Abstract
Objective
Arterial stiffness and peripheral artery disease (PAD) are both associated with an elevated risk of major adverse cardiac events (MACE); however, the association between arterial stiffness and PAD is less well characterized. The goal of the present study was to examine the association between parameters of radial artery tonometry, a non-invasive measure of arterial stiffness, and PAD.
Methods
We conducted a cross-sectional study of 134 vascular surgery outpatients (controls=33, PAD=101) using arterial applanation tonometry. Central augmentation index normalized to 75bpm (central AIX) and peripheral augmentation index (peripheral AIX) were measured using radial artery pulse wave analysis (PWA). Pulse wave velocity (PWV) was recorded at the carotid and femoral arteries. PAD was defined as symptomatic claudication with an ankle-brachial index (ABI) of <0.9 or a history of peripheral revascularization. Controls had no history of atherosclerotic vascular disease and an ABI≥0.9.
Results
Among the 126 participants with high quality tonometry data, compared to controls (n=33), patients with PAD (n=93) were older, with higher rates of hypertension, hyperlipidemia, diabetes, and smoking (P<.05). Patients with PAD also had greater arterial stiffness as measured by central AIX, peripheral AIX, and PWV (P<.05). In a multivariable model, each 10-unit increase in central and peripheral AIX was associated with significantly increased odds of PAD (OR 2.1, 95% CI 1.1–3.9, P=.03 and OR 1.9, 95% CI 1.2–3.2, P=.01, respectively). Additionally, central and peripheral AIX were highly correlated (r(120)=.76, P<.001).
Conclusions
In a cross-sectional analysis, arterial stiffness as measured by the augmentation index is independently associated with PAD, even when adjusting for several atherosclerotic risk factors. Further prospective data is needed to establish whether radial artery tonometry could be a tool for risk stratification in the PAD population.
INTRODUCTION
Peripheral artery disease (PAD) is a global health problem with increasing incidence,1 high economic burden,2 and poor patient outcomes; these include a strong association with cardiovascular disease risk,3 disability,4 and low quality of life.5 Furthermore, PAD is associated with a significantly increased risk of death, particularly from coronary heart disease (CHD).6, 7 Therefore, the early identification and risk stratification of PAD patients is extremely important for risk factor modification and targeted therapeutic interventions.
Arterial stiffness develops in association with the pathophysiological processes of arteriosclerosis and atherosclerosis.8 Evidence demonstrates the relationship between arterial stiffness, and CHD progression and mortality.9, 10 The most common method for quantifying arterial stiffness is based on the characteristics of the pulse wave generated with each heartbeat. Measuring the pulse-wave at two points of known distance, usually the common carotid and femoral arteries, yields the pulse-wave velocity (PWV). Because less wall compliance translates into higher velocity, PWV is considered the gold-standard measure of arterial stiffness.11–13 As each pulse wave travels forward, some of it gets reflected in a retrograde fashion.14 Pulse wave analysis (PWA) examines each waveform, which is the combination of a forward and reflected pulse wave. Stiffer arteries will reflect waves back earlier in diastole, leading to a higher augmented pressure, which refers to the increase in pressure from the reflected wave.13, 15, 16 Augmentation index (AIX) is the ratio of the augmented pressure, which is the difference between the first and second systolic peaks, and the pulse pressure, expressed as a percentage.17 A higher AIX is an indirect measure of greater arterial stiffness.12, 18 Although PWV and AIX are both accepted noninvasive methods, AIX measured by radial artery tonometry requires only one measurement site.12, 19
While many studies have linked arterial stiffness to CHD, the relationship between arterial stiffness and PAD is less well characterized. Furthermore, compared to PWV even fewer studies have examined the link between AIX, as measured by radial artery tonometry, and PAD. The goals of the present study were to investigate the relationship between PAD and arterial stiffness as measured by the AIX, as well as to identify the covariates related to this association. We hypothesize that the central and peripheral AIX are independently associated with PAD. Concurrently, we hypothesize that PWV is also associated with the presence of PAD.
METHODS
Study participants
A cross-sectional sample of 134 patients was recruited from a vascular surgery outpatient clinic between February 2012 and September 2016. Patients were considered to have PAD if they exhibited symptomatic claudication with an abnormal ABI (<0.9) or if they had a history of symptomatic PAD and had previously undergone peripheral revascularization (n=101). Controls were patients with normal ABI (>0.9), and no history of PAD, coronary artery disease, or cerebrovascular disease (n=33). To be eligible for inclusion, all participants had to be at least 35 years old to be representative of the PAD population at the San Francisco Veterans Affairs Medical Center. Individuals with significant renal (creatinine ≥ 2), hepatic (Child-Pugh class ≥ B), non-vascular inflammatory (e.g. rheumatoid arthritis or those requiring immunosuppressive medications), or concurrent severe acute disease were excluded.
Basic demographic variables collected on all participants included age, sex, race, and comorbidities. Blood pressure was assessed at the brachial artery and an ABI was calculated for each lower extremity using standard techniques.20 Participants were asked to complete the PTSD checklist - civilian version (PCL-C), with PTSD defined as a PCL ≥ 40.21 Depression was assessed using the patient health questionnaire (PHQ-9), with depression defined as a score ≥ 10.22 Current medications including aspirin, ace-inhibitor, beta-blocker, and statin were also recorded. A blood sample was obtained for standard clinical laboratory tests including lipid panel (total cholesterol, LDL, HDL, and triglycerides), estimated glomerular filtration rate (eGFR), hemoglobin A1c, and high-sensitivity C-reactive protein (hsCRP). The investigator-initiated protocol was approved by the University of California, San Francisco (UCSF) Committee on Human Research as well as the SFVA Research and Development Office. All participants gave written informed consent.
Pulse wave analysis
Pulse wave analysis (PWA) was performed using the SphygmoCor® applanation tonometer (AtCor Medical, Sydney, Australia). Patients were positioned supine on an exam table and allowed to rest for a minimum of five minutes. The brachial artery blood pressure was measured and the right arm was abducted 15 degrees with the hand supinated for access to the radial artery. The tonometer was applied to the radial artery and SphygmoCor’s proprietary software calculated the augmentation index as well as a quality index. Augmentation index (AIX) is defined as [augmented pressure/(systolic blood pressure - diastolic blood pressure)]; while peripheral AIX is calculated directly, this software uses established transfer equations to approximate the central artery waveforms using recordings from the radial artery in a process that has been previously validated by invasive methods.23 Because central AIX varies as a function of heart rate, all measurements were normalized to 75 beats per minute (bpm), consistent with previous studies.11, 17 The quality index is a summary measure calculated by the proprietary SphygmoCor software and based on variability in the recorded waveforms (e.g. pulse height variation, etc.). A higher quality index reflects less waveform variability and is assumed to be of higher quality. Multiple recordings (up to four) were conducted for each patient with the goal of achieving at least one measurement with a quality index >80. The measurement with the highest quality index was recorded.
Pulse wave velocity
Patients were positioned supine on an exam table and allowed to rest for a minimum of five minutes. The brachial artery blood pressure was measured and three EKG leads were applied. The distances from the sternal notch to the right common carotid and right common femoral arteries were measured and recorded. Using the SyphmoCor® applanation tonometer, waveforms were captured at both the common carotid and common femoral artery sites to calculate a carotid-femoral pulse-wave velocity (PWV). The exam with the lowest pulse-transit standard deviation (PTT-SD) was recorded.
Quality control
To ensure that only the highest quality tonometry data were used, all PWA and PWV data were reviewed and rated following a strict protocol by two reviewers (GZ and KS). Quality criteria were established in accordance with manufacturer guidelines. These reviewers had 100% interrater agreement. For PWA, data that had a quality index <80 were discarded. Minor quality criteria included a pulse height ≥ 80, height variation ≤ 5%, and diastolic variation ≤ 5%. To be considered for inclusion, at least two of the minor criteria also had to be met. For PWV, a carotid-femoral PTT-SD ≤ 10% was required for inclusion.
Eight participants had tonometry data of insufficient quality and were dropped from further analysis. Therefore, high quality PWA data for central AIX were obtained on 126 participants (PAD=93, control=33). Peripheral AIX data were missing for two participants and were dropped for two others because their values were >three standard deviations above the mean. This resulted in peripheral AIX data on 122 patients (PAD=89, control=33). Additionally, quality PWV data were obtained on 33 patients (PAD=16, control=17).
Statistical analysis
Data analysis was performed using Stata version 14 (StataCorp, College Station, Texas). Two-tailed unpaired t-tests and Fisher’s exact test were used to test for differences between PAD and control groups on variables of interest. Log-values of CRP were used due to its right-skewed distribution. Multivariable logistic regression models were used to test whether central AIX and peripheral AIX were independently associated with PAD after adjustment for potential confounders. To determine which variables to include in the multivariable model, a univariate screening analysis was performed with PAD as the outcome, while adjusting for either central AIX at 75bpm or peripheral AIX as each covariate was added. Covariates with a P<.10 were included in the final model. All variables in Table I were screened for inclusion in the multivariable model except for CAD and prior peripheral revascularization since these variables were used to separate participants into controls and PAD patients, thus were completely collinear with PAD. ABI was also excluded for similar reasons. In instances where related variables met criteria for inclusion (e.g. systolic blood pressure and hypertension), the underlying disorder (e.g. hypertension) was preferentially selected to avoid overfitting the model.
TABLE I.
Characteristics of Patients with PAD and Controls
| Characteristics* | PAD (n=93) | Controls (n=33) | P-valuea | ||
|---|---|---|---|---|---|
| n/mean | %/(SD) | n/mean | %/(SD) | ||
| Demographics | |||||
| Age | 69.9 | (6.7) | 66.3 | (9.7) | .02 |
| Male Sex | 90 | 96.8% | 32 | 97.0% | 1.00 |
| Caucasian | 64 | 69.6% | 21 | 63.6% | .52 |
| Comorbidites and Risk Factors | |||||
| Pack Years | 43.6 | (30.3) | 19.8 | (24.3) | <.001 |
| Hypertension | 85 | 91.4% | 20 | 60.6% | <.001 |
| Hyperlipidemia | 78 | 83.9% | 21 | 63.6% | .03 |
| Diabetes Mellitus | 34 | 36.6% | 5 | 15.2% | .03 |
| Coronary Artery Disease | 38 | 40.9% | 0 | 0.0% | <.001b |
| Depression (PHQ-9 score ≥ 10) | 12 | 15.2% | 2 | 8.3% | .51 |
| PTSD (PCL-C ≥ 40) | 16 | 20.5% | 2 | 8.7% | .23 |
| Systolic Blood Pressure (mm Hg) | 145.1 | (20.8) | 135.2 | (14.5) | .01 |
| Diastolic Blood Pressure (mm Hg) | 78.4 | (10.7) | 81.5 | (7.9) | .14 |
| Ankle Brachial Index (ABI) | 0.73 | (0.15) | 1.13 | (0.15) | <.001 |
| History of Revascularization | 38 | 41% | 0 | 0.0% | <.001b |
| Medications | |||||
| Aspirin | 71 | 76.3% | 19 | 57.6% | .05 |
| Ace-inhibitor | 46 | 49.5% | 12 | 36.4% | .23 |
| Beta-blocker | 56 | 60.2% | 6 | 18.2% | <.001 |
| Statin | 77 | 82.8% | 18 | 54.6% | <.01 |
| Laboratory Studies | |||||
| Total Cholesterol (mg/dL) | 162.1 | (42.1) | 175.9 | (35.0) | .11 |
| LDL (mg/dL) | 85.8 | (34.3) | 104.4 | (29.0) | <.01 |
| HDL (mg/dL) | 48.9 | (13.8) | 47.3 | (13.0) | .58 |
| Triglycerides (mg/dL) | 135.2 | (94.8) | 125.8 | (111.1) | .65 |
| eGFR (mL/min) | 75.9 | (22.9) | 87.2 | (23.4) | .02 |
| HgbA1C (%) | 6.18 | (1.31) | 5.93 | (1.52) | .39 |
| hsCRP (mg/L) | 3.61 | (3.76) | 3.88 | (4.10) | .73 |
Continuous characteristics are summarized by mean (SD) with between-groups p-values calculated using a t-test. Categorical variables were summarized by number (%) with p-values calculated using Fisher’s exact test.
By definition control subjects did not have CAD or revascularization.
RESULTS
Compared to controls (n=33), subjects with PAD (n=93) were significantly older, more likely to have hypertension, hyperlipidemia, and diabetes, and had smoked a greater number of pack years. Those with PAD also had significantly higher systolic blood pressure, lower LDL, and lower eGFR. The paradoxically lower LDL levels in the PAD group likely corresponded to their higher rate of statin use. Additionally, subjects with PAD were more likely to take aspirin and beta-blockers. Of note, the controls, who were recruited from an outpatient vascular surgery clinic, had a relatively high prevalence of various atherosclerotic risk factors. For example, 61% of controls had hypertension and 64% had hyperlipidemia, while there was no between-group difference for CRP (Table I).
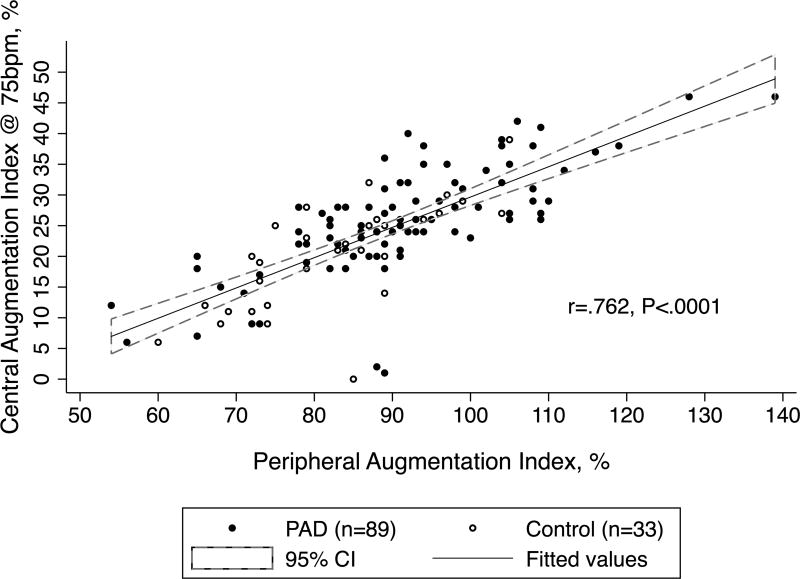
Analysis of the PWA data demonstrates that subjects with PAD had significantly higher central AIX at 75bpm (25.5 ± 8.9 vs. 19.6 ± 8.7, P=.002) and peripheral AIX (91.3 ± 14.3 vs. 81.8 ± 11.5, P=<.001) (Table II). A sub-group analysis of non-diabetic participants (PAD=59, control=28) found a similarly elevated AIX in PAD versus controls (central AIX 26.3 ± 8.5 vs. 18.8 ± 9.0, P<.001 and peripheral AIX 93.5 ± 13.8 vs. 81.6 ± 11.5, P=<.001). A Pearson correlation between central and peripheral AIX revealed a high degree of inter-relatedness between the two measures (r(120)=.762, P<.001), with variation in one explaining 58% of the variability in the other (Figure I). For the 33 subjects with high-quality PWV data (PAD=16, controls=17), subjects with PAD had a higher velocity (9.8 ± 2.7 vs. 7.6 ± 3.0 m/s, P=.034), consistent with stiffer central arteries.
TABLE II.
Tonometry Parameters in Patients with PAD and Controls
| Characteristicsa | PAD (n=93) | Controls (n=33) |
P-value | ||
|---|---|---|---|---|---|
| mean | (SD) | mean | (SD) | ||
| Central Artery Stiffness | |||||
| Central Systolic Pressure (mm/Hg) | 135.7 | (20.5) | 126.9 | (16.1) | .03 |
| Central Diastolic Pressure (mm/Hg) | 78.9 | (13.4) | 82.9 | (7.7) | .11 |
| Central Augmentation Index | 31.5 | (9.3) | 25.8 | (8.9) | <.01 |
| Central Augmentation Index at 75bpm | 25.5 | (8.9) | 19.6 | (8.7) | <.01 |
| Peripheral Artery Stiffnessb | |||||
| Peripheral Systolic Pressure (mm/Hg) | 146.4 | (20.4) | 136.5 | (15.7) | .01 |
| Peripheral Diastolic Pressure (mm/Hg) | 79.1 | (11.3) | 81.9 | (7.6) | .19 |
| Peripheral Mean Pressure (mm/Hg) | 101.6 | (13.2) | 101.2 | (8.9) | .89 |
| Peripheral Augmentation Index | 91.3 | (14.3) | 81.8 | (11.5) | <.001 |
Continuous characteristics are summarized by mean (SD) with between-groups p-values calculated using a t-test.
Peripheral tonometry data summarizes all values after two outliers >3SD from the mean were dropped.
Figure I.
Relationship between Peripheral and Central Aix (n=122).
To determine whether central AIX and peripheral AIX were associated with PAD independent of potential confounders, a series of univariate logistic regression analyses were performed between PAD and each potential confounder while adjusting for either central AIX or peripheral AIX. All covariates with a resulting P-value <.10 were added to the final model, and adjustment for age and race was also made since they are frequent confounders. The resulting multivariable model adjusted for several known risk factors for PAD and included the following: age, race, hypertension, diabetes, hyperlipidemia, pack years, and eGFR (Table III–IV). The multivariable models revealed that central AIX normalized to 75bpm (OR 1.08, 95% CI 1.01–1.15, P=.026) and peripheral AIX (OR 1.07, 95% CI 1.02–1.12, P=.009) were independently associated with higher odds of PAD. This corresponds to a 2.07-fold increased odds of PAD for each 10-unit increase in central AIX normalized to 75bpm (OR 2.07, 95% CI 1.09–3.93, P=.026) and a 1.9- fold increased odds of PAD for each 10-unit increase in peripheral AIX (OR 1.94, 95% CI 1.18–3.20, P=.009). Central AIX (OR 1.09, 95% CI 1.01–1.18, P=.021) and peripheral AIX (OR 1.08, 95% CI 1.02–1.14, P=.010) remained significantly associated with PAD when medication use was added to the multivariable models. The only other variables that remained significantly associated with PAD in the multivariable models were number of pack years smoked (both models) and hypertension (central AIX model only) (Table III–IV).
TABLE III.
Bivariate and Multivariable Associations between Central Augmentation Index at 75bpm and PAD (n=126)
| Predictor | Bivariate Analysisa | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Central AIX @ 75bpmb | 1.08 | (1.03–1.13) | <.01 | 1.08 | (1.01–1.15) | .03 |
| Age (years) | 1.05 | (.99–1.11) | .13 | 1.00 | (.92–1.09) | .96 |
| Caucasian | 1.58 | (0.65–3.83) | .31 | 1.86 | (0.57–6.04) | .30 |
| Hypertension | 6.43 | (2.25–18.4) | <.01 | 4.18 | (1.07–16.3) | .04 |
| Diabetes Mellitus | 3.49 | (1.18–10.3) | .02 | 2.04 | (0.55–7.52) | .29 |
| Hyperlipidemia | 3.34 | (1.28–8.73) | .01 | 2.52 | (0.66–9.67) | .18 |
| Pack Years | 1.03 | (1.01–1.06) | <.01 | 1.03 | (1.00–1.06) | .02 |
| eGFR (mL/min) | 0.98 | (0.96–1.00) | .02 | 0.98 | (0.96–1.00) | .07 |
The bivariate logistic regression model coefficient estimates the associated odds ratio (95% confidence interval) of PAD per one unit change in the predictor when controlling for central augmentation index at 75 bpm. All variables from table I were run through a bivariate analysis to screen for potential confounders. All variables with a p-value <.10 in the bivariate analysis were included in the multivariable model. Age and Caucasian race were also included, despite not achieving a p-value <.10 in the bivariate analysis, since they are common confounders. Sex was omitted due to the small number of female subjects.
Central AIX was run as a univariate logistic regression because it was the predictor in the bivariate analysis.
Table IV.
Bivariate and Multivariable Associations between Peripheral Augmentation Index and PAD (n=122)
| Predictor | Bivariate Analysisa | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Peripheral AIXb | 1.06 | (1.02–1.09) | <.01 | 1.07 | (1.02–1.12) | <.01 |
| Age (years) | 1.04 | (0.98–1.10) | .23 | 1.00 | (0.92–1.08) | .93 |
| Caucasian | 1.45 | (0.59–3.52) | .42 | 1.71 | (0.52–5.64) | .38 |
| Hypertension | 5.39 | (1.89–15.3) | <.01 | 3.63 | (0.92–14.2) | .07 |
| Diabetes Mellitus | 4.23 | (1.35–13.3) | .01 | 3.04 | 0.75–12.4) | .12 |
| Hyperlipidemia | 2.84 | (1.09–7.40) | .03 | 2.11 | (0.56–7.98) | .27 |
| Pack Years | 1.04 | (1.01–1.06) | <.01 | 1.03 | (1.01–1.06) | .01 |
| eGFR (mL/min) | 0.98 | (0.96–1.00) | .05 | 0.98 | (0.96–1.01) | .16 |
The bivariate logistic regression model coefficient estimates the associated odds ratio (95% confidence interval) of PAD per one unit change in the predictor when controlling for central augmentation index at 75 bpm. All variables from table I were run through a bivariate analysis to screen for potential confounders. All variables with a p-value <.10 in the bivariate analysis were included in the multivariable model. Age and Caucasian race were also included, despite not achieving a p-value <.10 in the bivariate analysis, since they are common confounders. Sex was omitted due to the small number of female subjects.
Peripheral AIX was run as a univariate logistic regression because it was the predictor in the bivariate analysis.
Finally, an analysis was performed to determine the extent to which the presence of hypertension influences the relationship between AIX and PAD. Participants with hypertension had higher central AIX (24.5 ± 9.1 vs. 21.2 ± 9.5, P=.14) and peripheral AIX (90.0 ± 14.3 vs. 82.4 ± 12.2, P=.02), but only the difference in peripheral AIX reached statistical significance. A univariate analysis of PAD and hypertension, adjusting for either central AIX (OR 6.43, 95% CI 2.25–18.4, P<.01) or peripheral AIX (OR 5.39, 95% CI 1.89–15.3, P<.01), found that hypertension was significantly associated with PAD. However, the results of the multivariable model demonstrate that central and peripheral AIX remain significantly associated with PAD, while hypertension only remains significantly associated with PAD in the model for central AIX (Table III & IV). Therefore, despite the influence of hypertension and several between-group differences in atherosclerotic risk factors that reached statistical significance (Table I), adjusting for these factors demonstrated that AIX remained significantly and independently associated with PAD.
DISCUSSION
In one of the largest studies in a PAD population, the present study found that central AIX normalized to 75bpm and peripheral AIX are associated with PAD, independent of other atherosclerotic risk factors, in a cohort of vascular surgery clinic patients. These results on arterial stiffness and AIX in PAD supports findings of prior research. Khaleghi et al. demonstrated in 475 participants, including 20 with PAD, that AIX was inversely correlated with ABI.24 A supporting study in 65 PAD patients by Mosimann et al. also found a negative correlation between AIX and ABI.25 The present study did not examine the relationship between ABI and AIX since several PAD patients who had undergone revascularization demonstrated improved ABI prior to tonometry assessment. Catalano et al. found that central AIX was higher in 97 patients with PAD, compared to controls.8 However, central AIX was not normalized to 75bpm as in the present study. Paapstel et al. found that a group of 38 PAD patients had a higher augmentation index relative to CAD patients and healthy controls, in an unadjusted analysis.26 Zagura et al. also saw a difference in PWV and augmentation index for PAD versus controls in an unadjusted analysis.27 Tsuchikura et al. found that 83 PAD patients had higher PWV than controls with no atherosclerotic disease, even when controlling for potential confounders, but they did not assess the augmentation index.28 In a prospective cohort of 145 PAD patients, Pradeepa et al. found an association between augmentation index and PAD, but this association was lost when adjusting for age.29 Finally, Beckmann et al. demonstrated a higher central AIX in abdominal aortic aneurysm and PAD patients compared to healthy controls, but their control group was significantly younger and healthier than controls in the present study, which also included patients with aneurysmal disease.17 Overall, the present study adds to the literature by demonstrating in one of the largest samples an independent association between AIX and PAD. This was robust enough to maintain significance despite comparison to controls with high prevalence of atherosclerotic risk factors.
With new research emphasizing the importance of studying arterial stiffness in vascular disease, the pathophysiological mechanisms underlying the development of arterial stiffness are becoming clearer. Stiffness is related to many CHD risk factors including age, diabetes, smoking, renal function, and lack of exercise.15, 30, 31 The arterial stiffness observed in PAD appears to be the result of both intrinsic changes in the quality of the arterial wall, as well as its thickness, though the intrinsic properties have a proportionally larger effect.32 Therefore, arterial stiffness is the result of more than hypertension and arterial thickness. Collagen and elastin contribute to the elastic properties of the arterial wall. Abnormalities in the matrix metallopeptidase 9 (MMP-9) and transforming growth factor β1 (TGF- β1) pathway, which controls collagen turnover, contributes to arterial stiffness.19 Stiffness has also been associated with inflammatory conditions and markers of such conditions, such as CRP.16 Inflammatory pathways are proposed to increase stiffness through several mechanisms including decreased nitric oxide production, elastin and collagen degradation, increased smooth muscle cell migration to the intima, and swelling of the extra-cellular matrix (ECM).33 Additionally, activation of the renin-angiotensin-aldosterone system (RAAS) along with physical forces mediate the association between hypertension and the development of arterial stiffness.15 However, the results of the present study demonstrate that hypertension cannot explain the entire association between arterial stiffness, as measured by AIX, and PAD. Finally, many of the biomarkers associated with stiffness are also associated with PAD, such as CRP, osteoprotegrin, and MMP-9.27, 34, 35
Non-invasive measures of arterial stiffness continue to show promise as clinical tools for risk stratification, but while these measures have shown usefulness in predicting CHD and CHD events,36 there is less evidence in the PAD population.11 Carotid-femoral PWV increases with higher Framingham cardiac risk scores37 and AIX is correlated with lower walking distance,38 which might make AIX a more accurate predictor of functional performance than ABI.14 Brand et al. (2013) found that arterial tonometry measures were associated with critical limb ischemia,39 the most advanced manifestation of PAD. Additionally, since arterial stiffness is strongly linked with the pathophysiological development of PAD, targeting stiffness for treatment and using tonometry to measure the treatment response could be of value. Measures of arterial stiffness have been shown to improve with reduction of traditional risk factors, increased physical activity, smoking cessation, and weight loss.9, 40 Anti-hypertensives have been shown to reduce PWV,41 anti-inflammatory agents reduce PWV and AIX,33 and revascularization leads to a modest reduction in AIX.42
Future research should examine the relationship between arterial stiffness and several inflammatory mediators as well as markers of resolution of inflammation, to determine the potential pathophysiological role of these pathways in the development of arterial stiffness and PAD. Additionally, prospective observational studies should be conducted in the PAD population to establish whether arterial tonometry can be used clinically for risk stratification as well as to measure the response of arterial stiffness to treatment. An examination of the association between AIX and ABI, comorbid disease burden, and functional performance would help to determine whether AIX adds to currently used methods for risk prediction and assessing treatment response. Lastly, while AIX is generally accepted as an indirect measure of arterial stiffness,43 some studies have found it correlates imperfectly with PWV,44 the gold-standard measure. Future studies should more carefully examine AIX as a marker of stiffness in PAD because of its relative ease of use compared to PWV, which may make it a better candidate for widespread clinical implementation.
Limitations
A major limitation of the present study is the use of a cross-sectional sample, which can only establish an association between arterial stiffness and PAD. Males also made up an overwhelming majority of the sample, so assessment of sex differences in arterial stiffness, which have been previously documented,15 could not be performed. Analysis of measures of exercise or walking distance were also not included, causing another possible limitation since exercise may confound or mediate the relationship between arterial stiffness and PAD. Additionally, the inclusion of PAD patients with prior revascularization excluded examination of the relationship between AIX and ABI. Furthermore, while this study went beyond much of the previous literature in screening the tonometry data for only the highest quality data, 68% of PWV data was dropped due to insufficient quality. However, this may be a limitation of the technique itself. Compared to PWA, PWV may require a greater learning curve. In a PAD population with aorto-iliac disease, carotid-femoral PWV could be subject to systematic error, whereas radial artery tonometry would be unaffected assuming there is no subclavian disease. In fact, PWV has been shown to be paradoxically decreased in advanced critical limb ischemia (CLI) due to severe aorto-iliac disease, whereas the central AIX remains elevated.39
CONCLUSION
The present study provides further evidence in support of studies that have demonstrated an independent association between augmentation index, as measured by radial artery tonometry, and PAD, expanding understanding of the physiological properties of vessels in a disease state. Additionally, the association was robust enough to maintain significance despite comparison to a control group with significant atherosclerotic risk factors as well as in multivariable models that controlled for these risk factors. Future studies are needed to examine the potential role of radial artery tonometry in the early identification of PAD and to stratify the risk of patients with known disease. One potential method for further characterizing the importance of AIX as a non-invasive measure for people with PAD is through comparison with other established measures such as flow-mediated dilation (FMD).
Acknowledgments
Disclosures: This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR001871 with additional student research support from the Society for Vascular Surgery Student Research Fellowship Award and the American Heart Association Student Scholarship (GZ). Furthermore, this work was supported by start-up funds from the University of California San Francisco and the Northern California Institute for Research and Education, by Award Number KL2RR024130 from the National Center for Research Resources, Award Number 1K23HL122446-01 from the National Institute of Health/NHLBI, and a Society for Vascular Surgery Seed Grant and Career Development Award (MG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funding organizations were not involved in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or preparation, review or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author conflict of interest: none.
Note to the editor: Abstract was presented at the Association for Clinical and Translational Science’s Translational Science 2017 conference in Washington DC, April 19th–21st, 2017.
References
- 1.Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. The Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642. doi: 10.1161/CIRCOUTCOMES.109.930735. [DOI] [PubMed] [Google Scholar]
- 3.Grenon SM, Vittinghoff E, Owens CD, Conte MS, Whooley M, Cohen BE. Peripheral artery disease and risk of cardiovascular events in patients with coronary artery disease: Insights from the Heart and Soul Study. Vascular Medicine. 2013;18:176–184. doi: 10.1177/1358863X13493825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson UKA, Fowkes FGR, McDermott MM, Criqui MH, Aboyans V, Norman PE, et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Global Heart. 2014;9:158.e21. doi: 10.1016/j.gheart.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vascular Medicine. 2008;13:15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 6.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 7.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease. Circulation. 2006;114:688. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 8.Catalano M, Scandale G, Carzaniga G, Cinquini M, Minola M, Antoniazzi V, et al. Aortic augmentation index in patients with peripheral arterial disease. The Journal of Clinical Hypertension. 2014;16:782–787. doi: 10.1111/jch.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kals J, Lieberg J, Kampus P, Zagura M, Eha J, Zilmer M. Prognostic impact of arterial stiffness in patients with symptomatic peripheral arterial disease. European Journal of Vascular and Endovascular Surgery. 2014;48:308–315. doi: 10.1016/j.ejvs.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husmann M, Jacomella V, Thalhammer C, Amann-Vesti B. Markers of arterial stiffness in peripheral arterial disease. Vasa. 2015;44:341–348. doi: 10.1024/0301-1526/a000452. [DOI] [PubMed] [Google Scholar]
- 12.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell GF. Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk. Artery Research. 2009;3:56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutinho T, Rooke TW, Kullo IJ. Arterial dysfunction and functional performance in patients with peripheral artery disease: A review. Vascular Medicine. 2011;16:203–211. doi: 10.1177/1358863X11400935. [DOI] [PubMed] [Google Scholar]
- 15.Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. American Journal of Hypertension. 2002;15:1101–1108. doi: 10.1016/s0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 16.Duprez DA. Is vascular stiffness a target for therapy? Cardiovascular Drugs and Therapy. 2010;24:305–310. doi: 10.1007/s10557-010-6250-z. [DOI] [PubMed] [Google Scholar]
- 17.Beckmann M, Jacomella V, Kohler M, Lachat M, Salem A, Amann-Vesti B, et al. Risk stratification of patients with peripheral arterial disease and abdominal aortic aneurysm using aortic augmentation index. PLoS ONE. 2015;10:0139887. doi: 10.1371/journal.pone.0139887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brant LCC, Hamburg NM, Barreto SM, Benjamin EJ, Ribeiro ALP. Relations of digital vascular function, cardiovascular risk factors, and arterial stiffness: The Brazilian Longitudinal Study of Adult Health Cohort Study. J Am Heart Assoc. 2014;3(6):001279. doi: 10.1161/JAHA.114.001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikonomidis I, Makavos G, Lekakis J. Arterial stiffness and coronary artery disease. Current opinion in cardiology. 2015;30 doi: 10.1097/HCO.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 20.Grenon SM, Gagnon J, Hsiang Y. Ankle-brachial index for assessment of peripheral arterial disease. N Engl J Med. 2009;361:40. doi: 10.1056/NEJMvcm0807012. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL) Behaviour Research and Therapy. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C-H, Nevo E, Fetics B, Pak PH, Yin FCP, Maughan WL, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 24.Khaleghi M, Kullo IJ. Aortic augmentation index is associated with the ankle-brachial index: A community-based study. Atherosclerosis. 2007;195:248–253. doi: 10.1016/j.atherosclerosis.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosimann K, Jacomella V, Thalhammer C, Meier TO, Kohler M, Amann-Vesti B, et al. Severity of peripheral arterial disease is associated with aortic pressure augmentation and subendocardial viability ratio. The Journal of Clinical Hypertension. 2012;14:855–860. doi: 10.1111/j.1751-7176.2012.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paapstel K, Zilmer M, Eha J, Tootsi K, Piir A, Kals J. Association between fibulin-1 and aortic augmentation index in male patients with peripheral arterial disease. European Journal of Vascular and Endovascular Surgery. 2016;51:76–82. doi: 10.1016/j.ejvs.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Zagura M, Serg M, Kampus P, Zilmer M, Zilmer K, Eha J, et al. Association of osteoprotegerin with aortic stiffness in patients with symptomatic peripheral artery disease and in healthy subjects. American Journal of Hypertension. 2010;23:586–591. doi: 10.1038/ajh.2010.38. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchikura S, Shoji T, Kimoto E, Shinohara K, Hatsuda S, Koyama H, et al. Central versus peripheral arterial stiffness in association with coronary, cerebral and peripheral arterial disease. Atherosclerosis. 2010;211:480–485. doi: 10.1016/j.atherosclerosis.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 29.Pradeepa R, Chella S, Surendar J, Indulekha K, Anjana RM, Mohan V. Prevalence of peripheral vascular disease and its association with carotid intima-media thickness and arterial stiffness in type 2 diabetes: The Chennai Urban Rural Epidemiology Study (CURES 111) Diabetes and Vascular Disease Research. 2014;11:190–200. doi: 10.1177/1479164114524584. [DOI] [PubMed] [Google Scholar]
- 30.Strasser B, Arvandi M, Pasha EP, Haley AP, Stanforth P, Tanaka H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutrition, Metabolism and Cardiovascular Diseases. 2015;25:495–502. doi: 10.1016/j.numecd.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Sedaghat S, Dawkins Arce FG, Verwoert GC, Hofman A, Ikram MA, Franco OH, et al. Association of renal function with vascular stiffness in older adults: the Rotterdam study. Age and Ageing. 2014;43:827–833. doi: 10.1093/ageing/afu111. [DOI] [PubMed] [Google Scholar]
- 32.Claridge MW, Bate GR, Hoskins PR, Adam DJ, Bradbury AW, Wilmink AB. Measurement of arterial stiffness in subjects with vascular disease: Are vessel wall changes more sensitive than increase in intima-media thickness? Atherosclerosis. 2009;205:477–480. doi: 10.1016/j.atherosclerosis.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA. Inflammation and arterial stiffness in humans. Atherosclerosis. 2014;237:381–390. doi: 10.1016/j.atherosclerosis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of c-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 35.Berger JS, Ballantyne CM, Davidson MH, Johnson JL, Tarka EA, Lawrence D, et al. Peripheral artery disease, biomarkers, and darapladib. American Heart Journal. 2011;161:972–978. doi: 10.1016/j.ahj.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacroix V, Willemet M, Verhelst R, Beauloye C, Jacquet L, Astarci P, et al. Central and peripheral pulse wave velocities are associated with ankle-brachial pressure index. Artery Research. 2012;6:28–33. [Google Scholar]
- 38.Brewer LC, Chai H-S, Bailey KR, Kullo IJ. Measures of arterial stiffness and wave reflection are associated with walking distance in patients with peripheral arterial disease. Atherosclerosis. 2007;191:384–390. doi: 10.1016/j.atherosclerosis.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 39.Brand M, Woodiwiss AJ, Michel F, Booysen HL, Veller MG, Norton GR. A Mismatch between aortic pulse pressure and pulse wave velocity predicts advanced peripheral arterial disease. European Journal of Vascular and Endovascular Surgery. 2013;46:338–346. doi: 10.1016/j.ejvs.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Endes S, Schaffner E, Caviezel S, Dratva J, Autenrieth CS, Wanner M, et al. Physical activity is associated with lower arterial stiffness in older adults: results of the SAPALDIA 3 Cohort Study. European Journal of Epidemiology. 2016;31:275–285. doi: 10.1007/s10654-015-0076-8. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Keith JC, Jr, Struthers AD, Feuerstein GZ. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovascular Therapeutics. 2008;26:214–223. doi: 10.1111/j.1755-5922.2008.00051.x. [DOI] [PubMed] [Google Scholar]
- 42.Jacomella V, Shenoy A, Mosimann K, Kohler MK, Amann-Vesti B, Husmann M. The impact of endovascular lower-limb revascularisation on the aortic augmentation index in patients with peripheral arterial disease. European Journal of Vascular and Endovascular Surgery. 2013;45:497–501. doi: 10.1016/j.ejvs.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Stoner L, Young JM, Fryer S. Assessments of arterial stiffness and endothelial function using pulse wave analysis. International Journal of Vascular Medicine. 2012;2012:903107. doi: 10.1155/2012/903107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai M, Yamakado T, Kurachi H, Kato T, Kuroda K, Ishisu R, et al. The relationship between aortic augmentation index and pulse wave velocity: an invasive study. Journal of Hypertension. 2007;25 doi: 10.1097/HJH.0b013e3280115b7c. [DOI] [PubMed] [Google Scholar]