Abstract
Background
Food intake and use of drugs of abuse like cocaine share common central and peripheral physiological pathways. Appetitive hormones play a major role in regulating food intake; however, little is known about the effects of acute cocaine administration on the blood concentrations of these hormones in cocaine users.
Methods
We evaluated serum concentrations of six appetitive hormones: ghrelin (total and acyl-ghrelin), amylin, glucagon-like peptide-1 (GLP-1), insulin, leptin and peptide YY (PYY), as well as acute cardiorespiratory and subjective responses of 8 experienced cocaine users who received 25 mg intravenous (IV) cocaine.
Results
Serum concentrations of GLP-1 (p = 0.014) and PYY (p = 0.036) were significantly decreased one hour following IV cocaine administration; there was a trend towards a decrease for insulin (p = 0.055) and amylin (p = 0.063) concentrations, while no significant IV cocaine effect was observed for ghrelin (total or acyl-ghrelin) or leptin concentrations (p’s > 0.5). We also observed associations between hormone concentrations acutely affected by IV cocaine (GLP-1, PYY, insulin, amylin) and some cocaine-related cardiorespiratory and subjective responses (e.g., increased heart and respiratory rates; feeling high and anxious).
Discussion
These findings show a significant effect of acute IV cocaine administration on some appetitive hormones and suggest potential associations between these hormones and cocaine-related cardiorespiratory and subjective responses. Additional research is needed to further investigate the potential mechanisms underlining these associations.
Keywords: Appetitive hormones, IV cocaine administration, Cardiorespiratory responses, Subjective responses
1.0. Introduction
Appetitive behaviors are regulated by a myriad of hormones and neuropeptides that maintain the homeostasis between hunger and satiety (Suzuki et al., 2010). Some appetitive hormones like ghrelin, peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) are produced in the gastrointestinal tract; the latter is also produced centrally in the nucleus of the solitary tract (Delzenne et al., 2010). Additional hormones with key roles in regulating appetite and food intake include leptin, primarily produced by adipose tissue, as well as insulin and amylin, both produced primarily by the pancreas (Delzenne et al., 2010). These hormones, through reciprocal cross-talks and gut-brain interactions, play key roles in regulating glucose, energy homeostasis and body weight through the modulation of appetite and food intake (Ahima and Antwi, 2008). Specifically, while ghrelin stimulates food intake, GLP-1, PYY, insulin, amylin and leptin are anorexigenic and thus inhibit food intake (Camilleri, 2015; Suzuki et al., 2010). Such appetitive hormones contribute to the regulation of food intake and appetite by manipulating subjective responses to hunger, fullness and satiety (Blundell et al., 2010; Chaudhri et al., 2006). These hormones also play important physiological roles in controlling metabolic processes. For example, insulin and amylin are co-secreted (Moore and Cooper, 1991), and GLP-1 plays a role in stimulating insulin secretion (Holst, 2007), thus making their roles interrelated. Specifically, the main function of GLP-1 is to promote insulin release from the pancreatic β-cells in response to increased glucose concentrations following food intake, thus decreasing insulin levels while inhibiting glucagon secretion (Holst, 2007). Insulin subsequently promotes glucose absorption into fat, liver and skeletal muscles, while additionally controlling protein, fat and carbohydrate metabolism (Sonksen and Sonksen, 2000).
Another potential function of these appetitive hormones is a central action via neurobiological pathways that regulate both hedonic- and reward-related seeking behaviors that characterize both obesity and addiction, including cocaine use disorder (Kenny, 2011; Tomasi et al., 2015; Volkow et al., 2013). Appetitive hormones may act directly on structures integral to dopaminergic reward processing, including the ventral tegmental area (VTA) and the nucleus accumbens (NAc) (Dagher, 2009; Engel and Jerlhag, 2014; Murray et al., 2014). Manipulations of such hormonal signaling may result in changes in cocaine-related behaviors. For example, systemic ghrelin administration facilitates cocaine-induced hyperlocomotion and conditioned place preference (CPP) in rodents (Dickson et al., 2011; Jerlhag et al., 2010; Wellman et al., 2013). Conversely, blockade of the ghrelin receptor via genetic (Abizaid et al., 2011) or pharmacological (Clifford et al., 2012) manipulations attenuates cocaine-induced hyperlocomotion. Furthermore, the presence of or even the expectancy of cocaine depresses leptin concentrations, while leptin administration inhibits cocaine-induced seeking behavior and reward (You et al., 2016). The administration of the GLP-1 analog exendin-4 to rodents reduces the rewarding effects of cocaine, cocaine self-administration and cocaine-induced CPP (Egecioglu et al., 2013; Graham et al., 2013; Reddy et al., 2016; Schmidt et al., 2016; Sørensen et al., 2015). In contrast, insulin enhances the function of cocaine-sensitive dopamine and norepinephrine transporters in the rat NAc by acting at the presynaptic level (Schoffelmeer et al., 2011).
In summary, evidence exists to support common mechanisms involved in both food- and cocaine-related behaviors, as observed by overlapping circuits and shared signaling pathways. Research suggests that these hormones may contribute to either reducing or increasing the rewarding effects, not only of food, but also drugs of abuse like cocaine. However, there is a paucity of human studies investigating the relationship between these appetitive hormones and cocaine use. Specifically, no human studies have investigated the effects of an acute cocaine administration on the neuroendocrine signals discussed above (ghrelin, amylin, GLP-1, insulin, leptin and PYY). Furthermore, acute cocaine administration causes changes in cardiorespiratory (e.g., increased heart rate and blood pressure) and subjective (e.g., “euphoria”, “high”) responses (Ellefsen et al., 2016; Fischman et al., 1976; Mendelson et al., 2002), which parallel acute activation of a key neuroendocrine system like the hypothalamic-pituitary-adrenal (HPA) axis (Calogero et al., 1989; Logrip et al., 2011). It has been suggested that appetitive hormones may play a role in cardiorespiratory parameters like blood pressure and heart rate (Beltowski, 2006; Grieve et al., 2009; Lambert et al., 2011; Playford et al., 1992). However, how these hormones relate to cocaine-related cardiorespiratory and subjective responses has not been studied. Therefore, the current exploratory clinical study aimed to analyze the effects of an acute intravenous (IV) cocaine administration on serum concentrations of ghrelin, PYY, GLP-1, leptin, insulin and amylin in current cocaine users, as well as the relationship between these appetitive hormones and the acute cardiorespiratory responses to cocaine (CRC) and subjective responses to IV cocaine administration (SRC).
2.0. Methods
2.1. Participants
Participants were part of a clinical protocol whose details were previously reported (Ellefsen et al., 2014; Ellefsen et al., 2016). Briefly, the protocol investigated pharmacodynamic and pharmacokinetic interactions of cocaine with oral acetazolamide or oral quinine following controlled IV cocaine administration. After providing written informed consent, 11 participants completed comprehensive medical, psychiatric, and psychological assessments to determine eligibility. Criteria included being 18 to 50 year-olds, in good physical health, who smoked or used IV cocaine for at least six months and at least three times per month during the 3 months prior to screening, no current physical dependence on any drug other than cocaine, caffeine, or nicotine, and not seeking treatment for their cocaine use (Ellefsen et al., 2014; Ellefsen et al., 2016). Out of the 11 individuals who participated in the main protocol, eight were enrolled in this study after it was amended to include measurement of appetitive hormones (see Table 1 for participant sociodemographic and cocaine use characteristics).
Table 1.
Sample Characteristics (N = 8)
| Age (mean ± SD) | 43.9 ± 4.5 |
| Gender | 1 F; 7 M |
| Weight (kg) | 77.3 ± 8.8 |
| Height (cm) | 175.5 ± 5.8 |
| BMI (Kg/m2) | 25.1 ± 2.7 |
| Ethnicity (n) | |
| - African American | 5 |
| - White | 3 |
| Lifetime duration of cocaine use (years) (mean ± SD) | 21.1 ± 9.0 |
| Age at first cocaine use (years) (mean ± SD) | 22.2 ± 7.5 |
| Mean cocaine use (smoked or IV) over 3 months prior to screening (days/week) | 2–7/week |
| Days used in 14 days prior to screening (smoked or IV) | 3.9 ± 4.9 |
2.2. Setting
This study was conducted at the NIDA IRP, with participants residing for 13 consecutive days at the Clinical Research Unit, Johns Hopkins Bayview Medical Center, Baltimore, MD, a secure, medically monitored inpatient research unit. The study was approved by the NIDA IRP IRB, monitored by a Data Safety and Monitoring Board, and conducted under a federal Certificate of Confidentiality. All participants provided written informed consent.
2.3. Procedures
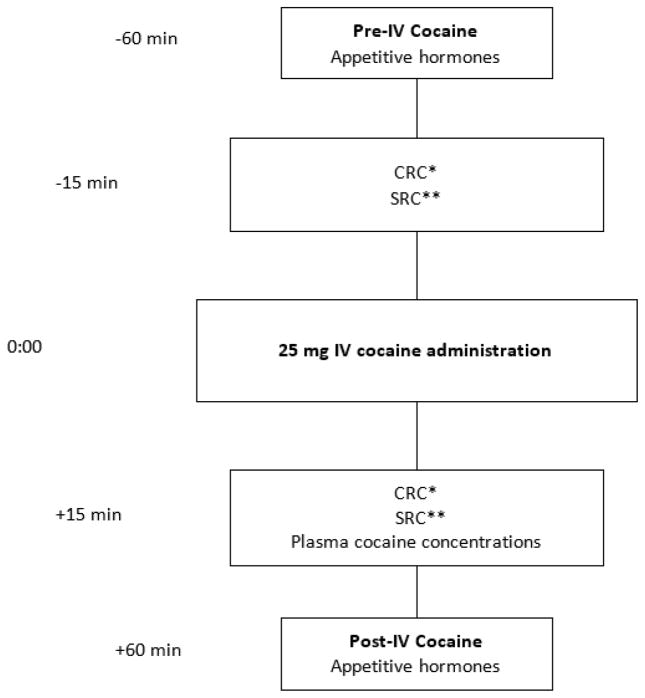
Participants were administered an IV cocaine injection, according to the National Advisory Council on Drug Abuse Guidelines for Administration of Drugs to Human Subjects (National Institute on Drug Abuse (NIDA), 2006). The use of IV cocaine was approved under the Food and Drug Administration IND 117,902. Intravenous cocaine was injected through an indwelling peripheral venous catheter on three separate days: Day 1 (cocaine alone), Day 5 (cocaine + oral acetazolamide) and Day 10 (cocaine + oral quinine) (Ellefsen et al., 2014; Ellefsen et al., 2016). Cocaine hydrochloride obtained from Mallinckrodt (St. Louis, MO) was dissolved in 2 mL 0.9% sterile saline (25 mg) and injected over 10 seconds to closely mirror cocaine injection among cocaine users. The 25-mg cocaine dose was selected to ensure measurable cardiorespiratory and subjective responses without producing clinically significant adverse effects (Ellefsen et al., 2014; Ellefsen et al., 2016). Peripheral venous blood samples for assay of the appetitive hormones ghrelin, PYY, GLP-1, leptin, insulin and amylin were collected 60 minutes before and after IV cocaine administration on Day 1 only, to avoid potential confounding by the presence of acetazolamide or quinine. Fig. 1. outlines the specific study procedures.
Fig. 1. Schematic of study assessments, blood collections for hormone measurements, subjective responses to cocaine (SRC) and cardiorespiratory responses to cocaine (CRC).
*CRC: Cardiorespiratory responses to cocaine include systolic and diastolic blood pressure, heart rate, and respiratory rate.
**SRC: Subjective Responses to Cocaine include “Crave Cocaine”, “Want Cocaine”, “Like Cocaine”, “Strong Drug Effect”, “Good Drug Effect”, “Bad Drug Effect”, “High”, “Rush”, “Elated”, “Stimulated”, “Suspicious”, “Anxious” and “Tired” assessed with 100 mm visual-analogue scales.
Cardiorespiratory (CRC; systolic and diastolic blood pressure, heart rate, respiratory rate) and subjective responses to cocaine (SRC) were measured 15 minutes before and after IV cocaine administration, estimated as the time of peak response (Ellefsen et al., 2016). For SRCs, participants were asked to rate, on 100 mm visual-analogue scales (VAS), anchored by “not at all” at left end and “most ever” at right end, their subjective responses to cocaine: 1) How strong is the drug effect? “Strong Drug Effect”; 2) Do you feel a good drug effect? “Good Drug Effect”; 3) Do you feel a bad drug effect? “Bad Drug Effect”; 4) How high do you feel? “High”; 5) Do you feel a rush? “Rush”; 6) Do you feel elated? “Elated”; 7) Do you feel stimulated? “Stimulated”; 8) Do you feel suspicious? “Suspicious”; 9) Do you feel anxious? “Anxious”; 10) Do you feel tired? “Tired”; 11) Do you want cocaine now? “Want Cocaine”; 12) Do you crave cocaine now? “Crave Cocaine”; 13) Do you like cocaine? “Like Cocaine”. Blood samples for plasma cocaine assays were collected 15 min after IV cocaine administration.
2.4. Hormonal Assays
Blood samples were collected to determine ghrelin, amylin, GLP-1, insulin, leptin and PYY serum concentrations. Ghrelin presents in the circulation as acyl-ghrelin and des-acyl-ghrelin; the latter is acylated by the enzyme ghrelin O-acyltransferase (GOAT) before coupling to its receptor (Delhanty et al., 2012). Consistent with kit manufacturer’s instructions, blood was collected into 2.5 mL red-white SST tubes containing inhibitors for serine protease (pefabloc), dipeptidyl peptidase IV and protease to prevent the metabolism of acyl-ghrelin, GLP-1, and amylin, respectively. Blood samples were centrifuged (1000 × g, 10 min at 25 °C) and serum was aliquoted into small storage tubes for storage at −80 °C until analysis. Total ghrelin (acyl + des-acyl) concentrations were measured using a commercial ELISA kit (EMD Millipore, Billerica, MA Cat. #EZGRT-89K). Samples were run in duplicate, per kit instructions, on a Glomax Multi spectrophotometer (Promega, Madison, WI, USA). Acyl-ghrelin, GLP-1, insulin, leptin, PYY, and amylin concentrations were measured using a commercial Milliplex kit and were prepared, in duplicate, according to the kit manufacturer’s instructions (Millipore Cat. #HMHMAG-34K). Data were collected on a Luminex MAGPIX system by the Xponent software (Luminex Corporation, Austin, TX, USA) and analyzed by Analyst software (Millipore). Values below the lower limit of quantitation (LLOQ) were set to ½ of the LLOQ (Beal, 2001); two, three and five values were below LLOQ for PYY (sensitivity <1.7), acyl-ghrelin (sensitivity <4.66) and amylin (sensitivity <2.91), respectively. Plasma cocaine concentrations were analyzed by liquid chromatography–tandem mass spectrometry (Ellefsen et al., 2016).
2.5. Statistical Approach
All data were examined for normality using the Shapiro-Wilk test. The change (Δ) (post- vs. pre-IV cocaine) in hormone concentration was assessed. If data were normally distributed, the Student’s paired-sample t test for location was reported; if the data were not normally distributed, the (non-parametric) Wilcoxon signed-rank test S for location was reported. We used a multivariate regression model to investigate potential predictors of appetitive hormone concentrations following IV cocaine administration. Potential predictors included in the model were only those cardiorespiratory and subjective responses that were significantly altered or showed a trend (>0.05, but <0.10) change (Sterne and Smith, 2001) after IV cocaine administration (Supplementary Figures S1–S31). This decision was further strengthened by taking into account the effect size using Cohen’s d (Hojat and Xu, 2004; Sullivan and Feinn, 2012). The multivariate regression model also included the specific hormone concentrations 60 min before IV cocaine administration and cocaine concentration 15min after IV cocaine administration. We used a stepwise regression approach, with an assumed entry and exit significance level of 0.15 (Beal, 2005; Budtz–Jørgensen et al., 2007; Hocking, 1976). Given the exploratory and hypotheses-generating nature of the study, this approach maximizes the power to detect effects on the dependent variable, by testing various combinations of potential predictor variables and identifying the best set of variables predicting the dependent variable. Resulting standardized beta estimates (β) for each predictor in each model are presented. Correlations were calculated using Spearman rank order (rho) due to the small sample size (Bishara and Hittner, 2012). All statistical analyses were performed using SAS Enterprise Guide version 5.1 for Windows run on SAS 9.3 Server (SAS Institute, Cary, NC, USA).
3.0. Results
3.1. Effects of An Acute IV Cocaine Administration on Appetitive Hormones
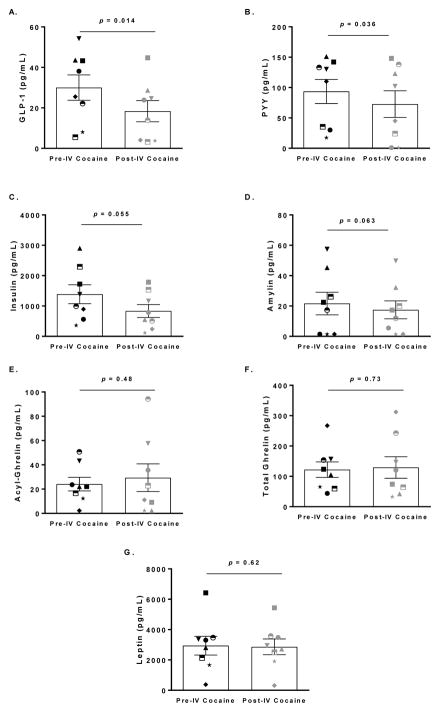
Both GLP-1 and PYY concentrations significantly decreased after IV cocaine administration (d = −1.15; t = −3.26; p = 0.014 and d = −0.92; t = −2.59; p = 0.036, respectively; Fig. 2A–B). A trend towards decreased insulin and amylin concentrations was also observed (d = −0.69; S = −14; p = 0.055 and d = −0.78; t = −2.20; p = 0.063 respectively; Fig. 2C–D). No significant changes were observed for acyl-ghrelin, total ghrelin or leptin (p’s > 0.05; Fig. 2E–G). Thus, only GLP-1, PYY, insulin and amylin were further investigated.
Fig. 2. Differences in appetitive hormone concentrations pre- and post-intravenous (IV) cocaine administration.
IV cocaine administration significantly decreased (A) GLP-1 (p = 0.014) and (B) PYY (p = 0.036) concentrations, induced a tendency for decrease for (C) Insulin (p = 0.055) and (D) Amylin (p = 0.063), but had no effect on (E) Acyl-Ghrelin (p = 0.48), (F) Total Ghrelin (p = 0.73), or (G) Leptin concentrations (p = 0.62). Data are presented as mean and standard error of the mean (SEM).
3.2. Predictors of Hormone Concentrations Post-IV Cocaine Administration
Based on our criterion of significant or trend change after IV cocaine administration, the following responses were included in the models: systolic blood pressure, heart rate and respiratory rate, “Rush”, “High”, “Stimulated”, “Elated”, “Anxious”, “Good Drug Effect”, “Strong Drug Effect” and “Want Cocaine” (Table 2). For additional details, see Supplementary Figures S1 and S22.
Table 2. Predictors of appetitive hormone concentrations post-IV cocaine administration.
Potential predictors include the cardiorespiratory responses to cocaine (CRC) and subjective responses to cocaine (SRC) secondary to intravenous (IV) cocaine administration, the specific hormone’s concentration pre-IV cocaine, and cocaine concentration +15 min following IV cocaine administration.
| Post-IV Cocaine concentrations: | ||||
|---|---|---|---|---|
| GLP-1 | PYY | Insulin | Amylin | |
| Δ Heart Rate | - | 0.20 (0.62; 2.01) | - | −0.03 (−0.05; −0.03) |
| Δ Systolic Blood Pressure | - | - | - | - |
| Δ Respiratory Rate | - | - | −1.18 (−161.48; −97.45) | −0.06 (−0.24; −0.21) |
| Δ “Want Cocaine” | - | - | - | - |
| Δ “Good Drug Effect” | - | - | - | - |
| Δ “Strong Drug Effect” | - | - | - | - |
| Δ “High” | −0.32 (−0.53; 0.02) | −0.48 (−2.74; −1.82) | - | - |
| Δ “Rush” | - | - | - | 0.18 (0.46; 0.48) |
| Δ “Elated” | - | - | - | - |
| Δ “Stimulated” | - | - | - | - |
| Δ “Anxious” | 0.46 (0.02; 0.63) | - | - | −0.04 (−0.06; −0.05) |
| Specific hormone concentrations pre-IV Cocaine | 0.90 (0.36; 0.75) | 1.05 (1.04; 1.28) | 0.36 (0.09; 0.29) | 0.92 (0.73; 0.74) |
| +15 min Cocaine Concentration | - | - | −0.48 (−12.52; −3.08) | - |
Δ refers to the acute change in the specific measure −15 min prior, and +15 min following 25 mg IV cocaine administration
The values represent standardized Beta estimates and 95% confidence limits: β (95% Cl)
3.3. Predictors of GLP-1 Concentrations Post-IV Cocaine Administration
The significant predictors of post-IV cocaine GLP-1 concentration were changes in the subjective feeling of being “Anxious”, feeling “High”, and pre-IV cocaine GLP-1 concentration. The final model shows that (F3,3 = 41.70, p = 0.006; Table 2), holding all other variables constant, for every unit increase in “Anxious”, “High”, or pre-IV cocaine GLP-1 concentration, there is, respectively, an expected 0.46 unit increase, −0.32 unit decrease and 0.90 unit increase in post-IV cocaine GLP-1 concentration.
3.4. Predictors of PYY Concentrations Post-IV Cocaine Administration
The significant predictors of PYY concentration post-IV cocaine administration were PYY concentration pre-IV cocaine, as well as changes in heart rate and the subjective feeling of being “High”. The final model shows that (F3,1 = 6115.18, p = 0.009; Table 2) holding all other variables constant, for every unit increase in heart rate, “High”, and pre-IV cocaine PYY concentration, there is, respectively, an expected 0.20 unit increase, a −0.48 unit decrease, and a 1.05 unit increase in post-IV cocaine PYY concentration.
3.5. Predictors of Insulin Concentration Post-IV Cocaine Administration
The significant predictors of post-IV cocaine insulin concentration were changes in respiratory rate, post-IV cocaine concentration, and pre-IV cocaine insulin concentration. The final model shows that (F3,3 = 93.02, p = 0.002; Table 2) holding all other variables constant, for every unit increase in respiratory rate, cocaine concentration +15 min post-IV, and pre-IV cocaine insulin concentration, respectively, there is an expected −1.18 decrease, a −0.48 unit decrease, and a 0.36 unit increase in post-IV cocaine insulin concentration.
3.6. Predictors of Amylin Concentrations Post-IV Cocaine Administration
The significant predictors of post-IV cocaine amylin concentration were changes in heart rate, respiratory rate, and the subjective feelings of being “Anxious”, feeling a “Rush”, as well as pre-IV cocaine amylin concentration. The final model shows that (F5,1 = 4125414, p = 0.0004; Table 2), holding all other variables constant, for every unit increase in heart rate, respiratory rate and the subjective feeling of being “Anxious”, there is respectively an expected −0.03, −0.06 and −0.04-unit decrease in post-IV cocaine amylin concentration. Finally, for every unit increase in “Rush” and pre-IV cocaine amylin concentration, there is an expected 0.18 and 0.92 unit increase in post-IV cocaine amylin concentration, respectively.
Each specific hormone’s pre-IV cocaine concentration was a predictor of its post-IV concentration across all models. This observation prompted us to evaluate the potential of all six appetitive hormones’ concentrations prior to IV cocaine administration as potential predictors of the acute changes in cardiorespiratory and subjective parameters following IV cocaine administration. The results of those models are detailed in Supplementary Tables S1 and S23.
4.0. Discussion
The present study, albeit exploratory in nature, is the first of which we are aware to investigate IV cocaine effects on appetitive hormones in a controlled human drug administration study. We found that an acute IV cocaine administration significantly decreased serum GLP-1 and PYY concentrations in cocaine users, with a trend towards decreased insulin and amylin concentrations. There was no effect on leptin or ghrelin concentrations. In addition, cardiorespiratory and subjective responses to IV cocaine predicted serum hormone concentrations.
For GLP-1, both physiological and pharmacological effects can explain the present results; possibly, these considerations may extend to other hormones of interest such as PYY. Elevated GLP-1 concentrations contribute to decreased hunger and promote postprandial satiety (Steinert et al., 2016). Although we did not assess such parameters, including craving for food, previous research shows that IV cocaine administration increased feelings of hunger without affecting craving for cocaine (Lynch et al., 2008), an observation consistent with the direction of change in our results, i.e. IV cocaine administration reduced serum concentrations of anorexigenic hormones. Such changes in appetitive hormones following cocaine intake, if sustained and repeated, could impact hunger in ways that significantly alter food intake and body weight. This hypothesis is contrary to the common observation of decreased body weight observed in populations with cocaine use disorder (Billing and Ersche, 2015). This difference may be due to the fact that our sample consisted of occasional, not chronic and frequent, cocaine users. From a pharmacological standpoint, we speculate that cocaine-induced reductions in GLP-1 concentrations might promote continued cocaine use, thereby further reducing GLP-1 concentrations and initiating a positive feedback loop that promotes cocaine use. This hypothesis is consistent with rodent work indicating that increasing GLP-1 signaling via the administration of GLP-1 analogs reduces cocaine intake (Egecioglu et al., 2013; Graham et al., 2013; Reddy et al., 2016; Sørensen et al., 2015).
To the best of our knowledge, no previous research has been conducted on the potential relationship between PYY and cocaine use. Our results appear consistent with previous work conducted on PYY in individuals with other substance use disorders. For example, cannabis use resulted in decreased blood PYY concentrations in HIV-positive men (Riggs et al., 2012), and a negative association between blood PYY concentrations and subjective ratings of cigarette craving was also previously reported (al’Absi et al., 2014). Taken together, these findings support the hypothesis that, in addition to promoting satiety, there may be a relationship between PYY signaling and intake of drugs of abuse like nicotine, cannabis and cocaine.
It is also noteworthy that GLP-1 and PYY are mainly involved in the short-term anorexigenic regulation of food intake, while IV cocaine administration did not affect concentrations of the long-term anorexigenic hormone leptin, nor concentrations of the only known orexigenic hormone, ghrelin. In other words, the effect of an acute IV cocaine administration seems to be specific to short-term signaling anorexigenic hormones, at least within the group of hormones that were assessed. This suggests several hypotheses for future research, such as a possible role in the rebound hyperphagia following cessation of cocaine use (Edge and Gold, 2011). It has been suggested that rebound hyperphagia could be a physiological attempt to ‘replenish’ the body’s neurotransmitter supplies, previously depleted by the over-activation of endogenous reward systems following cocaine (or other drug or food) intake (Hodgkins et al., 2004). However, it is commonly accepted that some individuals use cocaine as a way to suppress appetite and reduce body weight (Cochrane et al., 1998), also referred to as ‘cocaine-induced anorexia’ (Jonas and Gold, 1986). During abstinence from cocaine, the observed weight gain may be a direct result of increased food intake due to compensation for the absent rewarding effects of cocaine or to regain the weight that was lost (Hodgkins et al., 2004). New research challenges this long held belief, suggesting that there are profound metabolic perturbations in cocaine users (Ersche et al., 2013), such as low blood leptin concentrations and low body weight, despite high preference for and intake of high-fat, high-carbohydrate foods. Newly abstinent cocaine users keep the same eating behavior as during active drug use, but gain weight and exhibit an imbalanced fat storage profile, suggesting the ability of cocaine to blunt or prevent fat storage. Lastly, we know that cocaine administration leads to a stress response via activation of the HPA axis (Manetti et al., 2014). Consuming highly palatable foods eases this HPA-related stress response, a phenomenon also known as “self-medication” for stress relief (Ulrich-Lai, 2016). Thus, an additional working hypothesis is that the decrease in GLP-1 and PYY concentrations observed following cocaine administration could be an indication of the body’s physiological response to relief from the cocaine-induced emotional-affective (i.e., anxiety, depression) and stress responses (i.e., ACTH, cortisone) (Koob and Kreek, 2007; Painsipp et al., 2011). Therefore, the decreased concentrations of GLP-1 and/or PYY following cocaine administration could be a result of the system’s effort to buffer cocaine’s effects. While we were not able to measure HPA-related hormones in this study, this hypothesis is consistent with the anxiolytic properties of GLP-1 signaling (Bordnick et al., 2008; Kinzig et al., 2003; Moller et al., 2002), and with the present findings that feeling “anxious” was one of the GLP-1 predictors. On the other hand, the fact that heart rate was one of the PYY predictors is consistent with previous work indicating effects of PYY on the human cardiovascular system (Playford et al., 1992). Furthermore, “High” was a common subjective parameter associated with both PYY and GLP-1 concentrations in our study, a finding that further supports a relationship between appetitive signals and emotion regulation in the context of cocaine use (Macht, 2008). Finally, the fact that we found relationships between appetitive hormones and both objective (i.e., heart rate) and subjective (i.e., feeling anxious and high) responses to acute cocaine administration adds validity to the present findings.
This study was the first controlled human drug administration study, of which we are aware, assessing serum concentrations of appetitive hormones after an acute cocaine administration in cocaine users. It was conducted in a well-controlled setting, which allowed for regulating the IV cocaine dose and ensuring standardized physiological and subjective assessments, compliance to procedures across participants and excellent study safety. Furthermore, this is one of the few clinical studies to simultaneously assay several appetitive hormones with varying physiological functions. Although the sample size is small, it is important to acknowledge: a) the reasonably large effect sizes, even more so, given the experiment’s crossover design; b) the challenges of conducting such as clinical study with controlled drug administration; and c) the strict inclusion/exclusion criteria. However, this study also has several limitations. First, the inability to perform repeated blood sampling due to a strict timeline following IV cocaine administration, which might have prevented the observation of time-sensitive patterns of appetitive hormone changes. Such sample collection limitations were due to attaining maximum allowed blood draws in the participants to answer the primary protocol’s main aims. Second, IV cocaine administration was not done blind, i.e., with corresponding placebo administration. Third, given the exploratory nature of this work, many other hormones related to appetite, food intake and stress could not be measured. Finally, for ethical reasons, we could not include a cocaine-naïve control group to evaluate whether the effects observed are specific to cocaine users.
The present study describes associations and does not allow conclusions about mechanisms of action or causality. Furthermore, and although these findings are preliminary to directly guide research towards novel treatment approaches, they represent a step forward in understanding the intricate relationships between cocaine use and parameters related to food intake regulation in the context of an acute cocaine administration. Future studies are needed to address the limitations of the present study and provide more definitive information towards a better understanding of the relationship between cocaine use and neuroendocrine appetitive pathways, especially in patients with more severe symptoms of cocaine use disorder and who are seeking treatment for it.
In conclusion, this study reports novel findings on the effects of an acute IV cocaine administration on appetitive hormone concentrations like GLP-1 and PYY in cocaine users. The findings suggest a potential relationship between cocaine use and the appetitive processes mediated by these hormones.
Supplementary Material
Highlights.
Appetitive hormones were examined in cocaine users after intravenous (IV) cocaine administration
Glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) serum concentrations decreased 60 min after IV cocaine administration
Such changes were linked to cardiorespiratory and subjective responses to IV cocaine
Acknowledgments
Role of Funding Sources
This work was supported by the National Institute on Drug Abuse Intramural Research Program (SB, KE, MBS, SP, DAG, MAH, LL) and the National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research (SB, MBS, ES, LL). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors acknowledge the contributions of the clinical staff of the Intramural Research Program of the National Institute on Drug Abuse, and the Clinical Research Unit, Johns Hopkins Bayview Medical Center, as well as the University of Maryland, Baltimore, a member of the Graduate Partnership Program, National Institutes of Health (NIH). Furthermore, the authors thank Ms. April Le (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology, National Institute on Alcohol Abuse and Alcoholism and National Institute on Drug Abuse, NIH) for technical support during sample analyses and Ms. Karen Smith (National Institutes of Health Library) for bibliographic assistance.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Clinicaltrials.gov Registration
Conflict of interest
The authors report no conflicting biomedical financial interests.
Contributors
LL and MAH designed the study described in this paper; MAH and DAG designed the parent study and clinical protocol; LL and MAH provided funding; KNE, SP, DAG and MAH conducted the clinical protocol; SP and DAG provided medical supervision; ES provided technical support and supervision of the laboratory operations to generate the data; SB developed the data analysis plan; SB analyzed the data; SB, MBS and LL wrote the first draft of the manuscript. KNE and MAH contributed to the writing of the manuscript. All authors approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abizaid A, Mineur Y, Roth R, Elsworth J, Sleeman M, Picciotto M, Horvath T. Reduced locomotor responses to cocaine in ghrelin-deficient mice. Neuroscience. 2011;192:500–506. doi: 10.1016/j.neuroscience.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008;37:811–823. doi: 10.1016/j.ecl.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Lemieux A, Nakajima M. Peptide YY and ghrelin predict craving and risk for relapse in abstinent smokers. Psychoneuroendocrinology. 2014;49:253–259. doi: 10.1016/j.psyneuen.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal DJ. SAS® code to select the best multiple linear regression model for multivariate data using information criteria. Paper presented at: Southeast SAS Users Group Conference; Portsmouth, VA. 23–25 Oct. 2005; 2005. Paper SA01_05. [Google Scholar]
- Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- Beltowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24:789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- Billing L, Ersche KD. Cocaine-s appetite for fat and the consequences on body weight. Am J Drug Alcohol Abuse. 2015;41:115–118. doi: 10.3109/00952990.2014.966196. [DOI] [PubMed] [Google Scholar]
- Bishara AJ, Hittner JB. Testing the significance of a correlation with nonnormal data: comparison of Pearson, Spearman, transformation, and resampling approaches. Psychol Methods. 2012;17:399–417. doi: 10.1037/a0028087. [DOI] [PubMed] [Google Scholar]
- Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, van der Knaap H, Westerterp M. Appetite control: Methodological aspects of the evaluation of foods. Obes Rev. 2010;11:251–270. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordnick PS, Traylor A, Copp HL, Graap KM, Carter B, Ferrer M, Walton AP. Assessing reactivity to virtual reality alcohol based cues. Addict Behav. 2008;33:743–756. doi: 10.1016/j.addbeh.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Budtz–Jørgensen E, Keiding N, Grandjean P, Weihe P. Confounder selection in environmental epidemiology: assessment of health effects of prenatal mercury exposure. Ann Epidemiol. 2007;17:27–35. doi: 10.1016/j.annepidem.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Gallucci WT, Kling MA, Chrousos GP, Gold PW. Cocaine stimulates rat hypothalamic corticotropin-releasing hormone secretion in vitro. Brain Res. 1989;505:7–11. doi: 10.1016/0006-8993(89)90109-1. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology. 2015;148:1219–1233. doi: 10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187–1209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS, Rodriguez J, Schul D, Hughes S, Kniffin T, Hart N, Eitan S, Brunel L, Fehrentz JA, Martinez J. Attenuation of cocaine-induced locomotor sensitization in rats sustaining genetic or pharmacologic antagonism of ghrelin receptors. Addict Biol. 2012;17:956–963. doi: 10.1111/j.1369-1600.2011.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C, Malcolm R, Brewerton T. The role of weight control as a motivation for cocaine abuse. Addict Behav. 1998;23:201–207. doi: 10.1016/s0306-4603(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Dagher A. The neurobiology of appetite: Hunger as addiction. Int J Obes. 2009;33:S30–S33. doi: 10.1038/ijo.2009.69. [DOI] [PubMed] [Google Scholar]
- Delhanty PJ, Neggers SJ, van der Lely AJ. Mechanisms in endocrinology: Ghrelin: the differences between acyl-and des-acyl ghrelin. Eur J Endocrinol. 2012;167:601–608. doi: 10.1530/EJE-12-0456. [DOI] [PubMed] [Google Scholar]
- Delzenne N, Blundell J, Brouns F, Cunningham K, De Graaf K, Erkner A, Lluch A, Mars M, Peters HP, Westerterp-Plantenga M. Gastrointestinal targets of appetite regulation in humans. Obes Rev. 2010;11:234–250. doi: 10.1111/j.1467-789X.2009.00707.x. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340:80–87. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Edge PJ, Gold MS. Drug withdrawal and hyperphagia: lessons from tobacco and other drugs. Curr Pharm Des. 2011;17:1173–1179. doi: 10.2174/138161211795656738. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013;8:e69010. doi: 10.1371/journal.pone.0069010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefsen KN, Concheiro M, Beck O, Gorelick DA, Pirard S, Huestis MA. Quantification of cocaine and metabolites in exhaled breath by liquid chromatography-high-resolution mass spectrometry following controlled administration of intravenous cocaine. Anal Bioanal Chem. 2014;406:6213–6223. doi: 10.1007/s00216-014-8051-x. [DOI] [PubMed] [Google Scholar]
- Ellefsen KN, Concheiro M, Pirard S, Gorelick DA, Huestis MA. Pharmacodynamic effects and relationships to plasma and oral fluid pharmacokinetics after intravenous cocaine administration. Drug Alcohol Depend. 2016;163:116–125. doi: 10.1016/j.drugalcdep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E. Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs. 2014;28:875–886. doi: 10.1007/s40263-014-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Stochl J, Woodward JM, Fletcher PC. The skinny on cocaine: Insights into eating behavior and body weight in cocaine-dependent men. Appetite. 2013;71:75–80. doi: 10.1016/j.appet.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Resnekov L, Shick JFE, Krasnegor NA, Fennell W, Freedman DX. Cardiovascular and subjective effects of intravenous cocaine administration in humans. Arch Gen Psychiatry. 1976;33:983–989. doi: 10.1001/archpsyc.1976.01770080101010. [DOI] [PubMed] [Google Scholar]
- Graham DL, Erreger K, Galli A, Stanwood GD. GLP-1 analog attenuates cocaine reward. Mol Psychiatry. 2013;18:961. doi: 10.1038/mp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve DJ, Cassidy RS, Green BD. Emerging cardiovascular actions of the incretin hormone glucagon-like peptide-1: Potential therapeutic benefits beyond glycaemic control? Br. J Pharmacol. 2009;157:1340–1351. doi: 10.1111/j.1476-5381.2009.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking RR. A Biometrics invited paper. The analysis and selection of variables in linear regression. Biometrics. 1976;32:1–49. [Google Scholar]
- Hodgkins CC, Cahill KS, Seraphine AE, Frostpineda K, Gold MS. Adolescent drug addiction treatment and weight gain. J Addict Dis. 2004;23:55–65. doi: 10.1300/J069v23n03_05. [DOI] [PubMed] [Google Scholar]
- Hojat M, Xu G. A visitor’s guide to effect sizes: statistical significance versus practical (clinical) importance of research findings. Adv Health Sci Educ Theory Pract. 2004;9:241–249. doi: 10.1023/B:AHSE.0000038173.00909.f6. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Engel JA. Ghrelin receptor antagonism attenuates cocaine-and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl) 2010;211:415–422. doi: 10.1007/s00213-010-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JM, Gold MS. Cocaine abuse and eating disorders. Lancet. 1986;1:390–391. doi: 10.1016/s0140-6736(86)92360-3. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert E, Lambert G, Ika-Sari C, Dawood T, Lee K, Chopra R, Straznicky N, Eikelis N, Drew S, Tilbrook A, Dixon J, Esler M, Schlaich MP. Ghrelin modulates sympathetic nervous system activity and stress response in lean and overweight men. Hypertension. 2011;58:43–50. doi: 10.1161/HYPERTENSIONAHA.111.171025. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Koob GF, Zorrilla EP. Role of corticotropin-releasing factor in drug addiction. CNS Drugs. 2011;25:271–287. doi: 10.2165/11587790-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, Malison RT. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addict Biol. 2008;13:403–410. doi: 10.1111/j.1369-1600.2008.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht M. How emotions affect eating: A five-way model. Appetite. 2008;50:1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Manetti L, Cavagnini F, Martino E, Ambrogio A. Effects of cocaine on the hypothalamic–pituitary–adrenal axis. J Endocrinol Invest. 2014;37:701–708. doi: 10.1007/s40618-014-0091-8. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Mutschler N, Halpern J. Temporal concordance of cocaine effects on mood states and neuroendocrine hormones. Psychoneuroendocrinology. 2002;27:71–82. doi: 10.1016/s0306-4530(01)00036-1. [DOI] [PubMed] [Google Scholar]
- Mendelson MDJH, Sholar M, Mello PDNK, Teoh MDSK, Sholar JW. Cocaine tolerance: Behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacology. 1998;18:263–271. doi: 10.1016/S0893-133X(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Moller C, Sommer W, Thorsell A, Rimondini R, Heilig M. Anxiogenic-like action of centrally administered glucagon-like peptide-1 in a punished drinking test. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:119–122. doi: 10.1016/s0278-5846(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Moore CX, Cooper GJ. Co-secretion of amylin and insulin from cultured islet β-cells: Modulation by nutrient secretagogues, islet hormones and hypoglycemic agents. Biochem Biophys Res Commun. 1991;179:1–9. doi: 10.1016/0006-291x(91)91325-7. [DOI] [PubMed] [Google Scholar]
- Murray S, Tulloch A, Gold MS, Avena NM. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nature Rev Endocrinol. 2014;10:540–552. doi: 10.1038/nrendo.2014.91. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA) [accessed on 05.01.2017];National Advisory Council on Drug Abuse (NACDA) guidelines for administration of drugs to human subjects. 2006 http://drugabuse.gov/funding/hsguide.html.
- Painsipp E, Herzog H, Sperk G, Holzer P. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br J Pharmacol. 2011;163:1302–1314. doi: 10.1111/j.1476-5381.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford RJ, Benito-Orfila MA, Nihoyannopoulos P, Nandha KA, Cockcroft J, Todd S, Ghatei MA, Domin J, Bloom SR, Calam J. Effects of peptide YY on the human cardiovascular system: Reversal of responses to vasoactive intestinal peptide. Am J Physiol. 1992;263:E740–747. doi: 10.1152/ajpendo.1992.263.4.E740. [DOI] [PubMed] [Google Scholar]
- Reddy IA, Pino JA, Weikop P, Osses N, Sørensen G, Bering T, Valle C, Bluett RJ, Erreger K, Wortwein G, Reyes JG, Graham D, Stanwood GD, Hackett TA, Patel S, Fink-Jensen A, Torres GE, Galli A. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl Psychiatry. 2016;6:e809. doi: 10.1038/tp.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs PK, Vaida F, Rossi SS, Sorkin LS, Gouaux B, Grant I, Ellis RJ. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46–52. doi: 10.1016/j.brainres.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR. Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2016;41:1917–28. doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Drukarch B, De Vries TJ, Hogenboom F, Schetters D, Pattij T. Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior. J Neurosci. 2011;31:1284–1291. doi: 10.1523/JNEUROSCI.3779-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000;85:69–79. doi: 10.1093/bja/85.1.69. [DOI] [PubMed] [Google Scholar]
- Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, Galli A, Fink-Jensen A. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav. 2015;149:262–268. doi: 10.1016/j.physbeh.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert RE, Beglinger C, Langhans W. Intestinal GLP-1 and satiation: From man to rodents and back. Int J Obes. 2016;40:198–205. doi: 10.1038/ijo.2015.172. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Smith GD. Sifting the evidence—what’s wrong with significance tests? BMJ. 2001;322:226–231. doi: 10.1136/bmj.322.7280.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Feinn R. Using effect size—or why the p value is not enough. J Grad Med Educ. 2012;4:279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57:359–372. doi: 10.1507/endocrj.k10e-077. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: Association to striatal D2/D3 receptors. Hum Brain Mapp. 2015;36:120–136. doi: 10.1002/hbm.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM. Self-medication with sucrose. Curr Opin Behav Sci. 2016;9:78–83. doi: 10.1016/j.cobeha.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: Neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman PJ, Clifford PS, Rodriguez JA. Ghrelin and ghrelin receptor modulation of psychostimulant action. Front Neurosci. 2013;7:171. doi: 10.3389/fnins.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Liu QR, Wu Y, Otvos L, Wise RA. Reciprocal inhibitory interactions between the reward-related effects of leptin and cocaine. Neuropsychopharmacology. 2016;41:1024–1033. doi: 10.1038/npp.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.