Abstract
Sulfonate-containing hydrogels are of particular interest because of their tunable mechanical and swelling properties, as well as their biological effects. Polysulfonate copolymers were synthesized by reacting 2-acrylamido-2-methylpropanesulfonic acid (AMPS), acrylamide (AM), and acrylic acid (AA). We found that the incorporation rate of sulfonate-containing monomer and the molecular weight of the copolymer were significantly enhanced by increasing the ionic strength of the solution. We introduced thiol groups by modifying the pendant carboxylates or copolymerizing along with a disulfide-containing monomer. The thiol-containing copolymers were reacted with a 4-arm acrylamide-terminated poly(ethylene glycol) via a thiol–ene click reaction, which was mediated by a photoinitiator, a redox initiator, or a base-catalyzed Michael-Addition. We were able to tailor the storage modulus (33–1800 Pa) and swelling capacity (1–91 wt %) of the hydrogel by varying the concentration of the copolymers. We determined that the injectable sulfonate-containing hydrogels were biocompatible up to 20 mg/mL, as observed by an electric cell–substrate impedance sensing (ECIS) technique, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay using three different cell lines: human retinal pigment epithelial cells (ARPE-19), fibroblasts (NIH 3T3), and Chinese hamster ovary cells (CHO).
Graphical Abstract

1. INTRODUCTION
Hydrogels are chemically or physically cross-linked hydrophilic polymer networks, which are able to absorb and retain a large amount of water. Because of their high water content, softness, and other biomimetic properties, hydrogels have been widely used in tissue engineering, biomedical devices, and drug delivery.1 Sulfonate-containing hydrogels are of particular interest because of their tunable swelling2 and mechanical properties, as well as their ability to bind cationic drugs.3,4 Chondroitin sulfate and dermatin sulfate are important natural sulfonate-containing polysaccharides that have been used in cartilage tissue engineering,5 skin substitution,6 and treatment of joint diseases.7 Chondroitin sulfate has been incorporated into hydrogels to tailor their swelling,3 elasticity,3,8 and drug release rate,3,9,10 and to promote adhesion of cells to hydrogels.11 In addition, Matsusaki et al. reported that sulfonate-containing hydrogels have anticoagulant activity12 and can immobilize fibroblast growth factor-212 and vascular endothelial growth factor via its heparin-binding domains.13 Sulfonate-containing polymers inhibited not only the cytopathic effect of HIV at concentrations that are not toxic to the host cells,14 but angiogenesis, which is associated with many diseases, as well as the growth of solid tumors,15,16 arthritis,17 proliferative diabetic retinopathy,18 intervertebral disc regeneration, and pain.19 Hence, incorporating sulfonate groups into hydrogels to mitigate angiogenesis would be valuable in designing tissue substitutes that do not have or need blood vessels, such as the cornea, vitreous, and nucleus pulposus. Here we focus primarily on various synthetic polymer techniques for the development of injectable in situ gelling sulfonate-containing hydrogels.
We selected 2-acrylamido-2-methylpropanesulfonic acid (AMPS) as a monomer to provide sulfonate groups. Because the strongly ionizable sulfonate dissociates in a wide pH range, hydrogels containing AMPS exhibit pH-independent swelling behavior. PolyAMPS, which is nontoxic and the tested doses, has been reported to achieve dose-dependent inhibition of angiogenesis.20 In addition, polyAMPS has been studied as a prototypic molecule for the development of antimitogenic drugs for the treatment of neoplastic pathologies,21 as well as multitarget drugs for the treatment of HIV infection and AIDS-associated pathologies.22
Injectable hydrogels have gained much attention for their great potential in the biomedical area.23,24 In this technique, a premixed solution containing cross-linkable polymers, drugs, cells, and another ingredient is injected to fill native or potential cavities; a matrix, which may be difficult to prefabricate, forms in vivo and conforms to different shapes. Injectable hydrogels have considerable advantages in many biomedical applications, including injectable tissue engineering,25,26 minimally invasive drug delivery,27 and wound dressing.28,29 However, to the best of our knowledge, the synthesis of an injectable in situ gelling AMPS hydrogel for biomedical uses has not been reported. Here we describe several methods of preparing injectable sulfonate-containing hydrogels and test their biocompatibility.
First we investigated the effect of ionic strength on the reactivity of AMPS in the copolymerization of AMPS and acrylamide (AM). We then compared three methods to introduce thiol groups to the copoly(AM-co-AMPS). Subsequently, we used three techniques for cross-linking the pendant thiol groups on the copolymer with a cross-linker consisting of a 4-arm poly(ethylene glycol) (PEG) with terminal acrylamide groups via thiol–ene chemistry. Thus, the copolymer/PEG solution, when injected into the body cavity, could form a hydrogel in situ and exert permanent biomedical effects by the covalently bonded sulfonates. We were able to tune the swelling and mechanical properties of the hydrogel by varying the concentration of copolymer. We also determined that the biocompatibility of sulfonate-containing hydrogels on three different cell lines: human retinal pigment epithelial cells (ARPE-19), fibroblasts (NIH 3T3), and Chinese hamster ovary cells (CHO) by using electric cell–substrate impedance sensing (ECIS), a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and cell images.
2. EXPERIMENTAL SECTION
2.1. Materials
All chemicals were purchased from Sigma-Aldrich unless otherwise stated. 4,4′-Azo-bis(4-cyanopentanoic acid (AIBN, 98%) was purchased from MP Biomedicals. The retinal pigment epithelial (RPE) cell line ARPE-19, NIH 3T3 fibroblast cells, and Chinese hamster ovary (CHO) cells were purchased from American Type Culture Collection and cultured in DMEM/F12 with 10% FCS, 1% antibiotic/antimycotic/gentamicin, 0.1% amphotericin B.
2.2. Synthesis of Thiol- and Sulfonate-Containing Copolymers
2.2.1. Investigation of the Effect of Ionic Strength on the Reactivity of AMPS
The effect of ionic strength on the reactivity of AMPS was investigated by comparing the kinetic profile of the copolymerization of AMPS and AM at different concentrations of sodium chloride. 4,4′-Azobis(4-cyanovaleric acid) (V501; 16.9 mg, 0.060 mmol), sodium chloride (3.54 g, 0.060 mol), AMPS, and AM (total amount of AMPS and AM was 0.030 mol) were dissolved in 40 mL of water. A 10 wt % NaOH solution was added to adjust the pH to ~9. Water was then added to a final concentration of monomer at 0.5 M. The solution was purged with nitrogen at 22 °C for 30 min to remove dissolved oxygen. The mixture was stirred at 70 °C for 3 h. Aliquots were taken at ~25 min and at the end of polymerization, then characterized by gel permeation chromatography (GPC) and nuclear magnetic resonance spectrum (NMR). 1H NMR spectra were obtained on a Varian Unity Inova 500 MHz (Palo Alto, CA). Aliquots from the reaction were freeze-dried and redissolved in D2O (8 mg/mL). Each sample was scanned 128 times at 25 °C. The GPC system used a VE 1122 pump with a VE 7510 degasser (Viscotek/Malvern, Houston, TX) equipped with a TDA302 triple detector system that measured the refractive index (RI), multiangle laser light scattering, and viscosity. The column used was a G5000PWXL (Tosoh Biosep, Montgomeryville, PA). Viscotek Omnisec software was used to calculate the RI area, weight-averaged molecular weight, intrinsic viscosity, and hydrodynamic radius. Samples (100 μL) were injected at a concentration of 2 mg/mL. The column buffer (pH 7.6) contained 20 mM of sodium phosphate and 100 mM of NaCl. The flow rate was 0.8 mL/min. Measurements were conducted at 37 °C.
2.2.2. Synthesis of Copoly(AM-co-AMPS-co-BAC)
Thiol groups were introduced into the copolymer by incorporating a disulfide-containing bis-acrylamide-cystamine (BAC) cross-linker. The desired amounts of AM, AMPS, and BAC were dissolved in N,N-dimethylformamide (DMF) at 5 wt %. A stock AIBN/DMF solution (10 mg/mL) was injected into the vial and the solution was purged with nitrogen at 22 °C for 1 h to remove the dissolved oxygen. The solution was incubated at 60 °C for 5 h, yielding a chunk of white solid, which was suspended in DI water to form a hydrogel. A 10 M excess of dithiothreitol (DTT) was added to reduce the disulfide bonds to thiols. A 1 N NaOH solution was added to the hydrogel to adjust the pH to ~7.5. The mixture was stirred at 22 °C for 18 h, during which the suspension became clear and less viscous. DMF, any unreacted monomers, and excess DTT were removed by dialysis (MWCO = 12000–14000) against nitrogen-bubbled HCl solution (1 mM, 4 L × 6) for 3 days. The products were lyophilized, yielding white solid. The extent of thiolation was determined using a 2-nitro-5-thiosulobenzoate (NTSB) assay as previously reported.30
2.2.3. Synthesis and Derivatization of Copoly(AM-co-AMPS-co-AA)
We also incorporated thiol groups by copolymerization of acrylic acid (AA) with AM and AMPS, then derivatized the carboxylates with cystamine.
2.2.3.1. Synthesis of Copoly(AM-co-AMPS-co-AA)
The procedure for synthesizing copoly(AM-co-AMPS-co-AA) was similar to that for synthesizing copoly(AM-co-AMPS), except that the reaction time was 1 h. The mixture was dialyzed (MWCO = 12000–14000 kDa) against 4 L × 6 of DI water at 22 °C for 3 days. The solution was then lyophilized, yielding a white solid (~90%).
2.2.3.2. Derivatization of Copoly(AM-co-AMPS-co-AA) Using Carbodiimide-Based Condensation
Thiol groups were introduced to the copoly(AM-co-AMPS-co-AA) using a carbodiimide-based condensation. Various formulations were used and one of them is described. A copoly(AM-co-AMPS-co-AA) was dissolved in DI water at 1 wt %. Stirring the mixture at 22 °C for 2 h yielded a viscous solution. The desired amounts of cystamine dihydrochloride (cys), N-hydroxysuccinimide (NHS), and 1-ethyl-3-(3-(dimethylamino)-propyl)carbodiimide (EDC) were added to the vial. The pH was adjusted to 4.5 by adding a 1 N HCl aqueous solution. The solution was stirred at 22 °C for 20 h. Excess cys, EDC, and NHS were removed by dialysis (MWCO = 12000–14000) against DI water (4 L × 4) for 2 days. A 10 M excess of DTT comparing to the conjugated cys, as determined by a disulfide assay, was added to the product solution. The pH was adjusted to ~7.5 by adding 1 N NaOH solution. The mixture was stirred at 22 °C for 4 h to reduce the disulfide bonds. Excess DTT was removed by dialysis (MWCO = 12000–14000) against nitrogen-bubbled HCl solution (1 mM, 4 L × 6) for 3 days. The product was lyophilized, yielding a white solid.
2.2.3.3. Derivatization of Copoly(AM-co-AMPS-co-AA) Using Triazine-Based Condensation
Triazine-based condensation was also used to introduce thiol groups to the copoly(AM-co-AMPS-co-AA). A copoly(AM-co-AMPS-co-AA) was dissolved in 6 mL of DI water at 1.7 wt %. Acetonitrile and N-methylmorpholine (NMM) were added to the vial sequentially. A 1 M HCl aqueous solution was added to adjust the pH to 7. The mixture was cooled in an ice bath. 2-Chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) was added and the mixture was stirred at 22 °C for 1 h. Cystamine and sodium bicarbonate were added and the mixture was stirred at 22 °C for 20 h. As compared to the amount of cystamine, a ten-molar excess of DTT was added to the product solution. The pH was adjusted to ~7.5 by adding a 1 N NaOH solution. Most of the gels or precipitates were dissolved in 30 min, yielding clear solutions. The mixture was incubated at 22 °C for 4 h to fully reduce the disulfide bonds. Excess DTT was removed by dialysis (MWCO = 12000–14000) against nitrogen-bubbled HCl solution (1 mM, 4 L × 6) for 3 days. The products were lyophilized, yielding a white solid.
2.3. Synthesis of Injectable Sulfonate-Containing Hydrogels
Using three methods, the thiol- and sulfonate-containing copolymer was cross-linked with a 4-arm PEG-acrylamide to form a hydrogel.
2.3.1. Cross-Link by Photoinitiated Thiol–Ene Addition
A riboflavin stock solution (0.1 mg/mL) was bubbled with nitrogen gas for 30 min to remove oxygen. A 4-arm PEG-acrylamide (MW = 2 kDa) and copoly(AM-co-AMPS-co-BAC) was dissolved in the riboflavin stock solution at 10 and 40 mg/mL respectively, after which arginine in the solution was dissolved at 0.2 mg/mL. This mixed solution was irradiated with UV light (wavelength = 360 nm, intensity = 3000 μW/cm2) until a hydrogel was formed. Copolymers were tested for gelling by tipping the dishes at a 45° angle. When the material resisted flowing, they were considered to have gelled.
2.3.2. Cross-Link by Redox-Initiated Thiol–Ene Addition
A 4-arm PEG-acrylamide (MW = 2k Da) and copoly(AM-co-AMPS-co-BAC) was dissolved in oxygen-free water at 10 and 40 mg/mL respectively. Ammonium persulfate (APS, 1.4 mg/mL) and tetramethylethylenediamine (TEMED, 7 μL/mL) were dissolved in the solution, which was kept at room temperature until a hydrogel was formed.
2.3.3. Cross-Link by Base-Initiated Michael Addition
A 4-arm PEG-acrylamide (MW = 2k Da) and copoly(AM-co-AMPS-co-BAC) were dissolved in oxygen-free water at the desired concentrations. Triethylamine (TEA; 2.8 μL/mL) was dissolved in the solution and the solution was allowed to gel.
2.4. Test of Swelling Capacity and Rheology of the Sulfonate-Containing Hydrogels
Copolymer (15, 20, 30, and 40 mg/mL)/riboflavin (0.1 mg/mL) solutions were prepared for hydrogel formation. The solutions were mixed with 4-arm PEG-acrylamide (3.75, 5, 7.5, and 10 mg/mL) and arginine (0.2 mg/mL) and pipetted into weighed 35 mm dishes. The mixtures were irradiated with UV light for 30 min to form hydrogels. The initial weights of the hydrogels were recorded. 1× PBS was added to the dishes containing hydrogels, and the gels were left at 37 °C. At certain time points (as shown in Figure 7), excess PBS was poured off and the hydrogels were gently blotted dry and weighed. Swelling was determined by the change in weight from the initial weight. The rheological properties of the sulfonate-containing hydrogels were analyzed using a modular compact rheometer (MCR rheometer; Anton Paar, TX). The swollen hydrogel in PBS in the 35 mm dishes were cut with a custom-made cutter 21 mm in diameter. The dishes containing hydrogels of 21 mm diameter were placed on the plate of the rheometer in contact with the measuring system (20 mm plate). A force of 0.2 N was applied to each of these gels to ensure good contact. Samples were measured at a 2% strain and a constant temperature of 37 °C at a range of frequencies.
Figure 7.
Hydrogels with a higher concentration of sulfonate-containing copoly(AM-co-AMPS-co-BAC) (AM/AMPS/BAC = 7.5:2:0.5) swelled more and took a longer time to equilibrate.
2.5. Test of Cytotoxicity of the Sulfonate-Containing Hydrogels
We tested the biocompatibility of sulfonate-containing hydrogels with ARPE-19, NIH 3T3, and CHO by using ECIS, MTT assay, and cell images. For both MTT and ECIS, all three cell lines were seeded at 20000 cells/well. The time required for cells to reach confluency is cell-type dependent. Retinal cells can become confluent by the end of the 24th hour after seeding, whereas 3T3 and CHO cells require almost 50 h to reach confluency. Thus, the time point for the addition of copolymer/PEG solutions to 3T3 and CHO cells was different from the time point for ARPE-19 cells. After the addition of these solutions, cell media were aspirated and the copolymer/PEG solutions were directly added to the cell monolayers. The wells were exposed to UV light at 365 nm for 1 min, so that hydrogels were formed in situ where they were in direct contact with the cells. We used this procedure throughout the entire study. After the addition of hydrogel, media change was no longer possible. MTT tests and imaging were done after the cells and hydrogels had been in direct contact for at least 96 h.
Cell characteristics were analyzed using electric-cell–substrate impedance sensing (ECIS), a noninvasive technique that continuously measures the impedance across gold electrodes present at the bottom of tissue-culture wells, using various frequencies of alternating current. The 96-well 10idf ECIS arrays were prepared according to the manufacturer’s instruction. Arrays were pretreated with 10 mM sterile cysteine in water, which provides a coating for the gold electrodes by interaction of the –SH groups in cysteine with the gold surface, which increases the reproducibility of cell attachment and spreading. Tissue-culture medium was then added to the wells for protein adsorption to the gold electrode surface. Resistance measurements were chosen over complex impedance measurements because the polymer has a capacitance that complicates impedance readings. A frequency of 4000 Hz was used to monitor growth and adherence, since at this frequency the resistance for the hydrogels was minimum.
MTT assays were done in treated 96-well tissue-culture plates. After 96 h, the gels were removed and the cell culture medium with 1 mg/mL MTT was added to each well in 100 μL. The plates were incubated for 5 h, after which the MTT was removed and 100 μL of dimethyl sulfoxide (DMSO) was added. Optical density at 540 nm was measured in a 96-well plate reader. Both positive and negative controls were used. Using the optical density readings, the viability of cells exposed to the hydrogels was expressed as a percentage of viability compared to control cells which had not been exposed to the hydrogels. The results were normalized based on the negative control readings.
Light microscopy images of the cells were obtained using an Olympus CKX41 (Tokyo, Japan) inverted microscope in conjunction with Olympus Cell Imaging System software. The images were processed with Picture Frame Application 3.0.
3. RESULTS AND DISCUSSION
The sulfonate-containing hydrogel is of great interest for use in the biomedical field due to its broad biomedical effects. It has anticoagulant, antiangiogenic, and antimitogenic properties; it also inhibits the cytopathicity of HIV. The sulfonate groups could also be used for binding cationic drugs, as well as tuning swelling capacity and mechanical properties of the hydrogel. It will be extremely valuable to develop an injectable sulfonate-containing hydrogel that can be injected into body cavities without destroying the polymeric network and maintain the integrity and mechanical property of the hydrogel. The in situ formed hydrogel would provide long-term, localized, biomedical effects by covalently bonded sulfonate groups and, thus, slow diffusion of therapeutic agent,3,9,10 thus, reducing both the need for periodic injections and potential complications. This paper describes three different methods for synthesizing and modifying sulfonate-containing copolymers and in situ hydrogels that have tunable swelling and mechanical properties that are compatible with three different cell lines.
3.1. Effect of Ionic Strength on the Reactivity of Sulfonate-Containing Monomer
The properties of a copolymer are largely affected by its microstructure. It is critical to investigate whether the monomers incorporated into copolymer are statistically close to the feed ratio. AMPS is a strong acid and mostly dissociates in aqueous solutions, so its reactivity is probably affected by the ionic strength in the reaction medium. To investigate this effect, AMPS and AM were copolymerized with or without NaCl (1 M) in the reaction mixture. Aliquots were taken during polymerization (25 min) and after all monomers were completely polymerized (3 h). The samples were characterized by 1H NMR and GPC to compare the reaction rates, as well as the compositions and molecular weights of copolymers obtained at different ionic strengths.
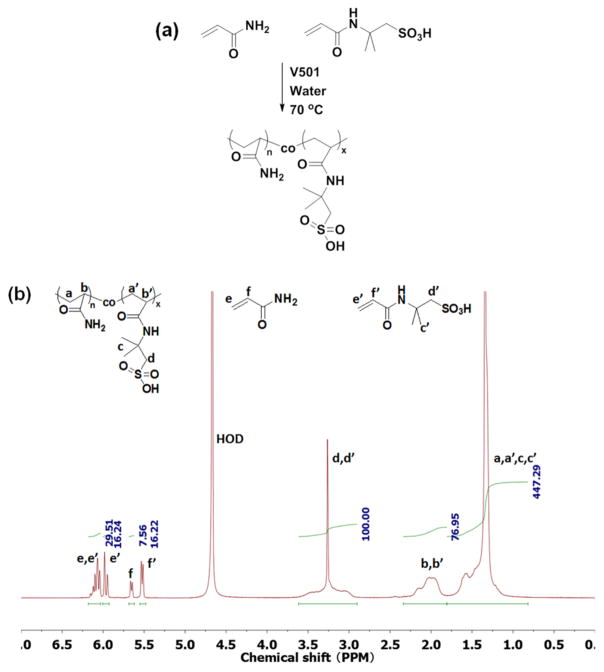
The conversions of AMPS and AM and the compositions of the copolymers were calculated from the 1H NMR spectra (Figure 1). The resonance at 2.9–3.7 ppm was assigned to the methylene group next to the sulfonate in the copolymer; the sharp peak at 3.26 ppm was assigned to this methylene group in the unreacted AMPS monomer. Since the total amount of the methylene group next to sulfonate (polymer plus monomer) remained constant during polymerization, the integration from 2.9 to 3.7 ppm was used as an internal standard for calculation. The peaks at 5.52 and 5.65 ppm were assigned, respectively, to the vinyl protons next to the carbonyl groups in the unreacted AMPS and AM monomers. Thus, the conversion of monomers and the composition of the copolymer could be calculated by comparing the amounts of unreacted monomers with the fed amounts of monomers. The results obtained from 1H NMR spectra are listed in Table 1.
Figure 1.
(a) Copolymerization of AM and AMPS in an aqueous solution. (b) Characteristic 1H NMR spectrum of the reaction mixture. This sample was taken at 25 min of polymerization with a molar feed ratio of 50:50 AMPS/AM.
Table 1.
Effect of NaCl on the Reactivity of AMPS and AM
| feed ratio (AMPS/AM) | reaction time (min) | polymerized AMPS (conversion) | polymerized AM (conversion) | total conversion (%) |
|---|---|---|---|---|
| 80:20 | 25 | 51.4 (64.3%) | 17.8 (89.0%) | 69.2 |
| 180 | 78.2 (97.8%) | 20.0 (100%) | 98.2 | |
| 80:20 (NaCl) | 25 | 72.3 (90.4%) | 19.7 (98.5%) | 92.0 |
| 180 | 79.5 (99.4%) | 20.0 (100%) | 99.5 | |
| 50:50 | 25 | 33.8 (67.6%) | 42.4 (84.8%) | 76.2 |
| 180 | 48.0 (96.0%) | 50.0 (100%) | 98.0 | |
| 50:50 (NaCl) | 25 | 42.5 (85.0%) | 46.3 (92.6%) | 88.8 |
| 180 | 49.3 (98.6%) | 50.0 (100%) | 99.3 | |
| 20:80 | 25 | 12.8 (64.0%) | 60.1 (75.1%) | 72.9 |
| 180 | 18.9 (94.5%) | 78.9 (98.6%) | 97.8 | |
| 20:80 (NaCl) | 25 | 16.3 (81.5%) | 68.7 (85.9%) | 85.0 |
| 180 | 19.7 (98.5%) | 79.0 (98.8%) | 98.7 |
Both AMPS and AM were completely reacted after 3 h under all experimental conditions. The conversions of AMPS and AM at 25 min of each batch of polymerization were compared in Figure 2. AMPS was found to incorporate into the polymer more slowly than did AM in each polymerization batch. This is likely due to the fact that the negatively charged AMPS in the growing chain impedes the incorporation of negatively charged AMPS monomers by electrostatic repulsion. Therefore, the addition of salts was expected to screen the charges and reduce the electrostatic repulsion between the growing chain and AMPS. In fact, the conversion of AMPS at 25 min of polymerization was significantly enhanced with the addition of NaCl because of the two reasons mentioned earlier. Moreover, as expected, the effect of NaCl was greater in polymerization containing more AMPS, confirming that the lower reactivity of AMPS was caused by negative charges. Since NaCl enhanced the polymerization rate and diminished the difference in the reactivity of AMPS and AM, it was used in the following polymerizations done in an aqueous medium.
Figure 2.
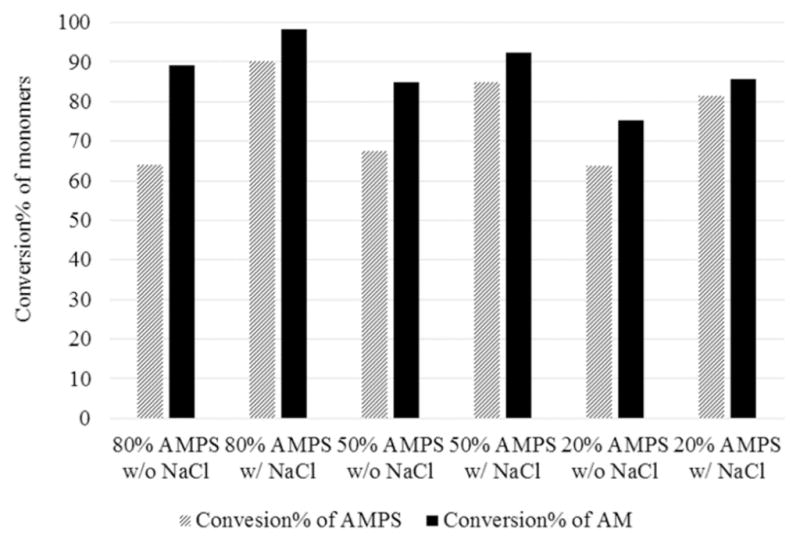
Conversions of AMPS and AM at 25 min of polymerization under different conditions showed that the presence of NaCl enhanced the reactivities of both monomers and reduced the difference between their reactivities.
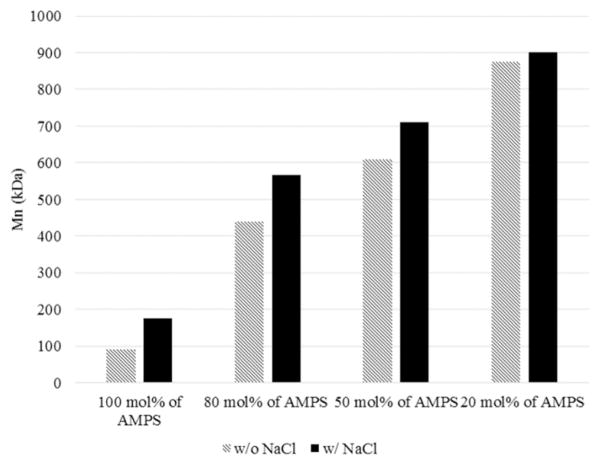
The copolymers were also characterized by GPC. The molecular weights of the copoly(AM-co-AMPS) are higher than that of the PAMPS homopolymer and increased with decreasing molar percentages of AMPS. This occurred because AMPS is less reactive than AM, as discussed. Also, the molecular weights increased in the presence of NaCl in the polymerization (Figure 3), especially in the copolymer with more AMPS. Therefore, the observations based on GPC characterization supported the inference from the reaction rates.
Figure 3.
Addition of NaCl and AM increased the molecular weight of copolymers.
3.2. Synthesis of Thiol- and Sulfonate-Containing Copolymers
An injectable sulfonate-containing hydrogel is of particular interest because it could easily be injected into body cavities that are difficult to access by surgery, eliminate complicated surgical procedures for implanting a preformed hydrogel, avoid mechanical damage when injecting a preformed hydrogel through a small-gauge needle, and express long-term localized bioactivities that are provided by the covalently bonded sulfonate groups. A variety of techniques are used for implementing these in situ-forming gels, including redox polymerization,31 photopolymerization,32,33 click reaction,32 thiol–ene reaction,34 ionic attractions,35 thermosensitive gelation,36 enzymatic gelation,28 formation of Schiff base,26 and others. Thiol-modified polymers are of great interest for making injectable hydrogels because of their ability to cross-link via either thiol–ene addition or the formation of disulfide bonds by oxidation.
3.2.1. Synthesis of Copoly(AM-co-AMPS-co-BAC)
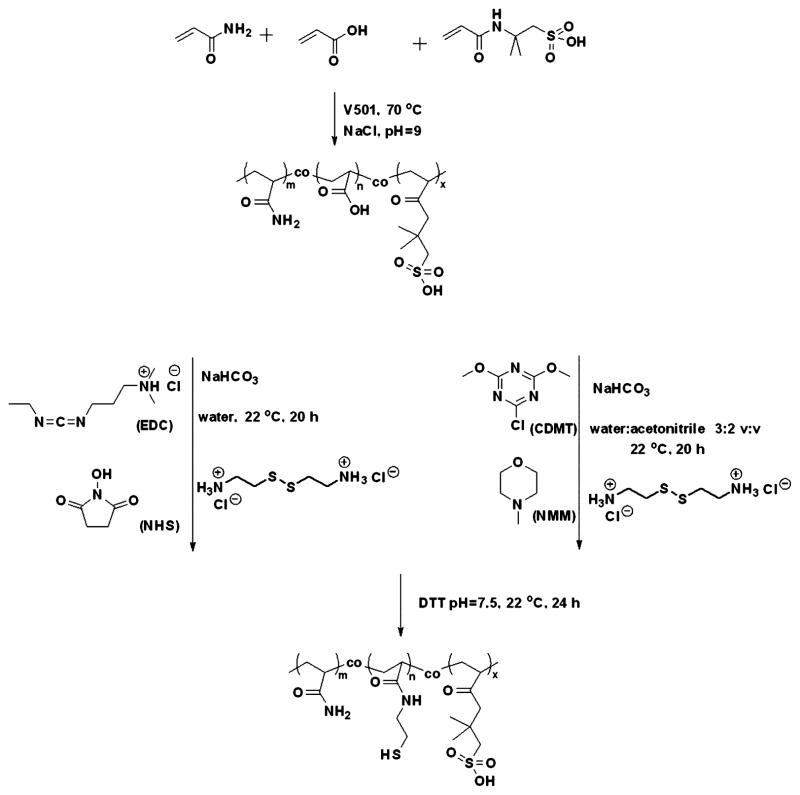
To introduce thiol groups to the copoly(AM-co-AMPS), we copolymerized a disulfide cross-linker with AM and AMPS. This is a one-step synthesis that allows stoichiometric incorporation of thiol groups into the copolymer. A disulfide-containing cross-linker, BAC, was copolymerized with AM and AMPS to form a cross-linked copolymer. The copolymer was then reduced to generate the pendant thiol groups (Figure 4).
Figure 4.
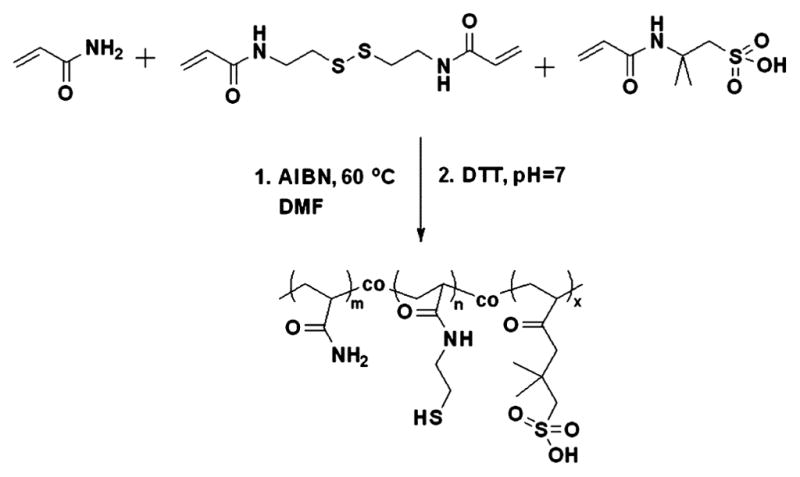
Synthesis of a thiol- and sulfonate-containing copolymer by copolymerization of AM, AMPS, and BAC in DMF, followed by reduction of the disulfide bonds using DTT.
Since BAC is not soluble in water, in this polymerization we used DMF as a solvent and AIBN as an initiator. Sodium chloride and sodium hydroxide were not used because the sulfonic acids are relatively less disassociated in DMF. By using the conventional free-radical polymerization, we were able to obtain copolymers with a molecular weight (Mn = 194 kDa) that, according to our previous work, is suitable for making hydrogels.37 The thiol content (5.0–5.1 mol %), which was measured by a 2-nitro-5-thiosulobenzoate (NTSB) assay, was very close to the feed ratio (5.0 mol %), suggesting stoichiometric incorporation of thiol groups into the copolymer. The content of AMPS (20.6 mol %) was also close to the feed ratio (20 mol %), as determined by 1H NMR. A reversible addition–fragmentation chain-transfer (RAFT) polymerization of AM, AMPS, and BAC was also done.
A 4-cyanopentanoic acid-4-dithiobenzoate chain transfer agent was also added to achieve a RAFT living polymerization. We kept the molar ratio of monomer to the chain-transfer agent at 1000 and 2000 to obtain copolymers with molecular weights of ~100 and 200 kDa. The RAFT living polymerizations were carried out at 70 °C for 2 days, during which they steadily formed precipitation. However, the molecular weights of copolymers were only ~25% of the theoretical values, suggesting that RAFT living polymerization is not suitable for synthesis of copoly(AM-co-AMPS-co-BAC) with high molecular weight.
3.2.2. Thiolation of Copoly(AM-co-AMPS-co-AA)
Although the method of preparing copoly(AM-co-AMPS-co-BAC) is a convenient one-step synthesis and allows stoichiometric incorporation of thiol groups, a hazardous organic solvent, DMF, was used. To minimize the consumption of organic solvent, we developed a more environmentally friendly method of preparing thiol- and sulfonate-containing copolymer in the aqueous phase. We copolymerized AA with AM and AMPS, then derivatized the carboxylates with cystamine. These two steps were both done in aqueous solution. Copoly(AM-co-AMPS-co-AA) (AM/AMPS/AA = 7:2:1) were synthesized with varied amounts of V501 initiator to obtain different molecular weights (Table 2). As expected, we were able to alter the molecular weight by varying the molar ratio of the initiator. However, the molecular weight distributions were also increased with increasing the amount of initiator as a consequence of side reactions caused by the higher concentration of free radicals.
Table 2.
GPC Characterization of the Copoly(AM-co-AMPS-co-AA)
| mol % of V501 | Mn (kDa) | Mw (kDa) | PDI | yield (%) |
|---|---|---|---|---|
| 0.008 | 468 | 885 | 1.89 | 91 |
| 0.006 | 666 | 997 | 1.50 | 89 |
| 0.004 | 826 | 1153 | 1.40 | 90 |
| 0.002 | 935 | 1267 | 1.36 | 91 |
Two methods, carbodiimide- and triazine-based condensations, have been used to derivatize the carboxylates on the copoly(AM-co-AMPS-co-AA) (Figure 4).
Carbodiimide-based condensation is a well-known method for derivatization of carboxylates.38 We used EDC as a carbodiimide activator for aqueous synthesis. In addition, NHS was used to form a stable NHS-ester intermediate and thus achieve more efficient derivatization. By using different formulations, we successfully derivatized 23%–39% of the carboxylates in copoly(AM-co-AMPS-co-AA) (Table 3). However, the efficiency of EDC, calculated by the molar ratio of (modified COOH)/(feed EDC), was only 3.9–5.8% due to the instability of EDC and intermediate in aqueous solution.
Table 3.
Molar Ratios of the Reactants and Modification% of the Carboxylates Using a Carbodiimide-Based Condensation
| –COOH | EDC | NHS | –NH2 | modification (%) | efficiency of EDCa (%) |
|---|---|---|---|---|---|
| 1 | 4 | 2 | 3 | 23 | 5.8 |
| 1 | 6 | 3 | 4 | 30 | 5.0 |
| 1 | 10 | 5 | 5 | 39 | 3.9 |
Calculated by the molar ratio of (modified COOH)/(feed EDC).
Although the carbodiimide method is widely used, there is still room for improvement to overcome the low efficiency of carbodiimide. A triazine-based coupling method, which involves an intermediate postulated in the concept of “superactive ester,” has gained much attention.39 Because of its excellent efficiency and low cost, the triazine-based coupling method has been used to synthesize or modify polysaccharides,40,41 peptides,42,43 and nucleic acids.44–46 In this study, we used CDMT as a triazinebased activator to introduce the thiol group to the copoly(AM-co-AMPS-co-AA). Two formulations were tried; the modification percentages were 24 and 43% (Table 4). The efficiencies of CDMT were 16 and 14%, which were higher than that of EDC.
Table 4.
Molar Ratios of the Reactants and Modification% of the Carboxylates Using a Triazine-Based Condensation
| –COOH | CDMT | NHS | –NH2 | modification (%) | efficiency of CDMT (%) |
|---|---|---|---|---|---|
| 1 | 1.5 | 1.5 | 1.1 | 24 | 16 |
| 1 | 3 | 3 | 1.1 | 43 | 14 |
3.3. Synthesis of Injectable Sulfonate-Containing Hydrogels via Thiol–Ene Click Reaction or Michael Addition
To make a hydrogel, the copolymer can be cross-linked by making the thiol react with ene groups either by thiol–ene click reaction in the presence of a free radical initiator or by Michael addition with the base. We tried to directly introduce side allyl groups to the copolymers as sites for cross-link by a carbodiimide or triazine coupling method. However, the product suffered from low yield and low purity. Therefore, we used a 4-arm PEG-acrylamide as the ene-containing cross-linker to make hydrogels (Figure 5). Three different cross-linking methods were used (Table 5): thiol–ene reaction with redox initiators APS and TEMED; Michael addition with an organic base, TEA; and thiol–ene reaction with photoinitiators, riboflavin, and arginine, a well-known combination for photoinitiation.47 All three methods resulted in the formation of gels. However, other possible side reactions could also form gels, which include a cross-link between the copolymer and the 4-arm PEG, free radical polymerization of the acrylamide, and oxidation of the thiol groups.
Figure 5.
Thiolation of copoly(AM-co-AMPS-co-AA) via carbodiimide and triazine methods.
Table 5.
Formation of Gels Made with a 4-Arm PEG-Acrylamide and a Thiol/Sulfonate-Containing Copolymer
| 4-arm PEG | thiol/sulfonate copolymer | initiator | gelation time |
|---|---|---|---|
| 10 mg/mL | 0 | APS/TEMED | no gel |
| 0 | 40 mg/mL | none | no gel |
| 10 mg/mL | 40 mg/mL | APS/TEMED | <1 min |
| 10 mg/mL | 40 mg/mL | TEA | ~30 min |
| 10 mg/mL | 40 mg/mL | riboflavin/arginine/UV | <1 min |
To determine the major mechanism leading to the formation of gels, solutions containing only the copolymer or the 4-arm PEG and redox initiator were prepared. None of the one-component solutions formed a gel, suggesting that the gels were formed by cross-link between the copolymer and the 4-arm PEG. Although three different methods were used to investigate the reactivity of the cross-link reaction, photo-initiation with riboflavin and arginine would be the most biocompatible one in vivo. Photo-cross-link has been extensively reported for making in situ forming hydrogels using transdermal illumination,48,49 transarterial illuminations, 50,51 laparoscopic devices,52,53 or cathether.54,55 Thus, only photoinitiation was used for the rest of this work.
3.4. Swelling and Mechanical Properties of the Injectable Sulfonate-Containing Hydrogels
The most important characteristic of hydrogels is their ability to absorb a significant amount of water. This provides hydrogels with the permeability of nutrients and metabolites, biomimetic elastic, and surface properties, which are highly desirable for tissue engineering.
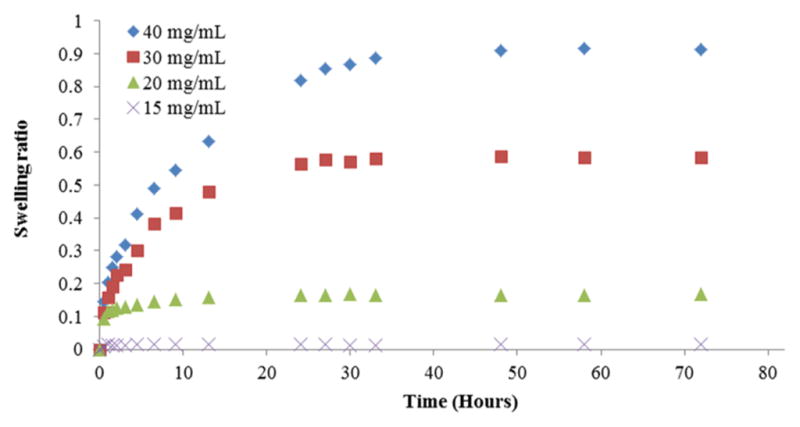
We assessed the water absorbance of our sulfonate-containing hydrogels with different concentrations of the copolymer by measuring their kinetic swelling profiles in 1× PBS at 37 °C (Figure 6). The swelling ratio increased with increasing concentrations of copoly(AM-co-AMPS-co-BAC) (AM/AMPS/BAC = 7.5:2:0.5) in the hydrogel. Hydrogels containing 15 mg/mL of copolymer reached equilibrium swelling (1.3%) within 1 h. Hydrogels containing 20 mg/mL of copolymer swelled ~16% in 12 h. Hydrogels with 30 mg/mL of copolymer swelled ~58% in about 1 day, while hydrogels with 40 mg/mL of copolymer swelled ~91% in 2 days. We attribute this to increased proximity of electrostatic repulsion, which drives the expansion in the polymeric network. In addition, more ionic groups make a hydrogel more hydrophilic and thus able to absorb more water.
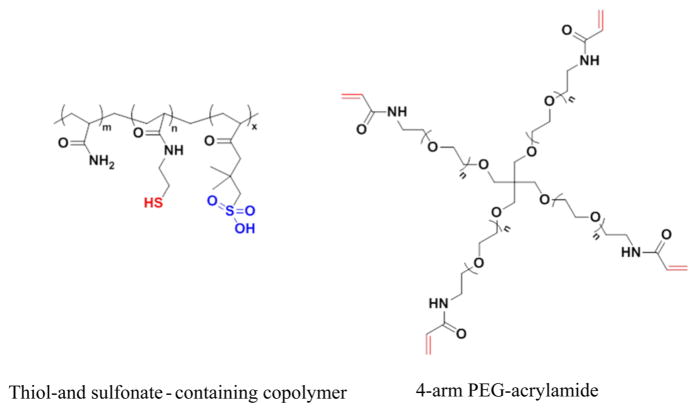
Figure 6.
Structures of the components of sulfonate-containing hydrogel: the thiol- and sulfonate-containing copolymer and the 4-arm PEG-acrylamide.
One critical parameter for designing hydrogels in tissue engineering is to mimic the mechanical properties of natural tissues. To replace a natural tissue, a hydrogel needs to provide appropriate stiffness and deformation properties.56 We obtained wide range of storage moduli for sulfonate-containing hydrogels by varying the concentration of copolymer (Figure 7). It is noteworthy that by increasing the concentration of the two copolymers from 10 to 40 mg/mL, the storage moduli increased dramatically from 33 to 1800 Pa. Therefore, the mechanical properties of a gel could be adjusted by varying the concentration of copolymer and the density of the cross-linker and, thus, match the mechanical properties of natural tissues.
3.5. Cytotoxicity of the Injectable Sulfonate-Containing Hydrogels
The biocompatibility of the sulfonate-containing hydrogel was tested using ECIS and MTT assay. ECIS provides electrical information, which can be interpreted in terms of cell physiology. This real-time, noninvasive method measures the impedance across electrodes by using alternating current at various frequencies. When cells are growing on the electrodes, the impedance is affected by the number, morphology and attachment of cells covering the electrodes. Other publications have favorably compared the results of MTT and ECIS.37,57,58 ECIS produces abundant data and provides unbiased information on changes in impedance or resistance for the time course of the cellular response. Responses are based on cell density and changes in cell morphology associated with cell death. The response can be modeled to show adhesion and barrier responses in addition to cell growth.
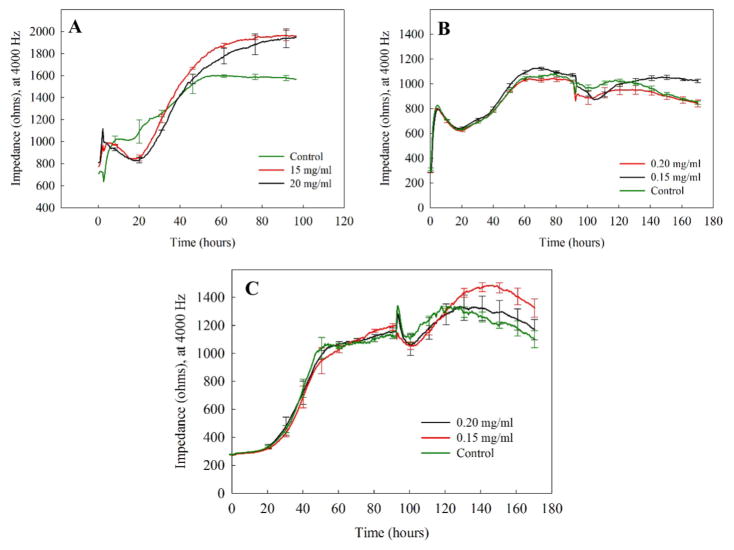
We chose three different cell lines, epithelial (ARPE-19), NIH 3T3, and CHO cells, as our modeling cells because of our interest in using hydrogels as a vitreous substitute. The tested concentrations of copolymers were 15 and 20 mg/mL, based on the mechanical properties of natural vitreous59,60 as compared to the hydrogels. For retinal cells, as shown in Figure 8A, the spikes at 24 h in the ECIS resistance curve were due to media change and did not reflect a cell response. As compared to the control, the addition of hydrogel caused a decrease in resistance during 24–42 h, after which resistance recovered rapidly. The initial decline, which is commonly observed,37,6–63 occurred as the cells adapted to the presence of the hydrogel. As explained previously, the time requirement for 3T3 cells and CHO cells to reach confluency is more than that required by ARPE-19 cells. It took both 3T3 and CHO cells almost 50 h to become confluent (Figure 8B,C). By the end of the 90th hour, the hydrogels were added to 3T3 and CHO cells. Similarly, the spikes at 90 h in the ECIS resistance curve were due to media change and did not reflect cell response.
Figure 8.
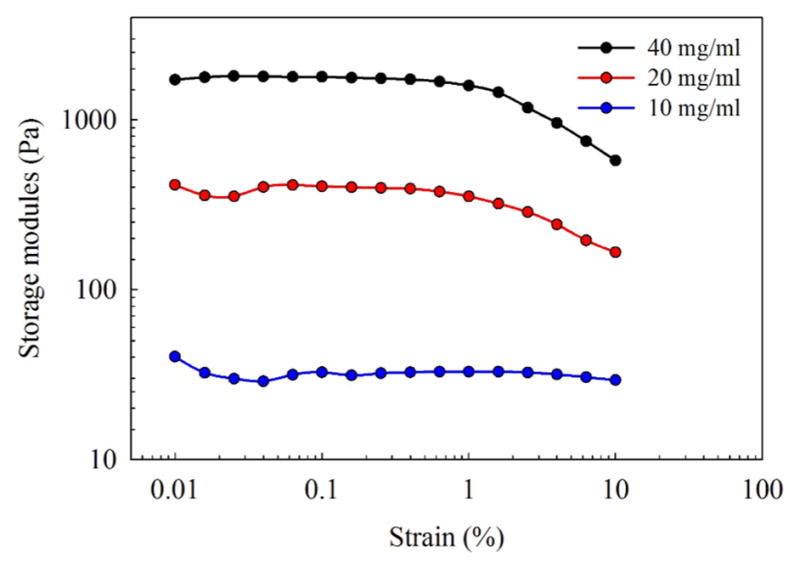
Sulfonate-containing hydrogel with increasing concentrations of copolymer (10–40 mg/mL) showed increasing storage moduli.
According to the ECIS resistance curves (Figure 8), no cells exposed to sulfonate-containing hydrogel at the tested concentrations showed a decline in resistance, indicating that the hydrogels did not adversely affect morphology or cell attachment.
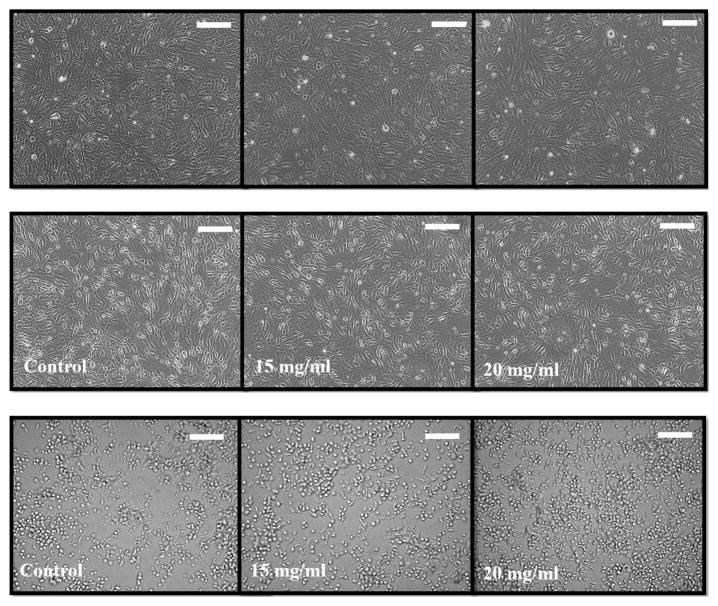
The ECIS results were substantiated by MTT assay of all three cell lines (Figure 9), which showed greater than 80% viability for the hydrogels. CHO cells were slightly more compatible with the hydrogels (both 15 and 20 mg/mL) than were 3T3 and ARPE-19 cells. The images of cells exposed to the hydrogels at both tested concentrations showed similar morphology and cell number compared to control cells (Figure 10). Cells did not appear to be rounding up or sloughing off, which would indicate cell death. It can be concluded that both hydrogel concentrations, 15 and 20 mg/mL, were compatible with all three cell types tested (Figure 11).
Figure 9.
Resistance measurements of (A) ARPE-19, (B) NIH 3T3, and (C) CHO cells plated at 20000 cells/well and exposed to sulfonate-containing hydrogel at 15 and 20 mg/mL showed excellent recovery and a minor lag phase. Unlike ARPE-19 cells, NIH 3T3, and CHO cells require almost 50 h to reach confluency; therefore, the hydrogel addition points for NIH 3T3 and CHO cells were different from that for ARPE-19 cells. Because media change is not possible following the addition of the hydrogels, a slight decrease in cell attachment was observed. Overall, the presence of hydrogels did not show any toxic effect on the cell types tested. Values are expressed in mean ± SEM, with each condition tested (n = 4).
Figure 10.
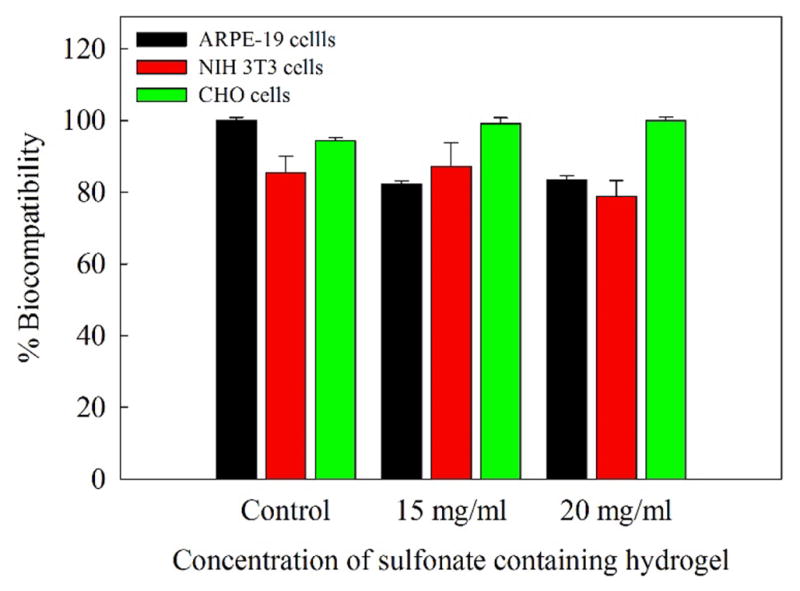
Overall, the viability of ARPE-19, NIH 3T3, and CHO cells exposed to sulfonate-containing hydrogels at 15 and 20 mg/mL for 96 h were greater than 80%. CHO cells were the least affected. Each column represents the average of four wells. Error bars represent the standard error as established by the standard deviation divided by the square root of the well number (n = 4).
Figure 11.
Inverted light microscopy images of (A) ARPE-19, (B) NIH 3T3, and (C) CHO cells exposed for 72 h to sulfonate-containing hydrogel at different concentrations showed similar morphology and cell number compared to control cells. The images of confluent cell monolayers were obtained using an Olympus CKX41 inverted microscope with ×10 magnification. The visualization window is 60 × 60 μm; the scale bars correspond to 15 μm.
4. CONCLUSIONS
We have described the various ways of synthesizing injectable sulfonate-containing hydrogels based on AMPS copolymers. It was found that AMPS incorporated into the polymer chain more slowly than did AM. In addition, increasing the molar percentage of AMPS to the copolymer decreased the molecular weight. It was also determined that the addition of sodium chloride to the polymerization enhanced the reactivity of AMPS, with a corresponding increase in molecular weight. We introduced thiol groups to the copolymers by functionalizing the carboxylates by carbodiimide or triazine coupling reaction or by directly copolymerizing a disulfide-containing monomer. We cross-linked the copolymers with a 4-arm acrylamide PEG to make hydrogels and were able to alter the swelling and mechanical properties of the sulfonate-containing hydrogels. Preliminarily, the sulfonate-containing copolymers were compatible with ARPE-19, 3T3, and CHO cells based on ECIS and MTT assays and cell images. The biological activity will be explored in future studies.
Acknowledgments
This research was supported by the NIH Grant EY021620 and Department of Veterans Affairs Rehab Merit Review Grant RX000657-01 to N.R. Also, Research to Prevent Blindness, Inc. and NIH Core Grant P30 EY02687 facilities supported parts of the research. We thank Mr. Paul Hamilton for conducting the GPC test.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Delivery Rev. 2002;54(1):3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 2.Liu XX, Tong Z, Hu O. Swelling Equilibria of Hydrogels with Sulfonate Groups in Water and in Aqueous Salt-Solutions. Macromolecules. 1995;28(11):3813–3817. [Google Scholar]
- 3.Fajardo AR, Silva MB, Lopes LC, Piai JF, Rubira AF, Muniz EC. Hydrogel based on an alginate-Ca2+/chondroitin sulfate matrix as a potential colon-specific drug delivery system. RSC Adv. 2012;2(29):11095–11103. [Google Scholar]
- 4.Hussain T, Ansari M, Ranjha NM, Khan IU, Shahzad Y. Chemically Cross-Linked Poly(acrylic-co-vinylsulfonic) Acid Hydrogel for the Delivery of Isosorbide Mononitrate. Sci World J. 2013;2013:1. doi: 10.1155/2013/340737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh JKF, Matthew HWT. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 6.Phillips TJ. New skin for old: developments in biological skin substitutes. Arch Dermatol. 1998;134(3):344–9. doi: 10.1001/archderm.134.3.344. [DOI] [PubMed] [Google Scholar]
- 7.Hardingham T. Chondroitin sulfate and joint disease. Osteoarthr Cartilage. 1998;6:3–5. doi: 10.1016/s1063-4584(98)80004-6. [DOI] [PubMed] [Google Scholar]
- 8.Khanlari A, Detamore MS, Gehrke SH. Increasing Cross-Linking Efficiency of Methacrylated Chondroitin Sulfate Hydrogels by Copolymerization with Oligo(Ethylene Glycol) Diacrylates. Macromolecules. 2013;46(24):9609–9617. [Google Scholar]
- 9.Oprea AM, Profire L, Lupusoru CE, Ghiciuc CM, Ciolacu D, Vasile C. Synthesis and characterization of some cellulose/chondroitin sulphate hydrogels and their evaluation as carriers for drug delivery. Carbohydr Polym. 2012;87(1):721–729. doi: 10.1016/j.carbpol.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 10.Oprea AM, Ciolacu D, Neamtu A, Mungiu OC, Stoica B, Vasile C. Cellulose/Chondroitin Sulfate Hydrogels: Synthesis, Drug Loading/Release Properties and Biocompatibility. Cell Chem Technol. 2010;44(9):369–378. [Google Scholar]
- 11.Lee CT, Kung PH, Lee YD. Preparation of poly(vinyl alcohol)-chondroitin sulfate hydrogel as matrices in tissue engineering. Carbohydr Polym. 2005;61(3):348–354. [Google Scholar]
- 12.Matsusaki M, Serizawa T, Kishida A, Akashi M. Novel functional biodegradable polymer. III. The construction of poly-(gamma-glutamic acid)-sulfonate hydrogel with fibroblast growth factor-2 activity. J Biomed Mater Res, Part A. 2005;73A(4):485–491. doi: 10.1002/jbm.a.30305. [DOI] [PubMed] [Google Scholar]
- 13.Christman KL, Vazquez-Dorbatt V, Schopf E, Kolodziej CM, Li RC, Broyer RM, Chen Y, Maynard HD. Nanoscale Growth Factor Patterns by Immobilization on a Heparin-Mimicking Polymer. J Am Chem Soc. 2008;130(49):16585–16591. doi: 10.1021/ja803676r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohan P, Schols D, Baba M, Declercq E. Sulfonic-Acid Polymers as a New Class of Human-Immunodeficiency-Virus Inhibitors. Antiviral Res. 1992;18(2):139–150. doi: 10.1016/0166-3542(92)90034-3. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 16.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 17.Marrelli A, Cipriani P, Liakouli V, Carubbi F, Perricone C, Perricone R, Giacomelli R. Angiogenesis in rheumatoid arthritis: a disease specific process or a common response to chronic inflammation? Autoimmun Rev. 2011;10(10):595–8. doi: 10.1016/j.autrev.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Boehm BO, Lang G, Feldmann B, Kurkhaus A, Rosinger S, Volpert O, Lang GK, Bouck N. Proliferative diabetic retinopathy is associated with a low level of the natural ocular antiangiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor. a pilot study. Horm Metab Res. 2003;35(6):382–6. doi: 10.1055/s-2003-41362. [DOI] [PubMed] [Google Scholar]
- 19.Silva-Correia J, Miranda-Goncalves V, Salgado AJ, Sousa N, Oliveira JM, Reis RM, Reis RL. Angiogenic Potential of Gellan-Gum-Based Hydrogels for Application in Nucleus Pulposus Regeneration: In Vivo Study. Tissue Eng, Part A. 2012;18(11–12):1203–1212. doi: 10.1089/ten.TEA.2011.0632. [DOI] [PubMed] [Google Scholar]
- 20.Liekens S, Neyts J, Degreve B, DeClercq E. The sulfonic acid polymers PAMPS [poly(2-acrylamido-2-methyl-1-propanesulfonic acid)] and related analogues are highly potent inhibitors of angiogenesis. Oncol Res. 1997;9(4):173–181. [PubMed] [Google Scholar]
- 21.Garcia-Fernandez L, Aguilar MR, Fernandez MM, Lozano RM, Gimenez G, San Roman J. Antimitogenic Polymer Drugs Based on AMPS: Monomer Distribution-Bioactivity Relationship of Water-Soluble Macromolecules. Biomacromolecules. 2010;11(3):626–634. doi: 10.1021/bm901194e. [DOI] [PubMed] [Google Scholar]
- 22.Bugatti A, Urbinati C, Ravelli C, De Clercq E, Liekens S, Rusnati M. Heparin-mimicking sulfonic acid polymers as multitarget inhibitors of human immunodeficiency virus type 1 tat and gp120 proteins. Antimicrob Agents Chemother. 2007;51(7):2337–2345. doi: 10.1128/AAC.01362-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko DY, Shinde UP, Yeon B, Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog Polym Sci. 2013;38(3–4):672–701. [Google Scholar]
- 24.Yu L, Ding JD. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37(8):1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 25.Cao LP, Cao B, Lu CJ, Wang GW, Yu L, Ding JD. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J Mater Chem B. 2015;3(7):1268–1280. doi: 10.1039/c4tb01705f. [DOI] [PubMed] [Google Scholar]
- 26.Wan WB, Li QT, Gao HY, Ge LP, Liu YQ, Zhong W, Ouyang J, Xing M. BMSCs laden injectable aminodiethoxypropane modified alginate-chitosan hydrogel for hyaline cartilage reconstruction. J Mater Chem B. 2015;3(9):1990–2005. doi: 10.1039/c4tb01394h. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Wang L, Deng LD, Hu RJ, Dong AJ. Performance optimization of injectable chitosan hydrogel by combining physical and chemical triple crosslinking structure. J Biomed Mater Res, Part A. 2013;101(3):684–693. doi: 10.1002/jbm.a.34364. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, Bae JW, Lee JW, Suh W, Park KD. Enzyme-catalyzed in situ forming gelatin hydrogels as bioactive wound dressings: effects of fibroblast delivery on wound healing efficacy. J Mater Chem B. 2014;2(44):7712–7718. doi: 10.1039/c4tb01111b. [DOI] [PubMed] [Google Scholar]
- 29.Sakai S, Tsumura M, Inoue M, Koga Y, Fukano K, Taya M. Polyvinyl alcohol-based hydrogel dressing gellable on-wound via a co-enzymatic reaction triggered by glucose in the wound exudate. J Mater Chem B. 2013;1(38):5067–5075. doi: 10.1039/c3tb20780c. [DOI] [PubMed] [Google Scholar]
- 30.Thannhauser TW, Konishi Y, Scheraga HA. Analysis for Disulfide Bonds in Peptides and Proteins. Methods Enzymol. 1987;143:115–119. doi: 10.1016/0076-6879(87)43020-6. [DOI] [PubMed] [Google Scholar]
- 31.Guo X, Park H, Liu GP, Liu W, Cao YL, Tabata Y, Kasper FK, Mikos AG. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials. 2009;30(14):2741–2752. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adzima BJ, Tao YH, Kloxin CJ, DeForest CA, Anseth KS, Bowman CN. Spatial and temporal control of the alkyne-azide cycloaddition by photoinitiated Cu(II) reduction. Nat Chem. 2011;3(3):256–259. doi: 10.1038/nchem.980. [DOI] [PubMed] [Google Scholar]
- 33.Haines LA, Rajagopal K, Ozbas B, Salick DA, Pochan DJ, Schneider JP. Light-activated hydrogel formation via the triggered folding and self-assembly of a designed peptide. J Am Chem Soc. 2005;127(48):17025–17029. doi: 10.1021/ja054719o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truong VX, Barker IA, Tan M, Mespouille L, Dubois P, Dove AP. Preparation of in situ-forming poly(5-methyl-5-allyloxycarbonyl-1,3-dioxan-2-one)-poly(ethylene glycol) hydrogels with tuneable swelling, mechanical strength and degradability. J Mater Chem B. 2013;1(2):221–229. doi: 10.1039/c2tb00148a. [DOI] [PubMed] [Google Scholar]
- 35.Bakota EL, Wang Y, Danesh FR, Hartgerink JD. Injectable Multidomain Peptide Nanofiber Hydrogel as a Delivery Agent for Stem Cell Secretome. Biomacromolecules. 2011;12(5):1651–1657. doi: 10.1021/bm200035r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon HJ, Ko DY, Park MH, Joo MK, Jeong B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem Soc Rev. 2012;41(14):4860–4883. doi: 10.1039/c2cs35078e. [DOI] [PubMed] [Google Scholar]
- 37.Liang J, Struckhoff JJ, Du H, Hamilton PD, Ravi N. Synthesis and characterization of in situ forming anionic hydrogel as vitreous substitutes. J Biomed Mater Res, Part B. 2016 doi: 10.1002/jbm.b.33632. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santhanam S, Liang J, Baid R, Ravi N. Investigating thiol-modification on hyaluronan via carbodiimide chemistry using response surface methodology. J Biomed Mater Res, Part A. 2015;103(7):2300–2308. doi: 10.1002/jbm.a.35366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunishima M, Kitamura M, Tanaka H, Nakakura I, Moriya T, Hioki K. Study of 1,3,5-Triazine-Based Catalytic Amide-Forming Reactions: Effect of Solvents and Basicity of Reactants. Chem Pharm Bull. 2013;61(8):882–886. doi: 10.1248/cpb.c13-00368. [DOI] [PubMed] [Google Scholar]
- 40.Worthen AJ, Lapitsky Y. Stabilization of bioderived surfactant/polyelectrolyte complexes through surfactant conjugation to the biopolymer. Colloid Polym Sci. 2011;289(14):1589–1596. [Google Scholar]
- 41.Farkas P, Bystricky S. Efficient activation of carboxyl polysaccharides for the preparation of conjugates. Carbohydr Polym. 2007;68(1):187–190. [Google Scholar]
- 42.Higashibayashi S, Kohno M, Goto T, Suziki K, Mori T, Hashimoto K, Nakata M. Synthetic studies on thiostrepton family of peptide antibiotics: synthesis of the pentapeptide segment containing dihydroxyisoleucine, thiazoline and dehydroamino acid. Tetrahedron Lett. 2004;45(19):3707–3712. [Google Scholar]
- 43.Tachibana Y, Monde K, Nishimura SI. Sequential glycoproteins: Practical method for the synthesis of antifreeze glycoprotein models containing base labile groups. Macromolecules. 2004;37(18):6771–6779. [Google Scholar]
- 44.Gartner ZJ, Kanan MW, Liu DR. Expanding the reaction scope of DNA-templated synthesis. Angew Chem, Int Ed. 2002;41(10):1796–1800. doi: 10.1002/1521-3773(20020517)41:10<1796::aid-anie1796>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 45.Chhabra R, Sharma J, Liu Y, Yan H. Addressable molecular tweezers for DNA-templated coupling reactions. Nano Lett. 2006;6(5):978–983. doi: 10.1021/nl060212f. [DOI] [PubMed] [Google Scholar]
- 46.Li XY, Gartner ZJ, Tse BN, Liu DR. Translation of DNA into synthetic N-acyloxazolidines. J Am Chem Soc. 2004;126(16):5090–5092. doi: 10.1021/ja049666+. [DOI] [PubMed] [Google Scholar]
- 47.Encinas MV, Rufs AM, Bertolotti S, Previtali CM. Free radical polymerization photoinitiated by riboflavin/amines. Effect of the amine structure. Macromolecules. 2001;34(9):2845–2847. [Google Scholar]
- 48.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci U S A. 1999;96(6):3104–3107. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valenta C, Auner BG. The use of polymers for dermal and transdermal delivery. Eur J Pharm Biopharm. 2004;58(2):279–289. doi: 10.1016/j.ejpb.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 50.An YJ, Hubbell JA. Intraarterial protein delivery via intimally-adherent bilayer hydrogels. J Controlled Release. 2000;64(1–3):205–215. doi: 10.1016/s0168-3659(99)00143-1. [DOI] [PubMed] [Google Scholar]
- 51.Lyman MD, Melanson D, Sawhney AS. Characterization of the formation of interfacially photopolymerized thin hydrogels in contact with arterial tissue. Biomaterials. 1996;17(3):359–364. doi: 10.1016/0142-9612(96)85574-8. [DOI] [PubMed] [Google Scholar]
- 52.Hillwest JL, Chowdhury SM, Dunn RC, Hubbell JA. Efficacy of a Resorbable Hydrogel Barrier, Oxidized Regenerated Cellulose, and Hyaluronic-Acid in the Prevention of Ovarian Adhesions in a Rabbit Model. Fertil Steril. 1994;62(3):630–634. doi: 10.1016/s0015-0282(16)56956-8. [DOI] [PubMed] [Google Scholar]
- 53.Hillwest JL, Chowdhury SM, Sawhney AS, Pathak CP, Dunn RC, Hubbell JA. Prevention of Postoperative Adhesions in the Rat by in-Situ Photopolymerization of Bioresorbable Hydrogel Barriers. Obstet Gynecol. 1994;83(1):59–64. [PubMed] [Google Scholar]
- 54.Hillwest JL, Chowdhury SM, Slepian MJ, Hubbell JA. Inhibition of Thrombosis and Intimal Thickening by in-Situ Photopolymerization of Thin Hydrogel Barriers. Proc Natl Acad Sci U S A. 1994;91(13):5967–5971. doi: 10.1073/pnas.91.13.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West JL, Hubbell JA. Separation of the arterial wall from blood contact using hydrogel barriers reduces intimal thickening after balloon injury in the rat: The roles of medial and luminal factors in arterial healing. Proc Natl Acad Sci U S A. 1996;93(23):13188–13193. doi: 10.1073/pnas.93.23.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao YH, Friis EA, Gehrke SH, Detamore MS. Mechanical Testing of Hydrogels in Cartilage Tissue Engineering: Beyond the Compressive Modulus. Tissue Eng, Part B. 2013;19(5):403–412. doi: 10.1089/ten.teb.2012.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kling B, Bucherl D, Palatzky P, Matysik FM, Decker M, Wegener J, Heilmann J. Flavonoids, Flavonoid Metabolites, and Phenolic Acids Inhibit Oxidative Stress in the Neuronal Cell Line HT-22 Monitored by ECIS and MTT Assay: A Comparative Study. J Nat Prod. 2014;77(3):446–454. doi: 10.1021/np400518k. [DOI] [PubMed] [Google Scholar]
- 58.Yun YH, Dong ZY, Tan ZQ, Schulz MJ. Development of an electrode cell impedance method to measure osteoblast cell activity in magnesium-conditioned media. Anal Bioanal Chem. 2010;396(8):3009–3015. doi: 10.1007/s00216-010-3521-2. [DOI] [PubMed] [Google Scholar]
- 59.Nickerson CS, Park J, Kornfield JA, Karageozian H. Rheological properties of the vitreous and the role of hyaluronic acid. J Biomech. 2008;41(9):1840–1846. doi: 10.1016/j.jbiomech.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Nickerson CS, Karageozian HL, Park J, Kornfield JA. Internal tension: A novel hypothesis concerning the mechanical properties of the vitreous humor. Macromol Symp. 2005;227:183–189. [Google Scholar]
- 61.Santhanam S, Liang J, Struckhoff J, Hamilton PD, Ravi N. Biomimetic hydrogel with tunable mechanical properties for vitreous substitutes. Acta Biomater. 2016;43:327–337. doi: 10.1016/j.actbio.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karakoçak BB, Raliya R, Davis JT, Chavalmane S, Wang WN, Ravi N, Biswas P. Biocompatibility of gold nanoparticles in retinal pigment epithelial cell line. Toxicol In Vitro. 2016;37:61–69. doi: 10.1016/j.tiv.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Xiao CD, Lachance B, Sunahara G, Luong JHT. Assessment of cytotoxicity using electric cell-substrate impedance sensing: Concentration and time response function approach. Anal Chem. 2002;74(22):5748–5753. doi: 10.1021/ac025848f. [DOI] [PubMed] [Google Scholar]