead to malfunction of cardiac tissue (Britz-Cunningham et al., 1995; Kruger et al., 2000). In the heart, GJIC plays a critical role in cardiac homeostasis by allowing conduction of electrical signals throughout the myocardium (Davis et al., 1995).
GJIC occurs between different cell types including antigen-presenting Langerhans cells/T lymphocytes (Concha et al., 1993), and leukocytes/endothelial cells (Oviedo-Orta et al., 2002). Furthermore, the process of transmigration of monocytes between dysfunctional endothelial cells may contribute to the development of atherosclerosis (Wong et al., 2004). Using genetically modified mouse models, specific connexin knockouts, or specific connexin antibodies or peptides, our knowledge about gap junctional function has been substantially improved (Garcia et al., 2016).
2.4.2. Non-coupling connexin function
Connexin proteins perform physiological functions other than classical gap junctional activity. These non-coupling functions involve hemichannels, connexin interacting proteins (gap junction proteome), mtCx43, and channel-independent function in cell survival/death signals.
Hemichannels are extra-junctional connexons located in an unapposed cell membrane. They allow the exchange of molecules between intracellular and extracellular milieu (Goodenough and Paul, 2003). More details of connexin hemichannels will be in Section 4 of this review.
Connexins are known as multifaceted proteins, which can manifest coupling-independent actions (Laird, 2010). Gap junction proteome includes tight junctions, adherens junctions, cytoskeletal proteins, various kinases, phosphatases, and other proteins such as calmodulin and caveolin (Giepmans, 2004; Herve et al., 2012). For example, the PDZ-2 domain of ZO-1 was found to interact with the C-terminal of Cx43 (Sorgen et al., 2004). It was also reported that Cx43 is physically associated and interacts with the amino-terminal domain of ZO-1 in cardiac myocytes (Toyofuku et al., 1998). ZO-1 was also suggested to provide temporary docking for connexins at the cell boundary (Toyofuku et al., 1998). Without this binding, the level of Cx43 will be reduced by 30–40% compared to wild type (Hunter et al., 2005; Toyofuku et al., 2001). Transfection of Cx32 in Cx32-deficient mice hepatocytes was associated with induction of ZO-1, occludin and claudin-1, reinforcing tight junctions (Kojima et al., 2002). Cx43 also co-localizes and co-precipitates with tight junction proteins occludin, ZO-1, and ZO-2 (Laing et al., 2005; Nagasawa et al., 2006; Singh et al., 2005) (Figure 1). Moreover, ZO-1 can modulate gap junction assembly by mediating Cx43 delivery from lipid raft domains to gap junction plaques (Laing et al., 2005). Furthermore, Cx43 was found to be critical in maintaining the tight junction assembly at the blood testis barrier (Li et al., 2010). Interestingly, blocking gap junction activity in brain and lung endothelial cells resulted in impaired barrier function of tight junctions (Nagasawa et al., 2006). The fact that gap junction uncouplers or depressors can inhibit tight junction barrier function in endothelial cells suggests connexins may be necessary to maintain endothelial barrier functions (Nagasawa et al., 2006; Tien et al., 2013). Altogether, the reciprocal influence of connexins and gap junction proteome has expanded our understanding of connexin functions.
Figure 1. Schematic representation of a gap junction and a tight junction organization.
Gap junction proteins, such as Cx43, can directly interact with tight junction proteins and thereby impact barrier characteristics.
Gap junction and hemichannel-independent suppression of cell proliferation was first discovered in HeLa cells transfected with Cx26 (Mesnil et al., 1995). Other studies suggest that the C-terminal portion of Cx43 is mainly involved in cell proliferation and that this portion of Cx43 is present in both the cytosol and nucleus (Dang et al., 2003; Moorby and Patel, 2001). Interestingly, a study has shown that Cx43 inhibits the expression of S phase kinase associated protein 2 (Skp2) to affect cell cycle progression (Zhang et al., 2003). Largely unknown, it has been suggested that connexin can affect gene transcription since the entire length of Cx43 and C-terminal portion of Cx43 were found in the cell nucleus to mediate cell growth and death (Vinken et al., 2012). Cx50 promotes lens fiber differentiation, a function independent of its role in forming gap junctions and hemichannels (Banks et al., 2007; Gu et al., 2003). Moreover, this action is mediated through a direct interaction between the C-terminus of Cx50 and Skp2, an E3 ligase. This interaction retains Skp2 in the cytosol and prevents its nuclear translocation, which results in stabilization of p27/p57 and exit of cell-cycle (Shi et al., 2015).
MtCx43 was first discovered in endothelial cells (Li et al., 2002). Cx43 localization in mitochondria occurs via translocation through the outer membrane, which involves chaperone binding (Rodriguez-Sinovas et al., 2006). During heart preconditioning and intermittent ischemia, an increase in cardiac mtCx43 was reported (Boengler et al., 2005; Lu et al., 2010). In addition, inhibition of mtCx43 translocation may reduce mtCx43’s cardio-protective effects (Boengler et al., 2005). On the contrary, HG reduces mtCx43 in retinal endothelial cells (Mohammad and Kowluru, 2011; Trudeau et al., 2012). Since cardiac mtCx43 regulates apoptosis (Denuc et al., 2016; Goubaeva et al., 2007), HG-induced mtCx43 inhibition may contribute to accelerated cell death in the diabetic retina. More details of the involvement of mitochondrial connexin in diabetes and its complications are provided in Section 3 of this review.
2.5. Connexin phosphorylation
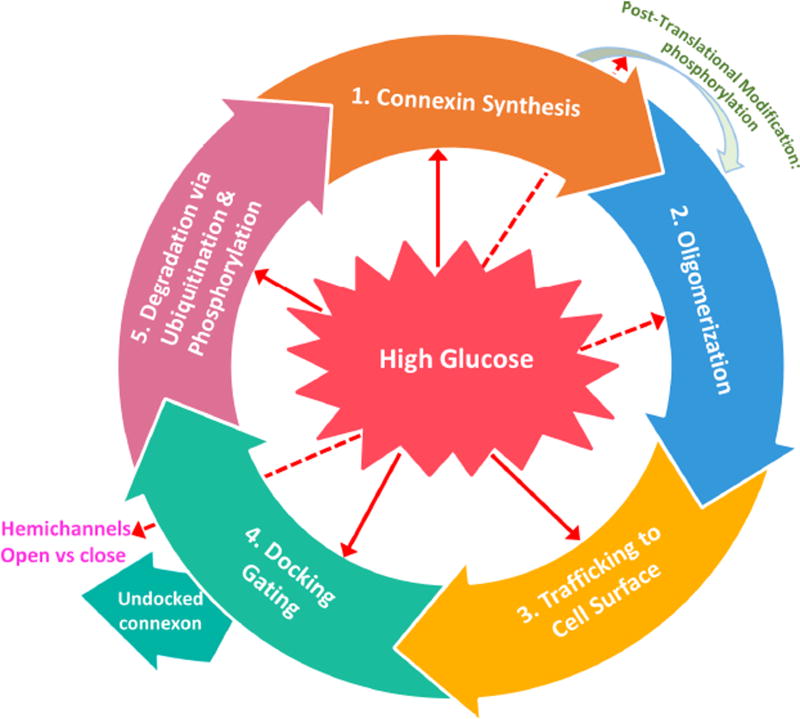
Connexin biosynthesis is a complex process involving specific connexin gene transcription, transport, assembly, and the formation and removal of gap junctions from the cell surface. Cx43 phosphorylation promotes decreased gap junction assembly and can result in Cx43 degradation (Fernandes et al., 2004). Additionally, phosphorylation at specific sites of Cx43 may play a regulatory role in Cx43 channel internalization and degradation, and thus impact gap junctional communication. Also, the role of connexin phosphorylation may differ among cell types, stages of the cell cycle, extracellular matrix interactions, and other functions (Laird, 2005). It should be noted that connexin degradation is not always linked to their phosphorylation status, and that the degradation process for some connexin species may differ. In fact, studies indicate that connexin phosphorylation is not a generic prerequisite for connexins to be targeted for degradation; Cx26 exists in a non-phosphorylated state, and the disease-linked Cx32 mutants degrade with little or no phosphorylation (Laird, 2005).
3. Connexin hemichannels
3.1. General introduction of hemichannels
For over a decade or so, connexin hemichannels have been demonstrated to exhibit specific roles, independent of the function of gap junctions (Goodenough and Paul, 2003). These channels mediate the passage of molecules and communication between intracellular and the extracellular microenvironment. In contrast to GJIC, connexin hemichannels display very low open probability and/or low membrane permeability to small molecules (less than 1 kDa) (e.g., ATP, NAD+, prostaglandins and small fluorescent dyes) and ions in cells under “rest” conditions (Saez et al., 2010). However, the activation or opening of these channels is involved in autocrine/paracrine signaling, which provides a pathway for release of ATP (Cotrina et al., 1998), glutamate (Ye et al., 2003), NAD+ (Bruzzone et al., 2001) and prostaglandins (Cherian et al., 2005), and mediate various aspects of cell signaling and function. Hemichannel activity is regulated by posttranslational modifications of connexin subunits, e.g., S-nitrosylation, phosphorylation and dephosphorylation (Contreras et al., 2003; Retamal et al., 2006), membrane physiochemical conditions, e.g., membrane voltage, pH, mechanical stimulation and concentrations of monovalent and divalent cations (Burra et al., 2010; Schalper et al., 2010; Siller-Jackson et al., 2008) and by extracellular signaling molecules, e.g., ATP, FGF1/2 and lipophilic molecules (Garre et al., 2010; Kar et al., 2012; Retamal et al., 2011; Schalper et al., 2012). In addition to connexin hemichannels, pannexins also form channels with similar molecular stoichiometry and permeability properties as connexin hemichannels. Several lines of evidence suggest important roles of the pannexin channels (hemichannels) under normal physiological and pathophysiological conditions (for review, see (Penuela et al., 2014)). Here we will primarily focus on connexin hemichannels including its function, regulation and the involvement in diseases and cellular and tissue injury.
3.2. Function and regulation
Although the open probability of connexin hemichannels is low under normal physiological condition, hemichannels are over-activated under certain stimulatory conditions, including tissue injury, inflammation and pathological conditions. Abnormal activity of hemichannels has been associated with secondary tissue injury including edema, and compromised vascular integrity and neuronal death (for review see (Bennett et al., 2012) and for the review of retinal injury see (Danesh-Meyer et al., 2016)). Therefore, over-activating connexin hemichannels under pathological conditions mostly result in the disruption of normal homeostasis and cellular function. For instance, in heart cells, functional hemichannels in isolated adult rabbit ventricular myocytes were detected (John et al., 1999; Kondo et al., 2000). Work by Kondo et al demonstrated removal of extracellular [Ca2+] and metabolic inhibition can activate non-selective electrical current, which may impact ionic fluxes, and promote arrhythmias and myocardial infarction (Kondo et al., 2000). The activity of phosphatases and protein kinases is elevated whereas Cx43 is dephosphorylated during myocardial ischemia (Armstrong and Ganote, 1992; Beardslee et al., 2000). In other studies, mitogen-activated protein kinases (MAPK), PKC and PTK signaling pathways are reported to be involved in cardiac protection during ischemia (Weinbrenner et al., 1998). Hemichannels are kept closed when Cx43 is phosphorylated at the MAPK sites under normal extracellular [Ca2+] condition. However, dephosphorylation of those sites by phosphatases activated by biological stress like hyperosmolarity and metabolic stress opens the hemichannels, allowing the influx of extracellular ions which compromises the physiological function of heart cells (John et al., 2003).
Connexin hemichannels form an integrated network of channels present in the astrocytes and neurons. The majority of the cell types in the central nervous system (CNS) exhibit hemichannel activity but the physiological function of hemichannels remains largely unresolved. Majority of the studies focuses on Cx43 hemichannels in astrocytes. Cx43 hemichannels in astrocytes and C6 glioma cells mediate the release of glutamate and ATP. Several reports show that Cx43 expression and [Ca2+]-deficient solutions potentiate ATP release from C6 gliomas, which is inhibited by connexin channel blockers (Contreras et al., 2004; Retamal et al., 2006; Ye et al., 2003). In other study, ATP released from astrocytes is thought to be mediated by the purinergic receptor, P2X7 and not by the Cx43 hemichannels since ATP release was absent in P2X7−/− spinal cord astrocytes but not those from Cx43−/− mice (Suadicani et al., 2006). Connexin hemichannels observed in the different cells of the nervous system are associated pathological implications. The role of Cx45 hemichannels is implicated under pathological conditions like ischemia and epilepsy when extracellular [Ca2+] is absent. Cx30 is expressed in astrocytes (Nagy et al., 1999) and cochlea (Lautermann et al., 1998), and the mutations in Cx30 causes deafness (Rabionet et al., 2000). Interestingly, two mutations in Cx30 (A88V and G11R) formed ATP-permeable, leaky hemichannels (Essenfelder et al., 2004). Another study showed that prenatal nicotine exposure in pregnant mice fed with a high fat/cholesterol increased the opening of Cx43 hemichannels in astrocytes and pannexin 1 channels, and inhibition of these hemichannels reduced ATP and glutamate release in hippocampal slides of nicotine-exposed offspring (Orellana et al., 2014). Additionally, multiple lines of evidences suggest uncontrolled hemichannel activities impair glial cell function, neuronal transmission and survival, and blocking hemichannels in glial cells and/or neurons of the inflamed CNS might prevent neurovascular dysfunction and neurodegeneration (for review, see (Orellana et al., 2011)). It is postulated that glial cell functions are disturbed in metabolic syndrome (MS), a disorder characterized by the multiple physiological syndromes including abdominal fat, insulin resistance, high triglycerides, low HDL and high blood pressure and inflammation. Impairment of glial-to-neuron due to hemichannel dysfunction is implicated as a possible cause of MS progression as summarized in Del Rio et al. (2015) (Del Rio et al., 2015). Currently, although ample evidence supports the role of connexin hemichannels in neuronal cells and astrocytes, more research is necessary, particularly in the in vivo models, to support studies done in cultured cells.
The beneficial effect and cell protective function of connexin hemichannels started to emerge, especially under normal physiological conditions. Connexin hemichannel is an important player in the maintenance of cellular homeostasis. In bone tissues, hemichannels are richly present in osteocytes, the most abundant bone cell type. Bone formation and remodeling is influenced by hemichannels that facilitate transmission of signals elicited by mechanical stimulation to other bone cells and the extracellular matrix (for review, see (Jiang et al., 2007; Plotkin, 2014)). In mouse models of Cx43 knockout and transgenic expressing dominant negative mutants of Cx43 with compromised hemichannels, osteocyte apoptosis and cell death are observed (Plotkin et al., 2008; Xu et al., 2015), which provides support of the protective role of Cx43 hemichannels in bone cells. In response to mechanical stimulation, Cx43 hemichannels mediate the release of bone anabolic factors, such as prostaglandin E2 (Cherian et al., 2005), which acts in an autocrine/paracrine manner to promote anabolic function of the bone. Moreover, the opening of Cx43 hemichannels by mechanical stimulation and bisphosphonates in osteocytes activates Src and ERK kinases and promotes cell survival (Plotkin et al., 2002; Plotkin et al., 2005). Connexin hemichannels have also been proposed to influence the neurite growth of CNS and cell proliferation. ATP through hemichannels acts on purinergic receptors and enhances neurite outgrowth (Belliveau et al., 2006). Cx43 hemichannels stimulate cell proliferation via NAD+ release, which results in reduced S phase in mouse fibroblast cells and increased calcium concentration (Franco et al., 2001). In addition, hemichannels could possibly be an important requirement for the normal inter-kinetic nuclear movement of the retinal progenitor cells during the G1 and G2 phase of the cell cycle. Targeting the connexin hemichannel activity in RPE cells by a mimetic peptide Gap26 slows the normal inter-kinetic movement and the duration of the cell cycle in retinal progenitor cells (Pearson et al., 2005). Moreover, Cx43 hemichannel signaling and ATP release are involved in the initiation of early innate immune response in the endothelium when stimulated by peptidoglycan derived from Staphylococcus epidermidis (Robertson et al., 2010).
Several studies have demonstrated the physiological roles of Cx46 and Cx50 hemichannel in eye lens. Cx46-forming hemichannels are gated by calcium and voltage, and are also mechanosensitive (Bao et al., 2004; Zhang et al., 2006). Due to their mechanosensitive nature, Cx46 hemichannels are implicated in assisting accommodation of the lens by providing transit path for volume flow as the lens changes shape (Bao et al., 2004). Cx50 hemichannels are sensitive to monovalent cations and the density of Cx50 hemichannels is directly proportional to the magnitude of the Cx50 Ca2+-sensitive current. A study from Jiang’s laboratory has shown that PKA activation enhanced both Cx50 gap junctions as well as hemichannel functions due to a direct phosphorylation of Cx50 at Ser395 site (Liu et al., 2011). The functional connexin hemichannels are demonstrated in retina. Cx26 forms hemichannels in horizontal cell dendrites near glutamate-releases site of the cones. Cx26 forms hemichannels at the location where a negative feedback pathway exists from horizontal cells to cones in the first synapse of the retina. Interestingly, all feedback-mediated responses are abolished upon blockage of Cx26 hemichannels. It is suggested that activity of the Ca2+ channels and glutamate release of the cones are modulated by these hemichannels (Kamermans et al., 2001). However, the precise functions and regulatory mechanism of connexin hemichannels in the optical tissues and cells still remain largely obscure.
3.3. Connexin hemichannels in DR
The role of Cx43 and gap junctions in central nervous system injury is thoroughly reviewed in a previously published paper (Chew et al., 2010). The retinal ischemia reperfusion is considered as one of the major models to study DR in animals. The involvement of Cx43 hemichannels in ischemia-reperfusion injury was investigated during in vitro simulated ischemia under oxygen-glucose deprivation. Transient Cx43 hemichannel opening and increase in intracellular [Ca2+] due to oxygen-glucose deprivation are inhibited in the presence of a connexin-channel inhibitory peptide called Gap26 and lanthanum chloride as is the reduction in cell viability. These results suggest that transient connexin hemichannel opening during oxygen-glucose deprivation is responsible for cell injury (Shintani-Ishida et al., 2007). The study by Kerr et al (2012) has shown that unilateral retinal ischemic injury in Wistar rats significantly increases Cx43 expression in retinal astrocytes, Müller cells and vascular endothelium of both the ischemic and contralateral retinas, but not in injured sham controls (Kerr et al., 2012). The study from the same group further shows that the application of a Cx43 mimetic peptide, Peptide 5 that blocks hemichannels reduces vascular leakage induced by ischemia reperfusion damage and increases astrocyte cell survival (Danesh-Meyer et al., 2012). The in vitro cell study from the same paper confirms that the endothelial cell death is mediated by opening of Cx43 hemichannels. Due to a potential challenge of delivering natural peptides due to poor stability, a follow-up study is reported by using Cx43 mimetic peptides encapsulated into slow release nano- and microparticles (Chen et al., 2015b). The treatment with the above nanoparticles results in retinal ganglion cell rescue, while microparticles exhibit a delayed effect on retinal ganglion cell preservation albeit the initial inflammatory response due to the size of microparticles. To enhance the stability of the native peptide in vivo, Peptide 5 is chemically modified to C12-C12-Peptide 5 and the treatment with this modified peptide reduces vessel leakage, inflammation, and protects retinal ganglion cell after ischemic injury (Chen et al., 2015a). These studies point to Cx43 hemichannels as a potential, pharmacological target for the treatment of ischemic retinal damage and DR, and inhibition of hemichannel function could provide new opportunities for therapeutic intervention.
3.4 Connexin hemichannels in tissue injury and other diseases
Cx43 hemichannels are present on the sarcolemma of cardiomyocytes and remain closed under physiological conditions but under ischemic stress, these channels may open resulting in irreversible tissue damage and cell death. This study confirms that blockade of connexin hemichannel opening by Gap26 confers protection to intact heart against ischemia-reperfusion injury regardless of the administration before or after the occurrence of ischemia (Hawat et al., 2010). Another study also shows that opening of Cx43 hemichannels during ischemia-reperfusion appears to be an important mechanism for ischemia-reperfusion injury in the heart given that Gap26 significantly reduces the infarct/risk ratio (Johansen et al., 2011). However, the alternative hemichannel, pannexin channel is not involved. Therefore, Cx43 hemichannels are postulated to play an important part in the ischemic preconditioning of the heart intercepted with brief periods of ischemia and timely closure of the hemichannels by protein phosphorylation by specific kinases may delay the hemichannel opening as well as associated deleterious consequences. Given that hemichannels open in response to injury and inflammatory factors with release of small factors such as ATP to promote and sustain uncontrolled inflammation, inhibition of hemichannel opening is proposed to be a plausible therapeutic strategy to prevent tissue damage. The role of Cx43 hemichannels in the inflammasome pathway in microglia, astrocytes and endothelial cells has been detailed described (Kim et al., 2016a).
In brain endothelial cells, inflammatory peptide bradykinin triggers Ca2+ oscillations and increases endothelial permeability, and these responses are partially blocked by Gap27 or connexin channel inhibitor carbenoxolone (De Bock et al., 2011). They concluded that disruption of endothelial connexin hemichannels can be a novel approach to limiting blood brain barrier (BBB) permeability. BBB is compromised in acute ischemic stroke leading to neuronal damage. Hemichannels formed by Cx43 and pannexin 1 are induced open in the absence of Ca2+, a condition mimicking acute stroke, in a human BBB endothelial cell line hCMEC/D3. Clinically used drugs that have neuroprotective effect also inhibit hemichannels formed by Cx43, implying the potential role of hemichannels in dysregulation of BBB during acute ischemia (Kaneko et al., 2015). On the other hand, controlled opening of hemichannels plays an important role in neurovascular coupling, a process to maintain brain function with proper blood flow. Based on experimental data, it is suggested that neurovascular coupling may activate nitric oxide production which leads to Ca2+ influx through hemichannels leading to the propagation of the signal to end feet of astrocytes to enhance the neurovascular coupling as reviewed in (Munoz et al., 2015). Also, nitric oxide released by the astrocytic hemichannels at end feet may contribute to the vasodilation.
Several connexin mutants with abnormal hemichannel activities are associated with human diseases. For example, hearing sensitivity may be influenced by the release of ATP that could influence outer hair cells through hemichannels (Anselmi et al., 2008; Zhao, 2005). One deafness-linked Cx26 mutation, G45E, associated with a fatal form of keratitis-ichthyosis-deafness syndrome (Griffith et al., 2006; Janecke et al., 2005), causes leaky hemichannels, which leads to cell death (Stong et al., 2006). Cx43G138R mutation is identified in human oculodentodigital dysplasia inherited disorder (ODDD) with anomalies of the face, fingers and toes, eyes, and teeth. This mutation results in a leaky Cx43 hemichannels with the excessive level of ATP release (Dobrowolski et al., 2008).
Activation of Cx43 hemichannels is involved in the pathogenesis of renal ischemic lesions. Moderate ATP depletion activates Cx43 hemichannels in human renal proximal tubule cells and increases cell death, possibly through dissipation of solute fluxes across the cell membrane leading to impairment of the recovery from ischemic insult (Vergara et al., 2003). Ovarian granulosa cells express abundant Cx43 hemichannels. These hemichannels respond to mechanical stimulation such as reduced levels of extracellular divalent cations which can trigger opening of Cx43 hemichannels and subsequent ATP release in folliculogenesis (Tong et al., 2007).
Cx43 expression is upregulated at chronic wound margins and down regulation of Cx43 is important for the process of wound healing. Inhibition of Cx43 channels by Gap27, a connexin mimetic peptide, promotes skin cell migration of keratinocytes and fibroblasts (Wright et al., 2009). Gap27 decreases hemichannel activity with enhanced migration and proliferation in non-diabetic cells, while cells of diabetic origin are less susceptible to Gap27 during early passages, but showed comparable effects to non-diabetic cells in late passages. The cause of the differences between diabetic and non-diabetic cells is correlated with decreased hemichannel activity in diabetic cells (Pollok et al., 2011). A recent study by Kim et al. shows that Peptide 5 which acts on second extracellular loop domain, and prevents lesion spread and vascular permeability in CNS injury, blocks hemichannels and ATP release at low dosage (Kim et al., 2016b). Another mimetic peptide (JM2), which targets the microtubule-binding domain of Cx43, is shown to inhibit ATP released by hemichannels in cultured endothelial cells. Moreover, this peptide suppresses early inflammatory response to submuscular silicone implants (Calder et al., 2015). Danesh-Meyer et al (Danesh-Meyer et al., 2012) reports that a Cx43 mimetic peptide that blocks Cx43 hemichannels can significantly reduce vascular leakage and injury caused by retinal ischemia in male Wistar rats and endothelial cell death following hypoxia in vitro. This study suggests that blocking hemichannels following injury offers new opportunities for treating ischemia in CNS. Studies in bovine corneal endothelial cells show that RhoA is a key player that mediates thrombin-induced inhibition of Cx43 hemichannels, consequently the inhibition of ATP-dependent paracrine signaling under pathophysiological stress (Ponsaerts et al., 2012). Using the same cell line, this group also reports that the interaction between intracellular loop domain and C-terminal tail domain plays a key role in controlling the activity of Cx43 hemichannels (D'Hondt et al., 2014). This knowledge may contribute to the development of new peptide drugs that can specifically inhibit Cx43 hemichannels. Therefore, the hemichannel-blocking peptides are postulated to be developed into potential treatment alternatives for injury, inflammatory and chronic disease. The role of Cx43 hemichannels has drawn significant attention, especially in the pathogenesis of chronic inflammation in the eye (Zhang et al., 2014). They hypothesize that vascular disruption mimics that seen in tumor and can be prevented with the modulation of connexin hemichannels and further suggest that maintaining or restoring cancer vasculature, in contrast to current opinion, leads to reduced tumor hypoxia and promotes the survival of normal cells. Refer to Table 1 for a summary of recently published studies on impacts of HG or diabetes-induced changes on GJIC and hemichannels.
Table 1.
Summary of recently published studies on impacts of HG or diabetes-induced changes on GJIC and hemichannels
| Connexin | Cell/Tissue | Results | References |
|---|---|---|---|
| Cx43 | T cells in non-obese diabetic (NOD) mice | Age-dependent loss of suppressor functions in T(regs) cells of NOD mice is associated with reduced GJIC and Cx43. | (Kuczma et al., 2015) |
| Cx43 | Pericytes and endothelial cell (EC) | Diabetic pericytes are unable to sustain EC growth arrest in coculture and reduces Cx43. | (Durham et al., 2015) |
| Cx43 | Rat retinal endothelial cells (RRECs) | HG downregulates Cx43 and GJIC, and downregulated Cx43 reduces tight junction protein ZO-1; Cx43 and GJIC may be involved in breakdown endothelial barrier in DR. | (Tien et al., 2013) |
| Cx30.2 | Rat retinal endothelial cells (RRECs) | HG reduces Cx30.2 protein and diminishes GJIC; Cx30.2 knockout increases acelluar capillaries and pericyte loss, suggesting downregulation of Cx30.2 and GJIC in retinal vascular lesions in early DR. | (Manasson et al., 2013) |
| Cx43 | Primary human dermal fibroblasts | Cx43 mimetic peptide Gap27 enhances scrape-wound closure in diabetic conditions by inhibiting GJIC; irrespective of the IGF-1:IGFBP-5 balance. | (Wright et al., 2013) |
| Cx43 | Rat retinal endothelial cells (RRECs) | HG downregulates Cx43 in mitochondria; inhibition of gap junctions by β-GA induces mitochondrial fragmentation and increase cytochrome c release. | (Trudeau et al., 2012) |
| Cx43 | Fibroblasts from the human chronic diabetic foot ulcers (DFU) and NIH3T3 cells | HG increases Cx43 protein and GJIC, and represses filopodial extensions and migration rates; upregulation of Cx43 in fibroblasts in DFUs correlates with inhibition of fibroblast migration. | (Mendoza-Naranjo et al., 2012) |
| Cx43 | Human kidney (HK) 2 and human proximal tubule cells | TGF-β1 induced EMT leads to loss of E-cadherin, and reduces Cx43 and GJIC in the proximal tubules under diabetic conditions. | (Hills et al., 2012) |
| Cx37, Cx40 and Cx43 | Human omental arteries and veins | In both vessels, responses to bradykinin are partially blocked by gap junction inhibitor carbenoxolone. GJIC is involved in endothelium-dependent hyperpolarizing (EDH) in response to bradykinin. | (Hammond et al., 2011) |
| Cx26, Cx30 and Cx43 | Astrocytes and brain tissues | Reduced Cx30 and Cx43, not Cx26 are seen in cultured astrocytes by HG and in inferior colliculus of STZ-diabetic rats, not in cerebral cortex, and decreased GJIC in astrocytes by HG. | (Ball et al., 2011) |
| Not specified | Rabbit optic never head (ONH) | ONH blood flow is decreased in diabetic, not healthy rabbits, with reduced ocular perfusion pressure (OPP); inhibition of GJIC by octanol or Gap27 reduces ONH blood flow even in the healthy rabbits. | (Shibata et al., 2011) |
| Cx43 | Ex vivo human skin tissue | Diabetic cells are less susceptible to Gap27 in cell migration and proliferation in early passages, and similar response in late passages and correlates with connexin hemichannel activity. | (Pollok et al., 2011) |
3.5. Conclusion and Perspective
Prior studies point to the specific role of hemichannels in various aspects of homeostasis of different organ systems under normal physiology and pathophysiology. Although great advances have been achieved to understand roles of hemichannels injury repair and wound healing, the specific function of hemichannels in diabetes and DR is largely speculative. Development of mouse model for testing the hypothesis associated with mutations resulting in hemichannel dysfunction needs attention. In addition, the possible involvement of pannexin channels also cannot be ignored (Panchin et al., 2000). Multiple studies suggest that these two types of hemichannels have a close relationship and work coordinately in the CNS that differentially regulates the ionic and metabolic fluxes and cellular function (Decrock et al., 2015; Jiang and Penuela, 2016).
4. Effect of HG and/or diabetes on connexin expression
4.1. Connexin abnormalities in non-retinal tissues and organs
Many diabetic complications have been shown to have differential expression of connexin proteins in various tissues. Among the twenty-one connexins discovered in humans (Sohl and Willecke, 2003), Cx43 is the most ubiquitous. In this section, altered Cx43 in diabetes is discussed. Studies of Cx43 expression and GJIC activity in hyperglycemic conditions and diabetes revealed new understanding of diabetic pathology in molecular level.
4.1.1. CNS complications related to connexin abnormalities
Some epidemiological studies report that type 2 diabetes mellitus is a risk factor for cognitive impairment (Biessels et al., 2006; Kalmijn et al., 1995; Manschot et al., 2007). Though not conclusive, disruption of BBB, microvascular disease and glucose toxicity are considered as possible contributing factors (Biessels et al., 2006; Mogi and Horiuchi, 2011). Current concept of neurovascular unit in the brain implies the coordination between neurons, astrocytes, endothelial cells and pericytes to maintain homeostasis. Cx43 expression in neurons, astrocytes (Nadarajah et al., 1996; Orthmann-Murphy et al., 2008), endothelium, and pericytes suggests its role in the function of neurovascular units in metabolic coupling, vascular control and maintenance of BBB (Salmina et al., 2014).
Impaired GJIC activity and Cx43 downregulation were observed in astrocytes and in brain slices of inferior colliculus in streptozotocin (STZ)-induced diabetic rat brains (Ball et al., 2011; Gandhi et al., 2010). These findings may have an effect on neurovascular unit function in diabetes. Ischemia-reperfusion of brain in Akita diabetic mice revealed marked reduction in Cx43 immunoreactivity whereas in control mice, ischemia-reperfusion brought increased Cx43 immunoreactivity, which may imply that diabetes decreases glial activation after an ischemic insult and renders less neuronal support and more diabetic stroke severity (Kalani et al., 2015).
4.1.2. Macrovascular complications related to connexin abnormalities
Diabetes is an independent risk factor of cardiovascular disease. Type I diabetes carries a 10-fold increase in risk of cardiovascular disease compared to age-matched non-diabetic population (Laing et al., 2003). Cx37, Cx40 and Cx43 are the main gap junction proteins present in aorta and coronary arteries (van Kempen and Jongsma, 1999). Cx40, Cx43 and Cx45 are the dominant isoforms in the heart (Davis et al., 1995). Moreover, myoendothelial gap junctions between vascular endothelial cells and smooth muscle cells have been reported in rat mesentery arteries, allowing transfer of currents and small molecules to coordinate arterial functions (Sandow et al., 2009). These findings suggest that connexin expression plays a role in the regulation of vascular tone, blood flow and pressure.
Downregulation of Cx43 was observed in diabetic aortic endothelial and smooth muscle cells, and excessive phosphorylation of Cx43 impaired ventricular conduction (Inoguchi et al., 2001). Decreased Cx43 is involved in the susceptibility of diabetic rats to hypokalemia-induced ventricular fibrillation (Okruhlicova et al., 2002). In parallel, Cx40, Cx43 and Cx45 mRNA is increased in sino-atrial node in STZ-induced diabetic rats with prolonged conduction (Howarth et al., 2007). Total and phosphorylated Cx43 levels are increased in ventricular muscles of STZ-induced diabetic rats without mRNA change. The phosphorylation of Cx43 possibly associates with GJIC suppression and slowing down of ventricular conduction (Howarth et al., 2008; Joshi et al., 2015). Taken together, these findings suggest that differential expression of gap junctions in diabetes contributes to the development of diabetic cardiomyopathy.
4.1.3. Renal complications related to connexin abnormalities
Diabetic nephropathy is a well-known complication of diabetes. Nine connexins have been found in the kidney (Wright et al., 2012). A study reported that gap junctions are involved in tubuloglomerular feedback (Ren et al., 2002). Electric coupling through gap junctions in the vessels (Segal and Duling, 1989) for conduction of vasomotor response is strongly suggested in nephrons because of the distribution of Cx37, Cx40 and Cx43 in afferent arterioles and only Cx43 in post-glomerular efferent arterioles (Zhang and Hill, 2005). An increase in Cx40 in pre-glomerular arterioles and a decrease in Cx43 in post-glomerular arterioles were observed in STZ-treated diabetic mice. In addition, connexins may participate in the regulation of glomerular blood flow and filtration rate. In HG condition, downregulation of Cx43 promotes the senescence of glomerular mesangial cells. Enhanced phosphorylation of Cx43 and Cx37 downregulation was reported in renin-secreting cells in Zucker diabetic fatty rats associated with glomerular hyperfiltration (Takenaka et al., 2011). Increased Cx43 expression and increased calcium ion concentration were observed in human collecting duct cells grown in HG medium (Hills et al., 2006). Interestingly, Cx43 downregulation in podocytes is closely associated with the progression of diabetic nephropathy (Sawai et al., 2006). These findings imply HG and diabetes influence renal function greatly through altered connexin expression and GJIC activity. However, more studies are needed to better understand the role of connexins in influencing renal functions under hyperglycemic conditions.
4.1.4. Genitourinary complications related to connexin abnormalities
Erectile and bladder dysfunction are more common in diabetic patients compared to non-diabetic age-matched individuals. Cx43 gap junctions were found to be the predominant gap junction in the corpus cavernosum (Campos de Carvalho et al., 1993). Corporal smooth muscles exhibits increased Cx43 permeability in diabetic rats. This may contribute to increased contractility and decreased relaxation of penile corpora, resulting in erectile dysfunction (Brink et al., 2006). Significant decrease of Cx43 expression in penile corpora in diabetic rats (Suadicani et al., 2009; Traish et al., 2013) will likely reduce coupling and coordination, which can be prevented from insulin therapy (Suadicani et al., 2009). Cx43 is localized in lamina propia myofibroblasts and interstitial cells of bladder, suggesting that Cx43 may play a role in coordinating detrusor muscle bundles (Ikeda et al., 2007). Increased Cx43 expression in bladder of diabetic rats may confer more gap junction-mediated signaling and activity in the detrusor muscle (Suadicani et al., 2009). These findings provide a molecular understanding of detrusor over-activity in diabetes.
4.1.5. Other complications related to connexin abnormalities
Diabetic patients have problems with delayed wound healing, which may result in chronic foot ulcer and lead to amputation. Multiple connexins are found in the skin, of which Cx43 is the most abundant in the epidermis. In diabetic rat skin, Cx43 is downregulated in the epidermis while it is upregulated in the dermis. Re-epithelialization begins at the time when Cx43 levels decline (Wang et al., 2007). Increased Cx43 and GJIC activity in diabetic fibroblasts correlate to decreased proliferation (Abdullah et al., 1999). Overexpression of Cx43 in human diabetic foot ulcers promotes fibroblast migration and contributes to impaired wound healing (Mendoza-Naranjo et al., 2012). Interestingly, a Cx43 antisense-oligodeoxynucleotide strategy has demonstrated increased re-epithelialization (Qiu et al., 2003; Wang et al., 2007). In fact, a clinical trial of Cx43-based peptide has recently launched in chronic diabetic foot ulcer patients (Grek et al., 2015a).
Cx43 is the predominantly expressed connexin in granulosa cells, and decreases in cumulus cell-enclosed oocyte in diabetic mice (Ratchford et al., 2008). Cx43 is also the most dominant connexin expressed in the blastocyst period (Hardy et al., 1996). Additionally, a high level of Cx43 expression in germ cells of healthy rats is indicative of high fertilization rate (Aktug et al., 2013). Moreover, Cx43 expression in human cumulus cells is correlated with pregnancy outcome during in vitro fertilization (Winterhager and Kidder, 2015). These clues may help elucidate the mechanisms of diabetic infertility involving cell-cell communication. Refer to Table 2 for a summary on differential changes in connexins in diabetes.
Table 2.
Differential changes of connexins in diabetes, ocular diseases and DR
| Connexin | Ocular diseases | Diabetes affected tissues | Diabetic Retinopathy | References |
|---|---|---|---|---|
|
| ||||
| Cx26 | Keratitis-Ichthyosis-Deafness syndrome | Astrocyte ↓ | (Mhaske, Levit et al. 2013) | |
| (Ly, Yee et al. 2011) | ||||
|
| ||||
| Cx30.2 | Endothelial cell ↓ | (Manasson, Tien et al. 2013) | ||
|
| ||||
| Cx32 | Perineurium ↓ | (Pitre, Seifert et al. 2001) | ||
|
| ||||
| Cx37 | Renin secreting cell ↓ | (Takenaka, Inoue et al. 2011) | ||
| Oocyte ↓ | (Ratchford, Esguerra et al. 2008) | |||
|
| ||||
| Cx40 | Coronary endothelial cell ↓ | (Makino, Platoshyn et al. 2008) | ||
|
| ||||
| Oculodentodigital dysplasia | (Huang, Shao et al. 2013) | |||
| Brain astrocyte ↓ | (Gandhi, Ball et al. 2010) | |||
| Inferior colliculus of brain ↓ | (Ball, Harik et al. 2011) | |||
| Heart ↓ | (Lin, Ogawa et al. 2006, Ball, Harik et al. 2011) | |||
| Aortic smooth muscle ↓ | (Inoguchi, Yu et al. 2001) | |||
| Bronchial epithelial cell ↓ | (Yu, Yang et al. 2016) | |||
| Renal mesangial cell ↓ | (Zhang, Chen et al. 2006) | |||
| Renal collecting duct ↑ | (Hills, Bland et al. 2009) | |||
| Skin ↑ | (Abdullah, Luthra et al. 1999, Sutcliffe, Chin et al. 2015) | |||
| Germ cell ↓ | (Aktug, Bozok Cetintas et al. 2013) | |||
| Cx43 | Penile corpora ↓ | (Suadicani, Urban-Maldonado et al. 2009, Traish, Stottrup et al. 2013) | ||
| Detrusor smooth muscle ↑ | (Suadicani, Urban-Maldonado et al. 2009) | |||
| Detrusor interstitial cell ↓ | (Canda, Dogan et al. 2014) | |||
| Müller cell ↓ | (Muto, Tien et al. 2014) | |||
| Endothelial cell ↓ | (Sato, Haimovici et al. 2002, Fernandes, Girao et al. 2004) | |||
| Pericyte ↓ | (Li, Sato et al. 2003, Durham, Dulmovits et al. 2015) | |||
| Astrocyte ↓ | (Ly, Yee et al. 2011) | |||
| RPE cell ↓ | (Losso, Truax et al. 2010) | |||
|
| ||||
| Cx45 | Sino-atrial node ↑ | (Howarth, Nowotny et al. 2007) | ||
|
| ||||
| Cx46 | Congenital cataract | (Tong, Sohn et al. 2013) | ||
|
| ||||
| Cx50 | Congenital cataract | (Berthoud, Minogue et al. 2013) | ||
4.2. Retina
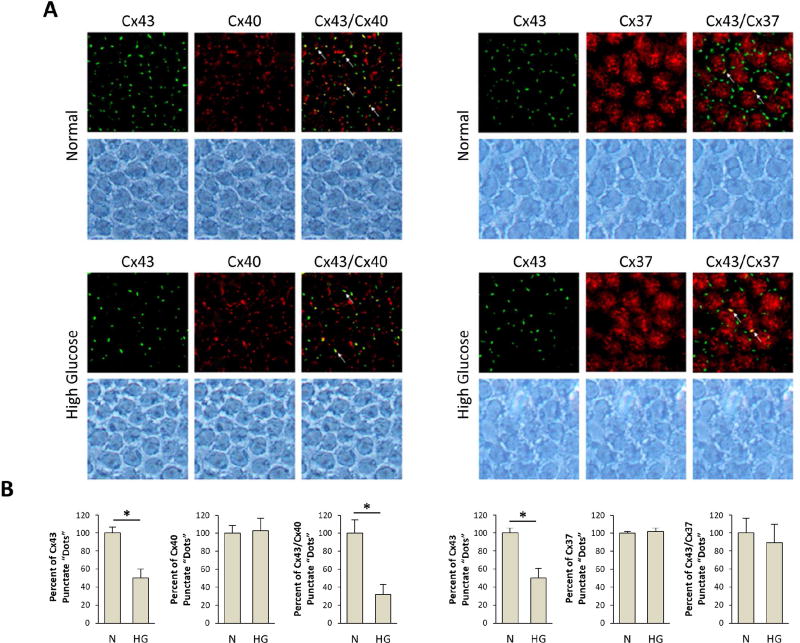
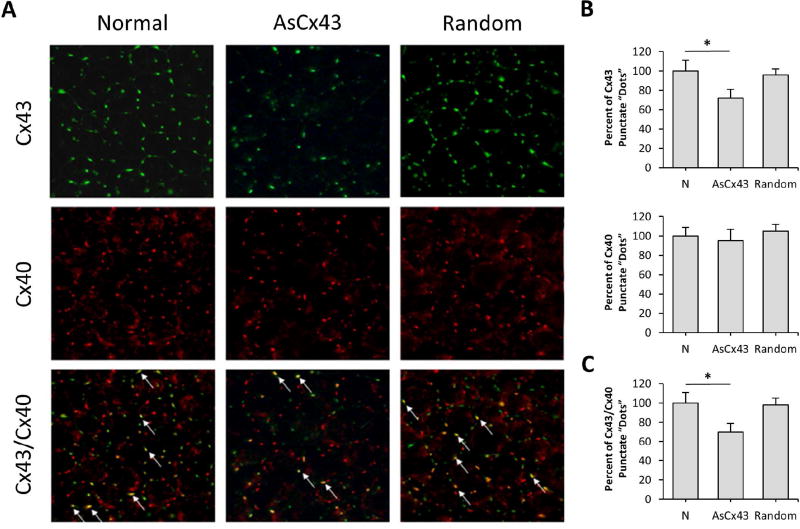
Disruption of retinal vascular homeostasis involving compromised GJIC activity is associated with the development and progression of DR. Studies have demonstrated that Cx43 is abundantly expressed in the retina (Janssen-Bienhold et al., 1998), contributing to the maintenance of the blood retinal barrier (BRB) and plays a central role in retinal vasculature homeostasis (Figueroa et al., 2004). Furthermore, endothelial cells and pericytes possess gap junctions and exhibit junctional transfer both in vitro (Larson et al., 1990) and in vivo (Little et al., 1995; Oku et al., 2001). In addition, morphometric analysis of human retinal capillaries by electron microscopy has shown that endothelial cells and pericytes come in contact with each other through thinned regions of the basement membrane (Carlson, 1989). Interestingly, a study demonstrated that advanced glycation end products downregulated Cx43 expression in a dose-dependent manner and mainly through reduced Cx43 transcription, suggesting that hyperglycemic stress may interfere with the connexin synthesis (Wang et al., 2011). To better understand how HG impacts connexin expression and its functionality in the context of microvascular endothelial cells, a study was undertaken to determine whether HG condition alters expression of endothelial-specific connexins (Cx37, Cx40, and Cx43), connexin phosphorylation pattern, and GJIC activity in microvascular endothelial cells (Sato et al., 2002). A significant downregulation of Cx43 expression at both the mRNA and protein levels in cells grown in HG conditions was observed; however, Cx37 and Cx40 expression levels were not altered by HG. In addition, three forms of Cx43 were identified using alkaline phosphatase and Western blot analyses: a non-phosphorylated form (P0) and two phosphorylated forms (P1 and P2) and all three forms were downregulated by HG condition. Immunofluorescence microscopy revealed reduced Cx43 plaques, or sites of contact between adjacent cells, in cells grown in HG medium. Furthermore, these cells exhibited reduced GJIC activity, which was correlated with decreased Cx43 expression (r=0.9). This study showed for the first time that HG condition not only impaired Cx43 synthesis but also compromised its functional GJIC activity in microvascular endothelial cells (Sato et al., 2002). A study supporting these findings was reported by Fernandes et al. (2004) showing that HG reduces Cx43 expression not only at the transcriptional level but also through accelerated degradation of Cx43 by a proteasome-dependent mechanism, leading to endothelial cell dysfunction associated with the breakdown of the BRB in DR (Fernandes et al., 2004). Interestingly, intracellular trafficking of Cx43 from the cytoplasm to the cell surface may also play a critical role in maintaining functional GJIC activity. Under HG condition, intracellular trafficking of Cx43 was compromised in retinal endothelial cells (Stottrup and Roy, 2012), thereby disrupting the docking of hemichannels and thus inhibiting GJIC activity.
While studies have established that HG reduces Cx43 expression and thereby inhibits cell-cell communication, the direct consequences of compromised GJIC activity are not well understood. Previous studies have reported that changes in GJIC activity can impact cell survival and also promote apoptosis (Krysko et al., 2004; Nakase et al., 2003; Yasui et al., 2000). To determine whether HG-induced Cx43 downregulation and impaired GJIC activity impact cell survival, microvascular endothelial cells grown in HG condition or transfected with antisense-Cx43 oligonucleotides were assessed for apoptosis (Li and Roy, 2009). Interestingly, DNA ladder assay, Terminal Deoxynucleotidyl Transferase-Mediated Uridine 5′-Triphosphate-Biotin Nick End Labeling (TUNEL) assay, and differential dye staining assay revealed that there was a significant increase in the number of apoptotic cells in both cells grown in HG and in cells treated with antisense-Cx43 oligonucleotides that exhibited reduced Cx43 expression, lower number of Cx43 plaques and compromised GJIC activity. In addition, cells treated with β-GA, a GJIC inhibitor, showed accelerated apoptosis compared to that in control cells. These findings demonstrated for the first time that a specific downregulation of Cx43 expression alone concomitant with reduced GJIC activity promotes the development of apoptosis in microvascular endothelial cells. Likewise, HG-induced Cx43 downregulation may be one of the mechanisms that trigger apoptosis associated with the development of DR. The exact mechanisms by which HG promotes retinal vascular cell apoptosis are not fully understood. However, this phenomenon may be explained at the level of gap junction activity. Retinal vascular cells connected to each other by gap junction channels exchange various ions and small metabolites including cAMP, Ca2+, and other necessary molecules essential for cell survival, growth, proliferation, and regulation of homeostasis (Andrade-Rozental et al., 2000; Vinken et al., 2006). Therefore, maintenance of GJIC activity is, at least in part, necessary for retinal endothelial cell survival.
In addition to its conventional role in maintaining GJIC activity, Cx43 proteins have been implicated in regulating tight junction barrier characteristics. Findings from previous studies underscore the functional interdependences between Cx43, a gap junction protein, and ZO-1 and occludin, tight junction proteins (Laing et al., 2005; Nagasawa et al., 2006). While ZO-1 (Gardner, 1995) and Cx43 expression levels (Fernandes et al., 2004; Li and Roy, 2009; Sato et al., 2002) are reduced under HG conditions, the molecular interaction and functional relationship between these gap junction and tight junction proteins are not well understood. Therefore, a study was performed to determine whether HG-induced Cx43 downregulation alters occludin and ZO-1 expression in rat retinal endothelial cells (RRECs), and consequently impact their barrier characteristics (Tien et al., 2013). In RRECs, immunostaining assay revealed an extensive network of Cx43 localization near the cell surface (Figure 2). Western blot analysis revealed that both cells grown in HG medium or cells transfected with Cx43 small interfering RNA (siRNA) exhibited reduced Cx43 expression. More importantly, this reduction in Cx43 expression is associated with reduced ZO-1 and occludin expression. Decreased expression of these tight junction proteins is concomitant with increased cell monolayer permeability, indicative of impaired tight junction barrier functionality. In addition, Cx43 downregulation mediated by HG or using a Cx43 siRNA strategy results in loss of GJIC activity. Interestingly, plasmid-mediated Cx43 upregulation has a protective effect in preventing HG-induced excess cell monolayer permeability. Findings from this study suggest that reduced Cx43 level exacerbates cell monolayer permeability, at least in part, by reducing ZO-1 and occludin protein expression and compromising their barrier functionalities. Taken together, maintaining Cx43 levels is necessary not only in regulating GJIC activity, but also in strengthening barrier characteristics associated with tight junction proteins.
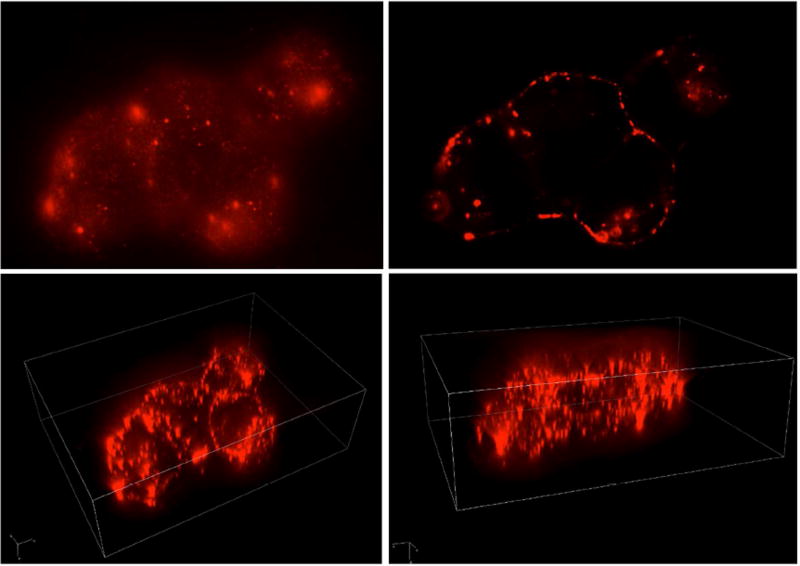
Figure 2. Cx43 localization in Rat Retinal Endothelial Cells (RRECs).
Images show Cx43 immunostaining in RRECs photographed through X, Y, and Z planes.
VEGF, the primary angiogenic cytokine known to drive vasopermeability in DR, is also involved in altering junctional complexes such as gap junctions or tight junctions. Several retinal cell types including endothelial cells, pericytes and RPE cells release VEGF in response to hypoxia, leading to angiogenesis and vascular hyperpermeability (Miller et al., 2013). Interestingly, VEGF was found to disrupt GJIC by phosphorylating Cx43 via activation of MAP kinase in endothelial cells (Nimlamool et al., 2015; Suarez and Ballmer-Hofer, 2001). Specifically, diabetes-induced elevation of VEGF can result in activation of PKC, which can subsequently increase phosphorylation of Cx43, ZO-1, and occludin (Antonetti et al., 1999; Nimlamool et al., 2015). Increased phosphorylation of ZO-1 and occludin can promote vasopermeability while phosphorylated Cx43 can lead to decreased GJIC and eventually apoptosis. However, further studies are necessary to elucidate the relationship between VEGF and connexin channels in the context of DR.
In addition to Cx43, it has been reported that a novel retinal connexin called Cx30.2, also known as gap junction protein delta 3 (GJD3), may be involved in facilitating GJIC activity (Muller et al., 2010). Cx30.2 shares 79% identical residues and 84% similar residues with human Cx31.9 protein (Nielsen and Kumar, 2003). Furthermore, it is expressed in the heart, vascular smooth muscle, testes, brain, (Nielsen and Kumar, 2003) and retina (Muller et al., 2010). In the mouse retina, Cx30.2 was shown to regulate cell-cell coupling between retinal ganglion cells and amacrine cells (Muller et al., 2010). While Cx30.2 was reported to be expressed in the vasculature of different tissues, the effect of HG or the diabetic milieu on Cx30.2 expression and its functional activity was not well known. Therefore, a study was conducted to determine whether Cx30.2 is expressed in the retinal vasculature and whether its expression and/or activity are altered by HG condition. Additionally, Cx30.2 −/− knockout mice are used to assess whether Cx30.2 deficiency promotes the development of AC and PL in the retinas of Cx30.2 −/− mice. Cx30.2 expression was identified both in retinal endothelial cells in vitro and in vascular cells of mouse and rat retinal capillaries in vivo (Manasson et al., 2013). Similar to findings on Cx43, retinal endothelial cells grown in HG medium exhibited reduced Cx30.2 expression, decreased Cx30.2 plaques, and consequently had reduced GJIC activity compared to those grown in N medium. Interestingly, when cells grown in N medium were transfected with Cx30.2 siRNA, reduced GJIC activity was observed. Furthermore, retinas of Cx30.2 −/− mice exhibited a significant increase in the number of AC and PL. These findings indicate that downregulating Cx30.2 alone is sufficient to compromise GJIC activity and thereby trigger retinal vascular cell death associated with DR. This also highlights the fact that not only one form of connexin is altered in HG or diabetic condition but rather a network of connexin subtypes are altered, which can ultimately contribute to the disruption of retinal vascular homeostasis.
An event early in the course of DR is the loss of pericytes (Cogan et al., 1961), which are cells positioned on the abluminal surface of a retinal vessel. Gap junctions provide direct intercellular connections and allow cell-cell communication between the membranes of endothelial cells and pericytes (Bergers and Song, 2005; Hirschi and D'Amore, 1996). Additionally, a study by Oku et al. reported that shortly after the onset of STZ-induced diabetes in rats, cell-cell coupling was markedly reduced in retinal pericytes, assessed using a gap junction-permeant tracer, Neurobiotin, and analyzed by immunohistochemical and electrophysiological methods (Oku et al., 2001). However, not much is known on which family of connexins is impacted by diabetes and whether HG condition interferes with connexin synthesis. Therefore, a study was performed to investigate whether HG condition alters Cx43 expression and compromise GJIC activity in retinal pericytes. There was a significant downregulation of Cx43 expression in human or bovine retinal pericytes grown in HG condition while Cx37 and Cx40 levels were not altered (Li et al., 2003). Furthermore, there was a marked reduction in the relative number of Cx43 plaques between adjacent pericytes in HG condition. Similarly, when pericytes and endothelial cells were co-cultured, the number of Cx43 plaques was reduced in HG condition compared to those grown in normal glucose medium. Moreover, cells with decreased Cx43 levels exhibited reduced GJIC activity in retinal pericytes. Taken together, these results demonstrate that HG condition downregulates Cx43 expression and compromises GJIC activity in pericytes as well as in endothelial cells.
Studies indicate that vascular cells may not be the only cells affected by diabetes in the retina. Müller cells, the principal glial cells of the retina, are also known to be affected by diabetes. These cells span the entire thickness of the retina from the inner limiting membrane to the outer limiting membrane, perform diverse functions (Sarthy et al., 1998), and their processes ensheath retinal neurons. Electron microscopy revealed Müller cell processes often wrap around the retinal vasculature (Hollander et al., 1991). Importantly, these cells form end feet on retinal capillaries (Distler and Dreher, 1996) and therefore can interact with the vascular cells. End feet of retinal Müller cells are known to participate in mediating potassium (Brew et al., 1986), which could compromise Müller cell activity associated with DR. Additionally, aquaporins, present in the end feet of retinal Müller cells, maintain the balance of water-electrolyte metabolism and are altered in diabetes (Zhang et al., 2011). In particular, increased aquaporin4 level in Müller cells is known to promote apoptosis (Da and Verkman, 2004). A key function of Müller cells is the release of glutamate via vesicular exocytosis, which is part of an autocrine glutamatergic-purinergic signaling cascade that prevents osmotic swelling of the cells (Reichenbach and Bringmann, 2013). In addition, Müller cells contribute to the maintenance of the inner BRB (Choi and Kim, 2008) as Müller cells share the ability of astrocytes to induce the formation of barrier properties by vascular endothelial cells, and Müller cells play a major role in the formation of barrier properties in retinal vessels (Tout et al., 1993). Furthermore, studies report that HG condition promotes retinal Müller cell apoptosis in both in vitro and in vivo models of DR (Xi et al., 2005; Yego et al., 2009). Since glial cells impart barrier properties in retinal vascular cells, HG- or diabetes-induced retinal Müller cell death could lead to a loss of endothelial cell barrier properties (Barber et al., 1998). Moreover, a study reported that under hypoxic condition, Müller cells secrete VEGF that can induce MMP-2 expression and activity thus promoting retinal neovascularization in proliferative DR (Rodrigues et al., 2013), suggesting that there is a complex interplay between retinal Müller cells and the retinal vasculature.
A study using confocal microscopy demonstrated that the Müller cells were in close apposition with one another at every level of the retina. However, Cx43 immunoreactivity was heaviest at the outer limiting membrane, where the apical processes of Müller cells are located (Ball and McReynolds, 1998). Cx43 is the major gap junctional protein between Müller cells in lower vertebrates (Sohl et al., 2000). Additionally, Cx43 immunoreactivity was detected in Müller cells labeled with glutamine synthetase in human retinas (Kerr et al., 2010). Furthermore, as connexin hemichannels are involved in the release of ATP from murine Müller cells, calcium signaling appears to be involved in trafficking of connexin hemichannel-containing vesicles towards the plasma membrane and/or in the calcium-dependent opening of connexin hemichannels (Bruckner et al., 2012).
Müller to Müller cell communication through Cx43 channels has been shown to be necessary for their survival. (Zahs and Ceelen, 2006). Müller cell processes can wrap around retinal blood vessels and thereby make frequent contacts with pericytes, which are located abluminally on the vessels (Hosoya and Tomi, 2005). While studies have demonstrated that Müller cells communicate to Müller cells and other cells via gap junction channels, not much was known whether HG condition influences Cx43-mediated GJIC activity between Müller cells and vascular cells. Therefore, a study was conducted to investigate whether HG alters Cx43 expression and GJIC activity in retinal Müller cells, and promotes Müller cell and pericyte apoptosis. In this study, rMC-1, an immortalized Müller cell line isolated from adult rat retina (Sarthy et al., 1998), was grown as a monoculture in normal or HG condition. Additionally, to determine whether reduced Cx43 level in retinal Müller cells affect retinal vascular cells, rMC-1 transfected with Cx43 siRNA were co-cultured with pericytes. In parallel, rMC-1 transfected with Cx43 plasmid were grown in HG. In monocultures of rMC-1 and co-cultures of rMC-1 and pericytes, Cx43 protein expression, number of Cx43 plaques, GJIC activity, and AKT phosphorylation were significantly reduced by HG. Number of TUNEL-positive cells was also significantly increased in rMC-1 monocultures and in rMC-1 and pericyte co-cultures grown in HG medium, indicating increased HG-induced apoptosis. Importantly, when rMC-1 transfected with Cx43 siRNA were grown as co-cultures with pericytes, a significant decrease in GJIC activity, and increase in TUNEL-positive cells was observed, concomitant with decreased AKT phosphorylation. Interestingly, upregulation of Cx43 by transfection with Cx43 plasmid rescued rMC-1 from HG-induced apoptosis. This study demonstrated for the first time that retinal Müller cells grown in HG medium exhibit reduced numbers of Cx43 gap junctions at the site of contact between adjacent Müller cells. Furthermore, HG reduces Cx43 expression and the number of Cx43 plaques in Müller cells and in co-cultures of Müller cells and pericytes. Consequently, GJIC activity is significantly reduced in monocultures of Müller cells as well as in co-cultures of Müller cells and pericytes under HG condition. These results provided the first evidence that communication among Müller cells and between Müller cells and pericytes is compromised under HG condition. Furthermore, compromised GJIC activity promoted apoptosis in these cells, similar to previous reports in retinal vascular cells. Therefore, targeting HG-induced abnormal Cx43 downregulation in retinal Müller cells may provide beneficial effects against the disruption of neurovascular homeostasis associated with DR.
In addition to retinal Müller cells, studies suggest that astrocytes may contribute to the pathogenesis of DR. Specifically, the close proximity of astrocytes to the retinal vasculature and ganglion cell layer, two cell types known to be altered during diabetes, in addition to their role in retinal blood vessel formation, neurovascular coupling, and modulation of pathologic neovascularization, makes them a critical regulator of early diabetic retinal change. Given their role in retinal neuronal and blood vessel function, and the finding that they are lost early in diabetes, astrocytes may play an early and essential role in the development of neuronal dysfunction before overt retinal vessel changes in DR. In addition, gap junction coupling between retinal astrocytes and retinal Müller cells has been established by the intercellular transfer of gap junction permeant tracers (Zahs and Ceelen, 2006; Zahs and Newman, 1997).
Moreover, Cx43 is the predominant gap junctional protein between astrocytes in higher vertebrates (Sohl et al., 2000). Cx43 immunoreactivity was also present in the human retina on glial fibrillary acidic protein-positive astrocytes in the retinal ganglion cell layer (Kerr et al., 2010). Cx43 is expressed primarily on astrocyte processes surrounding blood vessels and chemical synapses, helping in the maintenance of local homeostasis by redistributing excess extracellular K+ ions through the cytoplasm to a network of bordering astrocytes connected by gap junctions (Nagy et al., 1996). However, it is not well understood whether HG or diabetes affects the connexin channels in retinal astrocytes. A study by Ly et al. (2011) aimed to investigate whether astrocytes may play an early role in the development of DR (Ly et al., 2011). Findings from the study indicated that retinal astrocyte Cx26 and Cx43 mRNA and protein expression decreased after 4 weeks of STZ-induced diabetes in rat retinas, before significant astrocyte loss. Concurrently, the retina became hypoxic, with increased HIF-1α expression in the ganglion cell layer. These events led to a decrease in ganglion cell function. Early changes in astrocytes are associated with inner retinal hypoxia and ganglion cell functional deficits. In parallel, retinal Müller cell gliosis and more extensive decreases in neuronal function were observed at a later time point in the development of DR pathophysiology. Moreover, a study by Gandhi et al showed that HG and diabetes impair gap junctional communication in brain astrocytes, at least in part, by downregulation of Cx43 expression (Gandhi et al., 2010). These findings suggest that diabetes-mediated downregulation of Cx43 channels compromise gap junction formation between astrocytes, hindering cell-cell communication, and thereby inhibiting cell survival ultimately leading to astrocyte loss seen early in diabetes. Furthermore, findings suggest that astrocytes may play an early and critical role in changes in retinal vasculature and inner retinal dysfunction in diabetes (Ly et al., 2011).
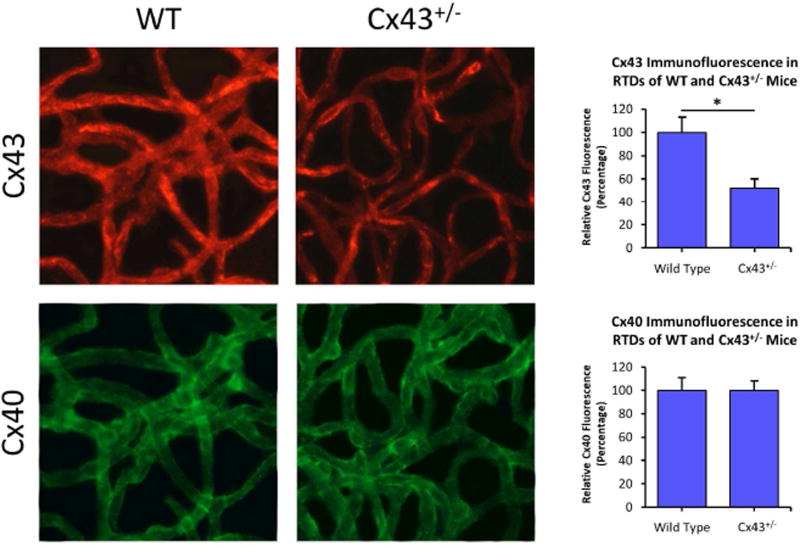
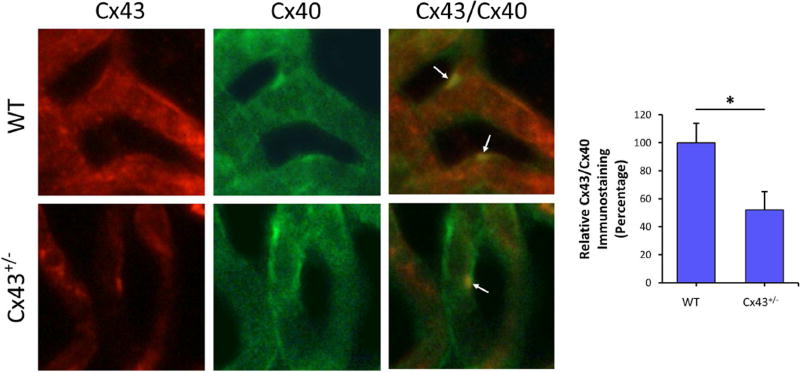
Altered connexin expression in diabetes is known to contribute to complications in pancreas, heart, liver, perineurium, kidney, bladder, and other tissues (Hamelin et al., 2009; Inoguchi et al., 2001; Makino et al., 2008; Pitre et al., 2001; Poladia et al., 2005; Wright et al., 2012; Zhang and Hill, 2005). More specifically, Cx43 deficiency was associated with increased apoptosis in Cx43 +/− mice, and in tissues of Cx43 null mice (Furlan et al., 2001; Nakase et al., 2003; Nakase et al., 2004). However, currently the role of Cx43 in promoting vascular lesions in the diabetic retina is unclear. Therefore, a study was performed to investigate whether diabetes alters Cx43 and thereby promotes retinal vascular cell loss associated with DR. STZ-induced diabetic mice and Cx43 +/− mice were used as animal models to directly determine whether diabetes reduces Cx43 expression in the retina, and to assess the consequence of reduced Cx43 levels in retinal vascular cell loss by apoptosis (Bobbie et al., 2010). The retinal capillary networks of both diabetic and Cx43 +/− mice exhibited decreased Cx43 expression as well as reduced Cx43 immunostaining compared to those in control mice retinas. Interestingly, both the diabetic and Cx43 +/− mouse retinas revealed an increase in the amount of acellular capillaries (AC), pericyte loss (PL), and TUNEL-positive cells, indicating signs of accelerated vascular cell loss by apoptosis. These findings demonstrate that diabetes reduces Cx43 expression in the retina and thereby this reduction in Cx43 level promotes the development of AC and PL by apoptosis associated with DR.
While the retinal vascular endothelium forms the inner BRB, the RPE cells form the outer BRB. Furthermore, the RPE cells are known to be altered in DR (Xu et al., 2011). In chronic hyperglycemic stress, the RPE cells may undergo slow and progressive degradation, leading to compromised outer BRB and central vision loss. Importantly, the tight junctions located in the apical side of RPE cells regulate barrier characteristics. However, the ultrastructural integrity of the tight junctions is compromised by diabetes in the RPE, leading to excess vascular leakage (Xu et al., 2011). Interestingly, most gap junctions are located within the tight junction in RPE cells, suggesting that gap junction-tight junction interactions may be of importance in RPE (Rizzolo et al., 2011). Specifically, strong Cx43 immunoreactivity and extensive gap junction expression were noted in the RPE of human retinas (Kerr et al., 2010). Furthermore, Cx43 contributes to RPE differentiation via cAMP signaling (Kojima et al., 2008). In addition, HG significantly reduced the total cellular Cx43 level (Losso et al., 2010; Malfait et al., 2001) and compromised GJIC activity in rat RPE cells (Himpens et al., 1999; Losso et al., 2010). Interestingly, a compound called trans-resveratrol was found to protect the RPE cells against HG-induced inflammation, HG-induced Cx43 downregulation and GJIC degradation (Losso et al., 2010). Therefore, targeting Cx43 downregulation in the RPE cells may be of therapeutic value to maintain retinal homeostasis and to regulate the outer BRB.
Previous studies have demonstrated that downregulation of Cx43 plays a crucial role in promoting vascular cell apoptosis and excess cell monolayer permeability (Li and Roy, 2009; Tien et al., 2013). To confirm whether these findings are present in vivo, a study was conducted to investigate whether diabetes-induced or siRNA-mediated Cx43 downregulation contributes to the development of AC, PL, and vascular leakage in rat retinas (Tien et al., 2014). Results indicated that there was a significant decrease in Cx43 protein level and Cx43 immunostaining in the retinas of diabetic rats and those intravitreally injected with Cx43 siRNA compared to the retinas of control rats. Interestingly, Cx43 downregulation seen in the diabetic rats and rats intravitreally injected with Cx43 siRNA were concomitant with an increase in the number of AC, PL, TUNEL-positive cells, retinal thickness, and vascular leakage compared to those of control rats. These results demonstrated for the first time that downregulation of Cx43 expression alone is sufficient to promote apoptosis in the retinal vascular cells, which is subsequently evident as ACs and PL in the diabetic retina. Furthermore, directly inhibiting Cx43 expression in rat retinas induced vascular leakage in the retinal capillary networks. These findings suggest that diabetes-induced Cx43 downregulation directly compromises retinal vascular cell survival and contributes to the breakdown of BRB, ultimately promoting the development of retinal vascular lesions associated with DR.
Several studies indicate that Cx43 downregulation contributes to development of AC and PL associated with DR. These studies are based on various animal models including animal models of diabetes (Bobbie et al., 2010; Tien et al., 2014) namely Cx43 +/− mice and STZ-induced diabetic mice. Only recently a study investigating the role of Cx43 in human DR revealed an association between abnormal Cx43 levels in the retina and the developments of retinal vascular lesions in DR (Tien et al., 2016). Specifically, the study focused on understanding whether altered Cx43 expression and reduced GJIC promotes retinal vascular lesions in the human diabetic retina. Donor eye samples represented specific stages in the development of DR. Retinal tissues from human donor eyes of diabetic individuals were compared to those of non-diabetic individuals for assessment of Cx43 plaques, AC, and PL, characteristic hallmarks of DR. Results indicated that Cx43 protein expression was significantly reduced in the diabetic retinas compared to non-diabetic retinas. Additionally, a significant decrease in the number of Cx43 plaques per unit length of retinal capillaries was observed in the diabetic retinas. Similarly, the number of AC and PL was significantly increased in diabetic retinas compared to those in non-diabetic retinas. A strong inverse relationship between retinal Cx43 protein expression and the relative number of AC and the relative number of PL was reported. The results of this study showed for the first time that Cx43 expression is downregulated in human diabetic retinas, and that the extent of Cx43 downregulation influences the severity of retinal vascular lesions. This underscores the importance of cell-cell communication and maintenance of retinal vascular homeostasis. Moreover, enhancing Cx43 synthesis by a plasmid approach showed promising results as did pharmaceutical agents that improve cell coupling. Therefore, improvement of cell-cell coupling in the retinal capillaries may represent a novel therapeutic strategy in preventing retinal vascular cell death in DR (Table 3, Figure 3). Taken together, these studies provide evidence that HG may affect Cx43 at various stages of its connexin life cycle (Figure 4).
Table 3.
Summary of recently published studies on effects of HG or diabetes on connexins
| Connexin | Cell/tissue | Results | References |
|---|---|---|---|
| Cx43 | Airway epithelium | HG decreases Cx43, disassociates Cx43 interaction with tight junction and increases permeabilityof airway epithelial cells. | (Yu et al., 2016) |
| Cx43 | Pericytes and endothelial cell (EC) | Diabetic pericytes are unable to sustain EC growth arrest in coculture and reduce Cx43. | (Durham et al., 2015) |
| Cx43 | Foot | Cx43 mimetic peptide ACT1 accelerates the healing of chronic diabetic foot ulcers in patients. | (Grek et al., 2015) |
| Cx43 | Vascular endothelial cell(VSMC) | Diabetes and hyperlipidemia-induced inflammatory response upregulate the expression of Cx43 through selective downregulation of miRNAs. | (Li et al., 2015) |
| Cx43 | Endothelia cells | Cx43 expression is independent of shear stress and is upregulated by irreversibly glycated advanced glycation end-products (AGE). | (Maria et al., 2014) |
| Cx43 and Cx40 | Heart of type 1 diabetes (T1D) rats treated with streptozotocin (STZ) | Cx43 expression increases, distribution changes and tyrosine phosphorylation decreases in STZ-induced diabetic rats; Cx40 has no change. | (Joshi et al., 2015) |
| Cx43 | Cornea | Alpha-carboxy terminus 1 (αCT1) peptide modified from part of C-terminus suppresses inflammatory response and increases wound healing rate of corneal wound closure in STZ type-I diabetes rat model. | (Moore et al., 2014) |
| Cx43 | Glomeruli of diabetic nephropathy and glomerular mesangial cells (GMCs) | HG decreases Cx43 and p-AMPK; Activating AMPK prevents the downregulation of Cx43 through the inhibition of mTOR. A dominant-negative AMPK reduces Cx43 expression and induces GMC senescence. | (Guo et al., 2014) |
| Cx43 | Heart | STZ-diabetic rats benefit from n-3 polyunsaturated fatty acids (PUFA) intake likely through increased Cx43 protein (not mRNA) level by attenuating myocardial Cx43 abnormalities and improving cardiac function. | (Anna et al., 2014) |
| Cx43 | Human corpus cavernosum (HCC) tissue | The number of Cx43 plaques is reduced in HCC tissues from diabetic or hypogonadal subjects, which may contribute to diminished erectile physiology. | (Traish et al., 2013) |
| Cx43 | Germ cells | Gene expression of Cx43 is significantly reduced in germ cells from diabetic rats along with cell adhesion molecules. | (Aktug et al., 2013) |
| Cx32, Cx26 and Cx43 | Sciatic nerves | COMP-Ang-1 reduces blood glucose and cholesterol in ob/ob mice and improves expression of Cx32 and Cx26, but suppress TNFα and Cx43. | (Kosacka et al., 2012) |
| Cx43 | Human dermal fibroblasts and keratinocytes | Cx mimetic peptide Gap27 regulates extracellular matrix gene expression and improves cell migration in hyperglycemia/hyperinsulinemia, but has less influence in diabetic cells. | (Wright et al., 2012) |
| Cx26, 30.3, 31 and 43 | Wound mouse skin | mRNAs of these four Cx subtypes are elevated in diabetic wounds and selenium downregulates Cx expression, and improves angiogenesis and wound healing. | (Bajpai et al., 2011) |
| Cx37, Cx40 and Cx43 | Rat renal tissue | Cx43 phosphorylation is enhanced in Zucker diabetic fatty (ZDF); Cx43 Gap27 peptide impairs renal autoregulation in Zucker lean (ZL) rate, but not in ZDF rat model of type 2 diabetes. | (Takenaka et al., 2011) |
| Cx43 | Retinal endothelial cells | HG increased matrix metalloproteinase-2 (MMP2) and decreases Cx43 in mitochondria. Overexpression of MnSOD protects retinal mitochondriaby decreasing MMP2 and increasing HSp60 and Cx43. | (Mohammad and Kowluru, 2011) |
| Cx43 | Rat renal tissue | The increase of Cx43 in renal tissue of diabetic rat is alleviated by argirein compound along with reduction of NADPH oxidase and ER stress. | (Hu et al., 2011) |
Figure 3. Effects of diabetes- or HG-induced Cx43 downregulation in DR.
Diabetes- or HG-induced Cx43 downregulation in retinal endothelial cells, pericytes, Müller cells, astrocytes, and RPE cells ultimately lead to accelerated retinal cell death, impaired retinal blood flow, increased ischemia, compromised BRB integrity, and angiogenesis, altogether contributing to the development of DR.
Figure 4. HG affects Cx43 at various stages of connexin life cycle.
HG is known to directly inhibit Cx43 synthesis (1), and thereby downregulate Cx43 expression. HG also alters post-translational modification and oligomerization (2) of connexin subunits, hinders intracellular trafficking of connexin proteins to the cell surface (3), affects connexon docking to form gap junction channels and channel gating (4) or influences open vs close status of hemichannels. Hemichannel formation is impeded by HG, causing a disruption in cell-extracellular retinal microenvironment. Eventually, HG accelerates degradation of Cx43via ubiquitination (5). Dotted arrows indicate areas under investigation.
5. Role of mitochondrial Cx43 (mtCx43) in DR
The functional role of mtCx43 in different cell types, and how it may affect pathological development in diseases is not well understood. The presence of Cx43 in the mitochondria of various cell types has gained attention only recently (Azarashvili et al., 2011; Kiec-Wilk et al., 2012; Kowluru, 2005; Ma et al., 2012; Miro-Casas et al., 2009; Nishikawa and Araki, 2007; Rottlaender et al., 2010). Inhibition of mtCx43 expression is known to influence mitochondrial metabolism, and thereby play a role in the initiation of apoptosis (Wasilewski and Scorrano, 2009). Increased mtCx43 is associated with ischaemic preconditioning and cardioprotection, likely through regulation of mitochondrial ion homeostasis and swelling (Schulz et al., 2007). Additionally, a study indicated mtCx43’s involvement in apoptosis initiation, where inhibition of mtCx43 channels promoted Ca2+ and cytochrome c release from myocyte mitochondria (Goubaeva et al., 2007). Studies have shown localization of Cx43 in the mitochondria and investigated how HG affects mtCx43 expression, and whether altered mtCx43 channel activity is involved in promoting apoptosis in retinal endothelial cells. MtCx43 localization was determined using immunostaining, green fluorescent protein-tagged Cx43 followed by confocal imaging, and Western blot analysis using mitochondrial protein from retinal endothelial cells. The results indicated mtCx43 channel inhibition affects mitochondrial morphology. Western blot analysis performed with protein fractions containing inner and outer mitochondrial membrane indicated Cx43 to be predominantly localized in the inner mitochondria membrane. Similar reports confirmed Cx43 to be localized at the inner membrane of myocardial subsarcolemmal mitochondria (Boengler et al., 2012; Soetkamp et al., 2014). When mitochondria isolated from these cells were treated with β-glycyrrhetinic acid (β-GA), an inhibitor of connexin channels, this resulted in increased cytochrome c release. Furthermore, blockage of mitochondrial connexin channels resulted in mitochondrial fragmentation. Although the exact role of mtCx43 in retinal vascular cells remains unclear, a recent study indicated that mtCx43 confers cardioprotection by reducing cytosolic and mitochondrial reactive oxygen species production, mitochondrial calcium overload, mitochondrial membrane depolarization, and cytochrome c release, and thereby play an important role in the protection of cardiac cells (Pecoraro et al., 2015). Additionally, since inhibition of mtCx43 affects mitochondrial respiration in cardiomyocytes (Boengler et al., 2012), it is possible that HG-induced reduced oxygen consumption in retinal vascular cells (Trudeau et al., 2010; Trudeau et al., 2011) may be linked to decreased mtCx43. Overall, these findings indicate that HG-induced downregulation of mtCx43 protein contributing to decreased Cx43 channel activity, altered mitochondrial morphology, and cytochrome c release. This could provide a framework for a novel mechanism underlying apoptotic retinal vascular cell death in DR.
6. Effect of HG or diabetes on Cx43-Cx40 and Cx43-Cx37 heterotypic gap junctions
Cx43, Cx40, and Cx37 are vascular specific connexins (De Maio et al., 2002; Foote et al., 1998; Guldenagel et al., 2000) and Cx43 amongst the three is the most abundantly expressed connexin in the microvascular endothelial cells (Janssen-Bienhold et al., 1998; Sohl et al., 2000). The docking of dissimilar connexons results in the formation of heterotypic gap junctions and it is likely that differential expression of different connexins influences the formation of heterotypic gap junctions. We have studied the localization, distribution, and expression of heterotypic gap junctions, Cx43/Cx40 and Cx43/Cx37, in rat microvascular endothelial cells, and examined whether the distribution of heterotypic gap junctions and whether the ratio of the connexons is altered by HG condition. Results suggested that in cells grown in normal medium, the ratio of Cx43 to Cx40 plaque count was approximately 60:40 i.e. 1.6 (600±48 vs 370±75, p<0.05); and the ratio of plaque count for Cx43 to Cx37 was 65:35 i.e. 1.9 (540±40 vs 300±12, p<0.05) indicating that the contribution from Cx43 plaques is significantly higher than those of either Cx40 or Cx37 (Figure 5). When cells were grown in HG medium, the ratio of Cx43 to Cx40 and that of Cx43 to Cx37 were reduced to 55:45 i.e. 1.2 (384±35 vs. 318±20, p<0.05) and 55:45 i.e. 1.2 (360±42 vs 300±24, p<0.05), respectively. The number of Cx43/Cx40 heterotypic gap junctions in cells grown in HG medium significantly decreased (Cx43/Cx40: 6.4±2.0 vs 2.1±1.0, p<0.05). Additionally, the distribution pattern of the immunofluorescent plaques of Cx40 and Cx37 was different from that of Cx43. The Cx43 plaques primarily localized at the cell surface, whereas Cx40 and Cx37 were present within the cells. Furthermore, the number of Cx43/Cx40 heterotypic gap junctions was reduced in microvascular endothelial cells transfected with antisense Cx43 oligonucleotides (Figure 6) suggesting that reduction in the expression of Cx43 has a direct effect on the formation of heterotypic gap junctions between Cx43 and Cx40. In conclusion, the result suggests that HG may significantly affect the presence of heterotypic gap junctions, in particular, Cx43/Cx40, in microvascular endothelial cells.
Figure 5. Cx43, Cx40, Cx37 immunostaining in RMECs grown in normal (N) or HG medium.
(A) The number of immunostained Cx43 dots was reduced in cells grown in HG condition, whereas those of Cx40 and Cx37 were not changed. Arrows indicate “yellow” dots representing colocalization of Cx43/Cx40 or Cx43/Cx37 immunostaining. Note the number of Cx43/Cx40 yellow dots is reduced in RMECs grown in HG medium. Corresponding to bright field images are shown directly below the immunostained images. Cx37 distribution is cytoplasmic and on the cell boundaries, compared to Cx43 and Cx40 distribution that was mostly on the cell boundaries only. (B) Graphical representation of Cx43, Cx40, and Cx37 immunostaining observed as punctate “dots”. The number of Cx43 dots was reduced in HG medium compared to those grown in N medium, while the number of Cx40 and Cx37 dots showed no change. Furthermore, the number of Cx43/Cx40 heterotypic gap junctions was reduced, whereas the number of Cx43/Cx37 did not change in cells grown in HG medium compared to that of cells grown in N medium. *p<0.05, n=4.
Figure 6. Cx43 and Cx40 immunostaining in RMECs grown in normal (N) medium, and transfected with antisense Cx43 (AsCx43) or random oligonucleotides.
(A) Images showing Cx43, Cx40, and Cx43/Cx40 immunostaining in RMECs. The number of Cx43 immunostained dots was reduced in cells transfected with AsCx43 oligonucleotides. Arrows indicate “yellow” dots representing colocalization of Cx43/Cx40 immunostaining. The number of Cx43/Cx40 heterotypic gap junctions was also reduced in cells transfected with AsCx43 oligonucleotides. (B) Graphical representation of Cx43 and Cx40 immunostaining. As expected, the expression of Cx43 was reduced in cells transfected with AsCx43 oligonucleotides, and Cx40 showed no change. The observation that Cx43 expression was reduced by AsCx43 oligonucleotides but not the Cx40 expression, demonstrates specificity of the AsCx43 oligonucleotides. * p<0.05, n=4. (C) Graphical representation of the number of Cx43/Cx40 heterotypic gap junctions. The number of Cx43/Cx40 heterotypic gap junctions was significantly reduced in cells transfected with AsCx43 oligonucleotides. *p<0.05, n=4.
As a follow up to the studies using HG cell culture model, experiments were carried out to determine whether reduced Cx43 expression in Cx43+/− knockout mice affects Cx43/Cx40 colocalization and the number of heterogeneous gap junctions in vascular cells of retinal capillaries. Results indicate that Cx43+/− mice exhibit reduced number of heterogeneous gap junctions in vascular cells of retinal capillaries. Since diabetes or HG has been shown to downregulate Cx43 expression and GJIC, this study provides insight into how hyperglycemia might influence the formation and functionality of heterotypic gap junctions in the vasculature of diabetic retinas. Western Blot analysis exhibited significant Cx43 downregulation in the Cx43+/− mice compared to that of control mice (52±7% of control, p<0.05). In addition, Cx43+/− mice exhibited reduced Cx43 immunostaining in the retinal vessels compared to that of control mice (53±7% of control, p<0.05) while there was no change in Cx40 immunostaining (93±9% of control). Furthermore, Cx40 localization showed a more pericellular appearance than that of Cx43 (Figure 7). Cx43+/− mice also expressed reduced number of Cx43/Cx40 heterogeneous gap junction plaques compared to those of control mice (42±22% of control, P=0.001; Figure 8). The finding from this study indicates that modulation of Cx43 expression can directly affect the number of Cx43/Cx40 heterogeneous gap junction plaques, and that Cx43 expression regulates the formation of Cx43/Cx40 heterogeneous gap junctions in the retinal capillaries. In conclusion, the decrease in the number of Cx43/Cx40 heterotypic gap junctions under HG condition may affect exchange of specific metabolites and contribute to disruption of vascular homeostasis associated with DR. Further study is necessary to identify specific cellular and biochemical changes mediated by reduced number of heterotypic gap junctions and their impact on the pathogenesis of DR.
Figure 7. Immunofluorescence of Cx43 and Cx40 in WT and Cx43 +/− mice retinal capillary networks.
Cx43+/− mice compared to that of WT mice exhibited reduced Cx43 immunostaining in the retinal vessels while Cx40 immunostaining showed no change. *p<0.05, n=4. Moreover, Cx40 localization appeared more pericellular than that of Cx43, while Cx43 localization was more on cell boundaries.
Figure 8. Reduced Cx43/Cx40 heterotypic gap junction plaques in Cx43 +/− mice retinal capillary networks.
The number of Cx43/Cx40 heterogeneous gap junction plaques (indicated by arrows) was significantly reduced in the Cx43+/− mice compared to those of WT mice. *p=0.001, n=4.
7. Current therapeutic strategies targeting connexins, gap junctions and hemichannels
There are several strategies in the development of potential therapeutics targeting connexins, gap junctions and hemichannels. The target strategies include the enhancement of GJIC and the inhibition of aberrant opening of hemichannels. The earlier, less-specific strategy was the use of chemicals to upregulate GJIC, e.g., in the treatment of cancer including retinoids, vitamin D, carotenoids, and cAMP (Trosko and Chang, 2001). Later, more specific approaches have been developed including siRNA and short peptides with specific sequences homologous to intracellular and extracellular connexin domains, termed connexin mimetic peptides (Iyyathurai et al., 2013). ACT1 peptide targeting a portion of Cx43 C-terminus enhances GJIC for potential therapies in wound healing and cancer (Grek et al., 2015a; Grek et al., 2015b). Given that connexin hemichannels operate independently of gap junctions and also serve different roles in cell physiology and pathology, several peptides that target to extracellular loop regions of Cx43 have been developed and some of them exhibit stronger inhibitory effects against hemichannels than GJIC. Mimetic peptides, GAP26 and GAP27, are widely studied for their relevance to the E1 and E2 loop domains, respectively. These two peptides inhibit hemichannel activity and permeability in 3–4 min, while the significant inhibitory effect on GJIC inhibition takes much longer, over 30 min (Desplantez et al., 2012; Evans et al., 2012; Iyyathurai et al., 2013; Wang et al., 2012). Another peptide, Peptide5 has been reported that closes hemichannels at order of magnitude lower concertation than other peptides such as Gap26 and Gap27 (O'Carroll et al., 2008). Moreover, as described in the above Section 3.3, this peptide has been successfully applied to in vivo animal models, including DR (intravitreal and systemic, natural peptide, sustained release and stabilized), dry form of age-related macular degeneration (intravitreal), spinal cord injury (intrathecal and systemic delivery models), chronic pain, perinatal sheep brain ischemia, preterm sheep asphyxia, etc. At the doses used for hemichannel blockage Peptide5 does not inhibit gap junction coupling (Kim et al., 2017). Currently several of Cx43 mimetic peptides are under active, pre-clinical, clinical development and different stages of clinical trials for multiple clinical indications, including skin and diabetic foot ulcers and eye injuries.
The routes of delivery of connexin mimetic peptides to retina have been described. In a paper by Danesh-Meyer et al., (2012), Cx43 mimetic peptide, Peptide5 is delivered into the retina through intraperitoneal injection at the start of reperfusion using a retinal ischemic model in rat (Danesh-Meyer et al., 2012). Blocking Cx43 hemichannels by this mimetic peptide reduces vascular leakage and increases retinal ganglion cell survival after ischemic injury. In another study, Peptide5 is similarly delivered via intraperitoneal injection in albino rat model and this treatment reduces neuronal damages likely by reducing choroid inflammation caused by light damage (Guo et al., 2016). The approaches for new peptide drug formulation and delivery are reported in two papers; in the first paper, Cx43 mimetic peptides are encapsulated into slow-release poly(lactic-co-glycolic) acid nanoparticles and microparticles and a triphasic release is shown in vitro with an initial burst release and following a slow release (Chen et al., 2015b). The sustained intravitreal delivery with these Cx43 mimetic peptide-containing nano- and microparticles increases retinal ganglion cell viability. In the second paper, Cx43 mimetic peptide is loaded into hyaluronic acid nanoparticles coated with human serum albumin (Huang et al., 2017). HA-coated nanoparticles enhance cell uptake and ex vivo retinal penetration mediated by the binding of HA to CD44 receptor on the cell surface. Moreover, nanoparticle delivery stabilizes Cx43 peptide, sustains its release and prolongs its bioactivity. Recently, Cx43 mimetic peptide, αCT1 is delivered to mouse retinal pigment epithelium via topical delivery through eye drop in two mouse models (Obert et al., 2017). This peptide targets tight junction protein ZO-1, enhances tight junction function, and reduces VEGF-dependent retinal pigment epithelial dysfunction. Further studies are required to identify the specific mechanism of these blocking peptides so that the specificity and binding kinetics of these pharmacological peptides can be well characterized. Antibody approach provides a more effective alternative for specifically targeting hemichannels, not gap junctions due to the bulky size of the antibody and potential inaccessibility of gap junctional plaques (Riquelme et al., 2013). Moreover, antibody therapeutics offers several advantages over traditional chemical and peptide drugs with regards to drug stability, specificity and lesser side-effects, which could be one of the future directions for drug development targeting connexin and connexin channels.
8. Conclusion / Future Directions
Background DR and its progression to proliferative stages are currently processes without treatment. Despite progress in understanding the cellular and molecular events, a link between compromised vascular homeostasis and development of early vascular lesions i.e., vascular cell loss and increased vascular leakage, in DR remains unclear. It is clear that GJIC plays a critical role in the maintenance of vascular homeostasis in the retina. However, it is unknown how diabetes compromises such cell-cell communication and what are the consequences that lead to the pathogenesis of DR. Future research directions in this field will certainly investigate the mechanistic underpinning(s) of reduced cell-cell communication in diabetic retinal capillaries. The future challenge is to identify the specific connexin subtype(s) and gap junctions and/or hemichannels that are affected under hyperglycemia, given multiple connexins are expressed in the retina and endothelial cells. Finally, identification of key molecules that traverse these connexin channels and play critical roles in the development and progression of the retinal pathology would be important for development of new therapeutic strategies in treating DR.
Acknowledgments
Research was supported by NIH grants R01 EY018218 (SR), EY025528 (SR), EY012085 (JXJ) and Welch Foundation grant AQ-1507 (JXJ).
Appendix A
Abbreviations used in the article.
| AC | Acellular capillaries |
| β-GA | β-glycyrrhetinic acid |
| BBB | Blood Brain Barrier |
| BRB | Blood Retinal Barrier |
| CNS | Central Nervous System |
| Cx | Connexin |
| DR | Diabetic Retinopathy |
| GJIC | Gap Junction Intercellular Communication |
| HG | High Glucose |
| mtCx43 | Mitochondrial Cx43 |
| PL | Pericyte Loss |
| RPE | Retinal Pigment Epithelial |
| rMC-1 | Rat Retinal Müller Cells |
| RRECs | Rat Retinal Endothelial Cells |
| siRNA | Small Interfering RNA |
| STZ | Streptozotocin |
| TUNEL | Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling |
| VEGF | Vascular Endothelial Growth Factor |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah KM, Luthra G, Bilski JJ, Abdullah SA, Reynolds LP, Redmer DA, Grazul-Bilska AT. Cell-to-cell communication and expression of gap junctional proteins in human diabetic and nondiabetic skin fibroblasts: effects of basic fibroblast growth factor. Endocrine. 1999;10:35–41. doi: 10.1385/ENDO:10:1:35. [DOI] [PubMed] [Google Scholar]
- Ahsan H. Diabetic retinopathy--biomolecules and multiple pathophysiology. Diabetes & metabolic syndrome. 2015;9:51–54. doi: 10.1016/j.dsx.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Aktug H, Bozok Cetintas V, Uysal A, Oltulu F, Yavasoglu A, Akarca SO, Kosova B. Evaluation of the Effects of STZ-Induced Diabetes on In Vitro Fertilization and Early Embryogenesis Processes. Journal of diabetes research. 2013;2013:603813. doi: 10.1155/2013/603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33(Suppl 1):S62–69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Rozental AF, Rozental R, Hopperstad MG, Wu JK, Vrionis FD, Spray DC. Gap junctions: the "kiss of death" and the "kiss of life". Brain research. Brain research reviews. 2000;32:308–315. doi: 10.1016/s0165-0173(99)00099-5. [DOI] [PubMed] [Google Scholar]
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. The Journal of biological chemistry. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- Armstrong SC, Ganote CE. Effects of the protein phosphatase inhibitors okadaic acid and calyculin A on metabolically inhibited and ischaemic isolated myocytes. J Mol Cell Cardiol. 1992;24:869–884. doi: 10.1016/0022-2828(92)91100-j. [DOI] [PubMed] [Google Scholar]
- Azarashvili T, Baburina Y, Grachev D, Krestinina O, Evtodienko Y, Stricker R, Reiser G. Calcium-induced permeability transition in rat brain mitochondria is promoted by carbenoxolone through targeting connexin43. Am J Physiol Cell Physiol. 2011;300:C707–720. doi: 10.1152/ajpcell.00061.2010. [DOI] [PubMed] [Google Scholar]
- Baldridge WH, Vaney DI, Weiler R. The modulation of intercellular coupling in the retina. Seminars in cell & developmental biology. 1998;9:311–318. doi: 10.1006/scdb.1998.0235. [DOI] [PubMed] [Google Scholar]
- Ball AK, McReynolds JS. Localization of gap junctions and tracer coupling in retinal Muller cells. The Journal of comparative neurology. 1998;393:48–57. doi: 10.1002/(sici)1096-9861(19980330)393:1<48::aid-cne5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ball KK, Harik L, Gandhi GK, Cruz NF, Dienel GA. Reduced gap junctional communication among astrocytes in experimental diabetes: contributions of altered connexin protein levels and oxidative-nitrosative modifications. Journal of neuroscience research. 2011;89:2052–2067. doi: 10.1002/jnr.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks EA, Yu XS, Shi Q, Jiang JX. Promotion of lens epithelial-fiber differentiation by the C-terminus of connexin 45.6 a role independent of gap junction communication. Journal of cell science. 2007;120:3602–3612. doi: 10.1242/jcs.000935. [DOI] [PubMed] [Google Scholar]
- Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. American journal of physiology. Cell physiology. 2004;287:C1389–1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circulation research. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- Belliveau DJ, Bani-Yaghoub M, McGirr B, Naus CC, Rushlow WJ. Enhanced neurite outgrowth in PC12 cells mediated by connexin hemichannels and ATP. The Journal of biological chemistry. 2006;281:20920–20931. doi: 10.1074/jbc.M600026200. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Garre JM, Orellana JA, Bukauskas FF, Nedergaard M, Saez JC. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012;1487:3–15. doi: 10.1016/j.brainres.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. The Lancet. Neurology. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Bobbie MW, Roy S, Trudeau K, Munger SJ, Simon AM, Roy S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Investigative ophthalmology & visual science. 2010;51:3758–3763. doi: 10.1167/iovs.09-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F, Heusch G, Schulz R. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovascular research. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Boengler K, Ruiz-Meana M, Gent S, Ungefug E, Soetkamp D, Miro-Casas E, Cabestrero A, Fernandez-Sanz C, Semenzato M, Di Lisa F, Rohrbach S, Garcia-Dorado D, Heusch G, Schulz R. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. Journal of cellular and molecular medicine. 2012;16:1649–1655. doi: 10.1111/j.1582-4934.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew H, Gray PT, Mobbs P, Attwell D. Endfeet of retinal glial cells have higher densities of ion channels that mediate K+ buffering. Nature. 1986;324:466–468. doi: 10.1038/324466a0. [DOI] [PubMed] [Google Scholar]
- Brink PR, Valiunas V, Wang HZ, Zhao W, Davies K, Christ GJ. Experimental diabetes alters connexin43 derived gap junction permeability in short-term cultures of rat corporeal vascular smooth muscle cells. The Journal of urology. 2006;175:381–386. doi: 10.1016/S0022-5347(05)00007-8. [DOI] [PubMed] [Google Scholar]
- Britz-Cunningham SH, Shah MM, Zuppan CW, Fletcher WH. Mutations of the Connexin43 gap-junction gene in patients with heart malformations and defects of laterality. N Engl J Med. 1995;332:1323–1329. doi: 10.1056/NEJM199505183322002. [DOI] [PubMed] [Google Scholar]
- Bruckner E, Grosche A, Pannicke T, Wiedemann P, Reichenbach A, Bringmann A. Mechanisms of VEGF- and glutamate-induced inhibition of osmotic swelling of murine retinal glial (Muller) cells: indications for the involvement of vesicular glutamate release and connexin-mediated ATP release. Neurochem Res. 2012;37:268–278. doi: 10.1007/s11064-011-0606-z. [DOI] [PubMed] [Google Scholar]
- Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, Jiang JX. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13648–13653. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder BW, Matthew Rhett J, Bainbridge H, Fann SA, Gourdie RG, Yost MJ. Inhibition of connexin 43 hemichannel-mediated ATP release attenuates early inflammation during the foreign body response. Tissue Eng Part A. 2015;21:1752–1762. doi: 10.1089/ten.tea.2014.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos de Carvalho AC, Roy C, Moreno AP, Melman A, Hertzberg EL, Christ GJ, Spray DC. Gap junctions formed of connexin43 are found between smooth muscle cells of human corpus cavernosum. The Journal of urology. 1993;149:1568–1575. doi: 10.1016/s0022-5347(17)36455-8. [DOI] [PubMed] [Google Scholar]
- Carlson EC. Fenestrated Subendothelial Basement-Membranes in Human Retinal Capillaries. Investigative ophthalmology & visual science. 1989;30:1923–1932. [PubMed] [Google Scholar]
- Chen YS, Green CR, Teague R, Perrett J, Danesh-Meyer HV, Toth I, Rupenthal ID. Intravitreal injection of lipoamino acid-modified connexin43 mimetic peptide enhances neuroprotection after retinal ischemia. Drug Deliv Transl Res. 2015a;5:480–488. doi: 10.1007/s13346-015-0249-8. [DOI] [PubMed] [Google Scholar]
- Chen YS, Green CR, Wang K, Danesh-Meyer HV, Rupenthal ID. Sustained intravitreal delivery of connexin43 mimetic peptide by poly(D,L-lactide-co-glycolide) acid micro- and nanoparticles--Closing the gap in retinal ischaemia. Eur J Pharm Biopharm. 2015b;95:378–386. doi: 10.1016/j.ejpb.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SS, Johnson CS, Green CR, Danesh-Meyer HV. Role of connexin43 in central nervous system injury. Exp Neurol. 2010;225:250–261. doi: 10.1016/j.expneurol.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Choi YK, Kim KW. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41:345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Archives of ophthalmology. 1961;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- Concha M, Vidal A, Garces G, Figueroa CD, Caorsi I. Physical interaction between Langerhans cells and T-lymphocytes during antigen presentation in vitro. The Journal of investigative dermatology. 1993;100:429–434. doi: 10.1111/1523-1747.ep12472117. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Veliz LP, Bukauskas FF, Bennett MV, Saez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain research. Brain research reviews. 2004;47:290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hondt C, Iyyathurai J, Himpens B, Leybaert L, Bultynck G. Cx43-hemichannel function and regulation in physiology and pathophysiology: insights from the bovine corneal endothelial cell system and beyond. Frontiers in physiology. 2014;5:348. doi: 10.3389/fphys.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da T, Verkman AS. Aquaporin-4 gene disruption in mice protects against impaired retinal function and cell death after ischemia. Investigative ophthalmology & visual science. 2004;45:4477–4483. doi: 10.1167/iovs.04-0940. [DOI] [PubMed] [Google Scholar]
- Danesh-Meyer HV, Kerr NM, Zhang J, Eady EK, O'Carroll SJ, Nicholson LF, Johnson CS, Green CR. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain. 2012;135:506–520. doi: 10.1093/brain/awr338. [DOI] [PubMed] [Google Scholar]
- Danesh-Meyer HV, Zhang J, Acosta ML, Rupenthal ID, Green CR. Connexin43 in retinal injury and disease. Prog Retin Eye Res. 2016;51:41–68. doi: 10.1016/j.preteyeres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Dang X, Doble BW, Kardami E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Molecular and cellular biochemistry. 2003;242:35–38. [PubMed] [Google Scholar]
- Davis LM, Rodefeld ME, Green K, Beyer EC, Saffitz JE. Gap junction protein phenotypes of the human heart and conduction system. Journal of cardiovascular electrophysiology. 1995;6:813–822. doi: 10.1111/j.1540-8167.1995.tb00357.x. [DOI] [PubMed] [Google Scholar]
- De Bock M, Culot M, Wang N, Bol M, Decrock E, De Vuyst E, da Costa A, Dauwe I, Vinken M, Simon AM, Rogiers V, De Ley G, Evans WH, Bultynck G, Dupont G, Cecchelli R, Leybaert L. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:1942–1957. doi: 10.1038/jcbfm.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio A, Vega VL, Contreras JE. Gap junctions, homeostasis, and injury. Journal of cellular physiology. 2002;191:269–282. doi: 10.1002/jcp.10108. [DOI] [PubMed] [Google Scholar]
- Decrock E, De Bock M, Wang N, Bultynck G, Giaume C, Naus CC, Green CR, Leybaert L. Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology? Cellular and molecular life sciences : CMLS. 2015;72:2823–2851. doi: 10.1007/s00018-015-1962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Schultz K, Pieper M, Dirks P, Maxeiner S, Willecke K, Weiler R, Janssen-Bienhold U. Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. The European journal of neuroscience. 2006;24:1675–1686. doi: 10.1111/j.1460-9568.2006.05052.x. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Quintanilla RA, Orellana JA, Retamal MA. Neuron-Glia Crosstalk in the Autonomic Nervous System and Its Possible Role in the Progression of Metabolic Syndrome: A New Hypothesis. Frontiers in physiology. 2015;6:350. doi: 10.3389/fphys.2015.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmar M. Connexin diversity: discriminating the message. Circulation research. 2002;91:85–86. doi: 10.1161/01.res.0000028342.56448.9f. [DOI] [PubMed] [Google Scholar]
- Denuc A, Nunez E, Calvo E, Loureiro M, Miro-Casas E, Guaras A, Vazquez J, Garcia-Dorado D. New protein-protein interactions of mitochondrial connexin 43 in mouse heart. Journal of cellular and molecular medicine. 2016;20:794–803. doi: 10.1111/jcmm.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplantez T, Verma V, Leybaert L, Evans WH, Weingart R. Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol Res. 2012;65:546–552. doi: 10.1016/j.phrs.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Current diabetes reports. 2012;12:346–354. doi: 10.1007/s11892-012-0283-6. [DOI] [PubMed] [Google Scholar]
- Distler C, Dreher Z. Glia cells of the monkey retina--II. Muller cells. Vision research. 1996;36:2381–2394. doi: 10.1016/0042-6989(96)00005-3. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, Bukauskas FF, Civitelli R, Lewalter T, Fleischmann BK, Willecke K. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Human molecular genetics. 2008;17:539–554. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essenfelder GM, Bruzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P, Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Human molecular genetics. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- Evans WH, Bultynck G, Leybaert L. Manipulating connexin communication channels: use of peptidomimetics and the translational outputs. J Membr Biol. 2012;245:437–449. doi: 10.1007/s00232-012-9488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, Martin PE. Gap junctions: structure and function (Review) Molecular membrane biology. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- Fernandes R, Girao H, Pereira P. High glucose down-regulates intercellular communication in retinal endothelial cells by enhancing degradation of connexin 43 by a proteasome-dependent mechanism. The Journal of biological chemistry. 2004;279:27219–27224. doi: 10.1074/jbc.M400446200. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology. 2004;19:277–284. doi: 10.1152/physiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Foote CI, Zhou L, Zhu X, Nicholson BJ. The pattern of disulfide linkages in the extracellular loop regions of connexin 32 suggests a model for the docking interface of gap junctions. The Journal of cell biology. 1998;140:1187–1197. doi: 10.1083/jcb.140.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco L, Zocchi E, Usai C, Guida L, Bruzzone S, Costa A, De Flora A. Paracrine roles of NAD+ and cyclic ADP-ribose in increasing intracellular calcium and enhancing cell proliferation of 3T3 fibroblasts. The Journal of biological chemistry. 2001;276:21642–21648. doi: 10.1074/jbc.M010536200. [DOI] [PubMed] [Google Scholar]
- Furlan F, Lecanda F, Screen J, Civitelli R. Proliferation, differentiation and apoptosis in connexin43-null osteoblasts. Cell communication & adhesion. 2001;8:367–371. doi: 10.3109/15419060109080755. [DOI] [PubMed] [Google Scholar]
- Gandhi GK, Ball KK, Cruz NF, Dienel GA. Hyperglycaemia and diabetes impair gap junctional communication among astrocytes. ASN neuro. 2010;2:e00030. doi: 10.1042/AN20090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia IE, Prado P, Pupo A, Jara O, Rojas-Gomez D, Mujica P, Flores-Munoz C, Gonzalez-Casanova J, Soto-Riveros C, Pinto BI, Retamal MA, Gonzalez C, Martinez AD. Connexinopathies: a structural and functional glimpse. BMC cell biology. 2016;17(Suppl 1):17. doi: 10.1186/s12860-016-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TW. Histamine, ZO-1 and increased blood-retinal barrier permeability in diabetic retinopathy. Trans Am Ophthalmol Soc. 1995;93:583–621. [PMC free article] [PubMed] [Google Scholar]
- Garre JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Saez JC, Bennett MV, Abudara V. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22659–22664. doi: 10.1073/pnas.1013793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. The Journal of biological chemistry. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nature reviews. Molecular cell biology. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Goubaeva F, Mikami M, Giardina S, Ding B, Abe J, Yang J. Cardiac mitochondrial connexin 43 regulates apoptosis. Biochemical and biophysical research communications. 2007;352:97–103. doi: 10.1016/j.bbrc.2006.10.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grek CL, Prasad GM, Viswanathan V, Armstrong DG, Gourdie RG, Ghatnekar GS. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2015a;23:203–212. doi: 10.1111/wrr.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grek CL, Rhett JM, Bruce JS, Abt MA, Ghatnekar GS, Yeh ES. Targeting connexin 43 with alpha-connexin carboxyl-terminal (ACT1) peptide enhances the activity of the targeted inhibitors, tamoxifen and lapatinib, in breast cancer: clinical implication for ACT1. BMC Cancer. 2015b;15:296. doi: 10.1186/s12885-015-1229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith AJ, Yang Y, Pryor SP, Park HJ, Jabs EW, Nadol JB, Jr, Russell LJ, Wasserman DI, Richard G, Adams JC, Merchant SN. Cochleosaccular dysplasia associated with a connexin 26 mutation in keratitis-ichthyosis-deafness syndrome. Laryngoscope. 2006;116:1404–1408. doi: 10.1097/01.mlg.0000224549.75161.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Ek-Vitorin JF, Taffet SM, Delmar M. Coexpression of connexins 40 and 43 enhances the pH sensitivity of gap junctions: a model for synergistic interactions among connexins. Circulation research. 2000;86:E98–E103. [PubMed] [Google Scholar]
- Gu S, Yu XS, Yin X, Jiang JX. Stimulation of lens cell differentiation by gap junction protein connexin 45.6. Investigative ophthalmology & visual science. 2003;44:2103–2111. doi: 10.1167/iovs.02-1045. [DOI] [PubMed] [Google Scholar]
- Guldenagel M, Sohl G, Plum A, Traub O, Teubner B, Weiler R, Willecke K. Expression patterns of connexin genes in mouse retina. The Journal of comparative neurology. 2000;425:193–201. [PubMed] [Google Scholar]
- Guo CX, Mat Nor MN, Danesh-Meyer HV, Vessey KA, Fletcher EL, O'Carroll SJ, Acosta ML, Green CR. Connexin43 Mimetic Peptide Improves Retinal Function and Reduces Inflammation in a Light-Damaged Albino Rat Model. Investigative ophthalmology & visual science. 2016;57:3961–3973. doi: 10.1167/iovs.15-16643. [DOI] [PubMed] [Google Scholar]
- Hamelin R, Allagnat F, Haefliger JA, Meda P. Connexins, diabetes and the metabolic syndrome. Current protein & peptide science. 2009;10:18–29. doi: 10.2174/138920309787315167. [DOI] [PubMed] [Google Scholar]
- Han Y, Massey SC. Electrical synapses in retinal ON cone bipolar cells: subtype-specific expression of connexins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13313–13318. doi: 10.1073/pnas.0505067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K, Warner A, Winston RM, Becker DL. Expression of intercellular junctions during preimplantation development of the human embryo. Molecular human reproduction. 1996;2:621–632. doi: 10.1093/molehr/2.8.621. [DOI] [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Quarterly reviews of biophysics. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- Hawat G, Benderdour M, Rousseau G, Baroudi G. Connexin 43 mimetic peptide Gap26 confers protection to intact heart against myocardial ischemia injury. Pflugers Arch. 2010;460:583–592. doi: 10.1007/s00424-010-0849-6. [DOI] [PubMed] [Google Scholar]
- Herve JC, Derangeon M, Sarrouilhe D, Giepmans BN, Bourmeyster N. Gap junctional channels are parts of multiprotein complexes. Biochimica et biophysica acta. 2012;1818:1844–1865. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Hills CE, Bland R, Wheelans DC, Bennett J, Ronco PM, Squires PE. Glucose-evoked alterations in connexin43-mediated cell-to-cell communication in human collecting duct: a possible role in diabetic nephropathy. American journal of physiology. Renal physiology. 2006;291:F1045–1051. doi: 10.1152/ajprenal.00344.2005. [DOI] [PubMed] [Google Scholar]
- Himpens B, Stalmans P, Gomez P, Malfait M, Vereecke J. Intra- and intercellular Ca2+ signaling in retinal pigment epithelial cells during mechanical stimulation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13(Suppl):S63–68. doi: 10.1096/fasebj.13.9001.s63. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovascular research. 1996;32:687–698. [PubMed] [Google Scholar]
- Hollander H, Makarov F, Dreher Z, van Driel D, Chan-Ling TL, Stone J. Structure of the macroglia of the retina: sharing and division of labour between astrocytes and Muller cells. The Journal of comparative neurology. 1991;313:587–603. doi: 10.1002/cne.903130405. [DOI] [PubMed] [Google Scholar]
- Hosoya K, Tomi M. Advances in the cell biology of transport via the inner blood-retinal barrier: establishment of cell lines and transport functions. Biol Pharm Bull. 2005;28:1–8. doi: 10.1248/bpb.28.1. [DOI] [PubMed] [Google Scholar]
- Howarth FC, Chandler NJ, Kharche S, Tellez JO, Greener ID, Yamanushi TT, Billeter R, Boyett MR, Zhang H, Dobrzynski H. Effects of streptozotocin-induced diabetes on connexin43 mRNA and protein expression in ventricular muscle. Molecular and cellular biochemistry. 2008;319:105–114. doi: 10.1007/s11010-008-9883-5. [DOI] [PubMed] [Google Scholar]
- Howarth FC, Nowotny N, Zilahi E, El Haj MA, Lei M. Altered expression of gap junction connexin proteins may partly underlie heart rhythm disturbances in the streptozotocin-induced diabetic rat heart. Molecular and cellular biochemistry. 2007;305:145–151. doi: 10.1007/s11010-007-9537-z. [DOI] [PubMed] [Google Scholar]
- Huang D, Chen YS, Rupenthal ID. Hyaluronic Acid Coated Albumin Nanoparticles for Targeted Peptide Delivery to the Retina. Mol Pharm. 2017;14:533–545. doi: 10.1021/acs.molpharmaceut.6b01029. [DOI] [PubMed] [Google Scholar]
- Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. American journal of physiology. Renal physiology. 2007;293:F1018–1025. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Yu HY, Imamura M, Kakimoto M, Kuroki T, Maruyama T, Nawata H. Altered gap junction activity in cardiovascular tissues of diabetes. Medical electron microscopy : official journal of the Clinical Electron Microscopy Society of Japan. 2001;34:86–91. doi: 10.1007/s007950170002. [DOI] [PubMed] [Google Scholar]
- Iyyathurai J, D'Hondt C, Wang N, De Bock M, Himpens B, Retamal MA, Stehberg J, Leybaert L, Bultynck G. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels. Neuropharmacology. 2013;75:491–505. doi: 10.1016/j.neuropharm.2013.04.050. [DOI] [PubMed] [Google Scholar]
- Janecke AR, Hennies HC, Gunther B, Gansl G, Smolle J, Messmer EM, Utermann G, Rittinger O. GJB2 mutations in keratitis-ichthyosis-deafness syndrome including its fatal form. Am J Med Genet A. 2005;133A:128–131. doi: 10.1002/ajmg.a.30515. [DOI] [PubMed] [Google Scholar]
- Janssen-Bienhold U, Dermietzel R, Weiler R. Distribution of connexin43 immunoreactivity in the retinas of different vertebrates. The Journal of comparative neurology. 1998;396:310–321. [PubMed] [Google Scholar]
- Jiang JX, Penuela S. Connexin and pannexin channels in cancer. BMC cell biology. 2016;17(Suppl 1):12. doi: 10.1186/s12860-016-0094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–1462. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen D, Cruciani V, Sundset R, Ytrehus K, Mikalsen SO. Ischemia induces closure of gap junctional channels and opening of hemichannels in heart-derived cells and tissue. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;28:103–114. doi: 10.1159/000331719. [DOI] [PubMed] [Google Scholar]
- John S, Cesario D, Weiss JN. Gap junctional hemichannels in the heart. Acta Physiol Scand. 2003;179:23–31. doi: 10.1046/j.1365-201X.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. The Journal of biological chemistry. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- Joshi MS, Mihm MJ, Cook AC, Schanbacher BL, Bauer JA. Alterations in connexin 43 during diabetic cardiomyopathy: competition of tyrosine nitration versus phosphorylation. Journal of diabetes. 2015;7:250–259. doi: 10.1111/1753-0407.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Tyagi N. Diabetic Stroke Severity: Epigenetic Remodeling and Neuronal, Glial, and Vascular Dysfunction. Diabetes. 2015;64:4260–4271. doi: 10.2337/db15-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmijn S, Feskens EJ, Launer LJ, Stijnen T, Kromhout D. Glucose intolerance, hyperinsulinaemia and cognitive function in a general population of elderly men. Diabetologia. 1995;38:1096–1102. doi: 10.1007/BF00402181. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Tachikawa M, Akaogi R, Fujimoto K, Ishibashi M, Uchida Y, Couraud PO, Ohtsuki S, Hosoya K, Terasaki T. Contribution of pannexin 1 and connexin 43 hemichannels to extracellular calcium-dependent transport dynamics in human blood-brain barrier endothelial cells. The Journal of pharmacology and experimental therapeutics. 2015;353:192–200. doi: 10.1124/jpet.114.220210. [DOI] [PubMed] [Google Scholar]
- Kar R, Batra N, Riquelme MA, Jiang JX. Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys. 2012;524:2–15. doi: 10.1016/j.abb.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr NM, Johnson CS, de Souza CF, Chee KS, Good WR, Green CR, Danesh-Meyer HV. Immunolocalization of gap junction protein connexin43 (GJA1) in the human retina and optic nerve. Invest Ophthalmol Vis Sci. 2010;51:4028–4034. doi: 10.1167/iovs.09-4847. [DOI] [PubMed] [Google Scholar]
- Kerr NM, Johnson CS, Zhang J, Eady EK, Green CR, Danesh-Meyer HV. High pressure-induced retinal ischaemia reperfusion causes upregulation of gap junction protein connexin43 prior to retinal ganglion cell loss. Exp Neurol. 2012;234:144–152. doi: 10.1016/j.expneurol.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Kiec-Wilk B, Czech U, Janczarska K, Knapp A, Goralska J, Cialowicz U, Malecki MT, Dembinska-Kiec A. Connexin 43 and metabolic effect of fatty acids in stressed endothelial cells. Genes Nutr. 2012;7:257–263. doi: 10.1007/s12263-011-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Davidson JO, Gunn KC, Phillips AR, Green CR, Gunn AJ. Role of Hemichannels in CNS Inflammation and the Inflammasome Pathway. Adv Protein Chem Struct Biol. 2016a;104:1–37. doi: 10.1016/bs.apcsb.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Kim Y, Griffin JM, Harris PW, Chan SH, Nicholson LF, Brimble MA, O'Carroll SJ, Green CR. Characterizing the mode of action of extracellular Connexin43 channel blocking mimetic peptides in an in vitro ischemia injury model. Biochimica et biophysica acta. 2016b;1861:68–78. doi: 10.1016/j.bbagen.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Kim Y, Griffin JM, Harris PW, Chan SH, Nicholson LF, Brimble MA, O'Carroll SJ, Green CR. Characterizing the mode of action of extracellular Connexin43 channel blocking mimetic peptides in an in vitro ischemia injury model. Biochimica et biophysica acta. 2017;1861:68–78. doi: 10.1016/j.bbagen.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Kojima A, Nakahama K, Ohno-Matsui K, Shimada N, Mori K, Iseki S, Sato T, Mochizuki M, Morita I. Connexin 43 contributes to differentiation of retinal pigment epithelial cells via cyclic AMP signaling. Biochemical and biophysical research communications. 2008;366:532–538. doi: 10.1016/j.bbrc.2007.11.159. [DOI] [PubMed] [Google Scholar]
- Kojima T, Spray DC, Kokai Y, Chiba H, Mochizuki Y, Sawada N. Cx32 formation and/or Cx32-mediated intercellular communication induces expression and function of tight junctions in hepatocytic cell line. Experimental cell research. 2002;276:40–51. doi: 10.1006/excr.2002.5511. [DOI] [PubMed] [Google Scholar]
- Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005;7:1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- Krutovskikh V, Yamasaki H. Connexin gene mutations in human genetic diseases. Mutat Res. 2000;462:197–207. doi: 10.1016/s1383-5742(00)00037-5. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Mussche S, Leybaert L, D'Herde K. Gap junctional communication and connexin43 expression in relation to apoptotic cell death and survival of granulosa cells. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2004;52:1199–1207. doi: 10.1369/jhc.3A6227.2004. [DOI] [PubMed] [Google Scholar]
- Laing JG, Chou BC, Steinberg TH. ZO-1 alters the plasma membrane localization and function of Cx43 in osteoblastic cells. Journal of cell science. 2005;118:2167–2176. doi: 10.1242/jcs.02329. [DOI] [PubMed] [Google Scholar]
- Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46:760–765. doi: 10.1007/s00125-003-1116-6. [DOI] [PubMed] [Google Scholar]
- Laird DW. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochimica et biophysica acta. 2005;1711:172–182. doi: 10.1016/j.bbamem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Laird DW. The gap junction proteome and its relationship to disease. Trends in cell biology. 2010;20:92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Larson DM, Haudenschild CC, Beyer EC. Gap junction messenger RNA expression by vascular wall cells. Circulation research. 1990;66:1074–1080. doi: 10.1161/01.res.66.4.1074. [DOI] [PubMed] [Google Scholar]
- Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell and tissue research. 1998;294:415–420. doi: 10.1007/s004410051192. [DOI] [PubMed] [Google Scholar]
- Li AF, Roy S. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Investigative ophthalmology & visual science. 2009;50:1400–1407. doi: 10.1167/iovs.07-1519. [DOI] [PubMed] [Google Scholar]
- Li AF, Sato T, Haimovici R, Okamoto T, Roy S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Investigative ophthalmology & visual science. 2003;44:5376–5382. doi: 10.1167/iovs.03-0360. [DOI] [PubMed] [Google Scholar]
- Li H, Brodsky S, Kumari S, Valiunas V, Brink P, Kaide J, Nasjletti A, Goligorsky MS. Paradoxical overexpression and translocation of connexin43 in homocysteine-treated endothelial cells. Am J Physiol Heart Circ Physiol. 2002;282:H2124–2133. doi: 10.1152/ajpheart.01028.2001. [DOI] [PubMed] [Google Scholar]
- Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 is critical to maintain the homeostasis of the blood-testis barrier via its effects on tight junction reassembly. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17998–18003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. The American journal of physiology. 1995;268:H729–739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- Liu J, Ek-Vitorin JF, Weintraub ST, Gu S, Shi Q, Burt JM, Jiang JX. Phosphorylation of connexin 50 by protein kinase A enhances gap junction and hemichannel function. The Journal of biological chemistry. 2011;286:16914–16928. doi: 10.1074/jbc.M111.218735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losso JN, Truax RE, Richard G. trans-resveratrol inhibits hyperglycemia-induced inflammation and connexin downregulation in retinal pigment epithelial cells. Journal of agricultural and food chemistry. 2010;58:8246–8252. doi: 10.1021/jf1012067. [DOI] [PubMed] [Google Scholar]
- Lu G, Haider H, Porollo A, Ashraf M. Mitochondria-specific transgenic overexpression of connexin-43 simulates preconditioning-induced cytoprotection of stem cells. Cardiovascular research. 2010;88:277–286. doi: 10.1093/cvr/cvq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, Fletcher EL. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Investigative ophthalmology & visual science. 2011;52:9316–9326. doi: 10.1167/iovs.11-7879. [DOI] [PubMed] [Google Scholar]
- Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and beta-cell failure in type 2 diabetes mellitus. Experimental diabetes research. 2012;2012:703538. doi: 10.1155/2012/703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol. 2008;295:C221–230. doi: 10.1152/ajpcell.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait M, Gomez P, van Veen TA, Parys JB, De Smedt H, Vereecke J, Himpens B. Effects of hyperglycemia and protein kinase C on connexin43 expression in cultured rat retinal pigment epithelial cells. The Journal of membrane biology. 2001;181:31–40. doi: 10.1007/s0023200100082. [DOI] [PubMed] [Google Scholar]
- Manasson J, Tien T, Moore C, Kumar NM, Roy S. High glucose-induced downregulation of connexin 30.2 promotes retinal vascular lesions: implications for diabetic retinopathy. Investigative ophthalmology & visual science. 2013;54:2361–2366. doi: 10.1167/iovs.12-10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschot SM, Biessels GJ, de Valk H, Algra A, Rutten GE, van der Grond J, Kappelle LJ Utrecht Diabetic Encephalopathy Study, G. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50:2388–2397. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Naranjo A, Cormie P, Serrano AE, Wang CM, Thrasivoulou C, Sutcliffe JE, Gilmartin DJ, Tsui J, Serena TE, Phillips AR, Becker DL. Overexpression of the gap junction protein Cx43 as found in diabetic foot ulcers can retard fibroblast migration. Cell biology international. 2012;36:661–667. doi: 10.1042/CBI20110628. [DOI] [PubMed] [Google Scholar]
- Mese G, Richard G, White TW. Gap junctions: basic structure and function. The Journal of investigative dermatology. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Krutovskikh V, Piccoli C, Elfgang C, Traub O, Willecke K, Yamasaki H. Negative growth control of HeLa cells by connexin genes: connexin species specificity. Cancer research. 1995;55:629–639. [PubMed] [Google Scholar]
- Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120:106–114. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- Miro-Casas E, Ruiz-Meana M, Agullo E, Stahlhofen S, Rodriguez-Sinovas A, Cabestrero A, Jorge I, Torre I, Vazquez J, Boengler K, Schulz R, Heusch G, Garcia-Dorado D. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovascular research. 2009;83:747–756. doi: 10.1093/cvr/cvp157. [DOI] [PubMed] [Google Scholar]
- Mogi M, Horiuchi M. Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circulation journal : official journal of the Japanese Circulation Society. 2011;75:1042–1048. doi: 10.1253/circj.cj-11-0121. [DOI] [PubMed] [Google Scholar]
- Mohammad G, Kowluru RA. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Investigative ophthalmology & visual science. 2011;52:3832–3841. doi: 10.1167/iovs.10-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorby C, Patel M. Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Experimental cell research. 2001;271:238–248. doi: 10.1006/excr.2001.5357. [DOI] [PubMed] [Google Scholar]
- Muller LP, Dedek K, Janssen-Bienhold U, Meyer A, Kreuzberg MM, Lorenz S, Willecke K, Weiler R. Expression and modulation of connexin 30.2, a novel gap junction protein in the mouse retina. Visual neuroscience. 2010;27:91–101. doi: 10.1017/S0952523810000131. [DOI] [PubMed] [Google Scholar]
- Munoz MF, Puebla M, Figueroa XF. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca(2+) signaling. Front Cell Neurosci. 2015;9:59. doi: 10.3389/fncel.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Thomaidou D, Evans WH, Parnavelas JG. Gap junctions in the adult cerebral cortex: regional differences in their distribution and cellular expression of connexins. The Journal of comparative neurology. 1996;376:326–342. doi: 10.1002/(SICI)1096-9861(19961209)376:2<326::AID-CNE13>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Nagasawa K, Chiba H, Fujita H, Kojima T, Saito T, Endo T, Sawada N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. Journal of cellular physiology. 2006;208:123–132. doi: 10.1002/jcp.20647. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Hossain MZ, Hertzberg EL, Marotta CA. Induction of connexin43 and gap junctional communication in PC12 cells overexpressing the carboxy terminal region of amyloid precursor protein. J Neurosci Res. 1996;44:124–132. doi: 10.1002/(SICI)1097-4547(19960415)44:2<124::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Patel D, Ochalski PA, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999;88:447–468. doi: 10.1016/s0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- Nakase T, Fushiki S, Naus CC. Astrocytic gap junctions composed of connexin 43 reduce apoptotic neuronal damage in cerebral ischemia. Stroke. 2003;34:1987–1993. doi: 10.1161/01.STR.0000079814.72027.34. [DOI] [PubMed] [Google Scholar]
- Nakase T, Sohl G, Theis M, Willecke K, Naus CC. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. The American journal of pathology. 2004;164:2067–2075. doi: 10.1016/S0002-9440(10)63765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen PA, Kumar NM. Differences in expression patterns between mouse connexin-30.2 (Cx30.2) and its putative human orthologue, connexin-31.9. FEBS letters. 2003;540:151–156. doi: 10.1016/s0014-5793(03)00252-7. [DOI] [PubMed] [Google Scholar]
- Nimlamool W, Andrews RM, Falk MM. Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated gap junction internalization. Mol Biol Cell. 2015;26:2755–2768. doi: 10.1091/mbc.E14-06-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxidants & redox signaling. 2007;9:343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- O'Carroll SJ, Alkadhi M, Nicholson LF, Green CR. Connexin 43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell communication & adhesion. 2008;15:27–42. doi: 10.1080/15419060802014164. [DOI] [PubMed] [Google Scholar]
- Obert E, Strauss R, Brandon C, Grek C, Ghatnekar G, Gourdie R, Rohrer B. Targeting the tight junction protein, zonula occludens-1, with the connexin43 mimetic peptide, alphaCT1, reduces VEGF-dependent RPE pathophysiology. J Mol Med (Berl) 2017;95:535–552. doi: 10.1007/s00109-017-1506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okruhlicova L, Tribulova N, Misejkova M, Kucka M, Stetka R, Slezak J, Manoach M. Gap junction remodelling is involved in the susceptibility of diabetic rats to hypokalemia-induced ventricular fibrillation. Acta histochemica. 2002;104:387–391. doi: 10.1078/0065-1281-00675. [DOI] [PubMed] [Google Scholar]
- Oku H, Kodama T, Sakagami K, Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Investigative ophthalmology & visual science. 2001;42:1915–1920. [PubMed] [Google Scholar]
- Orellana JA, Busso D, Ramirez G, Campos M, Rigotti A, Eugenin J, von Bernhardi R. Prenatal nicotine exposure enhances Cx43 and Panx1 unopposed channel activity in brain cells of adult offspring mice fed a high-fat/cholesterol diet. Front Cell Neurosci. 2014;8:403. doi: 10.3389/fncel.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Figueroa XF, Sanchez HA, Contreras-Duarte S, Velarde V, Saez JC. Hemichannels in the neurovascular unit and white matter under normal and inflamed conditions. CNS Neurol Disord Drug Targets. 2011;10:404–414. doi: 10.2174/187152711794653869. [DOI] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. Journal of molecular neuroscience : MN. 2008;35:101–116. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A. Structure and closure of connexin gap junction channels. FEBS letters. 2014 doi: 10.1016/j.febslet.2014.01.042. [DOI] [PubMed] [Google Scholar]
- Oviedo-Orta E, Errington RJ, Evans WH. Gap junction intercellular communication during lymphocyte transendothelial migration. Cell biology international. 2002;26:253–263. doi: 10.1006/cbir.2001.0840. [DOI] [PubMed] [Google Scholar]
- Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Current biology : CB. 2000;10:R473–474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Pecoraro M, Sorrentino R, Franceschelli S, Del Pizzo M, Pinto A, Popolo A. Doxorubicin-Mediated Cardiotoxicity: Role of Mitochondrial Connexin 43. Cardiovasc Toxicol. 2015;15:366–376. doi: 10.1007/s12012-014-9305-8. [DOI] [PubMed] [Google Scholar]
- Penuela S, Harland L, Simek J, Laird DW. Pannexin channels and their links to human disease. The Biochemical journal. 2014;461:371–381. doi: 10.1042/BJ20140447. [DOI] [PubMed] [Google Scholar]
- Pitre DA, Seifert JL, Bauer JA. Perineurium inflammation and altered connexin isoform expression in a rat model of diabetes related peripheral neuropathy. Neuroscience letters. 2001;303:67–71. doi: 10.1016/s0304-3940(01)01696-2. [DOI] [PubMed] [Google Scholar]
- Plotkin LI. Connexin 43 hemichannels and intracellular signaling in bone cells. Front Physiol. 2014;5:131. doi: 10.3389/fphys.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res. 2008;23:1712–1721. doi: 10.1359/JBMR.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. The Journal of biological chemistry. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. American journal of physiology. Cell physiology. 2005;289:C633–643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- Poladia DP, Schanbacher B, Wallace LJ, Bauer JA. Innervation and connexin isoform expression during diabetes-related bladder dysfunction: early structural vs. neuronal remodelling. Acta diabetologica. 2005;42:147–152. doi: 10.1007/s00592-005-0194-y. [DOI] [PubMed] [Google Scholar]
- Pollok S, Pfeiffer AC, Lobmann R, Wright CS, Moll I, Martin PE, Brandner JM. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. Journal of cellular and molecular medicine. 2011;15:861–873. doi: 10.1111/j.1582-4934.2010.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsaerts R, D'Hondt C, Hertens F, Parys JB, Leybaert L, Vereecke J, Himpens B, Bultynck G. RhoA GTPase switch controls Cx43-hemichannel activity through the contractile system. PLoS One. 2012;7:e42074. doi: 10.1371/journal.pone.0042074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Coutinho P, Frank S, Franke S, Law LY, Martin P, Green CR, Becker DL. Targeting connexin43 expression accelerates the rate of wound repair. Current biology : CB. 2003;13:1697–1703. doi: 10.1016/j.cub.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Rabionet R, Gasparini P, Estivill X. Molecular genetics of hearing impairment due to mutations in gap junction genes encoding beta connexins. Hum Mutat. 2000;16:190–202. doi: 10.1002/1098-1004(200009)16:3<190::AID-HUMU2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Rackauskas M, Kreuzberg MM, Pranevicius M, Willecke K, Verselis VK, Bukauskas FF. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophysical journal. 2007;92:1952–1965. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratchford AM, Esguerra CR, Moley KH. Decreased oocyte-granulosa cell gap junction communication and connexin expression in a type 1 diabetic mouse model. Molecular endocrinology. 2008;22:2643–2654. doi: 10.1210/me.2007-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A. New functions of Muller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- Ren Y, Carretero OA, Garvin JL. Role of mesangial cells and gap junctions in tubuloglomerular feedback. Kidney international. 2002;62:525–531. doi: 10.1046/j.1523-1755.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal MA, Evangelista-Martinez F, Leon-Paravic CG, Altenberg GA, Reuss L. Biphasic effect of linoleic acid on connexin 46 hemichannels. Pflugers Arch. 2011;461:635–643. doi: 10.1007/s00424-011-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme MA, Kar R, Gu S, Jiang JX. Antibodies targeting extracellular domain of connexins for studies of hemichannels. Neuropharmacology. 2013;75:525–532. doi: 10.1016/j.neuropharm.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolo LJ, Peng S, Luo Y, Xiao W. Integration of tight junctions and claudins with the barrier functions of the retinal pigment epithelium. Prog Retin Eye Res. 2011;30:296–323. doi: 10.1016/j.preteyeres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Robertson J, Lang S, Lambert PA, Martin PE. Peptidoglycan derived from Staphylococcus epidermidis induces Connexin43 hemichannel activity with consequences on the innate immune response in endothelial cells. The Biochemical journal. 2010;432:133–143. doi: 10.1042/BJ20091753. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Xin X, Jee K, Babapoor-Farrokhran S, Kashiwabuchi F, Ma T, Bhutto I, Hassan SJ, Daoud Y, Baranano D, Solomon S, Lutty G, Semenza GL, Montaner S, Sodhi A. VEGF secreted by hypoxic Muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013;62:3863–3873. doi: 10.2337/db13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, Konietzka I, Miro E, Totzeck A, Heusch G, Schulz R, Garcia-Dorado D. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circulation research. 2006;99:93–101. doi: 10.1161/01.RES.0000230315.56904.de. [DOI] [PubMed] [Google Scholar]
- Rottlaender D, Boengler K, Wolny M, Michels G, Endres-Becker J, Motloch LJ, Schwaiger A, Buechert A, Schulz R, Heusch G, Hoppe UC. Connexin 43 acts as a cytoprotective mediator of signal transduction by stimulating mitochondrial KATP channels in mouse cardiomyocytes. J Clin Invest. 2010;120:1441–1453. doi: 10.1172/JCI40927. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Roy S, Kern TS, Song B, Stuebe C. Mechanistic Insights into Pathological Changes in the Diabetic Retina: Implications for Targeting Diabetic Retinopathy. Am J Pathol. 2017;187:9–19. doi: 10.1016/j.ajpath.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Trudeau K, Roy S, Behl Y, Dhar S, Chronopoulos A. New insights into hyperglycemia-induced molecular changes in microvascular cells. J Dent Res. 2010;89:116–127. doi: 10.1177/0022034509355765. [DOI] [PubMed] [Google Scholar]
- Saez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Experimental cell research. 2010;316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Salmina AB, Morgun AV, Kuvacheva NV, Lopatina OL, Komleva YK, Malinovskaya NA, Pozhilenkova EA. Establishment of neurogenic microenvironment in the neurovascular unit: the connexin 43 story. Reviews in the neurosciences. 2014;25:97–111. doi: 10.1515/revneuro-2013-0044. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What's where and why at a vascular myoendothelial microdomain signalling complex. Clinical and experimental pharmacology & physiology. 2009;36:67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Muller cell line. Investigative ophthalmology & visual science. 1998;39:212–216. [PubMed] [Google Scholar]
- Sato T, Haimovici R, Kao R, Li AF, Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes. 2002;51:1565–1571. doi: 10.2337/diabetes.51.5.1565. [DOI] [PubMed] [Google Scholar]
- Sawai K, Mukoyama M, Mori K, Yokoi H, Koshikawa M, Yoshioka T, Takeda R, Sugawara A, Kuwahara T, Saleem MA, Ogawa O, Nakao K. Redistribution of connexin43 expression in glomerular podocytes predicts poor renal prognosis in patients with type 2 diabetes and overt nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:2472–2477. doi: 10.1093/ndt/gfl260. [DOI] [PubMed] [Google Scholar]
- Schalper KA, Riquelme MA, Branes MC, Martinez AD, Vega JL, Berthoud VM, Bennett MV, Saez JC. Modulation of gap junction channels and hemichannels by growth factors. Mol Biosyst. 2012;8:685–698. doi: 10.1039/c1mb05294b. [DOI] [PubMed] [Google Scholar]
- Schalper KA, Sanchez HA, Lee SC, Altenberg GA, Nathanson MH, Saez JC. Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am J Physiol Cell Physiol. 2010;299:C1504–1515. doi: 10.1152/ajpcell.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Boengler K, Totzeck A, Luo Y, Garcia-Dorado D, Heusch G. Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev. 2007;12:261–266. doi: 10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? The American journal of physiology. 1989;256:H838–845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- Shi Q, Gu S, Yu XS, White TW, Banks EA, Jiang JX. Connexin Controls Cell-Cycle Exit and Cell Differentiation by Directly Promoting Cytosolic Localization and Degradation of E3 Ligase Skp2. Dev Cell. 2015;35:483–496. doi: 10.1016/j.devcel.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani-Ishida K, Uemura K, Yoshida K. Hemichannels in cardiomyocytes open transiently during ischemia and contribute to reperfusion injury following brief ischemia. Am J Physiol Heart Circ Physiol. 2007;293:H1714–1720. doi: 10.1152/ajpheart.00022.2007. [DOI] [PubMed] [Google Scholar]
- Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, Jiang JX. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. The Journal of biological chemistry. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Solan JL, Taffet SM, Javier R, Lampe PD. Connexin 43 interacts with zona occludens-1 and -2 proteins in a cell cycle stage-specific manner. The Journal of biological chemistry. 2005;280:30416–30421. doi: 10.1074/jbc.M506799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetkamp D, Nguyen TT, Menazza S, Hirschhauser C, Hendgen-Cotta UB, Rassaf T, Schluter KD, Boengler K, Murphy E, Schulz R. S-nitrosation of mitochondrial connexin 43 regulates mitochondrial function. Basic research in cardiology. 2014;109:433. doi: 10.1007/s00395-014-0433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl G, Guldenagel M, Traub O, Willecke K. Connexin expression in the retina. Brain research. Brain research reviews. 2000;32:138–145. doi: 10.1016/s0165-0173(99)00074-0. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10:173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovascular research. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. The Journal of biological chemistry. 2004;279:54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- Stong BC, Chang Q, Ahmad S, Lin X. A novel mechanism for connexin 26 mutation linked deafness: cell death caused by leaky gap junction hemichannels. Laryngoscope. 2006;116:2205–2210. doi: 10.1097/01.mlg.0000241944.77192.d2. [DOI] [PubMed] [Google Scholar]
- Stottrup C, Roy S. Upregulation of rab20 Expression by High Glucose Reduces Gap Junction Activity and Accelerated Death of Retinal Endothelial Cells, Diabetologia; 48th EASD Annual Meeting; 2012. E-Abstract 1120. [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani SO, Urban-Maldonado M, Tar MT, Melman A, Spray DC. Effects of ageing and streptozotocin-induced diabetes on connexin43 and P2 purinoceptor expression in the rat corpora cavernosa and urinary bladder. BJU international. 2009;103:1686–1693. doi: 10.1111/j.1464-410X.2008.08337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez S, Ballmer-Hofer K. VEGF transiently disrupts gap junctional communication in endothelial cells. J Cell Sci. 2001;114:1229–1235. doi: 10.1242/jcs.114.6.1229. [DOI] [PubMed] [Google Scholar]
- Takenaka T, Inoue T, Okada H, Ohno Y, Miyazaki T, Chaston DJ, Hill CE, Suzuki H. Altered gap junctional communication and renal haemodynamics in Zucker fatty rat model of type 2 diabetes. Diabetologia. 2011;54:2192–2201. doi: 10.1007/s00125-011-2175-8. [DOI] [PubMed] [Google Scholar]
- Tien T, Barrette KF, Chronopoulos A, Roy S. Effects of high glucose-induced Cx43 downregulation on occludin and ZO-1 expression and tight junction barrier function in retinal endothelial cells. Investigative ophthalmology & visual science. 2013;54:6518–6525. doi: 10.1167/iovs.13-11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien T, Muto T, Barrette K, Challyandra L, Roy S. Downregulation of Connexin 43 promotes vascular cell loss and excess permeability associated with the development of vascular lesions in the diabetic retina. Molecular vision. 2014;20:732–741. [PMC free article] [PubMed] [Google Scholar]
- Tien T, Muto T, Zhang J, Sohn EH, Mullins RF, Roy S. Association of reduced Connexin 43 expression with retinal vascular lesions in human diabetic retinopathy. Exp Eye Res. 2016;146:103–106. doi: 10.1016/j.exer.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Tong D, Li TY, Naus KE, Bai D, Kidder GM. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. Journal of cell science. 2007;120:4016–4024. doi: 10.1242/jcs.011775. [DOI] [PubMed] [Google Scholar]
- Tout S, Chan-Ling T, Hollander H, Stone J. The role of Muller cells in the formation of the blood-retinal barrier. Neuroscience. 1993;55:291–301. doi: 10.1016/0306-4522(93)90473-s. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. The Journal of biological chemistry. 2001;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. The Journal of biological chemistry. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- Traish AM, Stottrup C, van Renterghem K, Achten R, Roy S. Density and distribution of connexin 43 in corpus cavernosum tissue from diabetic and hypogonadal patients with erectile dysfunction. Hormone molecular biology and clinical investigation. 2013;13:7–12. doi: 10.1515/hmbci-2013-0001. [DOI] [PubMed] [Google Scholar]
- Trosko JE, Chang CC. Mechanism of up-regulated gap junctional intercellular communication during chemoprevention and chemotherapy of cancer. Mutat Res. 2001;480–481:219–229. doi: 10.1016/s0027-5107(01)00181-6. [DOI] [PubMed] [Google Scholar]
- Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: implications for diabetic retinopathy. The American journal of pathology. 2010;177:447–455. doi: 10.2353/ajpath.2010.091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau K, Molina AJ, Roy S. High glucose induces mitochondrial morphology and metabolic changes in retinal pericytes. Investigative ophthalmology & visual science. 2011;52:8657–8664. doi: 10.1167/iovs.11-7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau K, Muto T, Roy S. Downregulation of mitochondrial connexin 43 by high glucose triggers mitochondrial shape change and cytochrome C release in retinal endothelial cells. Investigative ophthalmology & visual science. 2012;53:6675–6681. doi: 10.1167/iovs.12-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Beyer EC, Brink PR. Cardiac gap junction channels show quantitative differences in selectivity. Circulation research. 2002;91:104–111. doi: 10.1161/01.res.0000025638.24255.aa. [DOI] [PubMed] [Google Scholar]
- van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol. 1999;112:479–486. doi: 10.1007/s004180050432. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Weiler R. Gap junctions in the eye: evidence for heteromeric, heterotypic and mixed-homotypic interactions. Brain research. Brain research reviews. 2000;32:115–120. doi: 10.1016/s0165-0173(99)00070-3. [DOI] [PubMed] [Google Scholar]
- Vergara L, Bao X, Cooper M, Bello-Reuss E, Reuss L. Gap-junctional hemichannels are activated by ATP depletion in human renal proximal tubule cells. The Journal of membrane biology. 2003;196:173–184. doi: 10.1007/s00232-003-0636-9. [DOI] [PubMed] [Google Scholar]
- Vinken M, Decrock E, Leybaert L, Bultynck G, Himpens B, Vanhaecke T, Rogiers V. Non-channel functions of connexins in cell growth and cell death. Biochimica et biophysica acta. 2012;1818:2002–2008. doi: 10.1016/j.bbamem.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Vinken M, Vanhaecke T, Papeleu P, Snykers S, Henkens T, Rogiers V. Connexins and their channels in cell growth and cell death. Cellular signalling. 2006;18:592–600. doi: 10.1016/j.cellsig.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Wang CM, Lincoln J, Cook JE, Becker DL. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes. 2007;56:2809–2817. doi: 10.2337/db07-0613. [DOI] [PubMed] [Google Scholar]
- Wang CY, Liu HJ, Chen HJ, Lin YC, Wang HH, Hung TC, Yeh HI. AGE-BSA down-regulates endothelial connexin43 gap junctions. BMC cell biology. 2011;12:19. doi: 10.1186/1471-2121-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, De Bock M, Antoons G, Gadicherla AK, Bol M, Decrock E, Evans WH, Sipido KR, Bukauskas FF, Leybaert L. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res Cardiol. 2012;107:304. doi: 10.1007/s00395-012-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewski M, Scorrano L. The changing shape of mitochondrial apoptosis. Trends Endocrinol Metab. 2009;20:287–294. doi: 10.1016/j.tem.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Weinbrenner C, Baines CP, Liu GS, Armstrong SC, Ganote CE, Walsh AH, Honkanen RE, Cohen MV, Downey JM. Fostriecin, an inhibitor of protein phosphatase 2A, limits myocardial infarct size even when administered after onset of ischemia. Circulation. 1998;98:899–905. doi: 10.1161/01.cir.98.9.899. [DOI] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. The Journal of cell biology. 1994;125:879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Paul DL, Goodenough DA, Bruzzone R. Functional analysis of selective interactions among rodent connexins. Molecular biology of the cell. 1995;6:459–470. doi: 10.1091/mbc.6.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AS. Gap junction-mediated intercellular signalling in health and disease. Bioessays. 1998;20:686–688. doi: 10.1002/(SICI)1521-1878(199808)20:8<686::AID-BIES13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT Global Diabetic Retinopathy Project, G. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- Winterhager E, Kidder GM. Gap junction connexins in female reproductive organs: implications for women's reproductive health. Human reproduction update. 2015;21:340–352. doi: 10.1093/humupd/dmv007. [DOI] [PubMed] [Google Scholar]
- Wong CW, Christen T, Kwak BR. Connexins in leukocytes: shuttling messages? Cardiovascular research. 2004;62:357–367. doi: 10.1016/j.cardiores.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Wright CS, van Steensel MA, Hodgins MB, Martin PE. Connexin mimetic peptides improve cell migration rates of human epidermal keratinocytes and dermal fibroblasts in vitro. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17:240–249. doi: 10.1111/j.1524-475X.2009.00471.x. [DOI] [PubMed] [Google Scholar]
- Wright JA, Richards T, Becker DL. Connexins and diabetes. Cardiology research and practice. 2012;2012:496904. doi: 10.1155/2012/496904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi X, Gao L, Hatala DA, Smith DG, Codispoti MC, Gong B, Kern TS, Zhang JZ. Chronically elevated glucose-induced apoptosis is mediated by inactivation of Akt in cultured Muller cells. Biochemical and biophysical research communications. 2005;326:548–553. doi: 10.1016/j.bbrc.2004.11.064. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. The Journal of comparative neurology. 1997;383:512–528. doi: 10.1002/(sici)1096-9861(19970714)383:4<512::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Xu H, Gu S, Riquelme MA, Burra S, Callaway D, Cheng H, Guda T, Schmitz J, Fajardo RJ, Werner SL, Zhao H, Shang P, Johnson ML, Bonewald LF, Jiang JX. Connexin 43 channels are essential for normal bone structure and osteocyte viability. J Bone Miner Res. 2015;30:436–448. doi: 10.1002/jbmr.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HZ, Song Z, Fu S, Zhu M, Le YZ. RPE barrier breakdown in diabetic retinopathy: seeing is believing. J Ocul Biol Dis Infor. 2011;4:83–92. doi: 10.1007/s12177-011-9068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K, Kada K, Hojo M, Lee JK, Kamiya K, Toyama J, Opthof T, Kodama I. Cell-to-cell interaction prevents cell death in cultured neonatal rat ventricular myocytes. Cardiovascular research. 2000;48:68–76. doi: 10.1016/s0008-6363(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M, Harris AL. Gap junction channel structure in the early 21st century: facts and fantasies. Current opinion in cell biology. 2007;19:521–528. doi: 10.1016/j.ceb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yego EC, Vincent JA, Sarthy V, Busik JV, Mohr S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Muller cells by IL-1beta and IL-6. Investigative ophthalmology & visual science. 2009;50:1920–1928. doi: 10.1167/iovs.08-2082. [DOI] [PubMed] [Google Scholar]
- Zahs KR, Ceelen PW. Gap junctional coupling and connexin immunoreactivity in rabbit retinal glia. Visual neuroscience. 2006;23:1–10. doi: 10.1017/S0952523806231018. [DOI] [PubMed] [Google Scholar]
- Zahs KR, Newman EA. Asymmetric gap junctional coupling between glial cells in the rat retina. Glia. 1997;20:10–22. [PubMed] [Google Scholar]
- Zhang J, Hill CE. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney international. 2005;68:1171–1185. doi: 10.1111/j.1523-1755.2005.00509.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, O'Carroll SJ, Henare K, Ching LM, Ormonde S, Nicholson LF, Danesh-Meyer HV, Green CR. Connexin hemichannel induced vascular leak suggests a new paradigm for cancer therapy. FEBS letters. 2014;588:1365–1371. doi: 10.1016/j.febslet.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zou T, Liu Y, Qi Y. The gating effect of calmodulin and calcium on the connexin50 hemichannel. Biol Chem. 2006;387:595–601. doi: 10.1515/BC.2006.076. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu G, Ling Q, Da C. Expression of aquaporin 4 and Kir4.1 in diabetic rat retina: treatment with minocycline. The Journal of international medical research. 2011;39:464–479. doi: 10.1177/147323001103900214. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Nakayama K, Nakayama K, Morita I. A novel route for connexin 43 to inhibit cell proliferation: negative regulation of S-phase kinase-associated protein (Skp 2) Cancer research. 2003;63:1623–1630. [PubMed] [Google Scholar]
- Zhao HB. Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signalling and metabolic communications. The European journal of neuroscience. 2005;21:1859–1868. doi: 10.1111/j.1460-9568.2005.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]