Abstract
Background
Clostridium difficile infection (CDI) is the most common infectious cause of nosocomial diarrhea and its prevention is an urgent public health priority. However, reduction of CDI is challenging, because of its complex pathogenesis, large reservoirs of colonized patients, and persistence of infectious spores. The literature lacks high quality evidence for evaluating interventions, and many hospitals have implemented bundled interventions to reduce CDI with variable results. Thus, we conducted a systematic review to examine the components of CDI bundles, their implementation processes, and their impact on CDI rates.
Methods
We conducted a comprehensive literature search of multiple computerized databases from their date of inception through April 30, 2016. The protocol was registered in PROSPERO. Bundle effectiveness, adherence, and study quality was assessed for each study meeting criteria for inclusion.
Results
In the 26 studies that met inclusion criteria for this review, we found that implementation and adherence factors to interventions were variably and incompletely reported, making study reproducibility and replicability challenging. Despite contextual differences and the variety of bundle components utilized, all 26 studies reported an improvement in CDI rates. However, given the lack of randomized controlled trials in the literature, assessing a causal relationship between bundled interventions and CDI rates is currently impossible.
Conclusions
Cluster randomized trials that include a rigorous assessment of the implementation of bundled interventions are urgently needed to causally test the effect of intervention bundles on CDI rates.
Background
Clostridium difficile (C. difficile) infection (CDI) is a major public health threat in the healthcare setting, and is associated with considerable morbidity, mortality, and economic costs.1–6 Reporting of CDIs has been mandatory in England’s National Health Service since 2004 and C. difficile is considered one of the top three urgent threat pathogens by the Centers for Disease Control and Prevention in the United States.7,8 Beginning in 2017, CDI rates will be included among the hospital-acquired complications used by the Centers for Medicare and Medicaid to penalize the lowest performing hospitals.9
Control of CDI is especially challenging, given multiple sources of transmission and a complex, poorly understood pathogenesis and set of risk factors.10 C. difficile has large reservoirs in the environment, including asymptomatic carriers that may account for over half of disease transmission.11,12 Its spores can persist on hard surfaces for up to 5 months, further complicating disease eradication.13
Bundled interventions targeting catheter-associated urinary tract infections (CAUTI) and central line-associated blood stream infections (CLABSI) have had success in reducing the rates of those device-associated HAIs. Seeking to continue this trend, hospitals have implemented targeted C. difficile intervention bundles. Unlike CAUTI and CLABSI however, the evidence for these bundles is far less robust. Few randomized clinical trials have examined interventions to reduce CDI incidence, and those that have all focused on single interventions such as patient hand hygiene,14 disposable equipment,15 daily chlorhexidine bathing,16 or environmental disinfection,17,18 rather than an intervention bundle.
Bundled interventions require a high degree of compliance to be effective.19 Yet, adherence with complex bundle components may be challenging and variable across settings. Given the lack of direct evidence for CDI bundle adherence and intervention outcomes, we undertook a systematic review to examine common bundle components, evaluate component adherence and study replicability, and assess the effectiveness of bundles on reducing hospital CDI rates.
Methods
For the purposes of this review, a bundle was defined as any set of multiple (>1) interventions focused on reducing CDI in the inpatient setting.
Search Strategies
We conducted a comprehensive search of four databases: the Cochrane Central Register of Controlled Trials, PubMed, Web of Science, and the Cumulative Index to Nursing and Allied Health. We sought to capture articles and abstracts published between each database’s date of inception and May 28th, 2015. Thus, the search start date was different for each database. Another search was run closer to publication to include articles available through April 30th, 2016. The search strategy was designed and conducted by an experienced librarian with input from the study team. The following keywords were used to search for bundled interventions aimed at reducing C. difficile infections: (c difficile OR c. difficile OR clostridium difficile OR "c diff" OR "c. diff") AND ("infection control" OR bundle OR bundled OR bundles OR "multiple control" OR "multiple controls" OR "control package" OR "control packages" OR "integrated control" OR "integrated controls" OR multipronged OR multi-pronged). We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement guidelines in conducting this systematic review.20 The protocol was registered at the International prospective register of systematic reviews (PROSPERO), record number 2015:CRD42015023252.
All abstracts resulting from the search strategy were screened and potentially relevant articles for a full text review were identified. Bibliographies were manually inspected to identify relevant studies not previously identified by our database search.
Inclusion/exclusion criteria
We included all inpatient studies that examined the effectiveness of a CDI-specific intervention bundle and provided data on the rates of CDI before and after intervention implementation. Single intervention studies (non-bundle) and studies that did not provide data to evaluate effectiveness were excluded, as were abstracts, review articles, and editorials. There were no language restrictions.
Data Abstraction
The primary outcome of this review was the mean difference in the rates of hospital-acquired CDI. Rates were measured at the hospital level, in units defined by each article. For every study we abstracted the following: the interventions included in each bundle; C. difficile case definition; infection rates before and after intervention implementation; C. difficile outbreak status; hospital setting; study population; study design; and intervention adherence rates.
All studies were abstracted and screened independently by two reviewers (AB and CN). For disagreements regarding article inclusion, resolution was reached by discussion between the two reviewers.
Assessing bundle effectiveness and adherence
We assessed effectiveness of the bundles by extracting the reported point estimates and calculating the difference in infection rates before and after intervention implementation. Bundled interventions were categorized into 10 primary components: antibiotic stewardship, contact precautions, dedicated equipment, staff education, patient education, environmental cleaning, hand hygiene, isolation and/or cohorting, proton pump inhibitor stewardship, and systems and workflow changes.
Adherence has been previously defined as the extent to which specified program components are delivered as outlined in a program manual.21 We evaluated adherence by identifying and quantifying the number of adherence measures within a given component of the CDI prevention bundle. A study was considered to have assessed adherence if it reported a method of measuring compliance for one or more bundle elements, such as direct observation or tracking glove usage.
TiDier Checklist
We assessed replicability of each study included in the final analysis using the Template for Intervention Description and Replication (TiDier) checklist.22 This tool consists of twelve dichotomous items that assess the description of an intervention and evaluate its replicability. The score reflects the number of items that a given intervention addressed, with higher scores indicating better replicability. The maximum score was twelve.
Bias Assessment
To assess the quality and risk of bias for each study, we used a modified version of the Checklist for Measuring Quality instrument developed by Downs and Black.23,24 This tool contains 27 dichotomous items regarding reporting, external validity, bias, confounding, and power. The maximum possible score of the modified instrument is 28, with the assessment of power modified from a 0–5 scale to 0–1.
Results
The search strategy above identified 1242 distinct articles, of which 1181 were excluded based on abstract information. The remaining 61 full text articles were reviewed, and 26 were determined to meet inclusion criteria. This is summarized in the PRISMA flow diagram (Figure 1).
Figure 1.
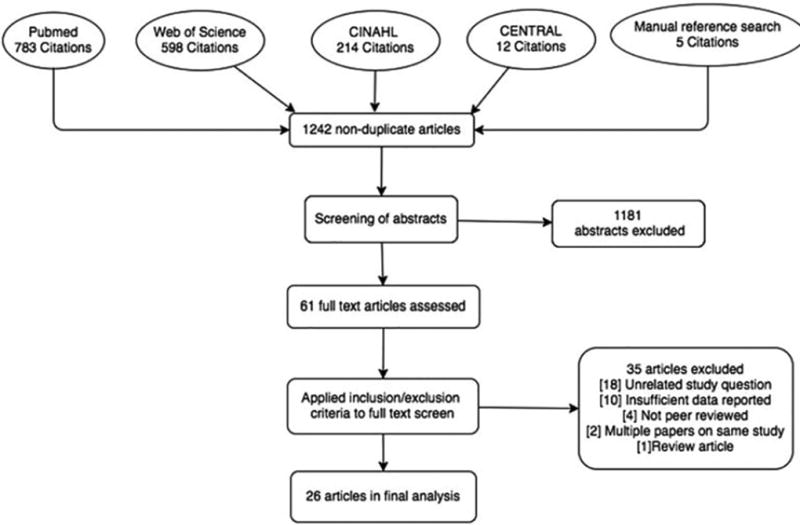
Characteristics of included studies
While details of the case definitions for CDI differ between studies, they were generally consistent. Most required clinical symptoms and a positive C. difficile test. A comprehensive description of the studies is listed in Table 1. The study locations varied, including nine in Europe,25–33 three in Asia,34–36 and fourteen in North America.37–50 Most studies examined all hospital wards (65%, 17/26). Others analyzed specific wards or patient populations only: Bone marrow transplant,41,48 medical ICU,36,48 geriatric ward,31 surgical inpatients,49 all units except psychiatry and pediatrics,37 all units except neonatal,32 hip-fracture patients,25and patients over age two.26
Table 1.
Study characteristics
| Author, Year | Study design | Bundle start date(s) |
Setting | Study populationa |
CDI definition | Context |
|---|---|---|---|---|---|---|
| Abbett et al., 200940 | Interrupted time series | 1/06–4/06 | Brigham and Women's Hospital, Boston, USA; 750-bed tertiary, academic | All inpatients | + toxin | Rates trending upward pre-intervention |
| Apisarnthanarak et al., 200448 | Pre-post intervention | 6/02–8/02 | Barnes- Jewish hospital, St. Louis, USA; academic | BMT and MICU | + toxin | Outbreak |
| Bishop et al., 201349 | Interrupted time series | 9/08 | Stamford Hospital, Stamford, USA; academic | All surgical patients (n = 39,093) | + toxin or pseudomembranous colitis | Endemic rates |
| Brakovich et al., 201350 | Interrupted time series | 12/09 | Long-term acute care hospital, southeast USA | All inpatients | + combined antigen markers, glutamate dehydrogenase, toxin A/B | Endemic rates |
| Cheng et al., 201534 | Interrupted time series | Q2,10 | 1 acute and 3 extended care hospitals; 3,200 beds; Hong Kong | All inpatients | Diarrhea, + toxin, and + stool culture | Rates trending upward pre-intervention |
| Gulihar et al., 200925 | Interrupted time series | 8/06–5/07 | University Hospitals of Leicester NHS Trust; UK | Adults with femur fracture (n = 3,417) | Diarrhea and + toxin | Endemic rates |
| Hanna et al., 200041 | Pre-post intervention | 5/95 | University of Texas MD Anderson Cancer Center, Houston, USA | BMT patients | Diarrhea and + toxin | Outbreak |
| Lai et al., 199747 | Interrupted time series | 10/91–2/92 | University of Massachusetts Memorial Medical Center, Worcester, USA; 370-bed, tertiary, academic | All inpatients | Diarrhea and + toxin | Outbreak |
| Marufu et al., 201526 | Interrupted time series | 2/03–10/11 | King’s College Hospital NHS Foundation Trust, London, UK | Inpatients over age 2 (n = 704,654) | Loose stools and + toxin test or pseudomembranous and CDI histopathology | Endemic rates |
| Mattner et al., 200827 | Interrupted time series | 5/07–4/08 | Northern Germany; academic | All inpatients | Diarrhea and + toxin | Outbreak |
| Mermel et al., 201344 | Interrupted time series | Q4,06-Q3,12 | Rhode Island Hospital, Providence, USA; 719-bed, tertiary, academic | All inpatients | Diarrhea or toxic megacolon and + toxin or pseudomembranous colitis | Rates trending upward pre-intervention |
| Muto et al., 200738 | Interrupted time series | 7/00–7/03 | University of Pittsburgh Medical Center–Presbyterian, Pittsburgh, USA; 834-bed, tertiary, academic | All inpatients | + toxin or pseudomembranous colitis | Outbreak |
| Oleastro et al., 201428 | Interrupted time series | 4/12 | Centro Hospitalar do Algarve, Lagos, Portugal; 330-bed secondary hospital and 40 bed internal medicine center | All inpatients | + toxin | Outbreak |
| Power et al., 201029 | Interrupted time series | 2/07–12/07 | Salford Royal NHS Foundation Trust, England, UK; 850 bed, academic | All inpatients | Diarrhea and + toxin | Endemic rates |
| Price et al., 201030 | Interrupted time series | 1/08 | Brighton and Sussex University Hospitals NHS trust, UK; 820-bed acute secondary and tertiary, academic | All inpatients (n = 200,245) | Diarrhea and + toxin | Rates trending upward pre-intervention |
| Salgado et al., 200946 | Interrupted time series | 11/04 | Medical University of South Carolina hospital, 610 bed, tertiary | All inpatients | Diarrhea and + toxin | Outbreak |
| Stone et al., 199831 | Interrupted time series | 7/95 | Royal Free NHS Trust, London, England, UK; academic | Non-MRSA geriatrics patients (n = 2,467) | Diarrhea and + toxin | Rates trending upward pre-intervention |
| Struelens et al., 199132 | Pre-post intervention | 8/88–7/89 | University of Brussles Hospital Erasme, Bruselles, Belgium; 840-bed, tertiary | All except neonatal | Diarrhea or pseudomembraneous colitis and + stool culture or + toxin | Outbreak |
| Suzuki et al., 201335 | Pre-post intervention | 10/11 | Tsukuba Medical Center Hospital, Tsukuba, Japan; 409-bed, tertiary, academic | All inpatients | + toxin | Endemic rates |
| Valiquette et al., 200737 | Interrupted time series | 9/03–10/04 | Centre Hospitalier Universitaire de Sherbrooke, CA; 683-bed secondary/tertiary | All except psychiatry, pediatrics | + toxin, pseudomembranous/ antibiotic associated/ C. difficile colitis | Outbreak |
| Weiss et al., 200939 | Interrupted time series | 6/05–11/05 | Maisonneuve-Rosemont Hospital, Montreal, CA; 554-bed acute care, tertiary, academic | All inpatients | Diarrhea and + toxin | Outbreak |
| Whitaker et al., 200742 | Interrupted time series | 10/03–7/04 | University Community Hospital, Tampa, USA; 469-bed tertiary | All inpatients | + toxin | Rates trending upward pre-intervention |
| White, et al., 201633 | Interrupted time series | 08/06 | University Hospitals of Leicester (UHL), 2000-bed acute UK NHS Trust | All inpatients | Diarrhea and + toxin | Rates trending upward pre-intervention |
| Wong-mcclure et al., 201343 | Interrupted time series | 1/09–5/09 | Costa Rica; 685 bed, tertiary | All inpatients | Diarrhea, + toxin, and + stool culture | Outbreak |
| You et al., 201436 | Pre-post intervention | 4/12 | Wonkwang University Hospital, Iksan, South Korea; academic | MICU (n = 567) | Diarrhea and + toxin | Endemic rates |
| Zafar et al., 199845 | Pre-post intervention | 4/90–93 | Columbia Arlington Hospital, Arlington, USA; 350-bed acute care community teaching | All inpatients | Diarrhea and + toxin | Endemic rates |
The total sample size is reported for all studies that provided this information; BMT: Bone marrow transplant, C. difficile: Clostridium difficile, CDI: C. difficile infection, MICU: Medical intensive care unit, NHS: National Health System, UK: United Kingdom, USA: United States of America
Our literature search did not identify any randomized controlled trials. Among included studies, 20 were interrupted time series25–31,33,34,37–40,42–44,46,47,49,50 and 6 were quasi-experimental pre-post intervention studies.32,35,36,41,45,48 Eleven were conducted in the midst of a C. difficile outbreak,27,28,32,37–39,41,43,46–48 eight as quality improvement projects to reduce endemic rates,25,26,29,35,36,45,49,50 and seven in the context of upwardly trending CDI rates.30,31,33,34,40,42,44
Intervention bundles components
The type of interventions implemented as part of a CDI bundle varied widely across studies (Table 2). Among the 10 bundle components, hand hygiene and environmental cleaning components were the most common interventions employed. Both were included in 88.5% (23/26) of the studies. These were followed by isolation and/or cohorting (77%, 20/26). Contact precautions, antibiotic stewardship, and staff education were each included in 73% (19/26) of studies. System and workflow changes were in 54% (14/26), dedicated equipment, 27%, (7/26), patient education, 19%, (5/26), and proton pump inhibitor stewardship, 12% (3/26).
Table 2.
Intervention details
| First Author | Intervention | Component Details |
|---|---|---|
| Abbett | Antibiotic Stewardship | Discontinue nonessential antimicrobials when suspected case |
| Contact Precautions | Enhanced; gowns, gloves, alcohol gel before, soap and water after patient contact | |
| Dedicated Equipment | Stethoscope in patient rooms | |
| Education- Staff | CDI and prompt responses; nurses, doctors, physician assistants, environmental services, and administration | |
| Environmental Cleaning | Hyperchlorite disinfectant at discharge | |
| Hand Hygiene | Described in contact precautions | |
| Isolation and/or Cohorting | Suspected cases in single room | |
| Systems and Workflow | Infection control for suspected cases, communication improved between lab and nurses, infection preventionists and environmental services, infection preventionists sent daily CDI list and confirm prevention practices, electronic medical record flagging, standardize CDI treatment | |
| Apisarn-thanarak | Contact Precautions | Not Specified |
| Education- Staff | Regarding contact precaution | |
| Environmental Cleaning | Patient rooms and staff areas cleaned with 10% hypochlorite, carpeted areas cleaned | |
| Hand Hygiene | Signage | |
| Bishop | Antibiotic Stewardship | Prophylactic antibiotics regulated, fluoroquinolones limited |
| Contact Precautions | Limit patient contact to 1 member of surgical team, lab coats provided, glove changes | |
| Environmental Cleaning | Terminal cleaning focused on immediate patient environment | |
| Hand Hygiene | Before and after gloving, increased education, increased monitoring, facility improvements, | |
| Proton Pump Inhibitor Stewardship | Limited to intensive care unit or specific clinical indications | |
| Systems and Workflow | Resident rounding to limit staff exposures | |
| Brakovich | Environmental Cleaning | New cleaning equipment (microfiber mops vs. cotton), decontaminate more frequently, hydrogen peroxide vapor decontamination |
| Antibiotic Stewardship | Lower frequency and duration of antimicrobials, restrict clindamycin and cephalosporin | |
| Education - Staff | Hands on training for environmental services | |
| Hand Hygiene | Recommend soap and water, reminder stickers, staff and visitors | |
| Isolation and/or Cohorting | CDI patient isolate to private room | |
| Contact Precautions | Contact precautions for all CDI patients | |
| Systems and Workflow | Improved diagnostic testing, cleaning checklist for environmental services staff | |
| Cheng | Antibiotic Stewardship | Immediate concurrent feedback, and focus on broad-spectrum intravenous antibiotics |
| Contact Precautions | Gloves and gowns | |
| Dedicated Equipment | Bedpans and commodes | |
| Education- Staff | Train cleaning staff, emphasizing high-touch areas, training ward staff quarterly | |
| Environmental Cleaning | Clean rooms twice daily, 1,000 parts per million sodium hypochlorite, curtain change at discharge | |
| Hand Hygiene | Soap and water | |
| Isolation and/or Cohorting | Nursed as cohort, preferably in single rooms | |
| Guilhar | Antibiotic Stewardship | Five-day antibiotic stop policy, approval for high-risk antibiotics, surgery prophylaxis changed from cefuroxime to co-amoxiclav or vancomycin |
| Education- Patient | Focus on hand hygiene | |
| Education- Staff | Focus on hand hygiene | |
| Environmental Cleaning | Sodium-dichloroisocyanurate for environmental cleaning | |
| Hand Hygiene | Alcohol gel on rounds, soap/water before and after ward and isolation bays, new sinks | |
| Isolation and/or Cohorting | Rapid isolation of diarrheal patients in side rooms or isolation bays | |
| Hanna | Contact Precautions | Enteric precautions for all diarrheal patients, disposable gowns, gloves in CDI rooms |
| Dedicated Equipment | Mercury thermometers | |
| Education- Staff | On-ward sessions on CDI | |
| Environmental Cleaning | Daily, routine, and terminal cleaning with 1:100 bleach | |
| Hand Hygiene | Chlorhexidine gluconate before and after patient care, individual rolls of paper towels | |
| Isolation and/or Cohorting | Not specified | |
| Lai | Contact Precautions | Universal precautions |
| Education- Staff | Intense education on modes of transmission, prevention, and control | |
| Environmental Cleaning | New commode cleaning, new commodes | |
| Hand Hygiene | Emphasized for staff, new soap dispensers in patient bathrooms, towelettes before meals | |
| Isolation and/or Cohorting | CDI patients cohorted | |
| Marufu | Antibiotic Stewardship | Microbiologist-led antibiotic rounds, restrictive antibiotic policy, audits |
| Dedicated Equipment | Disposable bedpans and macerators | |
| Education- Staff | Infection control training consults, ongoing notices for staff | |
| Environmental Cleaning | Clean equipment and environment with hypochlorite, new cleaning strategy group | |
| Hand Hygiene | WHO Clean your hands campaign | |
| Isolation and/or Cohorting | Isolation unit introduced | |
| Systems and Workflow | Infection control scorecard, new infection control strategy team, review meetings, CDI feedback to all wards, Saving Lives toolkit, United Kingdom infection control code, diarrhea care plan and action cards, CDI ward rounds | |
| Mattner | Education- Staff | Occupational groups trained |
| Environmental Cleaning | Sporicidal disinfection done more frequently | |
| Hand Hygiene | Recommend gloves, hand wash, disinfection | |
| Isolation and/or Cohorting | Introduced | |
| Mermel | Antibiotic Stewardship | Audit antibiotic use, provide feedback, use electronic drug orders, pre-authorization requirements, streamline therapy based on labs, optimize doses, intravenous to oral conversion, increase narrow spectrum antibiotic use, limit quinolones, clindamycin |
| Contact Precautions | Easily accessible, many size gloves, gowns, masks in isolation rooms, empty trash often | |
| Dedicated Equipment | Blood pressure cuff, thermometer, stethoscope in isolation rooms | |
| Education- Staff | Annual infection control education, include antibiotic policy | |
| Environmental Cleaning | Hire more housekeepers, hypochlorite-based cleaning of isolation rooms, dedicate team monitor cleaning supplies, enhanced daily room cleaning | |
| Hand Hygiene | Soap and water use encouraged | |
| Systems and Workflow | New tool to identify high-risk patients, nurses order CDI test and initiate isolation, improve CDI test sensitivity, increase testing frequency, develop management guidelines | |
| Muto | Antibiotic Stewardship | Clindamycin, ceftriaxone, levofloxacin, broad spectrum antimicrobials require approval |
| Contact Precautions | Sustained for duration of hospitalization | |
| Education- Staff | Printed material, lecture at staff meetings on epidemiology, risk factors, clinical findings, control measures, and rates | |
| Environmental Cleaning | Daily cleaning with bleach (1:100) of high-touch surfaces, later increased to 1:10 | |
| Hand Hygiene | Soap and water (not alcohol) for CDI patients | |
| Isolation and/or Cohorting | Cohorting facilitated by EMR | |
| Systems and Workflow | Nurses can order lab test, EMR flag high-risk patients and email alert physicians, establish CDI management team for rapid evaluation, real-time lab notifications | |
| Olestro | Antibiotic Stewardship | Limit quinolones and 3rd generation cephalosporins by time and indication |
| Contact Precautions | Gloves and aprons | |
| Education- Staff | Educate on treatment, prevention, diagnosis | |
| Education- Patients | Distribute written material | |
| Environmental Cleaning | Clean ward and equipment every 8-hours with 5,000 parts per million hypochlorite, with peroxide hydrogen vaporization after discharge | |
| Hand Hygiene | Soap and Water | |
| Isolation and/or Cohorting | Admit to individual room, cohort cases | |
| Proton Pump Inhibitor Stewardship | Limited to those clinically indicated | |
| Systems and Workflow | Report outbreak to authorities, nurses, ward leaders, establish Regional Infection Control Group network, protocol for early diagnosis and treatment, type toxin positive samples | |
| Power | Antibiotic Stewardship | Antimicrobial management team developed new guidelines to restrict certain antibiotics |
| Education- Staff | Focused and systematic education for all staff, target knowledge gaps identified by questionnaire. | |
| Education- Patients | Symptom reporting, poster campaign | |
| Environmental Cleaning | Clean seals used for equipment, disposable washbowls, bed linens stored centrally, identify key surfaces | |
| Hand Hygiene | Practices studied and improved, common errors identified, strict enforcement, hand washing rounds for patient initiated | |
| Isolation and/or Cohorting | Isolated at start of suspected symptoms | |
| Price | Antibiotic Stewardship | Cephalosporin and quinolone restrictions |
| Contact Precautions | Scrubs, gloves, and aprons changed between patient contacts | |
| Isolation and/or Cohorting | Phase 1: All diarrheal patients isolated in side rooms, Phase 2: CDI patients in CDI cohort ward within 24 hours of CDI diagnosis, kept until discharge. Dedicated nursing staff. | |
| Salgado | Contact Precautions | Keep until CDI ruled out as cause of diarrhea, CDI patients kept in contact precaution for duration of hospitalization: gown, gloves, private rooms |
| Environmental Cleaning | Use bleach in areas occupied by CDI patients | |
| Hand Hygiene | Require soap and water, not alcohol gel | |
| Stone | Antibiotic Stewardship | Limit antibiotics to seven day course, restrict the use of cephalosporins |
| Hand Hygiene | Emphasized between patients, 4% chlorhexidine scrub if prolonged contact, 0.5% chlorhexidine rub otherwise, dispensers at each bay and side room | |
| Systems and Workflow | Providers alerted to new cases, quarterly rates discussed at teaching sessions, nurses informed | |
| Struelens | Antibiotic Stewardship | Alternatives to clindamycin |
| Contact Precautions | Gloves and gowns for fecal contact | |
| Environmental Cleaning | Daily furniture and floor cleaning (0.04% formaldehyde, 0.03% glutaraldehyde), dedicated utensils, single use towels | |
| Hand Hygiene | Soap and water between patient contact | |
| Isolation and/or Cohorting | Single rooms for those with diarrhea, cohorting of infected patients | |
| Systems and Workflow | Early diagnostic testing | |
| Suzuki | Antibiotic Stewardship | Carbapenem use restricted |
| Contact Precautions | In place beginning with diarrhea | |
| Systems and Workflow | Previous microbiology results of all admissions chart reviewed by infection preventionists, MDRO information provided to ward staff, infection control rounds within two days of new MDRO or hospital admission of patient with previous MDRO infection or colonization | |
| Valiquette | Antibiotic Stewardship | Decrease use of second and third generation cephalosporin, ciprofloxin, clindamycin, and macrolides, decreased course of treatment |
| Dedicated Equipment | Rectal thermometers | |
| Education- Staff | Lectures on isolation, disinfection, cleaning, antibiotic guidelines | |
| Environmental Cleaning | Hypochlorite sodium for terminal disinfection, comprehensive ward sodium hypochlorite disinfection for wards with less than three cases | |
| Isolation and/or Cohorting | Isolate suspected cases until discharge | |
| Weiss | Antibiotic Stewardship | Change antibiotic use according to Quebec guidelines |
| Contact Precautions | Contact isolation for test-positive patients, routine gloving in CDI wards | |
| Education- Patient | CDI hand hygiene handout | |
| Education- Staff | Sixty-minute lecture on CDI transmission, epidemiology, hand hygiene, and isolation. Regular education on wards with more than two cases | |
| Environmental Cleaning | 1:50 bleach/water solution used for cleaning (down from 1:10) | |
| Hand Hygiene | Soap and water encouraged over alcohol gel before/after visit patient room, 85 new sinks | |
| Isolation and/or Cohorting | Dedicated CDI ward | |
| Systems and Workflow | Low turnover, dedicated CDI ward housekeeping team trained, rapid enzyme immunoassay diagnostic test on first liquid stool, hire four infection preventionists | |
| Whitaker | Antibiotic Stewardship | Formulary restriction for high-risk antibiotics |
| Contact Precautions | Gowns, gloves, soap and water hand hygiene only until ruled CDI negative | |
| Education- Patient | Flyer on CDI and prevention | |
| Education- Staff | Information on antibiotic use, clinical signs, prescriptive patterns, and awareness | |
| Environmental Cleaning | 10% hypochlorite disinfection in patient rooms, nursing units, horizontal surfaces, and medical equipment | |
| Hand Hygiene | Soap and water | |
| Isolation and/or Cohorting | Not specified | |
| Systems and Workflow | Automated report of MDR organism history at admission, standardized nursing units for isolation, lab results shared immediately, | |
| White | Antibiotic Stewardship | Five-day duration policy for the treatment of most common infections, limitation on the use of common classes of broad spectrum agents, “Prescription codes” to sanction the use of restricted antibiotics |
| Education- Staff | Mandatory training program for clinical staff- an online or face-to-face module on infection prevention matters, a module on antimicrobial prescribing for all medical staff and nurse prescribers | |
| Environmental Cleaning | Additional housekeeping staff, individual wards were vacated and deep cleaned before being treated with aerosolised hydrogen peroxide | |
| Hand Hygiene | Colorful signs throughout the hospital, computer screensavers, audio messages, “naked from the elbow down” policy, prohibition of white coats, and wrist and hand jewelry. | |
| Isolation and/or Cohorting | A 22-bed combined isolation and cohort ward, with six single rooms, and the remainder arranged in four bedded bays, patient cohorting | |
| Proton Pump Inhibitor Stewardship | Limited the use of proton pump inhibitors within the hospital and mandated regular review of prescriptions | |
| Systems and Workflow | “Paper care pathway,” new Infection Control Operational Group, individual Directorate Infection Control Groups, and a dedicated infection prevention nurse post in CDI | |
| Wong-McClure | Antibiotic Stewardship | Broad spectrum antibiotics restricted |
| Contact Precautions | Enforced for suspected cases, single use personal protective equipment | |
| Environmental Cleaning | Clean affected wards with 1:10 hypochlorite solution, clean equipment with 1:10 quaternary ammonium. | |
| Hand Hygiene | Enforcement campaign for staff and patients | |
| Isolation and/or Cohorting | Strict isolation for confirmed cases | |
| You | Contact Precautions | Gloves and gowns |
| Education- Staff | Lecture for all medical staff on baseline data | |
| Environmental Cleaning | Twice daily disinfection with 1000ppm sodium hypochlorite | |
| Hand Hygiene | 0.3% triclosan soap and water before and after contact with CDI patients | |
| Isolation and/or Cohorting | CDI patients in isolation zone, 2.2 meters between beds and sink, isolation until 48 hours symptom free | |
| Zafar | Contact Precautions | Gloves and gowns required in CDI rooms |
| Dedicated Equipment | Equipment dedicated to individual patients and gas sterilized | |
| Education- Staff | Monthly lecture program, videos, handouts, posters | |
| Environmental Cleaning | Phenol-containing disinfectant for surfaces contaminated with body fluids, cart wash sterilizer installed on wheelchairs, stretchers | |
| Hand Hygiene | 0.03% Triclosan soap and water required, education | |
| Isolation and/or Cohorting | Cohort patients and nurses, restrict patient movement | |
| Systems and Workflow | Centralize processing department, infection preventionist on rounds, regular meetings between infection preventionists and nurses, CDI rates disseminated monthly |
CDI: Clostridium difficile infection, MDRO: multi-drug resistant organism
Within each category, the interventions were multifaceted. Hand hygiene measures included sink installation, improving signage, and education initiatives. Hand hygiene referred to a variety of practices across studies, including increased use of pure alcohol-based hand rubs, soap and water, and chlorhexidine scrubs.
Environmental cleaning interventions included a diverse range of practices and agents. Some focused on increasing cleaning frequency, including enhanced daily decontamination, cleaning at discharge, and environmental cleaning for patients meeting symptomatic and diagnostic criteria. Others expanded the types of surfaces to be cleaned. The most common agent used for cleaning was sodium hypochlorite.
For isolation and cohorting interventions, CDI patients were often assigned single or side rooms and nursed as a cohort after a positive lab test. Some studies isolated symptomatic patients before case confirmation. Isolation was typically required until 48 hours after resolution of symptoms or continued until discharge. Enhanced contact precautions included expanding precautions to suspected cases and continuing these practices throughout the duration of hospitalization.
Antibiotic stewardship programs involved formulary restrictions, monitoring physician prescribing, and tracking hospital antibiotic consumption and purchasing. Antibiotic specific education initiatives promoted shortened treatment courses, limits on non-essential medications, and the timely de-escalation of empiric therapy.
Staff education initiatives included information on CDI treatment, prevention, diagnosis, transmission, etiology, and epidemiology, as well as contact precaution and isolation policies, hand hygiene, and antibiotic use. These interventions were disseminated through both ongoing and one-time programs. Patient education was conducted primarily via handouts, flyers, and signs that stressed the importance of hand hygiene in preventing C. difficile transmission.
Systems and workflow interventions aimed to change hospital practices to optimize prompt CDI diagnosis, and upon diagnosis, to rapidly involve infection prevention teams and start appropriate CDI patient care. Most interventions improved communication between diagnostic labs and healthcare providers using electronic medical record flagging or email notifications. Two studies addressed patients who are asymptomatic C. difficile carriers.34,41 Use of dedicated equipment included stethoscopes, thermometers, blood pressure cuffs, and bedpans. Finally, proton pump inhibitor interventions restricted these medications according to specific clinical indications.
Adherence to bundle components
The measures used to assess adherence varied across studies. Evaluation of contact precautions included direct observations of staff, availability and quantity of personal protective equipment, and glove usage. Antibiotic stewardship programs were quantified by the reduction of antibiotic use and typically reported reduction at the single antibiotic level. Hand hygiene adherence was measured by direct observation and alcohol-based gel rub use. Surface swabbing and usage of cleaning materials was tracked to assess environmental decontamination.
Almost all articles reported measuring adherence for at least one component in the bundle (96.2%, 25/26) and 46.2% measured adherence for each component (12/26, Supplementary S1). However, most studies only stated that they had evaluated adherence to a bundle component, without reporting compliance results. For example, adherence to antibiotic stewardship was assessed in 89.5% studies (17/19). However, only three reported the actual results of their adherence data(Supplementary S1).31,32,39 Furthermore, because all three studies used different adherence measures, average antibiotic stewardship compliance could not be determined.
TiDier scores
The level of detail with which interventions were described varied widely both between and within studies. The average TiDier score across all interventions was 6.0, ranging from 1.027 to 10.4.45 Two studies provided comprehensive descriptions for a single bundle component, obtaining the maximum score of 12 for these interventions.37,44 Only 9 studies scored 10 or more on any single intervention(Supplementary S1).29,33,34,37–39,44,45,49 The average TiDer score for a given bundle component was 5.7, ranging from 8.2 for systems and workflow interventions to 3.0 for proton pump inhibitor stewardship. Most interventions had an average TiDier score between 5 and 7. Each intervention component evaluated an average of 2.1 adherence measures, ranging from 3.3 for systems and workflow interventions to 1.3 for dedicated equipment.
Improvement in C. difficile Rates
All 26 studies showed a decrease in the rate of CDIs after bundle implementation (Table 3). The improvement was significant at the 0.05 level for the 15 studies reporting p-values (60%, 15/25).25,30,31,35–41,43,45,48–50 The odd ratio for developing CDI under the intervention bundle compared to the control period was reported in three studies and ranged from 0.29 to 0.38.36,38,39 The relative risk was 0.60 in the single study reporting this measure.40
Table 3.
CDI bundle effectiveness
| First author | Pre-intervention CDI rate (cases/1,000 PD) |
Post-intervention CDI rate (cases/1,000 PD) |
CDI rate reduction (%) |
|---|---|---|---|
| Eight interventions in bundlea | |||
| Abbett40 | 1.1 (95% CI: 1.00–1.21) | 0.66 (95% CI: 0.60–0.72) | 40%; RR: 0.60 (95% CI: 0.52 – 0.68), p<0.001 |
| Oleastro28 | 0.823b | 0.119 | 85.5% |
| Seven interventions in bundle | |||
| Brakovich50 | 5.652 | 3.151 | 44.2%, p<0.001 |
| Cheng34 | Increase 17.0% per quarter | Decrease 6.1% per quarter | |
| Marufu26 | 5.2 /1,000 admissions | 1.1 /1,000 admissions | 78.7% |
| Mermel44 | 12.2/1,000 dischargesb | 3.6/1,000 discharges | 70.5% |
| Muto38 | 10.4/1,000 dischargesb | 3.0/1,000 discharges | 71%; OR: 0.286 (95% CI: 0.185, 0.435), p<0.001 |
| Weiss39 | 37.28 /1000 admissionsb | 14.48 /1000 admissions | 61%, OR 0.379 (95% CI: 0.331–0.435), p<0.001 |
| Whitaker42 | 1.33 | 0.45 | 66% |
| White33 | 170 cases/month | 2–11 cases/month | 80% |
| Zafar45 | 155/year | 65/year | 60%, p<0.05 |
| Six interventions in bundle | |||
| Bishop49 | 4.13/month (SD: 2.64) | 1.93/month (SD: 1.56) | 53%, p=0.03 |
| Hanna41 | 60% attack rateb | 17% attack rate | 72%, p<0.05 |
| Struelens32 | 0.178b | 0.034 | 77.3% |
| Five interventions in bundle | |||
| Gulihar25 | 7.1% patients develop CDI | 1.5% patients develop CDI | 79%, p <0.001 |
| Lai47 | 22.5/1,000 dischargesb | 13.2/1,000 discharges | 41.3% |
| Power29 | 2.60 (95% CI: 2.11–3.17) intervention; 1.15 (95% CI: 1.03–1.29) control | 0.69 (95%CI: 0.50–0.91) Intervention; 0.51 (95% CI: 0.44–0.60) control | 73.46% intervention; 55.65% control |
| Valiquette37 | 2.03b | 0.82 | 60%, p=0.007 |
| Wong-McClure43 | 2.96b | 2.12 | 28.4%, p=0.001 |
| You36 | 4.70 | 1.53 | 67%; OR: 0.36 (95% CI: 0.13 – 0.85), p=0.012 |
| Four interventions in bundle | |||
| Apisarnthanarak48 | 5.8 MICU; 8.0 BMTb | 2.1 MICU; 4.2 BMT | MICU 63.8%, p=0.05; BMT 47.5%, p=0.04 |
| Mattner27 | 1.08b | Data in bar graph | 8 wards significantly reduced CDI |
| Three interventions in bundle | |||
| Price30 | 1.30 | 0.69 | 46.9%, p=0.03 |
| Salgado46 | 5.52b | 1.24 | 77.5% |
| Stone31 | 33.5 /1,000 admissions | 19.4 /1,000 admissions | 42%, p<0.05 |
| Suzuki35 | 0.471b | 0.108 | 77%, p<0.001 |
Education is considered one intervention, whether it includes staff and/or patient components;
Pre-intervention data collected during CDI outbreak
BMT: Bone marrow transplant, CDI: Clostridium difficile infection, CI: confidence interval, MICU: Medical intensive care unit, OR: Odds ratio, PD: patient days, SD: Standard deviation, RR: Relative risk
Study Quality
The average study quality score, as assessed by the modified Downs and Black checklist,25 was 15.2 out of 28 (Supplementary S2). Total scores ranged from 13 to 18. All studies performed well on questions regarding external validity and poorly on confounding.
Discussion
The overarching goal of bundled interventions is to implement combinations of evidence-based strategies that complement each other and work synergistically. In the case of CDI, the paucity of evidence-based interventions for prevention lead to considerable variation among the choice of bundle elements. The lack of adherence data and consistently low TiDier scores among many of these studies indicate that details on intervention implementation were poorly reported. This makes it challenging to corroborate and compare results among studies.
According to the Society for Healthcare Epidemiology of America and Infectious Diseases Society of America’s 2014 CDI prevention strategies compendium,51 none of the 10 interventions compiled in this review were supported by level I evidence for CDI prevention. Antibiotic stewardship and contact precautions using gloves were designated as having a moderate quality evidence of effectiveness (level II), referring to either a small number of supporting studies, moderate study limitations, or variation in results between studies. Contact precautions using gowns, hand hygiene, isolation and cohorting, environmental cleaning, patient and staff CDI education, dedicated equipment, and several systems and workflow interventions were rated as having low grade evidence (III), used when studies have major flaws, considerable variation, or are based on expert consensus. The recommendation for proton pump inhibitor stewardship was considered unresolved. This lack of strong evidence for any single intervention is likely related to the heterogeneity we found in the selection of bundle components.
Bundle implementation was associated with a decline in CDI rates in all 26 studies published in the literature, making this a potentially promising approach for reducing CDI. However, methodological limitations preclude the assessment of a causal relationship between bundled interventions and CDI rates.
Our systematic review extends and updates the findings of a prior review.52 In 2014, Yakob, et al. conducted a review of 21 articles on CDI interventions and their effectiveness, from which they identified 6 eligible studies. In our review we incorporated 5 articles from this prior review,38–40,46,49 as well as 21 additional articles. We did not include one article from Yakob’s study, because pre- and post-intervention rates were not clearly reported.53 Given the rapidly changing epidemiology of C. difficile and use of CDI bundles, our up-to-date analysis is highly relevant to the current state of CDI control.
Many hospitals have not experienced declines in CDI rates, despite intensive efforts to implement prevention strategies.54,55 While neither of these studies implemented a CDI bundle, they are otherwise similar to many of the studies that met our inclusion criteria. Our review sheds light on three potential reasons for a lack of decline in CDI rates. First, compliance to interventions may be below the threshold necessary to be effective. A 2011 study by Furuya, et al. found that the effect of a CLABSI bundle was not observed until compliance with at least one bundle element reached 95%.19 If adherence to bundle elements was low in the reviewed studies, the potential impact of C. difficile bundles may be underestimated.
Second, the lack of infection control strategies focusing on asymptomatic carriers may have contributed to the lack of declines in CDI rates. Only 8% of CDI bundles included surveillance of asymptomatic carriers. Yet, focusing exclusively on patients with clinical CDI neglects the much larger reservoir of colonized asymptomatic hospital patients.56 Asymptomatic patients are an important reservoir of infectious C. difficile spores and the impact of interventions focusing on colonized patients should be rigorously evaluated in future studies.
Finally, hand hygiene is especially complex in the context of CDI. Alcohol-based hand rub is an essential component of most horizontal hand hygiene interventions, and its benefits are widely reported.57–59 Horizontal, or broad based, approaches aim to reduce all infections, instead of targeting specific pathogens. The use of alcohol gels is typically counted in adherence data, but pure alcohol-based hand rubs are not active against C. difficile spores.60 Chlorhexidine mixed gels have some efficacy,61 thus distinguishing between them, pure alcohol-based rubs, and soap and water is essential in the context of CDI bundles. Hand hygiene compliance data that include the use of pure alcohol-based rubs may provide hospitals with an inaccurate assessment of CDI prevention efforts.
There are several limitations to this review. First, given the heterogeneity of bundles, it is unclear if CDI reduction can be attributed to a similar mechanism across all studies. We attempted to mitigate this by loosely defining a bundle as any infection control rollout with more than one intervention, hypothesizing that successful bundle implementation is itself a significant factor regardless of the specific components implemented. Second, the majority of studies in this review lacked rigorous statistical testing assessing the significance of the decline in CDI rates post-intervention. This was especially common among studies implementing interventions mid-outbreak, which often lacked definitive comparison rates. Outbreak rates, without contemporaneous controls, can appear statistically significant even when the observed effect is due to regression to the mean. When pre-intervention CDI rates were unclear, we have presented the highest level of CDI incidence during the outbreak prior to bundle implementation. In addition, publication bias may have favored the publication of studies in which bundles showed a beneficial effect. If studies reporting no reduction in CDI rates after bundle introduction were less likely to be published, then our findings could over-estimate the positive impact of bundle introduction. Finally, it was impossible to quantify the overall effectiveness of bundle implementation and intervention adherence, given the range of outcome and adherence measures employed. The variety of outcome measures used and the paucity of reported error measurements reported made it impossible to undertake a meta-analysis. The development and use of standard intervention-specific adherence measures would facilitate comparisons across future studies.
Ultimately, this review draws from a wide range of hospital types, locations, and infection control contexts. Given that CDI rates improved across all studies despite contextual differences and the variety of bundle components, a tailored bundle approach may be effective. However, this approach should be tested using cluster randomized clinical trials in multiple sites and settings with attention to implementation and process factors to facilitate replication and generalizability.
Supplementary Material
Acknowledgments
We acknowledge health sciences librarian Lia Vellardita, MA, at the University of Wisconsin-Madison for her assistance with our literature search.
Funding
Dr. Safdar is supported by a National Institutes of Health, Veterans Affairs Merit Award, and a Veterans Affairs Patient Safety Center award.
Anna Barker is supported under NIH awards UL1TR000427 and TL1TR000429, administered by the University of Wisconsin-Madison’s Institute for Clinical and Translational Research.
This project was supported by grant number R03HS023791 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research.
Footnotes
The authors report no conflict of interest.
References
- 1.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related Mortality Rates, United States, 1999–2004. Emerg Infect Dis. 2007;13:417–419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuijper EJ, van Dissel JT, Wilcox MH. Clostridium difficile: changing epidemiology and new treatment options. Curr Opin Infect Dis. 2007;20:376–383. doi: 10.1097/QCO.0b013e32818be71d. [DOI] [PubMed] [Google Scholar]
- 3.Freeman JBM, Baines SD, Corver J, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deneve C, Janoir C, Poilane I, Fantinato C, Collignon A. New trends in Clostridium difficile virulence and pathogenesis. Int J Antimicrob Agents. 2009;33:S24–28. doi: 10.1016/S0924-8579(09)70012-3. [DOI] [PubMed] [Google Scholar]
- 5.Lessa FC, Yi M, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriel L, Beriot-Mathiot A. Hospitalization stay and costs attributable to Clostridium difficile infection a critical review. J Hosp Infect. 2014;88:12–21. doi: 10.1016/j.jhin.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed April 4, 2016];Inclusion criteria for reporting C. difficile infection to the surveillance system. Health Protection Agency of the United Kingdom website. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/328334/c_difficile_inclusion_criteria_for_reporting.pdf Published 2014.
- 8.Antibiotic Resistance Threats in the United States. [Accessed November 12, 2015];Center for Disease Control website. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Published 2013.
- 9. [Accessed April 2, 2016];CMS to improve quality of care during hospital inpatient stays. Centers for Medicare & Medicaid Services website https://www.cms.gov/newsroom/mediareleasedatabase/fact-sheets/2014-fact-sheets-items/2014-08-04-2.html Published 2014.
- 10.Hedge DD, Strain JD, Heins JR, Farver DK. New advances in the treatment of Clostridium difficile infection (CDI) Ther Clin Risk Manag. 2008;4:949–964. doi: 10.2147/tcrm.s3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyre DW, Cule ML, Wilson DJ, et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. NEJM. 2013;369:1195–1205. doi: 10.1056/NEJMoa1216064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker AS, Eyre D, Wyllie DH, et al. Characterization of Clostridium difficile hospital ward-based transmission using extensive epidemiological data and moleculat typing. PLOS Med. 2012;9:e1001172. doi: 10.1371/journal.pmed.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer ASI, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:30. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kundrapu S, Venkata S, Jury I, Deshpande A, Donskey CJ. A randomized trial of soap and water hand wash versus alcohol hand rub for removal of Clostridium difficile spores from hands of patients. Infect Control Hosp Epidemiol. 2014;35:204–206. doi: 10.1086/674859. [DOI] [PubMed] [Google Scholar]
- 15.Jernigan JA, Siegman-Igra Y, Guerrant RC, Farr BM. A randomized crossover study of disposable thermometers for prevention of Clostridium difficile and other nosocomial infections. Infect Control Hosp Epdemiol. 1998;19:494–499. doi: 10.1086/647855. [DOI] [PubMed] [Google Scholar]
- 16.Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections. JAMA. 2015;313:369–378. doi: 10.1001/jama.2014.18400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kundraphu S, Sunkelsula V, Jury LA, Sitzlar BM, Donskey CJ. Daily disinfection of high-touch surfaces in isolation rooms to reduce contamination of healthcare workers' hands. Infect Control Hosp Epidemiol. 2012;33:1039–1042. doi: 10.1086/667730. [DOI] [PubMed] [Google Scholar]
- 18.Barbut F, Menuet D, Verachten M, Girou E. Comparison of the efficacy of a hydrogen peroxide dry-mist disinfection system and sodium hypochlorite solution for eradication of Clostridium difficile spores. Infect Control Hosp Epidemiol. 2009;30:507–514. doi: 10.1086/597232. [DOI] [PubMed] [Google Scholar]
- 19.Furuya EY, Dick A, Perencevich EN, Pogorzelska M, Goldmann D, Stone PW. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One. 2011;6:e15452. doi: 10.1371/journal.pone.0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151:264–169. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Dane AV, Schneider B. Program integrity in primary and early secondary prevention: are implementation effects out of control? Clin Psychol Rev. 1998;18:23–45. doi: 10.1016/s0272-7358(97)00043-3. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann T, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 23.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J epidemiol community health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor SR, Tully MA, Ryan B, Bradley JM, Baxter GD, McDonough SM. Failure of numerical quality assessment scale to identify potential risk of bias in a systematic review: a comparison study. BMC Res Notes. 2015;8:224. doi: 10.1186/s13104-015-1181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulihar A, Nixon M, Jenkins D, Taylor GJ. Clostridium difficile in hip fracture patients: prevention, treatment and associated mortality. Injury. 2009;40:746–751. doi: 10.1016/j.injury.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Marufu O, Desai N, Aldred D, Brown T, Eltringham I. Analysis of interventions to reduce the incidence of Clostridium difficile infection at a London teaching hospital trust, 2003–2011. J Hosp Infect. 2015;89:38–45. doi: 10.1016/j.jhin.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Mattner F, Winterfeld I, Oswald B, Solbach W. Successful bundle of prevention measures against a high CDAD incidence at a university hospital. Hyg Med. 2008;33:346–352. [Google Scholar]
- 28.Oleastro M, Coelho M, Giao M, et al. Outbreak of Clostridium difficile PCR ribotype 027--the recent experience of a regional hospital. BMC Infect Dis. 2014;14:209. doi: 10.1186/1471-2334-14-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Power M, Wigglesworth N, Donaldson E, Chadwick P, Gillibrand S, Goldmann D. Reducing Clostridium difficile infection in acute care by using an improvement collaborative. BMJ. 2010;341:c3359. doi: 10.1136/bmj.c3359. [DOI] [PubMed] [Google Scholar]
- 30.Price J, Cheek E, Lippett S, et al. Impact of an intervention to control Clostridium difficile infection on hospital- and community-onset disease; an interrupted time series analysis. Clin Microbiol Infect. 2010;16:1297–1302. doi: 10.1111/j.1469-0691.2009.03077.x. [DOI] [PubMed] [Google Scholar]
- 31.Stone SP, Beric V, Quick A, Balestrini AA, Kibbler CC. The effect of an enhanced infection-control policy on the incidence of Clostridium difficile infection and methicillin-resistant Staphyloccocus aureus colonization in acute elderly medical patients. Age Ageing. 1998;27:561–568. doi: 10.1093/ageing/27.5.561. [DOI] [PubMed] [Google Scholar]
- 32.Struelens MJ, Maas A, Nonhoff C, et al. Control of nosocomial transmission of Clostridium difficile based on sporadic case surveillance. Am J Med. 1991;91:138S–144S. doi: 10.1016/0002-9343(91)90359-6. [DOI] [PubMed] [Google Scholar]
- 33.White H, Wiselka M, Bell D. A Multi-Faceted Approach of One Teaching Hospital NHS Trust during the Clostridium difficile Epidemic-Antibiotic Management and Beyond. Antibiotics Basel. 2016;5:E13. doi: 10.3390/antibiotics5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng VC, Chau P, So SY, et al. Containment of Clostridium difficile infection without reduction in antimicrobial use in Hong Kong. Eur J Clin Microbiol Infect Dis. 2015;34:1381–1386. doi: 10.1007/s10096-015-2362-5. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Senda J, Yamashita K, et al. Impact of intensive infection control team activities on the acquisition of methicillin-resistant staphylococcus aureus, drug-resistant Pseudomonas aeruginosa and the incidence of Clostridium difficile-associated disease. J Infect Chemother. 2013;19:1047–1052. doi: 10.1007/s10156-013-0621-x. [DOI] [PubMed] [Google Scholar]
- 36.You E, Song H, Cho J, Lee J. Reduction in the incidence of hospital-acquired Clostridium difficile infection through infection control interventions other than the restriction of antimicrobial use. Int J Infect Dis. 2014;22:9–10. doi: 10.1016/j.ijid.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Valiquette L, Cossette B, Garant MP, Diab H, Pepin J. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis. 2007;45:S112–121. doi: 10.1086/519258. [DOI] [PubMed] [Google Scholar]
- 38.Muto CA, Blank M, Marsh JW, et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin Infect Dis. 2007;45:1266–1273. doi: 10.1086/522654. [DOI] [PubMed] [Google Scholar]
- 39.Weiss K, Boisvet A, Chagnon M, et al. Multipronged intervention strategy to control an outbreak of Clostridium difficile infection (CDI) and its impact on the rates of CDI from 2002 to 2007. Infect Control Hosp Epidemiol. 2009;30:156–162. doi: 10.1086/593955. [DOI] [PubMed] [Google Scholar]
- 40.Abbett SK, Yokoe D, Lipsitz SR, et al. Proposed checklist of hospital interventions to decrease the incidence of healthcare-associated Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30:1062–1069. doi: 10.1086/644757. [DOI] [PubMed] [Google Scholar]
- 41.Hanna H, Raad I, Gonzalez V, et al. Control of nosocomial Clostridium difficile transmission in bone marrow transplant patients. Infect Control Hosp Epidemiol. 2000;21:226–228. doi: 10.1086/501751. [DOI] [PubMed] [Google Scholar]
- 42.Whitaker J, Brown B, Vidal S, Calcaterra M. Designing a protocol that eliminates Clostridium difficile: a collaborative venture. Am J Infect Control. 2007;35:310–314. doi: 10.1016/j.ajic.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Wong-McClure RA, Ramirez-Salas E, Mora-Brenes N, et al. Long term effect of infection control practices and associated factors during a major Clostridium difficile outbreak in Costa Rica. J Infect Dev Ctries. 2013;7:914–921. doi: 10.3855/jidc.2854. [DOI] [PubMed] [Google Scholar]
- 44.Mermel LA, Jefferson J, Blanchard K, et al. Reducing Clostridium difficile incidence, colectomies, and mortality in the hospital setting: a successful multidisciplinary approach. Jt Comm J Qual Patient Saf. 2013;39:298–305. doi: 10.1016/s1553-7250(13)39042-4. [DOI] [PubMed] [Google Scholar]
- 45.Zafar AB, Gaydos LA, Furlong WB, Nguyen MH, Mennonna PA. Effectiveness of infection control program in controlling nosocomial Clostridium difficile. Am J Infect Control. 1998;26:588–593. doi: 10.1053/ic.1998.v26.a84773. [DOI] [PubMed] [Google Scholar]
- 46.Salgado CD, Mauldin PD, Fogle PJ, Bosso JA. Analysis of an outbreak of Clostridium difficile infection controlled with enhanced infection control measures. Am J Infect Control. 2009;37:458–464. doi: 10.1016/j.ajic.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Lai KK, Melvin ZS, Menard MJ, Kotilainen HR, Baker S. Clostridium difficile-associated diarrheal epidemiology, risk factors, and infection control. Infect Control Hosp Epidemiol. 1997;18:628–632. doi: 10.1086/647687. [DOI] [PubMed] [Google Scholar]
- 48.Apisarnthanarak A, Zack JE, Mayfield JL, et al. Effectiveness of environmental nad infection control programs to reduce transmission of Clostridium difficile. Clin Infect Dis. 2004;39:601–602. doi: 10.1086/422523. [DOI] [PubMed] [Google Scholar]
- 49.Bishop J, Parry MF, Hall T. Decreasing Clostridium difficile infections in surgery: impact of a practice bundle incorporating a resident rounding protocol. Conn Med. 2013;77:69–75. [PubMed] [Google Scholar]
- 50.Brakovich B, Bonham E, van Brackle L. War on the spore: Clostridium difficile disease among patients in a long-term acute care hospital. J Healthc Qual. 2013;35:15–21. doi: 10.1111/j.1945-1474.2011.00182.x. [DOI] [PubMed] [Google Scholar]
- 51.Dubberke E, Carling P, Carrico R, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:628–645. doi: 10.1086/676023. [DOI] [PubMed] [Google Scholar]
- 52.Yakob L, Riley TV, Paterson DL, Marquess J, Clements AC. Assessing control bundles for Clostridium difficile: a review and mathematical model. Emerg Microbes Infect. 2014;3:e43. doi: 10.1038/emi.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koll BS, Ruiz R, Calfee DP, et al. Prevention of hospital-onset Clostridium difficile infection in the New York Metropolitan Region using a collaborative intervention model. J Health Qual. 2014;36:35–45. doi: 10.1111/jhq.12002. [DOI] [PubMed] [Google Scholar]
- 54.Kamboj M, Sheahan A, Sun J, et al. Transmission of Clostridium difficile during hospitalization for allogeneic stem cell transplant. Infect Control Hosp Epidemiol. 2016;37:8–15. doi: 10.1017/ice.2015.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roth VR. The more we learn, the less we know. Infect Control Hosp Epidemiol. 2016;(37):16–18. doi: 10.1017/ice.2015.257. [DOI] [PubMed] [Google Scholar]
- 56.Gerding D, Johnson S, Peterson L, Mulligan M, Silva J. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16:459–477. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 57.Larson EL, and 1992, 1993, and 1994 APIC Guidelines Committee APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23:251–269. doi: 10.1016/0196-6553(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 58. [Accessed March 12, 2016];WHO guidelines on hand hygiene in health care: First global patient safety challenge, clean care is safer care. World Health Organzation website. http://www.who.int/gpsc/5may/tools/9789241597906/en/ Published 2009. [PubMed]
- 59.Cohen SH, Gerding D, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infections Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 60.Jabbar U, Leischner J, Kasper D, et al. Effectiveness of alcohol-based hand rubs for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemiol. 2010;31:565–570. doi: 10.1086/652772. [DOI] [PubMed] [Google Scholar]
- 61.Gerding DN, Muto C, Owens RC. Measures to control and prevent Clostridium difficile infection. Clin Infect Dis. 2008;46:S43–S49. doi: 10.1086/521861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


