Abstract
Background
The safety and efficacy of intraventricular fibrinolysis (IVF) in intraventricular hemorrhage (IVH) patients are unclear. We aimed to determine these issues and to evaluate whether there are differences between rt-PA and urokinase according to subgroup analyses.
Methods
Meta-Analysis of randomized controlled trials (RCTs) in IVH patients comparing the administration of rt-PA or urokinase through extraventricular drainage (EVD) with normal saline through EVD or EVD placement alone.
Results
Six RCTs involving 607 IVH patients were included; 2 trials investigated urokinase and 4 rt-PA. IVF reduced death from any cause at the end of follow-up (RR 0.63, 95% CI 0.47 to 0.83), which was mostly driven by rt-PA (RR 0.65, 95% CI 0.48 to 0.86). Urokinase did not reduce mortality (RR 0.30, 95% CI 0.06 to 1.53). However, rt-PA did not reduce the proportion of survivors with poor functional outcome (RR 1.36, 95% CI 1.04 to 1.77), or the composite endpoint of death and poor functional outcome (RR 0.96, 95% CI 0.83 to 1.11). IVF neither reduced the need for shunt placement (RR 1.06, 95% CI 0.75 to 1.49) nor increased ventriculitis (RR 0.57, 95% CI 0.35 to 0.93) and rebleeding (RR 1.65, 95% CI 0.79 to 3.45).
Conclusions
Although the use of IVF in IVH patients appears generally safe, its benefit is limited to a reduction in mortality at the expense of an increased number of survivors with moderately severe to severe disability. Subgroup analyses do not suggest an advantage of IVF with urokinase over rt-PA.
Keywords: intraventricular hemorrhage, urokinase, recombinant tissue-plasminogen activator, fibrinolytics, systematic review
Introduction
Intraventricular hemorrhage (IVH) is seen in approximately 45% of patients presenting with non-traumatic spontaneous intracerebral hemorrhage (ICH) 1, 2. It is a significant cause of morbidity, mortality, and poor outcome. Without specific treatment, the risk of poor outcome is 90% and the risk of death is 78%1. IVH can result in obstructive hydrocephalus and mass effect exerted by the blood clot, which can lead to elevated intracranial pressure and brain herniation1.
The management of IVH often requires the placement of an external ventricular drainage catheter (EVD)3. However, EVD could increase the risk of infection with incidence reported from 0% to 22% [3] and EVD may be complicated by catheter occlusion with blood clots4. In recent years, the administration of fibrinolytic agents, such as recombinant tissue-plasminogen activator (rt-PA) or urokinase, through the EVD into the ventricles has been proposed as an effective way to maintain catheter patency, increase blood clearance and clot removal, decrease ventricular enlargement and delayed hydrocephalus, and ameliorate inflammation caused by blood and its toxic products5, 6. However, the efficacy of intraventricular fibrinolysis (IVF) in IVH has been debatable. A Cochrane review in 2002 did not find sufficient good quality evidence from randomized trials to determine whether IVF does more good than harm7. A subsequent meta-analysis in 2014 including 8 randomized trials and 16 cohort studies, found that IVF significantly reduces mortality, improves functional outcome, and decreases shunt dependence in IVH8. However, the included trials were underpowered to support concrete recommendations about the use of IVF in IVH. Most recently, the results of the largest randomized trial of intraventricular rt-PA in IVH (CLEAR-III trial), were published9. Therefore, we undertook the current systematic review and meta-analysis to test, if rt-PA or urokinase applied by a ventricular catheter improve outcome in comparison to placebo and if the side effects are different in these three arms in patients with non-traumatic IVH.
In CLEAR III trial, IVF reduced mortality after IVH but did not improve functional outcomes. Preclinical studies suggest that intraventricular rt-PA may have pro-inflammatory and neurotoxic effects10–12, and that urokinase, but not rt-PA, significantly improves functional outcome after IVH13. This prompted us to perform subgroup analysis of the main meta-analysis based on the fibrinolytic agent used to examine any potential differences in efficacy and safety of rt-PA vs. urokinase in IVH, although urokinase is no longer available for clinical use in the USA and several other countries.
Methods
The methods and reporting of the systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for meta-analyses of interventional studies14.
Search methods for identification of studies
We searched the following electronic sources in order to identify published studies: MEDLINE (OVID, 1946 to January 20, 2017) and EMBASE (OVID, 1974 to January 20, 2017). Appendix 1 lists the search strategies used. We applied the Cochrane sensitivity-maximizing RCT filter to MEDLINE and adaptations of it to EMBASE15. In an effort to identify additional published, unpublished, and ongoing studies, we searched the following ongoing trials registers: WHO International Clinical Trial Registry Platform (ICTRP) (http://apps.who.int/trialsearch/, last accessed on January 20, 2017), and ClinicalTrials.gov (http://www.clinicaltrial.gov/, last accessed on January 20, 2017). We also searched all reference lists from relevant articles and reviews.
Criteria for study selection
We included RCTs in which IVF through EVD was compared with placebo or EVD alone. We considered any RCTs with at least one clinical outcome for this review. Eligible RCTs included participants with non-traumatic IVH aged 16 years or older. We accepted drug treatments and other interventions provided that they were given to both IVF-treated and comparator arms. There were no restrictions based on the timing of follow-up, dosing or choice of fibrinolytic agent (urokinase or rt-PA), frequency of drug administration, or language of publication. We extracted from each trial the number of participants randomly allocated to each intervention group to allow an intention-to-treat analysis. In both groups, we considered the number of participants who had one of the outcomes listed below during the specified follow-up period. We excluded uncontrolled studies, case reports/series, and trials that were not truly randomized, such as open-label dose-range-finding studies, and studies with only laboratory outcomes.
Outcome measures
The primary outcome measure was the composite end point of death and poor functional outcome, defined as moderately severe to severe disability on modified Rankin Scale (mRS) or Glasgow Outcome Scale (GOS) at the end of follow-up, as appropriate. The secondary outcome measures were: 1. Death at the end of follow-up; 2. Poor functional outcome at the end of follow-up; and 3. Adverse events including: shunt dependence, ventriculitis, and rebleeding.
Data collection and analysis
We merged the search results using Endnote X3, and removed duplicate records of the same report. We independently screened each title and abstract to exclude irrelevant reports, then retrieved full text of potentially relevant reports and examined the full texts for eligibility. We included duplicate publication just once. Two authors (DW and JL) independently extracted data from each report (including details of the study, number of participants, interventions, and outcome results) on electronic data collection forms. We resolved any disagreements among the authors by discussion. When the disagreement was due to a difference in interpretation, a third author (MS) acted as an arbitrator.
We assessed the risk of bias in all included studies using the recommended tool by The Cochrane Collaboration16. We made judgments of ‘low risk’, ‘high risk’, or ‘unclear risk’ of bias for the following six domains: 1. Random sequence generation (selection bias); 2. Allocation concealment (selection bias); 3. Blinding of participants and personnel (performance bias); 4. Blinding of outcome assessment (detection bias); 5. Incomplete outcome data (attrition bias); and 6. Selective outcome reporting (reporting bias). Two authors (DW and JL) independently performed the assessment of risk of bias in included studies. Any disagreements arising at any stage were resolved through discussion or by involving third author (MS) when necessary.
We expressed results for dichotomous outcomes as risk ratios with 95% confidence intervals, and expressed results for continuous outcomes as mean difference (if the same scale for each trial was available) or standardized mean difference (if different scales were used). We used RevMan 5.3 for all data entry and analysis. If data were missing, we considered both best-case and worst-case scenarios. We assessed statistical heterogeneity by using the I2 statistic16. If the I2 was less than 30%, we considered heterogeneity to be not important; 30% to 50% moderate; and more than 50% substantial. In cases where there was substantial statistical heterogeneity, we looked for the potential source(s) of the heterogeneity (that is clinical or methodological heterogeneity). If I2 statistic was less than 50%, we used fixed-effect meta-analysis. If heterogeneity was substantial, we undertook both fixed-effect and random-effect meta-analysis, and reported the most conservative result.
Results
Study selection
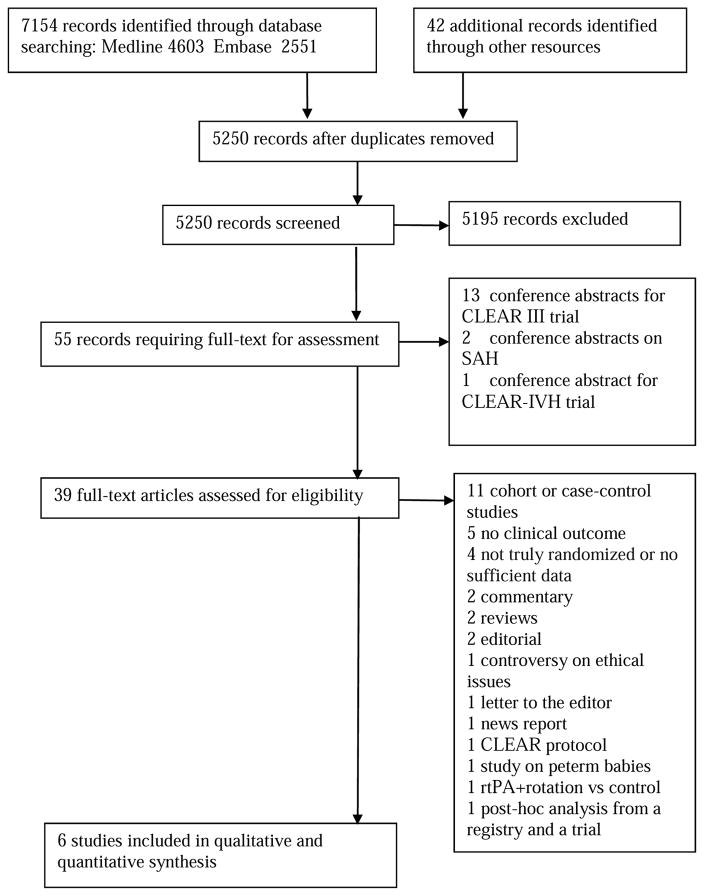
We identified 7196 records upon initial search. However, we only included 6 RCTs involving a total of 607 participants in this meta-analysis; 4 involving the use of rt-PA9, 17–19 and 2 urokinase20, 21. Figure 1 details the results of our literature search. Among excluded studies were 4 trials of urokinase22–25. These trials were not truly randomized and used biased or alternate allocation strategies. We also excluded 3 articles that used data from CLEAR IVH study26–28 and did not report clinical outcomes.
Figure 1.
Study flow diagram
Study characteristics
Table 1 shows the details of the included studies. All are RCTs with parallel-group design. All included trials were conducted in non-Asian countries except one21, which was conducted in Singapore. Two trials used urokinase and 4 used rt-PA as the intervention(s). Of the two urokinase trials, one was a randomized, double-blinded, multicenter trial which was terminated early due to the manufacturer withdrawing urokinase from the market20. The second was a single-center, double-blinded, randomized trial, which was conducted in Singapore21. Of the 4 rt-PA trials, 2 were multicenter, randomized, blinded, placebo-controlled trials9, 19, whereas the other 2 were single-center17, 18.
Table 1.
Details of included studies
| Trial | Trial period | Country | No. of centers | Participant | Intervention group | Control group | Primary outcome | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Naff 200420 | NR | NR (mainly USA) | NR | spontaneous supratentorial ICH <30 ml with IVH large enough to require EVD for obstructive hydrocephalus | N=7; No.(%) men:5(71.4) Mean±SD age (yrs): 49.6±14.5 Urokinase via EVD; 25000 IU q12h |
N=5; No.(%) men: 3(60%) Mean (SD) age (years):55.2±4.6 Normal saline via EVD |
clot resolution rate | 30 days |
| King 201221 | 2006–2008 | Singapore | 1 | supratentorial ICH of <30 mL with IVH of any volume; and acute obstructive hydrocephalus requiring EVD | N=7; No.(%) men: 3(42.9) Median age (yrs): 57.5 Urokinase via EVD; 25000 IU q12h |
N=9; No.(%) men: 7(77.8) Median age (yrs): 54 Normal saline via EVD |
infection rates, length of stay in ICU, survival, NIHSS; mRS | 6 months |
| Naff 201119 | NR | USA | 14 | small supratentorial ICH (≤30 mL) with massive IVH with an EVD already placed for obstructive hydrocephalus | N=26; No.(%) men: 19(73.1) Mean±SD age (yrs): 54.1±2.4 rtPA via EVD; 3 mg q12h |
N=22; No.(%) men: 7(31.8) Mean±SD age (yrs): 56.5±1.6 Normal saline via EVD |
mortality, ventriculitis, and bleeding events | 30 days |
| Litrico 201317 | 2005–2009 | France | 1 | aneurysmal SAH with severe IVH obstructing third and fourth ventricles requiring EVD | N=11; No.(%) men: 7(63.6) Mean±SD age (yrs): 52±11.2 rtPA via EVD; 3 mg q12h (maximum: 36mg) |
N=8; No.(%) men: 5(62.5) Mean±SD age (yrs): 60±6.9 EVD |
Mortality at 30 days | 1 year |
| Kramer 201418 | 2009–2012 | Canada | 1 | aneurysmal SAH with IVH and post-hemorrhagic hydrocephalus requiring EVD | N=6; No.(%) men: 2(33.3) Median (IQR) age (yrs): 55.5(50–63) rtPA via EVD; 2 mg q12h (maximum: 10mg) |
N=6; No.(%) men: 1(16.7) Median (IQR) age (yrs): 63(51–79) Normal saline via EVD |
feasibility, safety, and the rate of intracranial blood clearance | 6 months |
| CLEAR III 20169 | 2009–2015 | Brazil, Canada, Germany, Hungary, Israel, Spain, United Kingdom, USA | 73 | IVH obstructing third and/or fourth ventricles, with or without spontaneous supratentorial ICH ≤30 ml | N=249; No.(%) men: 144(57.8) Median (IQR) age (yrs): 59(51–66) rtPA via EVD; 1 mg q8h, up to 12 doses |
N=251; No.(%) men: 134(53.4) Median (IQR) age (yrs): 59(51–67) Normal saline via EVD |
mRS≤3 at 180 days | 12 months |
NR- not reported; ICH – intracerebral hemorrhage; IVH – intraventricular hemorrhage; ICU – intensive care unit; GOS - Glasgow Outcome Scale; EVD- external ventricular drainage; SD - standard deviation; IQR - interquartile range; NIHSS-National Institutes of Health Stroke Scale; mRS – modified Rankin Scale; SAH - Subarachnoid hemorrhage
The urokinase and 2 multicenter rt-PA trials were limited to IVH patients with obstructive hydrocephalus with or without supratentorial ICH ≤ 30 ml. The 2 single-center rt-PA trials were limited to patients with aneurysmal SAH with IVH and obstructive hydrocephalus. The number of participants across the included studies ranged from 12 to 500. The urokinase trials included 14 participants in the urokinase group and 14 participants in the control group in total. The rt-PA trials included a total of 292 participants in the rt-PA group and 287 participants in the control group. Overall, the age between treatment arms across studies was similar. The sex distribution was similar between the treatment groups in 3 studies9, 17, 20, while the male proportion in rt-PA group was about 2 fold greater than that in control group in 2 studies18, 19 and the reverse was true in 1 of the urokinase studies21.
Both of the urokinase studies compared urokinase (25000 IU of urokinase every 12 hours) with normal saline via EVD. Three of the 4 rt-PA studies compared rt-PA with normal saline via EVD, while the remaining study compared rt-PA via EVD with only EVD17. As Table 1 illustrates, the dose and frequency of rt-PA administration varied between the studies; from 1 mg to 3mg of rt-PA every 8 to 12 hours for a maximum of 5 to 12 doses.
Outcome data were reported at 30 days in 5 studies9, 17–20, 3 months in 2 study9, 17, 6 months in 3 studies9, 18, 21; and 12 months in 1study17. Although the follow-up in CLEAR III trial extended to 12 months, the 1-year follow-up outcome data are yet to be reported.
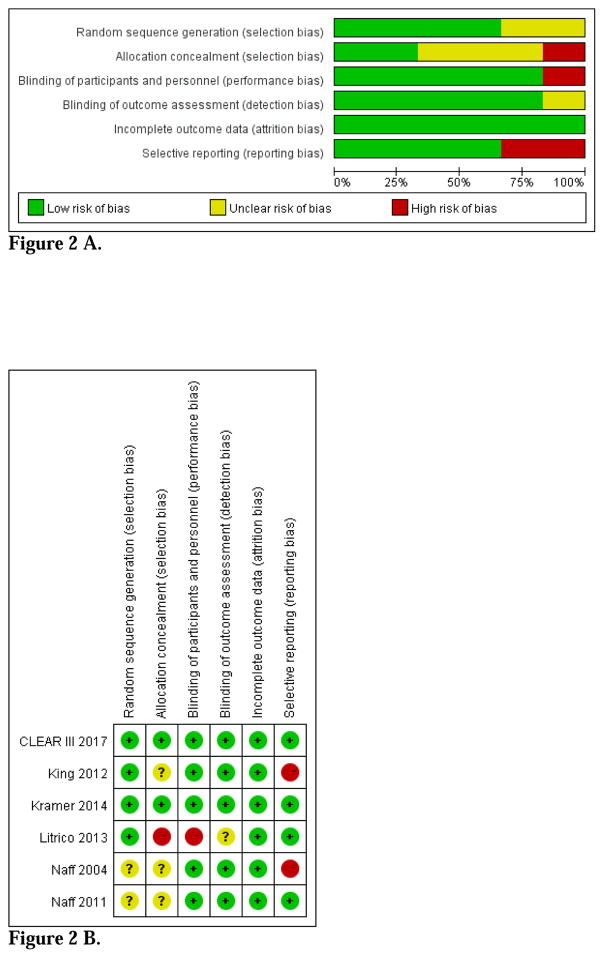
Risk of bias within studies
The overall risk of bias was more than moderate across studies (Figure 2 A–B). None of the urokinase trials reported the details of allocation concealment and only one reported the details of random sequence generation21. Although both participants and outcome assessors were blinded and these trials reported no loss to follow-up or withdrawal, none of both trials included functional outcomes. Therefore, we categorized both trials as ‘high risk’ for selective outcome reporting.
Figure 2.
Figure 2 A. Risk of bias graph
Figure 2 B. Risk of bias summary
All the 4 rt-PA trials were randomized, but one trial didn’t report the details of random sequence generation or allocation concealment19. Another trial was not placebo-controlled and did not report the details of allocation concealment and blinding17. We categorized it as ‘high risk’ for allocation concealment and blinding of participants and personnel and as ‘unclear risk’ for blinding of outcome assessment. The other three trials were categorized as ‘low risk’ for blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting9, 18, 19.
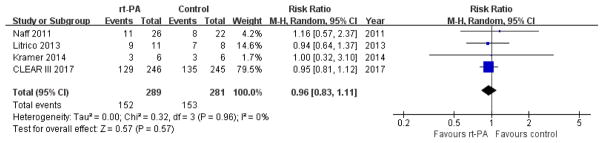
Death and poor functional outcome (Primary outcome)
Both urokinase trials did not report the details of functional outcomes. Although one trial used median mRS at 30 days as one comparator 21, no additional data was provided to determine the rates of survivors with poor or good outcomes. The rt-PA trials used different scales and different time points to define poor functional outcome. For example, Naff et al19 used GOS at 30 days, Litrico et al17 used GOS at 3 months, while Kramer et al18 and CLEAR III9 used mRS at 6 months. We merged these data and performed a meta-analysis using random-effect modeling to minimize heterogeneity. The use of rt-PA was not associated with a reduction in the combined endpoint of death and poor functional outcome (RR 0.96, 95% CI 0.83 to 1.11; participants = 570; studies = 4; I2 = 0%; Figure 3). Because 3 patients in rt-PA group and 6 patients in control group were lost to follow up at 6 months in CLEAR III trial, we performed ITT meta-analysis according to best-case and worst-case scenarios. The results were unchanged; best-case (RR 0.93, 95% CI 0.81 to 1.08; participants = 579; studies = 4; I2 = 0%) and worst-case (RR 0.99, 95% CI 0.85 to 1.14; participants = 579; studies = 4; I2 = 0%).
Figure 3.
Forest plot of death and poor functional outcome at the end of follow up
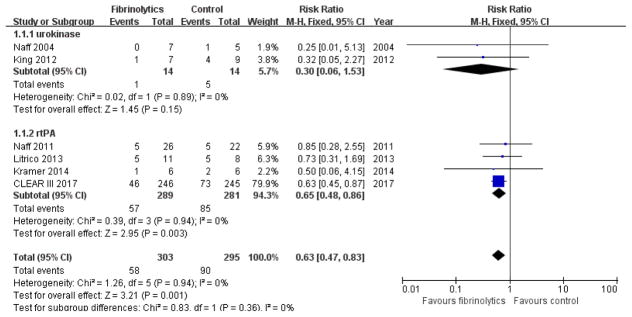
Death at the end of follow-up
All 6 trials reported data on death from any cause. One reported death in hospital18; 3 reported death at 30 days 17, 19, 20; 1 reported death at 6 months 21; and 1 reported death at 30 days and 6months9. Meta-analysis performed using fixed-effect modeling showed that IVF was associated with a reduction in mortality from any cause at the end of follow-up period (RR 0.63, 95% CI 0.47 to 0.83; participants = 598; studies = 6; I2 = 0%; Figure 4). Subgroup analysis of different fibrinolytic agents showed that urokinase did not result in reduction of death (RR 0.30, 95% CI 0.06 to 1.53; participants = 28; studies = 2; I2 = 0%). Use of rt-PA, on the other hand, resulted in significant reduction of death (RR 0.65, 95% CI 0.48 to 0.86; participants = 570; studies = 4; I2 = 0%).
Figure 4.
Forest plot of death at the end of follow up
Survivor with poor functional outcome at the end of follow-up
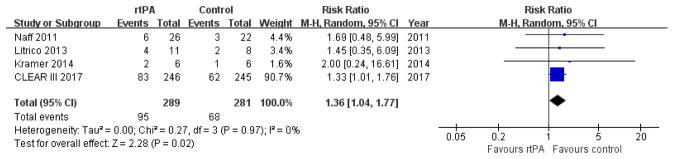
No data could be extracted from the urokinase trials. We merged the data from the rt-PA trials and performed a meta-analysis using random-effect modeling. The use of rt-PA was not associated with a reduction in the proportion of survivors with moderately severe or severe disability at the end of follow-up (RR 1.36, 95% CI 1.04 to 1.77; participants = 570; studies = 4; I2 = 0%; Figure 5).
Figure 5.
Forest plot of poor functional outcome at the end of follow-up
Adverse events
Shunt Dependence
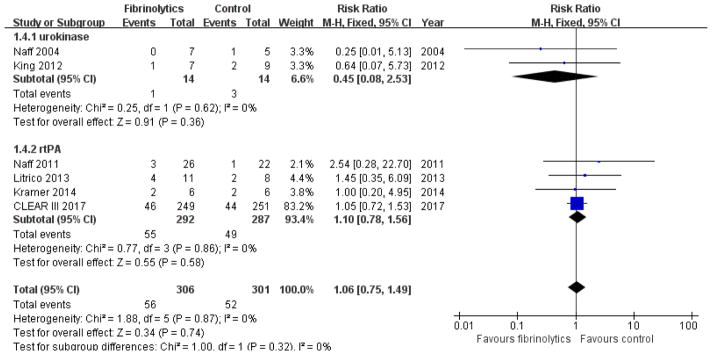
Naff et al did not report on the need for ventriculoperitoneal (VP) shunt placement19. Instead, they reported that 4 patients underwent craniotomy for uncontrolled intracranial hypertension. The other 5 trials reported on the requirement for VP shunt. Meta-analysis using fixed-effect modeling found no association between IVF and shunt placement (RR 1.06, 95% CI 0.75 to 1.49; participants = 607; studies = 6; I2 = 0%; Figure 6). Subgroup analysis showed no difference between the urokinase (RR 0.45, 95% CI 0.08 to 2.53; participants = 28; studies = 2; I2 = 0%) and rt-PA subgroups (RR 1.10, 95% CI 0.78 to 1.56; participants = 579; studies = 4; I2 = 0%).
Figure 6.
Forest plot of craniotomy or shunt dependency
Ventriculitis
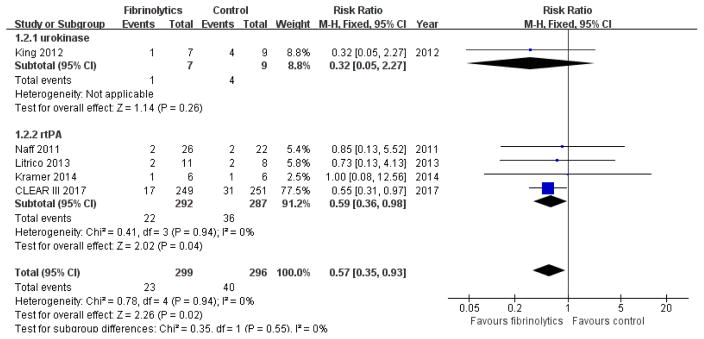
One of the urokinase trials reported only one event of ventriculitis without reference to the treatment group20. All other trials reported the rates of ventriculitis during the treatment and follow-up period. Meta-analysis using fixed-effect modeling showed that the likelihood of ventriculitis in the IVF group is low compared with control group (RR 0.57, 95% CI 0.35 to 0.93; participants = 595; studies = 5; I2 = 0%; Figure 7). Subgroup analysis showed no difference in the incidence of ventriculitis between urokinase and control groups (RR 0.32, 95% CI 0.05 to 2.27; participants = 16; studies = 1; I2 = 0%), while the likelihood of ventriculitis was much lower in the rt-PA group compared with controls (RR 0.59, 95% CI 0.36 to 0.98; participants = 579; studies = 4; I2 = 0%).
Figure 7.
Forest plot of ventriculitis during treatment and follow-up period
Rebleeding
No rebleeding occurred in one of the urokinase trials20. The second urokinase trial did not report the incidence or rate of rebleeding during the trial21. All of the rt-PA trials reported the rates of rebleeding. However, CLEAR III trial only reported the symptomatic rebleeding rate without specifying its definition9. Meta-analysis of rt-PA trials using fixed-effect modeling showed no difference in rebleeding between rt-PA and the control groups (RR 1.65, 95% CI 0.79 to 3.45; participants = 579; studies = 4; I2 = 0%; Figure 8).
Figure 8.
Forest plot of rebleeding
Other Serious Adverse Events
Two trials9, 19 reported other serious adverse events (SAE). Naff et al reported SAE in 61.5% of patients who received rt-PA vs. 36.4% in controls. However, this difference was not statistically significant (p=0.147)19. In CLEAR III, the rates of SAE were less in the rt-PA group than in the control group (46% vs. 60%; RR 0.76 [95% CI 0.64–0.90]; p=0.002)9. Meta-analysis using random-effect modeling found no association between rt-PA and SAE (RR 1.07, 95% CI 0.49 to 2.32; participants = 548; studies = 2; I2 = 83%).
Discussion
Our meta-analysis confirms that the currently used regimens of IVF in patients with IVH result in a reduction in mortality. However, the reduction in mortality is accompanied by an increase in the number of patients living with moderately severe and severe disability, and IVF does not increase the proportion of survivors with long-term good recovery and functional outcome. Furthermore, the use of IVF does not result in a decrease in the need for VP shunt placement in IVH patients who require an extraventricular drain. Overall, the use of IVF in IVH appears safe. It does not increase the risks of infection, ventriculitis, rebleeding, or serious adverse events. In subgroup analysis based on the use of rt-PA vs. urokinase for IVF, both agents have similar safety profile. However, unlike rt-PA, urokinase does not seem to reduce mortality.
Dissimilar to our findings, a previous meta-analysis of 24 studies reported that IVF increases the likelihood of good functional outcome after IVH and decreases the need for VP shunt dependence8. There are several reasons for these disparate results. While the meta-analysis by Khan et al. included randomized trials, and prospective and retrospective case cohort studies8, we only limited the current meta-analysis to randomized, controlled trials to minimize bias. We excluded 4 urokinase trials that were included in their meta-analysis, because they were not truly randomized due to biased or alternate allocation22–25. In addition, we included the results from CLEAR III9, the largest IVF trial, in our pooled analysis.
Contrary to preclinical studies showing that rt-PA may have pro-inflammatory and neurotoxic effects10–12, our meta-analysis shows that the likelihood of ventriculitis is lower in IVF (particularly rt-PA)-treated patients compared with controls. Kramer et al.29 reported that IVF with rt-PA in patients with ruptured brain aneurysms and IVH produces a transient local inflammatory response, the severity of which is associated with the degree of fibrinolysis, suggesting it may be induced by release of hemoglobin degradation products, rather than rt-PA itself. This suggests that accelerated removal of IVH by IVF might decrease the incidence of ventriculitis by decreasing the accumulation of blood degradation products in the cerebrospinal fluid, and raises an intriguing question as to whether intraventricular co-administration of drugs which activate Heme Oxygenase-1 (which catalyzes the degradation of heme), such as deferoxamine mesylate could enhance the efficacy of IVF in IVH30–32.
Gaberelet al13 reported that urokinase resulted in a better functional outcome than rt-PA in a rodent model of IVH, and attributed this to the potential pro-inflammatory and toxic effects of rt-PA. This prompted us to perform a subgroup analysis based on the fibrinolytic agent used to examine any potential differences in efficacy and safety between IVF with rt-PA vs. urokinase in IVH patients. The findings from our meta-analysis are not concordant with pre-clinical results. Compared to controls, IVF with urokinase had no effect on mortality or the incidence of ventriculitis, while rt-PA was associated with a reduction in mortality and ventriculitis after IVH.
Post-hoc analyses of CLEAR III trial suggest that patients with initial IVH volume ≥20 ml achieved better functional outcomes with rt-PA and that the probability of good functional recovery increased when IVH clearance was >80%9. Our meta-analysis could not explore these subgroups of IVF treated patients.
This meta-analysis provides the most up-to-date review of IVF in IVH following the completion of CLEAR III trial. We included only truly randomized trials with at least one clinical outcome to enhance the evidence quality of our study. However, our restrictive selection criteria led to the inclusion of a relatively small number of subjects and trials in this pooled analysis, especially for IVF with urokinase trials. Only 2 urokinase trials with 28 participants were included; their overall risk of bias was moderate; and none reported functional outcomes. As a result of these limitations, subgroup analyses were not adequately powered to evaluate the differences between IVF with urokinase vs. rt-PA and no firm conclusions can be made. Another limitation is that although all of the included rt-PA trials reported functional outcomes, they used different scales and cut-off values to define good outcome. Lastly, our results were likely influenced by the high number of subjects included from one trial, CLEAR III.
In summary, the use of IVF, particularly with rt-PA, in most patients with IVH is unlikely to benefit their functional recovery. While IVF appears to be safe, its benefit is limited to a reduction in mortality at the expense of increased number of survivors with moderately severe to severe disability. These results are in concordance with those from CLEAR III, and suggest that: 1) the decision to use IVF in IVH patients should not be routinely recommended in clinical practice and if used should be based on the patients’/family’s attributes towards survival & dependency, or preferably in the setting of future clinical trials; and 2) future trials investigating IVF, in particular rt-PA, should have a strong rationale as to why the results are likely to differ from the currently available evidence, and should consider innovative pharmacological approaches such as local co-induction of Heme Oxygenase-1 transcription.
Acknowledgments
Funding
DW receives research grant support from the National Natural Science Foundation of China (Grant No.81400964). ML receives research grant support from the National Natural Science Foundation of China (Grant No. 81620108009). MS is partly supported by the National Institute of Neurological Disorders and Stroke (Grant No. U01 NS 074425) and the American Heart Association Collaborative Science Award (Grant No. 15CSA24540001). These agencies did not have any role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Abbreviations
- IVF
intraventricular fibrinolysis
- IVH
intraventricular hemorrhage
- RCTs
randomized controlled trials
- EVD
extraventricular drainage
- ICH
intracerebral hemorrhage
- rt-PA
recombinant tissue-plasminogen activator
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ICTRP
WHO International Clinical Trial Registry Platform
- mRS
modified Rankin Scale
- GOS
Glasgow Outcome Scale
- RR
relative risk
- CI
confidence interverval
- VP
ventriculoperitoneal
- SAE
serious adverse events
- NR
not reported
- ICU
intensive care unit
- SD
standard deviation
- IQR
interquartile range
- NIHSS
National Institutes of Health Stroke Scale
- SAH
Subarachnoid hemorrhage
Appendix 1
MEDLINE (OVID) search strategy
exp cerebral hemorrhage/
Intracranial hemorrhage, hypertensive/or Intracranial hemorrhages/
Exp Cerebral ventricles/bs or exp *cerebral ventricles/or IVH.tw.
((brain or cerebral or intraventricular or intracranial or intracerebral) adj10 (haemorrhage$ or hemorrhage$ or bleed$)).tw.
1 or 2 or 3 or 4
Thrombolytic therapy/
Exp Fibrinolytic agents
Fibrinolysis/
(thrombolys$ or fibrinolys$ or clot lysis).tw.
(plasminogen or plasmin or t-PA or rt-PA or rtPA).tw.
(urokinase or plasminogen activator or pro?urokinase or streptokinase or alteplase or anistreplase or saruplase or rt-pa or r-tpa or rtpa or tpa or t-pa).tw.
or/6–11
5 and 12
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21
exp animals/not humans.sh.
22 not 23
13 and 24
EMBASE (OVID) search strategy
exp brain hemorrhage/
exp brain ventricle/
bleeding/
2 and 3
(ivh or pivh).tw.
(intraventricular or ventricle$ or intracerebral or periventricular or brain or intracranial).tw.
(hemorrhag$ or haemorrhag$ or bleed$).tw.
6 and 7
brain ventricle dilatation/
1 or 4 or 5 or 8 or 9
exp fibrinolytic agent/
fibrinolytic therapy/
fibrinolysis/
blood clot lysis/
fibrinogenolysis/
(thromboly$ or fibrinoly$ or antithromb$).tw.
(urokinase or plasminogen activator or pro?urokinase or Streptokinase or alteplase or anistreplase or saruplase or rt-pa or r-tpa orrtpa or tpa or t-pa).tw.
intracerebroventricular drug administration/
clot lys$.tw.
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
10 and 20
random$.tw.
factorial$.tw.
crossover$.tw.
cross over$.tw.
cross-over$.tw.
placebo$.tw.
(doubl$ adj blind$).tw.
(singl$ adj blind$).tw.
assign$.tw.
allocat$.tw.
volunteer$.tw.
crossover procedure/
double blind procedure/
randomized controlled trial/
single blind procedure/
22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36
(animal/or nonhuman/) not human/
37 not 38
21 and 39
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaberel T, Magheru C, Emery E. Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev. 2012;35:485–494. doi: 10.1007/s10143-012-0399-9. discussion 494–485. [DOI] [PubMed] [Google Scholar]
- 2.Hemphill JC, 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 3.Dey M, Jaffe J, Stadnik A, Awad IA. External ventricular drainage for intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2012;12:24–33. doi: 10.1007/s11910-011-0231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carhuapoma JR. Thrombolytic therapy after intraventricular hemorrhage: Do we know enough? J Neurol Sci. 2002;202:1–3. doi: 10.1016/s0022-510x(02)00242-3. [DOI] [PubMed] [Google Scholar]
- 5.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery. 1986;19:553–572. doi: 10.1227/00006123-198610000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 2. In vivo safety study of intraventricular urokinase. Neurosurgery. 1986;19:547–552. doi: 10.1227/00006123-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Lapointe M, Haines S. Fibrinolytic therapy for intraventricular hemorrhage in adults. Cochrane Database of Systematic Reviews. 2002:CD003692. doi: 10.1002/14651858.CD003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan NR, Tsivgoulis G, Lee SL, Jones GM, Green CS, Katsanos AH, et al. Fibrinolysis for intraventricular hemorrhage: An updated meta-analysis and systematic review of the literature. Stroke. 2014;45:2662–2669. doi: 10.1161/STROKEAHA.114.005990. [DOI] [PubMed] [Google Scholar]
- 9.Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: Results of the randomised, multicentre, multiregion, placebo-controlled clear iii trial. Lancet. 2017;09:09. doi: 10.1016/S0140-6736(16)32410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tpa) increases neuronal damage after focal cerebral ischemia in wild-type and tpa-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 11.Thiex R, Kuker W, Muller HD, Rohde I, Schroder JM, Gilsbach JM, et al. The long-term effect of recombinant tissue-plasminogen-activator (rt-pa) on edema formation in a large-animal model of intracerebral hemorrhage. Neurol Res. 2003;25:254–262. doi: 10.1179/016164103101201463. [DOI] [PubMed] [Google Scholar]
- 12.Goto H, Fujisawa H, Oka F, Nomura S, Kajiwara K, Kato S, et al. Neurotoxic effects of exogenous recombinant tissue-type plasminogen activator on the normal rat brain. J Neurotrauma. 2007;24:745–752. doi: 10.1089/neu.2006.0183. [DOI] [PubMed] [Google Scholar]
- 13.Gaberel T, Montagne A, Lesept F, Gauberti M, Lemarchand E, Orset C, et al. Urokinase versus alteplase for intraventricular hemorrhage fibrinolysis. Neuropharmacology. 2014;85:158–165. doi: 10.1016/j.neuropharm.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre c, manheimer e, glanville j. Chapter 6: Searching for studies. In: Higgins jpt, green s., editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated march 2011] The cochrane collaboration; 2011. Available from www.Cochrane-handbook.Org. [Google Scholar]
- 16.Higgins jpt, green s., editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated march 2011] The cochrane collaboration; 2011. Available from www.Cochrane-handbook.Org. [Google Scholar]
- 17.Litrico S, Almairac F, Gaberel T, Ramakrishna R, Fontaine D, Sedat J, et al. Intraventricular fibrinolysis for severe aneurysmal intraventricular hemorrhage: A randomized controlled trial and meta-analysis. Neurosurgical Review. 2013;36:523–530. doi: 10.1007/s10143-013-0469-7. discussion 530–521. [DOI] [PubMed] [Google Scholar]
- 18.Kramer AH, Roberts DJ, Holodinsky J, Todd S, Hill MD, Zygun DA, et al. Intraventricular tissue plasminogen activator in subarachnoid hemorrhage patients: A prospective, randomized, placebo-controlled pilot trial. Neurocritical Care. 2014;21:275–284. doi: 10.1007/s12028-014-9965-z. [DOI] [PubMed] [Google Scholar]
- 19.Naff N, Williams MA, Keyl PM, Tuhrim S, Bullock MR, Mayer SA, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: The intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42:3009–3016. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naff NJ, Hanley DF, Keyl PM, Tuhrim S, Kraut M, Bederson J, et al. Intraventricular thrombolysis speeds blood clot resolution: Results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54:577–583. doi: 10.1227/01.neu.0000108422.10842.60. discussion 583–574. [DOI] [PubMed] [Google Scholar]
- 21.King NKK, Lai JL, Tan LB, Lee KK, Pang BC, Ng I, et al. A randomized, placebo-controlled pilot study of patients with spontaneous intraventricular haemorrhage treated with intraventricular thrombolysis. Journal of Clinical Neuroscience. 2012;19:961–964. doi: 10.1016/j.jocn.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 22.Gubucz I, Kakuk I, Major O, Szegedi N, Barsi P, Panczel G, et al. effectiveness and safety of intraventricular fibrinolysis in secondary intraventricular hemorrhages (a prospective, randomized study) Orvosi Hetilap. 2004;145:1609–1615. [PubMed] [Google Scholar]
- 23.Tung MY, Ong PL, Seow WT, Tan KK. A study on the efficacy of intraventricular urokinase in the treatment of intraventricular haemorrhage. British Journal of Neurosurgery. 1998;12:234–239. doi: 10.1080/02688699845050. [DOI] [PubMed] [Google Scholar]
- 24.Naff NJ, Carhuapoma JR, Williams MA, Bhardwaj A, Ulatowski JA, Bederson J, et al. Treatment of intraventricular hemorrhage with urokinase : Effects on 30-day survival. Stroke. 2000;31:841–847. doi: 10.1161/01.str.31.4.841. [DOI] [PubMed] [Google Scholar]
- 25.Akdemir H, Selcuklu A, Pasaoglu A, Oktem IS, Kavuncu I. Treatment of severe intraventricular hemorrhage by intraventricular infusion of urokinase. Neurosurgical Review. 1995;18:95–100. doi: 10.1007/BF00417665. [DOI] [PubMed] [Google Scholar]
- 26.Ziai WC, Muschelli J, Thompson CB, Keyl PM, Lane K, Shao S, et al. Factors affecting clot lysis rates in patients with spontaneous intraventricular hemorrhage. Stroke. 2012;43:1234–1239. doi: 10.1161/STROKEAHA.111.641050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziai WC, Melnychuk E, Thompson CB, Awad I, Lane K, Hanley DF. Occurrence and impact of intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Critical Care Medicine. 2012;40:1601–1608. doi: 10.1097/CCM.0b013e318241e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb AJS, Ullman NL, Mann S, Muschelli J, Awad IA, Hanley DF. Resolution of intraventricular hemorrhage varies by ventricular region and dose of intraventricular thrombolytic: The clot lysis: Evaluating accelerated resolution of ivh (clear ivh) program. Stroke. 2012;43:1666–1668. doi: 10.1161/STROKEAHA.112.650523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer AH, Jenne CN, Zygun DA, Roberts DJ, Hill MD, Holodinsky JK, et al. Intraventricular fibrinolysis with tissue plasminogen activator is associated with transient cerebrospinal fluid inflammation: A randomized controlled trial. J Cereb Blood Flow Metab. 2015;35:1241–1248. doi: 10.1038/jcbfm.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selim M. Deferoxamine mesylate: A new hope for intracerebral hemorrhage: From bench to clinical trials. Stroke. 2009;40:S90–91. doi: 10.1161/STROKEAHA.108.533125. [DOI] [PubMed] [Google Scholar]
- 31.LeBlanc RH, 3rd, Chen R, Selim MH, Hanafy KA. Heme oxygenase-1-mediated neuroprotection in subarachnoid hemorrhage via intracerebroventricular deferoxamine. J Neuroinflammation. 2016;13:244. doi: 10.1186/s12974-016-0709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of iron in brain injury after intraventricular hemorrhage. Stroke. 2011;42:465–470. doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]