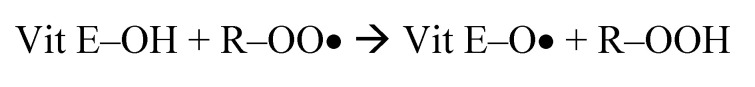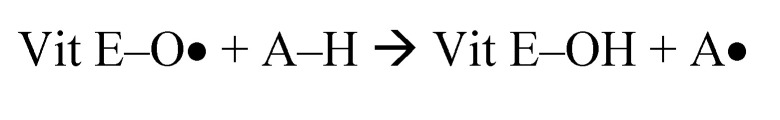Abstract
α-Tocopherol belongs to the group of vitamin E vitamers. Recent years findings indicate that α-tocopherol is more than just a simple fat-soluble anti-oxidant as it was found that it can also regulate gene expression. From all vitamin E vitamers human body preferentially retains α-tocopherol, but the reasons for this preference are still elusive. Different studies indicated that human body, through the action of two hepatic proteins, α-tocopherol transfer protein (α-TTP) and cytochrome P450 4F2 (CYP4F2), is able to make subtle structural differences between different vitamin E forms. This is an example of stereochemistry used as a discrimination factor between molecules with different biological activities.
1. Introduction
Vitamin E is the generic name used to designate eight chemically related compounds which differ in the number and positions of methyl groups on the chromanol ring and in the saturation and stereochemistry of the phytyl tail. Human body is able to concentrate and retain from food sources of α-tocopherol. This is accomplished through the action of two hepatic proteins α-TTP and CYP4F2, respectively. α-TTP acts as a vitamin E retention factor, whereas selectivity among the different vitamin E vitamers is driven by the substrate specificity of CYP4F2, which catalyzes the first step in vitamin E metabolism.
2. Vitamin E structure and functions
All vitamin E vitamers have a chromanol ring with different extents of substitution. There are four tocopherols designated α-,β-,γ-, and δ-tocopherol and their four corresponding tocotrienols.
Fig. 1.
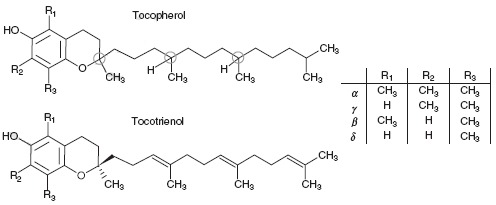
Chemical structures of tocopherols and tocotrienols. The circles mark the three chiral centers in tocopherols (2, 4’, 8’).
The tocopherols have a phytyl tail inserted on the chromanol ring and differ in respect to their methylation degree [1]. The tocotrienols have an unsaturated tail with three double bonds two of which have trans configuration. Each one of the four tocopherols has three chiral centers, represented by carbon atoms 2, 4’ and 8’ respectively. As a consequence of this for each tocopherol there are eight possible stereoisomers (RRR, RSR, RRS, RSS, SRR, SSR, SRS, and SSS). RRR-α-tocopherol is the naturally occurring form of α-tocopherol. It was found that natural tocopherols have the R configuration at all chiral centers and that stereoisomers with R configuration at C2 are more biologically active that those with S configuration [2,3]. The synthetic vitamin E is called all-rac-α-tocopherol and consists of a mixture of equal amounts of the eight possible stereoisomers (rac=racemic).
Vitamin E functions can be divided into anti-oxidant (free radical chain-breaking antioxidant) and non-antioxidant (mediated through protein kinase C and tocopherol-associated proteins and tocopherol-binding proteins) [4,5].
Vitamin E, alone and in conjunction with other compounds (vitamin C, polyphenols, and selenium), is one of the components of the first line of defense against lipid peroxidation. α-Tocopherol acts as a chain-breaking antioxidant preventing thus auto-oxidation of polyunsaturated fatty acids (PUFAs) from plasma membrane phospholipids and from lipoprotein particles. Peroxyl radicals (ROO•) tend to react faster with α-tocopherol (vit. E-OH) than with hydrogen atoms from bis-allylic positions within PUFAs [6].
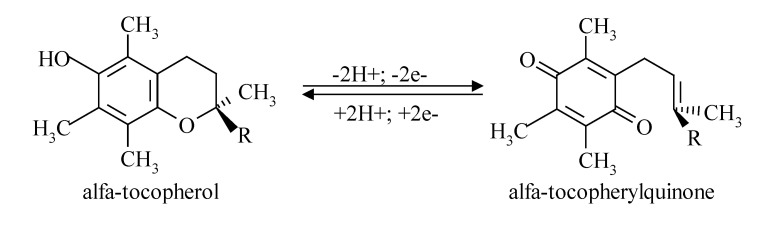
The main factor that enables α-tocopherol to act as a chain-breaking anti-oxidant is represented by its ability to reversible interconvert between α-tocopherol and α-tocopherolquinone forms (Figure 2).
Fig. 2.
α-Tocopherol acting as a chain-breaking anti-oxidant.
α-Tocopherol will be regenerated from the α-tocopheroxyl radical (vit E–O•) in a subsequent reaction with a reducing agent such as vitamin C or glutathione [7-9].
Recent data suggest that α-tocopherol can inhibit free radical generation in a manner independent of its „scavenger radical” properties. α-Tocopherol is able to limit superoxide production through inhibition of NADPH oxidase activity [10]. Also, α-tocopherol can inhibit phospholipase A2 and cyclooxygenase activities with a concomitant increase in α-glutamylcysteinil synthetase activity resulting in an overall diminution of free radicals production [11-13].
There are many studies to indicate that α-tocopherol influences gene expression independent of its anti-oxidant functions (Table I).
Table 1.
Effects of α-tocopherol upon expression of genes.
| Up-regulated genes | OBSERVATIONS |
|---|---|
| • α-tocopherol transfer protein (α-TTP) gene, | α-TTP is responsible for the specific uptake of α-tocopherol and for the control of α-tocopherol plasma level |
| • CYP3A4 and CYP3A5 genes | Involved in liver tocopherols catabolism [14] |
| • SR-B1 scavenger receptor gene | α-tocopherol-depleted rats show increased expression of scavenger receptor SR-B1 [15] |
| • α-tropomyosin gene | Extracellular protein [16] |
| • Connective tissue growth factor (CTGF) gene | α-tocopherol induces CTGF gene expression 1,8 fold [17] |
| Down-regulated genes | |
| • CD36 scavenger receptor gene | At physiological concentration α-tocopherol down-regulates CD36 mRNA transcription and protein expression [18] |
| • SR-A scavenger receptor gene | The same as for CD36 scavenger receptor [19] |
| • Collagenase (MMP-1) gene | Extracellular protein [20] |
| • Collagen α1(I) gene | α-Tocopherol induces the decrease of liver collagen mRNA [21] |
3. Vitamin E metabolism
All vitamin E vitamers are absorbed with very similar rates. It is speculated that tocotrienols are absorbed better than the corresponding tocopherols [22]. Being a fat-soluble compound, vitamin E absorption depends upon bile acids and pancreatic secretion in order to form micelles for uptake by entherocytes. Inside the entherocytes, tocopherols and tocotrienols are assembled together with triacilglycerols, cholesterol, phospholipids, carotenoids and apolipoprotein B48 into chylomicrons [23]. There is no discrimination between different vitamin E vitamers during intestinal absorption [24,25].
In the circulation, chylomicrons undergo triacilglycerols lipolysis by lipoprotein lipase. There are some studies suggesting the fact that lipoprotein lipase is able to deliver vitamin E vitamers to different cells [26,27]. Some of the newly absorbed vitamin E is transferred to other circulating lipoproteins, while the rest remains in the composition of chylomicron remnants. The exchange of vitamin E vitamers between different plasma lipoprotein particles is catalyzed by the phospholipid transfer protein (PLTP) [28,29].
The liver takes up chylomicron remnants and secretes VLDL into circulation. Studies using deuterated tocopherols indicated that RRR-α-tocopherol is preferentially secreted by hepatocytes. There is evidence that α-tocopherol becomes associated with VLDL after its secretion, probably in the sinusoidal space [30]. VLDL particles are not essential for tissue distribution of α-tocopherol, because in mice lacking VLDL the α-tocopherol content of HDL significantly increases [31]. HDL particles are also involved in reverse transport of α-tocopherol from peripheral tissues back to the liver. They also deliver α-tocopherol to different tissues. The transfer of α-tocopherol from HDL particles to tissues involves a pathway related to SR-B1 [32].
Unlike other fat-soluble vitamins, vitamin E is not stored. The metabolism of vitamin E vitamers consists of an initial ω-hydroxylation. The hydroxyl group will be oxidized to a carboxyl group. The final step is represented by a sequence of β-oxidation leading to α-, β-, γ-, and δ-CEHC (2’-carboxyethyl-6-hydroxychromane). The same pathway is followed by the four tocotrienols. The initial ω-hydroxylation is carried out by CYP4F2 and probably CYP3A4 [33,34]. Prior to excretion in either the bile or the urine, CEHCs are sulfated or glucuronidated [35]. There is evidence that unmodified α-tocopherol is excreted into bile, a process which depends upon two ATP-binding cassette (ABC) transporters located in the canalicular membranes of hepatocytes (MDR1 and MDR3) [36]. It is speculated that MDR1 is involved in α-tocopherol bile excretion under conditions of high-dose supplementation [36].
4. Secretion of α-tocopherol by the liver
There is a disagreement between diet and plasma concentrations of α-tocopherol and γ-tocopherol. A typical US diet contains large amounts of soybean oil which is rich in γ-tocopherol (approximately 70mg), but scarce α-tocopherol (approximately 7mg) [38]. Plasma α-tocopherol concentrations in human range from 11-37μmol/L, while γ-tocopherol concentrations are roughly 2-5μmol/L [39]. This paradox is a consequence of (1) the selectivity of the hepatic α-TTP and (2) the regulation of vitamin E hepatic metabolism and excretion.
4.1. α-Tocopherol transfer protein selectively binds α-tocopherol
The central factor that regulates α-tocopherol concentration is α-TTP. α-TTP is a cytosolic protein with a molecular weight of 32kDa coded by a gene located on chromosome 8 (8q13.1-13-3) [40]. α-TTP belongs to the CRAL-TRIO family along with the cellular retinaldehyde binding protein (CRALBP), yeast phosphatidylinositol transfer protein (Sec14p), and supernatant protein factor (SPF) involved in cholesterol biosynthesis [41]. α-TTP was identified in hepatocytes, human brain and human placenta [23]. The affinities of α-TTP for various vitamin E vitamers and the biological activities of α-tocopherol stereoisomers are presented in Table II and III.
Table 2.
Affinities of α-TTP for vitamin E vitamers [42].
| RRR-α-tocopherol | 100% |
| β-tocopherol | 38% |
| γ-tocopherol | 9% |
| δ-tocopherol | 2% |
| α-tocopherol acetate | 2% |
| α-tocopherol quinone | 2% |
| SRR-α-tocopherol | 11% |
| α-tocotrienol | 12% |
| trolox | 9% |
Table 3.
Biological activities of α-tocopherol stereoisomers [3].
| RRR-α-tocopheryl acetate | 100% |
| RRS-α-tocopherol | 90% |
| RSS-α-tocopherol | 73% |
| SSS-α-tocopherol | 60% |
| RSR-α-tocopherol | 57% |
| SRS-α-tocopherol | 37% |
| SRR-α-tocopherol | 31% |
| SSR-α-tocopherol | 21% |
Crystallographic studies revealed that α-tocopherol is bounded by α-TTP inside a hydrophobic pocket through van der Waals contacts [43,44]. Inside the hydrophobic pocket there are also four water molecules: two are hydrogen-bonded to the hydroxyl group of the chromanol ring, one is hydrogen-bonded to the oxygen atoms of Val182 and Leu189, and the fourth water molecule is hydrogen-bonded to the hydroxyl group of the Ser140 [44].
Inside the hydrophobic pocket of α-TTP there is an indent generated by the side chains of Phe133, Val182 and Ile179. It plays an important role in discrimination among different stereoisomers of α-tocopherol as it can accommodate only the chiral C2 with R configuration [43].
The factors responsible for ligand discrimination of α-TTP are (1) the methylation degree of the chromanol ring, (2) the presence of the phytyl tail, and (3) the R configuration at carbon 2 where the phytyl tail attaches to the chromanol ring [41]. The extreme low affinity of α-TTP for tocotrienols is explained by the presence of the three double bonds with rigid configurations, which impede the unsaturated tail to accommodate in the hydrophobic pocket of the protein.
Inside the hepatocytes, α-TTP aquires α-tocopherol from endosomes and then moves to plasma membrane where α-tocopherol is released. Then, α-tocopherol can be incorporated into nascent VLDL particles.
4.2. Regulation of hepatic vitamin E metabolism and excretion
Hepatic metabolism and excretion of vitamin E vitamers is the second regulatory level by which human body selectively retains RRR-α-tocopherol.
The first step of the hepatic vitamin E metabolism is represented by the CYP4F2 ω-hydroxylation. CYP4F2 is more active toward γ-tocopherol than toward α-tocopherol [45]. It was found that the critical determinants of the rate of ω-hydroxylation are (1) the position of methyl groups on the chromanol ring, particularly at C5, and (2) unsaturation of the side chain [46]. The presence of a methyl group at C5 leads to decrease susceptibility of ω-hydroxylation. It was also found that CYP4F2 has allosteric properties as α-tocopherol acts as a positive allosteric effector that stimulates the ω-hydroxylation rate of other vitamin E vitamers.
5. Conclusions
There are several gaps in the knowledge about the regulation of vitamin E concentration. All performed studies indicate that there is a high preference for α-tocopherol, but the exact reason of this preference is still controversial and remains undetermined. Acting in conjunction, two hepatic proteins, α-TTP and CYP4F2 are greatly responsible for the selective retention by the human body of α-tocopherol. Further studies are needed to obtain a clearer image about the regulation of α-tocopherol metabolism.
References
- 1.Cercasov C, Manolescu B. Biochemical and therapeutical aspects of vitamin E. Rev. Roum. Chim. 2005;50(6):419–432. [Google Scholar]
- 2.Weiser H, Vecchi H. Stereoisomers of alpha-tocopheryl acetate. II. Biopotencies of all eight stereoisomers, individually or in mixtures, as determined by rat resorption-gestation tests. Int. J. Vitam. Nutr. Res. 1982;52(3):351–370. [PubMed] [Google Scholar]
- 3.Weiser H, Riss G, Kormann AW. Biodiscrimination of the eight α-tocopherol stereoisomers results in preferential accumulation of the four 2R forms in tissues and plasma of rats. J. Nutr. 1996;126:2539–2549. doi: 10.1093/jn/126.10.2539. [DOI] [PubMed] [Google Scholar]
- 4.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 5.Azzi A, Zingg J-M. Vitamin E: textbooks require updating. Biochem. Mol. Biol. Educat. 2005;33(3):184–187. doi: 10.1002/bmb.2005.494033032451. [DOI] [PubMed] [Google Scholar]
- 6.Burton GW, Doba T, Gabe EJ, et al. Autooxidation of biological molecules. 4. Maximizing the antioxidant activity of phenols. J. Am. Chem. Soc. 1985;107:7053–7065. [Google Scholar]
- 7.McCay PB. Vitamin E: interactions with free radicals and ascorbate. Ann. Rev. Nutr. 1985;5:323–340. doi: 10.1146/annurev.nu.05.070185.001543. [DOI] [PubMed] [Google Scholar]
- 8.Niki E. Antioxidants in relation to lipid peroxidation. Chem. Phys. Lipids. 1987;44:227–253. doi: 10.1016/0009-3084(87)90052-1. [DOI] [PubMed] [Google Scholar]
- 9.Wefers H, Sies H. The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur. J. Biochem. 1988;174:353–357. doi: 10.1111/j.1432-1033.1988.tb14105.x. [DOI] [PubMed] [Google Scholar]
- 10.Cachia O, Benna JE, Pedruzii E, et al. α-Tocopherol inhibits respiratory burst in human monocytes: attenuation of p47phox membrane translocation and phosphorylation. J. Biol. Chem. 1998;273:32801–32805. doi: 10.1074/jbc.273.49.32801. [DOI] [PubMed] [Google Scholar]
- 11.Chandra V, Jasti J, Kaur P, et al. First structural evidence of a specific inhibition of phospholipase A2 by α-tocopherol (vitamin E) and its implications in inflammation: crystal structure of the complex formed between phospholipase A2 and α-tocopherol at 1,8 Å resolution. J. Mol. Biol. 2002;320:215–222. doi: 10.1016/S0022-2836(02)00473-4. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q, Elson-Schwab I, Courtemanche C, et al. γ-Tocopherol and its major metabolite, in contrast to α-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mari M, Cederbaum I. Induction of catalase, α, and microsomal glutathione S-transferase in CYP2E1 overexpressing HepG2 cells and protection against short-term oxidative stress. Hepatology. 2001;33:652–661. doi: 10.1053/jhep.2001.22521. [DOI] [PubMed] [Google Scholar]
- 14.Landes N, Pfluger P, Birringer M, et al. Vitamin E activates hPXR-mediated gene expression in HepG2 cells. Free Radic. Biol. Med. 2002;33:S189–S194. [Google Scholar]
- 15.Kolleck I, Schlame M, Fechner H, et al. HDL is the major source of vitamin E for type II pneumocytes. Free Rad. Biol. Med. 1999;27:882–890. doi: 10.1016/s0891-5849(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 16.Aratri E, Spycher E, Breyer I, et al. Modulation of α-tropomyosin expression by α-tocopherol in rat vascular smooth muscle cells. FEBS Lett. 1999;447:91–94. doi: 10.1016/s0014-5793(99)00277-x. [DOI] [PubMed] [Google Scholar]
- 17.Villacorta L, Graca-Souza AV, Ricciarelli R, et al. α-Tocopherol induces expression of connective tissue growth factor and antagonizes tumor necrosis factor-α-mediated down-regulation in human smooth muscle cells. Circ. Res. 2003;92:104–110. doi: 10.1161/01.res.0000049103.38175.1b. [DOI] [PubMed] [Google Scholar]
- 18.Azzi A, Ricciarelli R, Zingg JM. Non-antioxidant molecular functions of α-tocopherol (vitamin E) FEBS Lett. 2002;519:8–10. doi: 10.1016/s0014-5793(02)02706-0. [DOI] [PubMed] [Google Scholar]
- 19.Teupser D, Thiery J, Seidel D. α-Tocopherol decreases CD36 expression in human monocyte-derived macrophages. J. Lipid Res. 2001;42:521–527. [PubMed] [Google Scholar]
- 20.Ricciarelli R, Maroni P, Ozer N, et al. Age-dependent increase of collagenase expression can be reduced by α-tocopherol via protein kinase C inhibition. Free Rad. Biol. Med. 1999;27:729–737. doi: 10.1016/s0891-5849(99)00007-6. [DOI] [PubMed] [Google Scholar]
- 21.Chojkier M, Houglum K, Lee KS, et al. Long- and short-term D-α-tocopherol supplementation inhibits liver collagen α1(I) gene expression. Am. J. Physiol. 1998;275:G1480–G1485. doi: 10.1152/ajpgi.1998.275.6.G1480. [DOI] [PubMed] [Google Scholar]
- 22.Yap SP, Yuen KH, Wong JW. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J. Pharm. Pharmacol. 2001;53:67–71. doi: 10.1211/0022357011775208. [DOI] [PubMed] [Google Scholar]
- 23.Traber MG. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 24.Traber MG, Kayden HJ. Preferential incorporation of v-tocopherol vs α-tocopherol in human lipoproteins. Am. J. Clin. Nutr. 49:517–526. doi: 10.1093/ajcn/49.3.517. [DOI] [PubMed] [Google Scholar]
- 25.Traber MG, Burton G, Hughes L, et al. Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism. J. Lipid Res. 1992;33:1171–1182. [PubMed] [Google Scholar]
- 26.Traber MG, Olivercrona T, Kayden HJ. Bovine milk lipoprotein lipase transfers tocopherol to human fibroblasts during triglyceride hydrolysis in vitro. J. Clin. Invest. 1985;75:1729–1734. doi: 10.1172/JCI111883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sattler W, Levak-Frank S, Radner H, et al. Muscle-specific overexpression of lipoprotein lipase in transgenic mice results in increased α-tocopherol levels in skeletal muscle. Biochem. J. 1996;318:15–19. doi: 10.1042/bj3180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostner GM, Oettl K, Juahiainen M, et al. Human plasma phospholipids transfer protein accelerates exchange/transfer of α-tocopherol between lipoproteins and cells. Biochem. J. 1995;305:659–667. doi: 10.1042/bj3050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang XC, Tall AR, Qin S, et al. Phospholipid transfer protein deficiency protects circulating lipoproteins from oxidation due to the enhanced accumulation of vitamin E. J. Biol. Chem. 2002;277:31850–31856. doi: 10.1074/jbc.M205077200. [DOI] [PubMed] [Google Scholar]
- 30.Arita M, Nomura K, Arai H, et al. α-Tocopherol transfer protein stimulates the secretion of α-tocopherol from a cultured liver cell line through a brefeldin A-insensitive pathway. Proc. Natl. Acad. Sci. USA. 1997;94:12437–12441. doi: 10.1073/pnas.94.23.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minehira-Castelli K, Leonard SW, Walker Q, et al. Absence of VLDL secretion does not affect α-tocopherol content in peripheral tissues. J. Lipid Res. 2006;47:1773–1778. doi: 10.1194/jlr.M600125-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Goti D, Reicher H, Malle E, et al. High-density lipoprotein (HDL3)-associated alpha-tocopherol is taken up by HepG2 cells via the selective uptake pathway and resecreted with endogenously synthesized apo-lipoprotein B-rich lipoprotein particle. Biochem. J. 1998;332:57–65. doi: 10.1042/bj3320057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker RS, Sontag TJ, Swanson JE. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem. Biophys. Res. Commun. 2000;277:531–534. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 34.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism: novel mechanism of regulation of vitamin E status. J. Biol. Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 35.Mustacich DJ, Bruno RS, Traber MG. Vitamin E. 2007;76:1–21. doi: 10.1016/S0083-6729(07)76001-6. [DOI] [PubMed] [Google Scholar]
- 36.Mustacich DJ, Shields J, Horton RA, et al. Biliary secretion of α-tocopherol and the role of mdr2 P-glycoprotein in rats and mice. Arch. Biochem. Biophys. 1998;350:183–192. doi: 10.1006/abbi.1997.0529. [DOI] [PubMed] [Google Scholar]
- 37.Mustacich DJ, Leonard SW, Devereaux MW, et al. α-Tocopherol regulation of hepatic cytochrome P450s and ABC transporters in rats. Free Radic. Biol. Med. 2006;41:1069–1078. doi: 10.1016/j.freeradbiomed.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 38.Eitenmiller R, Lee J. Vitamin E: Food Chemistry, Composition, and Analysis. New York: Marcel Dekker; 2004. p. 530. [Google Scholar]
- 39.O’Byrne D, Grundy S, Packer L, et al. Studies of LDL oxidation following alpha-, gamma-, or delta-tocotrienyl acetate supplementation of hypercholesterolemic humans. Free Radic. Biol. Med. 2000;29:834–845. doi: 10.1016/s0891-5849(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 40.Catignani GL. An alpha-tocopherol binding protein in rat liver cytoplasm. Biochem. Biophys. Res. Commun. 1975;67:66–72. doi: 10.1016/0006-291x(75)90283-1. [DOI] [PubMed] [Google Scholar]
- 41.Panagabko C, Morley S, Hernandez M, et al. Ligand specificity in the CRAL-TRIO protein family. Biochemistry. 2003;42:6467–6474. doi: 10.1021/bi034086v. [DOI] [PubMed] [Google Scholar]
- 42.Hosomi A, Arita M, Sato Y, et al. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogues. FEBS Lett. 1997;409:105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 43.Meier R, Tomizaki T, Schulze-Briese C, et al. The molecular basis of vitamin E retention: structure of human α-tocopherol transfer protein. J. Mol. Biol. 2003;331:725–734. doi: 10.1016/s0022-2836(03)00724-1. [DOI] [PubMed] [Google Scholar]
- 44.Min KC, Kovall RA, Hendrickson WA. Crystal structure of human α-tocopherol transfer protein bound to its ligand: implications for ataxia with vitamin E deficiency. Proc. Natl. Acad. Sci. USA. 2003;100:14713–14718. doi: 10.1073/pnas.2136684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism: novel mechanismof regulation of vitamin E status. J. Biol. Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 46.Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their ω-oxidation by tocopherol-ω-hydroxylase. J. Lipid Res. 2007;48:1090–1098. doi: 10.1194/jlr.M600514-JLR200. [DOI] [PubMed] [Google Scholar]