Abstract
Objectives
Although HIV+ individuals may be at increased risk of alcohol-related cognitive impairment, the relations between drinking level and cognitive performance in these individuals are not well understood. We examined whether higher levels of recent drinking in HIV+ individuals were associated with poorer cognitive performance, particularly in executive functioning (EF) and memory.
Methods
We administered a comprehensive cognitive battery to 120 seropositive subjects (101 men) who reported alcohol consumption in the preceding 90 days. Participants were excluded if they were seeking alcohol treatment or showed evidence of dementia. Using the computerized CogState battery, we measured performance in EF, verbal learning/memory, visual learning/memory, attention, working memory, and psychomotor speed. The computerized Iowa Gambling Task was used to assess decision-making.
Results
The HIV+ subjects showed significantly slower psychomotor speed than a normative sample. Although across most domains, neurocognitive performance in our sample was not significantly associated with recent alcohol consumption, performance on the CogState measures of visual memory and attention was significantly poorer with a higher level of drinking in the past 3 months and a current alcohol use disorder, respectively.
Conclusions
Although cognitive weaknesses were detectable among these non-treatment-seeking HIV+ drinkers, the level of alcohol consumption was not a primary determinant of neurocognitive performance in this group. A comprehensive profile analysis may be most valuable for detecting cognitive strengths and weaknesses given the heterogeneity of this population. Longitudinal studies are needed to examine the potential additive or synergistic effects of heavy drinking and HIV seropositivity on cognitive performance.
Keywords: alcohol, HIV, neuropsychology, neurocognitive functioning, seropositivity
INTRODUCTION
HIV is associated with changes in neurocognitive functioning, the hallmark of which is frontostriatal deficits, as sub-cortical pathways involving the prefrontal cortex (PFC) appear especially susceptible to HIV-related damage. Thus, a central aspect of HIV-related cognitive impairment is a pattern of deficits in executive functioning (EF) (1, 2). In cross-sectional studies, individuals with HIV exhibited poorer performance on tasks of problem solving, cognitive flexibility, planning, inhibition, and decision making than healthy control groups (3–6). Mild-to-moderate learning and memory impairment with a mixed encoding and retrieval profile are also frequently observed in HIV+ individuals. consistent with frontostriatal dysfunction (3, 6–9). Learning and encoding difficulties likely reflect impairment in the mechanisms of EF, such as faulty search and retrieval strategies (e.g., failure to use semantic clustering on list-learning tasks) (3, 6). Even HIV+ individuals treated with highly active antiretroviral therapy (HAART), despite having acceptable CD4 counts and undetectable viral loads, can display cognitive dysfunction (10–12). Nonetheless, patterns of cognitive deficits tend to be heterogeneous across HIV+ individuals and may be moderated by other factors, including depression, head injury, age, premorbid IQ, education, and the use of alcohol and drugs (9).
Heavy drinking is common among HIV-infected persons and may reflect a premorbid risk factor for infection (13). Chronic heavy drinking, independent of HIV infection, is associated with deficits in learning, memory, and EF (14), and prior research suggests that HIV+ heavy drinkers may show greater deficits in EF, verbal and visuospatial learning and memory, and psychomotor speed than individuals with either HIV infection or alcohol use disorder (AUD) alone (15–18). However, studies examining the effect of alcohol use on neurocognitive functioning in HIV infection have produced variable results, or have been specific to a single cognitive domain.
Because heavy drinking and neurocognitive impairment are prevalent and debilitating factors in seropositive individuals, we examined the extent to which the level of alcohol consumed was associated with global cognitive functioning in HIV+ individuals. To do so, we used a computerized neuropsychological battery to examine the associations between recent alcohol use and cognitive performance in a sample of HIV+ individuals. In contrast to prior studies that examined performance in a single cognitive domain, we used a comprehensive battery to assess EF, visual and verbal memory, attention, psychomotor processing speed (reaction time), working memory, and decision-making. We also examined a range of demographic and premorbid characteristics that could affect cognition.
We hypothesized that, in this sample of HIV+ men and women, given the shared vulnerability to frontstriatal dysfunction associated with both HIV and alcohol use, performance on tests of EF and memory would be inversely related to the level of alcohol consumed in the three months preceding study participation.
MATERIALS AND METHODS
Overview
We used a cross-sectional design in which participants underwent a psychiatric diagnostic interview, completed self-report measures, and were administered a cognitive test battery. Prospective participants were screened by telephone to assess basic inclusion and exclusion criteria. Eligible individuals were invited for an in-person assessment and, if they had a zero breath alcohol concentration, were asked to give written, informed consent and underwent an interview and neuropsychological testing by a trained research evaluator. Participants were compensated for their time and transportation costs.
Participants
HIV+ individuals were included in the study if they were 18–70 years old, were currently taking highly active antiretroviral treatment (HAART), reported having consumed alcohol in the past 90 days, and had a verbal IQ of ≥80. All participants provided written informed consent to participate in the study, which was approved by the Institutional Review Board of the University of Pennsylvania Perelman School of Medicine.
Individuals were excluded from participation if, on the basis of history or psychiatric examination, they met criteria for a severe psychiatric disorder (psychosis, mania, active suicidal ideation or intent) or a current DSM-IV diagnosis of dependence on a drug other than nicotine or cannabis. To eliminate potential effects on cognitive performance, individuals taking antipsychotic, mood stabilizing, or sedative/hypnotic medications were also excluded. Lastly, individuals with significant cognitive impairment (based on MMSE score), a history of a traumatic brain injury, or another potential confounding neurological disorder (e.g., seizure disorder) were excluded.
Recruitment
The majority of subjects were identified through a database maintained by the University of Pennsylvania Center for AIDS Research. Other recruitment sources (in descending order of importance) were referrals from other HIV treatment settings in Philadelphia or from medical providers in the University of Pennsylvania Health System, word of mouth, flyers posted in healthcare and community settings, and media advertisements. We had initial telephone contact with 842 prospective participants, of whom 262 did not respond to subsequent phone calls and 107 declined to participate after hearing a description of the study protocol. Of the 473 remaining individuals, 259 did not meet eligibility criteria at screening because of current use of psychotropic medication (32%), drug dependence (21%), presence of a current DSM-IV disorder of psychosis or mania or suicidal risk (14%), absence of a definitive diagnosis of HIV infection (12%), no reported drinking in the preceding three months (11%), a co-occurring neurological disorder (7%), or other reasons (3%). Thirty-five participants that met study criteria did not attend the scheduled study visit. The reasons for exclusion at the study visit were: a current DSM-IV drug dependence diagnosis (29%), a verbal IQ score <80 (27%), a current, severe DSM-IV psychiatric disorder or suicidal risk (16%), a score of <25 on the Mini Mental State Examination (19) (13%), current use of a psychotropic medication (7%), a positive breath alcohol concentration (5%), or other reasons (3%). Of 179 participants who, at the study visit, were deemed eligible to participate, 122 completed the study.
Assessments
Sociodemographic information
Sociodemographic information included age, gender, race/ethnicity, household income, years of education, and marital status.
Reading ability and general intellectual function
The Wechsler Test of Adult Reading (WTAR; ref. 20), a well-validated, brief assessment of intellectual functioning, was used to estimate full-scale IQ based on irregularly spelled word reading. The WTAR full-scale IQ, which includes age and race adjustments, served as a measure of baseline intellectual functioning, as it is regarded as stable and generally unaffected by cognitive decline.
Gross cognitive function
The Mini-Mental State Exam (19) was used to screen for significant cognitive impairment, which was defined by a score of <25 (maximum possible = 30).
Psychiatric and drug use disorder diagnoses
Modules from the Mini International Neuropsychiatric Interview (21) were used to identify the presence of current and lifetime mania, psychotic disorders, suicidal risk, and drug use disorders according to DSM-IV criteria (22).
Alcohol use disorder diagnoses
The Structured Clinical Interview for DSM-IV (23) was used to diagnose lifetime and current DSM-IV alcohol abuse or dependence.
Alcohol-related measures
The Timeline Followback Interview (24) was used to estimate alcohol consumption and drinking patterns for the 90 days prior to the study visit. This widely used instrument shows high test-retest reliability and construct validity in the assessment of alcohol use (25, 26). We measured recent drinking based on evidence that a cross-sectional assessment of cognitive functioning is impacted most strongly by proximal heavy drinking (27).
Recent cannabis use
The TLFB (24) was adapted to measure the frequency of cannabis use during the 30 days prior to the interview. Because recent cannabis use can affect cognition (28, 29), we used this measure as a covariate in the analysis of cognitive performance.
HAART medication adherence
The TLFB (24) was also adapted to elicit retrospective self-reports of adherence to HAART in the 30 days prior to the study visit. We measured the total number of days with 100% medication adherence and the overall percentage of adherence in the past month, with the latter serving as a covariate in the analysis of cognitive performance.
Patient Health Questionnaire (PHQ-9)
The PHQ-9 is a 9-item, self-administered depression measure that is reliable and valid (30).
Cognitive/neuropsychological testing
We administered the computerized CogState battery (see Appendix for task descriptions), which is sensitive to cognitive impairment in individuals with HIV without dementia (31). The CogState scales used were the Detection Task (Psychomotor Speed/Reaction Time), Identification Task (Basic Attention), One-Back Memory Task (Working Memory), Shopping List Task–Immediate and Delayed Recall Trials (Verbal Learning and Memory), Card Learning Task (Visual Learning and Memory), and Groton Maze Learning Test (GMLT) (Executive Function).
Decision-making
We used the Iowa Gambling Task (32), a computerized gambling task, to simulate real-world decision making. In this task, the participant begins with $2,000 of “play” money and is instructed to maximize profit over 100 trials by selecting cards from any of four decks. Based on profit and loss potential, two of the decks are termed “advantageous” and two are “disadvantageous.” We examined the overall difference between total advantageous and total disadvantageous selections across five 20-card blocks. A positive score was indicative of advantageous decision making, while a negative score represented disadvantageous decision making. This was also evaluated as an outcome measure.
Analytic Approach
First, we assessed each variable for normality of distribution using the Shapiro-Wilk test. Consistent with CogState guidelines, two participants were removed from the analysis for having not completed the GMLT. Because CogState automatically log10 transforms reaction time, we reverse transformed the outcomes of the detection and identification tasks to facilitate their interpretation.
To provide a comparison group of healthy controls with which to compare CogState performance in our sample, we used a large database of published CogState scores previously collected from healthy individuals (Cogstate Ltd., Cogstate Normative Data for Adults; October, 2013). The norms were divided into four age bins: 18–34, 35–49, 50–59, and 60–69. Using the population mean and standard deviation provided for each CogState task, we created age-adjusted z-scores for each participant within each of the cognitive domains assessed in the current study: psychomotor speed, attention, working memory, visual memory, verbal learning/memory, and executive function. Within each cognitive domain, we calculated the mean z-score and standard deviation for our sample.
Drinking level was defined in two ways: (1) self-reported heavy drinking days (HDDs) in the previous 90 days (defined as >3 drinks/day for women and >4 drinks/day for men) and (2) presence of current alcohol abuse or dependence (AUD). Due to the highly skewed distribution of HDDs, the sample was stratified into 3 groups: light (no HDDs), moderate (1–24 HDDs), and heavy drinkers (25+ HDDs). Presence or absence of a current AUD was used as a binary measure. Sample characteristics were examined across both drinking measures using Jonckheere-Terpstra tests for continuous measures and chi-square statistics for categorical variables.
Associations between drinking and cognitive performance were assessed in three ways. Cognitive characteristics were examined by the ordinal HDD drinking measure using Jonckheere-Terpstra tests for non-normal distributions taking into account the ordering of the outcome. The relationship between current AUD and cognitive performance was also assessed using Jonckheere-Terpstra tests. Lastly, multiple generalized linear models (GLMs) were used to determine the relation between drinking (predictor variable) and CogState task performance (outcome), adjusting for age, race, PHQ-9 score, WTAR IQ score, and recent cannabis use. For the models with a speed measure as the outcome (i.e., detection and identification tasks), GLMs with gamma distributions and log links were used. For the models with a count measure as the outcome (i.e., one-back memory, shopping list – immediate and delayed recall, card learning tasks, GMLT), either Poisson or negative binomial models with log links were used. The natural log of the total number of responses was used as the offset for these models, using Pearson estimates for dispersion scaling when necessary. The model with decision-making score as the outcome was fit with a normally distributed GLM. All analyses were conducted using SAS version 9.4 (Cary, North Carolina).
RESULTS
Sample Characteristics
A description of the study sample is provided in Table 1. Because the data were largely non-normal in distribution, we present medians with interquartile ranges [IQR], unless otherwise indicated. Participants were 21–66 years old, with a median of 49 years (IQR = 11.0). The sample was predominantly male (n = 101; 84%), and most self-identified as non-Hispanic black/African American (n = 84; 71%). Participants had completed a median of 13.5 years of education (IQR = 3.0) and their mean verbal IQ score was within the average range (mean = 97.3; SD = 12.6, median = 96.0; IQR = 19.0).
Table 1.
Sample characteristics stratified by current drinking status (n=120).
| Demographic characteristic | All (n=120) |
Drinking Level
|
p-value | Current Alcohol Abuse or Dependence
|
p-value | |||
|---|---|---|---|---|---|---|---|---|
| Lighta (n=28; 23.3%) |
Moderateb (n=63; 52.5%) |
Heavyc (n=29; 24.2%) |
Positive (n=44; 36.7%) |
Negative (n=76; 63.3%) |
||||
|
| ||||||||
| Sex | 0.764 | 0.194 | ||||||
| Male | 101 (84.2) | 25 (89.3) | 51 (81.0) | 25 (86.2) | 34 (77.3) | 66 (88.0) | ||
|
| ||||||||
|
Age (in years) (median, IQR) |
49.0 (11.0) | 52.5 (11.5) | 47.0 (17.0) | 49.0 (7.0) | 0.068 | 49.0 (8.5) | 49.0 (15.0) | 0.760 |
|
| ||||||||
| Race/Ethnicityd | 0.003 | 0.013 | ||||||
| African American | 84 (70.6) | 13 (48.2) | 46 (73.0) | 25 (86.2) | 37 (84.1) | 47 (63.5) | ||
| European American | 25 (21.0) | 12 (44.4) | 11 (17.5) | 2 (6.9) | 3 (6.8) | 21 (28.4) | ||
| Hispanic | 9 (7.6) | 1 (3.7) | 6 (9.5) | 2 (6.9) | 4 (9.1) | 5 (6.8) | ||
| Othere | 1 (0.8) | 1 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | ||
|
| ||||||||
| Marital Status | 0.021 | 0.369 | ||||||
| Single, never married | 77 (64.2) | 16 (57.1) | 48 (76.2) | 13 (44.8) | 28 (63.6) | 49 (65.3) | ||
| Married or partnered | 20 (16.7) | 9 (32.1) | 6 (9.5) | 5 (17.2) | 5 (11.4) | 14 (18.7) | ||
| Separated or divorced | 23 (19.2) | 3 (10.7) | 9 (14.3) | 11 (37.9) | 11 (25.0) | 12 (16.0) | ||
|
| ||||||||
| Education (in years) | 0.0006 | 0.004 | ||||||
| (median, IQR) | 13.5 (3.0) | 15.0 (4.5) | 13.0 (3.0) | 12.0 (2.0) | 12.0 (2.0) | 14.0 (4.0) | ||
|
| ||||||||
| Employment Status | <0.0001 | 0.092 | ||||||
| Unemployed | 35 (29.2) | 2 (7.1) | 17 (27.0) | 16 (55.2) | 17 (38.6) | 18 (24.0) | ||
| Otherf | 85 (70.8) | 26 (92.9) | 46 (73.0) | 13 (44.8) | 27 (61.4) | 57 (76.0) | ||
|
| ||||||||
| Total Yearly Income | 0.0004 | 0.003 | ||||||
| <$20,000 | 62 (53.5) | 7 (25.0) | 36 (58.1) | 19 (73.1) | 32 (74.4) | 30 (41.7) | ||
| $20,000–$39,999 | 28 (24.1) | 7 (25.0) | 17 (27.4) | 4 (15.4) | 7 (16.3) | 21 (29.2) | ||
| >$40,000 | 26 (22.4) | 14 (50.0) | 9 (14.5) | 3 (11.5) | 21 (29.2) | 21 (29.2) | ||
|
| ||||||||
|
WTAR Verbal IQ (median, IQR) |
96.0 (19.0) | 108.0 (28.5) | 94.0 (17.0) | 91.0 (12.0) | 0.012 | 92.0 (16.5) | 98.0 (21.0) | 0.097 |
|
| ||||||||
| PHQ-9 Depression | 0.0006 | <0.001 | ||||||
| Score (0−27) (median, IQR) | 3.0 (6.5) | 1.5 (3.0) | 4.0 (8.0) | 6.0 (5.0) | 6.0 (7.5) | 2.0 (5.0) | ||
|
| ||||||||
| HAART Adherence in Past Month | ||||||||
| # Days 100% Adherent | 29.5 (3.0) | 30.0 (1.5) | 30.0 (2.0) | 29.0 (6.0) | 0.008 | 28.5 (4.0) | 30.0 (2.0) | 0.021 |
| Overall % Adherence | 99.2 (8.5) | 100.0 (3.2) | 100.0 (7.0) | 93.3 (15.0) | 0.004 | 96.7 (13.0) | 100.0 (7.0) | 0.024 |
|
| ||||||||
| Alcohol Use in Past 3 Months | ||||||||
| Drinking Days | 29.0 (41.0) | 14.0 (24.5) | 25.0 (35.0) | 63.0 (42.0) | <0.001 | 42.0 (43.5) | 25.0 (33.0) | <0.001 |
| Drinks per Drinking Day | 3.8 (4.1) | 1.6 (1.0) | 3.8 (2.2) | 8.2 (4.8) | <0.001 | 6.0 (5.4) | 3.0 (2.5) | <0.001 |
| Heavy Drinking Days | 3.0 (19.0) | 0.0 (0.0) | 3.0 (9.0) | 47.0 (35.0) | <0.001 | 18.0 (36.0) | 1.0 (5.0) | <0.001 |
|
| ||||||||
| Cannabis Use in Past Month | ||||||||
| Number of Days Used | (2.0) | 0.0 (0.5) | 0.0 (1.0) | 0.0 (6.0) | 0.097 | 0.0 (2.0) | 0.0 (2.0) | 0.444 |
| Amount Used (# of joints) | 0.0 (2.0) | 0.0 (0.5) | 0.0 (2.0) | 0.0 (4.0) | 0.124 | 0.0 (2.0) | 0.0 (2.0) | 0.552 |
IQR=interquartile range; WTAR=Wechsler Test of Adult Reading; PHQ-9=Patient Health Questionnaire; HAART=highly active antiretroviral therapy.
Defined as no heavy drinking days (more than 3 drinks per day for women and more than 4 drinks per day for men) in the previous 90 days.
Defined as 1 to 24 heavy drinking days in the previous 90 days.
Defined as 25 or more heavy drinking days in the previous 90 days.
When used in multivariate modeling, this characteristic was collapsed into three due to small/zero cells for Hispanic and Other: non-Hispanic white/Caucasian, non-Hispanic black/African-American, other.
Other includes Filipino.
The sample was comprised primarily of moderate drinkers (n = 63; 53%), though individuals who engaged in light and very heavy drinking were also represented. Participants drank on a median of 29 days (IQR = 41.0) in the 90-day recall period prior to their study visit. The median number of drinks per drinking day was 3.8 (IQR = 4.1) and for HDDs it was 3 (IQR = 19.0), although 38% of the sample (n = 45) reported having 0 or 1 HDD. One-third of the sample had both a lifetime and a current diagnosis of alcohol dependence (n=40, 33.6%) and 3.4% had both a lifetime and a current diagnosis of alcohol abuse (n=4). Further, 16.8% had a lifetime, but no current diagnosis of alcohol dependence (n=20), and 11.8% had a lifetime, but no current diagnosis of alcohol abuse (n=14). Just over one-third of the sample (n=41; 34.5%) had neither a lifetime nor a current AUD diagnosis.
As would be expected, there was a significant association between a current AUD diagnosis (either abuse or dependence) and self-reported drinking level (p < 0.0001). Among light drinkers, 3.6% (n=1) had a current AUD diagnosis; among moderate drinkers, 38.1% (n=24) had a current AUD diagnosis; and among heavy drinkers 65.5% (n=19) had a current AUD diagnosis. Most participants had very low scores on the PHQ-9 (median = 3.0; IQR = 6.5), with 86% of the sample scoring in the minimal or mild range of depression, 8% in the moderate range, 4% in the moderately severe range, and 2% in the severely depressed range. Half of the sample reported very high (>99%) adherence to their HAART medication regimen (IQR = 8.5) and this was significantly inversely correlated with PHQ-9 depression score (Spearman’s rho [rs] = −0.37, p < 0.0001).
When stratified by self-reported drinking level, moderate and heavy drinkers were more likely to be African American and unemployed and to have fewer years of education, lower annual income, lower estimated premorbid IQ, greater depression scores, and poorer HAART adherence than light drinkers. The distribution of light drinkers was nearly equal in African Americans and non-Hispanic Caucasians. Comparing the subgroups based on a current AUD diagnosis, those with an AUD were also more likely to be African American and to have fewer years of education, lower annual income, greater depression scores, poorer decision-making skills, and poorer HAART adherence than those with a current AUD diagnosis.
Mean Cognitive Performance and CogState Normative Data
As shown in Figure 1, the lowest mean z-score in our sample, and the only cognitive task for which mean performance was more than 1 standard deviation below the mean, was for reaction time on the CogState Detection Task of psychomotor speed (mean = 1.16; SD = 1.07). Performance on the GMLT was the second lowest z-score (mean = 0.93; SD = 2.09), but at less than 1 SD below the mean, it was within normal limits. Overall, the pattern of neuropsychological performance in this sample of HIV+ drinkers was modestly lower than expected based on normative data.
Figure 1.
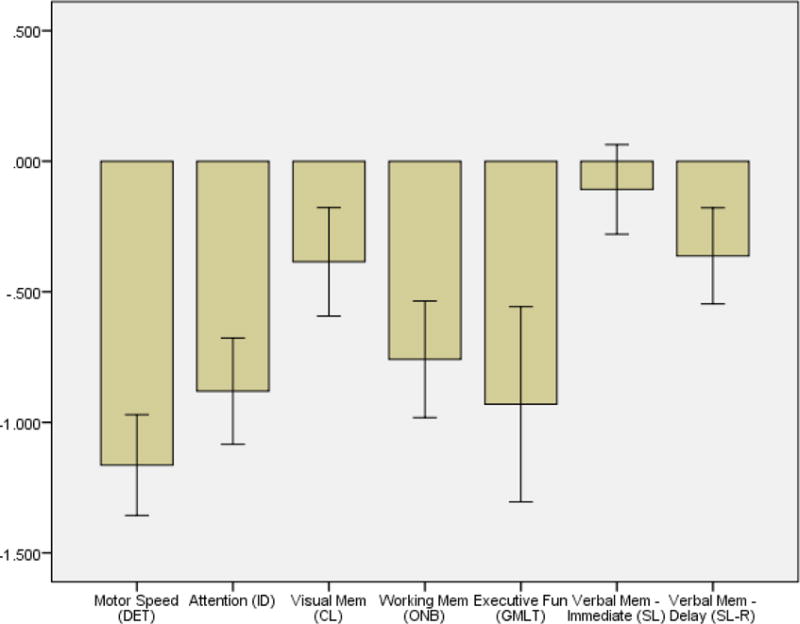
Mean z-scores for CogState tasks by domain. All z-scores were calculated using normative data collected from age-matched healthy controls who previously completed CogState testing. Error bars represent 95% confidence intervals.
Alcohol Use and Cognitive Performance
Self-reported drinking level was not associated with any of the measures of cognitive performance, though a current AUD diagnosis showed some associations in unadjusted analyses (Table 2). Individuals with a current AUD had a significantly slower median psychomotor speed, made significantly more errors on the GMLT, and were more likely to have poorer decision-making scores on the IGT than those without a current AUD diagnosis. However, these predictors were not significant in multivariable modeling (Table 3). Interestingly, after controlling for covariates, we found that individuals with a current AUD diagnosis performed significantly more poorly on the attention task (p = .01). After adjusting for age, race, IQ, PHQ-9 score, and recent cannabis use, self-reported drinking was significantly associated with cognitive functioning only in the visual memory model, where individuals with moderate drinking provided fewer correct responses than light drinkers (p = 0.02).
Table 2.
Neurocognitive performance according to self-reported and diagnostic drinking.
| Neurocognitive Tasks (median, IQR) |
All (n=120) |
Self-reported drinking level | p-value | Diagnosis of current abuse or dependence | ||||
|---|---|---|---|---|---|---|---|---|
| Lighta (n=28; 23.3%) |
Moderateb (n=63; 52.5%) |
Heavyc (n=29; 24.2%) |
Positive (n=44; 36.7%) |
Negative (n=76; 63.3%) |
p-value | |||
| Psychomotor Speed (ms) | 407.3 (151.5) |
382.0 (103.7) | 408.3 (166.2) | 417.9 (183.1) | 0.171 | 428.6 (156.2) |
385.3 (114.2) |
0.036 |
| Attention (ms) | 574.1 (139.0) |
571.4 (156.0) | 574.9 (141.1) | 554.3 (99.9) | 0.751 | 586.6 (189.2) |
557.2 (112.3) |
0.064 |
|
Working Memory (proportion correct) |
0.9 (0.1) | 0.91 (0.17) | 0.91 (0.15) | 0.93 (0.11) | 0.528 | 0.9 (0.2) | 0.9 (0.1) | 0.341 |
|
Visual Memory (proportion correct) |
0.7 (0.1) | 0.68 (0.15) | 0.64 (0.14) | 0.69 (0.13) | 0.805 | 0.7 (0.2) | 0.7 (0.1) | 0.991 |
|
Verbal Memory – Initial (# recalled) |
25.0 (6.0) | 25.0 (4.5) | 25.0 (6.0) | 24.0 (7.0) | 0.127 | 24.5 (7.0) | 25.0 (6.0) | 0.134 |
| Verbal Memory – Delayed (# recalled) | 8.0 (3.5) | 9.0 (2.5) | 8.0 (4.0) | 8.0 (3.0) | 0.148 | 8.0 (4.0) | 9.0 (4.0) | 0.086 |
| Executive Function (# of errors) | 65.5 (33.0) | 61.5 (33.5) | 65.0 (37.0) | 72.0 (25.0) | 0.142 | 74.5 (36.5) | 63.0 (32.0) | 0.021 |
| Decision-Making (scale) | −2.0 (31.0) | 8.0 (42.0) | −4.0 (32.0) | −2.0 (36.0) | 0.178 | −10.0 (32.0) | 2.0 (36.0) | 0.021 |
IQR=interquartile range; ms=milliseconds.
Boldface indicates significance ≤ .05.
Defined as no heavy drinking days (more than 3 drinks per day for women and more than 4 drinks per day for men) in the previous 90 days.
Defined as 1 to 24 heavy drinking days in the previous 90 days.
Defined as 25 or more heavy drinking days in the previous 90 days.
Table 3.
Multivariable neurocognitive tasks models.
| Covariate | Psychomotor speed Estimate (CI) | Attention Estimate (CI) | Working memory Estimate (CI) | Visual memory Estimate (CI) | Verbal memory – initial Estimate (CI) | Verbal memory – delayed Estimate (CI) | Executive function Estimate (CI) | Decision-making Estimate (CI) |
|---|---|---|---|---|---|---|---|---|
| Age | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (.99–1.00) | 1.00 (1.00–1.00) | 1.00 (.99–1.00) | 1.00 (.99–1.00) | 1.01 (1.00–1.02) | .10 (−.44–.65) |
| Non-Hispanic Caucasian | 1.12 (1.00–1.29) | 1.03 (.92–1.16) | 1.10 (.94–1.29) | 1.04 (.95–1.14) | 1.02 (.93–1.13) | 1.02 (.85–1.22) | 1.00 (.75–1.33) | −9.28 (−25.85–7.30) |
| Verbal IQ | 1.00 (.99–1.00) | 1.00 (.99–1.00) | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.01 (1.00–1.01) | 1.01 (1.00–1.01) | .98 (.97–.99) | .37 (−.19–.92) |
| PHQ-9 score | 1.00 (.99–1.01) | 1.00 (.99–1.00) | 1.01 (1.00–1.02) | 1.00 (1.00–1.01) | 1.00 (.99–1.01) | 1.00 (.99–1.01) | .99 (.97–1.01) | −.21 (−1.40–.99) |
| Current AUD dx | 1.07 (.98–1.18) | 1.11 (1.02–1.19) | .93 (.84–1.04) | .99 (.93–1.06) | .98 (.92–1.05) | .96 (.85–1.09) | 1.15 (.94–1.40) | −6.60 (−18.23–5.03) |
| Self-reported drinking level | ||||||||
| Light | REF | REF | REF | REF | REF | REF | REF | REF |
| Moderate | 1.07 (.95–1.20) | 1.04 (.95–1.14) | .94 (.83–1.06) | .92 (.86–.99) | .96 (.88–1.03) | .98 (.85–1.13) | 1.11 (.88–1.40) | −8.42 (−21.84–5.03) |
| Heavy | 1.04 (.92–1.19) | .98 (.88–1.09) | .98 (.85–1.13) | .99 (.91–1.07) | .94 (.86–1.03) | .95 (.81–1.13) | 1.07 (.82–1.41) | 3.59 (−11.97–19.15) |
| Cannabis use (past 30 days) |
1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (.99–1.01) | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (.99–1.01) | 1.01 (1.00–1.02) | −.27 (−1.04–.49) |
CI=Wald 95% confidence interval; REF=reference category; PHQ-9=Patient Health Questionnaire-9; AUD dx=alcohol use disorder diagnosis. Boldface indicates significance ≤ .05. Note: Each column provides the parameter estimates and corresponding errors for the model of a particular CogState task (indicated by the column heading). Current AUD diagnosis and self-reported drinking level variables were not included in the same model due to multicollinearity, so each model was run twice: once using AUD as the drinking measure and another using self-report as the drinking measure. All covariate parameter estimates displayed in the table (except for self-reported drinking level) are for the model with AUD as the drinking indicator, though there was very little variation in estimates from the models using self-reported drinking. For models fit using logarithmic links, shown estimates have been exponentiated.
DISCUSSION
We examined neurocognitive performance in an HIV+ sample that reported drinking during the 90 days prior to study entry, but were not seeking treatment for AUD and did not meet criteria for dementia, to examine the relations between drinking level or the presence of a current AUD and performance on a comprehensive cognitive assessment. Overall, the only areas in which neurocognitive performance in the sample was significantly associated with alcohol use were visual memory/learning and attention. Although psychomotor speed, executive function, and decision-making were significantly associated with the presence of a current AUD diagnosis in unadjusted analyses, these findings did not hold in multivariate modeling, potentially a consequence of low power due to a small sample size. Alternatively, it is possible that these data accurately reflect the lack of a relationship between alcohol and cognitive performance in HIV. The unadjusted findings, however, are consistent with previous studies showing an adverse effect of heavy alcohol use on cognition in HIV+ individuals (15–18, 33). The greater evidence of the effects on cognitive functioning of an AUD diagnosis than drinking level may also reflect an underreporting of drinking level among heavier drinkers. That is, individuals with a current AUD may have acknowledged alcohol-related symptoms, but underreported their drinking on the TLFB interview.
Future research should include an examination of specific cognitive processes, such as mechanisms of learning and memory, in larger samples of HIV+ heavy drinkers to permit the differentiation of HIV-related and alcohol-related effects. Clinically, cognitive changes in HIV+ individuals who drink alcohol are likely best detected with a comprehensive battery, allowing for intra-individual profile analysis of cognitive strengths and weaknesses. Advantages of using CogState and the IGT included their brief administration time, limited staff training/administration demands, and automated scoring and data collection with greater precision of measurement.
Surprisingly, our analyses did not support drinking-related differences in objective cognitive performance on a decision-making task (IGT), a finding that is inconsistent with prior research in both HIV and heavy drinking populations (4, 34, 35). However, the real-world significance of impaired IGT performance is unknown, as it has not shown a significant association with functional disability in daily living (36).
The present study had a number of other limitations. We did not have a healthy control group of non-drinkers or seronegative patients against which scores could be compared. To compensate for this, we compared overall patterns in CogState performance to a large sample of normative data collected from healthy individuals. This comparison provided information on the broad cognitive spectrum of HIV+ men and women who drink alcohol in relation to a non-HIV population. The cross-sectional data collection did not allow for inferences of causality. Because the sample lacked HIV disease markers (CD4 count, viral load), immunosuppression and/or uncontrolled neurotoxic processes could also have confounded the results. Further, we did not obtain information on the number of years since the infection was acquired. Thus, the direct effects of HIV disease progression on neurocognition (e.g., ref. 37) could have masked the relationship between cognition and both alcohol drinking level and AUD symptoms. It is also worth noting that our sample was recruited almost exclusively from medical settings and HIV treatment centers, and active engagement with a course of HAART treatment was an inclusion criterion for study participation. Together, these factors provide some support for the notion that the neurovirulent aspects of HIV were controlled to a greater degree than if recruitment took place from an untreated population. Future neuropsychological studies of the effects of alcohol in HIV+ individuals should include markers of infection and should assess multiple factors longitudinally.
Despite these limitations, the study had a number of important strengths. First, our study examined several participant characteristics and behaviors as potential predictors of cognitive performance, including depression score, premorbid intellectual functioning, and recent cannabis use. Second, we added to the relatively sparse body of literature on the effects of alcohol on cognition in HIV. We expanded the existing literature by utilizing a comprehensive battery of objective measures of cognitive performance in multiple domains, ranging from foundational tasks, such as basic attention and reaction time, to more complex tasks such as EF and decision-making. Third, we compared participants’ performance on the CogState battery to that of a normative sample, which showed generally that the HIV+ individuals’ neurocognitive performance was largely unimpaired, but that psychomotor speed was significantly lower than expected in view of the normative data. Finally, we used a reliable and valid measure of recent drinking, the TLFB (28–30), rather than a self-report questionnaire of unknown reliability or validity.
Further neuropsychological research is needed on the complex interplay among the course of HIV-related disease, alcohol consumption level, and premorbid/demographic characteristics. Study samples should be large enough to provide adequate statistical power and longitudinal designs should be tailored to allow a consideration of each factor’s unique contribution to variance in cognitive performance. Most importantly, the clinical and functional implications of detectable cognitive changes among individuals with HIV require further investigation.
Supplementary Material
Acknowledgments
The authors would like to thank Ms. Bridget Nolan, who provided assistance in the assessment of subjects in this study.
Funding: Dr. Kranzler has been an advisory board member, consultant, or CME speaker for Indivior and Lundbeck. He is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative (ACTIVE), which was supported in the last three years by AbbVie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, and XenoPort. The study was funded by NIH grants P30 AI045008 awarded to the University of Pennsylvania Center for AIDS Research and K24 AA013736 (to HRK).
Footnotes
Conflict of Interest: Dr. Douglas-Newman, Dr. Smith, Dr. Spiers, and Mr. Pond have no competing interests to report.
References
- 1.Cattie JE, Doyle K, Weber E, et al. Planning deficits in HIV-associated neurocognitive disorders: component processes, cognitive correlates, and implications for everyday functioning. J Clin Exper Neuropsychol. 2012;34:906–918. doi: 10.1080/13803395.2012.692772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joska JA, Gouse H, Paul RH, et al. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16:101–114. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- 3.Grant I. Neurocognitive disturbances in HIV. Int Review Psychiatry. 2008;20:33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- 4.Martin EM, Pitrak DL. Cognitive impulsivity and HIV serostatus in substance dependent males. J Int Neuropsychol Soc. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- 5.Thames AD, Streiff V, Patel SM, et al. The role of HIV infection, cognition, and depression in risky decision-making. J Neuropsychiatry Clin Neurosci. 2012;24:340–348. doi: 10.1176/appi.neuropsych.11110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods SP, Moore DJ, Weber E, et al. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley J, Ettenhofer M, Wright M, et al. Emerging issues in the neuropsychology of HIV infection. Curr HIV/AIDS Rep. 2008;5:204–11. doi: 10.1007/s11904-008-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peavy G, Jacobs D, Salmon DP, et al. Verbal memory performance of patients with human immunodeficiency virus infection: evidence of subcortical dysfunction. The HNRC group. J Clin Exper Neuropsychol. 1994;16:508–523. doi: 10.1080/01688639408402662. [DOI] [PubMed] [Google Scholar]
- 9.Reger M, Welsh R, Razani J, et al. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- 10.Grant I, Heaton RK, Atkinson JH. Neurocognitive disorders in HIV-1 infection. HNRC Group. HIV Neurobehavioral Research Center. Curr Top Microbiol Immunol. 1995;202:11–32. [PubMed] [Google Scholar]
- 11.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson-Papp J, Elliot KJ, Simpson DM. HIV-related neurocognitive impairment in the HAART era. Curr HIV/AIDS Rep. 2009;6:146–152. doi: 10.1007/s11904-009-0020-1. [DOI] [PubMed] [Google Scholar]
- 13.Samet JH, Horton NJ, Meli S, et al. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- 14.Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;1:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fama R, Rosenbloom MJ, Sassoon SA, et al. Remote semantic memory for public figures in HIV infection, alcoholism, and their comorbidity. Alcohol Clin Exp Res. 2011;35:265–276. doi: 10.1111/j.1530-0277.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green JE, Saveanu RV, Bornstein RA. The effect of previous alcohol abuse on cognitive function in HIV infection. Am J Psychiatry. 2004;161:249–254. doi: 10.1176/appi.ajp.161.2.249. [DOI] [PubMed] [Google Scholar]
- 17.Rothlind JC, Greenfield TM, Bruce AV, et al. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sassoon SA, Fama R, Rosenbloom MJ, et al. Component cognitive and motor processes of the digit symbol test: differential deficits in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 19.Folstein M, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Psychological Corporation Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Harnett-Sheehan K, et al. The M.I.N.I. International Neuropsychiatric Interview (M.I.N.I): The Development and Validation of a Structured Diagnostic Psychiatric Interview. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, DC: American Psychiatric Publishing; 1994. [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY Screen) New York: Biometric Research, New York State Psychiatric Institute; 2001. [Google Scholar]
- 24.Sobell LC, Sobell MB. Timeline Follow-back: a technique for assessing self-reported alcohol consumption. In: Allen J, Litten R, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods Clifton. NJ: Humana Press; 1992. pp. 41–65. [Google Scholar]
- 25.Searles JS, Helzer JE, Walter DE. Comparison of drinking patterns measured by daily reports and timeline follow back. Psychol Addict Behav. 2000;14:277–286. doi: 10.1037//0893-164x.14.3.277. [DOI] [PubMed] [Google Scholar]
- 26.Toll BA, Cooney NL, McKee SA, et al. Correspondence between Interactive Voice Response (IVR) and Timeline Followback (TLFB) reports of drinking behavior. Addict Behav. 2006;31:726–731. doi: 10.1016/j.addbeh.2005.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beatty WW, Tivis R, Stott HD, et al. Neuropsychological deficits in sober alcoholics; influences of chronicity and recent alcohol consumption. Alcohol Clin Exp Res. 2000;24:149–154. [PubMed] [Google Scholar]
- 28.Kelleher LM, Stough C, Sergejew AA, et al. The effects of cannabis on information-processing speed. Addict Behav. 2004;29:1213–1219. doi: 10.1016/j.addbeh.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 29.Pope HG, Gruber AJ, Hudson JI, et al. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42:41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Int Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overton ET, Kauwe JS, Paul R, et al. Performances on the CogState and standard neuropsychological batteries among HIV patients without dementia. AIDS Behav. 2011;15:1902–1909. doi: 10.1007/s10461-011-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bechara A, Damásio AR, Damásio H, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 33.Fama R, Rosenbloom MJ, Sassoon SA, Pfefferbaum A, Sullivan EV. Differential effect of alcoholism and HIV infection on visuomotor procedural learning and retention. Alcoholism: Clinical and Experimental Research. 2012;36(10):1738–1747. doi: 10.1111/j.1530-0277.2012.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brevers D, Bechara A, Cleeremans A, et al. Impaired decision-making under risk in individuals with alcohol dependence. Alcohol Clin Exp Res. 2014;38:1924–1931. doi: 10.1111/acer.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernández-Serrano MJ, Pérez-Garcia M, Schmidt Rio-Valle J, et al. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. 2010;24:1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- 36.Iudicello JE, Woods SP, Cattie JE, et al. Risky decision-making in HIV-associated neurocognitive disorders (HAND) Clin Neuropsychol. 2013;27:256–275. doi: 10.1080/13854046.2012.740077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bornstein RA, Nasrallah HA, Para MF, et al. Change in neuropsychological performance in asymptomatic HIV infection: 1-year follow-up. AIDS. 1993;7:1607–11. doi: 10.1097/00002030-199312000-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


