Abstract
Background: Patent foramen ovale (PFO) is an anatomic variant that may lead to several pathological conditions, notably right to left shunt, paradoxical embolism, hypoxemia, and cerebral fat embolism. Mechanical positive pressure ventilation may increase the prevalence of PFO opening in Intensive Care Unit (ICU) patients; however, the respiratory and hemodynamic determinants of PFO opening have been poorly investigated. Contrast-enhanced transesophageal echocardiogram (ce-TEE) is considered the gold standard for PFO detection. We prospectively performed a multicenter study using ce-TEE in order to determine the respiratory and hemodynamic factors that may lead to PFO opening.
Methods: One hundred and eight consecutive ICU adult patients under mechanical ventilation from three tertiary care hospitals, were included in the study. A standard multiplane ce-TEE was performed, and the dimensions and function of the right and left ventricle were studied. In each patient, the right ventricle (RV) end-diastolic area, RV end-systolic area, left ventricle (LV) end-diastolic area, and LV ejection fraction were measured using the modified Simpson’s rule and the four-chamber view. At least three bubble tests were performed to detect PFO opening. Ventilatory parameters such as tidal volume, plateau pressure, static lung compliance, and positive end-expiratory pressure were recorded during the bubble test.
Results: Data for 81 men and 27 women were analyzed. PFO was detected in 27 % of the study population. Statistical significance was found between the presence of PFO and plateau pressure (odds ratio 3.421, 95 % CI: 1.2-9.4, p =0.017). Additionally, the presence of right ventricular dilatation (RV>LV) was strongly associated with PFO opening (odds ratio 3.163, 95 % CI: 1.2-8.075, p =0.018).
Conclusions: In this group of mechanically ventilated, critically ill adult patients, right ventricular dilatation and plateau pressure above 26 mmHg were significantly associated with foramen ovale opening. Hippokratia 2016, 20(3): 209-213.
Keywords: Patent foramen ovale, mechanical ventilation, plateau pressure, positive end-expiratory pressure, right ventricle
Introduction
Fetal foramen ovale is an integral part of the normal fetal circulation. The anatomical closure of foramen ovale occurs around the second year of life in the majority of the population1. Autopsy and detailed contrast echocardiography studies demonstrate that anatomic closure is incomplete in approximately one in every four adults, with the frequency being similar in both sexes2. Patent foramen ovale (PFO) vary in size from one to 19 mm (mean: 4.9 mm)2. In about 25 % of individuals, the foramen ovale remains patent throughout life, providing a potential channel through which blood may shunt from right to left3. Echocardiographic techniques, especially contrast-enhanced transesophageal echocardiogram (ce-TEE), are the main methods for the clinical evaluation and diagnosis of PFO4. Furthermore, ce-TEE has a much higher detection rate than color Doppler alone5.
The presence of PFO may have significant clinical implications. It may lead to several pathological conditions, notably right to left shunt, paradoxical embolism, hypoxemia, and cerebral fat embolism6-9. It may open or close according to the interatrial pressure gradient. Mechanical ventilation, especially in patients with acute respiratory distress syndrome (ARDS) may stretch the pulmonary vasculature and right ventricle (RV), thus reversing the interatrial pressure gradient, leading to foramen ovale opening and a right to left shunt (RLS)10-12.
A PFO opening may have a detrimental effect on hypoxemic patients due to ARDS, and recruitment maneuvers further worsen hypoxemia13. The prevalence of PFO is reported between 16 % and 19 % even in ARDS patients mechanically ventilated with protective ventilation strategies14-16.
Interestingly, PFO opening has been reported when a left ventricle (LV) assist device was used. The increase in RV afterload induced by mechanical ventilation and the decrease in LV afterload by the assist device may reverse the pressure gradient between the two atria, thus increasing the prevalence of PFO opening17.
The respiratory and hemodynamic parameters that may influence PFO opening have been poorly investigated. Therefore, in a sample of 108 Intensive Care Unit (ICU) mechanically ventilated patients who underwent echo studies for hemodynamic assessment, we prospectively performed ce-TEE in order to determine the prevalence of PFO and investigate the correlated respiratory and hemodynamic factors, such as the plateau pressure (P-plateau) level, the positive end-expiratory pressure (PEEP) level, the compliance of the respiratory system, and the dimensions of the RV compared to the LV.
Methods
Patient population
All ICU adult patients under mechanical ventilation who underwent TEE for hemodynamic assessment, mainly for hemodynamic instability, over a period of two years (2013-2015) in the three participating tertiary care hospitals, were included in this study. The need for a TEE study for hemodynamic assessment was exclusively decided by the staff specialist responsible for the patient’s care, who was unaware of the study protocol. The ventilator settings were also at the discretion of the same physician, and the echocardiography specialist was responsible only for the TEE study.
Since TEE is routinely used to assess the circulatory status of mechanically ventilated patients in the three participating ICUs, TEE was considered as a component of standard care; therefore, the ethical committee waived the need for patients΄consent. However, written and oral information about the purpose of the study was provided to the patients’ families.
Description of procedure
The TEE examinations were performed by intensivists trained in critical care echocardiography. Three intensive care units participated in the study: ICU of Nicosia’s General Hospital, Cyprus, ICU of “George Papageorgiou” General Hospital, Thessaloniki, Greece and ICU of Limassol’s General Hospital, Cyprus.
All patients underwent the ce-TEE examination under sedation with fentanyl (2-3 mcg/kg) and midazolam (0.1 mg/kg) in the supine position using the GE Vivid -3 imaging system and a 4-7 MHz multiplane probe (GE ULTRASOUND EUROPE, Solingen Germany). A standard TEE study was performed, and the dimensions and function of the RV and LV were observed. In the four-chamber view, the RV end-diastolic area, RV end-systolic area, LV end-diastolic area, and LV ejection fraction were measured using the modified Simpson’s rule.
After hemodynamic assessment and hemodynamic stabilization of the patient according to the standard TEE study findings, a ce-TEE study for PFO detection was performed. The region of the fossa ovalis was examined at the midesophagus in the vertical plane, and the probe was rotated between 60⁰ and 90⁰ at the area of the bi-caval view. The presence of an atrial septal aneurysm or Chiari network was investigated. The bubble test was performed by using a contrast agent, 9.5 ml of a gelatin solution Haemaccel® (PlasmaSelect AG, Marburg, Germany) agitated with 0.5 ml of air, through the central venous catheter already in place. Three bubble tests with agitated Haemaccel® (PlasmaSelect AG, Marburg, Germany) were performed. A PFO was considered present when bubbles appeared in the left atrium during the first 3-5 heart cycles after full opacification of the right atrium in at least one examination4,5.
The injection of micro-bubbles through the central line was always performed at the same point in the respiratory cycle, that is, during the beginning of expiration after performing an inspiratory hold on the ventilator for 3-5 seconds. During the release of the positive pressure mechanical breath, the venous return to the right atrium through the inferior vena cava increases, facilitating visualization of bubbles through the RLS and thus enhancing PFO detection sensitivity. All the echocardiography specialists from the three units participating in the study were fully aware of the study protocol, especially the synchronization of the injection with the respiratory cycle.
The ventilator settings for each patient were set by the staff physician responsible for the patient according to the patient’s primary disease. However, the general practice regarding mechanical ventilation was to improve oxygenation by avoiding high tidal volumes, more than 8 ml/kg of the ideal body weight, and excessive levels of PEEP. In ARDS patients, low levels of PaO2 are tolerated (SpO2 ≥92 %) to avoid high PEEP levels and tidal volumes above 8 ml/kg. If levels of PEEP higher than 12 cm H2O were needed to improve oxygenation, a quasi-static pressure/volume curve of the total respiratory system was performed to detect the presence of a low inflection point and to set the PEEP level accordingly. In general practice, a P-plateau higher than 28 cm H2O was avoided if possible18.
Statistical analysis
Continuous variables are expressed as means ± standard deviations, while nominal variables are expressed as percentages. Cross-tabulation and Chi-square tests were performed to find the association between PFO detection by TEE and P-plateau (expressed as below 26 or above 26). The choice of cut-off point for P-plateau at the level of 26 was based on the study by Jardin et al who found that a plateau pressure below 27 (23-26) was associated with decreased mortality and less incidence of cor pulmonale18. Due to the use of a cut-off point, data are categorical, thus normal distribution does not apply and we used non-parametric test (χ2) that is more liberal. .Compliance [expressed as low (<40), medium (41-60), and high (>60)], and LV compared to RV (RV<LV vs. LV≤RV). Furthermore, multiple logistic regression models were performed to find the adjusted odds ratios (OR) of the statistically significant variables. The level of statistical significance was set at 5 % thus, p values less than 0.05 were considered statistically significant. The statistical package IBM SPSS Statistics version 20 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
One hundred and eight patients were included in the study. Patient demographic characteristics are presented in Table 1.
Table 1. Demographic characteristics of the 108 consecutive ICU adult patients under mechanical ventilation that were included in the study over a period of two years (2013-2015).
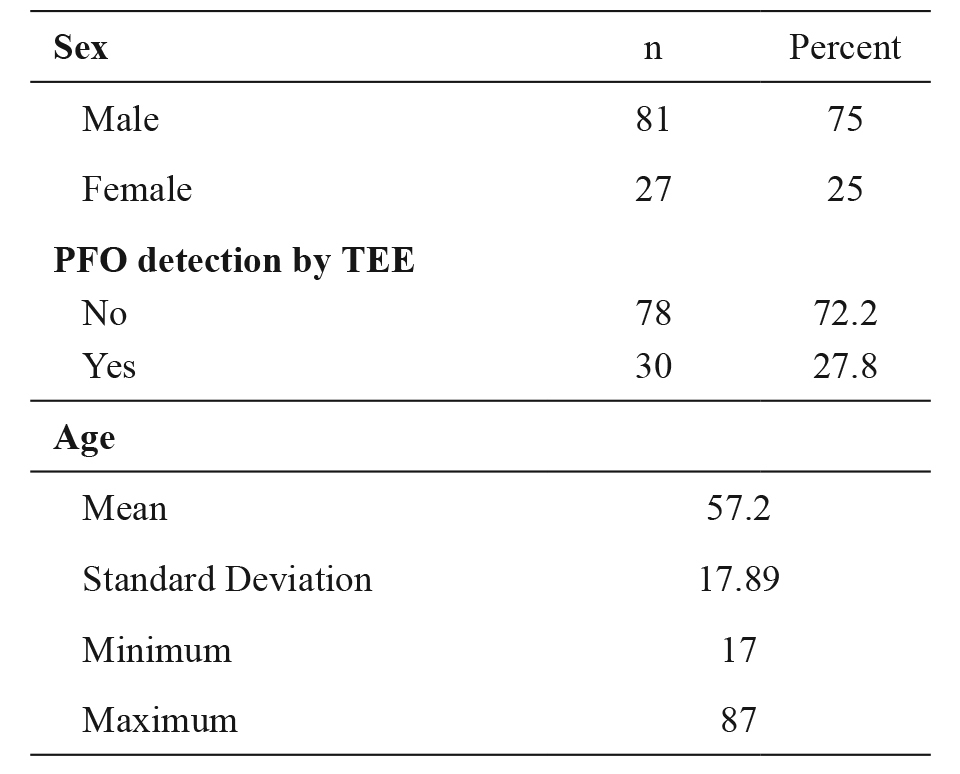
n: number of patients, ICU: Intensive Care Unit, n: number, PFO: patent foramen ovale, TEE: transesophageal echo.
Statistical significance was found between P-plateau and PFO detection (p =0.011). As a classification boundary, the value of 26 cm H2O was used18. Patients with values of less than 26 were classified as being at a low level, while patients with values above 26 were classified as being at a high level. PFO was detected in 20.5 % of the patients with a P-plateau <26 cm H2O and in 46.15 % of patients with a P-plateau >26 cm H2O (p =0.011) (Table 2).
Table 2. Statistical significance of PFO detection according to variables such as P-plateau, RV dimension, PEEP level, and compliance.
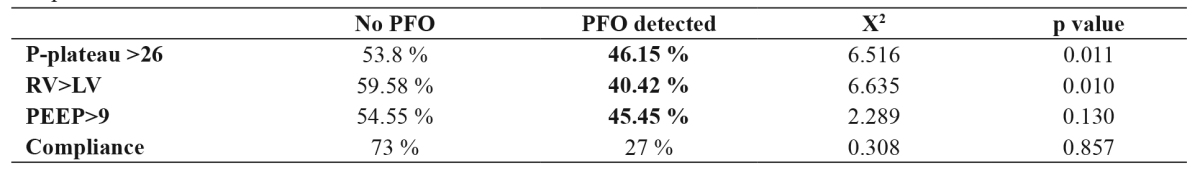
PFO: patent foramen ovale, RV: right ventricular PEEP: positive end-expiratory pressure, LV: left ventricular, P-plateau: plateau pressure.
Furthermore, statistical significance was found when comparing the RV dimensions with LV dimensions (p =0.010). In patients with an RV≥LV, the prevalence of PFO was 40.42 %, and of the patients with RV<LV, 18 % had PFO (Table 2).
There was no association between respiratory system compliance and PFO detection (p =0.857). In addition, no statistically significant association was found between the level of PEEP and PFO detection (p =0.113); however, an open foramen ovale was very common in patients with high levels of PEEP (Table 2).
Finally, a binary logistic regression was used to identify the possibility of PFO based only on less complicated indexes, such as P-plateau, PEEP, compliance, and LV/RV ratio. According to this model, the factors that influence the possibility of PFO opening were P-plateau >26 with an OR of 3.42 and RV≥LV with an OR of 3.13 (Table 3). Moreover, the analysis revealed that age and sex were not confounders.
Table 3. Results of the binary logistic regression, used to identify the possibility of PFO, indicating that the factors influencing the possibility of PFO opening are P-plateau >26 with an OR of 3.42 and RV≥LV with an OR of 3.13.
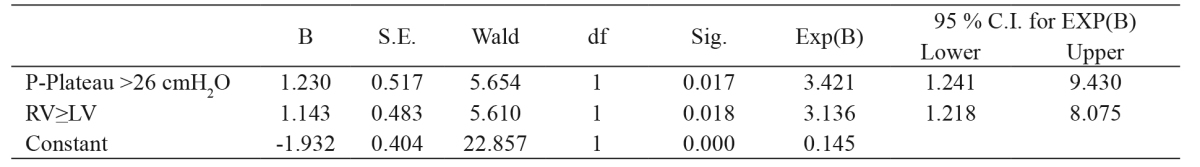
PFO: patent foramen ovale, CI: confidence interval, P-plateau: plateau pressure, LV: left ventricular, RV: right ventricular.
Discussion
The main finding of our prospective cohort study is that the presence of PFO in mechanically ventilated ICU patients is 27 %, approximately the same as that reported in the general population12. However, the distribution of PFO in these mechanically ventilated patients is not homogenous. Patients with a P-plateau >26 cm H2O have a PFO prevalence of 46 %, and they were 3.4 times more likely (OR: 3.4) to have an open foramen ovale compared to patients with a lower P-plateau (p =0.011), (Table 3). Similarly, patients with RV dilatation demonstrated an increased prevalence of PFO at 40 % (p =0.010), and they were also 3.1 times more likely to exhibit an open foramen ovale (OR: 3.13) compared with patients without RV dilatation (p =0.010).
According to literature reports, RV dilatation is considered to be related to a higher PFO rate since the RV dilates in response to acute or chronic volume and/or pressure overload18,19.
Furthermore, in mechanically ventilated patients, P-plateau increases when lung volume decreases. This is a consequence of the fact that, the tidal volume is distributed in a smaller lung volume. Lung over-distention increases pulmonary vascular resistance due to the increase of transpulmonary pressure causing partial or complete collapse of the pulmonary capillaries when the alveolar pressure becomes higher than the pulmonary arterial pressure. This RV afterload elevation, induced by mechanical ventilation, causes right heart chamber pressure raise, resulting in the opening of the foramen ovale.
Similarly, high levels of PEEP, like those used in ARDS patients aiming to improve oxygenation and reduce volutrauma and biotrauma by keeping the alveoli open, could possibly over-distend the normally aerated alveoli. We hypothesized that a high PEEP could increase RV afterload, which in turn would increase high RA pressure, hence, foramen ovale opening20,21. The fact that we only found a trend of correlation between PFO and high levels of PEEP could be a consequence of the small sample size. It is of note that, in patients with PEEP >9 cm H2O, the prevalence of PFO was 45 %, On the other hand, since we did not measure the pulmonary pressures, the role of a high PEEP per se in causing a direct elevation of RV afterload (and subsequent foramen ovale opening) in this particular sample of patients may well be questioned, since other confounders -related to the disease(s), severity, complications, or and management- could possibly be involved.
In Patients with ARDS, PFO shunting may worsen hypoxemia, and interventions like a recruitment maneuver may further worsen the oxygenation20. Hence, awareness of its existence is important for management decisions, and its high prevalence in this population should prompt complete evaluation of the cardiac anatomy whenever hypoxemia correlates poorly with the clinical scenarios, such as, lung compliance. On the other hand, Dessap et al reported that the prevalence of moderate-to-large PFO shunting in patients with moderate ARDS, was 19 % which is similar to the percentage reported from autopsy series. Nevertheless, this percentage is high enough to alert the clinician in the appropriate setting. In that cohort of patients (Dessap et al) those with right to left (RL) shunting were more likely to have larger RV dimensions and higher pulmonary artery systolic pressure. As that study shows, patients with PFO and RL shunt, had a poorer PaO2/FiO2 ratio, and a longer ICU stay16.
Overall, in our cohort, patients with a P-plateau >26 mmHg or with an RV/LV area ratio >1 were more likely to have an open foramen ovale. This finding may suggest that an open foramen ovale as well as an RV dilatation, may be consequent to the high P-plateau, hence, high alveolar pressure, the latter causing an elevated pulmonary artery systolic pressure and RV pressure overload. A final, practical application of our findings could be that in a mechanically ventilated patient with high P-plateau and/or PEEP levels, a drop in oxygenation ratio could potentially be related to an opening of a foramen ovale.
One limitation of our study is the small sample size. Furthermore, to the assumed explanations of our findings were not verified by a simultaneous recording of the right and left atrial pressures. Hence, our theory that a high plateau pressure causes interatrial pressure elevation, which in turn opens the FO, partly relies on the work of Lemaire et al, as well as the well-established related pathophysiological mechanisms that underlie these conditions13.
Our findings are consistent with the work of Papadopoulos et al, who suggested the use of a “provocation test”, during which, ventilation with 15 cm H2O of PEEP and high tidal volumes, increases the detection rate of PFOs with RLS, as examined by contrast TEE21. This study demonstrates that maneuvers aiming to increase the alveolar pressure may, indeed, open the FO by mechanisms of pulmonary pressure elevation, as explained above.
Conflict of Interest
The authors declare no conflicts of interest. No funding was obtained for this study.
References
- 1.Kutty S, Sengupta PP, Khandheria BK. Patent foramen ovale: the known and the to be known. J Am Coll Cardiol. 2012;59:1665–1671. doi: 10.1016/j.jacc.2011.09.085. [DOI] [PubMed] [Google Scholar]
- 2.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 3.Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol. 2001;38:613–623. doi: 10.1016/s0735-1097(01)01427-9. [DOI] [PubMed] [Google Scholar]
- 4.Mojadidi MK, Bogush N, Caceres JD, Msaouel P, Tobis JM. Diagnostic accuracy of transesophageal echocardiogram for the detection of patent foramen ovale: a meta‐analysis. Echocardiography. 2014;31:752–758. doi: 10.1111/echo.12462. [DOI] [PubMed] [Google Scholar]
- 5.Fisher DC, Fisher EA, Budd JH, Rosen SE, Goldman ME. The incidence of patent foramen ovale in 1,000 consecutive patients: A contrast transesophageal echocardiography study. Chest. 1995;107:1504–1509. doi: 10.1378/chest.107.6.1504. [DOI] [PubMed] [Google Scholar]
- 6.Seward JB, Hayes DL, Smith HC, Williams DE, Rosenow EC 3rd, Reeder GS, et al. Platypnea-orthodeoxia: clinical profile, diagnostic workup, management, and report of seven cases. Mayo Clin Proc. 1984;59:221–231. doi: 10.1016/s0025-6196(12)61253-1. [DOI] [PubMed] [Google Scholar]
- 7.Ranoux D, Cohen A, Cabanes L, Amarenco P, Bousser MG, Mas JL. Patent foramen ovale: is stroke due to paradoxical embolism? Stroke. 1993;24:31–34. doi: 10.1161/01.str.24.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Rimoldi SF, Ott S, Rexhaj E, de Marchi SF, Allemann Y, Gugger M, et al. Patent Foramen Ovale Closure in Obstructive Sleep Apnea Improves Blood Pressure and Cardiovascular Function. Hypertension. 2015;66:1050–1057. doi: 10.1161/HYPERTENSIONAHA.115.06303. [DOI] [PubMed] [Google Scholar]
- 9.Pell AC, Hughes D, Keating J, Christie J, Busuttil A, Sutherland GR. Brief report: fulminating fat embolism syndrome caused by paradoxical embolism through a patent foramen ovale. N Engl J Med. 1993;329:926–929. doi: 10.1056/NEJM199309233291305. [DOI] [PubMed] [Google Scholar]
- 10.Asrress KN, Marciniak M, Marciniak A, Rajani R, Clapp B. Patent foramen ovale: the current state of play. Heart. 2015;101:1916–1925. doi: 10.1136/heartjnl-2015-307639. [DOI] [PubMed] [Google Scholar]
- 11.Cujec B, Polasek P, Mayers I, Johnson D. Positive end-expiratory pressure increases the right-to-left shunt in mechanically ventilated patients with patent foramen ovale. Ann Intern Med. 1993;119:887–894. doi: 10.7326/0003-4819-119-9-199311010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Legras A, Caille A, Begot E, Lhéritier G, Lherm T, Mathonnet A, ARCO and CRICS network , et al. Acute respiratory distress syndrome (ARDS)-associated acute cor pulmonale and patent foramen ovale: a multicenter noninvasive hemodynamic study. Crit Care. 2015;19:174. doi: 10.1186/s13054-015-0898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemaire F, Richalet JP, Carlet J, Brun-Buisson C, MacLean C. Postoperative hypoxemia due to opening of a patent foramen ovale confirmed by a right atrium-left atrium pressure gradient during mechanical ventilation. Anesthesiology. 1982;57:233–236. doi: 10.1097/00000542-198209000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Lhéritier G, Legras A, Caille A, Lherm T, Mathonnet A, Frat JP, et al. Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: a multicenter study. Intensive Care Med. 2013;39:1734–1742. doi: 10.1007/s00134-013-3017-6. [DOI] [PubMed] [Google Scholar]
- 15.Boissier F, Katsahian S, Razazi K, Thille AW, Roche-Campo F, Leon R, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39:1725–1733. doi: 10.1007/s00134-013-2941-9. [DOI] [PubMed] [Google Scholar]
- 16.Dessap AM, Boissier F, Leon R, Carreira S, Campo FR, Lemaire F, Brochard L. Prevalence and prognosis of shunting across patent foramen ovale during acute respiratory distress syndrome. Crit Care Med. 2010;38:1786–1792. doi: 10.1097/CCM.0b013e3181eaa9c8. [DOI] [PubMed] [Google Scholar]
- 17.Marty P, Méjean S, Boudou N, Mayeur N, Minville V, Galinier M. Patent foramen ovale appearance with association of left ventricular assist device and mechanical ventilation. Am J Emerg Med. 2012;30:259. doi: 10.1016/j.ajem.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33:444–447. doi: 10.1007/s00134-007-0552-z. [DOI] [PubMed] [Google Scholar]
- 19.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 20.Koroneos A, Politis P, Malachias S, Manolis AS, Vassilakopoulos T. End-inspiratory occlusion maneuver during transesophageal echocardiography for patent foramen ovale detection in intensive care unit patients. Intensive Care Med. 2007;33:1458–1462. doi: 10.1007/s00134-007-0639-6. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulos G, Brock M, Eyrich K. [Intraoperative contrast echocardiography for detection of a patent foramen ovale using a provocation test and ventilation with PEEP respiration] Anaesthesist. 1996;45:235–239. doi: 10.1007/s001010050258. [DOI] [PubMed] [Google Scholar]


